Green Synthesis of Magnetite-Based Catalysts for Solar-Assisted Catalytic Wet Peroxide Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of MBCs
2.2. Characterization of MBCs
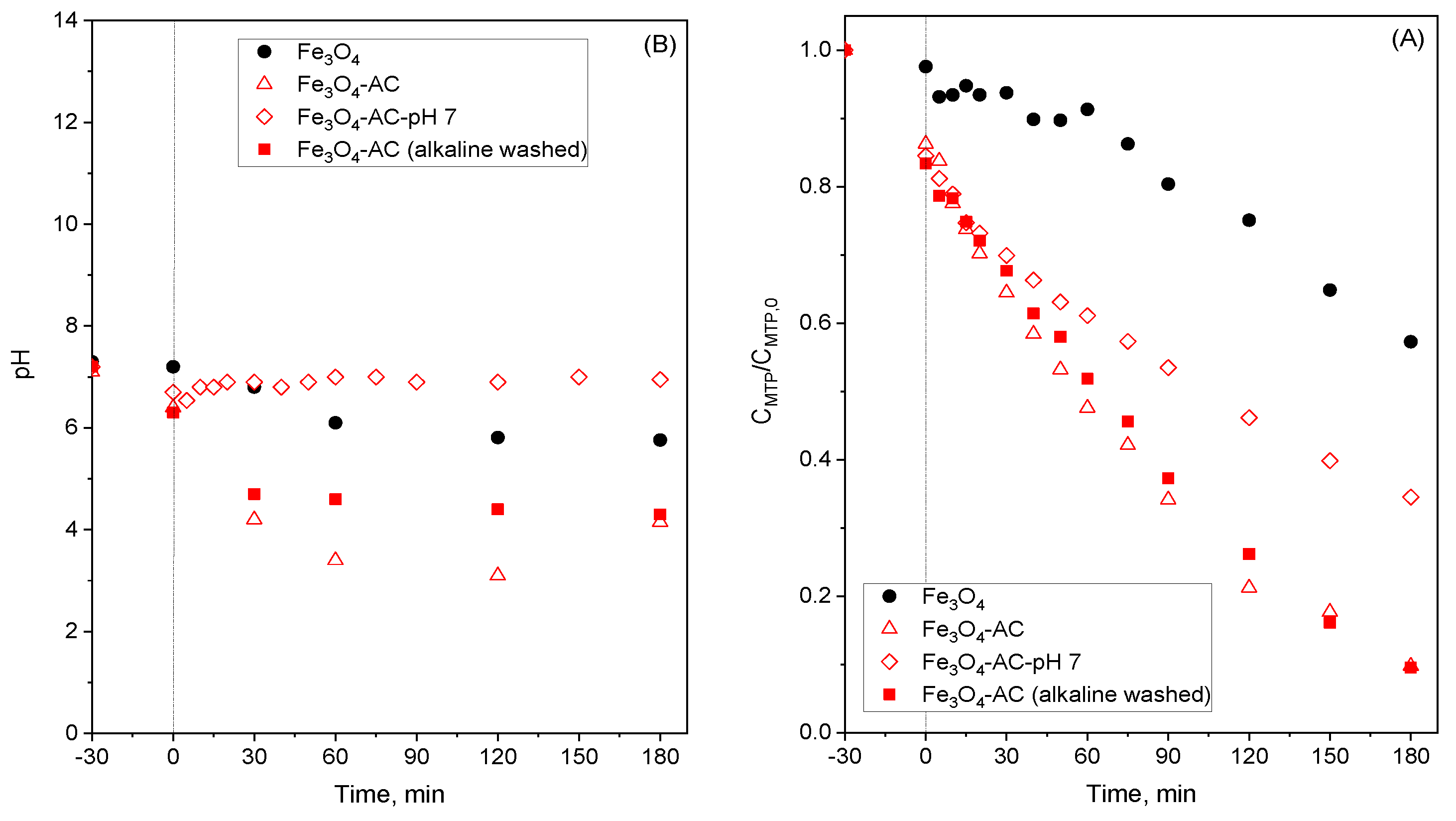
2.3. Stability of MBCs in Water
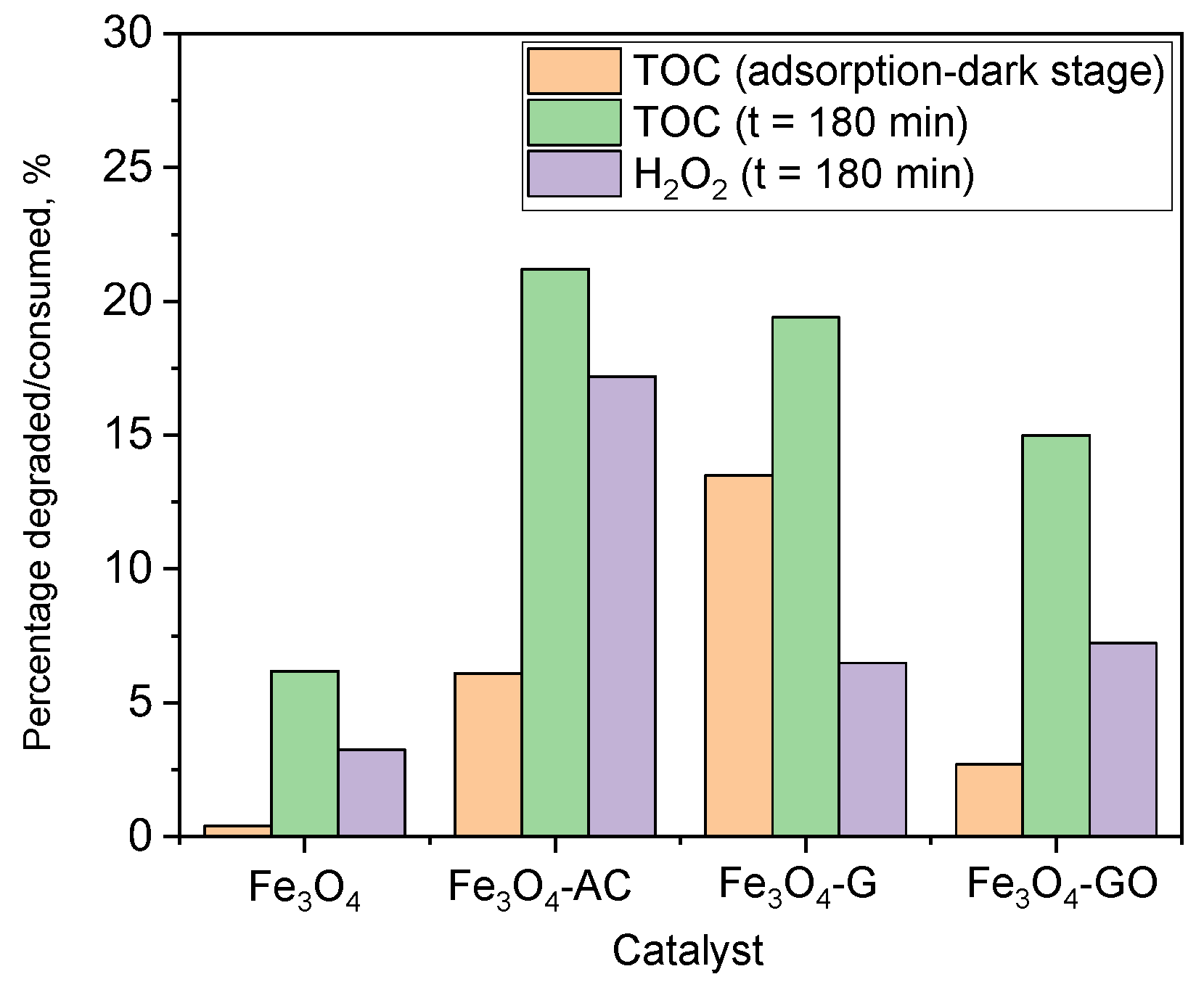
2.4. Catalytic Activity of MBCs in CWPO Processes
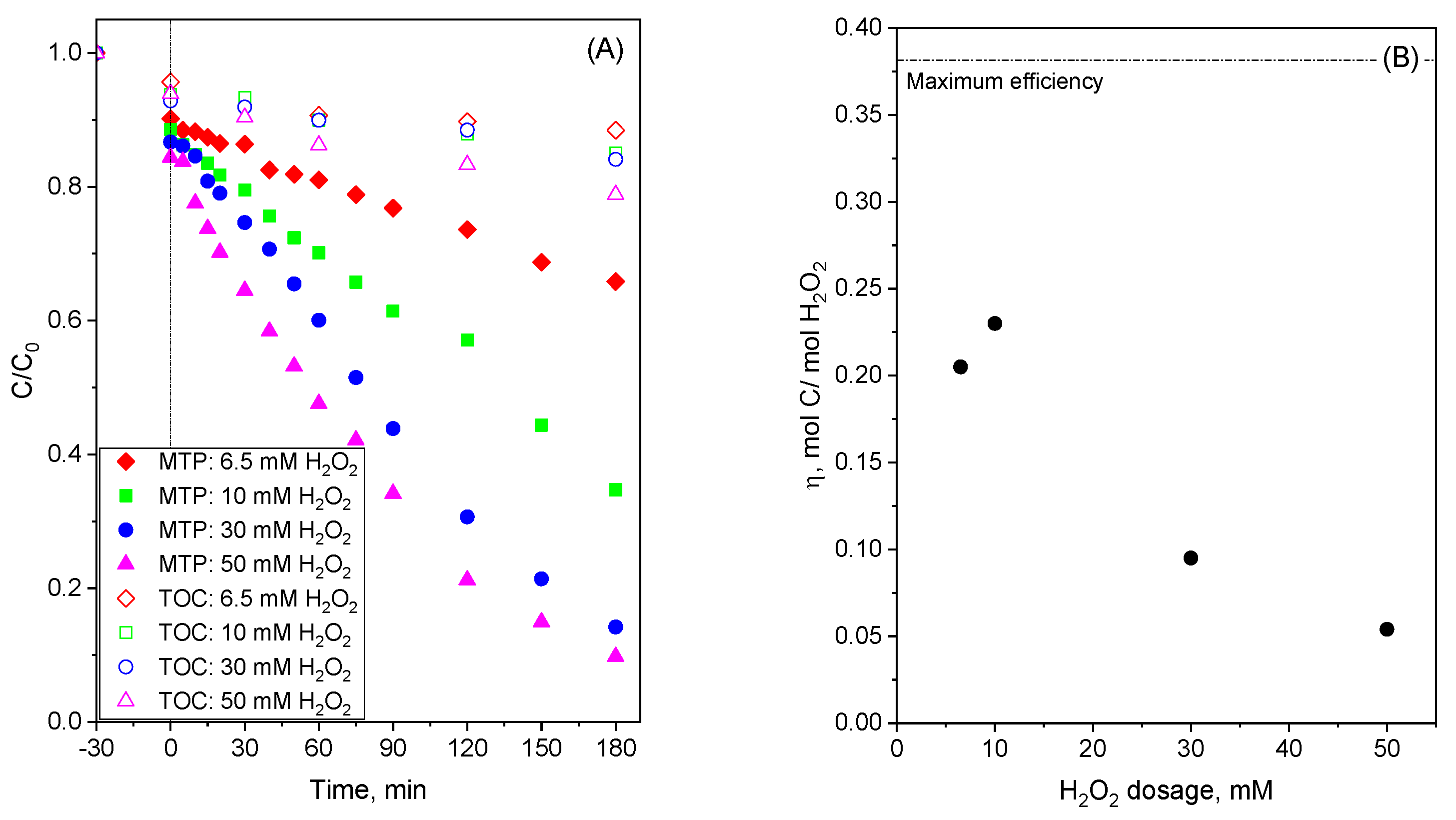
2.5. Improved Use of Fe3O4-AC Catalyst
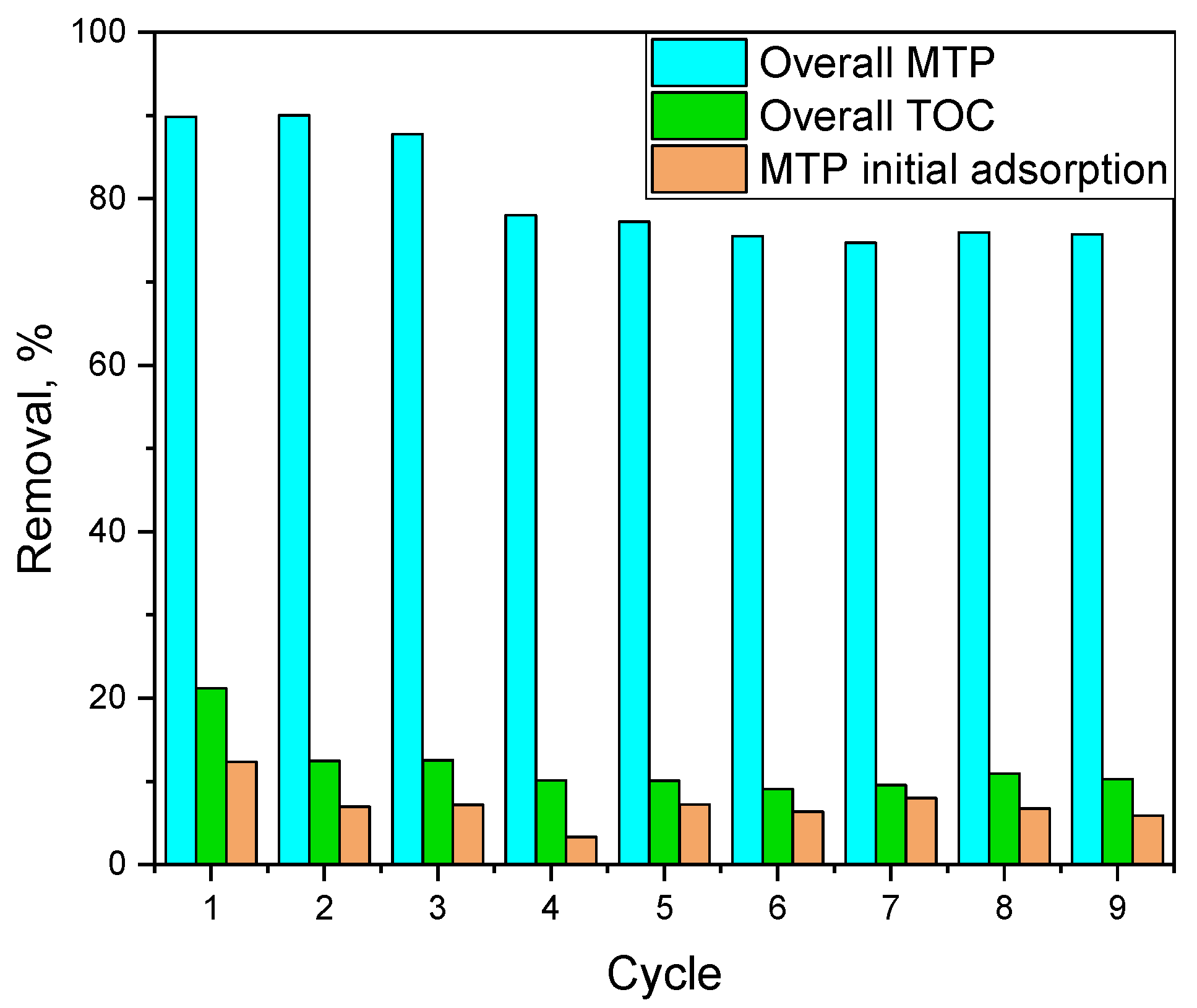
2.6. Reuse of Fe3O4-AC Catalyst
3. Materials and Methods
3.1. Materials
3.2. Synthesis of MBCs
3.3. Characterization of Catalysts
3.4. Stability Tests
3.5. Catalytic Activity Tests
3.6. Analytical Methods for Reaction Monitoring
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; De Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.K.; Gupta, M. Synthesis and Surface Engineering of Iron Oxide Nanoparticles for Biomedical Applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Cotin, G.; Piant, S.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Iron Oxide Nanoparticles for Biomedical Applications: Synthesis, Functionalization, and Application; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Nasir Baig, R.B.; Verma, S.; Nadagoud, M.N.; Varma, R.S. Advancing Sustainable Catalysis with Magnetite. Surface Modification and Synthetic Applications. Aldrichimica Acta 2016, 49, 35–41. [Google Scholar]
- Masudi, A.; Harimisa, G.E.; Ghafar, N.A.; Jusoh, N.W.C. Magnetite-Based Catalysts for Wastewater Treatment. Environ. Sci. Pollut. Res. 2020, 27, 4664–4682. [Google Scholar] [CrossRef]
- Muñoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodríguez, J.J. Preparation of Magnetite-Based Catalysts and Their Application in Heterogeneous Fenton Oxidation-A Review. Appl. Catal. B Environ. 2015, 176–177, 249–265. [Google Scholar] [CrossRef] [Green Version]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton Catalysts: A Review of Recent Advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.S.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Catalytic Wet Peroxide Oxidation: A Route towards the Application of Hybrid Magnetic Carbon Nanocomposites for the Degradation of Organic Pollutants. A Review. Appl. Catal. B Environ. 2016, 187, 428–460. [Google Scholar] [CrossRef]
- Lai, L.; He, Y.; Zhou, H.; Huang, B.; Yao, G.; Lai, B. Critical Review of Natural Iron-Based Minerals Used as Heterogeneous Catalysts in Peroxide Activation Processes: Characteristics, Applications and Mechanisms. J. Hazard. Mater. 2021, 416, 125809. [Google Scholar] [CrossRef] [PubMed]
- Teja, A.S.; Koh, P.Y. Synthesis, Properties, and Applications of Magnetic Iron Oxide Nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Chen, X.Y.; Wang, B.N.; Shi, C.W. Hydrothermal Synthesis and Self-Assembly of Magnetite (Fe3O4) Nanoparticles with the Magnetic and Electrochemical Properties. J. Cryst. Growth 2008, 310, 5453–5457. [Google Scholar] [CrossRef]
- Nidheesh, P.V. Heterogeneous Fenton Catalysts for the Abatement of Organic Pollutants from Aqueous Solution: A Review. RSC Adv. 2015, 5, 40552–40577. [Google Scholar] [CrossRef]
- Peng, S.; Wang, C.; Xie, J.; Sun, S. Synthesis and Stabilization of Monodisperse Fe Nanoparticles. J. Am. Chem. Soc. 2006, 128, 10676–10677. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Hu, Y.; Biasini, M.; Dong, C.; Guo, J.; Beyermann, W.P.; Yin, Y. One-Step Synthesis of Highly Water-Soluble Magnetite Colloidal Nanocrystals. Chem.-A Eur. J. 2007, 13, 7153–7161. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, H.; Bao, H.; Gao, M. One-Pot Reaction to Synthesize Water-Soluble Magnetite Nanocrystals. Chem. Mater. 2004, 16, 1391–1393. [Google Scholar] [CrossRef]
- Lu, X.; Niu, M.; Qiao, R.; Gao, M. Superdispersible PVP-Coated Fe3O4 Nanocrystals Prepared by a “One-Pot” Reaction. J. Phys. Chem. B 2008, 112, 14390–14394. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, Y.; Shuto, T.; Masahashi, N.; Tanabe, S. Preparation of Superparamagnetic Magnetite Nanoparticles by Reverse Precipitation Method: Contribution of Sonochemically Generated Oxidants. Ultrason. Sonochem. 2009, 16, 525–531. [Google Scholar] [CrossRef] [Green Version]
- Nedkov, I.; Merodiiska, T.; Slavov, L.; Vandenberghe, R.E.; Kusano, Y.; Takada, J. Surface Oxidation, Size and Shape of Nano-Sized Magnetite Obtained by Co-Precipitation. J. Magn. Magn. Mater. 2006, 300, 358–367. [Google Scholar] [CrossRef]
- Qu, S.; Yang, H.; Ren, D.; Kan, S.; Zou, G.; Li, D.; Li, M. Magnetite Nanoparticles Prepared by Precipitation from Partially Reduced Ferric Chloride Aqueous Solutions. J. Colloid Interface Sci. 1999, 215, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, T.; Toprak, M.S.; Baykal, A.; Kavas, H.; Köseoǧlu, Y.; Aktaş, B. Synthesis of Fe3O4 Nanoparticles at 100 °C and Its Magnetic Characterization. J. Alloys Compd. 2009, 472, 18–23. [Google Scholar] [CrossRef]
- Kobyliukh, A.; Olszowska, K.; Godzierz, M.; Kordyka, A.; Kubacki, J.; Mamunya, Y.; Pusz, S.; Stoycheva, I.; Szeluga, U. Effect of Graphene Material Structure and Iron Oxides Deposition Method on Morphology and Properties of Graphene/Iron Oxide Hybrids. Appl. Surf. Sci. 2022, 573, 151567. [Google Scholar] [CrossRef]
- Ioffe, M.; Long, M.; Radian, A. Systematic Evaluation of Activated Carbon-Fe3O4 Composites for Removing and Degrading Emerging Organic Pollutants. Environ. Res. 2021, 198, 111187. [Google Scholar] [CrossRef]
- Lopes, K.L.; de Oliveira, H.L.; Serpa, J.A.S.; Torres, J.A.; Nogueira, F.G.E.; de Freitas, V.A.A.; Borges, K.B.; Silva, M.C. Nanomagnets Based on Activated Carbon/Magnetite Nanocomposite for Determination of Endocrine Disruptors in Environmental Water Samples. Microchem. J. 2021, 168, 106366. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, S.; Ruíz, B.; Martínez-Blanco, D.; Sánchez-Arenillas, M.; Díez, M.A.; Marco, J.F.; Gorria, P.; Fuente, E. Towards Advanced Industrial Waste-Based Magnetic Activated Carbons with Tunable Chemical, Textural and Magnetic Properties. Appl. Surf. Sci. 2021, 551, 149407. [Google Scholar] [CrossRef]
- Stan, M.; Lung, I.; Soran, M.L.; Leostean, C.; Popa, A.; Stefan, M.; Lazar, M.D.; Opris, O.; Silipas, T.D.; Porav, A.S. Removal of Antibiotics from Aqueous Solutions by Green Synthesized Magnetite Nanoparticles with Selected Agro-Waste Extracts. Process Saf. Environ. Prot. 2017, 107, 357–372. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, W.N.; Biswas, P.; Fortner, J.D. Facile Aerosol Synthesis and Characterization of Ternary Crumpled Graphene-TiO2-Magnetite Nanocomposites for Advanced Water Treatment. ACS Appl. Mater. Interfaces 2014, 6, 11766–11774. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Kim, K.W.; Lee, S.W.; Kim, S.O.; Kim, J.S.; Lee, J.K. Synthesis and Characterization of TiO2-Coated Magnetite Clusters (NFe3O4@TiO2) as Anode Materials for Li-Ion Batteries. Curr. Appl. Phys. 2013, 13, 1923–1927. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Alemany, L.B.; Tour, J.M. Graphene Oxide. Origin of Acidity, Its Instability in Water, and a New Dynamic Structural Model. ACS Nano 2013, 7, 576–588. [Google Scholar] [CrossRef]
- Cabrera-Munguia, D.A.; León-Campos, M.I.; Claudio-Rizo, J.A.; Solís-Casados, D.A.; Flores-Guia, T.E.; Cano Salazar, L.F. Potential Biomedical Application of a New MOF Based on a Derived PET: Synthesis and Characterization. Bull. Mater. Sci. 2021, 44, 245. [Google Scholar] [CrossRef]
- Cosimbescu, L.; Merkel, D.R.; Darsell, J.; Petrossian, G. Simple but Tricky: Investigations of Terephthalic Acid Purity Obtained from Mixed PET Waste. Ind. Eng. Chem. Res. 2021, 60, 12792–12797. [Google Scholar] [CrossRef]
- Varaprasad, K.; Pariguana, M.; Raghavendra, G.M.; Jayaramudu, T.; Sadiku, E.R. Development of Biodegradable Metaloxide/Polymer Nanocomposite Films Based on Poly-ε-Caprolactone and Terephthalic Acid. Mater. Sci. Eng. C 2017, 70, 85–93. [Google Scholar] [CrossRef]
- Ball, G.L.; McLellan, C.J.; Bhat, V.S. Toxicological Review and Oral Risk Assessment of Terephthalic Acid (TPA) and Its Esters: A Category Approach. Crit. Rev. Toxicol. 2011, 42, 28–67. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, A.; Yanai, R.; Kuretani, K. Toxicity of Terephthalic Acid. Chem. Pharm. Bull. 1968, 16, 1655–1660. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.X.; Sun, S.L.; Zhang, Y.; Wu, B.; Zhang, Z.Y.; Liu, B.; Yang, L.Y.; Cheng, S.P. Toxicity of Purified Terephthalic Acid Manufacturing Wastewater on Reproductive System of Male Mice (Mus Musculus). J. Hazard. Mater. 2010, 176, 300–305. [Google Scholar] [CrossRef]
- Wang, D.; Yang, P.; Zhu, Y. Growth of Fe3O4 Nanoparticles with Tunable Sizes and Morphologies Using Organic Amine. Mater. Res. Bull. 2014, 49, 514–520. [Google Scholar] [CrossRef]
- Kumar, S.R.; Raja, M.M.; Mangalaraj, D.; Viswanathan, C.; Ponpandian, N. Surfactant Free Solvothermal Synthesis of Monodispersed 3D Hierarchical Fe3O4 Microspheres. Mater. Lett. 2013, 110, 98–101. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, R.; Liu, T.; Lv, H.; Zhou, L.; Zhang, X. One-Pot Synthesis of Grass-like Fe3O4 Nanostructures by a Novel Microemulsion-Assisted Solvothermal Method. Ceram. Int. 2014, 40 Pt A, 1059–1063. [Google Scholar] [CrossRef]
- Haw, C.Y.; Mohamed, F.; Chia, C.H.; Radiman, S.; Zakaria, S.; Huang, N.M.; Lim, H.N. Hydrothermal Synthesis of Magnetite Nanoparticles as MRI Contrast Agents. Ceram. Int. 2010, 4, 1417–1422. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the Surface Chemistry of Activated Carbons. Carbon N. Y. 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Likodimos, V.; Falaras, P.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. Role of Oxygen Functionalities on the Synthesis of Photocatalytically Active Graphene–TiO2 Composites. Appl. Catal. B Environ. 2014, 158–159, 329–340. [Google Scholar] [CrossRef]
- Ruíz-Baltazar, A.; Esparza, R.; Rosas, G.; Pérez, R. Effect of the Surfactant on the Growth and Oxidation of Iron Nanoparticles. J. Nanomater. 2015, 2015, 240948. [Google Scholar] [CrossRef]
- Pop, D.; Buzatu, R.; Moacă, E.A.; Watz, C.G.; Cîntă-Pînzaru, S.; Tudoran, L.B.; Nekvapil, F.; Avram, Ș.; Dehelean, C.A.; Crețu, M.O.; et al. Development and Characterization of Fe3O4@Carbon Nanoparticles and Their Biological Screening Related to Oral Administration. Materials 2021, 14, 3556. [Google Scholar] [CrossRef]
- Daou, T.J.; Grenèche, J.M.; Pourroy, G.; Buathong, S.; Derory, A.; Ulhaq-Bouillet, C.; Donnio, B.; Guillon, D.; Begin-Colin, S. Coupling Agent Effect on Magnetic Properties of Functionalized Magnetite-Based Nanoparticles. Chem. Mater. 2008, 20, 5869–5875. [Google Scholar] [CrossRef]
- Rizzuti, A.; Dassisti, M.; Mastrorilli, P.; Sportelli, M.C.; Cioffi, N.; Picca, R.A.; Agostinelli, E.; Varvaro, G.; Caliandro, R. Shape-Control by Microwave-Assisted Hydrothermal Method for the Synthesis of Magnetite Nanoparticles Using Organic Additives. J. Nanopart. Res. 2015, 17, 408. [Google Scholar] [CrossRef]
- Niemeyer, J.; Chen, Y.; Bollag, J.-M. Characterization of Humic Acids, Composts, and Peat by Diffuse Reflectance Fourier-Transform Infrared Spectroscopy. Soil Sci. Soc. Am. J. 1992, 56, 135. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; López-Ramón, M.V.; Carrasco-Marín, F. Changes in Surface Chemistry of Activated Carbons by Wet Oxidation. Carbon N. Y. 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Pakuła, M.; Świa̧tkowski, A.; Walczyk, M.; Biniak, S. Voltammetric and FT-IR Studies of Modified Activated Carbon Systems with Phenol, 4-Chlorophenol or 1,4-Benzoquinone Adsorbed from Aqueous Electrolyte Solutions. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 260, 145–155. [Google Scholar] [CrossRef]
- Komarneni, S.; Hu, W.; Noh, Y.D.; Van Orden, A.; Feng, S.; Wei, C.; Pang, H.; Gao, F.; Lu, Q.; Katsuki, H. Magnetite Syntheses from Room Temperature to 150 °C with and without Microwaves. Ceram. Int. 2012, 38, 2563–2568. [Google Scholar] [CrossRef]
- Mascolo, M.C.; Pei, Y.; Ring, T.A. Room Temperature Co-Precipitation Synthesis of Magnetite Nanoparticles in a Large Ph Window with Different Bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef] [Green Version]
- Ianos̗, R.; Păcurariu, C.; Mihoc, G. Magnetite/Carbon Nanocomposites Prepared by an Innovative Combustion Synthesis Technique—Excellent Adsorbent Materials. Ceram. Int. 2014, 40 Pt B, 13649–13657. [Google Scholar] [CrossRef]
- He, Y.T.; Traina, S.J. Transformation of Magnetite to Goethite under Alkaline pH Conditions. Clay Miner. 2007, 42, 13–19. [Google Scholar] [CrossRef]
- López, J.; Chávez, A.M.; Rey, A.; Álvarez, P.M. Insights into the Stability and Activity of MIL-53(Fe) in Solar Photocatalytic Oxidation Processes in Water. Catalysts 2021, 11, 448. [Google Scholar] [CrossRef]
- Sun, H.; Xie, G.; He, D.; Zhang, L. Ascorbic Acid Promoted Magnetite Fenton Degradation of Alachlor: Mechanistic Insights and Kinetic Modeling. Appl. Catal. B Environ. 2020, 267, 118383. [Google Scholar] [CrossRef]
- Carbajo, J.; Quintanilla, A.; Garcia-Costa, A.L.; González-Julián, J.; Belmonte, M.; Miranzo, P.; Osendi, M.I.; Casas, J.A. The Influence of the Catalyst on the CO Formation during Catalytic Wet Peroxide Oxidation Process. Catal. Today 2021, 361, 30–36. [Google Scholar] [CrossRef]
- Rey, A.; Quiñones, D.H.; Álvarez, P.M.; Beltrán, F.J.; Plucinski, P.K. Simulated Solar-Light Assisted Photocatalytic Ozonation of Metoprolol over Titania-Coated Magnetic Activated Carbon. Appl. Catal. B Environ. 2012, 111–112, 246–253. [Google Scholar] [CrossRef]
- Quiñones, D.H.; Rey, A.; Álvarez, P.M.; Beltrán, F.J.; Plucinski, P.K. Enhanced Activity and Reusability of TiO2 Loaded Magnetic Activated Carbon for Solar Photocatalytic Ozonation. Appl. Catal. B Environ. 2014, 144, 96–106. [Google Scholar] [CrossRef]
- Pliego, G.; Garcia-Muñoz, P.; Zazo, J.A.; Casas, J.A.; Rodriguez, J.J. Improving the Fenton Process by Visible LED Irradiation. Environ. Sci. Pollut. Res. 2016, 23, 23449–23455. [Google Scholar] [CrossRef]
- García-Muñoz, P.; Zussblatt, N.P.; Pliego, G.; Zazo, J.A.; Fresno, F.; Chmelka, B.F.; Casas, J.A. Evaluation of Photoassisted Treatments for Norfloxacin Removal in Water Using Mesoporous Fe2O3-TiO2 Materials. J. Environ. Manag. 2019, 238, 243–250. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S. Photo-Fenton Applied to the Removal of Pharmaceutical and Other Pollutants of Emerging Concern. Curr. Opin. Green Sustain. Chem. 2021, 29, 100458. [Google Scholar] [CrossRef]
- Domínguez, C.M.; Ocón, P.; Quintanilla, A.; Casas, J.A.; Rodriguez, J.J. Highly Efficient Application of Activated Carbon as Catalyst for Wet Peroxide Oxidation. Appl. Catal. B Environ. 2013, 140–141, 663–670. [Google Scholar] [CrossRef]
- Rey, A.; Hungria, A.B.; Duran-Valle, C.J.; Faraldos, M.; Bahamonde, A.; Casas, J.A.; Rodriguez, J.J. On the Optimization of Activated Carbon-Supported Iron Catalysts in Catalytic Wet Peroxide Oxidation Process. Appl. Catal. B Environ. 2016, 181, 249–259. [Google Scholar] [CrossRef]
- Rey, A.; Bahamonde, A.; Casas, J.A.; Rodríguez, J.J. Selectivity of Hydrogen Peroxide Decomposition towards Hydroxyl Radicals in Catalytic Wet Peroxide Oxidation (CWPO) over Fe/AC Catalysts. Water Sci. Technol. 2010, 61, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Maity, D.; Choo, S.G.; Yi, J.; Ding, J.; Xue, J.M. Synthesis of magnetite nanoparticles via a solvent-free thermal decomposition route. J. Magn. Magn. Mater. 2009, 321, 1256–1259. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; Mcintyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Chávez, A.M.; Rey, A.; Beltrán, F.J.; Álvarez, P.M. Solar Photo-Ozonation: A Novel Treatment Method for the Degradation of Water Pollutants. J. Hazard. Mater. 2016, 317, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, G.M. Colorimetric Determination of Hydrogen Peroxide. Ind. Eng. Chem.-Anal. Ed. 1943, 15, 327–328. [Google Scholar] [CrossRef]

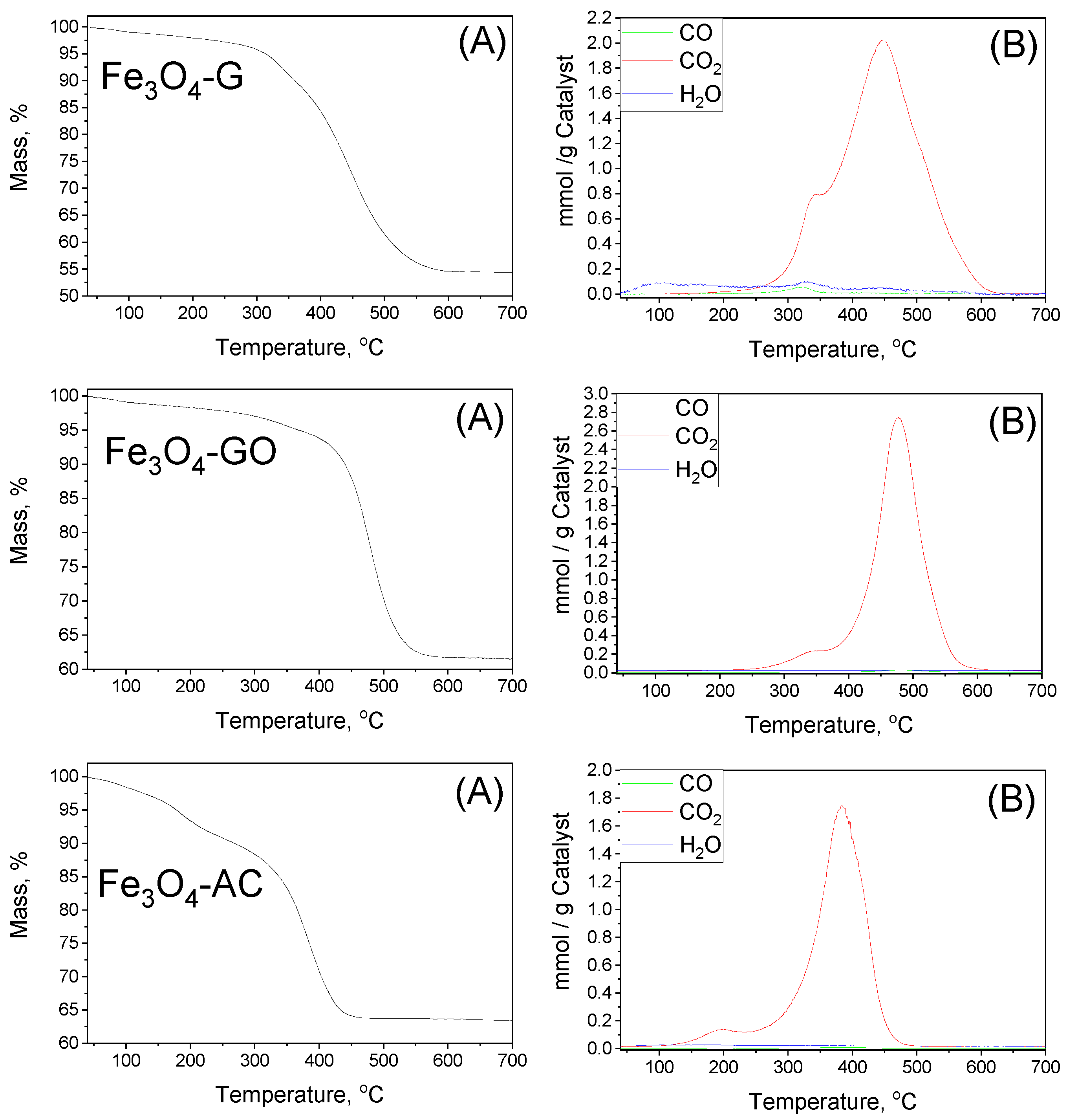

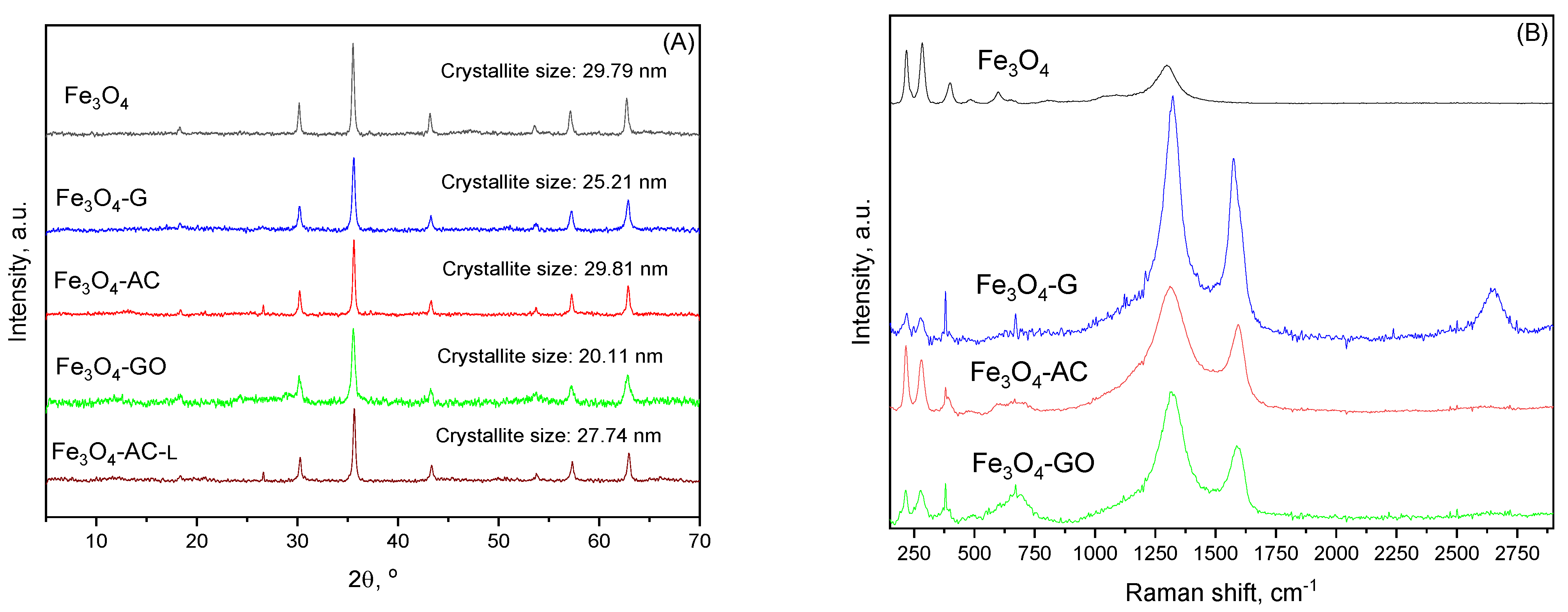

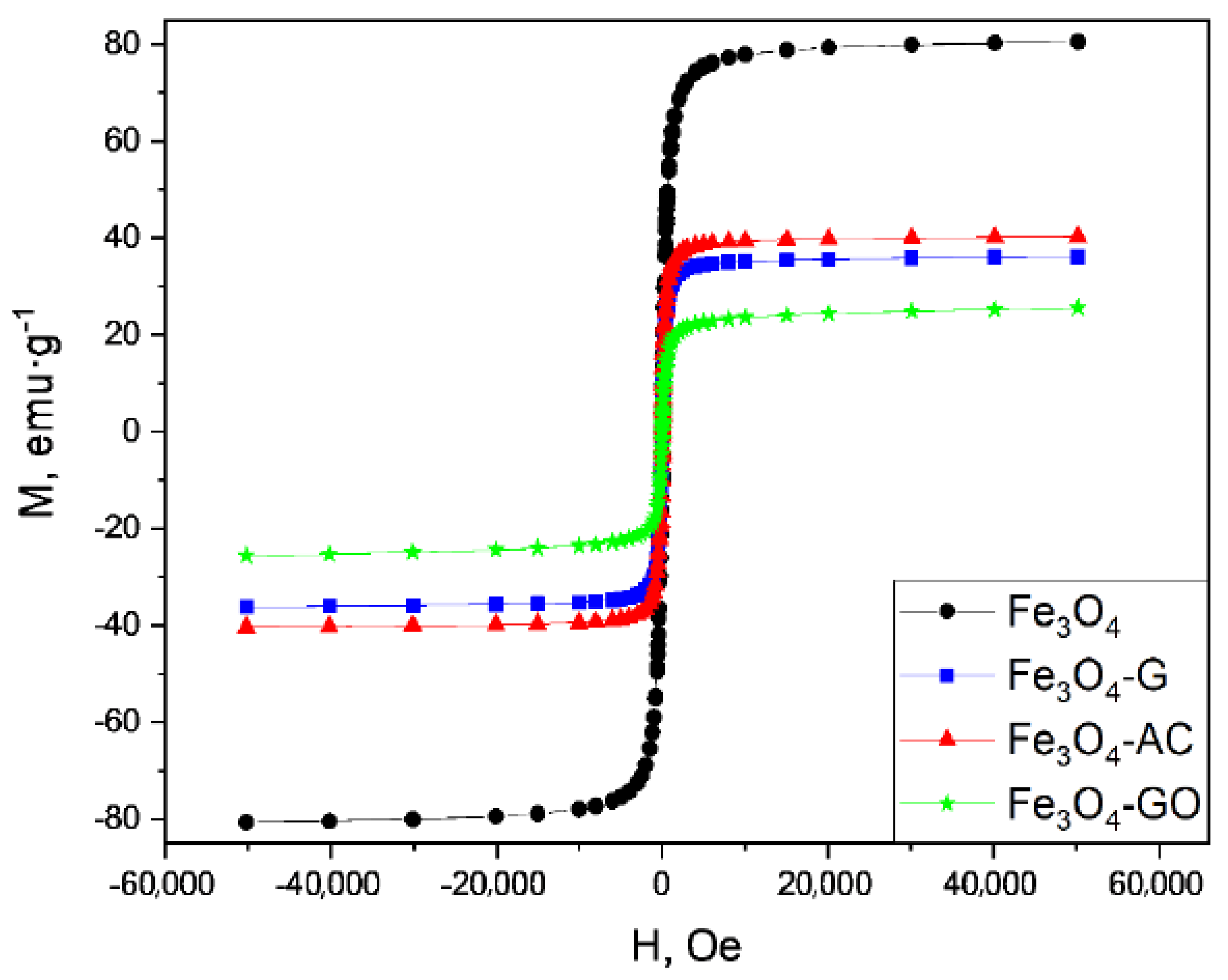
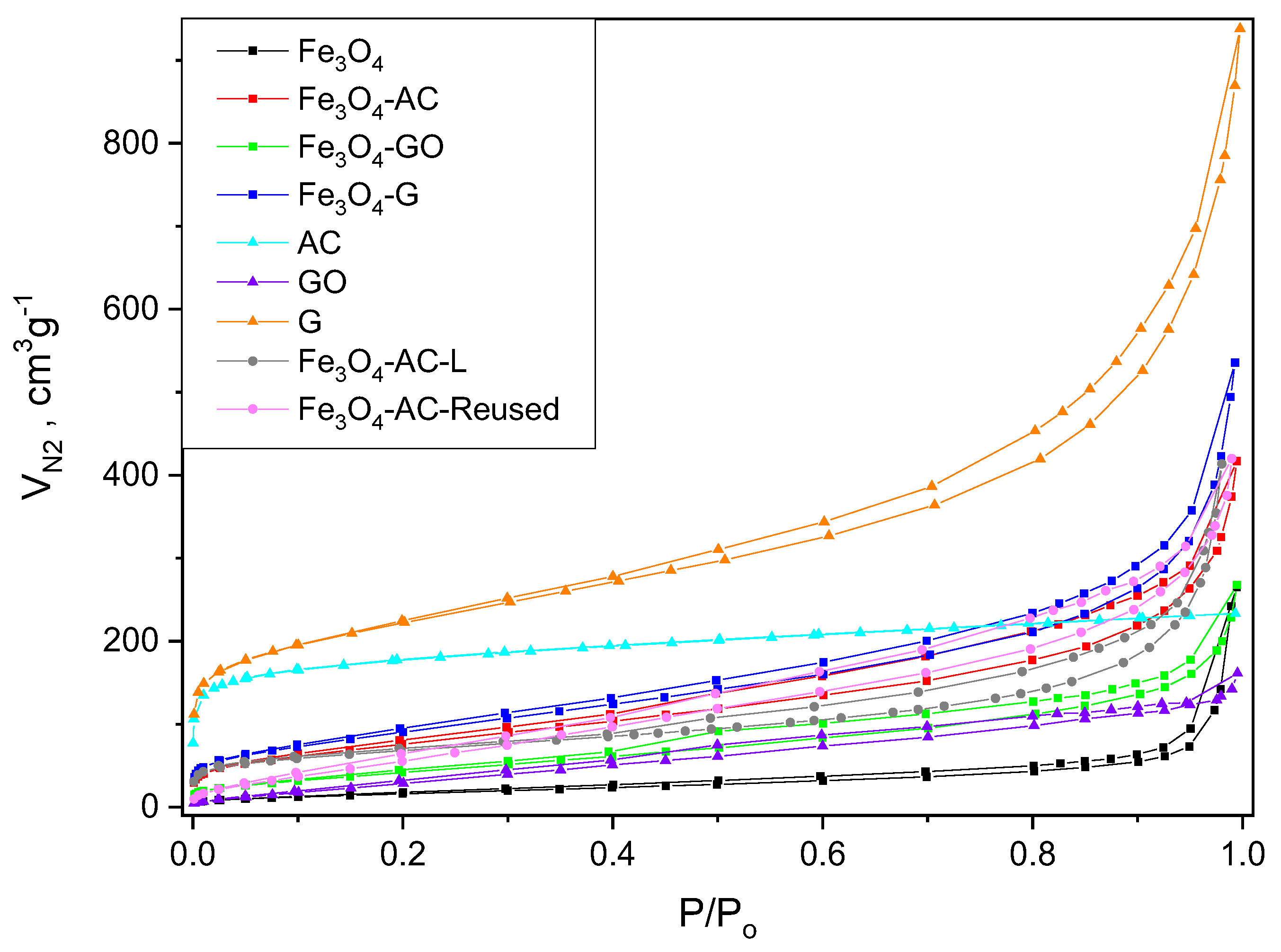



| MBC | Carbon Mass (g) | VNaOH (mL) | VTotal (mL) | H2BDC (g) | FeCl2·4H2O (g) | T (°C) | Yield (%) | (H2BDC)R (%) |
|---|---|---|---|---|---|---|---|---|
| Fe3O4 | - | 20 | 70 | 0.835 | 1.0 | 20 | 99.9 | 99 |
| Fe3O4-G | 0.3 | 20 | 70 | 0.835 | 1.0 | 20 | 99.5 | 98 |
| Fe3O4-GO | 0.3 | 20 | 70 | 0.835 | 1.0 | 20 | 97.1 | 97 |
| Fe3O4-AC | 0.3 | 20 | 70 | 0.835 | 1.0 | 20 | 99.9 | 96 |
| Fe3O4-ACR | 0.3 | 20 | 70 | 0.835 | 1.0 | 20 | 99.9 | 96 |
| Fe3O4-ACL | 6 | 400 | 1400 | 16.7 | 20 | 20 | 99.9 | 96 |
| Sample | C (wt.%) | H (wt.%) | N (wt.%) | O (wt.%) |
|---|---|---|---|---|
| H2BDC-t | 57.84 | 3.64 | 0.00 | 38.52 |
| H2BDC-R | 57.50 | 3.59 | 0.03 | 38.88 |
| Elemental Analysis | WDXRF | TGA-DTA-MS | ||||
|---|---|---|---|---|---|---|
| MBC | C (wt.%) | H (wt.%) | N (wt.%) | Fe (wt.%) | Fe3O4 (wt.%) | C (wt.%) |
| Fe3O4 | 0.73 | 0.00 | 0.00 | 72.36 | 100.00 | <0.5 |
| Fe3O4-G | 37.00 | 0.47 | 0.11 | 44.89 | 62.04 | 38.55 |
| Fe3O4-GO | 22.00 | 0.91 | 0.00 | 55.28 | 76.40 | 23.22 |
| Fe3O4-AC | 32.00 | 0.71 | 0.21 | 45.85 | 63.36 | 34.25 |
| Sample | SBET (m2g−1) | SEXT (m2g−1) | VMICRO (cm3g−1) | VTOTAL (cm3g−1) |
|---|---|---|---|---|
| G | 760 | 514 | 0.118 | 1.122 |
| GO | 142 | 122 | 0.024 | 0.220 |
| AC | 644 | 37 | 0.303 | 0.361 |
| Fe3O4 | 63 | 59 | 0.005 | 0.374 |
| Fe3O4-G | 337 | 271 | 0.048 | 0.764 |
| Fe3O4-GO | 164 | 131 | 0.036 | 0.353 |
| Fe3O4-AC | 282 | 213 | 0.052 | 0.578 |
| Fe3O4-AC-L | 240 | 181 | 0.029 | 0.641 |
| Fe3O4-AC-Reused | 255 | 255 | 0.001 | 0.801 |
| MBC | pH0 | pHf | H2BDC (mg L−1) | TOCH2BDC (mg L−1) | TOC (mg L−1) | (1) DiffTOC (mg L−1) | (2) Fe (mg L−1) |
|---|---|---|---|---|---|---|---|
| Fe3O4 | 4.00 | 4.61 | 0.93 | 0.54 | 0.92 | 0.38 | 0.18/0.12 |
| 6.00 | 6.13 | 1.04 | 0.60 | 0.58 | −0.02 | 0.37/0.28 | |
| 7.00 | 7.06 | 0.91 | 0.53 | 1.10 | 0.58 | 0.99/0.16 | |
| 8.00 | 7.96 | 0.98 | 0.57 | 0.81 | 0.24 | 1.34/0.26 | |
| 10.00 | 9.73 | 1.02 | 0.59 | 1.20 | 0.61 | 1.69/0.11 | |
| Fe3O4-G | 4.00 | 4.45 | 8.22 | 4.75 | 5.16 | 0.41 | 0.19/0.17 |
| 6.00 | 6.01 | 27.01 | 15.61 | 15.79 | 0.18 | 0.39/0.10 | |
| 7.00 | 6.93 | 31.53 | 18.22 | 18.36 | 0.14 | 0.42/0.16 | |
| 8.00 | 7.70 | 30.62 | 17.69 | 18.95 | 1.26 | >4.00/0.16 | |
| 10.00 | 9.57 | 31.74 | 18.34 | 19.85 | 1.51 | >4.00/0.17 | |
| Fe3O4-GO | 4.00 | 4.59 | 4.46 | 2.58 | 2.25 | −0.33 | >4.00/0.12 |
| 6.00 | 6.08 | 14.48 | 8.37 | 9.63 | 1.26 | >4.00/0.09 | |
| 7.00 | 6.90 | 19.60 | 11.32 | 13.93 | 2.61 | >4.00/0.10 | |
| 8.00 | 7.63 | 22.10 | 12.77 | 14.95 | 2.18 | >4.00/0.14 | |
| 10.00 | 8.43 | 20.77 | 12.00 | 16.18 | 4.18 | >4.00/0.11 | |
| Fe3O4-AC | 4.00 | 4.55 | 3.30 | 1.91 | 2.24 | 0.33 | 0.19/0.19 |
| 6.00 | 6.06 | 9.95 | 5.75 | 6.17 | 0.43 | 0.16/0.16 | |
| 7.00 | 7.02 | 13.25 | 7.66 | 8.17 | 0.51 | 0.28/0.17 | |
| 8.00 | 7.62 | 17.11 | 9.89 | 9.06 | −0.83 | 0.34/0.11 | |
| 10.00 | 8.65 | 19.48 | 11.25 | 10.61 | −0.64 | 0.43/0.11 |
| MBC | Test | Time (min) | H2BDC (mg L−1) | TOC (mg L−1) | pH |
|---|---|---|---|---|---|
| Fe3O4 | Rad+O2 | 0 | 0.23 | 0.12 | 7.38 |
| 180 | 0.29 | 0.52 | 7.35 | ||
| Rad+O2+H2O2 | 0 | 0.24 | 0.11 | 7.37 | |
| 180 | 0.00 | 0.55 | 7.30 | ||
| Fe3O4-G | Rad+O2 | 0 | 3.10 | 0.16 | 7.21 |
| 180 | 3.18 | 1.27 | 6.10 | ||
| Rad+O2+H2O2 | 0 | 3.14 | 0.17 | 7.20 | |
| 180 | 1.56 | 2.52 | 6.50 | ||
| Fe3O4-GO | Rad+O2 | 0 | 0.53 | 0.52 | 7.05 |
| 180 | 1.02 | 1.23 | 6.35 | ||
| Rad+O2+H2O2 | 0 | 0.60 | 0.54 | 7.03 | |
| 180 | 0.00 | 2.05 | 5.92 | ||
| Fe3O4-GO | Rad+O2 | 0 | 0.76 | 0.10 | 7.63 |
| 180 | 0.78 | 0.45 | 6.32 | ||
| Rad+O2+H2O2 | 0 | 1.27 | 0.11 | 7.65 | |
| 180 | 0.00 | 0.65 | 5.84 |
| Sample | Conditions | kapp-MTP (min−1) | R2 | η-TOC-H2O2 (mol C/mol H2O2) |
|---|---|---|---|---|
| Fe3O4 | 50 mM H2O2, pH0 7 | 2.5 × 10−3 | 0.956 | 0.074 |
| Fe3O4-G | 50 mM H2O2, pH0 7 | 6.0 × 10−3 | 0.997 | 0.129 |
| Fe3O4-GO | 50 mM H2O2, pH0 7 | 9.1 × 10−3 | 0.998 | 0.079 |
| Fe3O4-AC | 50 mM H2O2, pH0 7 | 9.6 × 10−3 | 0.997 | 0.054 |
| Fe3O4-AC | 50 mM H2O2, pH 7 (*) | 5.1 × 10−3 | 0.996 | 0.026 |
| Fe3O4-AC | 6.5 mM H2O2, pH0 7 | 1.7 × 10−3 | 0.998 | 0.205 |
| Fe3O4-AC | 10 mM H2O2, pH0 7 | 4.1 × 10−3 | 0.991 | 0.230 |
| Fe3O4-AC | 30 mM H2O2, pH0 7 | 7.4 × 10−3 | 0.974 | 0.095 |
| MBC | CAcetic acid (mg L−1) | CFormic acid (mg L−1) | COxalic acid (mg L−1) | Fe (mg L−1) | CH2BDC (mg L−1) |
|---|---|---|---|---|---|
| Fe3O4 | 0.44 | 2.85 | 0.86 | 0.12 | 0.23 |
| Fe3O4-G | 2.42 | 12.5 | 2.16 | 0.06 | 3.40 |
| Fe3O4-GO | 2.10 | 10.2 | 2.33 | 0.07 | 1.06 |
| Fe3O4-AC | 1.97 | 13.4 | 3.65 | 0.06 | 0.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, J.; Rey, A.; García-Araya, J.F.; Álvarez, P.M. Green Synthesis of Magnetite-Based Catalysts for Solar-Assisted Catalytic Wet Peroxide Oxidation. Catalysts 2022, 12, 271. https://doi.org/10.3390/catal12030271
López J, Rey A, García-Araya JF, Álvarez PM. Green Synthesis of Magnetite-Based Catalysts for Solar-Assisted Catalytic Wet Peroxide Oxidation. Catalysts. 2022; 12(3):271. https://doi.org/10.3390/catal12030271
Chicago/Turabian StyleLópez, Jorge, Ana Rey, Juan F. García-Araya, and Pedro M. Álvarez. 2022. "Green Synthesis of Magnetite-Based Catalysts for Solar-Assisted Catalytic Wet Peroxide Oxidation" Catalysts 12, no. 3: 271. https://doi.org/10.3390/catal12030271







