Catalytic Hydrodeoxygenation of Guaiacol to Cyclohexanol over Bimetallic NiMo-MOF-Derived Catalysts
Abstract
:1. Introduction
2. Result and Discussion
2.1. Catalyst Characterization
2.2. Catalytic Hydrodeoxygenation of Guaiacol
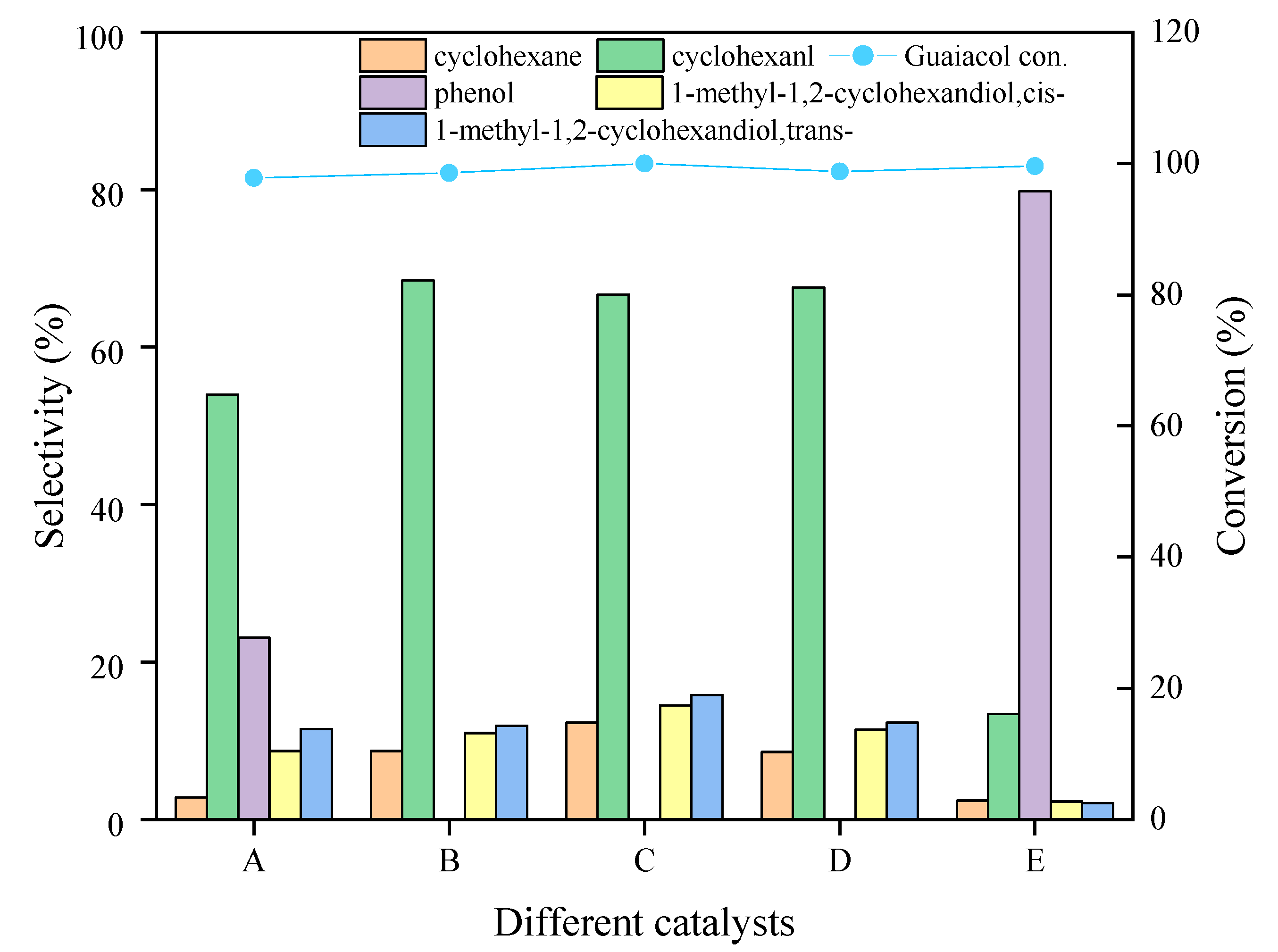
2.2.1. Effect of Metal Composition in Catalysts
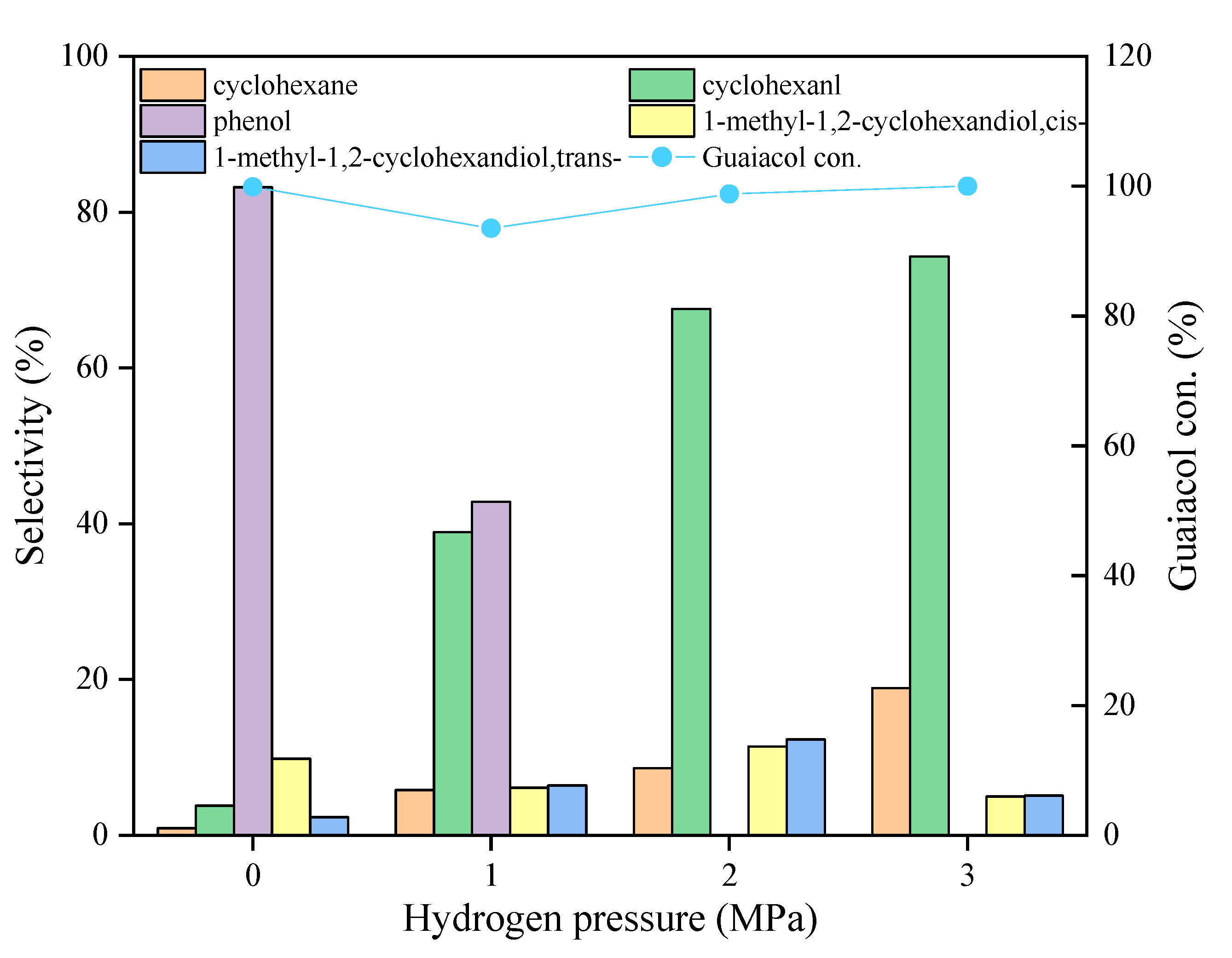
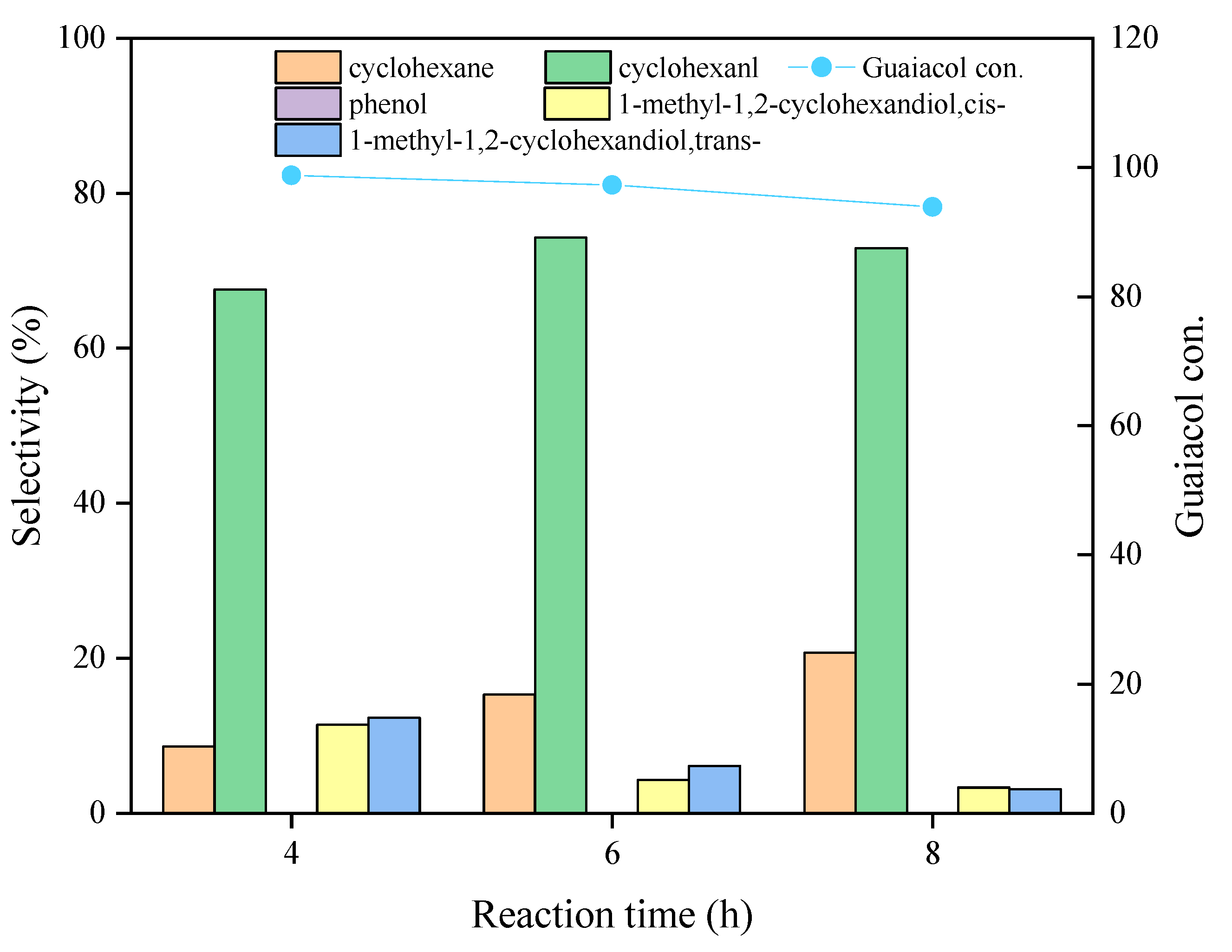
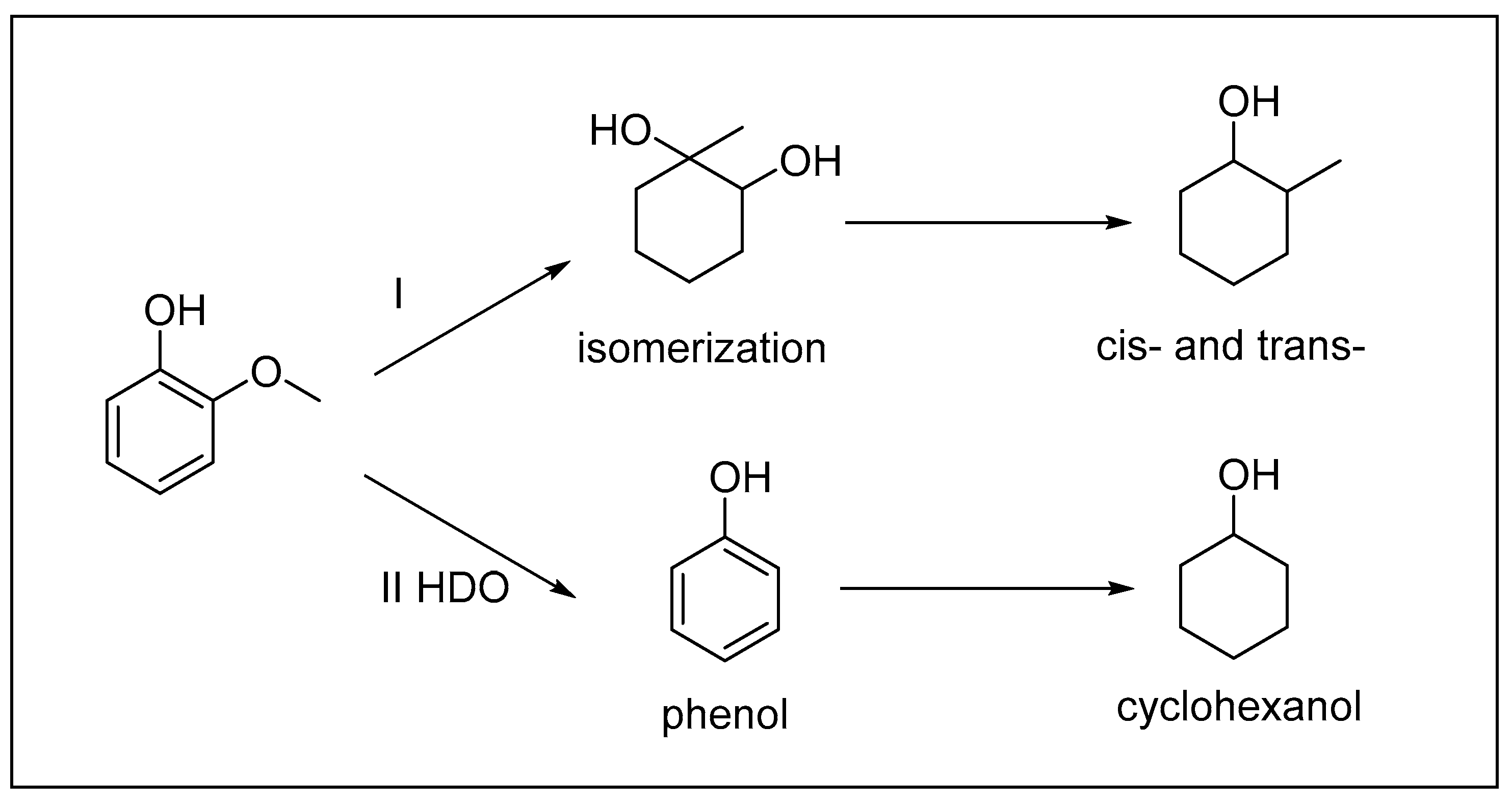
2.2.2. Product Distribution Studies over the Ni3Mo1@C Catalyst
3. Experimental
3.1. Chemicals and Materials
3.2. Synthesis of Catalysts. Ni@C Catalysts
3.3. Ni2Mo1@C, Ni3Mo1@C, Ni4Mo1@C, and Ni5Mo1@C Catalysts
3.4. Catalyst Characterization
3.5. Catalytical Hydrodeoxygenation of Guaiacol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Liu, P.; Xia, H.; Jiang, J.; Yang, X.; Zhou, M. Catalytic Conversion of Lignin to Liquid Fuels with an Improved H/Ceff Value over Bimetallic NiMo-MOF-Derived Catalysts. ACS Sustain. Chem. Eng. 2021, 9, 13937–13952. [Google Scholar] [CrossRef]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Bunrit, A.; Li, N.; Wang, F. Heteroatom-participated lignin cleavage to functionalized aromatics. Chem. Soc. Rev. 2020, 49, 3748–3763. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Zhou, M.H.; Ye, J.; Liu, P.; Xu, J.M.; Jiang, J.C. Water-Assisted Selective Hydrodeoxygenation of Guaiacol to Cyclohexanol over Supported Ni and Co Bimetallic Catalysts. ACS Sustain. Chem. Eng. 2017, 5, 8824–8835. [Google Scholar] [CrossRef]
- Zhai, Y.X.; Li, C.; Xu, G.Y.; Ma, Y.F.; Liu, X.H.; Zhang, Y. Depolymerization of lignin via a non-precious Ni-Fe alloy catalyst supported on activated carbon. Green Chem. 2017, 19, 1895–1903. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Ouyang, Y.; Xiao, G.; Zhao, Y.; Sarina, S.; Baeyens, J.; Su, H.; Zhu, H.-Y. Non-plasmonic Ni nanoparticles catalyzed visible light selective hydrogenolysis of aryl ethers in lignin under mild conditions. Green Chem. 2021, 23, 7780–7789. [Google Scholar] [CrossRef]
- Garedew, M.; Young-Farhat, D.; Bhatia, S.; Hao, P.C.; Jackson, J.E.; Saffron, C.M. Electrocatalytic cleavage of lignin model dimers using ruthenium supported on activated carbon cloth. Sustain. Energy Fuels 2020, 4, 1340–1350. [Google Scholar] [CrossRef]
- Zhou, M.; Tian, L.; Niu, L.; Li, C.; Xiao, G.; Xiao, R. Upgrading of liquid fuel from fast pyrolysis of biomass over modified Ni/CNT catalysts. Fuel Processing Technol. 2014, 126, 12–18. [Google Scholar] [CrossRef]
- Wang, S.Z.; Gao, W.; Xiao, L.P.; Shi, J.; Sun, R.C.; Song, G.Y. Hydrogenolysis of biorefinery corncob lignin into aromatic phenols over activated carbon-supported nickel. Sustain. Energy Fuels 2019, 3, 401–408. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Magallanes, G.; Karkas, M.D.; Bosque, I.; Lee, S.; Maldonado, S.; Stephenson, C.R.J. Selective C-O Bond Cleavage of Lignin Systems and Polymers Enabled by Sequential Palladium-Catalyzed Aerobic Oxidation and Visible-Light Photoredox Catalysis. ACS Catal. 2019, 9, 2252–2260. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Jensen, A.D. Screening of Catalysts for Hydrodeoxygenation of Phenol as a Model Compound for Bio-oil. ACS Catal. 2013, 3, 1774–1785. [Google Scholar] [CrossRef]
- Ly, H.V.; Kim, J.; Hwang, H.T.; Choi, J.H.; Woo, H.C.; Kim, S.-S. Catalytic Hydrodeoxygenation of Fast Pyrolysis Bio-Oil from Saccharina japonica Alga for Bio-Oil Upgrading. Catalysts 2019, 9, 1043. [Google Scholar]
- Ghadiryanfar, M.; Rosentrater, K.A.; Keyhani, A.; Omid, M. A review of macroalgae production, with potential applications in biofuels and bioenergy. Renew. Sustain. Energy Rev. 2016, 54, 473–481. [Google Scholar] [CrossRef]
- Zaheer, M.; Kempe, R. Catalytic Hydrogenolysis of Aryl Ethers: A Key Step in Lignin Valorization to Valuable Chemicals. ACS Catal. 2015, 5, 1675–1684. [Google Scholar] [CrossRef]
- Saidi, M.; Samimi, F.; Karimipourfard, D.; Nimmanwudipong, T.; Gates, B.C.; Rahimpour, M.R. Upgrading of lignin-derived bio-oils by catalytic hydrodeoxygenation. Energy Environ. Sci. 2014, 7, 103–129. [Google Scholar] [CrossRef]
- Nimmanwudipong, T.; Runnebaum, R.C.; Block, D.E.; Gates, B.C. Catalytic Conversion of Guaiacol Catalyzed by Platinum Supported on Alumina: Reaction Network Including Hydrodeoxygenation Reactions. Energy Fuels 2011, 25, 3417–3427. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, E.; Xu, X.; Sun, Y.; Tu, R. Hydrodeoxygenation of phenols, acids, and ketones as model bio-oil for hydrocarbon fuel over Ni-based catalysts modified by Al, La and Ga. Renew. Energy 2020, 146, 1991–2007. [Google Scholar] [CrossRef]
- Guan, W.; Chen, X.; Li, C.; Zhang, J.; Tsang, C.-W.; Hu, H.; Li, S.; Liang, C. Nb(Ta)-based solid acid modified Pt/CNTs catalysts for hydrodeoxygenation of lignin-derived compounds. Mol. Catal. 2019, 467, 61–69. [Google Scholar] [CrossRef]
- Dong, L.; Shao, Y.; Han, X.; Liu, X.; Xia, Q.; Parker, S.F.; Cheng, Y.; Daemen, L.L.; Ramirez-Cuesta, A.J.; Wang, Y.; et al. Comparison of two multifunctional catalysts [M/Nb2O5 (M = Pd, Pt)] for one-pot hydrodeoxygenation of lignin. Catal. Sci. Technol. 2018, 8, 6129–6136. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Qi, Z.; Li, X.; Ji, J.; Zhang, L.; Wang, H.; Liu, X.; Li, C. Cleavage of lignin C–O bonds over a heterogeneous rhenium catalyst through hydrogen transfer reactions. Green Chem. 2019, 21, 5556–5564. [Google Scholar] [CrossRef]
- Zhang, J.; Ibrahim, M.; Collière, V.; Asakura, H.; Tanaka, T.; Teramura, K.; Philippot, K.; Yan, N. Rh nanoparticles with NiOx surface decoration for selective hydrogenolysis of CO bond over arene hydrogenation. J. Mol. Catal. A Chem. 2016, 422, 188–197. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, C.; Liu, P.; Xia, H.; Li, J.; Sharma, B.K.; Jiang, J. Catalytic Hydrotreatment of β-O-4 Ether in Lignin: Cleavage of the C–O Bond and Hydrodeoxygenation of Lignin-Derived Phenols in One Pot. ACS Sustain. Chem. Eng. 2020, 8, 14511–14523. [Google Scholar] [CrossRef]
- Renom-Carrasco, M.; Lefort, L. Ligand libraries for high throughput screening of homogeneous catalysts. Chem. Soc. Rev. 2018, 47, 5038–5060. [Google Scholar] [CrossRef]
- Luo, Z.; Kong, J.; Ma, B.; Wang, Z.; Huang, J.; Zhao, C. Liquefaction and Hydrodeoxygenation of Polymeric Lignin Using a Hierarchical Ni Microreactor Catalyst. ACS Sustain. Chem. Eng. 2020, 8, 2158–2166. [Google Scholar] [CrossRef]
- Liu, S.; Bai, L.; van Muyden, A.P.; Huang, Z.; Cui, X.; Fei, Z.; Li, X.; Hu, X.; Dyson, P.J. Oxidative cleavage of β-O-4 bonds in lignin model compounds with a single-atom Co catalyst. Green Chem. 2019, 21, 1974–1981. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Z.; Chen, M.; Zhang, J.; Shi, J.; Wang, C.; Yang, Z.; Wang, J. Effect of Mo content in Mo/Sepiolite catalyst on catalytic depolymerization of Kraft lignin under supercritical ethanol. Energy Convers. Manag. 2020, 222, 113227. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, X.; Xin, J.; Liu, G.; Chen, J.; Wu, G.; Liu, T.; Zhang, J.; Kong, Z. Thiol–Ene Synthesis of Cysteine-Functionalized Lignin for the Enhanced Adsorption of Cu(II) and Pb(II). Ind. Eng. Chem. Res. 2018, 57, 7872–7880. [Google Scholar] [CrossRef]
- He, J.; Zhao, C.; Lercher, J.A. Ni-Catalyzed Cleavage of Aryl Ethers in the Aqueous Phase. J. Am. Chem. Soc. 2012, 134, 20768–20775. [Google Scholar] [CrossRef] [PubMed]
- Kordouli, E.; Pawelec, B.; Kordulis, C.; Lycourghiotis, A.; Fierro, J.L.G. Hydrodeoxygenation of phenol on bifunctional Ni-based catalysts: Effects of Mo promotion and support. Appl. Catal. B Environ. 2018, 238, 147–160. [Google Scholar] [CrossRef]
- Xia, H.H.; Li, J.; Chen, C.Z.; Wu, D.C.; Ren, J.R.; Jiang, J.C.; Zhou, M.H. Selective aqueous-phase hydrogenation of furfural to cyclopentanol over Ni-based catalysts prepared from Ni-MOF composite. Inorg. Chem. Commun. 2021, 133, 108894. [Google Scholar] [CrossRef]
- Rani, P.; Srivastava, R. Tailoring the catalytic activity of metal organic frameworks by tuning the metal center and basic functional sites. New J. Chem. 2017, 41, 8166–8177. [Google Scholar] [CrossRef]
- Liu, P.; Chen, C.Z.; Zhou, M.H.; Xu, J.M.; Xia, H.H.; Shang, S.B.; Jiang, J.C. Metal-organic framework-derived Ni-based catalyst for the hydrotreatment of triolein into green diesel. Sustain. Energy Fuels 2021, 5, 1809–1820. [Google Scholar] [CrossRef]
- Chen, C.Z.; Liu, P.; Zhou, M.H.; Li, J.; Xia, H.H.; Jiang, J.C. One-step catalytic hydrotreatment of lignin dimer model compounds to cycloalkane and cycloalcohol by spherical metal-organic framework derived NiLa bimetallic materials. J. Energy Inst. 2021, 99, 105–119. [Google Scholar] [CrossRef]
- Kang, X.C.; Liu, H.Z.; Hou, M.Q.; Sun, X.F.; Han, H.L.; Jiang, T.; Zhang, Z.F.; Han, B.X. Synthesis of Supported Ultrafine Non-noble Subnanometer-Scale Metal Particles Derived from Metal-Organic Frameworks as Highly Efficient Heterogeneous Catalysts. Angew. Chem. -Int. Ed. 2016, 55, 1080–1084. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Zhang, Z.; Li, M.; Yang, Q.; Yang, Y.; Bao, Z.; Ren, Q. M-Gallate (M = Ni, Co) Metal–Organic Framework-Derived Ni/C and Bimetallic Ni–Co/C Catalysts for Lignin Conversion into Monophenols. ACS Sustain. Chem. Eng. 2019, 7, 12955–12963. [Google Scholar] [CrossRef]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.A.; Zhou, H.C. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef]
- Espallargas, G.M.; Coronado, E. Magnetic functionalities in MOFs: From the framework to the pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.H.; Park, R.-s.; Kim, H.; Park, S.H.; Jung, S.-C.; Jeon, J.-K.; Kim, S.C.; Park, Y.-K. Hydrodeoxygenation of guaiacol over Pt loaded zeolitic materials. J. Ind. Eng. Chem. 2016, 37, 18–21. [Google Scholar] [CrossRef]
- Chen, M.-Y.; Huang, Y.-B.; Pang, H.; Liu, X.-X.; Fu, Y. Hydrodeoxygenation of lignin-derived phenols into alkanes over carbon nanotube supported Ru catalysts in biphasic systems. Green Chem. 2015, 17, 1710–1717. [Google Scholar] [CrossRef]
- Schutyser, W.; van den Bosch, S.; Dijkmans, J.; Turner, S.; Meledina, M.; van Tendeloo, G.; Debecker, D.P.; Sels, B.F. Selective Nickel- Catalyzed Conversion of Model and Lignin- Derived Phenolic Compounds to Cyclohexanone-Based Polymer Building Blocks. Chemsuschem 2015, 8, 1805–1818. [Google Scholar] [CrossRef]
- Chen, C.Z.; Zhou, M.H.; Liu, P.; Sharma, B.K.; Jiang, J.C. Flexible NiCo-based catalyst for direct hydrodeoxygenation of guaiacol to cyclohexanol. New J. Chem. 2020, 44, 18906–18916. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Ishikawa, M.; Tamura, M.; Tomishige, K. Selective production of cyclohexanol and methanol from guaiacol over Ru catalyst combined with MgO. Green Chem. 2014, 16, 2197–2203. [Google Scholar] [CrossRef]
- Schutyser, W.; van den Bossche, G.; Raaffels, A.; van den Bosch, S.; Koelewijn, S.-F.; Renders, T.; Sels, B.F. Selective Conversion of Lignin-Derivable 4-Alkylguaiacols to 4-Alkylcyclohexanols over Noble and Non-Noble-Metal Catalysts. ACS Sustain. Chem. Eng. 2016, 4, 5336–5346. [Google Scholar] [CrossRef]

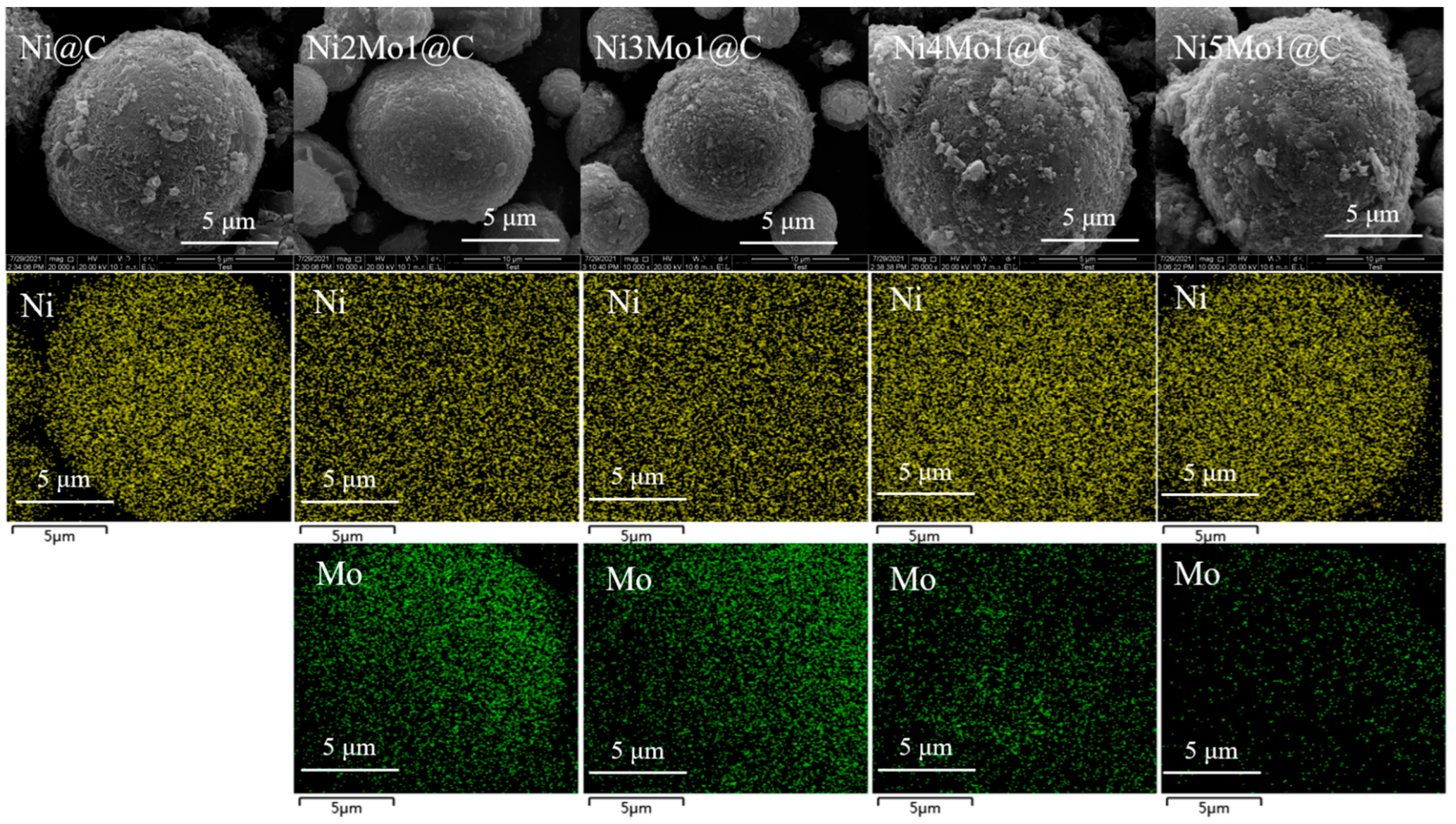
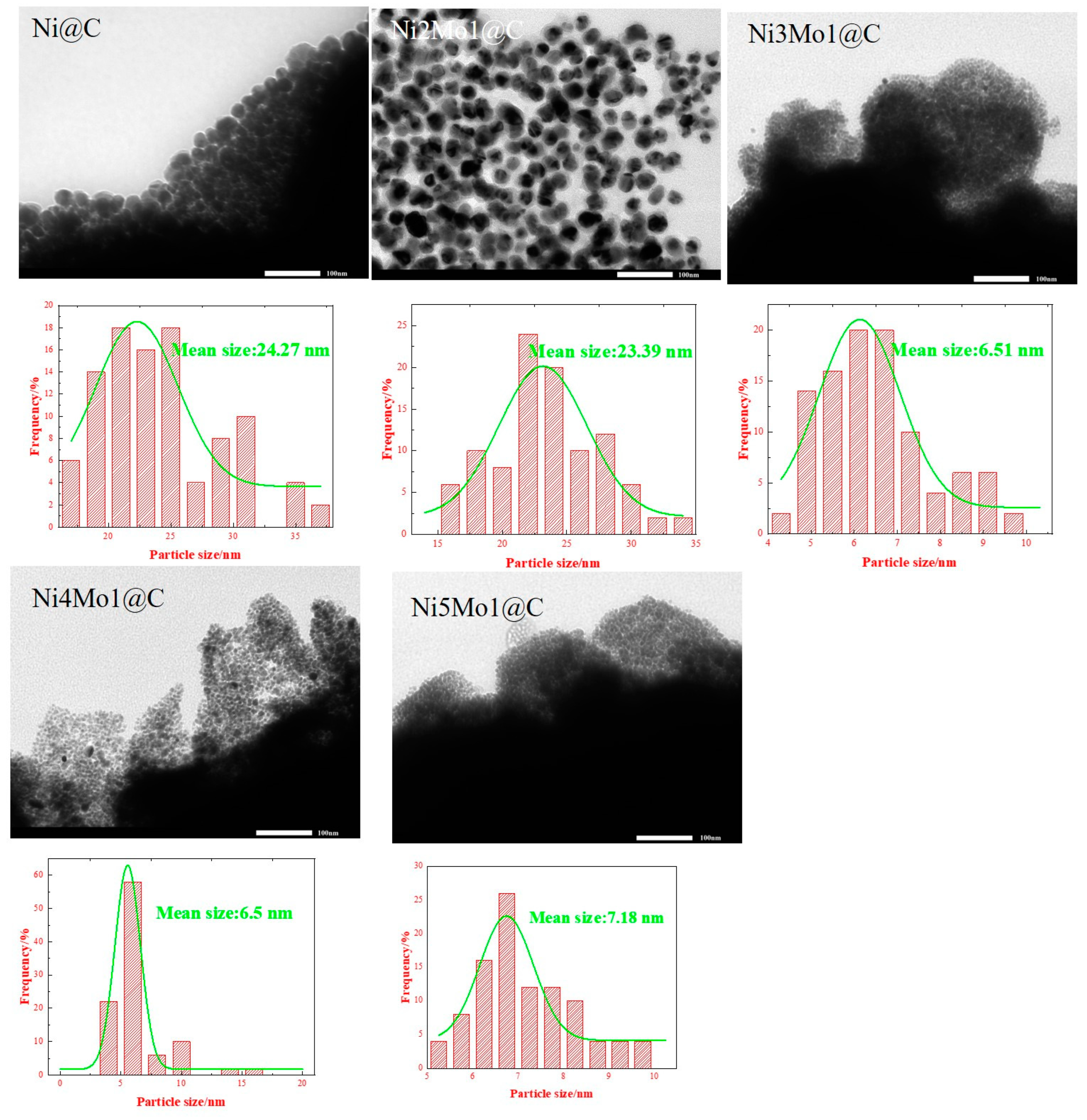


| Ni@C | Ni5Mo1@C | Ni4Mo1@C | Ni3Mo1@C | Ni2Mo1@C | |
|---|---|---|---|---|---|
| Ni amount (wt.%) | 59.88 | 49.12 | 48.89 | 44.86 | 40.91 |
| Mo amount (wt.%) | / | 10.08 | 12.17 | 15.48 | 21.98 |
| Ni/Mo mole ratio | / | 4.87 | 4.02 | 2.89 | 1.86 |
| Ni@C | Ni5Mo1@C | Ni4Mo1@C | Ni3Mo1@C | Ni2Mo1@C | |
|---|---|---|---|---|---|
| Total acidity a (mmol/g NH3) | 0.55 | 1.44 | 1.79 | 2.01 | 1.88 |
| Lewis acid b (μmol/g) | 142.32 | 190.02 | 245.81 | 275.06 | 225.36 |
| Bronsted acid b (μmol/g) | 3.25 | 4.56 | 5.68 | 6.88 | 6.00 |
| Entry | Substrate | T(℃)/t(h)/P(MPa) | Conversion | Selectivity of Products |
|---|---|---|---|---|
| 1 | 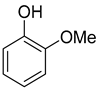 | 240/4/2 | 100% | 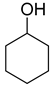 66% 66% |
| 2 | 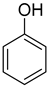 | 240/4/2 | 100% | 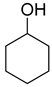 96% 96% |
| 3 |  | 240/4/2 | 79% | 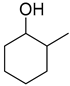 34% 34%  45% 45% |
| 4 | 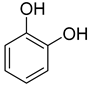 | 240/4/2 | 88% | 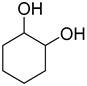 30% 30%  58% 58% |
| 5 | 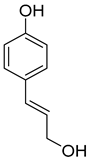 | 240/4/2 | 68% | 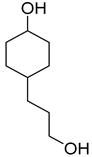 50% 50% 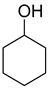 18% 18% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Ge, F.; Li, J.; Xia, H.; Liu, J.; Jiang, J.; Chen, C.; Zhao, J.; Yang, X. Catalytic Hydrodeoxygenation of Guaiacol to Cyclohexanol over Bimetallic NiMo-MOF-Derived Catalysts. Catalysts 2022, 12, 371. https://doi.org/10.3390/catal12040371
Zhou M, Ge F, Li J, Xia H, Liu J, Jiang J, Chen C, Zhao J, Yang X. Catalytic Hydrodeoxygenation of Guaiacol to Cyclohexanol over Bimetallic NiMo-MOF-Derived Catalysts. Catalysts. 2022; 12(4):371. https://doi.org/10.3390/catal12040371
Chicago/Turabian StyleZhou, Minghao, Fei Ge, Jing Li, Haihong Xia, Junli Liu, Jianchun Jiang, Changzhou Chen, Jun Zhao, and Xiaohui Yang. 2022. "Catalytic Hydrodeoxygenation of Guaiacol to Cyclohexanol over Bimetallic NiMo-MOF-Derived Catalysts" Catalysts 12, no. 4: 371. https://doi.org/10.3390/catal12040371







