The Catalytic Effect from Alkaline Elements on the Tar-Rich Coal Pyrolysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Thermal Characterisation for Coal Pyrolysis
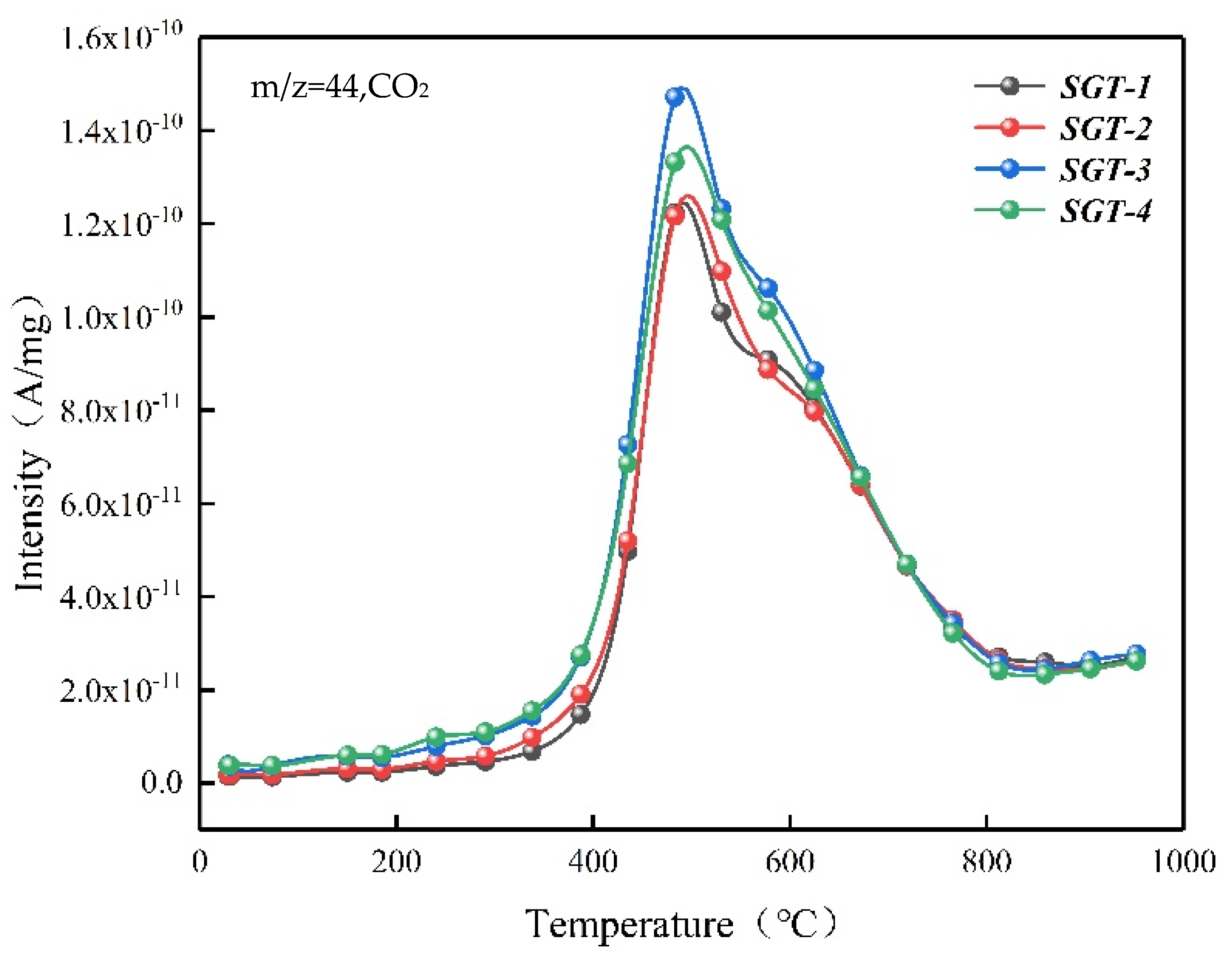
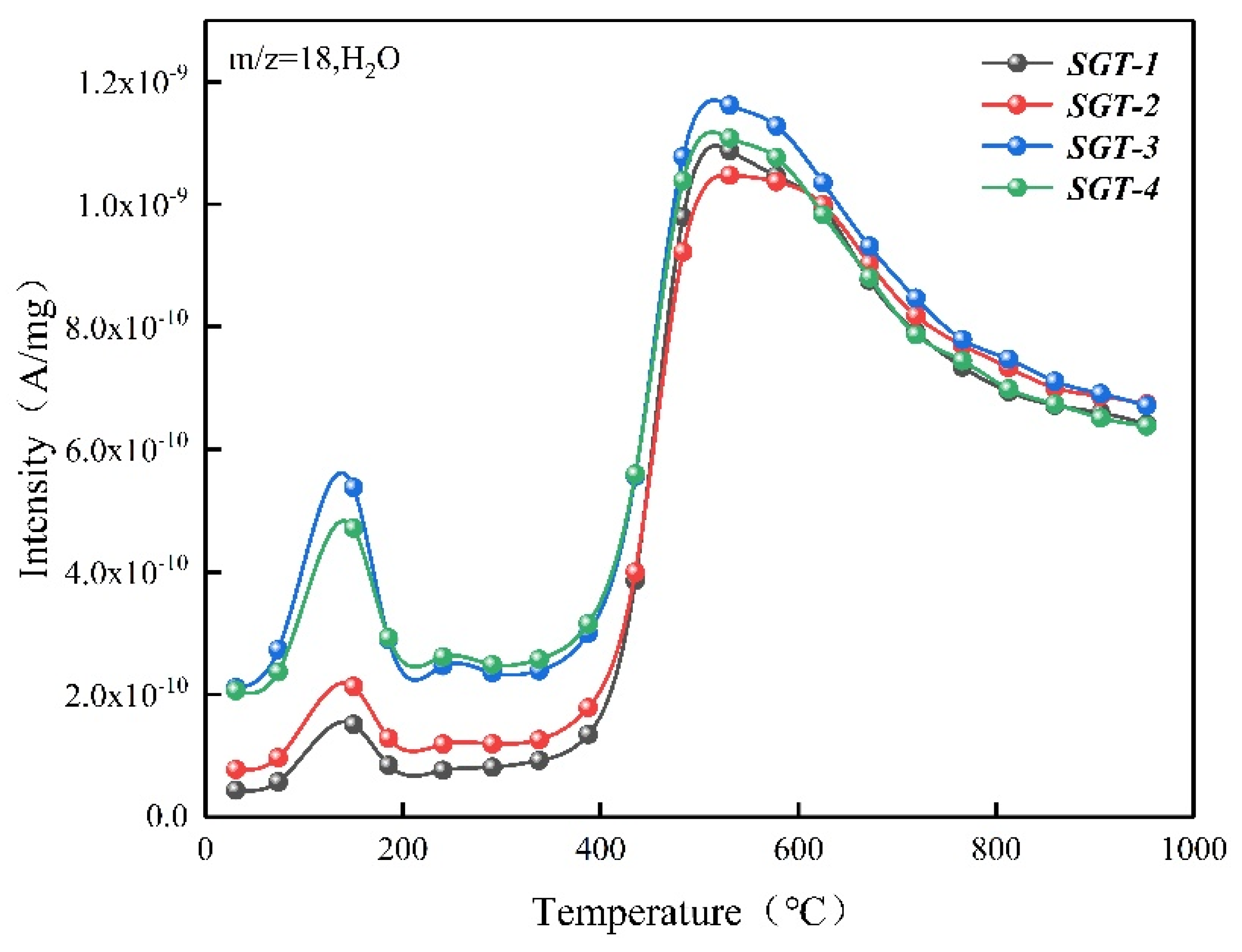
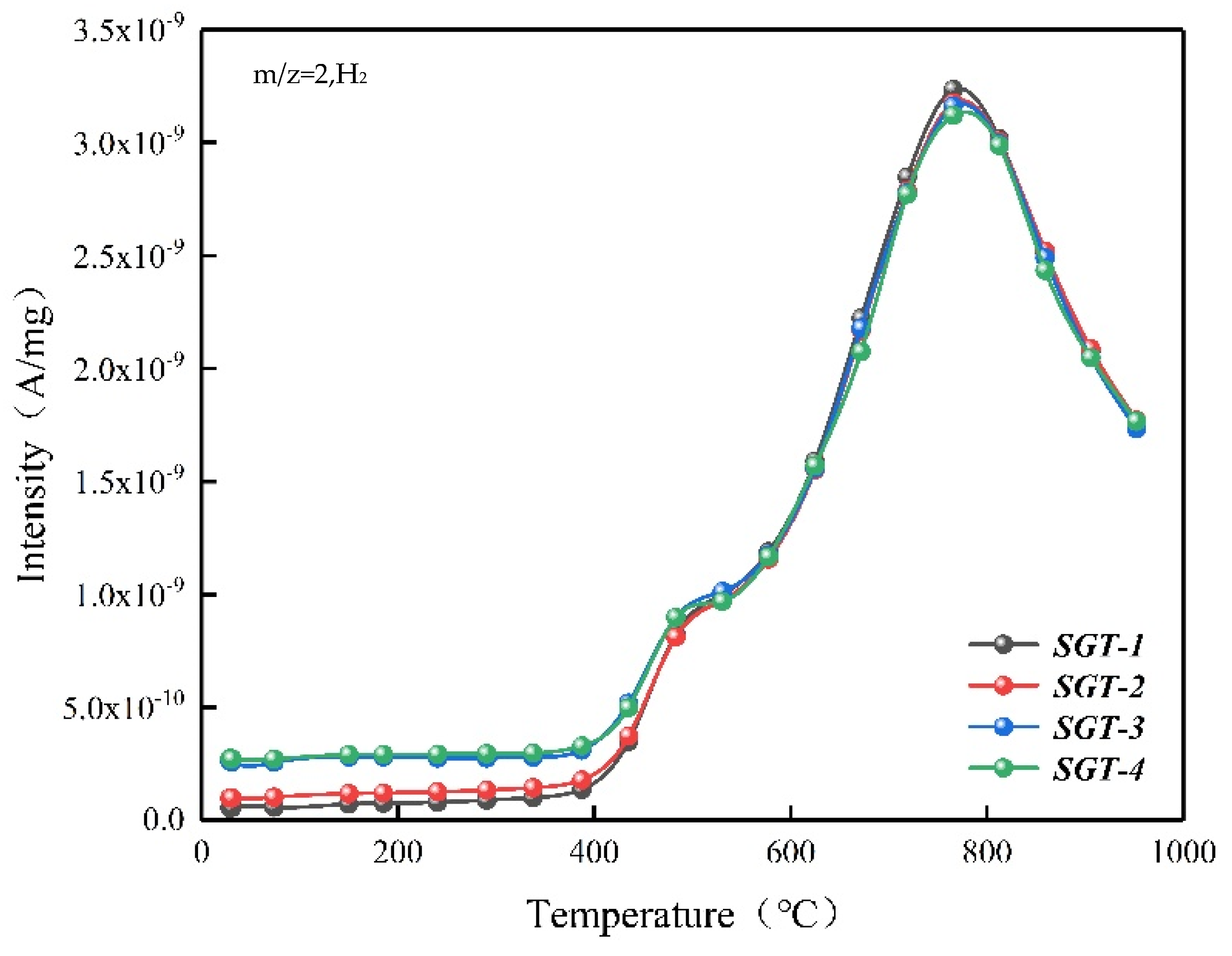
2.2. The Gas Composition for Pyrolysis
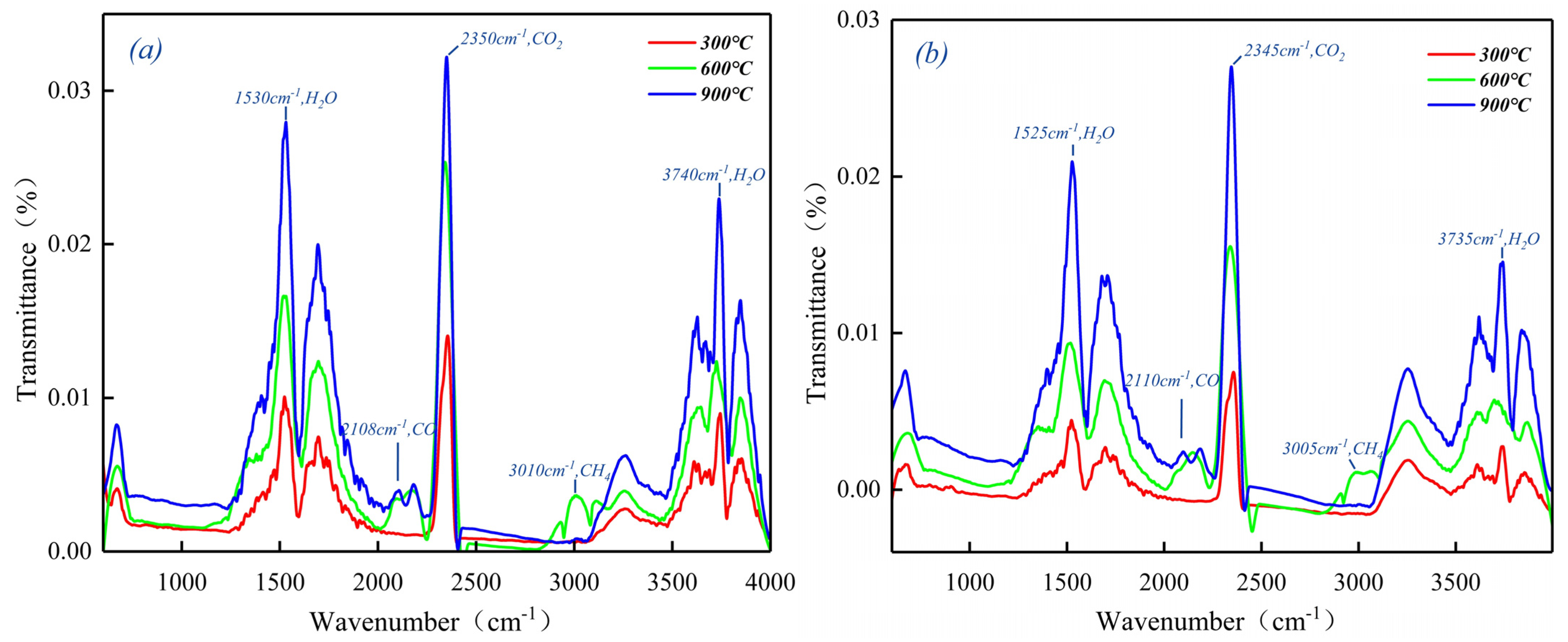
2.3. The Evolution of Carbon Compounds during Pyrolysis
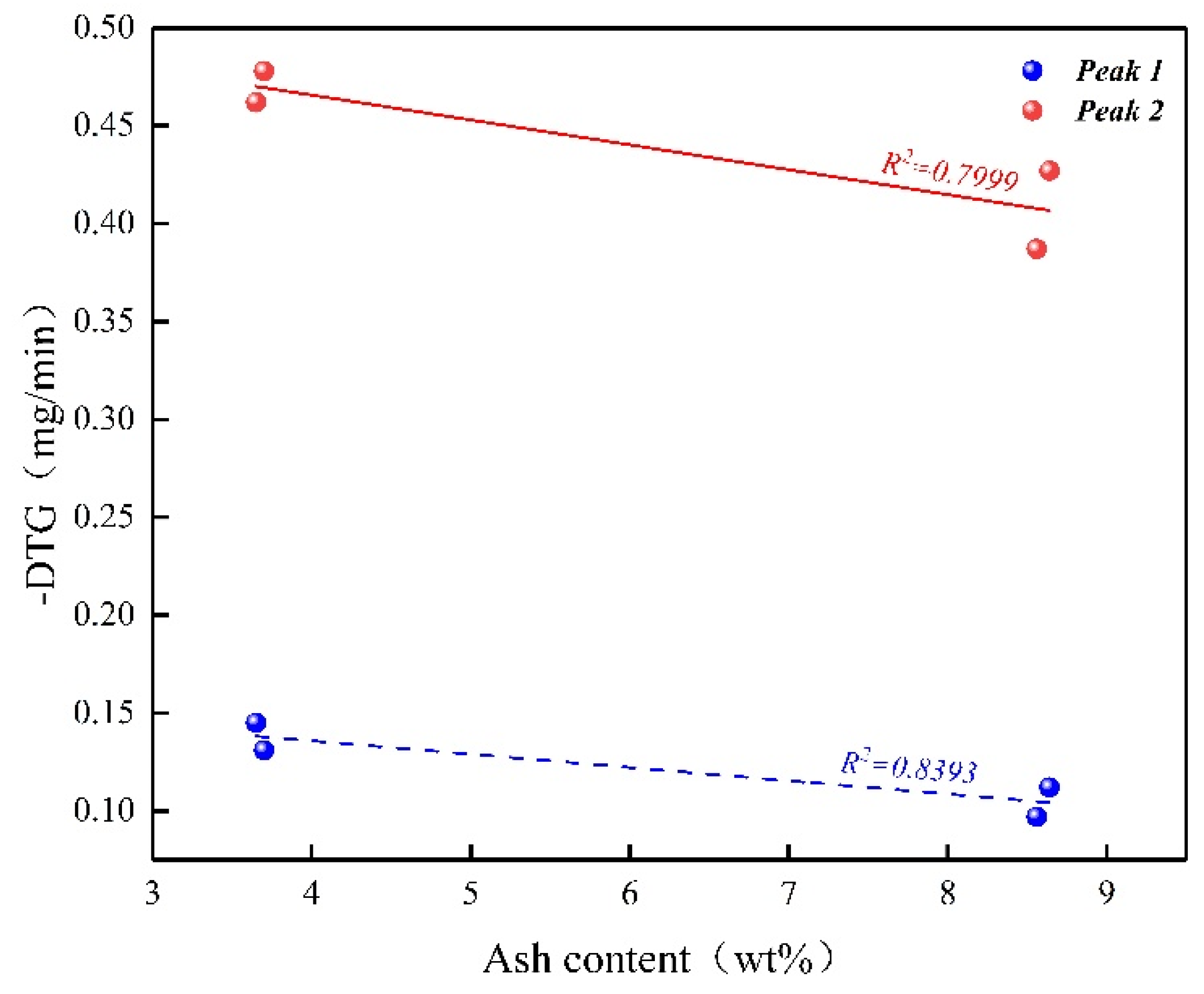
2.4. The Catalytic Effect from the Inorganic Compounds
2.5. The Mechanism for Thermal Pyrolysis of Tar-Rich Coal
3. Samples and Experimental Methods
3.1. Coal Samples
3.2. TG/MS/FTIR Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, H.; Ma, L. Study on distribution law and occurrence characteristics of rich oil in Zichang mining area. World Sci. Res. J. 2020, 6, 1–6. [Google Scholar]
- Ju, Y.; Zhu, Y.; Zhou, H.; Ge, S.; Xie, H. Microwave pyrolysis and its applications to the in situ recovery and conversion of oil from tar-rich coal: An overview on fundamentals, methods, and challenges. Energy Rep. 2021, 7, 523–536. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Q.; Wang, S.; Shen, J.; Sun, Q.; Cai, Y. Resource property and exploitation concepts with green and low-carbon of tar-rich coal as coal-based oil and gas. J. China Coal Soc. 2021, 46, 1365–1377. (In Chinese) [Google Scholar]
- Shen, J.; Wang, X.; Zhao, C.; Wang, S.; Guo, C.; Shi, Q.; Ma, W. Experimental study on multi-scale pore structure characteristics of tar-rich coal in Yushenfu mining area. Coal Geol. Explor. 2021, 49, 33–41. (In Chinese) [Google Scholar]
- Han, D.; Yang, Q. China Coalfield Geology; China Coal Industry Publishing House: Beijing, China, 1979. (In Chinese) [Google Scholar]
- Liu, L.; Gong, Z.; Wang, Z.; Zhang, H. Study on combustion and emission characteristics of chars from low-temperature and fast pyrolysis of coals with TG-MS. Environ. Eng. Res. 2020, 25, 522–528. [Google Scholar] [CrossRef] [Green Version]
- Jayaraman, K.; Kök, M.; Gokalp, I. Thermogravimetric and mass spectrometric (TG-MS) analysis and kinetics of coal-biomass blends. Renew. Energy 2017, 101, 293–300. [Google Scholar] [CrossRef]
- Vekemans, O.; Laviolette, J.; Chaouki, J. Co-combustion of coal and waste in pulverized coal boiler. Energy 2016, 94, 742–754. [Google Scholar] [CrossRef]
- Jayaraman, K.; Kök, M.; Gokalp, I. Pyrolysis combustion and gasification studies of different sized coal particles using TGA-MS. Appl. Therm. Eng. 2017, 125, 1446–1455. [Google Scholar] [CrossRef]
- Tromp, P.J.J. Coal Pyrolysis; Amsterdam University: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Wei, J.; Liu, X.; Guo, Q.; Chen, X.; Yu, G. A comparative study on pyrolysis reactivity and gas release characteristics of biomass and coal using TG-MS analysis. Energy Sources Part A 2018, 40, 1–7. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Huang, R.; Xiao, Y.; Mao, R.; Huang, J.; Zhou, C. Thermogravimetric study of the kinetics and characteristics of the pyrolysis of pulverized coal. Mater. Res. Express 2020, 7, 085604. [Google Scholar]
- He, C.; Tang, C.; Liu, W.; Dai, L.; Qiu, R. Co-pyrolysis of sewage sludge and hydrochar with coals: Pyrolytic behaviors and kinetics analysis using TG-FTIR and a discrete distributed activation energy model. Energy Convers. Manag. 2020, 203, 112226. [Google Scholar] [CrossRef]
- Lin, Y.; Tian, Y.; Xia, Y.; Fang, S.; Liao, Y.; Yu, Z.; Ma, X. General distributed activation energy model (G-DAEM) on co-pyrolysis kinetics of bagasse and sewage sludge. Bioresour. Technol. 2019, 273, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, M.; Li, Y.; Jin, L.; Hu, H. Integrated coal pyrolysis with steam reforming of propane to improve tar yield. J. Anal. Appl. Pyrolysis 2020, 147, 104805. [Google Scholar] [CrossRef]
- Yao, Q.; Ma, M.; Liu, Y.; He, L.; Sun, M.; Ma, X. Pyrolysis characteristics of metal ion-exchanged Shendong coal and its char gasification performance. J. Anal. Appl. Pyrolysis 2021, 155, 105087. [Google Scholar] [CrossRef]
- Li, Q.; Ye, H.; Wang, Z.; Zhou, H.; Wei, J. Characteristics and evolution of products under moderate and high temperature coal pyrolysis in drop tube furnace. J. Energy Inst. 2021, 96, 121–127. [Google Scholar] [CrossRef]
- Jayaraman, K.; Kök, M.; Gokalp, I. Combustion mechanism and model free kinetics of different origin coal samples: Thermal analysis approach. Energy 2020, 204, 117905. [Google Scholar] [CrossRef]
- Zhu, Y.; Wen, W.; Li, Y.; Lu, L.; Yang, J.; Xu, M.; Pan, Y. Pyrolysis study of Huainan coal with different particle sizes using TG analysis and online Py-PI-TOF MS. J. Energy Inst. 2020, 93, 405–414. [Google Scholar] [CrossRef]
- Jeong, T.; Isworo, Y.; Jeon, C. Evaluation of Char Characteristics and Combustibility of Low-Rank-Coal Blends with Different Reflectance Distributions. Energy Fuels 2019, 33, 8014–8025. [Google Scholar] [CrossRef]
- Yan, B.; Jiao, L.; Li, J.; Zhu, X.; Ahmed, S.; Chen, G. Investigation on microwave torrefaction: Parametric influence, TG-MS-FTIR analysis, and gasification performance. Energy 2021, 220, 119794. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.; Jiang, X.; Shen, J. An improved parallel reaction model applied to coal pyrolysis. Fuel Processing Technol. 2021, 211, 106608. [Google Scholar] [CrossRef]
- Pan, G. Characteristic Study of Some Different Kinds of Coal Particles Combustion with Online TG-MS-FTIR. IOP Conf. Ser. Earth Environ. Sci. 2018, 111, 012019. [Google Scholar] [CrossRef]
- Wang, S.; Tang, Y.; Schobert, H.; Guo, Y.; Gao, W.; Lu, X. FTIR and simultaneous TG/MS/FTIR study of Late Permian coals from Southern China. J. Anal. Appl. Pyrolysis 2013, 100, 75–80. [Google Scholar] [CrossRef]
- Pan, G. Characteristic Study of Shenmu Bituminous Coal Combustion with Online TG-MS-FTIR. IOP Conf. Ser. Earth Environm. Sci. 2018, 111, 012018. [Google Scholar] [CrossRef] [Green Version]
- Wall, T. Mineral matter transformations and ash deposition in pulverised coal combustion. Symp. Combust. 1992, 24, 1119–1126. [Google Scholar] [CrossRef]
- Burket, C.; Rajagopalan, R.; Foley, H. Overcoming the barrier to graphitization in a polymer-derived nanoporous carbon. Carbon 2008, 46, 501–510. [Google Scholar] [CrossRef]
- Strydom, C.; Bunt, J.; Schobert, H.; Raghoo, M. Changes to the organic functional groups of an inertinite rich medium rank bituminous coal during acid treatment processes. Fuel Process. Technol. 2011, 92, 764–770. [Google Scholar] [CrossRef]
- Dash, P.; Lingam, R.; Kumar, S.; Suresh, A.; Banerjee, P.; Ganguly, S. Effect of elevated temperature and pressure on the leaching characteristics of Indian coals. Fuel 2015, 140, 302–308. [Google Scholar] [CrossRef]
- Cuest, A.; Dhamelincourt, P.; Laureyns, J.; Martinez-Alonso, A.; Tascon, J. Raman microprobe studies on carbon materials. Carbon 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Shao, J.; Rong, Y.; Chen, H.; Yang, H.; Lee, D. Catalytic effect of metal oxides on pyrolysis of sewage sludge. Fuel Process. Technol. 2010, 91, 1113–1118. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, H.; Zhou, Q.; Zhu, S.; Chen, G. Effect of inorganic matter on reactivity and kinetics of coal pyrolysis. Fuel 2004, 83, 713–718. [Google Scholar] [CrossRef]
- Howard, D.; William, A.; Jack, B. Mineral matter effects on the rapid pyrolysis and hydropyrolysis of a bituminous coal: Effects of yields of char, tar, and light gaseous volatiles. Fuel 1981, 61, 155–160. [Google Scholar]
- Ahmad, T.; Awan, I.; Nisar, J.; Ahmad, I. Influence of inherent minerals and pyrolysis temperature on the yield of pyrolysates of some Pakistani coals. Energy Convers. Manag. 2009, 50, 1163–1171. [Google Scholar] [CrossRef]
- Sergey, V. Kinetic concepts of thermally stimulated reactions in solids: A view from a historical perspective. Int. Rev. Phys. Chem. 2000, 19, 45–60. [Google Scholar]
- Arenillas, A.; Rubiera, F.; Pis, J.; Cuesta, M.; Iglesias, M.; Jimenez, A.; Suarez-Ruiz, I. Thermal behavior during the pyrolysis of low rank perhydrous coals. J. Anal. Appl. Pyrolysis 2003, 68, 371–385. [Google Scholar] [CrossRef]
- Ofori, P.; Nguyen, A.V.; Firth, B.; Mcnally, C.; Hampton, M. The role of surface interaction forces and mixing in enhanced dewatering of coal preparation tailings. Fuel 2012, 97, 262–268. [Google Scholar] [CrossRef]
- Shi, L.; Liu, Q.; Guo, X.; Wu, W.; Liu, Z. Pyrolysis behavior and bonding information of coal-A TGA study. Fuel Process. Technol. 2013, 108, 125–132. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, S.; Zhao, W.; Liu, S. Co-pyrolysis of biomass and coal in a free fall reactor. Fuel 2007, 86, 353–359. [Google Scholar] [CrossRef]
- Wang, S.; Tang, Y.; Schobert, H.; Mitchell, G.; Liao, F.; Liu, Z. A thermal behavior study of Chinese coals with high hydrogen content. Int. J. Coal Geol. 2010, 81, 37–44. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, P.; Lu, X.; Wang, L.; Pan, T. Upgrading of low rank coal by hydrothermal treatment: Coal tar yield during pyrolysis. Fuel Process. Technol. 2016, 141, 117–122. [Google Scholar] [CrossRef]
- Ding, L.; Zhou, Z.; Dai, Z.; Yu, G. Effects of coal drying on the pyrolysis and in-situ gasification characteristics of lignite coals. Appl. Energy 2015, 155, 660–670. [Google Scholar] [CrossRef]
- Ahmed, I.; Gupta, A. Experiments and stochastic simulations of lignite coal during pyrolysis and gasification. Appl. Energy 2013, 102, 355–363. [Google Scholar] [CrossRef]
- Hodek, W.; Kirschstein, J.; Heek, K. Reactions of oxygen containing structures in coal pyrolysis. Fuel 1991, 70, 424–428. [Google Scholar] [CrossRef]
- Arenillas, A.; Rubiera, F.; Pis, J. Simultaneous thermogravimetric–mass spectrometric study on the pyrolysis behavior of different rank coals. J. Anal. Appl. Pyrolysis 1999, 50, 31–46. [Google Scholar] [CrossRef] [Green Version]
- Metzinger, T.; Hüttinger, K. Investigations on the cross-linking of binder pitch matrix of carbon bodies with molecular oxygen —Part I. Chemistry of reactions between pitch and oxygen. Carbon 1997, 35, 885–892. [Google Scholar] [CrossRef]
- Drbohlav, J.; Wtk, S. The oxidative stabilization and carbonization of a synthetic mesophase pitch, part II: The carbonization process. Carbon 1995, 33, 713–731. [Google Scholar] [CrossRef]
- Garcia, R.; Arenillas, A.; Crespo, J.; Pis, J.; Moinelo, S. A Comparative TG-MS Study of the Carbonization Behavior of Different Pitches. Energy Fuels 2002, 16, 935–943. [Google Scholar] [CrossRef]
- Chen, H.; Li, B.; Zhang, B. Effects of mineral matter on products and sulfur distributions in hydropyrolysis. Fuel 1999, 78, 713–719. [Google Scholar] [CrossRef]
- Heek, K.; Hodek, W. Structure and pyrolysis behavior of different coals and relevant model substances. Fuel 1994, 73, 886–896. [Google Scholar] [CrossRef]
- Zheng, L.; Furimsky, E. Assessment of coal combustion in O2 + CO2 by equilibrium calculations. Fuel Process. Technol. 2003, 81, 23–34. [Google Scholar] [CrossRef]
- Glarborg, P.; Bentzen, L. Chemical Effects of a High CO2 Concentration in Oxy-Fuel Combustion of Methane. Energy Fuels 2008, 22, 291–296. [Google Scholar] [CrossRef]
- Strugnell, B.; Patrick, J. Rapid hydropyrolysis studies on coal and maceral concentrates. Fuel 1996, 75, 183. [Google Scholar] [CrossRef]
- Bai, J.; Chen, X.; Shao, J.; Jia, C.; Wang, Q. Study of breakage of main covalent bonds during co-pyrolysis of oil shale and alkaline lignin by TG-FTIR integrated analysis. J. Energy Inst. 2019, 92, 512–522. [Google Scholar] [CrossRef]
- Yan, M.; Bai, Y.; Li, S.; Lin, H.; Yan, D.; Shu, C. Factors influencing the gas adsorption thermodynamic characteristics of low-rank coal. Fuel 2019, 248, 117–126. [Google Scholar] [CrossRef]
- Reichel, D.; Siegl, S.; Neubert, C.; Krzack, S. Determination of pyrolysis behavior of brown coal in a pressurized drop tube reactor. Fuel 2015, 158, 983–998. [Google Scholar] [CrossRef]
- Luo, L.; Liu, J.; Zhang, H.; Ma, J.; Wang, X.; Jiang, X. TG-MS-FTIR study on pyrolysis behavior of superfine pulverized coal. J. Anal. Appl. Pyrolysis 2017, 128, 67–74. [Google Scholar] [CrossRef]
- Xu, T.; Huang, X. Study on combustion mechanism of asphalt binder by using TG–FTIR technique. Fuel 2010, 89, 2185–2190. [Google Scholar] [CrossRef]
- Opata, Y.; Grivel, J. Synthesis and thermal decomposition study of dysprosium trifluoroacetate. J. Anal. Appl. Pyrolysis 2018, 132, S1035497363. [Google Scholar] [CrossRef] [Green Version]
- Scaccia, S. TG–FTIR and kinetics of devolatilization of Sulcis coal. J. Anal. Appl. Pyrolysis 2013, 104, 95–102. [Google Scholar] [CrossRef]
- Ahamad, T.; Alshehri, S. TG–FTIR–MS (Evolved Gas Analysis) of bidi tobacco powder during combustion and pyrolysis. J. Hazard. Mater. 2012, 199, 200–208. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, P.; Yuan, H.; Hu, K. Thermal degradation study of intumescent flame retardants by TG and FTIR: Melamine phosphate and its mixture with pentaerythritol. J. Anal. Appl. Pyrolysis 2009, 86, 207–214. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Dai, X.; Mei, Q.; Ding, G. Thermogravimetry–Fourier transform infrared spectrometry–mass spectrometry technique to evaluate the effect of anaerobic digestion on gaseous products of sewage sludge sequential pyrolysis. J. Anal. Appl. Pyrolysis 2017, 126, 288–297. [Google Scholar] [CrossRef]
- Brebu, M.; Tamminen, T.; Spiridon, I. Thermal degradation of various lignins by TG-MS/FTIR and Py-GC-MS. J. Anal. Appl. Pyrolysis 2013, 104, 531–539. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Zhao, W.; Zhou, J. TG-FTIR-MS study of pyrolysis products evolving from peat. J. Anal. Appl. Pyrolysis 2016, 117, 296–309. [Google Scholar] [CrossRef]
- Weng, J.; Liu, Y.; Zhu, Y.; Pan, Y.; Tian, Z. Online study on the co-pyrolysis of coal and corn with vacuum ultraviolet photoionization mass spectrometry. Bioresour. Technol. 2017, 244, 125–131. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Yuan, H.; Zhuang, X.; Yuan, S.; Yin, X.; Wu, C. Association of chemical structure and thermal degradation of lignins from crop straw and softwood. J. Anal. Appl. Pyrolysis 2018, 134, 25–34. [Google Scholar] [CrossRef]
- Liang, B.; Wang, J.; Hu, J.; Li, C.; Li, R.; Liu, Y.; Zeng, K.; Yang, G. TG-MS-FTIR study on pyrolysis behavior of phthalonitrile resin. Polymer Degradat. Stabil. 2019, 169, 108954. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Yang, H.; Chen, W.; Wang, X.; Chen, H. Fast pyrolysis of cotton stalk biomass using calcium oxide. Bioresour. Technol. 2017, 233, 15–20. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, J.; Zhang, Z. General roles of sludge ash, CaO and Al2O3 on the sludge pyrolysis toward clean utilizations. Appl. Energy 2019, 233, 412–423. [Google Scholar] [CrossRef]
- Li, C.; Sathe, C.; Kershaw, J.; Pang, Y. Fates and roles of alkali and alkaline earth metals during the pyrolysis of a Victorian brown coal. Fuel 2000, 79, 427–438. [Google Scholar] [CrossRef]
- Wang, M.; Fu, C.; Chang, L.; Xie, K. Effect of fractional step acid treatment process on the structure and pyrolysis characteristics of Ximeng brown coal. J. Fuel Chem. Technol. 2012, 40, 906–911. [Google Scholar]
- Wang, W. Chemical reactions in thermal decomposition of coal. Fuel Process. Technol. 1988, 20, 317–336. [Google Scholar]
- Solomon, P.; Serio, M.; Suuberg, E. Coal pyrolysis: Experiments, kinetic rates and mechanisms. Progress Energy Combust. Sci. 1992, 18, 133–220. [Google Scholar] [CrossRef]
- Painter, P. Fourier transform infrared study of acid-demineralized coal. Fuel 1978, 57, 125–126. [Google Scholar] [CrossRef]
- Painter, P.; Coleman, M.; Jenkins, R.; Whang, P.; Walker, J. Fourier transform infrared study of mineral matter in coal. A novel method for quantitative mineralogical analysis. Fuel 1978, 57, 337–344. [Google Scholar] [CrossRef]
- Painter, P.; Snyder, R.; Starsinic, M.; Coleman, M.; Kuehn, D.; Davis, A. Concerning the application of FT-IR to the study of coal: A critical assessment of band assignments and the application of spectral analysis programs. Appl. Spectrosc. 1981, 35, 475–485. [Google Scholar] [CrossRef]
- Geng, W.; Nakajima, T.; Takanashi, H.; Ohki, A. Analysis of carboxyl group in coal and coal aromaticity by fourier transform infrared (FT-IR) spectrometry. Fuel 2009, 88, 139–144. [Google Scholar] [CrossRef]





| Sample | Tmax | (dw/dτ)max | Wloss |
|---|---|---|---|
| °C | %/min | % | |
| SGT-1 | 460.07 | 0.427 | 25.11 |
| SGT-2 | 460.03 | 0.387 | 25.48 |
| SGT-3 | 460.81 | 0.462 | 26.45 |
| SGT-4 | 460.99 | 0.478 | 26.01 |
| Detected Gas | Functional Group | Vibration | Wavenumber (cm−1) |
|---|---|---|---|
| H2O | O-H | stretch | 4000–3500 |
| H-O-H | bending | 1875–1275 | |
| CH4 | C-H | stretch | 3000–2800 |
| CO2 | C=O | stretch | 2400–2240 |
| C=O | bending | 736–605 | |
| CO | C-O | stretch | 2240–2050 |
| Aromatics | C=C and benzene skeleton | stretch | 1690–1450 |
| HCN | C-H | bending | 815–615 |
| NH3 | N-H | stretch | 3340 |
| N-H | bending | 966,927 |
| SGT-1 | SGT-2 | SGT-3 | SGT-4 | |
|---|---|---|---|---|
| Ultimate analysis (wt %, daf) | ||||
| Cdaf | 75.91 | 76.03 | 76.45 | 76.33 |
| Hdaf | 3.67 | 3.61 | 3.89 | 3.88 |
| Odaf | 18.40 | 18.50 | 18.15 | 18.31 |
| Ndaf | 0.87 | 0.95 | 1.31 | 1.25 |
| St,d | 1.05 | 1.03 | 0.19 | 0.25 |
| H/C | 0.58 | 0.57 | 0.61 | 0.61 |
| O/C | 0.18 | 0.18 | 0.18 | 0.18 |
| Proximate analysis (wt %) | ||||
| Mad | 13.40 | 13.45 | 14.72 | 14.82 |
| Ad | 8.64 | 8.56 | 3.65 | 3.70 |
| Vdaf | 31.42 | 31.44 | 33.13 | 33.28 |
| FCd | 62.66 | 62.61 | 64.43 | 64.56 |
| Type | Composition Analysis of Ash (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| K2O | Na2O | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | TiO2 | MnO2 | P2O5 | |
| SGT-1 | 0.25 | 0.69 | 41.09 | 18.06 | 12.62 | 11.16 | 1.20 | 12.12 | 0.82 | 0.083 | 0.020 |
| SGT-2 | 0.27 | 0.75 | 40.95 | 18.01 | 12.65 | 11.16 | 1.25 | 12.05 | 0.85 | 0.082 | 0.021 |
| SGT-3 | 0.24 | 3.12 | 33.31 | 13.12 | 15.61 | 17.34 | 3.21 | 11.80 | 0.55 | 0.125 | 0.032 |
| SGT-4 | 0.23 | 3.11 | 33.31 | 13.07 | 15.60 | 17.33 | 3.11 | 11.70 | 0.57 | 0.127 | 0.032 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Z.; Li, W. The Catalytic Effect from Alkaline Elements on the Tar-Rich Coal Pyrolysis. Catalysts 2022, 12, 376. https://doi.org/10.3390/catal12040376
Du Z, Li W. The Catalytic Effect from Alkaline Elements on the Tar-Rich Coal Pyrolysis. Catalysts. 2022; 12(4):376. https://doi.org/10.3390/catal12040376
Chicago/Turabian StyleDu, Zhonghua, and Wu Li. 2022. "The Catalytic Effect from Alkaline Elements on the Tar-Rich Coal Pyrolysis" Catalysts 12, no. 4: 376. https://doi.org/10.3390/catal12040376
APA StyleDu, Z., & Li, W. (2022). The Catalytic Effect from Alkaline Elements on the Tar-Rich Coal Pyrolysis. Catalysts, 12(4), 376. https://doi.org/10.3390/catal12040376






