Protonated Chiral 1,2-Diamine Organocatalysts for N-Selective Nitroso Aldol Reaction
Abstract
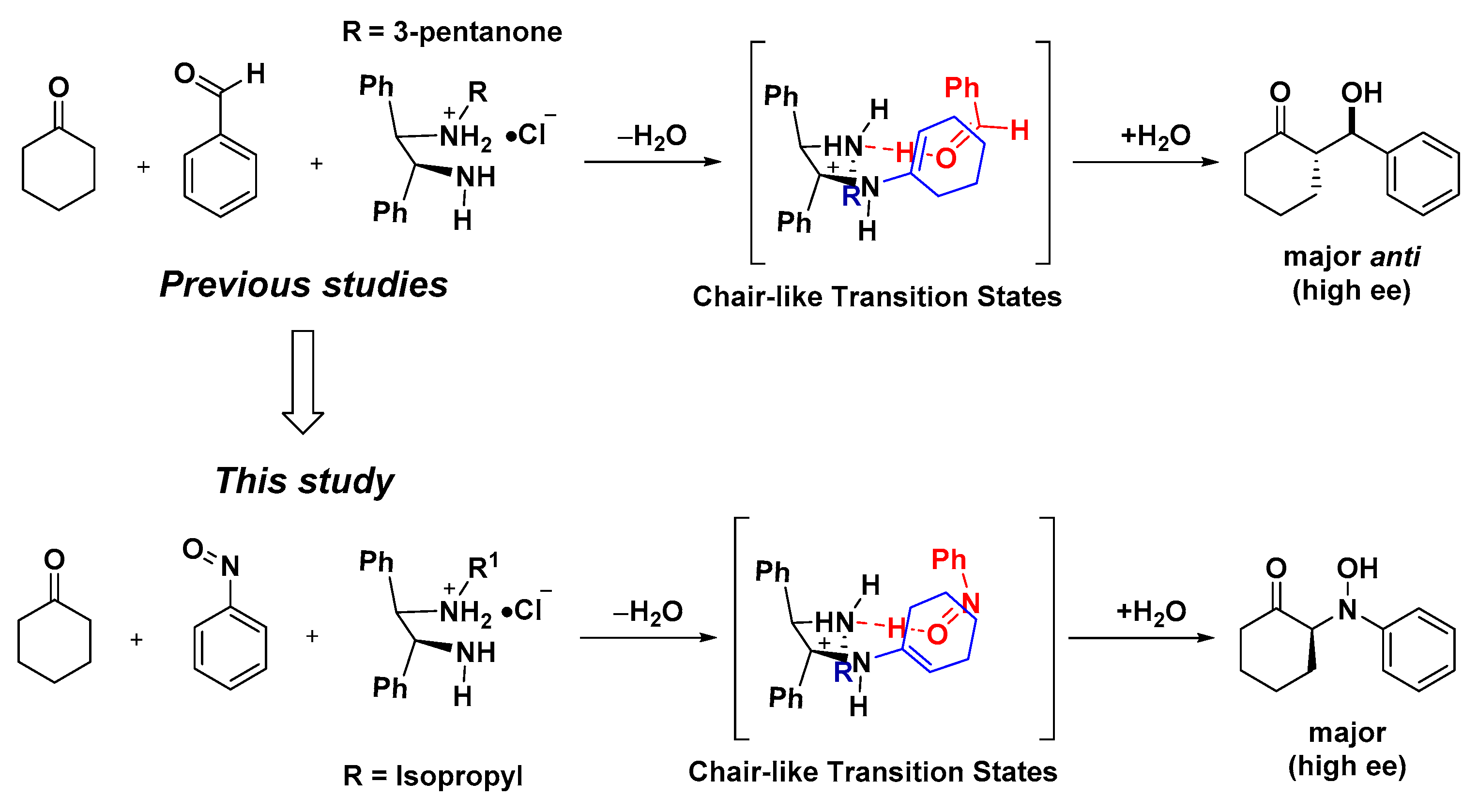
:1. Introduction
2. Results and Discussion
2.1. Nitroso Aldol Reaction Using DPEN
2.1.1. Effect of Solvents
2.1.2. Substituent Effects of DPEN Derivatives
2.1.3. Effects of Different Temperatures and Solvent Ratios
2.1.4. Proposed Mechanism including the Expected Transition State
2.2. Thermodynamic Energy Comparison of Solvent Effect and Mechanism through Quantum Calculations
Comparison of Gibbs Free Energy of Transition State by Catalyst and Type
2.3. Gibbs Free Energy of Each Mechanism
3. Materials and Methods
3.1. Instruments and Reagents
3.2. Experimental Method
3.2.1. Synthesis of Catalysts (1a–e); General Procedure
3.2.2. Asymmetric Nitroso Aldol Reaction of Nitrosobenzene and Cyclohexanone Using a Chiral DPEN Catalyst
3.3. Results of DFT Calculations and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fubini, B.; Otero Areán, C. Chemical Aspects of the Toxicity of Inhaled Mineral Dusts. Chem. Soc. Rev. 1999, 28, 373–381. [Google Scholar] [CrossRef]
- Dalko, P.I.; Moisan, L. Enantioselective Organocatalysis. Angew. Chem. Int. Ed. Engl. 2001, 40, 3726–3748. [Google Scholar] [CrossRef]
- Taylor, M.S.; Jacobsen, E.N. Asymmetric Catalysis by Chiral Hydrogen-Bond Donors. Angew. Chem. Int. Ed. 2006, 45, 1520–1543. [Google Scholar] [CrossRef] [PubMed]
- Raj, M.; Vishnumaya, V.; Ginotra, S.K.; Singh, V.K. Highly Enantioselective Direct Aldol Reaction Catalyzed by Organic Molecules. Org. Lett. 2006, 8, 4097–4099. [Google Scholar] [CrossRef]
- Ji-Mao, L.; Ben-Sheng, L. A Practical Method for the Preparation of Trimethylsilyl Enol Ethers. Synth. Commun. 1997, 27, 739–749. [Google Scholar] [CrossRef]
- Martinez, I.; Alford, P.E.; Ovaska, T.V. First Approach to the Frondosin C Ring System via a Tandem Cyclization/Claisen Rearrangement Sequence. Org. Lett. 2005, 7, 1133–1135. [Google Scholar] [CrossRef] [PubMed]
- Momiyama, N.; Yamamoto, H. Catalytic Enantioselective Synthesis of α-Aminooxy and α-Hydroxy Ketone Using Nitro sobenzene. J. Am. Chem. Soc. 2003, 125, 6038–6039. [Google Scholar] [CrossRef]
- Momiyama, N.; Yamamoto, H. Lewis Acid Promoted, O-Selective, Nucleophilic Addition of Silyl Enol Ethers to NdoublebondO Bonds. Angew. Chem. Int. Ed. Engl. 2002, 41, 2986–2988. [Google Scholar] [CrossRef]
- Mukaiyama, T.; Uchiro, H.; Kobayashi, S. A New Efficient Chiral Catalyst System. Combined Use of Tin(II) Oxide, Trime thylsilyl Triflate and Chiral Diamine in the Asymmetric Aldol Reaction. Chem. Lett. 1990, 19, 1147–1150. [Google Scholar] [CrossRef]
- Hollis, T.K.; Bosnich, B. Homogeneous Catalysis. Mechanisms of the Catalytic Mukaiyama Aldol and Sakurai Allylation Reactions. J. Am. Chem. Soc. 1995, 117, 4570–4581. [Google Scholar] [CrossRef]
- Surman, M.D.; Miller, M.J. Regio- and Stereochemically Controlled Formation of Hydroxamic Acid Containing Anti- or Syn-1,4-Cycloalkenols from Acylnitroso-Derived Diels-Alder Adducts. J. Org. Chem. 2001, 66, 2466–2469. [Google Scholar] [CrossRef] [PubMed]
- Adam, W.; Bottke, N. Hydroxy-Group Directivity in the Nitroso Ene Reaction: Diastereo- and Regioselective Amination of Chiral Allylic Alcohols. J. Am. Chem. Soc. 2000, 122, 9846–9847. [Google Scholar] [CrossRef]
- Shim, J.H.; Park, S.J.; Byung, K.A.; Lee, J.Y.; Kim, K.H.; Ha, D.C. Enantioselective Thiolysis and Aminolysis of Cyclic Anhydrides Using a Chiral Diamine-Derived Thiourea Catalyst. ACS Omega 2021, 6, 34501. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.R.; Momiyama, N.; Yamamoto, H. Metal-induced reactions of O-nitroso aldol products. Synlett 2006, 5, 705–708. [Google Scholar]
- Momiyama, N.; Yamamoto, H. Enantioselective O- and N-Nitroso Aldol Synthesis of Tin Enolates. Isolation of Three BINAP-Silver Complexes and Their Role in Regio- and Enantioselectivity. J. Am. Chem. Soc. 2004, 126, 5360–5361. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.; Candeias, N.R.; Barbas, C.F. III. Dimeric Quinidine-Catalyzed Enantioselective Aminooxygenation of Oxindoles: An Organocatalytic Approach to 3-Hydroxyoxindole Derivatives. J. Am. Chem. Soc. 2010, 132, 5574–5575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Cheng, L.; Liu, L.; Wang, D.; Chen, Y.J. Asymmetric Organocatalytic N-Nitroso-Aldol Reaction of Oxindoles. Tetrahedron Asymmetry 2010, 21, 2800–2806. [Google Scholar] [CrossRef]
- Shen, K.; Liu, X.H.; Wang, G.; Lin, L.L.; Feng, X.M. Facile and Efficient Enantioselective Hydroxyamination Reaction: Synthesis of 3-Hydroxyamino-2-Oxindoles Using Nitrosoarenes. Angew. Chem. Int. Ed. 2011, 50, 4684–4688. [Google Scholar] [CrossRef]
- Jia, L.N.; Huang, J.; Peng, L.; Wang, L.L.; Bai, J.F.; Tian, F.; He, G.Y.; Xu, X.Y.; Wang, L.X. Asymmetric Hydroxyamination of Oxindoles Catalyzed by Chiral Bifunctional Tertiary Amine Thiourea: Construction of 3-Amino-2-Oxindoles with Quaternary Stereocenters. Org. Biomol. Chem. 2012, 10, 236–239. [Google Scholar] [CrossRef]
- Companyó, X.; Valero, G.; Pineda, O.; Calvet, T.; Font-Bardía, M.; Moyano, A.; Rios, R. Enantioselective Organocatalytic Oxyamination of Unprotected 3-Substituted Oxindoles. Org. Biomol. Chem. 2012, 10, 431–439. [Google Scholar] [CrossRef]
- Mailhol, D.; Castillo, J.C.; Mohanan, K.; Abonia, R.; Coquerel, Y.; Rodriguez, J. Practical and Efficient Organocatalytic Enantioselective α-Hydroxyamination Reactions of β-Ketoamides. ChemCatChem 2013, 5, 1192–1199. [Google Scholar] [CrossRef]
- Sun, Q.S.; Zhu, H.; Chen, Y.J.; Yang, X.D.; Sun, X.W.; Lin, G.Q. Squaramide-Catalyzed Synthesis of Enantioenriched Spirocyclic Oxindoles via Ketimine Intermediates with Multiple Active Sites. Angew. Chem. Int. Ed. 2015, 54, 13253–13257. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; He, W.W.; Liu, X.Y.; Tan, B. Asymmetric Construction of Spirooxindoles by Organocatalytic Multicomponent Reactions Using Diazooxindoles. Angew. Chem. Int. Ed. 2015, 54, 9409–9413. [Google Scholar] [CrossRef] [PubMed]
- Mouri, S.; Chen, Z.H.; Mitsunuma, H.; Furutachi, M.; Matsunaga, S.; Shibasaki, M. Catalytic Asymmetric Synthesis of 3-Aminooxindoles: Enantiofacial Selectivity Switch in Bimetallic vs Monometallic Schiff Base Catalysis. J. Am. Chem. Soc. 2010, 132, 1255–1257. [Google Scholar] [CrossRef]
- Liu, L.W.; Wang, F.Y.; Tian, F.; Peng, L.; Wang, L.X. An Improved and Enantioselective Preparation of the Telaprevir Bicyclic [3.3.0] Proline Intermediate and Reuse of Unwanted Enantiomer. Org. Process Res. Dev. 2016, 20, 320–324. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhou, J. Organocatalytic Asymmetric Cyanation of Isatin Derived N-Boc Ketoimines. Chem. Commun. 2013, 49, 4421–4423. [Google Scholar] [CrossRef]
- Fu, J.Y.; Wang, Q.L.; Gui, Y.Y.; Wang, L.X. Direct Enantioselective Amination of α-Ketoester Catalyzed by Tertiary Amine Thiourea: A New Approach to Chiral α-Hydroxy-β-Amino Acid. Tetrahedron Letters. 2015, 56, 4220–4223. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.J.; Zhou, Q.Q.; Dong, L.; Chen, Y.C. Organocatalytic Asymmetric Allylic Amination of Morita-Baylis-Hillman Carbonates of Isatins. Beilstein J. Org. Chem. 2012, 8, 1241–1245. [Google Scholar] [CrossRef]
- Payette, J.N.; Yamamoto, H. Nitrosobenzene-Mediated C-C Bond Cleavage Reactions and Spectral Observation of an Oxazetidin-4-One Ring System. J. Am. Chem. Soc. 2008, 130, 12276–12278. [Google Scholar] [CrossRef] [Green Version]
- Momiyama, N.; Yamamoto, H. Bronsted Acid Catalysis of Achiral Enamine for Regio- and Enantioselective Nitroso Aldol Synthesis. J. Am. Chem. Soc. 2005, 127, 1080–1081. [Google Scholar] [CrossRef] [Green Version]
- Córdova, A. The Direct Catalytic Asymmetric Mannich Reaction. Acc. Chem. Res. 2004, 37, 102–112. [Google Scholar] [CrossRef] [PubMed]
- List, B.; Lerner, R.A.; Barbas, C.F., III. Proline-catalyzed direct asymmetric aldol reactions. J. Am. Chem. Soc. 2000, 122, 2395–2396. [Google Scholar] [CrossRef]
- Seayad, J.; List, B. Asymmetric Organocatalysis. Org. Biomol. Chem. 2005, 3, 719–724. [Google Scholar] [CrossRef]
- Mase, N.; Nakai, Y.; Ohara, N.; Yoda, H.; Takabe, K.; Tanaka, F.; Barbas, C.F. Organocatalytic Direct Asymmetric Aldol Reactions in Water. J. Am. Chem. Soc. 2006, 128, 734–735. [Google Scholar] [CrossRef] [PubMed]
- Momiyama, N.; Torii, H.; Saito, S.; Yamamoto, H. O-Nitroso Aldol Synthesis: Catalytic Enantioselective Route to α-Aminooxy Carbonyl Compounds via Enamine Intermediate. Proc. Natl. Acad. Sci. USA 2004, 101, 5374–5378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, Y.; Yamaguchi, J.; Sumiya, T.; Hibino, K.; Shoji, M. Direct Proline-Catalyzed Asymmetric Alpha-Aminoxylation of Aldehydes and Ketones. J. Org. Chem. 2004, 69, 5966–5973. [Google Scholar] [CrossRef] [PubMed]
- Córdova, A.; Sundén, H.; Bøgevig, A.; Johansson, M.; Himo, F. The Direct Catalytic Asymmetric Alpha-Aminooxylation Reaction: Development of Stereoselective Routes to 1,2-Diols and 1,2-Amino Alcohols and Density Functional Calculations. Chemistry 2004, 10, 3673–3684. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yamaguchi, J.; Hibino, K.; Sumiya, T.; Urushima, T.; Shoji, M.; Hashizume, D.; Koshino, H. A Highly Active 4-Siloxyproline Catalyst for Asymmetric Synthesis. Adv. Synth. Catal. 2004, 346, 1435–1439. [Google Scholar] [CrossRef]
- Shim, J.H.; Kim, M.J.; Lee, J.Y.; Kim, K.H.; Ha, D.C. Organocatalytic Asymmetric Aldol Reaction Using Protonated Chiral 1,2-Diamines. Tetrahedron Lett. 2020, 61, 152295. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. Mol. Phys. 1970, 19, 55341. [Google Scholar] [CrossRef]







| Entry | Solvent | Time | Yield (%) a | ee (%) b |
|---|---|---|---|---|
| 1 | Brine | 1 h | 65 | 94 |
| 2 | DMSO | 1 h | 58 | 91 |
| 3 | CH3CN | 1 h | 52 | 90 |
| 4 | MeOH | 1 h | 50 | 90 |
| 5 | - | 1 h | 46 | 92 |
| 6 | CH2Cl2 | 1 h | 42 | 81 |
| 7 | THF | 1 h | 40 | 94 |
| 8 | Toluene | 15 min | 25 | 86 |

| Entry | Cat | Yield (%) a | ee (%) b |
|---|---|---|---|
| 1 | 1a | 82 | 99 |
| 2 | 1b | 65 | 94 |
| 3 | 1c | 61 | 96 |
| 4 | 1d | 53 | 98 |
| 5 | 1e | 49 | 98 |

| Entry | Cyclohexanone: Nitrobenzene | Temp (°C) | Time (h) | Yield (%) a | ee (%) b |
|---|---|---|---|---|---|
| 1 | 1:1 | rt | 1 | 55 | 92 |
| 2 | 2:1 | 0 | 1.5 | 88 | 98 |
| 3 | 2:1 | –10 | 3 | 95 | 99 |
| 4c | 2:1 | –10 | 6 | 95 | 99 |
| 5d | 2:1 | –10 | 24 | 52 | 99 |
| 6 | 1:2 | –10 | 2 | 71 | 82 |
| 7 | 1:5 | –10 | 2 | 65 | 80 |
| 8e | 2:1 | –10 | 6 | 98 | 99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, J.H.; Lee, J.Y.; Kim, H.S.; Ha, D.-C. Protonated Chiral 1,2-Diamine Organocatalysts for N-Selective Nitroso Aldol Reaction. Catalysts 2022, 12, 435. https://doi.org/10.3390/catal12040435
Shim JH, Lee JY, Kim HS, Ha D-C. Protonated Chiral 1,2-Diamine Organocatalysts for N-Selective Nitroso Aldol Reaction. Catalysts. 2022; 12(4):435. https://doi.org/10.3390/catal12040435
Chicago/Turabian StyleShim, Jae Ho, Ji Yeon Lee, Hyeon Soo Kim, and Deok-Chan Ha. 2022. "Protonated Chiral 1,2-Diamine Organocatalysts for N-Selective Nitroso Aldol Reaction" Catalysts 12, no. 4: 435. https://doi.org/10.3390/catal12040435
APA StyleShim, J. H., Lee, J. Y., Kim, H. S., & Ha, D.-C. (2022). Protonated Chiral 1,2-Diamine Organocatalysts for N-Selective Nitroso Aldol Reaction. Catalysts, 12(4), 435. https://doi.org/10.3390/catal12040435






