Catalytic Oxidation of Chlorobenzene over Ce-Mn-Ox/TiO2: Performance Study of the Porous Structure
Abstract
:1. Introduction
2. Results and Discussion
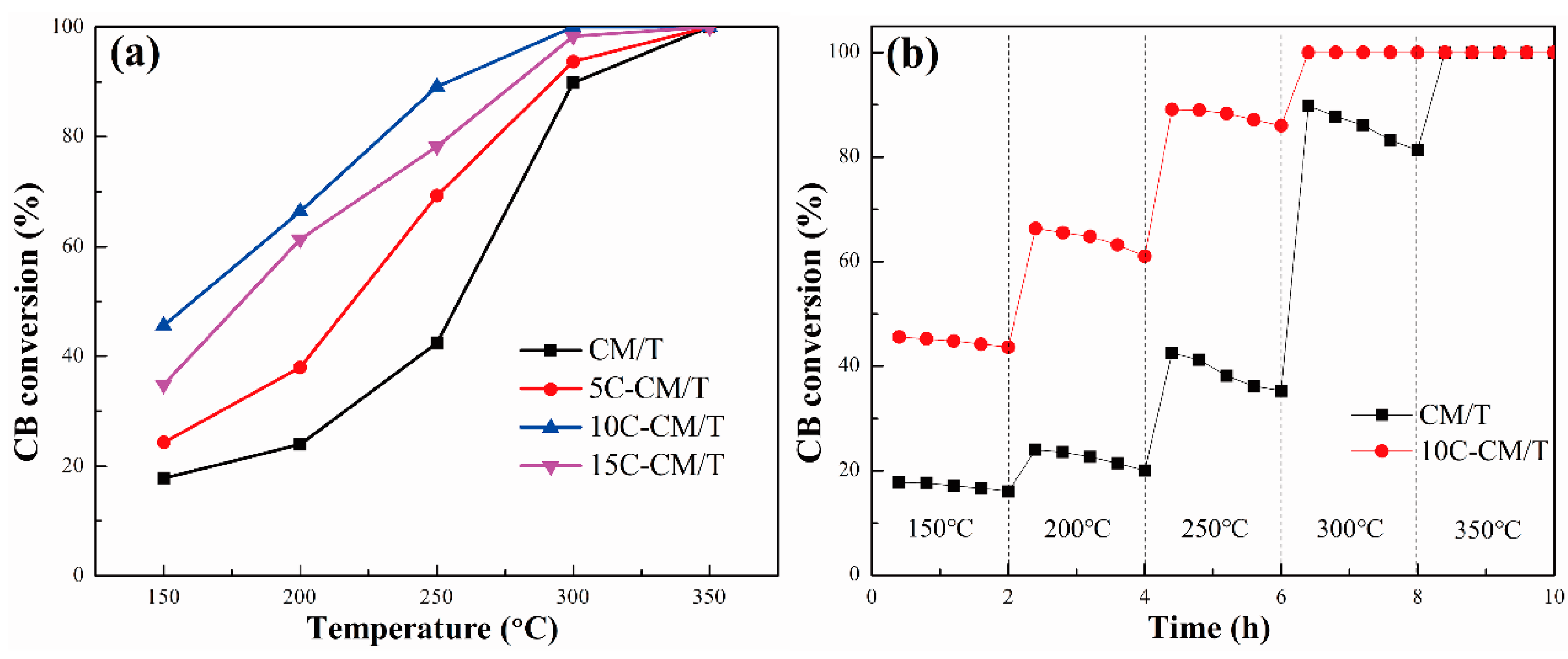
2.1. Catalytic Performance
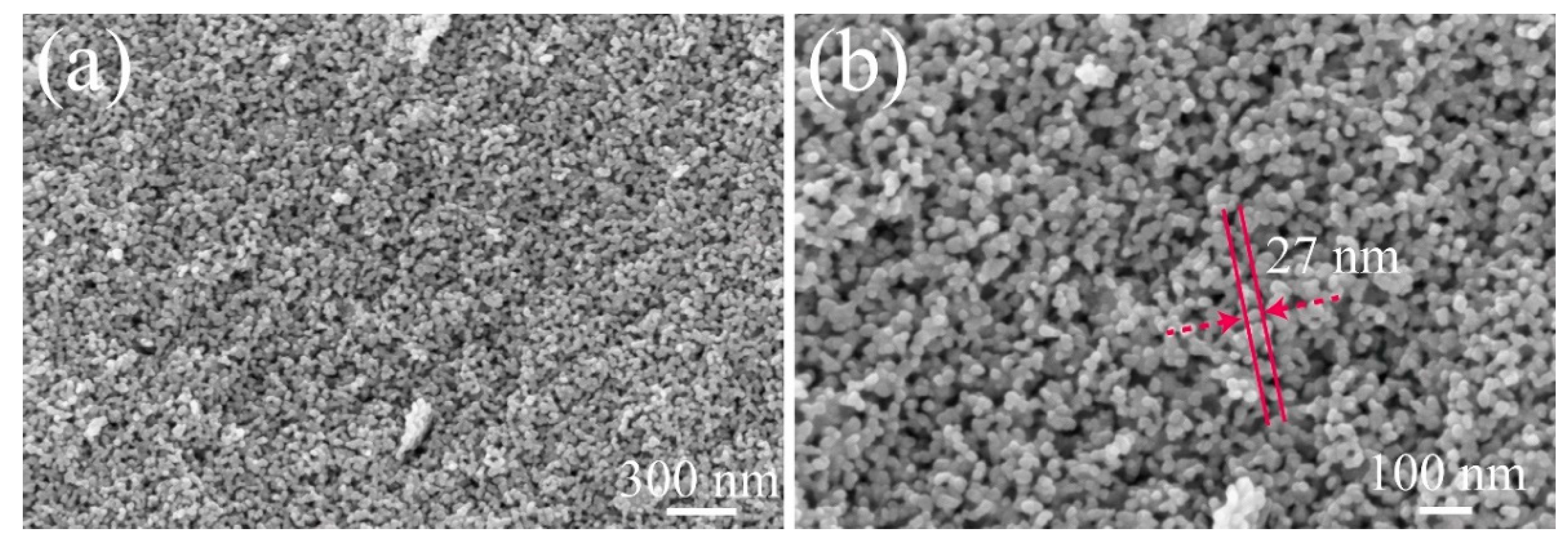
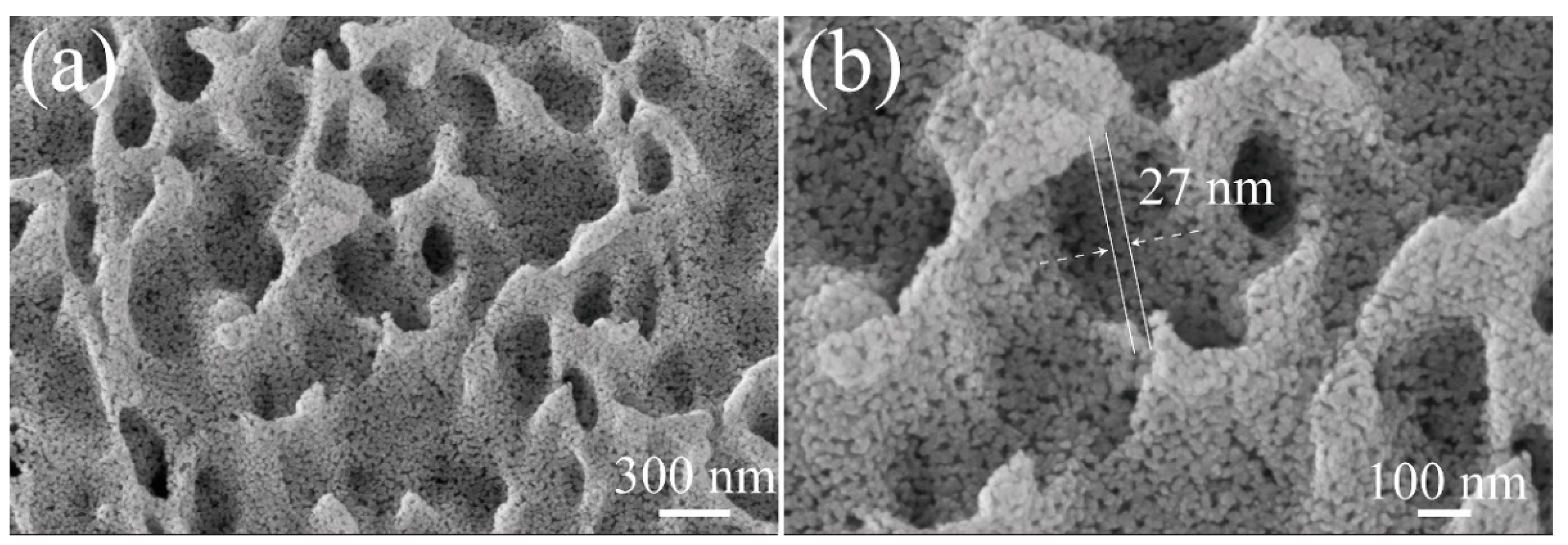
2.2. Structure Characterizations
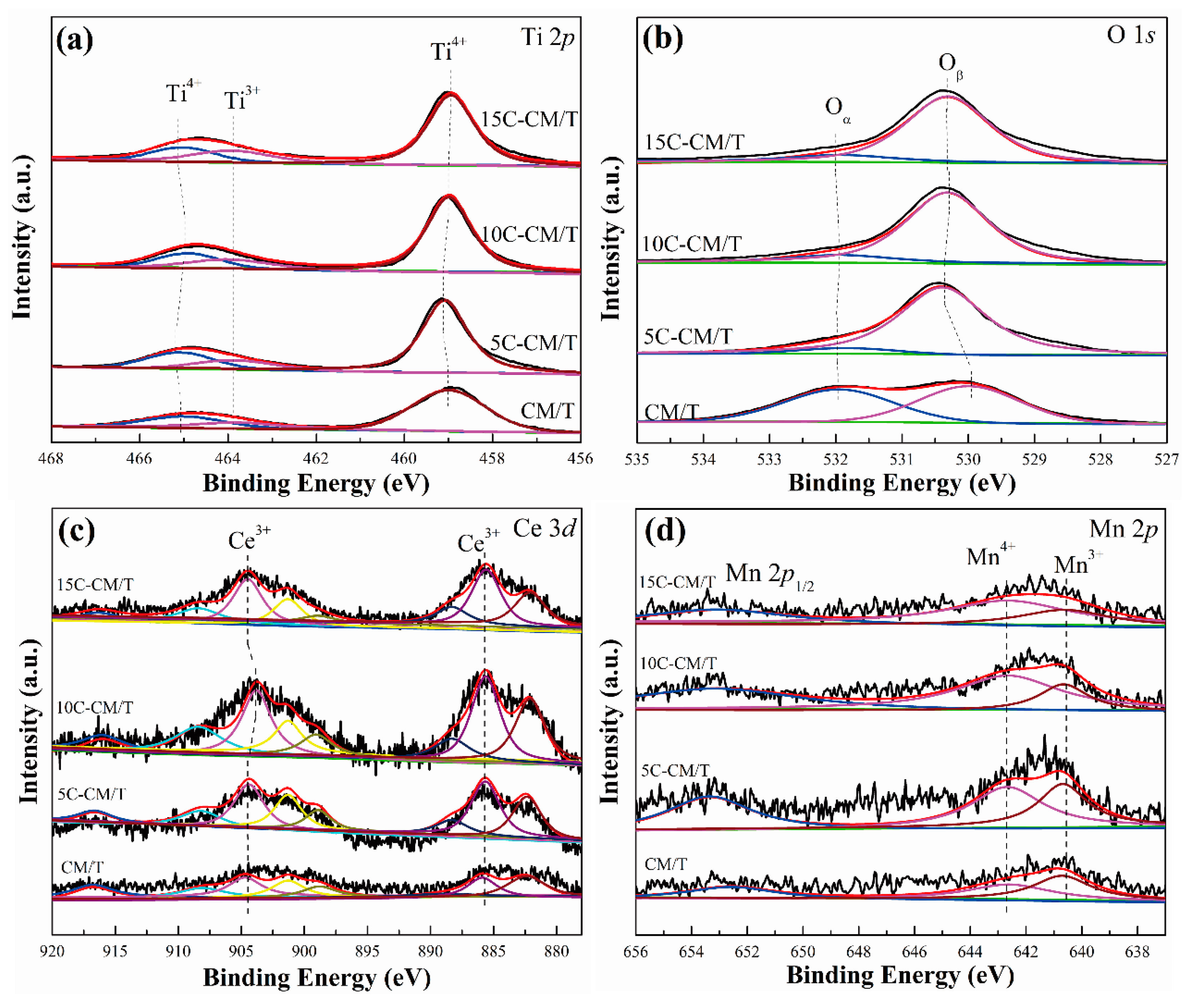
2.3. Surface Analysis
2.4. Reaction Mechanism




3. Experiment
3.1. Material Preparation
3.2. Catalytic Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Meng, Q.; Liu, J.; Jiang, W.; Pattisson, S.; Wu, Z. Catalytic Oxidation of Chlorinated Organics over Lanthanide Perovskites: Effects of Phosphoric Acid Etching and Water Vapor on Chlorine Desorption Behavior. Environ. Sci. Technol. 2018, 53, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lei, C.; Wei, C.; Zeng, G. Chlorinated volatile organic compounds (Cl-VOCs) in environment-sources, potential human health impacts, and current remediation technologies. Environ. Int. 2014, 71, 118–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, X.; Yan, D.; Jiang, M.; Xu, W.; Yu, H.; Jia, H. A facile strategy of enhancing interaction between cerium and manganese oxides for catalytic removal of gaseous organic contaminants. Appl. Catal. B Environ. 2019, 250, 396–407. [Google Scholar] [CrossRef]
- Zhang, T.; Lang, X.; Dong, A.; Wan, X.; Shan, G.; Wang, L.; Wang, L.; Wang, W. Difference of Oxidation Mechanism between Light C3-C4 Alkane and Alkene over Mullite YMn2O5 Oxides’ Catalyst. ACS Catal. 2020, 10, 7269–7282. [Google Scholar] [CrossRef]
- Liu, L.; Sun, J.; Ding, J.; Zhang, Y.; Sun, T.; Jia, J. Highly Active Mn3–xFexO4 Spinel with Defects for Toluene Mineralization: Insights into Regulation of the Oxygen Vacancy and Active Metals. Inorg. Chem. 2019, 58, 13241–13249. [Google Scholar] [CrossRef]
- Liu, L.; Sun, J.; Ding, J.; Zhang, Y.; Jia, J.; Sun, T. Catalytic Oxidation of VOCs over SmMnO3 Perovskites: Catalyst Synthesis, Change Mechanism of Active Species, and Degradation Path of Toluene. Inorg. Chem. 2019, 58, 14275–14283. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y.C. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Puértolas, B.; Smith, A.; Vázquez, I.; Dejoz, A.; Moragues, A.; Garcia, T.; Solsona, B. The different catalytic behaviour in the propane total oxidation of cobalt and manganese oxides prepared by a wet combustion procedure. Chem. Eng. J. 2013, 229, 547–558. [Google Scholar] [CrossRef]
- Ren, Z.; Wu, Z.; Song, W.; Xiao, W.; Guo, Y.; Ding, J.; Suib, S.L.; Gao, P.-X. Low temperature propane oxidation over Co3O4 based nano-array catalysts: Ni dopant effect, reaction mechanism and structural stability. Appl. Catal. B Environ. 2015, 180, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Xu, W.; Jiang, M.; Jia, H. Polyoxometallate functionalizing CeO2 via redox-etching precipitation to synergistically catalyze oxidation of gaseous chlorinated pollutants: From lab to practice. Appl. Catal. B Environ. 2020, 278, 119263. [Google Scholar] [CrossRef]
- Yang, W.; Su, Z.; Xu, Z.; Yang, W.; Peng, Y.; Li, J. Comparative study of alpha-, beta-, gamma- and delta-MnO2 on toluene oxidation: Oxygen vacancies and reaction intermediates. Appl. Catal. B-Environ. 2020, 260, 118150. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, S.; Wang, S.; Zhang, K.; Wang, H.; Huang, J.; Deng, S.; Wang, B.; Wang, Y.; Yu, G. Ball Milling Synthesized MnOx as Highly Active Catalyst for Gaseous POPs Removal: Significance of Mechanochemically Induced Oxygen Vacancies. Environ. Sci. Technol. 2015, 49, 4473–4480. [Google Scholar] [CrossRef]
- Li, K.; Chen, C.; Zhang, H.; Hu, X.; Sun, T.; Jia, J. Effects of phase structure of MnO2 and morphology of delta-MnO2 on toluene catalytic oxidation. Appl. Surf. Sci. 2019, 496, 143662. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, H.; Zhou, Y.; Zhang, L.; Wu, Z.; Yang, S.; Shi, H.; Zhu, Q.; Chen, Y.; Dai, S. Mesoporous MnCeOx solid solutions for low temperature and selective oxidation of hydrocarbons. Nat. Commun. 2015, 6, 8446. [Google Scholar] [CrossRef]
- Tang, X.F.; Li, Y.G.; Huang, X.M.; Xu, Y.D.; Zhu, H.Q.; Wang, J.G.; Shen, W.J. MnOx-CeO2 mixed oxide catalysts for complete oxidation of formaldehyde: Effect of preparation method and calcination temperature. Appl. Catal. B-Environ. 2006, 62, 265–273. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.; Rong, S.; Wang, H.; Zhang, P. Cerium modified birnessite-type MnO2 for gaseous formaldehyde oxidation at low temperature. Appl. Catal. B Environ. 2017, 211, 212–221. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, J.; Yuan, J.; Cai, T.; Xiao, B.; Liu, Z.; Zhao, K.; Yang, L.; He, D. Excellent low-temperature catalytic performance of nanosheet Co-Mn oxides for total benzene oxidation. Appl. Catal. Gen. 2018, 566, 104–112. [Google Scholar] [CrossRef]
- Hu, J.; Li, W.; Liu, R. Highly efficient copper-doped manganese oxide nanorod catalysts derived from CuMnO hierarchical nanowire for catalytic combustion of VOCs. Catal. Today 2018, 314, 147–153. [Google Scholar] [CrossRef]
- Dong, C.; Qu, Z.; Jiang, X.; Ren, Y. Tuning oxygen vacancy concentration of MnO2 through metal doping for improved toluene oxidation. J. Hazard. Mater. 2020, 391, 122181. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Xu, W.; Xu, Z.; Chen, J.; Jia, H.; Chen, J. Hydrolysis driving redox reaction to synthesize Mn-Fe binary oxides as highly active catalysts for the removal of toluene. Chem. Eng. J. 2017, 330, 281–293. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Chen, X.; Xu, W.; Xu, Z.; Jia, H.; Chen, J. Homogeneous introduction of CeOy into MnOx-based catalyst for oxidation of aromatic VOCs. Appl. Catal. B-Environ. 2018, 224, 825–835. [Google Scholar] [CrossRef]
- Mei, J.; Zhao, S.; Huang, W.; Qu, Z.; Yan, N. Mn-Promoted Co3O4/TiO2 as an efficient catalyst for catalytic oxidation of dibromomethane (CH2Br2). J. Hazard. Mater. 2016, 318, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Dutta, A.; Bhaumik, A. Highly Ordered Mesoporous TiO2–Fe2O3 Mixed Oxide Synthesized by Sol–Gel Pathway: An Efficient and Reusable Heterogeneous Catalyst for Dehalogenation Reaction. ACS Appl. Mater. Interfaces 2012, 4, 5022–5028. [Google Scholar] [CrossRef]
- Avijit, J.; John, M.; Parijat, B.; Sujan, M.; Asim, B.; Zhao, Y. Ruthenium bipyridyl tethered porous organosilica: A versatile, durable and reusable heterogeneous photocatalyst. Chem. Commun. 2015, 51, 10746–10749. [Google Scholar]
- Liao, Y.; Meng, X.; Yang, Y.; Shi, L.; Liu, N. Synthesis of diphenylamine by condensation of aniline catalyzed by microporous-mesoporous composite solid acid nanoreactor. Mol. Catal. 2022, 520, 112134. [Google Scholar] [CrossRef]
- Pi, X.; Qu, Z.; Sun, F.; Zhang, Z.; Gao, J. Catalytic activation preparation of nitrogen-doped hierarchical porous bio-char for efficient adsorption of dichloromethane and toluene. J. Anal. Appl. Pyrolysis 2021, 156, 105150. [Google Scholar] [CrossRef]
- Yan, D.; Chen, Z.; Ma, M.; Yu, Y.; Liu, Q.; He, C. Hierarchical Cu-Mn/ZSM-5 with boosted activity and selectivity for n-butylamine destruction: Synergy of pore structure and surface acidity. Appl. Catal. A Gen. 2022, 636, 118579. [Google Scholar] [CrossRef]
- Lietti, L.; Nova, I.; Tronconi, E.; Forzatti, P. Transient kinetic study of the SCR-DeNOx reaction. Catal. Today 1998, 45, 85–92. [Google Scholar] [CrossRef]
- Qu, W.; Liu, X.; Chen, J.; Dong, Y.; Tang, X.; Chen, Y. Single-atom catalysts reveal the dinuclear characteristic of active sites in NO selective reduction with NH3. Nat. Commun. 2020, 11, 1532. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.J.; He, G.Z.; Shan, W.P.; Yu, Y.B.; Huo, Y.L.; Zhang, Y.; Wang, M.; Yu, R.; Liu, S.S.; He, H. Introducing tin to develop ternary metal oxides with excellent hydrothermal stability for NH3 selective catalytic reduction of NOx. Appl. Catal. B 2021, 291, 120125. [Google Scholar] [CrossRef]
- Ning, P.; Song, Z.; Li, H.; Zhang, Q.; Liu, X.; Zhang, J.; Tang, X.; Huang, Z. Selective catalytic reduction of NO with NH3 over CeO2–ZrO2–WO3 catalysts prepared by different methods. Appl. Surf. Sci. 2015, 332, 130–137. [Google Scholar] [CrossRef]
- Jin, Q.; Shen, Y.; Ma, L.; Pan, Y.; Zhu, S.; Zhang, J.; Zhou, W.; Wei, X.; Li, X. Novel TiO2 catalyst carriers with high thermostability for selective catalytic reduction of NO by NH3. Catal. Today 2019, 327, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.Y.; Yu, Y.L.; Bi, F.; Sun, P.F.; Weng, X.L.; Wu, Z.B. Synergistic elimination of NOx and chloroaromatics on a commercial V2O5-WO3/TiO2 catalyst: Byproduct analyses and the SO2 effect. Environ. Sci. Technol. 2019, 53, 12657–12667. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.; Wang, Z.; Hardacre, C.; Liu, Z.; Chansai, S.; Stere, C. Promoting effect of Au on Pd/TiO2 catalyst for the selective catalytic reduction of NOx by H2. Catal. Today 2019, 332, 69–75. [Google Scholar] [CrossRef]
- Lee, K.; Choi, B.; Lee, C.; Oh, K. Effects of SiO2/Al2O3 ratio, reaction atmosphere and metal additive on de-NOx performance of HC-SCR over Cu-based ZSM-5. J. Ind. Eng. Chem. 2020, 90, 132–144. [Google Scholar] [CrossRef]
- Jin, Q.J.; Chen, M.M.; Tao, X.J.; Lu, B.X.; Shen, J.Y.; Shen, Y.S.; Zeng, Y.W. Component synergistic catalysis of Ce-Sn-W-Ba-Ox/TiO2 in selective catalytic reduction of NO with ammonia. Appl. Surf. Sci. 2020, 512, 145757. [Google Scholar] [CrossRef]
- Zhang, S.H.; Li, Y.Y.; Huang, J.H.; Lee, J.; Kim, D.H.; Frenkel, A.I.; Kim, T. Effects of molecular and electronic structures in CoOx/CeO2 catalysts on NO reduction by CO. J. Phys. Chem. C 2019, 123, 7166–7177. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Guo, R.-T.; Guan, Z.-Z.; Shi, X.; Pan, W.-G.; Fu, Z.-G.; Qin, H.; Liu, X.-Y. The promotion effect of Cr additive on CeZr2Ox catalyst for the low-temperature selective catalytic reduction of NOx with NH3. Appl. Surf. Sci. 2019, 485, 133–140. [Google Scholar] [CrossRef]
- Tian, X.; Lin, B.N.; Li, Y.P.; Wang, S.; Zhou, Y.H.; Zhong, H. CeO2-MnOx composite loaded on Al2O3 as a catalyst for HCl oxidation. Catal. Sci. Technol. 2020, 10, 4553–4561. [Google Scholar] [CrossRef]
- Yao, X.J.; Cao, J.; Chen, L.; Kang, K.K.; Chen, Y.; Tian, M.; Yang, F.M. Doping effect of cations (Zr4+, Al3+, and Si4+) on MnOx/CeO2 nano-rod catalyst for NH3-SCR reaction at low temperature. Chin. J. Catal. 2019, 40, 733–743. [Google Scholar] [CrossRef]
- Chen, L.; Yao, X.J.; Cao, J.; Yang, F.M.; Tang, C.J.; Dong, L. Effect of Ti4+ and Sn4+ co-incorporation on the catalytic performance of CeO2-MnOx catalyst for low temperature NH3-SCR. Appl. Surf. Sci. 2019, 476, 283–292. [Google Scholar] [CrossRef]
- Huang, X.; Wang, D.; Yang, Q.; Peng, Y.; Li, J. Multi-pollutant control (MPC) of NO and chlorobenzene from industrial furnaces using a vanadia-based SCR catalyst. Appl. Catal. B Environ. 2020, 285, 119835. [Google Scholar] [CrossRef]
- Smirnov, M.; Graham, G. Pd oxidation under UHV in a model Pd/ceria–zirconia catalyst. Catal. Lett. 2001, 72, 39–44. [Google Scholar] [CrossRef]
- Huang, H.; Gu, Y.F.; Zhao, J.; Wang, X.Y. Catalytic combustion of chlorobenzene over VOx/CeO2 catalysts. J. Catal. 2015, 326, 54–68. [Google Scholar] [CrossRef]
- Lichtenberger, J.; Amiridis, M.D. Catalytic oxidation of chlorinated benzenes over V2O5/TiO2 catalysts. J. Catal. 2004, 2223, 96–308. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Rivas, J.A.; Amiridis, M.D. Catalytic Oxidation of 1,2-Dichlorobenzene over Supported Transition Metal Oxides. J. Catal. 2000, 193, 264–272. [Google Scholar] [CrossRef]
- Li, L.; Shi, J.W.; Tian, M.J.; Chen, C.W.; Wang, B.R.; Ma, M.D.; He, C. In situ fabrication of robust three dimensional ordered macroporous γ-MnO2/LaMnO3.15 catalyst for chlorobenzene efficient destruction. Appl. Catal. B 2021, 282, 119565. [Google Scholar] [CrossRef]

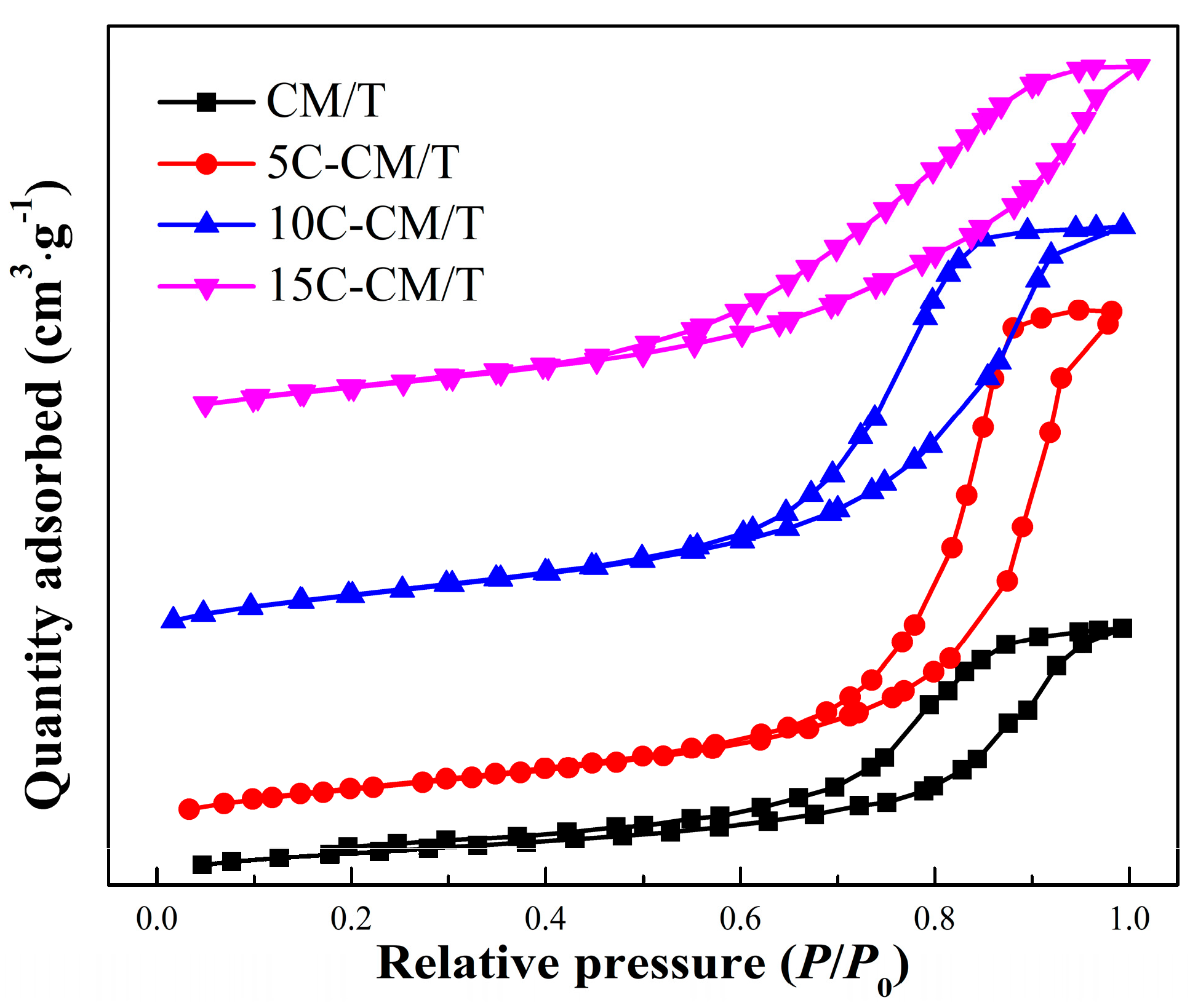

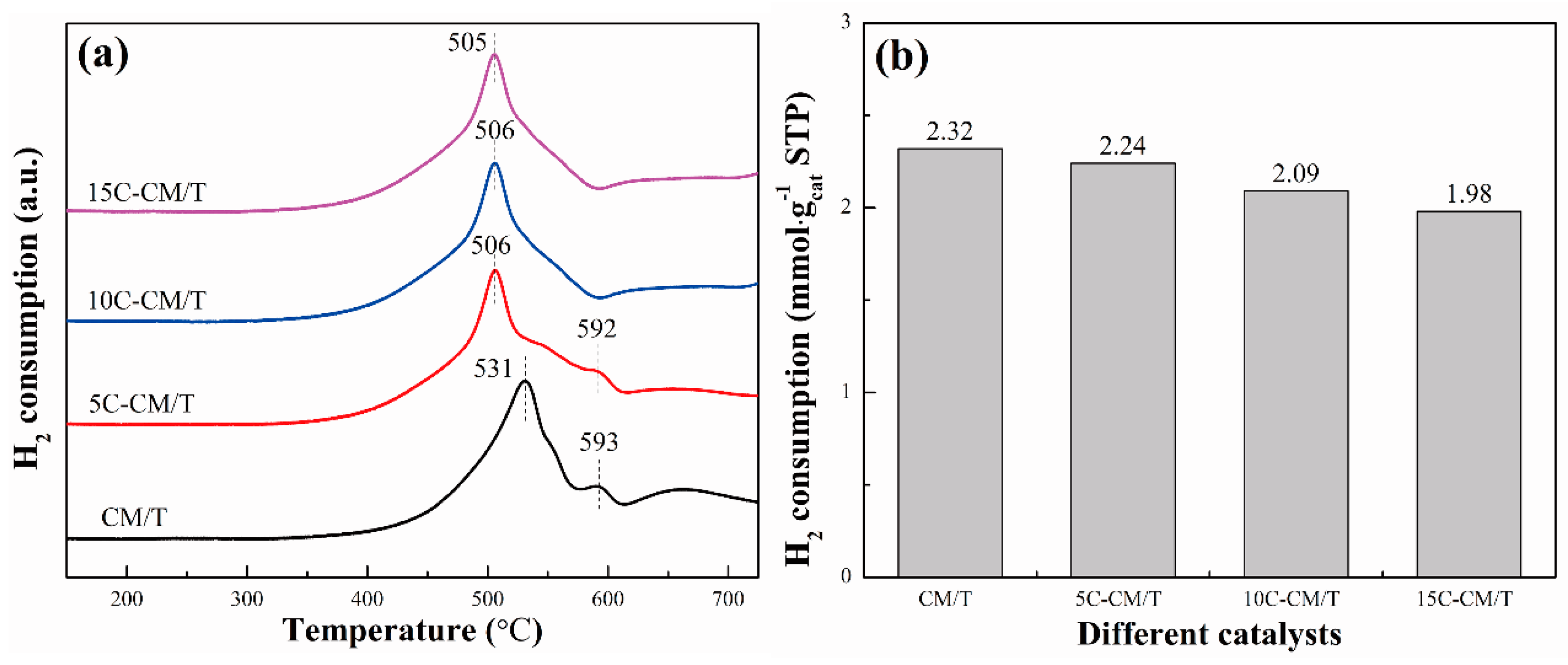

| Sample | BET Surface Area/(m2·g−1) | Pore Volume/(cm3·g−1) | Average Pore Diameter/nm |
|---|---|---|---|
| CM/T | 82.6 | 0.142 | 20.3 |
| 5C-CM/T | 101.4 | 0.465 | 21.8 |
| 10C-CM/T | 137.5 | 0.363 | 10.6 |
| 15C-CM/T | 123.4 | 0.284 | 9.9 |
| Sample | Ti3+/(Ti3+ + Ti4+) | Oα/(Oα + Oβ) | Ce3+/(Ce3+ + Ce4+) | Mn4+/(Mn3+ + Mn4+) |
|---|---|---|---|---|
| CM/T | 0.15 | 0.49 | 0.29 | 0.49 |
| 5C-CM/T | 0.12 | 0.10 | 0.39 | 0.59 |
| 10C-CM/T | 0.17 | 0.09 | 0.40 | 0.73 |
| 15C-CM/T | 0.18 | 0.07 | 0.43 | 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Ni, M.; Gu, Q.; Huang, Q.; Xu, L.; Chen, M.; Jin, Q.; Wang, Z. Catalytic Oxidation of Chlorobenzene over Ce-Mn-Ox/TiO2: Performance Study of the Porous Structure. Catalysts 2022, 12, 535. https://doi.org/10.3390/catal12050535
Yang B, Ni M, Gu Q, Huang Q, Xu L, Chen M, Jin Q, Wang Z. Catalytic Oxidation of Chlorobenzene over Ce-Mn-Ox/TiO2: Performance Study of the Porous Structure. Catalysts. 2022; 12(5):535. https://doi.org/10.3390/catal12050535
Chicago/Turabian StyleYang, Bo, Maosen Ni, Qiuxiang Gu, Qiong Huang, Leilei Xu, Mindong Chen, Qijie Jin, and Zhenhui Wang. 2022. "Catalytic Oxidation of Chlorobenzene over Ce-Mn-Ox/TiO2: Performance Study of the Porous Structure" Catalysts 12, no. 5: 535. https://doi.org/10.3390/catal12050535







