Yttria-Stabilized Zirconia of Balanced Acid-Base Pair for Selective Dehydration of 4-Methyl-2-pentanol to 4-Methyl-1-pentene
Abstract
:1. Introduction
2. Results and Discussion
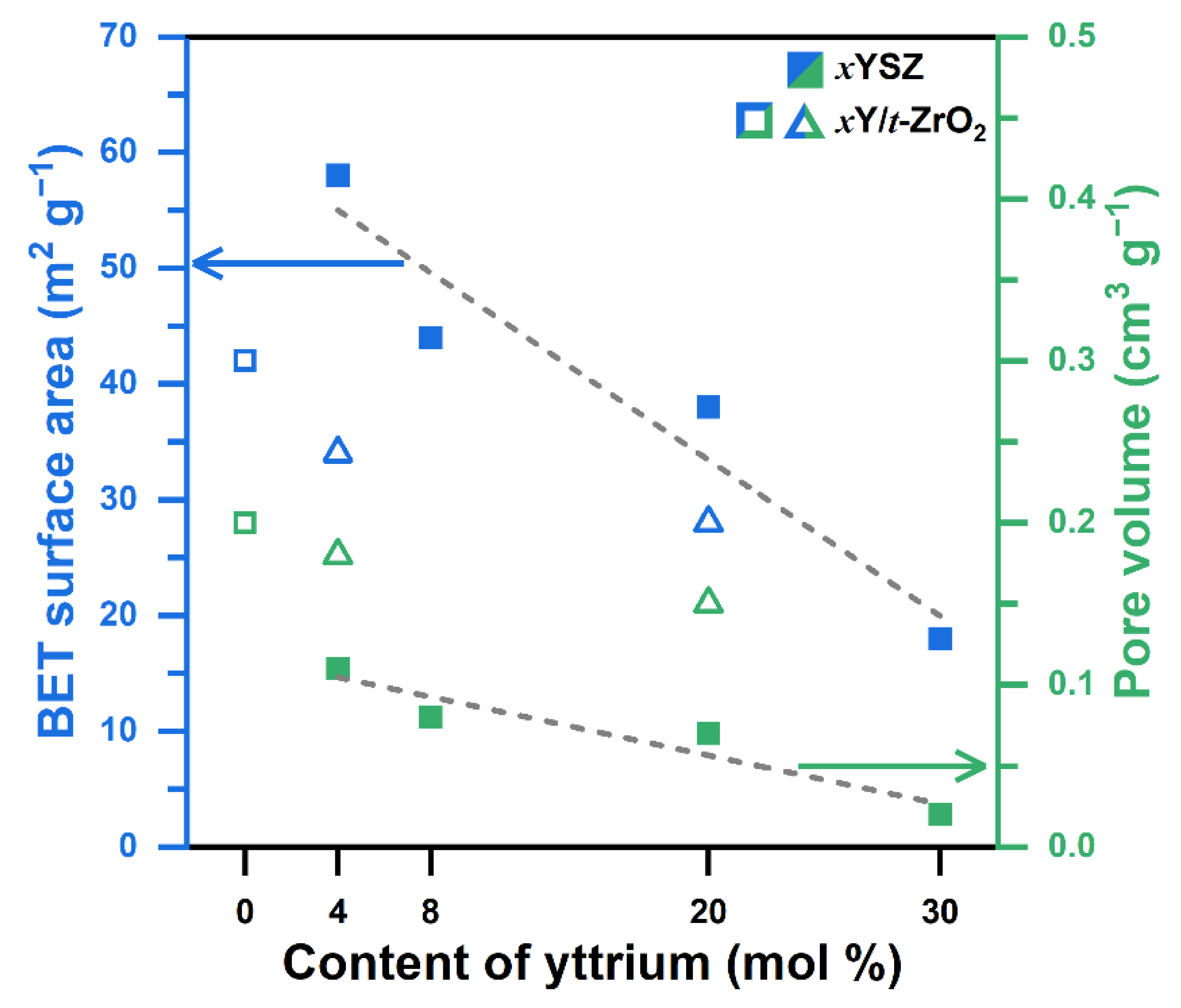
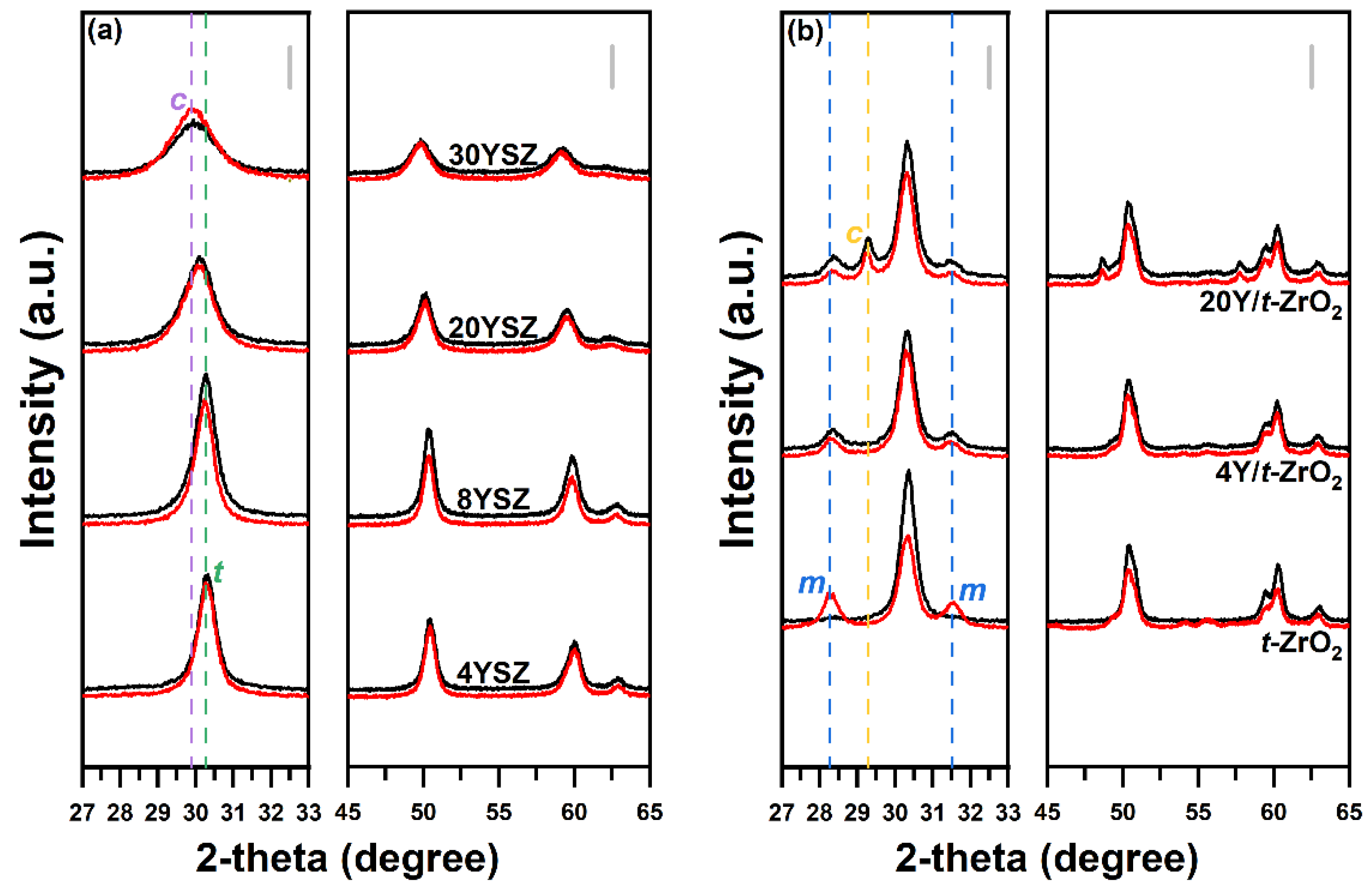
2.1. Physical Properties of YSZ Catalysts
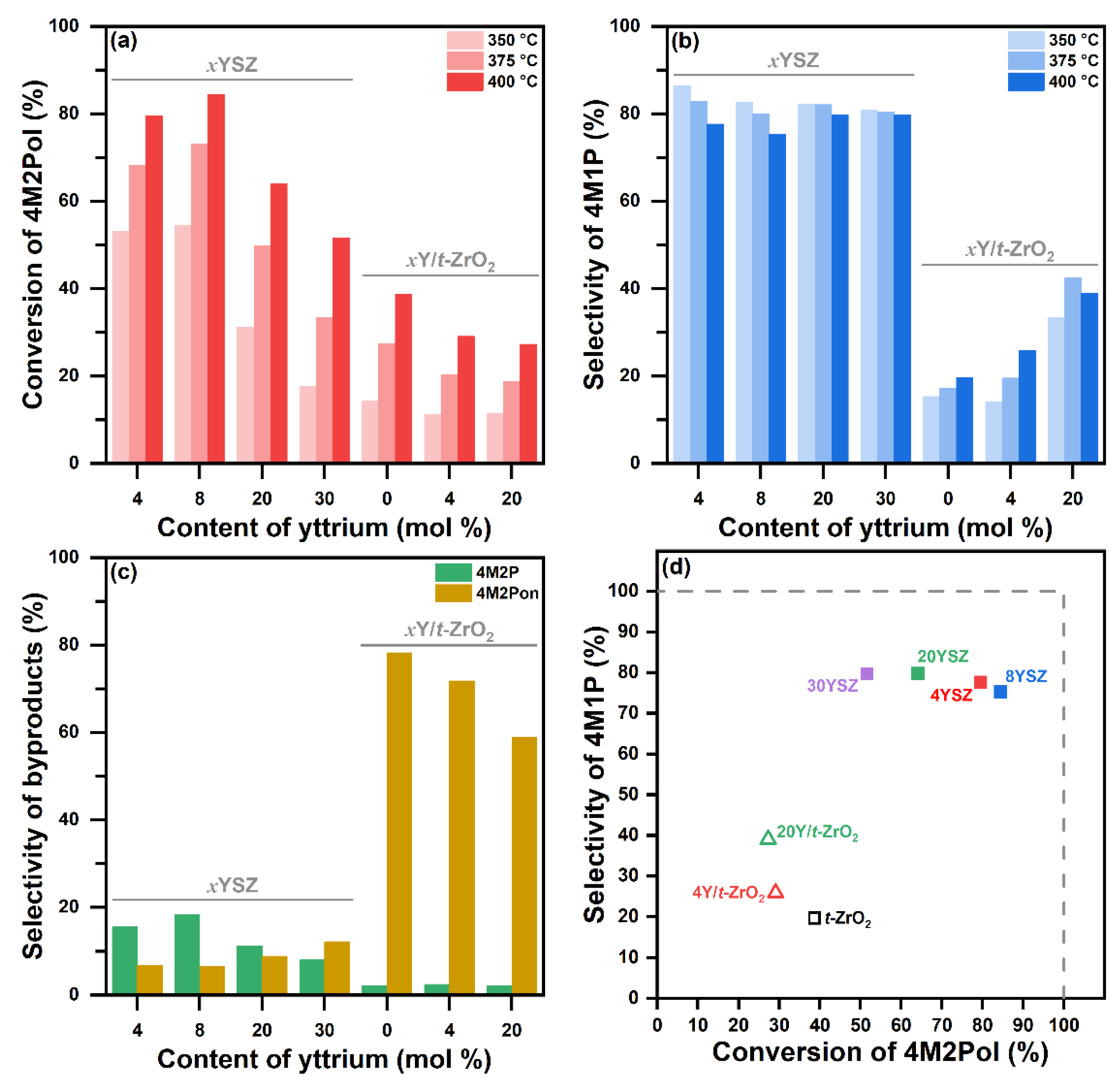
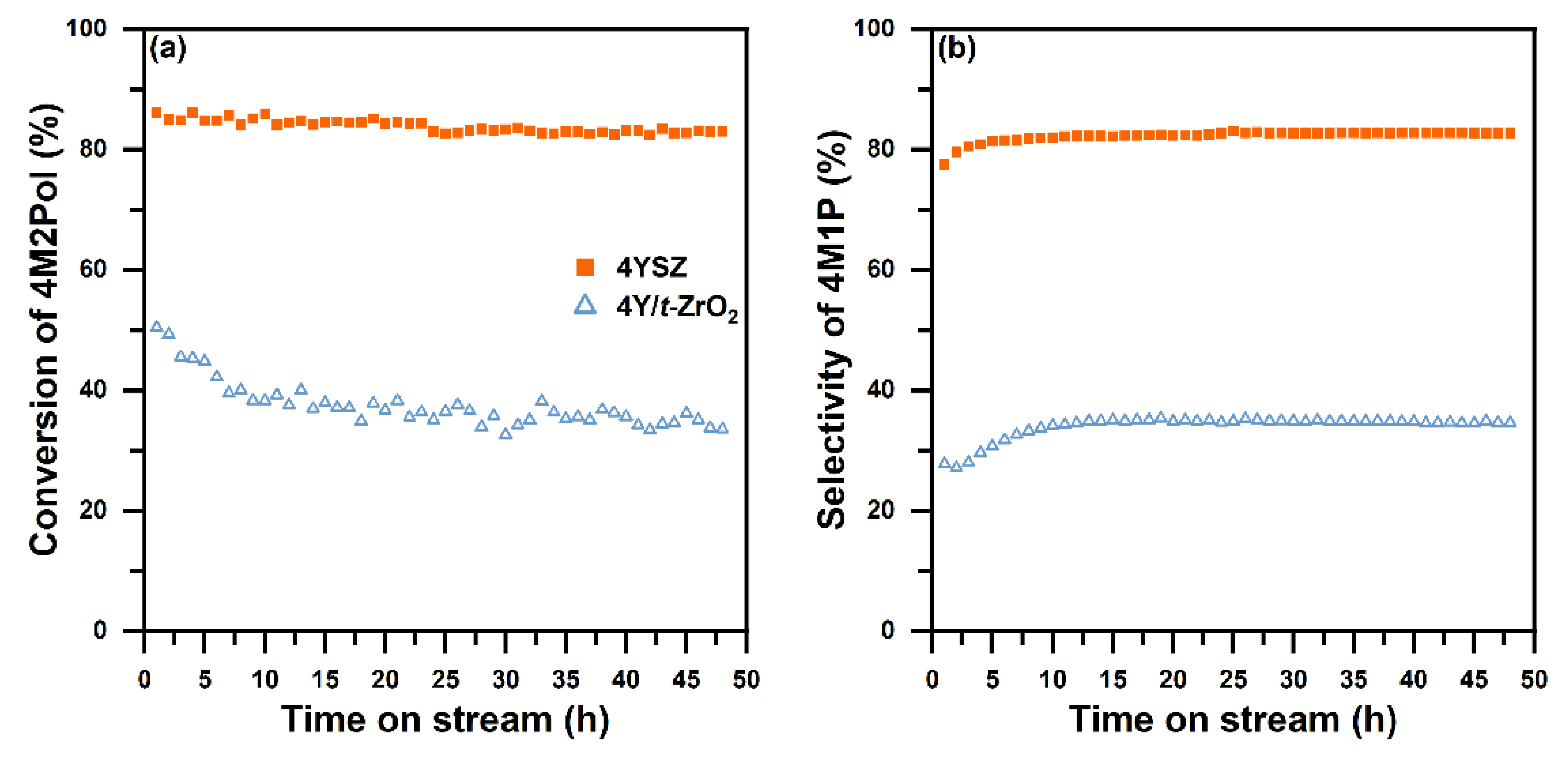
2.2. Catalytic Dehydration Performance of YSZ Catalysts
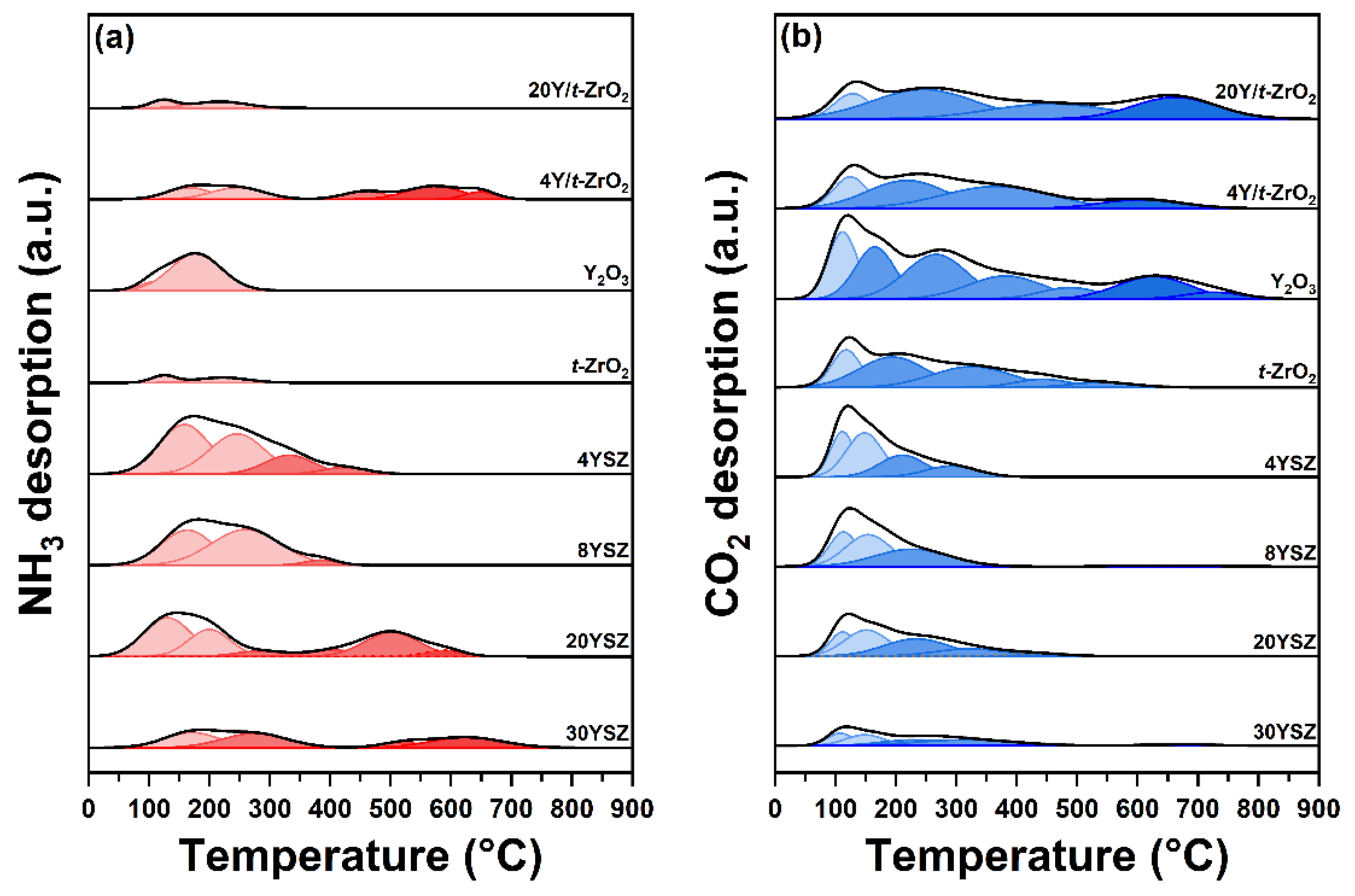
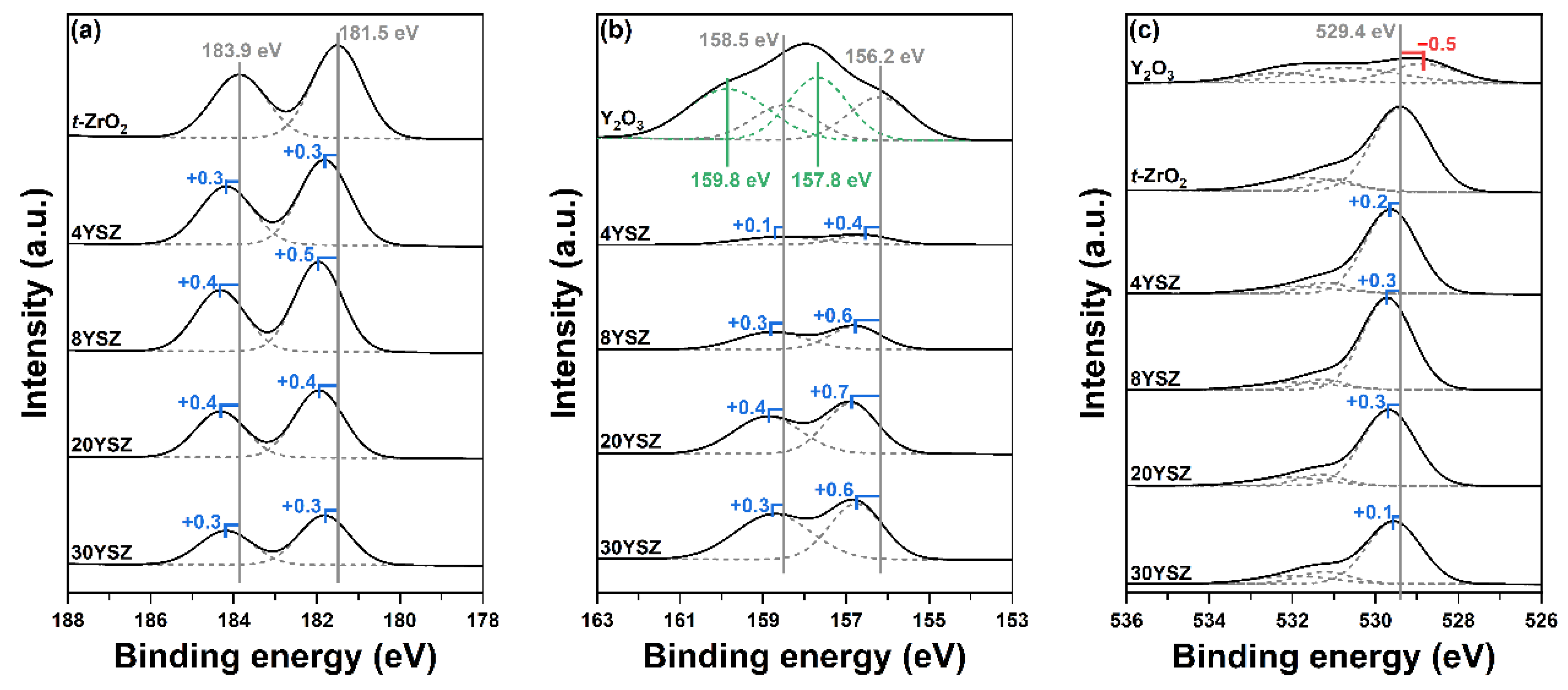
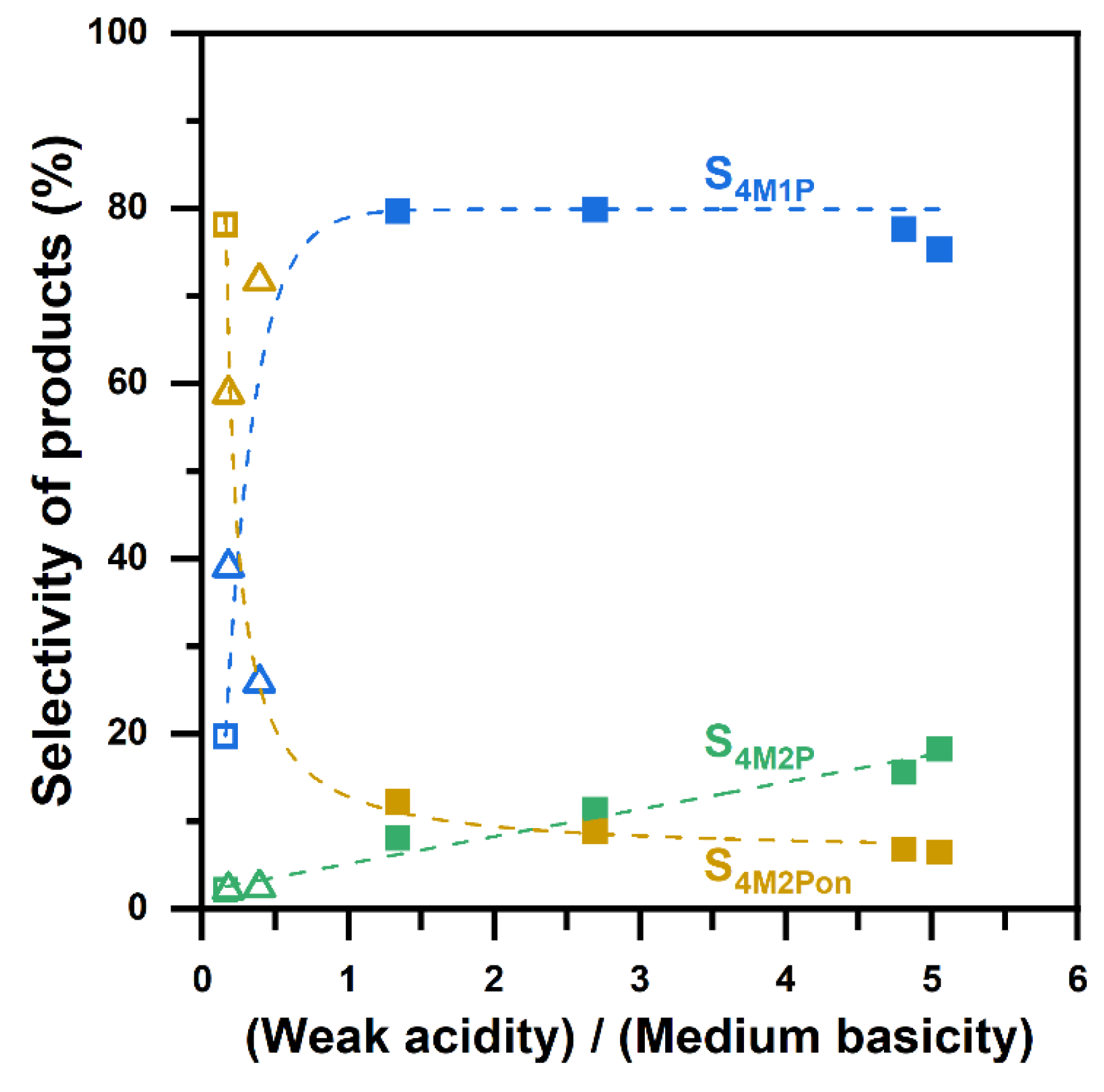
2.3. Acid–Base Characters of YSZ Catalysts
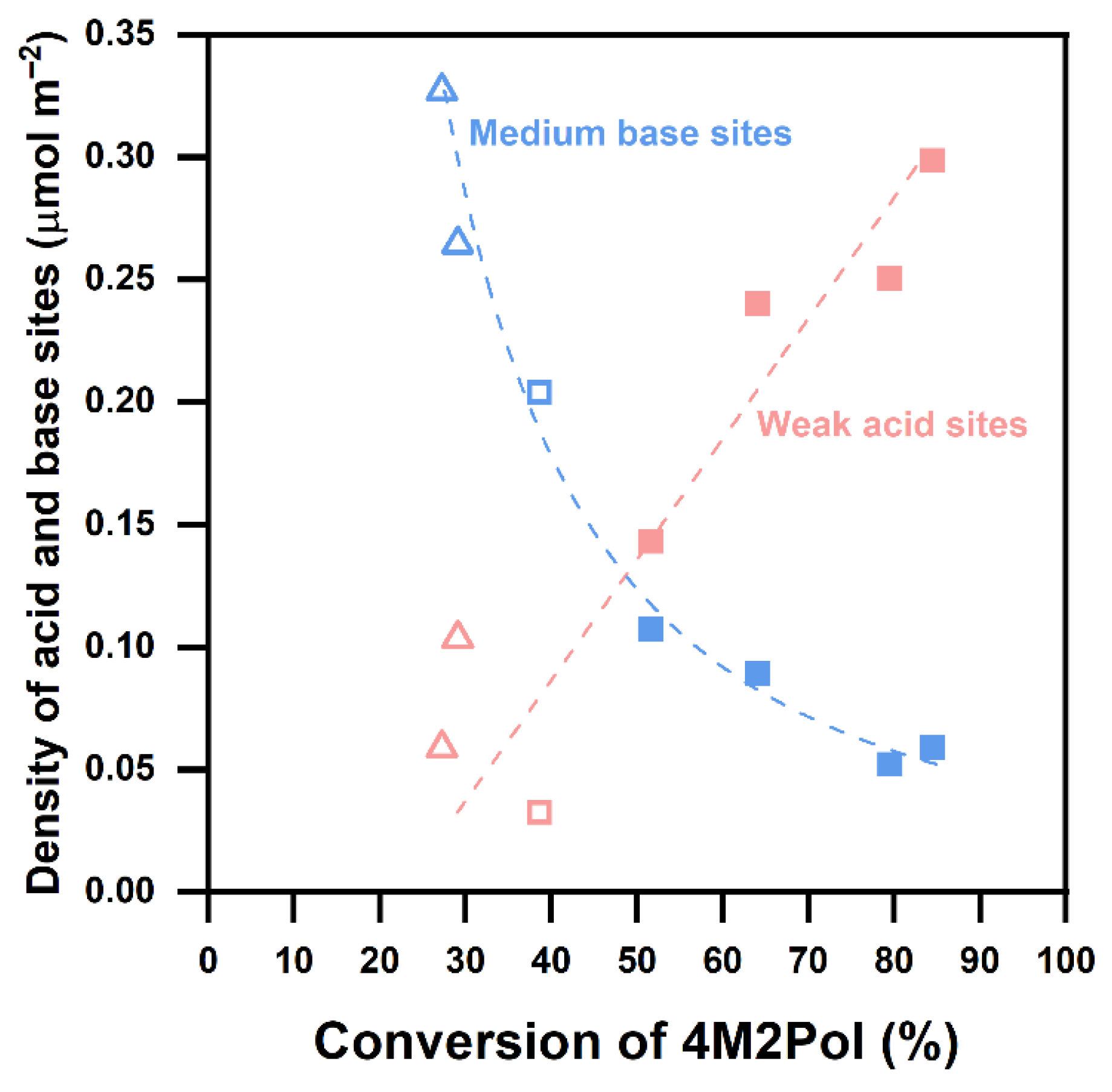
2.4. Understanding of Active Sites of YSZ Catalysts for 4M2Pol Dehydration
3. Materials and Methods
3.1. Preparation of Yttria-Stabilized Zirconia
3.2. Dehydration Experiments
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kashiwa, N.; Fukui, K. Process for Production of 4-methyl-1-pentene Polymer or Copolymer. U.S. Patent No. 4,659,792, 21 April 1987. [Google Scholar]
- Kashiwa, N.; Yoshitake, J. Polymerizations of α-olefins and styrene with MgCl2-supported titanium catalyst system: MgCl2/TiCl4/PhCO2Et with AlEt3PhCO2Et. Polym. Bull. 1984, 11, 485–489. [Google Scholar] [CrossRef]
- Lopez, L.C.; Wilkes, G.L.; Stricklen, P.M.; White, S.A. Synthesis, structure, and properties of poly(4-methyl-1-pentene). J. Macromol. Sci. Part. C 1992, 32, 301–406. [Google Scholar] [CrossRef]
- Cutrufello, M.G.; Ferino, I.; Monaci, R.; Rombi, E.; Solinas, V. Acid-base properties of zirconium, cerium and lanthanum oxides by calorimetric and catalytic investigation. Top. Catal. 2002, 19, 225–240. [Google Scholar] [CrossRef]
- Reddy, B.M.; Lakshmanan, P.; Bharali, P.; Saikia, P. Dehydration of 4-methylpentan-2-ol over CexZr1−xO2/SiO2 nano-composite catalyst. J. Mol. Catal. A Chem. 2006, 258, 355–360. [Google Scholar] [CrossRef]
- Reddy, B.M.; Thrimurthulu, G.; Saikia, P.; Bharali, P. Silica supported ceria and ceria–zirconia nanocomposite oxides for selective dehydration of 4-methylpentan-2-ol. J. Mol. Catal. A Chem. 2007, 275, 167–173. [Google Scholar] [CrossRef]
- Chen, X.J.; Khor, K.A.; Chan, S.H.; Yu, L.G. Influence of microstructure on the ionic conductivity of yttria-stabilized zirconia electrolyte. Mater. Sci. Eng.: A 2002, 335, 246–252. [Google Scholar] [CrossRef]
- Shimonosono, T.; Ueno, T.; Hirata, Y. Mechanical and thermal properties of porous yttria-stabilized zirconia. J. Asian Ceram. Soc. 2019, 7, 20–30. [Google Scholar] [CrossRef]
- Labaki, M.; Siffert, S.; Lamonier, J.-F.; Zhilinskaya, E.A.; Aboukaïs, A. Total oxidation of propene and toluene in the presence of zirconia doped by copper and yttrium: Role of anionic vacancies. Appl. Catal. B Environ. 2003, 43, 261–271. [Google Scholar] [CrossRef]
- Vlasenko, N.V.; Kyriienko, P.I.; Valihura, K.V.; Kosmambetova, G.R.; Soloviev, S.O.; Strizhak, P.E. Yttria-stabilized zirconia as a high-performance catalyst for ethanol to n-butanol Guerbet coupling. ACS Omega 2019, 4, 21469–21476. [Google Scholar] [CrossRef] [Green Version]
- Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Dou, B.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. Ni/Y2O3–ZrO2 catalyst for hydrogen production through the glycerol steam reforming reaction. Int. J. Hydrogen Energy 2020, 45, 10442–10460. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Nemoto, T.; Yotsumoto, R.; Yamada, Y.; Sato, F.; Takahashi, R.; Sato, S. Vapor-phase catalytic dehydration of butanediols to unsaturated alcohols over yttria-stabilized zirconia catalysts. Appl. Catal. A Gen. 2019, 575, 48–57. [Google Scholar] [CrossRef]
- Vasanthavel, S.; Kannan, S. Structural investigations on the tetragonal to cubic phase transformations in zirconia induced by progressive yttrium additions. J. Phys. Chem. Solids 2018, 112, 100–105. [Google Scholar] [CrossRef]
- Hattori, H. Solid base catalysts: Generation of basic sites and application to organic synthesis. Appl. Catal. A Gen. 2001, 222, 247–259. [Google Scholar] [CrossRef]
- Ray, J.C.; Pati, R.K.; Pramanik, P. Chemical synthesis and structural characterization of nanocrystalline powders of pure zirconia and yttria stabilized zirconia (YSZ). J. Eur. Ceram. Soc. 2000, 20, 1289–1295. [Google Scholar] [CrossRef]
- Albuquerque, E.M.; Borges, L.E.P.; Fraga, M.A.; Sievers, C. Relationship between acid–base properties and the activity of ZrO2-based catalysts for the Cannizzaro reaction of pyruvaldehyde to lactic acid. ChemCatChem 2017, 9, 2675–2683. [Google Scholar] [CrossRef]
- Wan Omar, W.N.N.; Amin, N.A.S. Biodiesel production from waste cooking oil over alkaline modified zirconia catalyst. Fuel Processing Technol. 2011, 92, 2397–2405. [Google Scholar] [CrossRef]
- Glazneva, T.S.; Kotsarenko, N.S.; Paukshtis, E.A. Surface acidity and basicity of oxide catalysts: From aqueous suspensions to in situ measurements. Kinet. Catal. 2008, 49, 859–867. [Google Scholar] [CrossRef]
- Bumajdad, A.; Nazeer, A.A.; Al Sagheer, F.; Nahar, S.; Zaki, M.I. Controlled synthesis of ZrO2 nanoparticles with tailored size, morphology and crystal phases via organic/inorganic hybrid films. Sci. Rep. 2018, 8, 3695. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.X.; Liu, A.; Meng, Y.; Shan, F.K.; Shin, B.C.; Lee, W.J.; Cho, C.R. Annealing dependence of solution-processed ultra-thin ZrOx films for gate dielectric applications. J. Nanosci. Nanotechnol. 2015, 15, 2185–2191. [Google Scholar] [CrossRef]
- Reddy, I.N.; Reddy, C.V.; Cho, M.; Kim, D.; Shim, J. Structural, optical, and XPS studies of doped yttria for superior water splitting under visible light illumination. J. Electroanal. Chem. 2019, 848, 113335. [Google Scholar] [CrossRef]
- Gu, H.; Ding, J.; Zhong, Q.; Zeng, Y.; Song, F. Promotion of surface oxygen vacancies on the light olefins synthesis from catalytic CO2 hydrogenation over FeK/ZrO2 catalysts. Int. J. Hydrog. Energy 2019, 44, 11808–11816. [Google Scholar] [CrossRef]
- Tan, J.; Cui, J.; Zhu, Y.; Cui, X.; Shi, Y.; Yan, W.; Zhao, Y. Complete aqueous hydrogenation of 5-hydroxymethylfurfural at room temperature over bimetallic RuPd/graphene catalyst. ACS Sustain. Chem. Eng. 2019, 7, 10670–10678. [Google Scholar] [CrossRef]
- Cole, K.M.; Kirk, D.W.; Thorpe, S.J. Y(OH)3 powder characterized by XPS. Surf. Sci. Spectra 2020, 27, 024007. [Google Scholar] [CrossRef]
- Hadjiivanov, K. Identification and characterization of surface hydroxyl groups by infrared spectroscopy. In Advances in Catalysis; Jentoft, F.C., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 57, pp. 99–318. [Google Scholar]
- Puriwat, J.; Chaitree, W.; Suriye, K.; Dokjampa, S.; Praserthdam, P.; Panpranot, J. Elucidation of the basicity dependence of 1-butene isomerization on MgO/Mg(OH)2 catalysts. Catal. Commun. 2010, 12, 80–85. [Google Scholar] [CrossRef]

| Catalyst | Acid Sites (µmol m−2) | Base Sites (µmol m−2) | ||||||
|---|---|---|---|---|---|---|---|---|
| Weak a | Medium b | Strong c | Total | Weak d | Medium e | Strong f | Total | |
| 20Y/t-ZrO2 | 0.06 | - | - | 0.06 | 0.06 | 0.33 | 0.12 | 0.51 |
| 4Y/t-ZrO2 | 0.10 | 0.03 | 0.09 | 0.22 | 0.06 | 0.26 | 0.04 | 0.37 |
| t-ZrO2 | 0.03 | - | - | 0.03 | 0.05 | 0.20 | - | 0.25 |
| 4YSZ | 0.25 | 0.06 | - | 0.31 | 0.09 | 0.05 | - | 0.14 |
| 8YSZ | 0.30 | 0.01 | - | 0.31 | 0.11 | 0.06 | - | 0.17 |
| 20YSZ | 0.24 | 0.16 | 0.02 | 0.42 | 0.09 | 0.09 | - | 0.18 |
| 30YSZ | 0.14 | 0.20 | 0.13 | 0.47 | 0.08 | 0.11 | 0.01 | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Kim, M.; Lim, S.; Mishra, D.K.; Suh, Y.-W. Yttria-Stabilized Zirconia of Balanced Acid-Base Pair for Selective Dehydration of 4-Methyl-2-pentanol to 4-Methyl-1-pentene. Catalysts 2022, 12, 559. https://doi.org/10.3390/catal12050559
Lee J-H, Kim M, Lim S, Mishra DK, Suh Y-W. Yttria-Stabilized Zirconia of Balanced Acid-Base Pair for Selective Dehydration of 4-Methyl-2-pentanol to 4-Methyl-1-pentene. Catalysts. 2022; 12(5):559. https://doi.org/10.3390/catal12050559
Chicago/Turabian StyleLee, Jae-Hong, Minseok Kim, Suhyun Lim, Dinesh Kumar Mishra, and Young-Woong Suh. 2022. "Yttria-Stabilized Zirconia of Balanced Acid-Base Pair for Selective Dehydration of 4-Methyl-2-pentanol to 4-Methyl-1-pentene" Catalysts 12, no. 5: 559. https://doi.org/10.3390/catal12050559
APA StyleLee, J.-H., Kim, M., Lim, S., Mishra, D. K., & Suh, Y.-W. (2022). Yttria-Stabilized Zirconia of Balanced Acid-Base Pair for Selective Dehydration of 4-Methyl-2-pentanol to 4-Methyl-1-pentene. Catalysts, 12(5), 559. https://doi.org/10.3390/catal12050559