Retention and Inactivation of Quality Indicator Bacteria Using a Photocatalytic Membrane Reactor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Photocatalytic Membranes Modified with TiO2 and Copper
2.2. Surface-Water Treatment Using a Submerged Hybrid Reactor
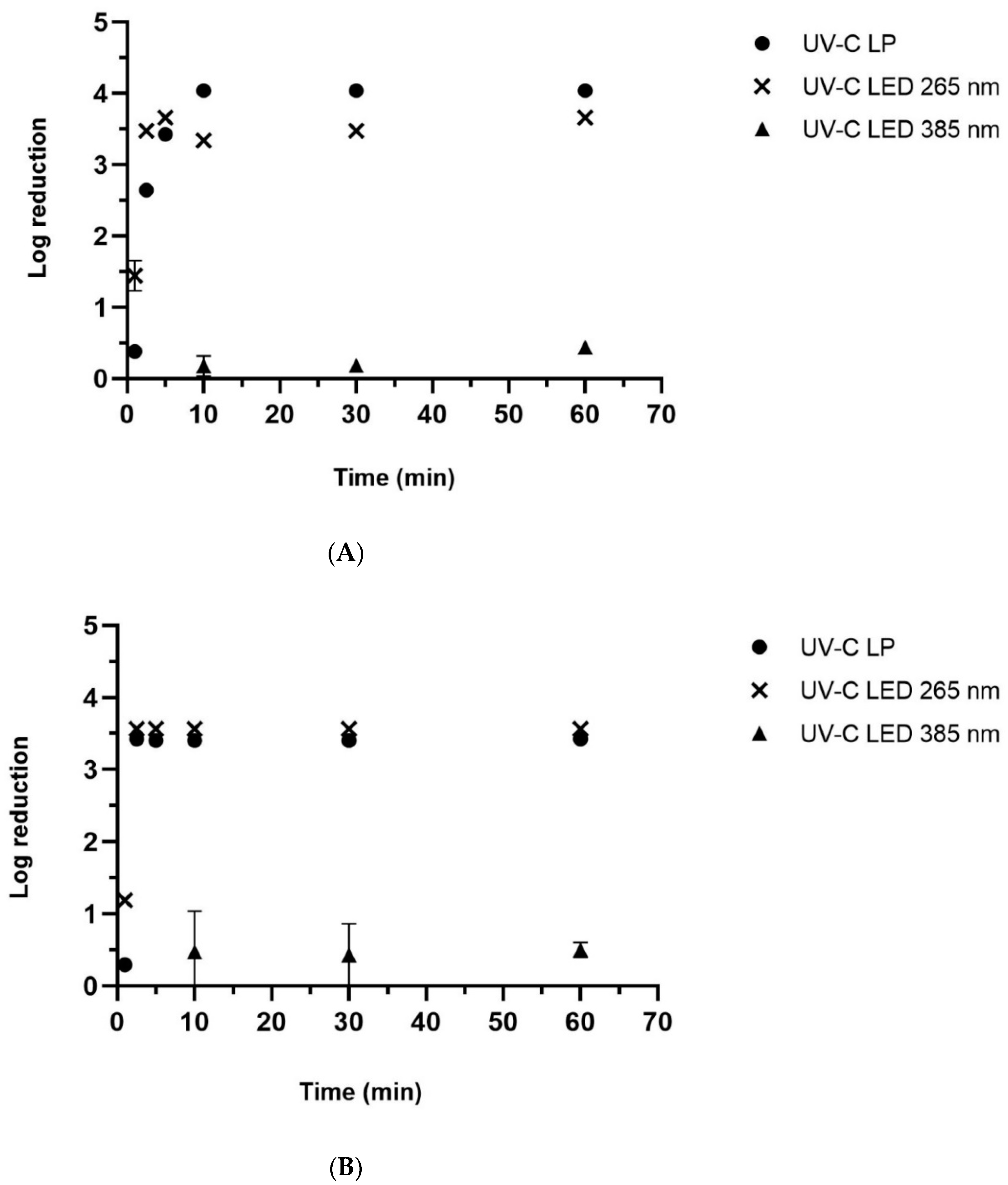
2.2.1. Direct Photolysis
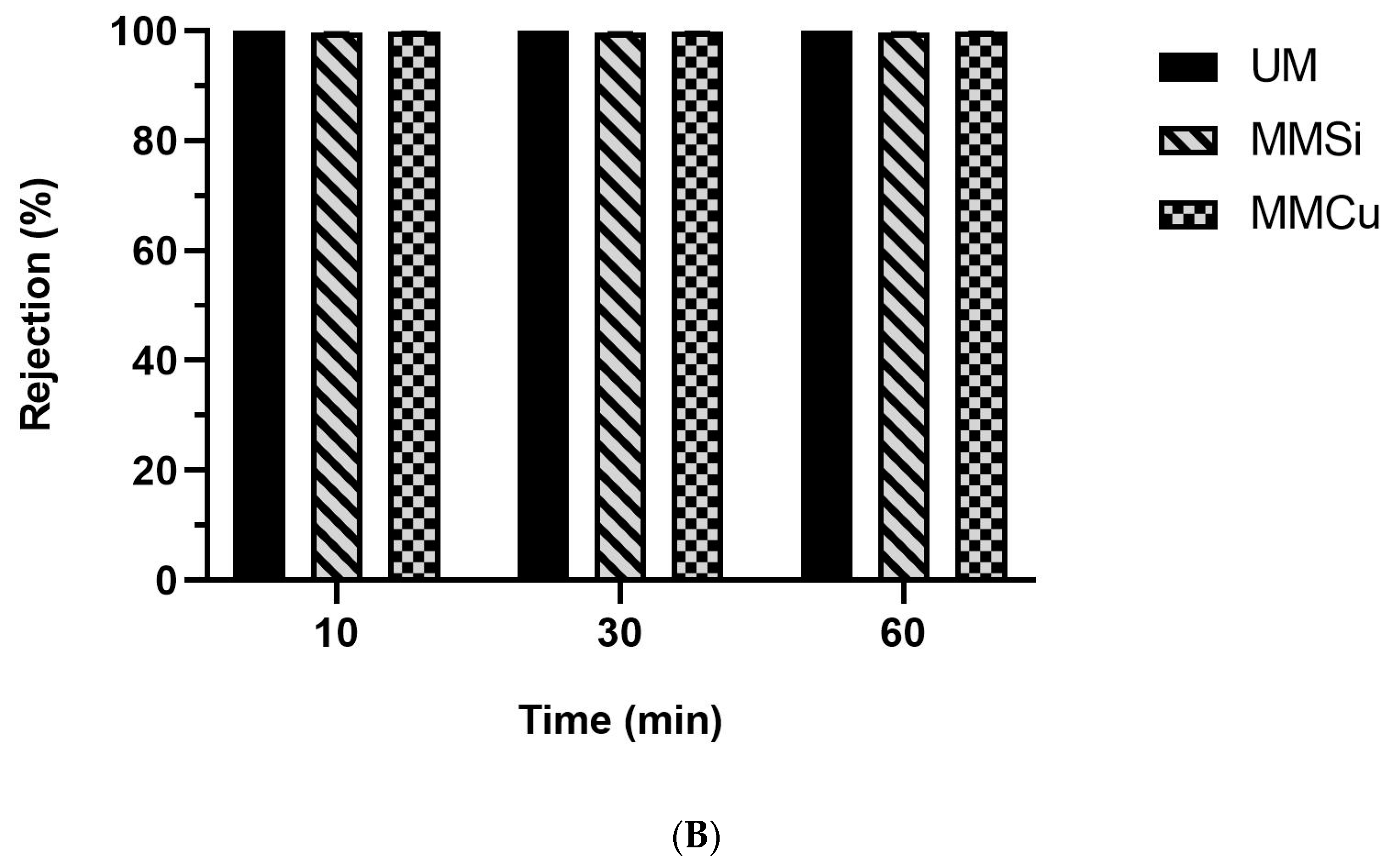
2.2.2. Membrane Filtration
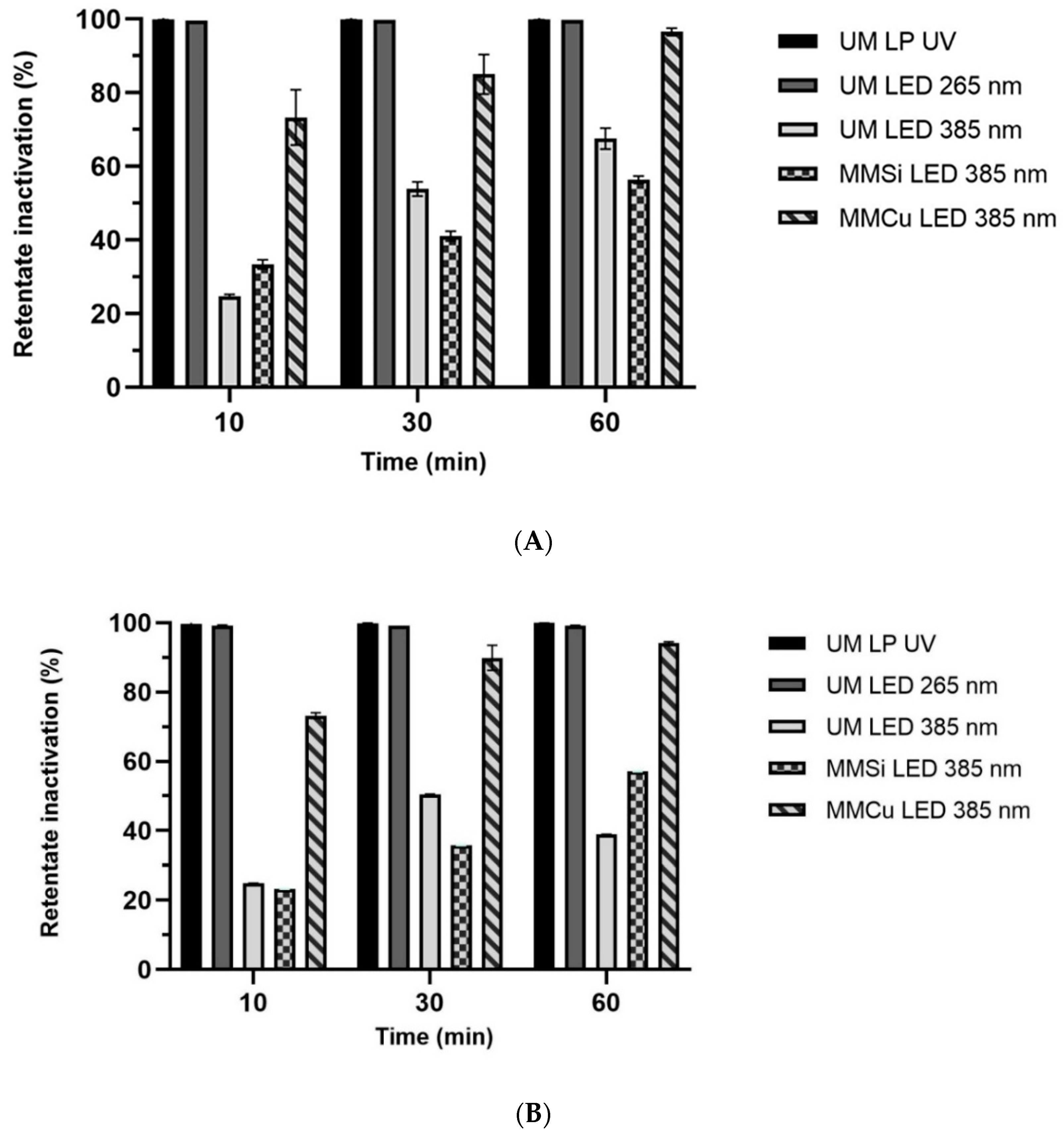
2.2.3. Membrane Filtration Combined with Photolysis
3. Materials and Methods
3.1. Water Sampling
3.2. Submerged Hybrid Reactor
- -
- two UV-C low-pressure mercury lamps (Puro TAP, UVC, 11 W, type GPH212T5L, New Zealand) placed submerged at 1.6 cm from each side of the membrane (Figure S5a); low-pressure mercury lamps are widely used in drinking and wastewater facilities since they are known to be extremely effective to achieve microbial inactivation.
- -
- two novel custom-made UV-C LED panels that emit light at 265 nm placed submerged at 2 cm from each side of the membrane (Figure S6b); these panels were built following previous studies that proved UV-C LEDs at 265 nm are extremely effective to achieve inactivation of different Aspergillus species and water-quality-indicator bacteria [9,32].
- -
- two novel custom-made UV-A LED panels that emit light at 385 nm placed submerged at 2 cm from each side of the membrane (Figure S6c); these panels were built following a study by Bernardo et al. [32] that showed that UV-A light sources could be used to activate photocatalytic surfaces and achieve inactivation through indirect photolysis.
3.2.1. Modification of the Membranes
- (a)
- a previously detailed solvent-free procedure [29] with silicon dioxide (SiO2) and TiO2 degussa nanoparticles. These photocatalytic microfiltration membranes were tested for treatment of olive mill wastewaters [34], and recently, small circular coupons were combined with three small ultraviolet A light-emitting diodes to ensure the retention and effective inactivation of the concentrated membrane retentate [32].
- (b)
- a solvent-free procedure with TiO2 and copper described below.
3.2.2. Characterization of the Membranes
Membrane Morphology
Membrane Hydrophilicity
3.3. Experimental Procedure
3.4. Bacteria Identification and Enumeration
3.4.1. Total Coliforms and E. coli
3.4.2. Enterococci
3.5. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergmann, H.; Lourtchouk, T.; Schöps, K.; Bouzek, K. New UV irradiation and direct electrolysis—Promising methods for water disinfection. Chem. Eng. J. 2002, 85, 111–117. [Google Scholar] [CrossRef]
- World Health Organization. Water, Sanitation, Hygiene and Health: A Primer for Health Professionals; License: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2019; Available online: https://apps.who.int/iris/handle/10665/330100 (accessed on 30 November 2021).
- World Health Organization & United Nations Children’s Fund (UNICEF). Progress on Sanitation and Drinking Water: 2014 Update; World Health Organization: Geneva, Switzerland, 2014; Available online: https://apps.who.int/iris/handle/10665/112727 (accessed on 30 November 2021).
- Song, K.; Taghipour, F.; Mohseni, M. Microorganisms inactivation by wavelength combinations of ultraviolet light-emitting diodes (UV-LEDs). Sci. Total Environ. 2019, 665, 1103–1110. [Google Scholar] [CrossRef]
- Oppenheimer, J.A.; Jacangelo, J.G.; Laine, J.-M.; Hoagland, J.E. Testing the equivalency of ultraviolet light and chlorine for disinfection of wastewater to reclamation standards. Water Environ. Res. 1997, 69, 14–24. [Google Scholar] [CrossRef]
- Betancourt, W.; Rose, J. Drinking water treatment processes for removal of Cryptosporidium and Giardia. Vet. Parasitol. 2004, 126, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Hijnen, W.A.M.; Beerendonk, E.F.; Medema, G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: A review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.; Roddick, F.; Linhua, F. Moving from the traditional paradigm of pathogen inactivation to controlling antibiotic resistance in water—Role of ultraviolet irradiation. Sci. Total Environ. 2019, 662, 923–939. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.R.; Crespo, M.T.B.; Pereira, V.J. Small but powerful: Light-emitting diodes for inactivation of Aspergillus species in real water matrices. Water Res. 2020, 168, 115108. [Google Scholar] [CrossRef]
- Oliveira, B.R.; Sanches, S.; Huertas, R.; Crespo, M.T.B.; Pereira, V.J. Treatment of a real drinking water matrix spiked with Aspergillus fumigatus using a photocatalytic membrane reactor. J. Membr. Sci. 2020, 598, 117788. [Google Scholar] [CrossRef]
- Oliveira, B.R.; Marques, A.P.; Muhammad, A.; Crespo, M.T.B.; Pereira, V.J. Light-emitting diodes effect on Aspergillus species in filtered surface water: DNA damage, proteome response and potential reactivation. Environ. Pollut. 2021, 287, 117553. [Google Scholar] [CrossRef]
- Oliveira, B.R.; Marques, A.P.; Ressurreição, M.; Moreira, C.J.S.; Silva Pereira, C.; Crespo, M.T.B.; Pereira, V.J. Inactivation of Aspergillus species in real water matrices using medium pressure mercury lamps. J. Photochem. Photobiol. B Biol. 2021, 221, 112242. [Google Scholar] [CrossRef]
- Goosen, N.; Moolenaar, G.F. Repair of UV damage in bacteria. DNA Repair 2008, 7, 353–379. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-K.; Kim, S.-J.; Kang, D.-H. Bactericidal effect of 266 to 279 nm wavelength UVC-LEDs for inactivation of Gram positive and Gram negative foodborne pathogenic bacteria and yeasts. Food Res. Int. 2017, 97, 280–287. [Google Scholar] [CrossRef]
- Nocker, A.; Shah, M.; Dannenmann, B.; Schulze-Osthoff, K.; Wingender, J.; Probst, A.J. Assessment of UV-C-induced water disinfection by differential PCR-based quantification of bacterial DNA damage. J. Microbiol. Methods 2018, 149, 89–95. [Google Scholar] [CrossRef]
- Banas, M.A.; Crawford, M.H.; Ruby, D.S.; Ross, M.P.; Nelson, J.S.; Allerman, A.A.; Boucher, R. Final LDRD Report: Ultraviolet Water Purification Systems for Rural Environments and Mobile Applications; United States Department of Energy: Washington, DC, USA, 2005.
- Shin, J.Y.; Kim, S.J.; Kim, D.K.; Kang, D.H. Fundamental characteristics of deep-UV light-emitting diodes and their application to control foodborne pathogens. Appl. Environ. Microbiol. 2016, 82, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.; Du, L.; Zubair, M.; Subedi, S.; Ullah, A.; Roopesh, M.S. Applications of light-emitting diodes (LEDs) in food processing and water treatment. Food Eng. Rev. 2020, 12, 268–289. [Google Scholar] [CrossRef]
- Wan, Q.; Wen, G.; Cao, R.; Xu, X.; Zhao, H.; Li, K.; Wang, J.; Huang, T. Comparison of UV-LEDs and LPUV on inactivation and subsequent reactivation of waterborne fungal spores. Water Res. 2020, 173, 115553. [Google Scholar] [CrossRef] [PubMed]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Borkowski, A.; Szala, M.; Cłapa, T. Adsorption Studies of the Gram-Negative Bacteria onto Nanostructured Silicon Carbide. Appl. Biochem. Biotechnol 2015, 175, 1448–1459. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Xu, P.; Wang, H. Photocatalytic membrane reactors for produced water treatment and reuse: Fundamentals, affecting factors, rational design, and evaluation metrics. J. Hazard. Mater. 2022, 424, 127493. [Google Scholar] [CrossRef]
- Argurio, P.; Fontananova, E.; Molinar, R.; Drioli, E. Photocatalytic Membranes in Photocatalytic Membrane Reactors. Processes 2018, 6, 162. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Ding, L.; Luo, J.; Michel, Y.; Jaffrin, M.Y.; Tang, B. Membrane fouling in photocatalytic membrane reactors (PMRs) for water and wastewater treatment: A critical review. Chem. Eng. J. 2016, 302, 446–458. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, Z.-P.; Shi, L.; Cheng, R.; Yuan, D.-H. Photocatalytic Membrane Reactors (PMRs) in Water Treatment: Configurations and Influencing Factors. Catalysts 2017, 7, 224. [Google Scholar] [CrossRef]
- Azaman, F.; Nor, M.A.A.M.; Abdullah, W.R.W.; Razali, M.H.; Zulkifli, R.C.; Zaini, M.A.A.; Ali, A. Review on natural clay ceramic membrane: Fabrication and application in water and wastewater treatment. Malays. J. Fundam. Appl. Sci. 2021, 17, 62–78. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Comparison of Polypropylene and Ceramic Microfiltration Membranes Applied for Separation of 1,3-PD Fermentation Broths and Saccharomyces cerevisiae Yeast Suspensions. Membranes 2021, 11, 44. [Google Scholar] [CrossRef]
- Tomczak, W.; Grubecki, I.; Gryta, M. The Use of NaOH Solutions for Fouling Control in a Membrane Bioreactor: A Feasibility Study. Membranes 2021, 11, 887. [Google Scholar] [CrossRef] [PubMed]
- Huertas, R.M.; Fraga, M.C.; Crespo, J.G.; Pereira, V.J. Solvent-Free Process for the Development of Photocatalytic Membranes. Molecules 2019, 24, 4481. [Google Scholar] [CrossRef] [Green Version]
- Chiang, L.-F.; Doong, R. Cu–TiO2 nanorods with enhanced ultraviolet- and visible-light photoactivity for bisphenol A degradation. J. Hazard. Mater. 2014, 277, 84–92. [Google Scholar] [CrossRef]
- Eraya, E.; Candelario, V.M.; Boffa, V.; Safafar, H.; Østedgaard-Munck, D.O.; Zahrtmann, N.; Kadrispahic, H.; Jørgensen, M.K. A roadmap for the development and applications of silicon carbide membranes for liquid filtration: Recent advancements, challenges, and perspectives. Chem. Eng. J. 2021, 414, 128826. [Google Scholar] [CrossRef]
- Bernardo, J.; Sério, J.; Oliveira, B.; Marques, A.P.; Huertas, R.; Crespo, J.G.; Pereira, V.J. Towards a Novel Combined Treatment Approach Using Light-Emitting Diodes and Photocatalytic Ceramic Membranes. Water 2022, 14, 292. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, T.; Kim, E.-R.; Cho, E.-B.; Jung, S.-C. Microwave-assisted synthesis of various Cu2O/Cu/TiO2 and CuxS/TiO2 composite nanoparticles towards visible-light photocatalytic applications. Mater. Chem. Phys. 2021, 259, 123986. [Google Scholar] [CrossRef]
- Fraga, M.C.; Huertas, R.M.; Crespo, J.G.; Pereira, V.J. Novel Submerged Photocatalytic Membrane Reactor for Treatment of Olive Mill Wastewaters. Catalysts 2019, 9, 769. [Google Scholar] [CrossRef] [Green Version]
- Huertas, R.M.; Fraga, M.C.; Crespo, J.G.; Pereira, V.J. Sol-gel membrane modification for enhanced photocatalytic activity. Sep. Purif. Technol. 2017, 180, 69–81. [Google Scholar] [CrossRef]
- Vilhunen, S.; Särkkä, H.; Sillanpää, M. Ultraviolet light-emitting diodes in water disinfection. Environ. Sci. Pollut. Res. 2009, 16, 439–442. [Google Scholar] [CrossRef]
- Chatterley, C.; Linden, K. Demonstration and evaluation of germicidal UV-LEDs for point-of-use water disinfection. J. Water Health 2010, 8, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Oguma, K.; Kita, R.; Sakai, H.; Murakami, M.; Takizawa, S. Application of UV light emitting diodes to batch and flow-through water disinfection systems. Desalination 2013, 328, 24–30. [Google Scholar] [CrossRef]
- Li, G.-Q.; Wang, W.-L.; Huo, Z.-Y.; Lu, Y.; Hu, H.-Y. Comparison of UV-LED and low pressure UV for water disinfection: Photoreactivation and dark repair of Escherichia coli. Water Res. 2017, 126, 134–143. [Google Scholar] [CrossRef]
- Thurman, R.B.; Gerba, C.P.; Bitton, G. The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses. Crit. Rev. Environ. Sci. Technol. 1989, 18, 295–315. [Google Scholar] [CrossRef]
- Xiong, P.; Hu, J. Inactivation/reactivation of antibiotic-resistant bacteria by a novel UVA/LED/TiO2 system. Water Res. 2013, 47, 4547–4555. [Google Scholar] [CrossRef]
- Claro, E.M.T.; Bidoia, E.D.; de Moraes, P.B. A high-performance doped photocatalysts for inactivation of total coliforms in superficial waters using different sources of radiation. J. Environ. Manag. 2016, 177, 264–270. [Google Scholar] [CrossRef] [Green Version]
- Fraga, M.C.; Sanches, S.; Crespo, J.G.; Pereira, V.J. Assessment of a New Silicon Carbide Tubular Honeycomb Membrane for Treatment of Olive Mill Wastewaters. Membranes 2017, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Biancullo, F.; Moreira, N.F.F.; Ribeiro, A.R.; Manaia, C.M.; Faria, J.L.; Nunes, O.C.; Silva, A.M.T. Heterogeneous photocatalysis using UVA-LEDs for the removal of antibiotics and antibiotic resistant bacteria from urban wastewater treatment plant effluents. Chem. Eng. J. 2019, 367, 304–313. [Google Scholar] [CrossRef]
- Kumar, S.R.; Pillai, S.C.; Hareesh, U.S.; Mukundan, P.; Warrier, K.G.K. Synthesis of thermally stable, high surface area anatase–alumina mixed oxides. Mater. Lett. 2000, 43, 286–290. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.B.; Keane, D.; Fernandez-Ibanez, P.; Colreavy, J.; Hinder, S.J.; Mcguigan, K.; Pillai, S.C. Nitrogen and copper doped solar light active TiO2 photocatalysts for water decontamination. Appl. Catal. B Environ. 2013, 130, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Masselin, I.; Durand-Bourlier, L.; Laine, J.-M.; Sizaret, P.-Y.; Xavier, C.; Lemordant, D. Membrane characterization using microscopic image analysis. J. Membr. Sci. 2001, 186, 85–96. [Google Scholar] [CrossRef]
- Warden, P.S.; DeSarno, M.S.; Volk, S.E.; Eldred, B.J. Evaluation of Colilert-18 for detection and enumeration of fecal coliform bacteria in wastewater using the U.S. Environmental Protection Agency Alternative Test Procedure Protocol. J. AOAC 2011, 94, 1573–1580. [Google Scholar] [CrossRef] [Green Version]
- Yakub, G.P.; Castric, D.A.; Stadterman-Knauer, K.L.; Tobin, M.J.; Blazina, M.; Heineman, T.N.; Yee, G.Y.; Frazier, L. Evaluation of Colilert and Enterolert Defined Substrate Methodology for Wastewater Applications. Water Environ. Res. 2002, 74, 131–135. [Google Scholar] [CrossRef]
- ISO 9308-2; Water Quality—Enumeration of Escherichia Coli and Coliform Bacteria—Part 2: Most Probable Number Method. ISO: Geneva, Switzerland, 2012.
- ISO 7899-1:2000/COR 1; Water Quality—Detection and Enumeration of Intestinal Enterococci—Part 1: Miniaturized Method (Most Probable Number) for Surface and Waste Water. ISO: Geneva, Switzerland, 2000.
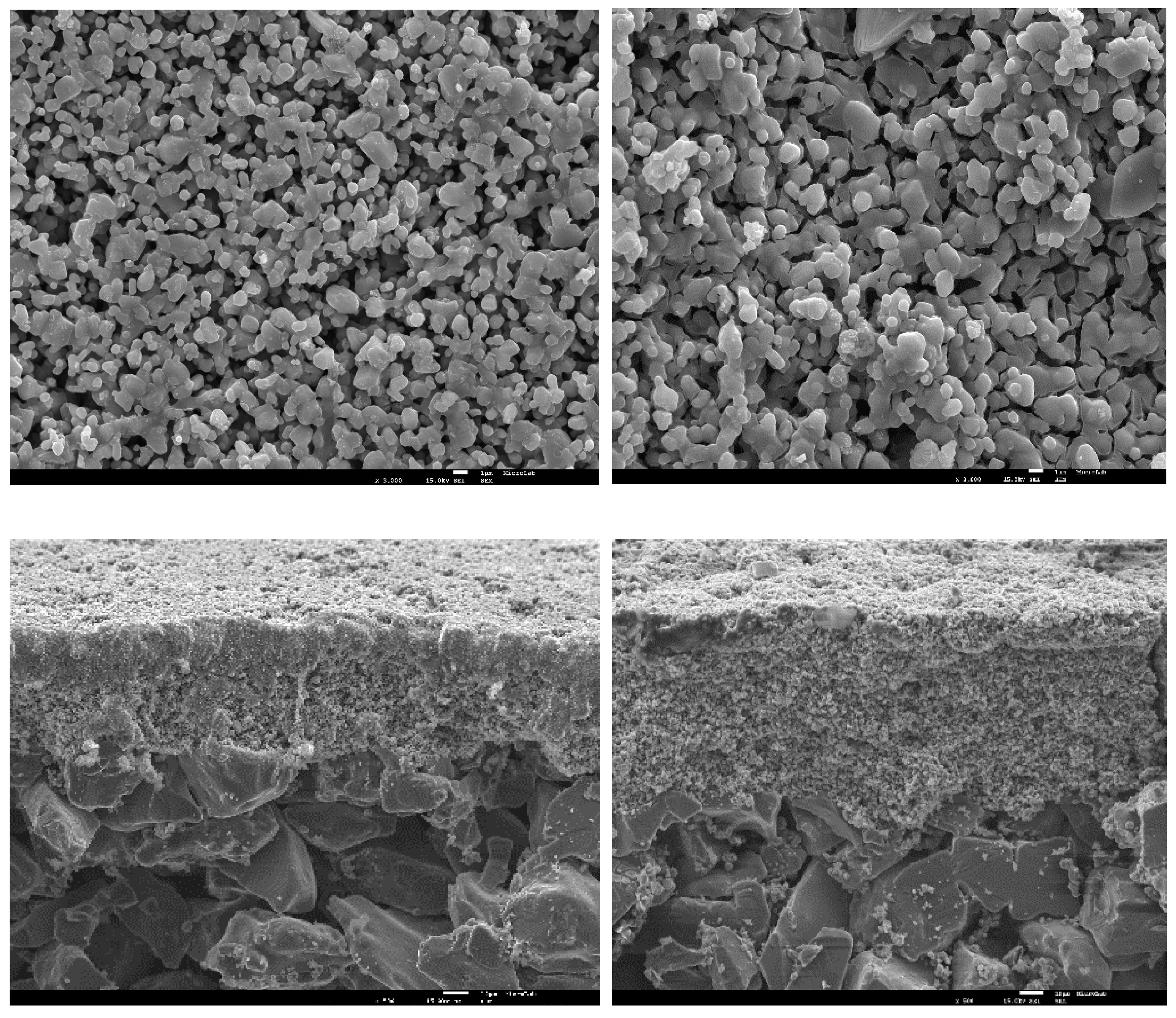

| UM | MMCu | |||
|---|---|---|---|---|
| Z1 (×3000) | Z2 (×3000) | Z1 (×3000) | Z2 (×3000) | |
| Porosity (%) | 6.2 | 6.6 | 6.5 | 6.3 |
| Pore density (µm−2) | 2.14 | 3.10 | 1.89 | 2.06 |
| Mean Pore Area (µm2) | 0.029 ± 0.122 | 0.021 ± 0.097 | 0.034 ± 0.158 | 0.031 ± 0.147 |
| Minimum Pore Area (µm2) | 0.0003 | 0.0002 | 0.0003 | 0.0003 |
| Maximum Pore Area (µm2) | 1.9160 | 2.2040 | 2.8450 | 2.5390 |
| Average circularity | 0.836 ± 0.267 | 0.830 ± 0.267 | 0.823 ± 0.273 | 0.807 ± 0.278 |
| Average Feret diameter (µm) | 0.15 ± 0.34 | 0.13 ± 0.29 | 0.17 ± 0.40 | 0.16 ± 0.39 |
| Maximum Feret diameter (µm) | 3.381 | 3.256 | 3.819 | 5.464 |
| Minimum Feret diameter (µm) | 0.025 | 0.021 | 0.024 | 0.024 |
| Experiment ID | Sampling Time (min) | Membrane Type | UV Light | Filtration | Objective | |
|---|---|---|---|---|---|---|
| 1 | LP-UV (254 nm) | 1, 2.5, 5, 10, 30, 60 | No | Yes | No | Evaluate direct photolysis using low-pressure mercury UV lamps |
| 2 | UV-C LED 265 nm | 1, 2.5, 5, 10, 30, 60 | No | Yes | No | Evaluate direct photolysis using light-emitting diode panels that emit light at 265 nm |
| 3 | UV-A LED 385 nm | 10, 30, 60 | No | Yes | No | Evaluate direct photolysis using light-emitting diode panels that emit light at 385 nm |
| 4 | UM | 10, 30, 60 | Unmodified (UM) | No | Yes | Evaluate the filtration performance of the unmodified silicon-carbide membrane |
| 5 | UM + LP-UV (254 nm) | 10, 30, 60 | Unmodified (UM) | Yes | Yes | Evaluate the combined effect (retention and inactivation) of the unmodified membrane and low-pressure mercury UV lamps |
| 6 | UM + UV-C LED 265 nm | 10, 30, 60 | Unmodified (UM) | Yes | Yes | Evaluate the combined effect of the unmodified membrane and light-emitting diode panels that emit light at 265 nm |
| 7 | UM + UV-A LED 385 nm | 10, 30, 60 | Unmodified (UM) | Yes | Yes | Evaluate the combined effect of the unmodified membrane and light-emitting diode panels that emit light at 385 nm |
| 8 | MM TiO2 + SiO2 | 10, 30, 60 | Modified (MM) | No | Yes | Evaluate the filtration performance of the membrane modified with TiO2 and SiO2 |
| 9 | MM TiO2 + SiO2 + UV-A LED 385 nm | 10, 30, 60 | Modified (MM) | Yes | Yes | Evaluate the combined effect of the membrane modified with TiO2 and SiO2 and light-emitting diode panels that emit light at 385 nm |
| 10 | MM TiO2 + Copper | 10, 30, 60 | Modified (MM) | No | Yes | Evaluate the filtration performance of the membrane modified with TiO2 and copper |
| 11 | MM TiO2 + Copper + UV-A LED 385 nm | 10, 30, 60 | Modified (MM) | Yes | Yes | Evaluate the combined effect of the membrane modified with TiO2 and copper and light-emitting diode panels that emit light at 385 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, A.P.; Huertas, R.; Bernardo, J.; Oliveira, B.; Crespo, J.G.; Pereira, V.J. Retention and Inactivation of Quality Indicator Bacteria Using a Photocatalytic Membrane Reactor. Catalysts 2022, 12, 680. https://doi.org/10.3390/catal12070680
Marques AP, Huertas R, Bernardo J, Oliveira B, Crespo JG, Pereira VJ. Retention and Inactivation of Quality Indicator Bacteria Using a Photocatalytic Membrane Reactor. Catalysts. 2022; 12(7):680. https://doi.org/10.3390/catal12070680
Chicago/Turabian StyleMarques, Ana Paula, Rosa Huertas, Jorge Bernardo, Beatriz Oliveira, João Goulão Crespo, and Vanessa Jorge Pereira. 2022. "Retention and Inactivation of Quality Indicator Bacteria Using a Photocatalytic Membrane Reactor" Catalysts 12, no. 7: 680. https://doi.org/10.3390/catal12070680
APA StyleMarques, A. P., Huertas, R., Bernardo, J., Oliveira, B., Crespo, J. G., & Pereira, V. J. (2022). Retention and Inactivation of Quality Indicator Bacteria Using a Photocatalytic Membrane Reactor. Catalysts, 12(7), 680. https://doi.org/10.3390/catal12070680







