Evaluation of Fe2+/Peracetic Acid to Degrade Three Typical Refractory Pollutants of Textile Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Experimental Procedures
2.3. Analytical Methods
3. Results and Discussions
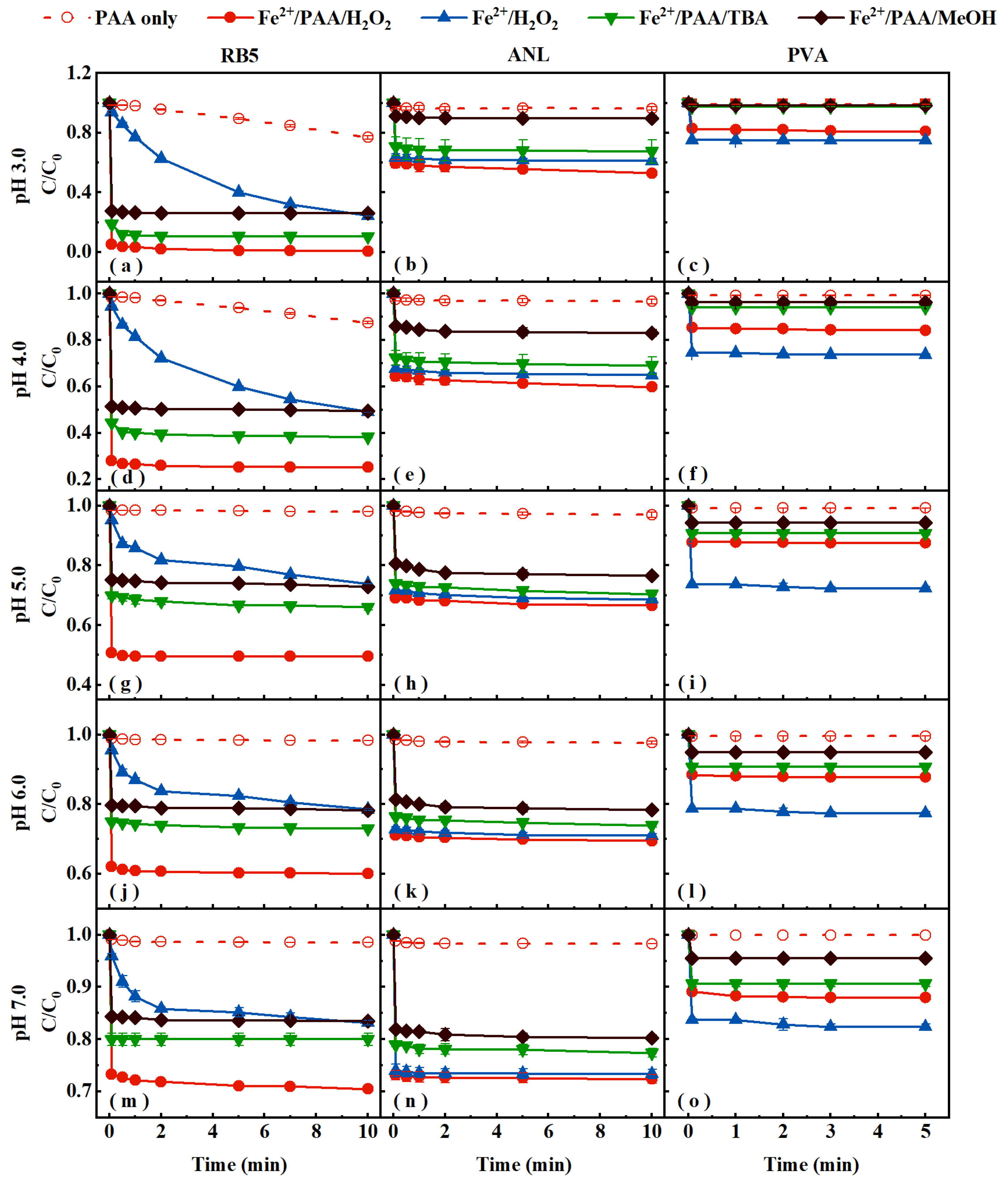
3.1. Process Degradation Efficiency Assessment
3.2. Effects of PAA and Fe2+ Dosages on Pollutants’ Removal
3.3. Effects of Coexisting Inorganic Anions and Humic Acid on Pollutants’ Removal
3.3.1. Effects of Coexisting SO42−, Cl−, and HCO3−
3.3.2. Effects of Background HA and Real Water Matrix
3.4. Acute Toxicity Evaluation
3.5. Intermediate Products and Proposed Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yukseler, H.; Uzal, N.; Sahinkaya, E.; Kitis, M.; Dilek, F.B.; Yetis, U. Analysis of the best available techniques for wastewaters from a denim manufacturing textile mill. J. Environ. Manag. 2017, 203, 1118–1125. [Google Scholar] [CrossRef]
- Chen, H.; Yu, X.; Wang, X.; He, Y.; Zhang, C.; Xue, G.; Liu, Z.; Lao, H.; Song, H.; Chen, W.; et al. Dyeing and finishing wastewater treatment in China: State of the art and perspective. J. Clean. Prod. 2021, 326, 129353. [Google Scholar] [CrossRef]
- Xue, G.; Wang, Q.; Qian, Y.; Gao, P.; Su, Y.; Liu, Z.; Chen, H.; Li, X.; Chen, J. Simultaneous removal of aniline, antimony and chromium by ZVI coupled with H2O2: Implication for textile wastewater treatment. J. Hazard. Mater. 2019, 368, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Khatri, A.; Peerzada, M.H.; Mohsin, M.; White, M. A review on developments in dyeing cotton fabrics with reactive dyes for reducing effluent pollution. J. Clean. Prod. 2015, 87, 50–57. [Google Scholar] [CrossRef]
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Nazli, Z.; Bhatti, H.N.; Nouren, S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018, 256, 395–407. [Google Scholar] [CrossRef]
- Martínez, C.M.; Celis, L.B.; Cervantes, F.J. Immobilized humic substances as redox mediator for the simultaneous removal of phenol and reactive red 2 in a UASB reactor. Appl. Microbiol. Biotechnol. 2013, 97, 9897–9905. [Google Scholar] [CrossRef]
- Hou, L.; Wu, Q.; Gu, Q.; Zhou, Q.; Zhang, J. Community structure analysis and biodegradation potential of aniline-degrading bacteria in biofilters. Curr. Microbiol. 2018, 75, 918–924. [Google Scholar] [CrossRef]
- Discharge Standards of Water Pollutants for Dyeing and Finishing of Textile Industry. 2013. Available online: https://english.mee.gov.cn/Resources/standards/water_environment/Discharge_standard/201301/t20130107_244749.shtml (accessed on 26 March 2022).
- Zhai, S.; Zheng, Q.; Ge, M. Nanosized mesoporous iron manganese bimetal oxides anchored on natural kaolinite as highly efficient hydrogen peroxide catalyst for polyvinyl alcohol degradation. J. Mol. Liq. 2021, 337, 116611. [Google Scholar] [CrossRef]
- Chou, W.; Wang, C.; Huang, K. Investigation of process parameters for the removal of polyvinyl alcohol from aqueous solution by iron electrocoagulation. Desalination 2010, 251, 12–19. [Google Scholar] [CrossRef]
- Sun, W.; Chen, L.; Wang, J. Degradation of PVA (polyvinyl alcohol) in wastewater by advanced oxidation processes. J. Adv. Oxid. Technol. 2017, 20, 20170018. [Google Scholar] [CrossRef]
- Eslami, A.; Moradi, M.; Ghanbari, F.; Mehdipour, F. Decolorization and COD removal from real textile wastewater by chemical and electrochemical Fenton processes: A comparative study. J. Environ. Health Sci. 2013, 11, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J.Q.; Wang, X.J.; Lin, X.L. Treatment of textile wastewater using facultative contact reactor-biological contact oxidation and ozone biological aerated filter. Adv. Mater. Res. 2013, 777, 318–325. [Google Scholar] [CrossRef]
- Aydin, M.I.; Yuzer, B.; Öngen, A.; Ökten, H.E.; Selcuk, H. Comparison of ozonation and coagulation decolorization methods in real textile wastewater. Desalination Water Treat. 2018, 103, 5–64. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Bessegato, G.G.; Zanoni, M.V.B. Efficiency comparison of ozonation, photolysis, photocatalysis and photoelectrocatalysis methods in real textile wastewater decolorization. Water Res. 2016, 98, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulta, K.; Aeli, G.K.; Chauhan, P.; Jasrotia, R.; Chauhan, P.K.; Ighalo, J.O. Multifunctional CuO nanoparticles with enhanced photocatalytic dye degradation and antibacterial activity. Sustain. Environ. Res. 2022, 32, 2. [Google Scholar] [CrossRef]
- Jasrotia, R.; Verma, A.; Verma, R.; Kumar, S.; Ahmed, J.; Krishan, B.; Kumari, S.; Tamboli, A.M.; Sharma, S.; Kalia, S. Nickel ions modified CoMg nanophotocatalysts for solar light-driven degradation of antimicrobial pharmaceutical effluents. J. Water Process Eng. 2022, 47, 102785. [Google Scholar] [CrossRef]
- Jasrotia, R.; Prakash, J.; Kumar, G.; Verma, R.; Kumari, S.; Kumar, S.; Singh, V.P.; Nadda, A.K.; Kalia, S. Robust and sustainable Mg1-xCexNiyFe2-yO4 magnetic nanophotocatalysts with improved photocatalytic performance towards photodegradation of crystal violet and rhodamine B pollutants. Chemosphere 2022, 294, 133706. [Google Scholar] [CrossRef]
- Kour, S.; Jasrotia, R.; Puri, P.; Verma, A.; Sharma, B.; Singh, V.P.; Kumar, R.; Kalia, S. Improving photocatalytic efficiency of MnFe2O4 ferrites via doping with Zn2+/La3+ ions: Photocatalytic dye degradation for water remediation. Environ. Sci. Pollut. Res. 2021, 1–16. [Google Scholar] [CrossRef]
- Ao, X.; Eloranta, J.; Huang, C.; Santoro, D.; Sun, W.; Lu, Z.; Li, C. Peracetic acid-based advanced oxidation processes for decontamination and disinfection of water: A review. Water Res. 2021, 188, 116479.1–116479.23. [Google Scholar] [CrossRef]
- Carlos, T.D.; Bezerra, L.B.; Vieira, M.M.; Sarmento, R.A.; Pereira, D.H.; Cavallini, G.S. Fenton-type process using peracetic acid: Efficiency, reaction elucidations and ecotoxicity. J. Hazard. Mater. 2021, 403, 23949.1–123949.8. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Emerging Technologies for Wastewater Treatment and in-Plant Wet Weather Management; US Environmental Protection Agency: Washington, DC, USA, 2013. [Google Scholar]
- Henao, L.D.; Turolla, A.; Antonelli, M. Disinfection by-products formation and ecotoxicological effects of effluents treated with peracetic acid: A review. Chemosphere 2018, 213, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Hassaballah, A.H.; Bhatt, T.; Nyitrai, J.; Dai, N.; Sassoubre, L. Inactivation of E. coli, Enterococcus spp., somatic coliphage, and cryptosporidium parvum in wastewater by peracetic acid (PAA), sodium hypochlorite, and combined PAA-ultraviolet disinfection. Environ. Sci.-Water Res. Technol. 2019, 6, 197–209. [Google Scholar] [CrossRef]
- Manoli, K.; Sarathy, S.; Maffettone, R.; Santoro, D. Detailed modeling and advanced control for chemical disinfection of secondary effluent wastewater by peracetic acid. Water Res. 2019, 153, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Liu, W.; Cao, H.; Zhao, H.; Huang, C.H. Oxidation of amino acids by peracetic acid: Reaction kinetics, pathways and theoretical calculations. Water Res. X 2018, 1, 100002. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, T.; Liu, W.; Du, P.; Dobson, J.T.; Huang, C. Advanced oxidation process with peracetic acid and Fe(II) for contaminant degradation Environ. Sci. Technol. 2019, 53, 13312–13322. [Google Scholar] [CrossRef]
- Lin, J.; Hu, Y.; Xiao, J.; Huang, Y.; Wang, M.; Yang, H.; Zou, J.; Yuan, B.; Ma, J. Enhanced diclofenac elimination in Fe(II)/peracetic acid process by promoting Fe(III)/Fe(II) cycle with ABTS as electron shuttle. Chem. Eng. J. 2021, 420, 129692. [Google Scholar] [CrossRef]
- De Laat, J.; Gallard, H.; Ancelin, S.; Legube, B. Comparative study of the oxidation of atrazine and acetone by H2O2/UV, Fe(III)/UV, Fe(III)/H2O2/UV and Fe(II) or Fe(III)/H2O2. Chemosphere 1999, 39, 2693–2706. [Google Scholar] [CrossRef]
- Kim, J.; Du, P.; Liu, W.; Luo, C.; Zhao, H.; Huang, C. Cobalt/peracetic acid: Advanced oxidation of aromatic organic compounds by acetylperoxyl radicals. Environ. Sci. Technol. 2020, 54, 5268–5278. [Google Scholar] [CrossRef]
- Li, R.; Manoli, K.; Kim, J.; Feng, M.; Huang, C.H.; Sharma, V.K. Peracetic acid–ruthenium(III) oxidation process for the degradation of micropollutants in water. Environ. Sci. Technol. 2021, 55, 9150–9160. [Google Scholar] [CrossRef]
- Cai, M.; Sun, P.; Zhang, L.; Huang, C. UV/peracetic acid for degradation of pharmaceuticals and reactive species evaluation. Environ. Sci. Technol. 2017, 51, 14217–14224. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, C. Modeling the kinetics of UV/peracetic acid advanced oxidation process. Environ. Sci. Technol. 2020, 54, 7579–7590. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Lu, C.; Yao, Y.; Sun, L.; Gong, F.; Li, D.; Pei, K.; Lu, W.; Chen, W. Activated carbon fibers as an effective metal-free catalyst for peracetic acid activation: Implications for the removal of organic pollutants. Chem. Eng. J. 2015, 281, 953–960. [Google Scholar] [CrossRef]
- Ghafoori, S.; Mehrvar, M.; Chan, P.K. Photoreactor scale-up for degradation of aqueous poly(vinyl alcohol) using UV/H2O2 process. Chem. Eng. J. 2014, 245, 133–142. [Google Scholar] [CrossRef]
- Chen, S.; Cai, M.; Liu, Y.; Zhang, L.; Feng, L. Effects of water matrices on the degradation of naproxen by reactive radicals in the UV/peracetic acid process. Water Res. 2019, 150, 153–161. [Google Scholar] [CrossRef]
- Lin, C.; Hsu, S. Performance of nZVI/H2O2 process in degrading polyvinyl alcohol in aqueous solutions. Sep. Purif. Technol. 2018, 203, 111–116. [Google Scholar] [CrossRef]
- HACH. Determination of Peracetic Acid (PAA) and Hydrogen Peroxide (H2O2) in Water; Application Note; HACH: Loveland, CO, USA, 2014. [Google Scholar]
- Klassen, N.V.; Marchington, D.; McGowan, H.C.E. H2O2 determination by the I3-method and by KMnO4 titration. Anal. Chem. 1994, 66, 2921–2925. [Google Scholar] [CrossRef]
- Liu, B.; Guo, W.; Wang, H.; Si, Q.; Zhao, Q.; Luo, H.; Ren, N. Activation of peroxymonosulfate by cobalt-impregnated biochar for atrazine degradation: The pivotal roles of persistent free radicals and ecotoxicity assessment. J. Hazard. Mater. 2020, 398, 122768. [Google Scholar] [CrossRef]
- Bhatt, D.K.; Patel, U.D. Photocatalytic degradation of reactive black 5 using Ag3PO4 under visible light. J. Phys. Chem. Solids 2021, 149, 109768. [Google Scholar] [CrossRef]
- Sharma, S.; Chokshi, N.P.; Ruparelia, J.P. Comparative studies for the degradation of reactive black 5 dye employing ozone-based AOPs. Nanotechnol. Environ. Eng. 2021. [Google Scholar] [CrossRef]
- Fadaei, S.; Noorisepehr, M.; Pourzamani, H.; Salari, M.; Moradnia, M.; Darvishmotevalli, M.; Mengelizadeh, N. Heterogeneous activation of peroxymonosulfate with Fe3O4 magnetic nanoparticles for degradation of reactive black 5: Batch and column study. J. Environ. Chem. Eng. 2021, 9, 105414. [Google Scholar] [CrossRef]
- Da Silva, M.P.; De Souza, A.C.A.; De Lima Ferreira, L.E.; Pereira Neto, L.M.; Nascimento, B.F.; De Araújo, C.M.B.; Fraga, T.J.M.; Da Motta Sobrinho, M.A.; Ghislandi, M.G. Photodegradation of reactive black 5 and raw textile wastewater by heterogeneous photo-Fenton reaction using amino-Fe3O4-functionalized graphene oxide as nanocatalyst. Environ. Adv. 2021, 4, 100064. [Google Scholar] [CrossRef]
- Li, L.; Guo, R.; Zhang, S.; Yuan, Y. Sustainable and effective degradation of aniline by sodium percarbonate activated with UV in aqueous solution: Kinetics, mechanism and identification of reactive species. Environ. Res. 2022, 207, 112176. [Google Scholar] [CrossRef] [PubMed]
- Shokri, A. Using NiFe2O4 as a nano photocatalyst for degradation of polyvinyl alcohol in synthetic wastewater. Environ. Chall. 2021, 5, 100332. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
- Grosjean, D.; Williams, E.L., II. Environmental persistence of organic compounds estimated from structure-reactivity and linear free-energy relationships. Unsaturated aliphatics. Atmos. Environ. Part A 1992, 26, 1395–1405. [Google Scholar] [CrossRef]
- Cope, V.W.; Schoen-Nan, C.; Hoffman, M.Z. Intermediates in the photochemistry of of carbonato-amine complexes of cobalt(III). Carbonate(-) radicals and the aquocarbonato complex. J. Am. Chem. Soc. 1973, 95, 3116–3121. [Google Scholar] [CrossRef]
- Yan, T.; Ping, Q.; Zhang, A.; Wang, L.; Dou, Y.; Li, Y. Enhanced removal of oxytetracycline by UV-driven advanced oxidation with peracetic acid: Insight into the degradation intermediates and N-nitrosodimethylamine formation potential. Chemosphere 2021, 274, 129726. [Google Scholar] [CrossRef]
- Du, P.; Wang, J.; Sun, G.; Chen, L.; Liu, W. Hydrogen atom abstraction mechanism for organic compound oxidation by acetylperoxyl radical in Co(II)/peracetic acid activation system. Water Res. 2022, 212, 118113. [Google Scholar] [CrossRef]
- Benito, A.; Penades, A.; Lluis Lliberia, J.; Gonzalez-Olmos, R. Degradation pathways of aniline in aqueous solutions during electro-oxidation with BDD electrodes and UV/H2O2 treatment. Chemosphere 2017, 166, 230–237. [Google Scholar] [CrossRef]
- Duan, X.; Chen, Y.; Liu, X.; Chang, L. Synthesis and characterization of nanometal-ordered mesoporous carbon composites as heterogeneous catalysts for electrooxidation of aniline. Electrochim. Acta 2017, 251, 270–283. [Google Scholar] [CrossRef]





| AOPs | Pollutants | Pollutant Concentration (mg/L) | Reaction Time (min) | Initial pH | Catalyst Dose (mg/L) | Oxidant Dose | Removal Efficiency (%) | References |
|---|---|---|---|---|---|---|---|---|
| Ag3PO4/Visible light | RB5 | 50 | 120 | 11.0 | 500 | 150 W | 91 | [41] |
| O3/Co-Ce-O | RB5 | 100 | 80 | 7.0 | 1000 | 60 LPH | 96 | [42] |
| Fe3O4/PMS | RB5 | 50 | 60 | 7.0 | 250 | 614.76 mg/L | 94.86 | [43] |
| AmGO/UV-A | RB5 | 100 | 120 | 8.0 | 5000 | 40 W | 75 | [44] |
| Fe2+/PAA/H2O2 | RB5 | 20 | 10 | 3.0 | 1.1 | 15/5 mg/L | 94 | This work |
| ANL | 10 | 10 | 3.0 | 2.2 | 47 | |||
| PVA | 10 | 5 | 3.0 | 2.2 | 20 | |||
| UV/SPC | ANL | 93.13 | 120 | 6.8 | 314 | 17.85 mw/cm2 | 54.25 | [45] |
| UV/NiFe2O4 | PVA | 25 | 140 | 6.0 | 300 | 15 W | 94.3 | [46] |
| No. | Retention Time (min) | Chemical Formula | Molecular Mass | Experimental Mass (m/z) | Proposed Structure |
|---|---|---|---|---|---|
| (1) | 5.72 | C18H16N3NaO13S4 | 633.58 | 634.15 |  |
| (2) | 5.72 | C22H15N5Na2O10S3 | 651.56 | 652.13 |  |
| (3) | 5.27 | C16H11N2NaO7S2 | 430.39 | 431.09 |  |
| (4) | 5.27 | C17H14N3NaO7S2 | 459.43 | 460.28 |  |
| (5) | 9.94 | C26H25N3O8S2 | 571.62 | 572.41 |  |
| (6) | 3.71 | C26H25N3O7S2 | 555.62 | 556.39 |  |
| (7) | 4.29 | C16H15N3O | 265.31 | 267.00 |  |
| (8) | 4.29 | C10H11N3O3 | 221.21 | 223.07 |  |
| (9) | 3.71 | C10H9NO3S | 223.25 | 224.07 |  |
| (10) | 4.63 | C10H10N2O | 174.20 | 175.11 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Shu, S.; Wang, Q.; Gao, N.; Zhu, Y. Evaluation of Fe2+/Peracetic Acid to Degrade Three Typical Refractory Pollutants of Textile Wastewater. Catalysts 2022, 12, 684. https://doi.org/10.3390/catal12070684
Yu J, Shu S, Wang Q, Gao N, Zhu Y. Evaluation of Fe2+/Peracetic Acid to Degrade Three Typical Refractory Pollutants of Textile Wastewater. Catalysts. 2022; 12(7):684. https://doi.org/10.3390/catal12070684
Chicago/Turabian StyleYu, Jiali, Shihu Shu, Qiongfang Wang, Naiyun Gao, and Yanping Zhu. 2022. "Evaluation of Fe2+/Peracetic Acid to Degrade Three Typical Refractory Pollutants of Textile Wastewater" Catalysts 12, no. 7: 684. https://doi.org/10.3390/catal12070684





