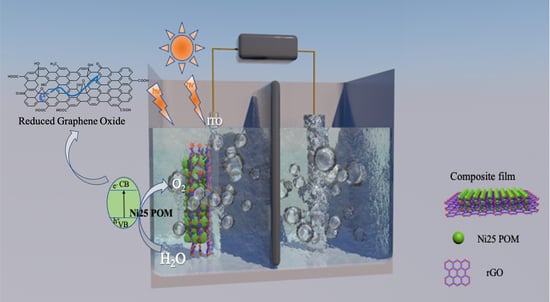
Application of Composite Film Containing Polyoxometalate Ni25 and Reduced Graphene Oxide for Photoelectrocatalytic Water Oxidation
Abstract
:1. Introduction
2. Result and Discussion
2.1. Electrochemical Property of Ni25 in Solution
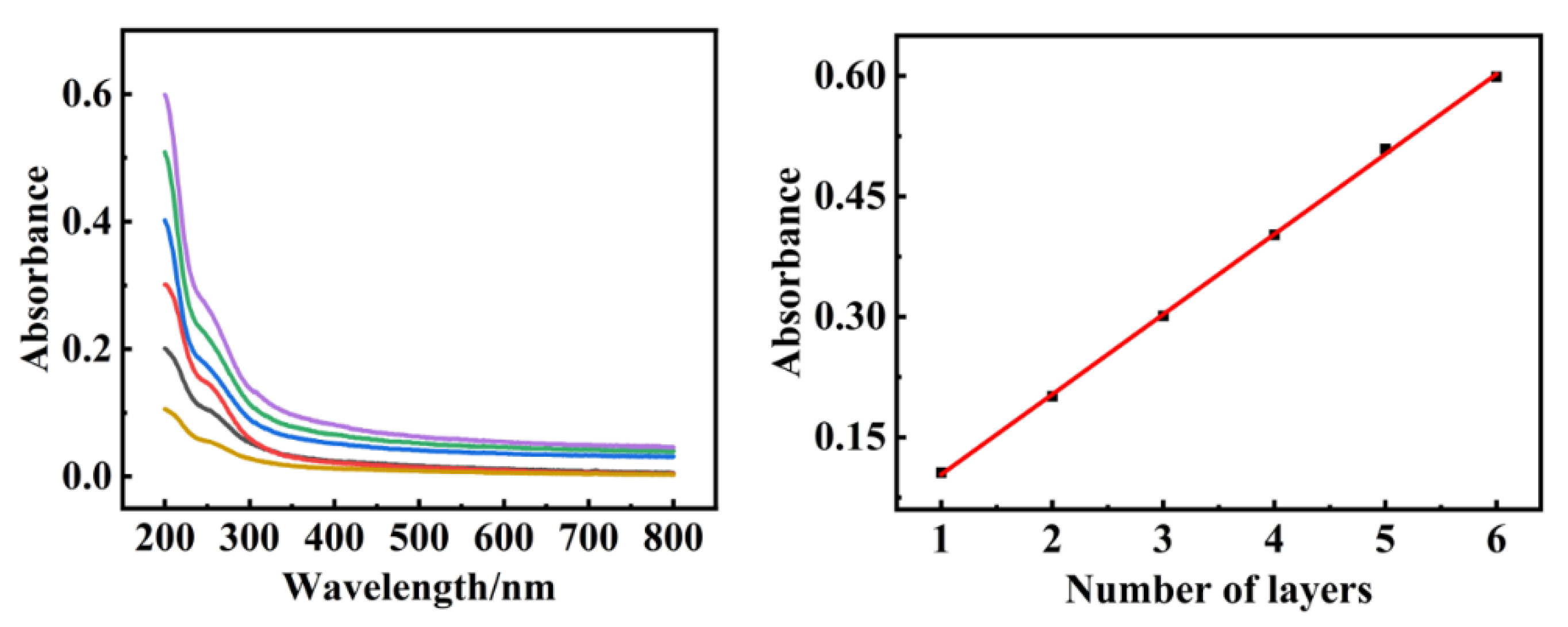
2.2. Characterization of the Composite Films Containing Ni25
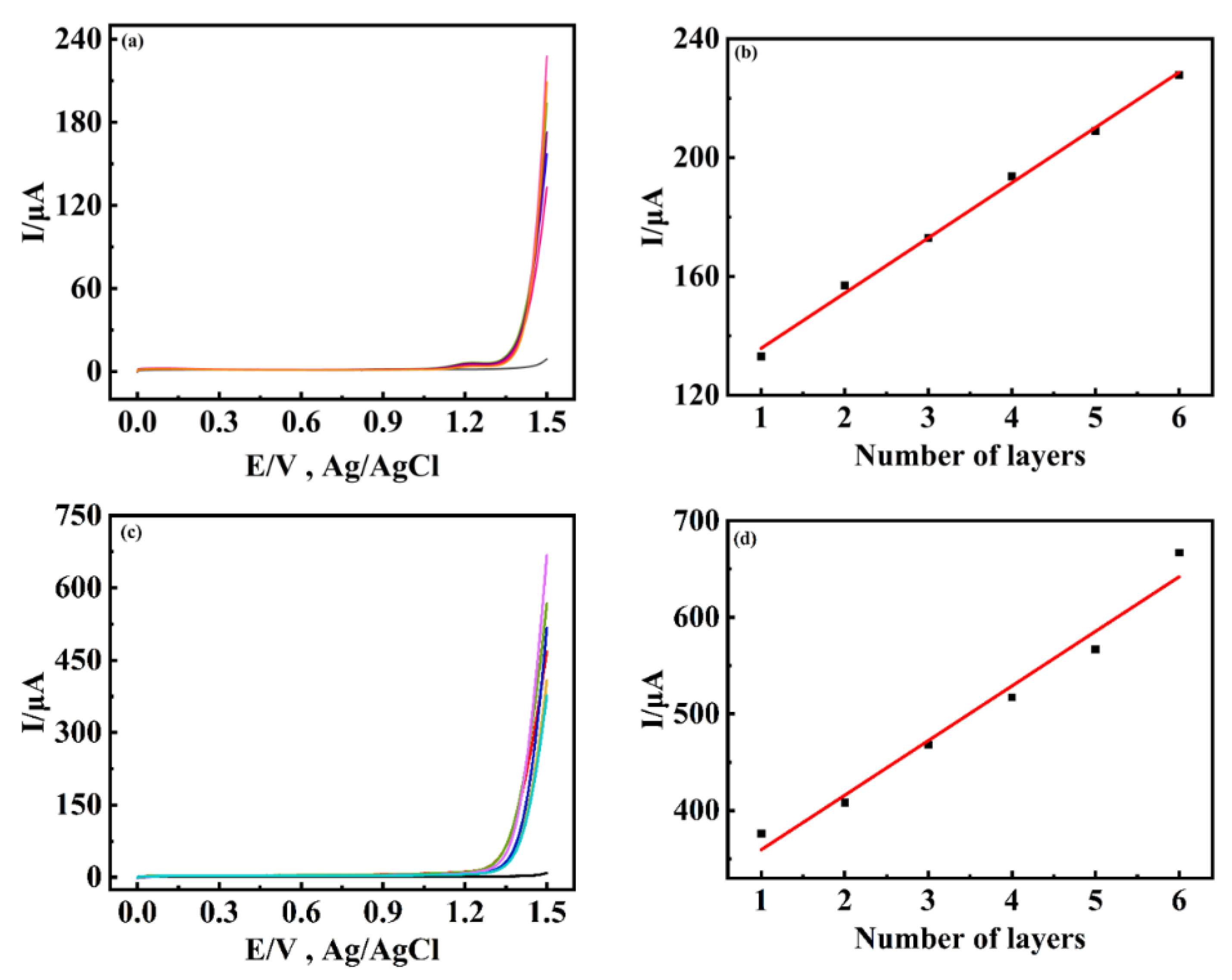
2.3. Study on Electrocatalytic Water Oxidation for Two Composite Films
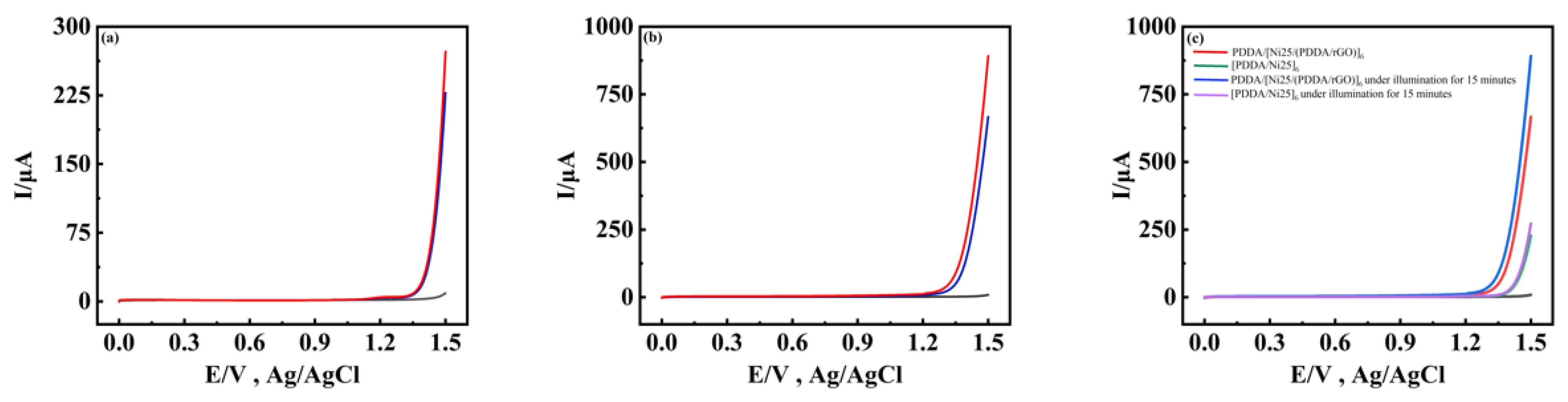
2.4. Visible-Light-Driven Photoelectrocatalytic Water Oxidation
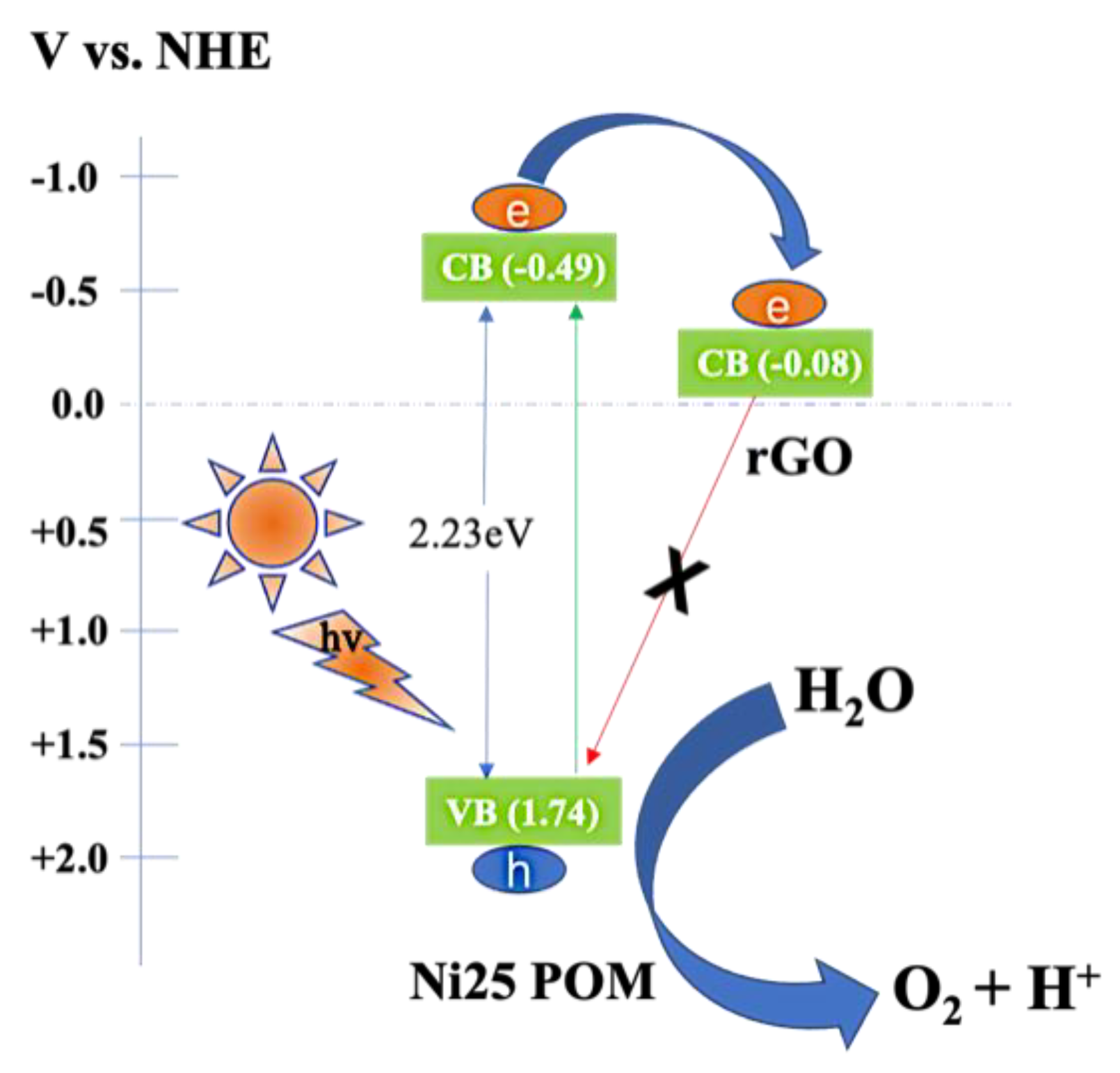
2.5. Plausible Mechanism of Photoelectrocatalytic Water Oxidation
2.6. Stability Study of Ni25 in Solution and on the Composite Films
3. Materials and Methods
3.1. Materials
3.2. LBL Assembled Composite Film
3.2.1. Preparation of the Composite Film [PDDA/Ni25]n
3.2.2. Preparation of the Composite Film PDDA/[Ni25/(PDDA–rGO)]n
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, N.S. Toward Cost-Effective Solar Energy Use. Science 2007, 315, 798–801. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.J.; Shi, J.Y.; Zhang, F.X.; Chen, Z.; Han, J.F.; Ding, C.M.; Chen, S.S.; Wang, Z.L.; Han, H.X.; Li, C. A Tantalum Nitride Photoanode Modified with a Hole-Storage Layer for Highly Stable Solar Water Splitting. Angew. Chem. Int. Ed. 2014, 53, 7295–7299. [Google Scholar] [CrossRef]
- Yang, L.; Nandakumar, D.K.; Miao, L.Q.; Suresh, L.; Zhang, D.W.; Xiong, T.; Vaghasiya, J.V.; Kwon, K.C.; Tan, S.C. Energy Harvesting from Atmospheric Humidity by a Hydrogel-Integrated Ferroelectric-Semiconductor System. Joule 2020, 4, 176–188. [Google Scholar] [CrossRef]
- Tachibana, Y.; Vayssieres, L.; Durrant, J.R. Artificial photosynthesis for solar water-splitting. Nat. Photonics 2012, 6, 511–518. [Google Scholar] [CrossRef]
- Zou, Z.; Ye, J.; Sayama, K.; Arakawa, H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Mater. Sustain. Energy 2010, 704, 293–296. [Google Scholar] [CrossRef]
- Kanan, M.W.; Nocera, D.G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 2008, 321, 1072–1075. [Google Scholar] [CrossRef] [Green Version]
- Kärkäs, M.D.; Verho, O.; Johnston, E.V.; Åkermark, B. Artificial Photosynthesis: Molecular Systems for Catalytic Water Oxidation. Chem. Rev. 2014, 114, 11863–12001. [Google Scholar] [CrossRef]
- Du, D.Y.; Qin, J.S.; Li, Y.G.; Li, S.L.; Lan, Y.Q.; Wang, X.L.; Saho, K.Z.; Su, Z.M.; Wang, E.B. An unprecedented 3D 8-connected pure inorganic framework based on nanosized ([Na12PO16H24]⊂[P4Mo6O31H6]4)15− clusters and zinc cations. Chem. Commun. 2011, 47, 2832–2834. [Google Scholar] [CrossRef]
- Hill, C.L. Introduction: PolyoxometalatesMulticomponent Molecular Vehicles To Probe Fundamental Issues and Practical Problems. Chem. Rev. 1998, 98, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Thiel, J.; Ritchie, C.; Streb, C.; Long, D.-L.; Cronin, L. Heteroatom-Controlled Kinetics of Switchable Polyoxometalate Frameworks. J. Am. Chem. Soc. 2009, 131, 4180–4181. [Google Scholar] [CrossRef]
- Stracke, J.J.; Finke, R.G. Electrocatalytic Water Oxidation Beginning with the Cobalt Polyoxometalate [Co4(H2O)2(PW9O34)2]10−: Identification of Heterogeneous CoOx as the Dominant Catalyst. J. Am. Chem. Soc. 2011, 133, 14872–14875. [Google Scholar] [CrossRef]
- Choi, Y.; Jeon, D.; Choi, Y.; Ryu, J.; Kim, B.S. Self-Assembled Supramolecular Hybrid of Carbon Nanodots and Polyoxometalates for Visible-Light-Driven Water Oxidation. ACS Appl. Mater. Interfaces 2018, 10, 13434–13441. [Google Scholar] [CrossRef]
- Tsuji, K.; Tomita, O.; Higashi, M.; Abe, R. Manganese-substituted polyoxometalate as an effective shuttle redox mediator in Z-scheme water splitting under visible light. ChemSusChem 2016, 9, 2201–2208. [Google Scholar] [CrossRef]
- Xing, X.L.; Wang, M.; Liu, R.J.; Zhang, S.S.; Zhang, K.; Li, B.; Zhang, G.J. Highly efficient electrochemically driven water oxidation by graphene-supported mixed-valent Mn-16-containing polyoxometalate. Green Energy Environ. 2016, 1, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Du, X.Q.; Ding, Y.; Chen, H.L.; Zhou, P.P. Efficient visible light-driven water oxidation catalyzed by an all-inorganic copper-containing polyoxometalate. Chem. Commun. 2015, 51, 17443–17446. [Google Scholar] [CrossRef]
- Su, X.F.; Guan, W.; Yan, L.K.; Su, Z.M. Tricopper-polyoxometalate catalysts for water oxidation: Redox-inertness of copper center. J. Catal. 2020, 381, 402–407. [Google Scholar] [CrossRef]
- Orlandi, M.; Argazzi, R.; Sartorel, A.; Carraro, M.; Scorrano, G.; Bonchio, M.; Scandola, F. Ruthenium polyoxometalate water splitting catalyst: Very fast hole scavenging from photogenerated oxidants. Chem. Commun. 2010, 46, 3152–3154. [Google Scholar] [CrossRef]
- Sokolov, M.N.; Adonin, S.A.; Mainichev, D.A.; Sinkevich, P.L.; Vicent, C.; Kompankov, N.B.; Gushchin, A.L.; Nadolinny, V.A.; Fedin, V.P. New {RuNO} Polyoxometalate [PW11O39RuII(NO)]4-: Synthesis and Reactivity. Inorg. Chem. 2013, 52, 9675–9682. [Google Scholar] [CrossRef]
- Haider, A.; Ibrahim, M.; Bassil, B.S.; Carey, A.M.; Viet, A.N.; Xing, X.; Ayass, W.W.; Miñambres, J.F.; Liu, R.; Zhang, G.; et al. Mixed-Valent Mn16-Containing Heteropolyanions: Tuning of Oxidation State and Associated Physicochemical Properties. Inorg. Chem. 2016, 55, 2755–2764. [Google Scholar] [CrossRef]
- Mitchell, S.G.; Molina, P.I.; Khanra, S.; Miras, H.N.; Prescimone, A.; Cooper, G.J.T.; Winter, R.S.; Brechin, E.K.; Long, D.-L.; Cogdell, R.J.; et al. A Mixed-Valence Manganese Cubane Trapped by Inequivalent Trilacunary Polyoxometalate Ligands. Angew. Chem. 2011, 123, 9320–9323. [Google Scholar] [CrossRef]
- Al-Oweini, R.; Sartorel, A.; Bassil, B.S.; Natali, M.; Berardi, S.; Scandola, F.; Kortz, U.; Bonchio, M. Photocatalytic Water Oxidation by a Mixed-Valent MnIII3MnIVO3 Manganese Oxo Core that Mimics the Natural Oxygen-Evolving Center. Angew. Chem. 2014, 126, 11364–11367. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, X.X.; Fu, Z.J.; Pei, J.Y.; Bi, L.H. Fabrication of Six Manganese Containing Polyoxometalate Modified Graphite C3N4 Nanosheets Catalysts Used to Catalyze Water Decomposition. Catalysts 2021, 11, 856. [Google Scholar] [CrossRef]
- Yan, J.; Kong, L.; Ji, Y.; White, J.; Li, Y.; Zhang, J.; An, P.; Liu, S.; Lee, S.-T.; Ma, T. Single atom tungsten doped ultrathin α-Ni(OH)2 for enhanced electrocatalytic water oxidation. Nat. Commun. 2019, 10, 2149. [Google Scholar] [CrossRef] [Green Version]
- Han, X.B.; Li, Y.G.; Zhang, Z.M.; Tan, H.Q.; Lu, Y.; Wang, E.B. Polyoxometalate-based nickel clusters as visible light-driven water oxidation catalysts. J. Am. Chem. Soc. 2015, 137, 5486–5493. [Google Scholar] [CrossRef]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Zhang, Z.; Tan, L.; Pang, H.; Ma, H. Facile fabrication of reduced graphene oxide and Keggin-type polyoxometalates nanocomposite film for high performance electrocatalytic oxidation of nitrite. J. Electroanal. Chem. 2017, 807, 97–103. [Google Scholar] [CrossRef]
- Cui, L.; Pu, T.; Liu, Y.; He, X. Layer-by-layer construction of graphene/cobalt phthalocyanine composite film on activated GCE for application as a nitrite sensor. Electrochim. Acta 2013, 88, 559–564. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Xie, J. Graphene in Photocatalysis: A Review. Small 2016, 12, 6640–6696. [Google Scholar] [CrossRef]
- Hadadian, M.; Correa-Baena, J.; Goharshadi, E.K.; Ummadisingu, A.; Seo, J.; Luo, J.; Gholipour, S.; Zakeeruddin, S.M.; Saliba, M.; Abate, A. Enhancing efficiency of perovskite solar cells via N-doped graphene: Crystal modification and surface passivation. Adv. Mater. 2016, 28, 8681–8686. [Google Scholar] [CrossRef] [Green Version]
- Finegold, L.; Cude, J.L. Biological sciences: One and two-dimensional structure of alpha-helix and beta-sheet forms of poly(L-Alanine) shown by specific heat measurements at low temperatures (1.5–20 K). Nature 1972, 238, 38–40. [Google Scholar] [CrossRef]
- Ye, S.; Ding, C.; Chen, R.; Fan, F.; Fu, P.; Yin, H.; Wang, X.; Wang, Z.; Du, P.; Li, C. Mimicking the Key Functions of Photosystem II in Artificial Photosynthesis for Photoelectrocatalytic Water Splitting. J. Am. Chem. Soc. 2018, 140, 3250–3256. [Google Scholar] [CrossRef] [PubMed]
- Mohamedkhair, A.K.; Drmosh, Q.A.; Qamar, M.; Yamani, Z.H. Tuning Structural Properties of WO3 Thin Films for Photoelectrocatalytic Water Oxidation. Catalysts 2021, 11, 381. [Google Scholar] [CrossRef]
- Xi, L.; Jin, Z.; Sun, Z.; Liu, R.; Xu, L. Enhanced photoelectrocatalytic performance for water oxidation by polyoxometalate molecular doping in BiVO4 photoanodes. Appl. Catal. A Gen. 2017, 536, 67–74. [Google Scholar] [CrossRef]
- Niu, P.; Hao, J. Photocatalytic degradation of methyl orange by titanium dioxide-decatungstate nanocomposite films supported on glass slides. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 431, 127–132. [Google Scholar] [CrossRef]
- Li, P.; Liu, H.; Yang, J.; Sun, D.; Chen, Y.; Zhou, Y.; Cai, C.; Lu, T. A ruthenium(III) phosphonate complex on polyallylamine functionalized carbon nanotube multilayer films: Self-assembly, direct electrochemistry, and electrocatalysis. J. Mater. Chem. B 2014, 2, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guo, S.; Ren, J.; Zhai, Y.; Dong, S.; Wang, E. Cyclodextrin functionalized graphene nanosheets with high supramolecular recognition capability: Synthesis and host-guest inclusion for enhanced electrochemical performance (ACS Nano (2010) 4 (4001–4010)). ACS Nano 2010, 4, 5512. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Liu, L.; Hu, J.; Cui, W. Highly ordered TiO2 nanotube arrays wrapped with g-C3N4 nanoparticles for efficient charge separation and increased photoelectrocatalytic degradation of phenol. J. Hazard. Mater. 2018, 344, 369–380. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Ghicov, A.; Räder, A.S.; Taveira, L.; Schmuki, P. Characterization of electronic properties of TiO2 nanotube films. Corros. Sci. 2007, 49, 203–210. [Google Scholar] [CrossRef]
- Jiao, J.; Zuo, J.; Pang, H.; Tan, L.; Chen, T.; Ma, H. A dopamine electrochemical sensor based on Pd-Pt alloy nanoparticles decorated polyoxometalate and multiwalled carbon nanotubes. J. Electroanal. Chem. 2018, 827, 103–111. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; You, W.; Liu, M.; Zhang, L.; Sang, X. Catalytic effects of [Ag(H2O)(H3PW11O39)]3- on a TiO2 anode for water oxidation. Cuihua Xuebao/Chinese J. Catal. 2018, 39, 534–541. [Google Scholar] [CrossRef]
- Mohanta, D.; Barman, K.; Jasimuddin, S.; Ahmaruzzaman, M. Encapsulating band gap engineered CoSnO3 mixed metal oxide nanocomposite in rGO matrix: A novel catalyst towards LED light induced photoelectrocatalytic water oxidation at neutral pH. J. Electroanal. Chem. 2021, 880, 114830. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, Z.; Shi, R.; Yang, X.; Zhang, T. Recent Advances in Conjugated Polymers for Visible-Light-Driven Water Splitting. Adv. Mater. 2020, 32, e1907296. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.D.; Batabyal, S.K.; Pramana, S.S.; Barber, J.; Wong, L.H.; Loo, S.C.J. A cuprous oxide-reduced graphene oxide (Cu2O-rGO) composite photocatalyst for hydrogen generation: Employing rGO as an electron acceptor to enhance the photocatalytic activity and stability of Cu2O. Nanoscale 2012, 4, 3875–3878. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-Layer Assembly of Ultrathin Composite Films from Micron-Sized Graphite Oxide Sheets and Polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]





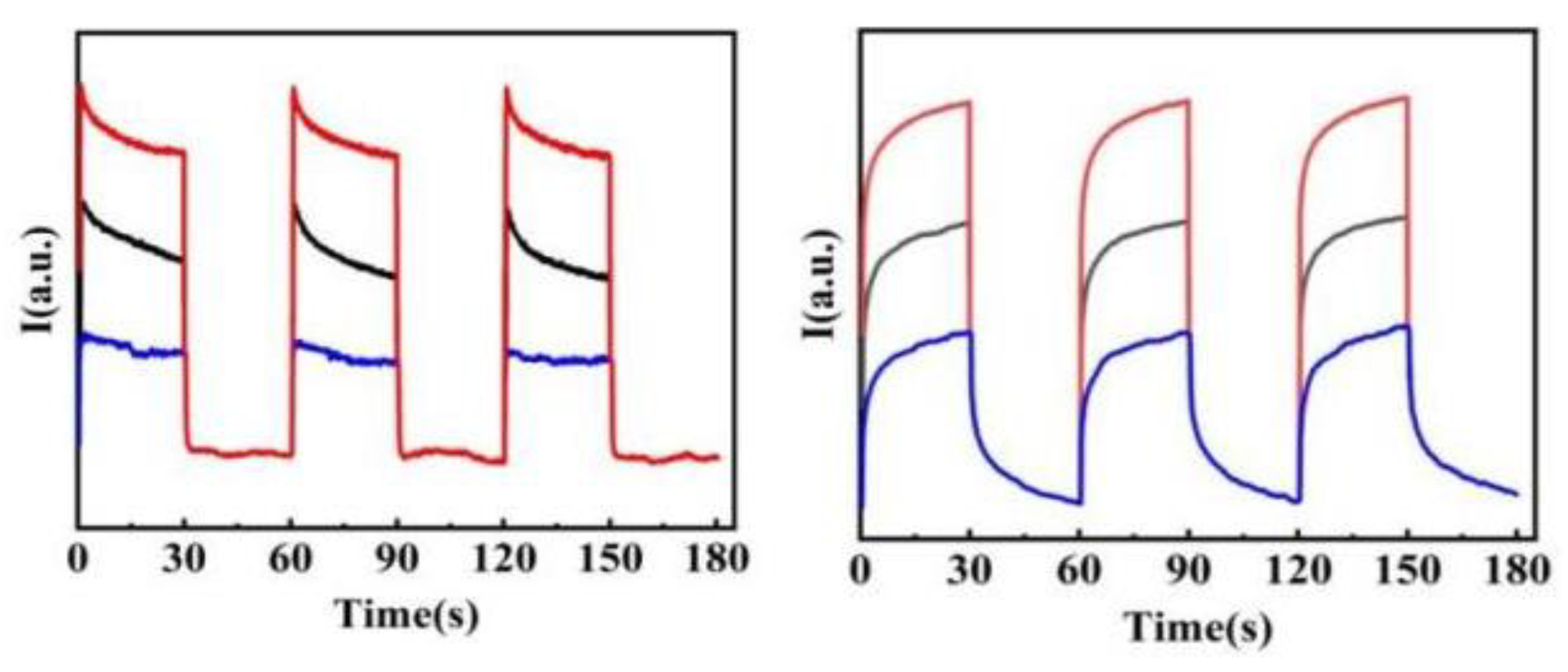


| Film | J@1.5V vs. RHE | IPCE |
|---|---|---|
| [PDDA/Ni25]6 | 0.60 mA cm−2 | 11.92% |
| PDDA/[Ni25/(PDDA–rGO)]6 | 2.1 mA cm−2 | 41.73% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, J.; Bi, L. Application of Composite Film Containing Polyoxometalate Ni25 and Reduced Graphene Oxide for Photoelectrocatalytic Water Oxidation. Catalysts 2022, 12, 696. https://doi.org/10.3390/catal12070696
Pei J, Bi L. Application of Composite Film Containing Polyoxometalate Ni25 and Reduced Graphene Oxide for Photoelectrocatalytic Water Oxidation. Catalysts. 2022; 12(7):696. https://doi.org/10.3390/catal12070696
Chicago/Turabian StylePei, Jianye, and Lihua Bi. 2022. "Application of Composite Film Containing Polyoxometalate Ni25 and Reduced Graphene Oxide for Photoelectrocatalytic Water Oxidation" Catalysts 12, no. 7: 696. https://doi.org/10.3390/catal12070696
APA StylePei, J., & Bi, L. (2022). Application of Composite Film Containing Polyoxometalate Ni25 and Reduced Graphene Oxide for Photoelectrocatalytic Water Oxidation. Catalysts, 12(7), 696. https://doi.org/10.3390/catal12070696