Protolytic Ring-Opening Cracking of Methylcyclohexane over Hierarchical High-Silica USY Zeolite: A Haag-Dessau Cracking
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure, Porosity and Morphology
2.2. Acidity
2.3. Diffusion Evaluation
2.4. Catalytic Reaction Evaluation
3. Materials and Methods
3.1. Zeolite Samples
3.2. Characterization Methods
3.3. Diffusion Study
3.4. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coronas, J. Present and future synthesis challenges for zeolites. Chem. Eng. J. 2010, 156, 236–242. [Google Scholar] [CrossRef]
- Li, K.; Valla, J.; Garcia-Martinez, J. Realizing the commercial potential of hierarchical zeolites: New opportunities in catalytic cracking. ChemCatChem 2014, 6, 2754–2775. [Google Scholar] [CrossRef] [Green Version]
- Primo, A.; Garcia, H. Zeolites as catalysts in oil refining. Chem. Soc. Rev. 2014, 43, 7548–7561. [Google Scholar] [CrossRef] [PubMed]
- Chal, R.; Gérardin, C.; Bulut, M.; van Donk, S. Overview and industrial assessment of synthesis strategies towards zeolites with mesopores. ChemCatChem 2011, 3, 67–81. [Google Scholar] [CrossRef]
- Hartmann, M.; Machoke, A.G.; Schwieger, W. Catalytic test reactions for the evaluation of hierarchical zeolites. Chem. Soc. Rev. 2016, 45, 3313–3330. [Google Scholar] [CrossRef] [Green Version]
- Schwieger, W.; Machoke, A.G.; Weissenberger, T.; Inayat, A.; Selvam, T.; Klumpp, M.; Inayat, A. Hierarchy concepts: Classification and preparation strategies for zeolite containing materials with hierarchical porosity. Chem. Soc. Rev. 2016, 45, 3353–3376. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Parmentier, T.E.; De Jong, K.P.; Zecevic, J. Tailoring and visualizing the pore architecture of hierarchical zeolites. Chem. Soc. Rev. 2015, 44, 7234–7261. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Ostraat, M.L. Innovations in hierarchical zeolite synthesis. Catal. Today 2016, 264, 3–15. [Google Scholar] [CrossRef]
- Jiao, W.Q.; Fu, W.H.; Liang, X.M.; Wang, Y.M.; He, M.Y. Preparation of hierarchically structured Y zeolite with low Si/Al ratio and its applications in acetalization reactions. RSC Adv. 2014, 4, 58596–58607. [Google Scholar] [CrossRef]
- García, J.R.; Bertero, M.; Falco, M.; Sedran, U. Catalytic cracking of bio-oils improved by the formation of mesopores by means of Y zeolite desilication. Appl. Catal. A Gen. 2015, 503, 1–8. [Google Scholar] [CrossRef]
- De Jong, K.P.; Zecevic, J.; Friedrich, H.; De Jongh, P.E.; Bulut, M.; Van Donk, S.; Kenmogne, R.; Finiels, A.; Hulea, V.; Fajula, F. Zeolite Y crystals with trimodal porosity as ideal hydrocracking catalysts. Angew. Chem. Int. Ed. 2010, 49, 10074–10078. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, J.; Li, K.; Krishnaiah, G. A mesostructured Y zeolite as a superior FCC catalyst—From lab to refinery. Chem. Commun. 2012, 48, 11841–11843. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, J.; Johnson, M.; Valla, J.; Li, K.; Ying, J.Y. Mesostructured zeolite Y—High hydrothermal stability and superior FCC catalytic performance. Catal. Sci. Technol. 2012, 2, 987–994. [Google Scholar] [CrossRef]
- Mihalyi, R.M.; Kollar, M.; Kiraly, P.; Karoly, Z.; Mavrodinova, V. Effect of extra-framework Al formed by successive steaming and acid leaching of zeolite MCM-22 on its structure and catalytic performance. Appl. Catal. A Gen. 2012, 417, 76–86. [Google Scholar] [CrossRef]
- Verboekend, D.; Vile, G.; Perez-Ramirez, J. Mesopore formation in USY and Beta zeolites by base leaching: Selection criteria and optimization of pore-directing agents. Cryst. Growth Des. 2012, 12, 3123–3132. [Google Scholar] [CrossRef]
- Kung, H.H.; Williams, B.A.; Babitz, S.M.; Miller, J.T.; Haag, W.O.; Snurr, R.Q. Enhanced hydrocarbon cracking activity of Y zeolites. Top Catal. 2000, 10, 59–64. [Google Scholar] [CrossRef]
- Williams, B.A.; Babitz, S.M.; Miller, J.T.; Snurr, R.Q.; Kung, H.H. The roles of acid strength and pore diffusion in the enhanced cracking activity of steamed Y zeolites. Appl. Catal. A Gen. 1999, 177, 161–175. [Google Scholar] [CrossRef]
- Lappas, A.A.; Patiaka, D.; Ikonomou, D. Separation and characterization of paraffins and naphthenes from FCC feedstocks. Ind. Eng. Chem. Res. 1997, 36, 3110–3115. [Google Scholar] [CrossRef]
- Townsend, A.T. FLuid catalytic cracking: Science and technology. Appl. Catal. A Gen. 1994, 109, N16–N17. [Google Scholar] [CrossRef]
- Abbot, J.; Wojciechowski, B.W. Kinetics of catalytic cracking of n-paraffins on HY zeolite. J. Catal. 1987, 104, 80–85. [Google Scholar] [CrossRef]
- Benazzi, E.; Chapus, T.H.; Cheron, T.; Cauffriez, H.; Marcilly, C.H. Relationship between zeolite structure and hydrogen-transfer reactions in naphthenes and paraffins cracking. Stud. Surf. Sci. Catal. 1994, 84, 1663–1669. [Google Scholar]
- Cerqueira, H.S.; Mihindou-Koumba, P.C.; Magnoux, P.; Guisnet, M. Methylcyclohexane transformation over HFAU, HBEA, and HMFI zeolites: I. reaction scheme and mechanisms. Ind. Eng. Chem. Res. 2001, 40, 1032–1041. [Google Scholar] [CrossRef]
- Corma, A.; Mocholi, F.; Orchilles, V.; Koermer, G.S.; Madon, R.J. Methylcyclohexane and methylcyclohexene cracking over zeolite Y catalysts. Appl. Catal. 1990, 67, 307–324. [Google Scholar] [CrossRef]
- Mihindou-Koumba, P.C.; Comparot, J.D.; Laforge, S.; Magnoux, P. Methylcyclohexane transformation over H-EU-1 zeolite: Selectivity and catalytic role of the acid sites located at the pore mouths. J. Catal. 2008, 255, 324–334. [Google Scholar] [CrossRef]
- Martins, G.V.A.; Berlier, G.; Bisio, C.; Coluccia, S.; Pastore, H.O.; Marchese, L. Quantification of Brønsted acid sites in microporous catalysts by a combined FTIR and NH3-TPD Study. J. Phys. Chem. C 2008, 112, 7193–7200. [Google Scholar] [CrossRef]
- Schuring, D.; Koriabkina, A.O.; De Jong, A.M.; Smit, B.; van Santen, R.A. Adsorption and diffusion of n-nexane/2-methylpentane mixtures in zeolite silicalite: Experiments and modeling. J. Phys. Chem. B 2001, 105, 7690–7698. [Google Scholar] [CrossRef] [Green Version]
- Vinh-Thang, H.; Huang, Q.L.; Ungureanu, A.; Eic, M.; Trong-On, D.; Kaliaguine, S. Structural and diffusion characterizations of steam-stable mesostructured zeolitic UL-ZSM-5 materials. Langmuir 2006, 22, 4777–4786. [Google Scholar] [CrossRef]
- Vinh-Thang, H.; Huang, Q.L.; Ungureanu, A.; Eic, M.; Trong-On, D.; Kaliaguine, S. Effect of the acid properties on the diffusion of C7 hydrocarbons in UL-ZSM-5 materials. Micropor. Mesopor. Mater. 2006, 92, 117–128. [Google Scholar] [CrossRef]
- Al-Sabawi, M.; De lasa, H. Kinetic modeling of catalytic conversion of methylcyclohexane over USY zeolites: Adsorption and reaction phenomena. AIChE J. 2009, 55, 1538–1558. [Google Scholar] [CrossRef]
- Kotrel, S.; Knözinger, H.; Gates, B.C. The Haag–Dessau mechanism of protolytic cracking of alkanes. Micropor. Mesopor. Mater. 2000, 35, 11–20. [Google Scholar] [CrossRef]
- Raichle, A.; Traa, Y.; Fuder, F.; Rupp, M.; Weitkamp, J. Haag-Dessau catalysts for ring opening of cycloalkanes. Angew. Chem. Int. Edit. 2001, 40, 1243–1246. [Google Scholar] [CrossRef]
- Borm, R.V.; Reyniers, M.-F.; Martens, J.A.; Marin, G.B. Catalytic cracking of methylcyclohexane on FAU, MFI, and bimodal porous materials: Influence of acid properties and pore topology. Ind. Eng. Chem. Res. 2010, 49, 10486–10495. [Google Scholar] [CrossRef]
- Martins, J.; Birot, E.; Guillon, E.; Lemos, F.; Ribeiro, F.R.; Magnoux, P.; Laforge, S. Sodium exchange over H-EU-1 zeolite. Part II: Catalytic properties. Micropor. Mesopor. Mater. 2013, 171, 238–245. [Google Scholar] [CrossRef]
- Corma, A.; Agudo, A.L. Isomerization, dehydrogenation and cracking of methylcyclohexane over HNaY zeolites. React. Kinet. Catal. Lett. 1981, 16, 253–257. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, W.; Xue, Z.; Ma, J.; Li, R. Diffusion of n-alkanes in mesoporous 5A zeolites by ZLC method. Adsorption 2013, 19, 201–208. [Google Scholar] [CrossRef]
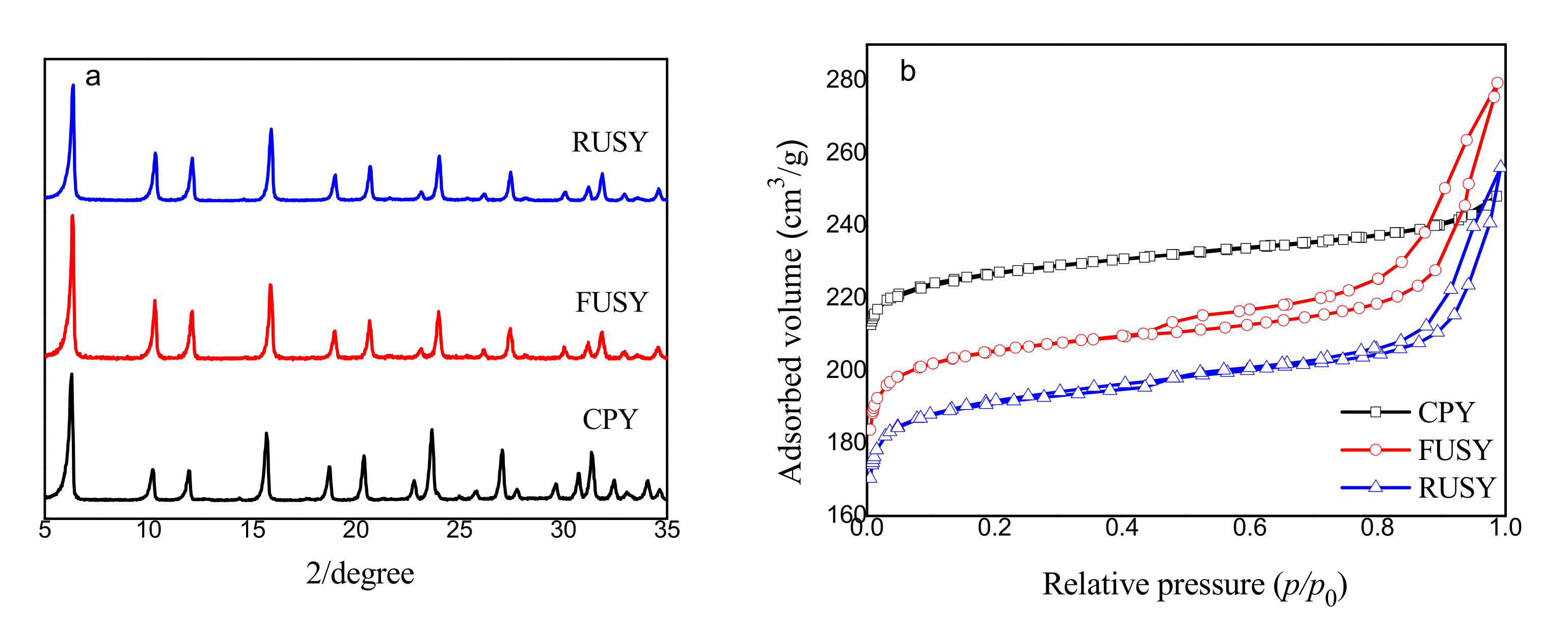

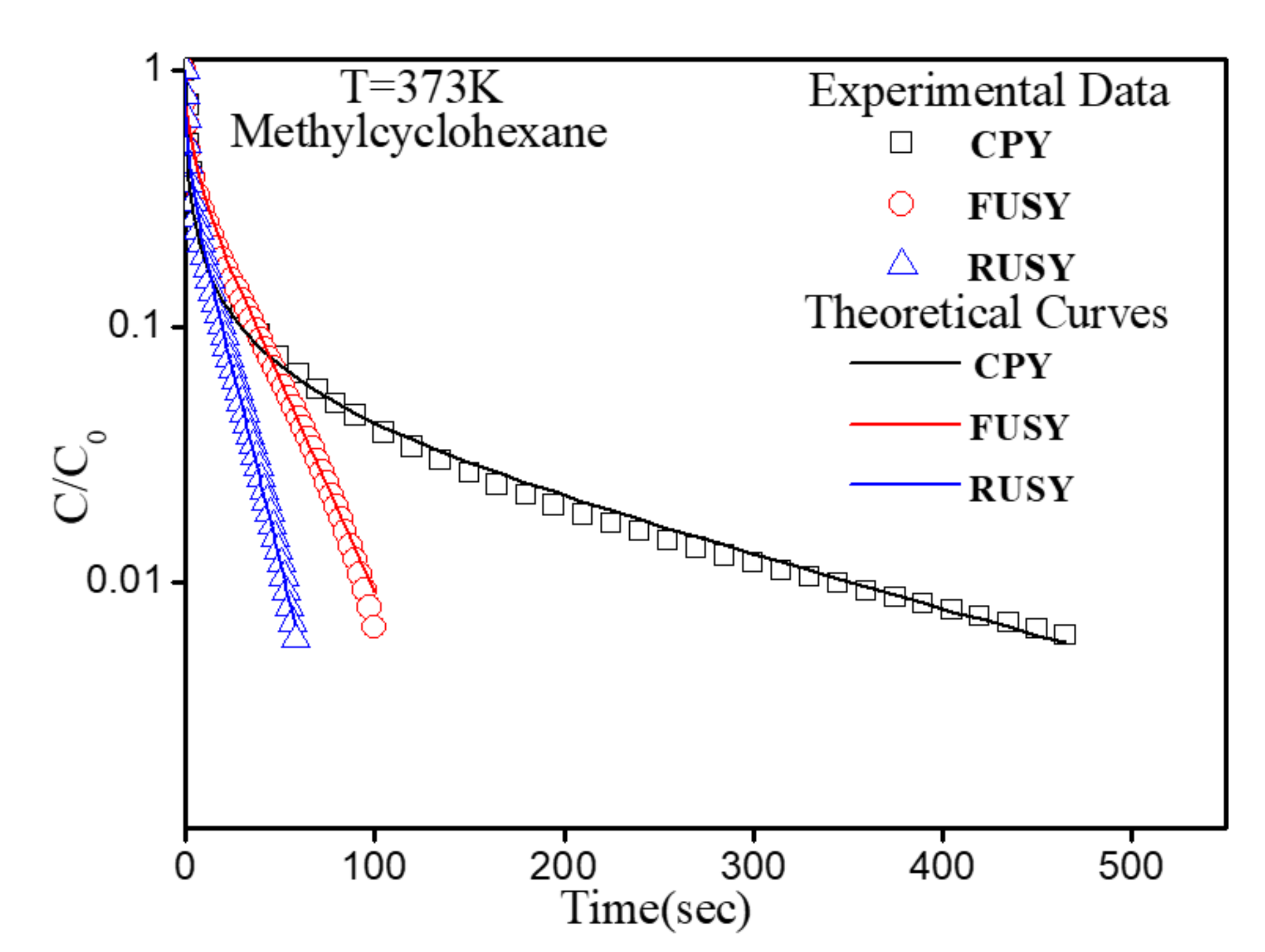
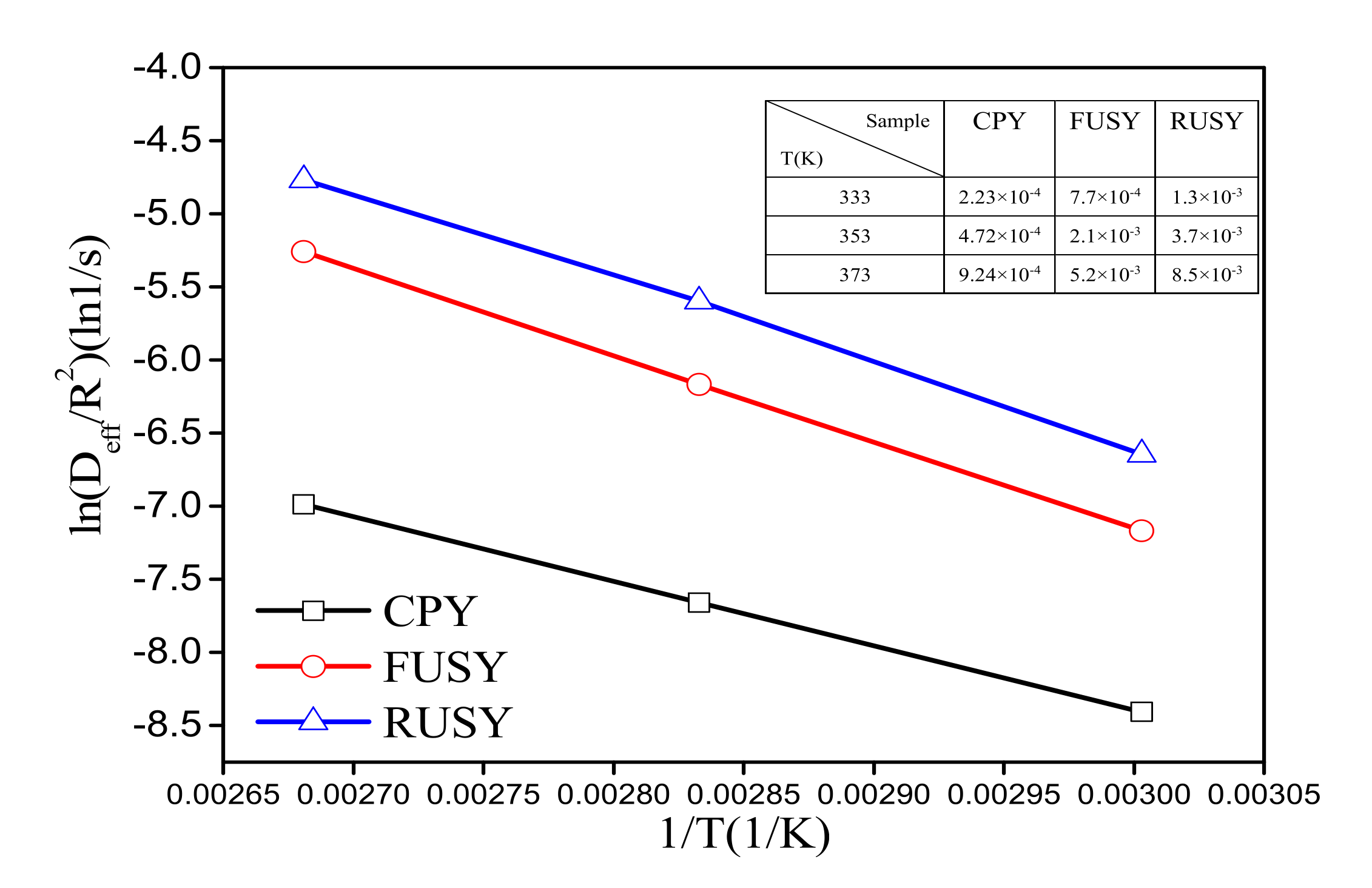

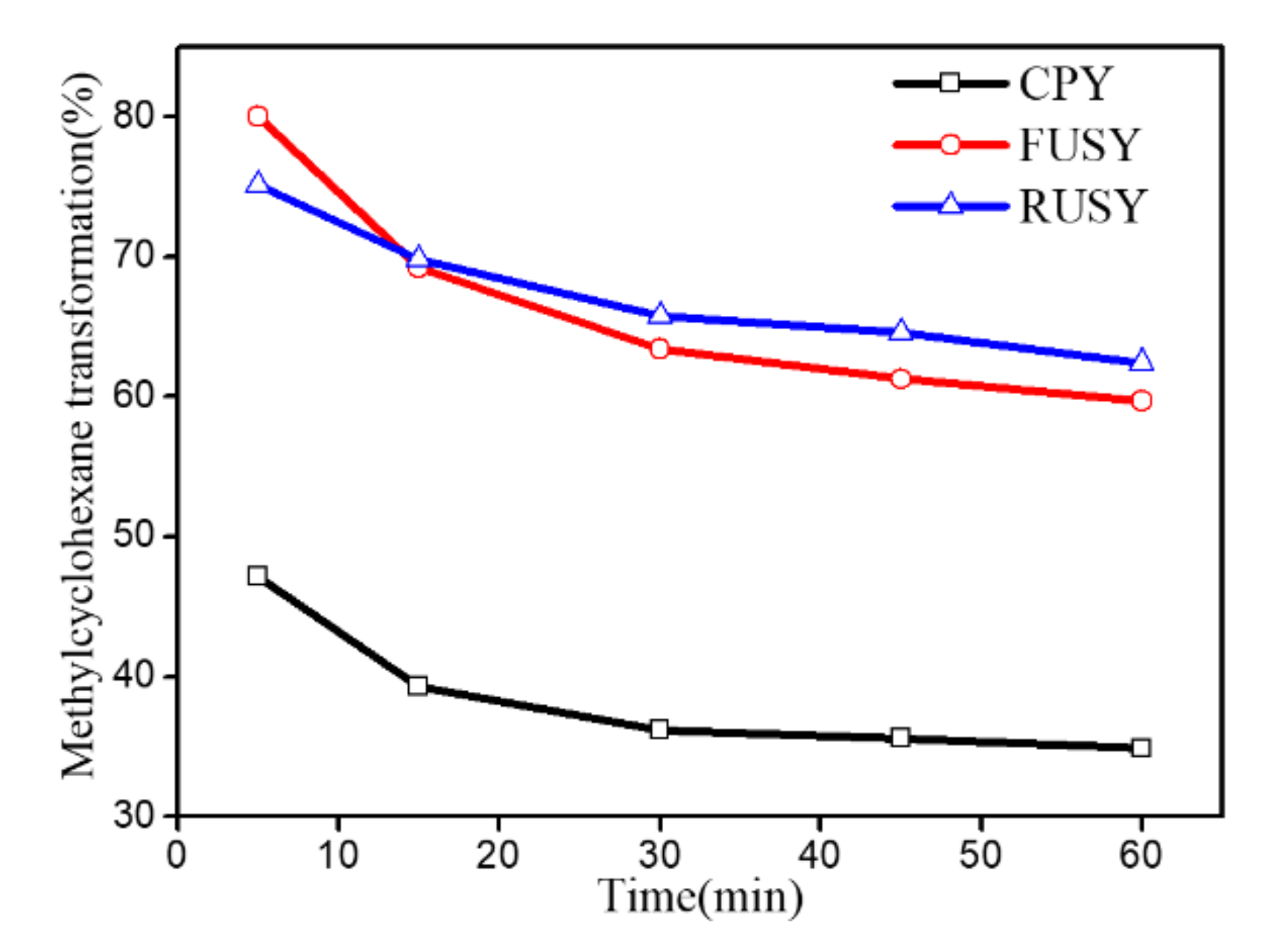
| Samples | SBET (m2/g) | Vmic (cm3/g) | Vmeso (cm3/g) | RC a (%) | nSi/nAlF b | nSi/nAlbulk c |
|---|---|---|---|---|---|---|
| CPY | 933 | 0.33 | 0.08 | 100 | 2.6 | 2.4 |
| FUSY | 756 | 0.28 | 0.13 | 70 | 5.5 | 4.6 |
| RUSY | 776 | 0.27 | 0.11 | 69 | 13.3 | 10.5 |
| Sample | T (K) | BAS (μmol/g) | LAS (μmol/g) | Total (μmol/g) |
|---|---|---|---|---|
| CPY | 423 | 322 | 298 | 620 |
| 523 | 292 | 194 | 486 | |
| 623 | 193 | 171 | 364 | |
| FUSY | 423 | 287 | 76 | 363 |
| 523 | 269 | 57 | 327 | |
| 623 | 225 | 46 | 271 | |
| RUSY | 423 | 246 | 65 | 311 |
| 523 | 231 | 50 | 282 | |
| 623 | 200 | 38 | 239 |
| Products | 5 min | 45 min | ||||
|---|---|---|---|---|---|---|
| CPY | FUSY | RUSY | CPY | FUSY | RUSY | |
| C1–C2 | 0.68 | 0.66 | 0.63 | 0.58 | 0.49 | 0.45 |
| 3.03 | 6.20 | 5.00 | 2.10 | 3.28 | 3.06 | |
| 0.64 | 0.47 | 0.38 | 0.49 | 0.38 | 0.31 | |
| 11.2 | 19.0 | 14.0 | 6.11 | 9.29 | 8.81 | |
| 1.68 | 3.39 | 2.80 | 1.10 | 1.79 | 1.70 | |
| 0.05 | 0.03 | 0.02 | 0.03 | 0.02 | 0.02 | |
| 0.23 | 0.14 | 0.13 | 0.17 | 0.13 | 0.11 | |
| 7.66 | 13.0 | 9.91 | 4.29 | 6.65 | 6.33 | |
| 0.07 | 0.04 | 0.03 | 0.05 | 0.03 | 0.03 | |
| 9.52 | 13.2 | 11.7 | 5.69 | 8.47 | 8.62 | |
| 1.25 | 2.61 | 1.82 | 0.73 | 1.24 | 1.05 | |
| 10.5 | 6.15 | 9.29 | 12.6 | 9.94 | 12.1 | |
| 3.90 | 3.69 | 3.74 | 3.58 | 2.77 | 4.14 | |
| Isomers | 48.2 | 27.6 | 35.6 | 58.2 | 51.6 | 49.4 |
| Benzene | 0.11 | 0.27 | 0.26 | 1.17 | 0.16 | 0.26 |
| Toluene | 1.37 | 3.64 | 4.67 | 3.06 | 3.78 | 3.61 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| Cracking (C) | 50.3 | 68.5 | 59.5 | 37.5 | 44.5 | 46.7 |
| Isomers (I) | 48.2 | 27.6 | 35.6 | 58.2 | 51.6 | 49.4 |
| Aromatics (A) | 1.48 | 3.91 | 4.93 | 4.23 | 3.94 | 3.87 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Qin, B.; Hao, W.; Du, Y.; Ma, J.; Li, R. Protolytic Ring-Opening Cracking of Methylcyclohexane over Hierarchical High-Silica USY Zeolite: A Haag-Dessau Cracking. Catalysts 2022, 12, 697. https://doi.org/10.3390/catal12070697
Li Y, Qin B, Hao W, Du Y, Ma J, Li R. Protolytic Ring-Opening Cracking of Methylcyclohexane over Hierarchical High-Silica USY Zeolite: A Haag-Dessau Cracking. Catalysts. 2022; 12(7):697. https://doi.org/10.3390/catal12070697
Chicago/Turabian StyleLi, Yaojie, Bo Qin, Wenming Hao, Yanze Du, Jinghong Ma, and Ruifeng Li. 2022. "Protolytic Ring-Opening Cracking of Methylcyclohexane over Hierarchical High-Silica USY Zeolite: A Haag-Dessau Cracking" Catalysts 12, no. 7: 697. https://doi.org/10.3390/catal12070697
APA StyleLi, Y., Qin, B., Hao, W., Du, Y., Ma, J., & Li, R. (2022). Protolytic Ring-Opening Cracking of Methylcyclohexane over Hierarchical High-Silica USY Zeolite: A Haag-Dessau Cracking. Catalysts, 12(7), 697. https://doi.org/10.3390/catal12070697








