Photocatalytic Remediation of Harmful Alexandrium minutum Bloom Using Hybrid Chitosan-Modified TiO2 Films in Seawater: A Lab-Based Study
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Fresh Hybrid Chitosan-Modified TiO2 Films
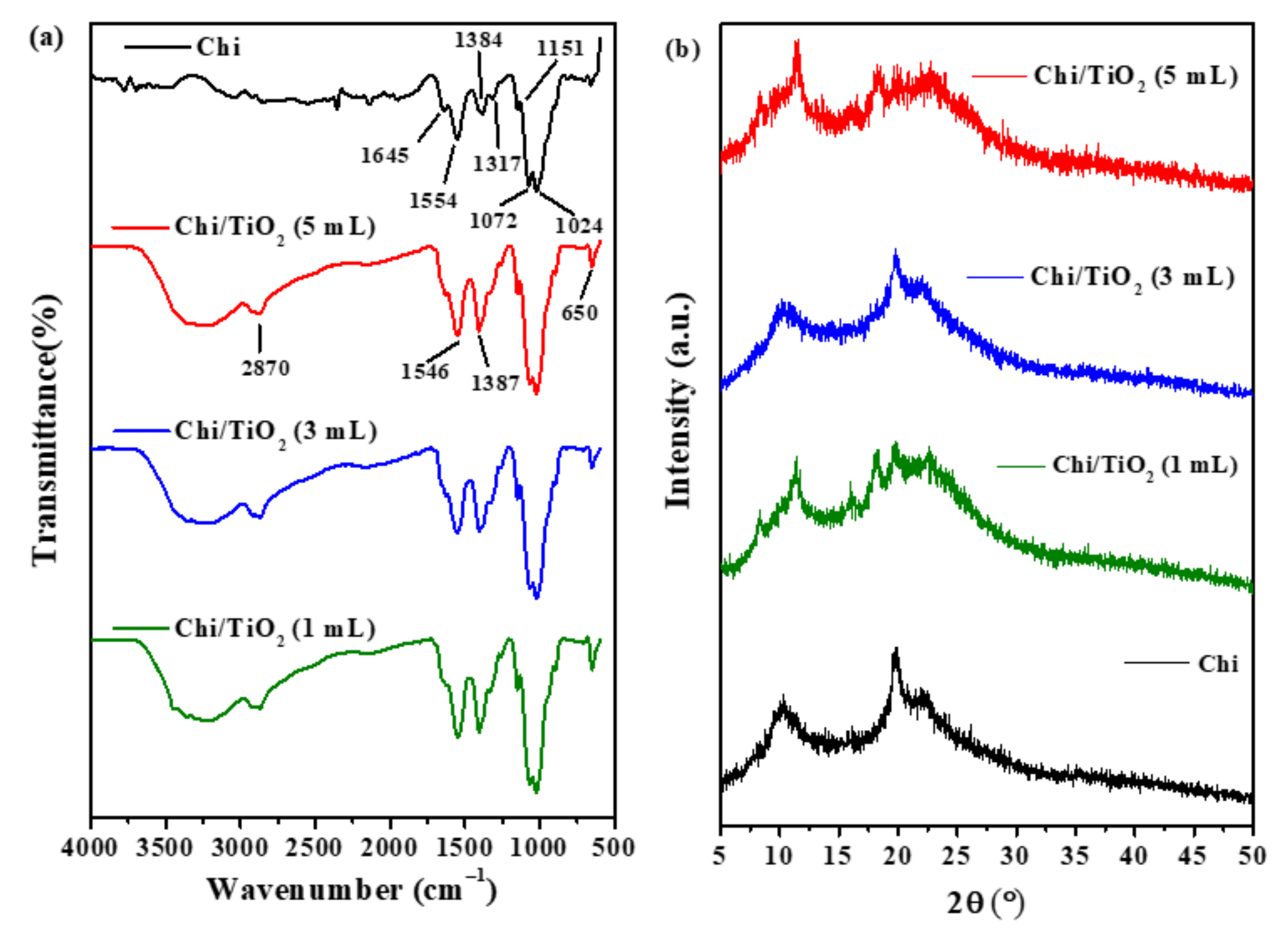
2.1.1. Attenuated Total Reflectance Fourier Transformed Infrared Spectroscopy (ATR-FTIR) Analysis
2.1.2. X-ray Diffraction (XRD) Analysis
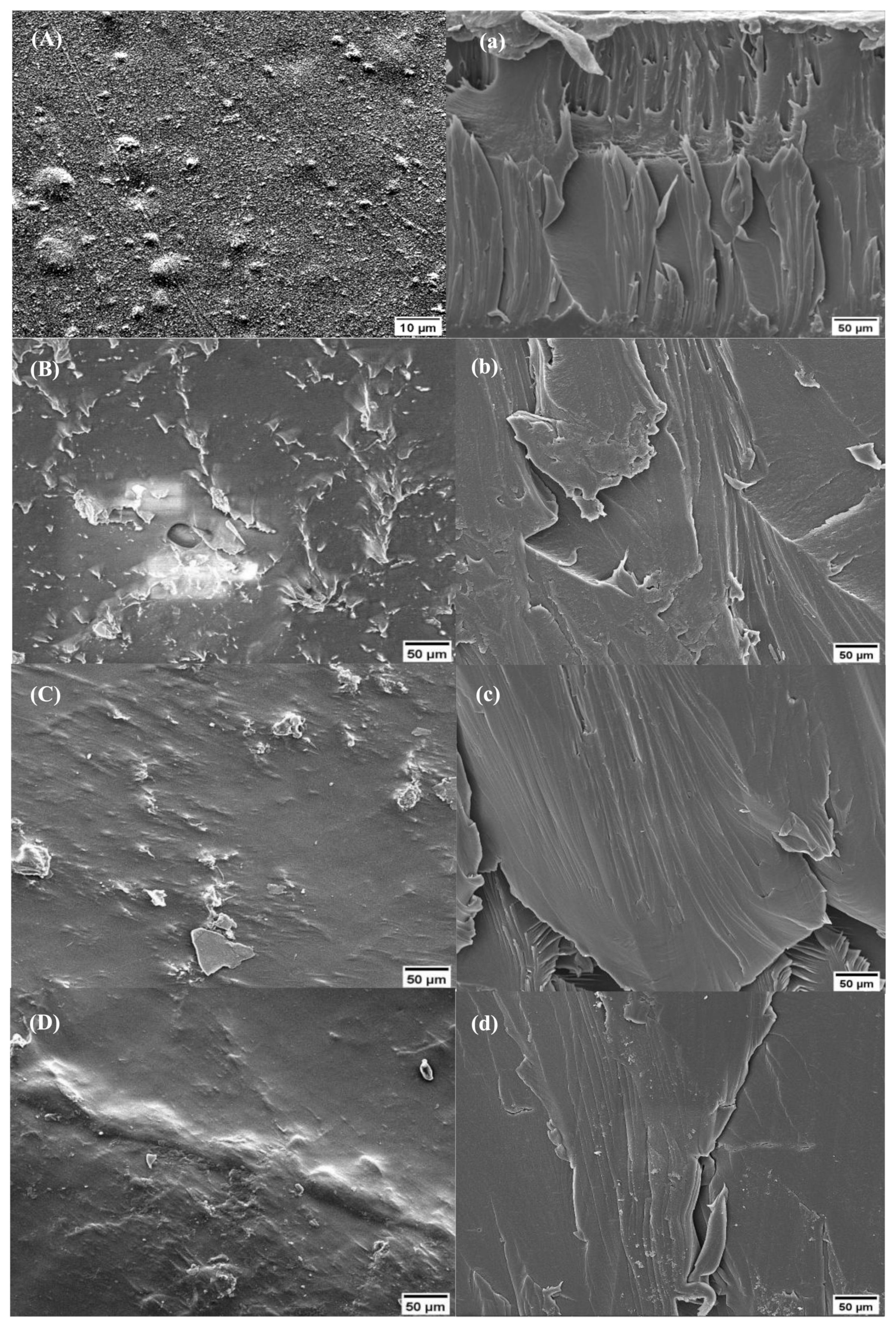
2.1.3. Scanning Electron Microscopy (SEM) Analysis
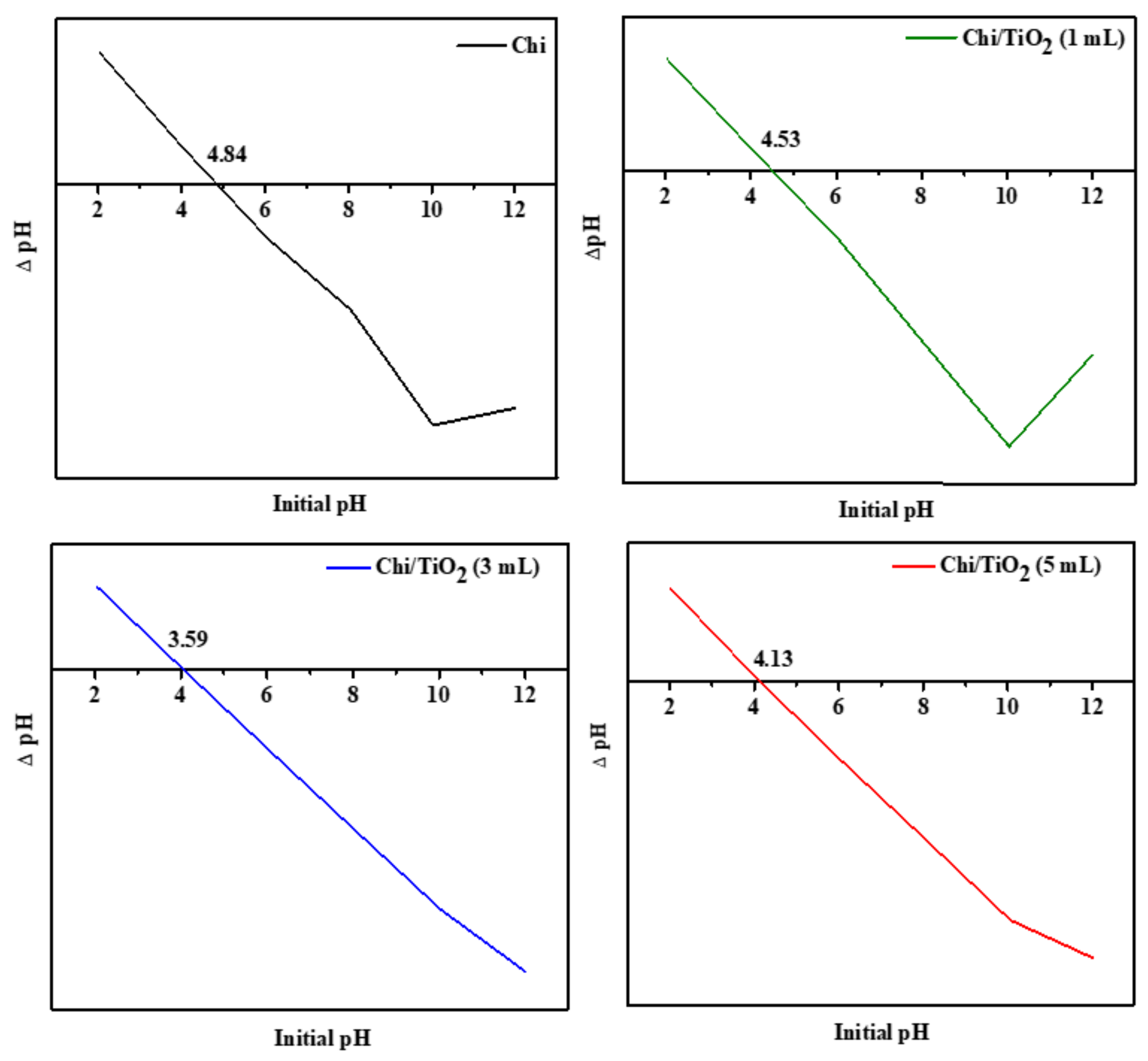
2.1.4. Point of Zero Charge (pHpzc)
2.1.5. Wettability and Swelling Index Analysis
2.2. Photocatalytic Mitigation Studies
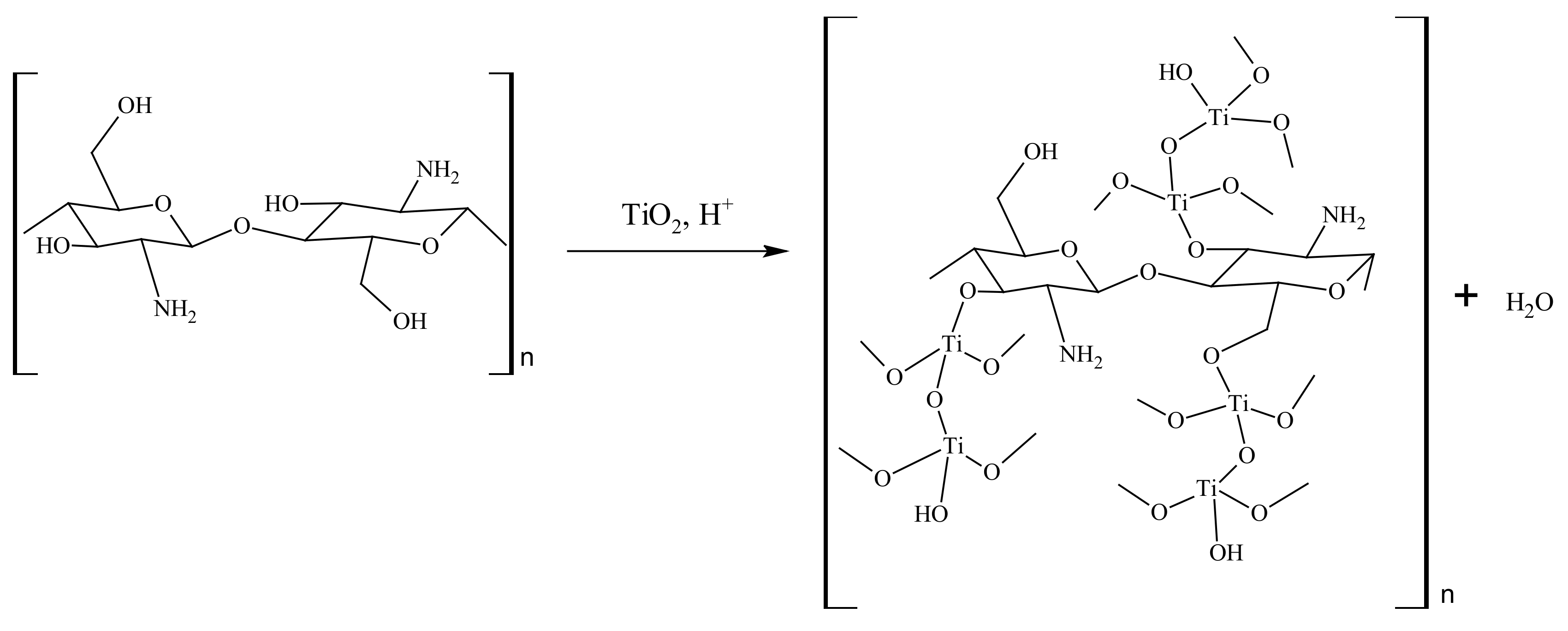
2.3. Proposed Reaction Mechanism
2.4. SEM and Digital Microscopy Analyses of the Films after Mitigation
2.5. Physical Appearance of Used Film Studies and Weight Change after Mitigation
3. Materials and Methods
3.1. Materials
3.2. Preparation of Hybrid Chitosan-Modified TiO2 Film
3.3. Characterization
3.3.1. Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) Spectroscopy
3.3.2. Scanning Electron Microscopy with Energy Dispersive X-ray (SEM-EDX)
3.3.3. Thermogravimetric Analysis (TGA)
3.3.4. X-ray Diffraction (XRD)
3.3.5. Swelling Index (SI)
3.3.6. Wettability Test (WS)
3.3.7. Point of Zero Charge (pzc)
3.3.8. Mitigation of Alexandrium Minutum
3.3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, D.M. Approaches to Monitoring, Control and Management of Harmful Algal Blooms (HABs). Ocean. Coast. Manag. 2009, 52, 342–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havens, K.E.; Paerl, H.W. Climate Change at a Crossroad for Control of Harmful Algal Blooms. Environ. Sci. Technol. 2015, 49, 12605–12606. [Google Scholar] [CrossRef] [PubMed]
- Shriwastav, A.; Thomas, J.; Bose, P. A Comprehensive Mechanistic Model for Simulating Algal Growth Dynamics in Photobioreactors. Bioresour. Technol. 2017, 233, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Madany, P.; Xia, C.; Bhattacharjee, L.; Khan, N.; Li, R.; Liu, J. Antibacterial Activity of Fe2O3 /TiO2 Nanoparticles on Toxic Cyanobacteria from a Lake in Southern Illinois. Water Environ. Res. 2021, 93, 2807–2818. [Google Scholar] [CrossRef]
- Berdalet, E.; Fleming, L.E.; Gowen, R.; Davidson, K.; Hess, P.; Backer, L.C.; Moore, S.K.; Hoagland, P.; Enevoldsen, H. Marine Harmful Algal Blooms, Human Health and Wellbeing: Challenges and Opportunities in the 21st Century. J. Mar. Biol. Ass. 2016, 96, 61–91. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, A.; Ahmad, N.; Mohammad Noor, N.; Wilson, L.D.; Ibrahim, N.H. Mitigation of Toxic Alexandrium Tamiyavanichii Using Chitosan-Silica Composite. Malays. J. Anal. Sci. 2019, 23, 31–39. [Google Scholar] [CrossRef]
- Hasija, V.; Raizada, P.; Sudhaik, A.; Sharma, K.; Kumar, A.; Singh, P.; Jonnalagadda, S.B.; Thakur, V.K. Recent Advances in Noble Metal Free Doped Graphitic Carbon Nitride Based Nanohybrids for Photocatalysis of Organic Contaminants in Water: A Review. Appl. Mater. Today 2019, 15, 494–524. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative Damage and Antioxidative System in Algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef]
- Baniamerian, H.; Tsapekos, P.; Alvarado-Morales, M.; Shokrollahzadeh, S.; Safavi, M.; Angelidaki, I. Anti-Algal Activity of Fe2O3–TiO2 Photocatalyst on Chlorella Vulgaris Species under Visible Light Irradiation. Chemosphere 2020, 242, 125119. [Google Scholar] [CrossRef]
- Song, J.; Li, C.; Wang, X.; Zhi, S.; Wang, X.; Sun, J. Visible-Light-Driven Heterostructured g-C3N4/Bi-TiO2 Floating Photocatalyst with Enhanced Charge Carrier Separation for Photocatalytic Inactivation of Microcystis aeruginosa. Front. Environ. Sci. Eng. 2021, 15, 129. [Google Scholar] [CrossRef]
- Fan, G.; Lin, X.; You, Y.; Du, B.; Li, X.; Luo, J. Magnetically Separable ZnFe2O4/Ag3PO4/g-C3N4 Photocatalyst for Inactivation of Microcystis aeruginosa: Characterization, Performance and Mechanism. J. Hazard. Mater. 2022, 421, 126703. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Akman, F.; Malyar, Y.N.; Issaoui, N.; Vasilieva, N.Y.; Karacharov, A.A. Synthesis Optimization, DFT and Physicochemical Study of Chitosan Sulfates. J. Mol. Struct. 2021, 1245, 131083. [Google Scholar] [CrossRef]
- Razzaz, A.; Ghorban, S.; Hosayni, L.; Irani, M.; Aliabadi, M. Chitosan Nanofibers Functionalized by TiO2 Nanoparticles for the Removal of Heavy Metal Ions. J. Taiwan Inst. Chem. Eng. 2016, 58, 333–343. [Google Scholar] [CrossRef]
- Saravanan, R.; Aviles, J.; Gracia, F.; Mosquera, E.; Gupta, V.K. Crystallinity and Lowering Band Gap Induced Visible Light Photocatalytic Activity of TiO2/CS (Chitosan) Nanocomposites. Int. J. Biol. Macromol. 2018, 109, 1239–1245. [Google Scholar] [CrossRef]
- Zhao, Y.; Tao, C.; Xiao, G.; Su, H. Controlled Synthesis and Wastewater Treatment of Ag2O/TiO2 Modified Chitosan-Based Photocatalytic Film. RSC Adv. 2017, 7, 11211–11221. [Google Scholar] [CrossRef] [Green Version]
- Abdullah Al Balushi, K.S.; Devi, G.; Saif Al Hudaifi, A.; Khamis Al Garibi, A.S.R. Development of Chitosan-TiO2 Thin Film and Its Application for Methylene Blue Dye Degradation. Int. J. Environ. Anal. Chem. 2021, 1–14. [Google Scholar] [CrossRef]
- Usup, G.; Pin, L.C.; Ahmad, A.; Teen, L.P. Alexandrium (Dinophyceae) Species in Malaysian Waters. Harmful Algae 2002, 1, 265–275. [Google Scholar] [CrossRef]
- Mahatmanti, F.W.; Nuryono, N.; Narsito, N. Physical Characteristics of Chitosan Based Film Modified with Silica and Polyethylene Glycol. Indones. J. Chem. 2014, 14, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Budnyak, T.M.; Pylypchuk, I.V.; Tertykh, V.A.; Yanovska, E.S.; Kolodynska, D. Synthesis and Adsorption Properties of Chitosan-Silica Nanocomposite Prepared by Sol-Gel Method. Nanoscale Res. Lett. 2015, 10, 87. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Wu, H.; Yu, A.; Wen, D. Biodegradable Polylactide/Chitosan Blend Membranes. Biomacromolecules 2006, 7, 1362–1372. [Google Scholar] [CrossRef]
- Duan, B.; Dong, C.; Yuan, X.; Yao, K. Electrospinning of Chitosan Solutions in Acetic Acid with Poly(Ethylene Oxide). J. Biomater. Sci. Polym. Ed 2004, 15, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Luo, Y.; Teng, Z.; Zhang, B.; Zhou, B.; Wang, Q. Development of Silver/Titanium Dioxide/Chitosan Adipate Nanocomposite as an Antibacterial Coating for Fruit Storage. LWT Food Sci. Technol. 2015, 63, 1206–1213. [Google Scholar] [CrossRef]
- Zhu, X.; Chang, Y.; Chen, Y. Toxicity and Bioaccumulation of TiO2 Nanoparticle Aggregates in Daphnia magna. Chemosphere 2010, 78, 209–215. [Google Scholar] [CrossRef]
- Farzana, M.H.; Meenakshi, S. Photo-Decolorization and Detoxification of Toxic Dyes Using Titanium Dioxide Impregnated Chitosan Beads. Int. J. Biol. Macromol. 2014, 70, 420–426. [Google Scholar] [CrossRef]
- Díaz-Visurraga, J.; Gutiérrez, C.; von Plessing, C.; García, A. Metal Nanostructures as Antibacterial Agents. In Science And Technology Against Microbial Pathogens: Research, Development and Evaluation; Méndez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2011. [Google Scholar]
- Wiącek, A.E.; Gozdecka, A.; Jurak, M. Physicochemical Characteristics of Chitosan–TiO2 Biomaterial. 1. Stability and Swelling Properties. Ind. Eng. Chem. Res. 2018, 57, 1859–1870. [Google Scholar] [CrossRef]
- Al-Taweel, S.S.; Saud, R.H. New Route for Synthesis of Pure Anatase TiO2 Nanoparticles via Utrasound-assisted Sol-Gel Method. J. Chem. Pharm. Res. 2016, 8, 620–626. [Google Scholar]
- Khan, A.; Khan, R.A.; Salmieri, S.; Le Tien, C.; Riedl, B.; Bouchard, J.; Chauve, G.; Tan, V.; Kamal, M.R.; Lacroix, M. Mechanical and Barrier Properties of Nanocrystalline Cellulose Reinforced Chitosan Based Nanocomposite Films. Carbohydr. Polym. 2012, 90, 1601–1608. [Google Scholar] [CrossRef]
- Theivasanthi, T.; Alagar, M. Titanium Dioxide (TiO2) Nanoparticles XRD Analyses: An Insight. arXiv 2013, arXiv:1307.1091. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Guo, X.; Li, W.; Chen, J.; Liu, Q.; Xu, Q.; Wang, Q.; Yang, H.; Shui, Y.; et al. Effects of Different TiO2 Nanoparticles Concentrations on the Physical and Antibacterial Activities of Chitosan-Based Coating Film. Nanomaterials 2020, 10, 1365. [Google Scholar] [CrossRef]
- López Calero, J.; Oquendo Berríos, Z.; Suarez, O.M. Biodegradable Chitosan Matrix Composite Reinforced with Titanium Dioxide for Biocidal Applications. In Renewable and Sustainable Composites; Pereira, A.B., Fernandes, F.A.O., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Shah, I.; Adnan, R.; Wan Ngah, W.S.; Mohamed, N. Iron Impregnated Activated Carbon as an Efficient Adsorbent for the Removal of Methylene Blue: Regeneration and Kinetics Studies. PLoS ONE 2015, 10, e0122603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullah, O.G.; Aziz, S.B.; Omer, K.M.; Salih, Y.M. Reducing the Optical Band Gap of Polyvinyl Alcohol (PVA) Based Nanocomposite. J. Mater. Sci. Mater. Electron. 2015, 26, 5303–5309. [Google Scholar] [CrossRef]
- Taspika, M.; Desiati, R.D.; Mahardika, M.; Sugiarti, E.; Abral, H. Influence of TiO2/Ag Particles on the Properties of Chitosan Film. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 015017. [Google Scholar] [CrossRef]
- Almeida, E.V.R.; Frollini, E.; Castellan, A.; Coma, V. Chitosan, Sisal Cellulose, and Biocomposite Chitosan/Sisal Cellulose Films Prepared from Thiourea/NaOH Aqueous Solution. Carbohydr. Polym. 2010, 80, 655–664. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of Chitosan-TiO2 Composite Film with Efficient Antimicrobial Activities under Visible Light for Food Packaging Applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef]
- Luo, Y.; Pan, X.; Ling, Y.; Wang, X.; Sun, R. Facile Fabrication of Chitosan Active Film with Xylan via Direct Immersion. Cellulose 2014, 21, 1873–1883. [Google Scholar] [CrossRef]
- Liu, H.; Adhikari, R.; Guo, Q.; Adhikari, B. Preparation and Characterization of Glycerol Plasticized (High-Amylose) Starch–Chitosan Films. J. Food Eng. 2013, 116, 588–597. [Google Scholar] [CrossRef]
- Clasen, C.; Wilhelms, T.; Kulicke, W.-M. Formation and Characterization of Chitosan Membranes. Biomacromolecules 2006, 7, 3210–3222. [Google Scholar] [CrossRef]
- Huang, L.; Dai, T.; Xuan, Y.; Tegos, G.P.; Hamblin, M.R. Synergistic Combination of Chitosan Acetate with Nanoparticle Silver as a Topical Antimicrobial: Efficacy against Bacterial Burn Infections. Antimicrob. Agents Chemother. 2011, 55, 3432–3438. [Google Scholar] [CrossRef] [Green Version]
- Palla-Rubio, B.; Araújo-Gomes, N.; Fernández-Gutiérrez, M.; Rojo, L.; Suay, J.; Gurruchaga, M.; Goñi, I. Synthesis and Characterization of Silica-Chitosan Hybrid Materials as Antibacterial Coatings for Titanium Implants. Carbohydr. Polym. 2019, 203, 331–341. [Google Scholar] [CrossRef]
- Jassby, D.; Farner Budarz, J.; Wiesner, M. Impact of Aggregate Size and Structure on the Photocatalytic Properties of TiO 2 and ZnO Nanoparticles. Environ. Sci. Technol. 2012, 46, 6934–6941. [Google Scholar] [CrossRef]
- Pellegrino, F.; Pellutiè, L.; Sordello, F.; Minero, C.; Ortel, E.; Hodoroaba, V.-D.; Maurino, V. Influence of Agglomeration and Aggregation on the Photocatalytic Activity of TiO2 Nanoparticles. Appl. Catal. B 2017, 216, 80–87. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Fan, G.; Chen, Z.; Wang, B.; Wu, S.; Luo, J.; Zheng, X.; Zhan, J.; You, Y.; Zhang, Z. Photocatalytic Removal of Harmful Algae in Natural Waters by Ag/AgCl@ZIF-8 Coating under Sunlight. Catalysts 2019, 9, 698. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Ao, Y.; Wang, P. Effective Inactivation of Microcystis aeruginosa by a Novel Z-Scheme Composite Photocatalyst under Visible Light Irradiation. Sci. Total Environ. 2020, 746, 141149. [Google Scholar] [CrossRef]
- Fan, G.; Chen, Z.; Yan, Z.; Du, B.; Pang, H.; Tang, D.; Luo, J.; Lin, J. Efficient Integration of Plasmonic Ag/AgCl with Perovskite-Type LaFeO3: Enhanced Visible-Light Photocatalytic Activity for Removal of Harmful Algae. J. Hazard. Mater. 2021, 409, 125018. [Google Scholar] [CrossRef]
- Hu, L.; Wang, R.; Wang, M.; Wang, C.; Xu, Y.; Wang, Y.; Gao, P.; Liu, C.; Song, Y.; Ding, N.; et al. The Inactivation Effects and Mechanisms of Karenia Mikimotoi by Non-Metallic Elements Modified TiO2 (SNP-TiO2) under Visible Light. Sci. Total Environ. 2022, 820, 153346. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Chen, H.; Chen, M.; Zhang, P.; Ding, N. Preparation of Floating BiOCl0.6I0.4/ZnO Photocatalyst and Its Inactivation of Microcystis aeruginosa under Visible Light. J. Environ. Sci. 2023, 125, 362–375. [Google Scholar] [CrossRef]
- Qun, G.; Ajun, W. Effects of Molecular Weight, Degree of Acetylation and Ionic Strength on Surface Tension of Chitosan in Dilute Solution. Carbohydr. Polym. 2006, 64, 29–36. [Google Scholar] [CrossRef]
- Bhalkaran, S.; Wilson, L. Investigation of Self-Assembly Processes for Chitosan-Based Coagulant-Flocculant Systems: A Mini-Review. IJMS 2016, 17, 1662. [Google Scholar] [CrossRef] [Green Version]
- Ahmad Barudin, N.H.; Sreekantan, S.; Ong, M.T.; Lai, C.W. Synthesis, Characterization and Comparative Study of Nano-Ag–TiO2 against Gram-Positive and Gram-Negative Bacteria under Fluorescent Light. Food Control 2014, 46, 480–487. [Google Scholar] [CrossRef]
- Sabzevari, M.; Cree, D.E.; Wilson, L.D. Mechanical Properties of Graphene Oxide-Based Composite Layered-Materials. Mater. Chem. Phys. 2019, 234, 81–89. [Google Scholar] [CrossRef]
- Kokinos, J.P.; Anderson, D.M. Morphological Development of Resting Cysts in Cultures of the Marine Dinoflagellate Lingulodinium polyedrum (= L. machaerophorum). Palynology 1995, 19, 143–166. [Google Scholar] [CrossRef]
- Kim, Z.-H.; Thanh, N.N.; Yang, J.-H.; Park, H.; Yoon, M.-Y.; Park, J.-K.; Lee, C.-G. Improving Microalgae Removal Efficiency Using Chemically-Processed Clays. Biotechnol. Bioproc. E 2016, 21, 787–793. [Google Scholar] [CrossRef]



| Film Sample 1 | Swelling Index (%) | Contact Angle (θ; °) |
|---|---|---|
| Chi | 63.7 ± 1.05 | 98.2 ± 0.84 |
| Chi/TiO2 (1 mL) | 143.8 ± 2.67 | 93.0 ± 0.54 |
| Chi/TiO2 (3 mL) | 150.7 ± 1.15 | 75.6 ± 0.03 |
| Chi/TiO2 (5 mL) | 144.2 ± 1.09 | 92.8 ± 0.05 |
| Mitigation Agent | HAB Species | Mitigation Approach | Removal Efficiency (%) | Lamp Intensity | Ref. |
|---|---|---|---|---|---|
| Fe2O3–TiO2 NPs | Chlorella vulgaris | Chemical | High removal rate of algal cells (99.8%) within 24 h was achieved under visible light irradiation. | 55 W/m2 | [9] |
| Ag/AgCl@ZIF-8 floating | Chlorophyll a M. aeruginosa Other algae | Physical | After 6 h of exposure to sunlight, the chlorophyll a degraded by 99.9%, Microcystis aeruginosa (92.6%) and biomass of the other algae decreased by about 80%. | Sunlight | [46] |
| Z-scheme g-C3N4-MoO3 (Mo-CN) composite photocatalysts | M. aeruginosa | Chemical | 15Mo-CN achieved a removal efficiency of 97% for the algal cells after 3 h of visible light irradiation. | 48.1 W/m2 | [47] |
| γFe2O3/TiO2 nanoparticle | M. aeruginosa A. circinalis | Physical | Within 1 h, M. aerugonisa (99.99%) and A.cricinalis (95.49) was removed. | 32 W/m2 | [4] |
| Ag/AgCl@LaFeO3 (ALFO) photocatalyst | Phytoplankton | Chemical | ALFO-20% had a higher photocatalytic activity with a near 100% removal rate of chlorophyll a within 150 min. | 10,000 W/m2 | [48] |
| g-C3N4/Bi-TiO2 floating photocatalyst | M. aeruginosa | Physical | Within 6 h of visible light illumination, 75.9% of M. aeruginosa was removed. | NA | [10] |
| Z-scheme Ag3PO4@PANI core–shell photocatalyst | Microcystis aeruginosa | Chemical | 99.2% was Microcystis aeruginosa was removed within 3 h. | NA | [11] |
| SNP-TiO2 | Karenia mikimotoi | Chemical | Under visible light irradiation, 81.8% was removed within 96 h. | NA | [49] |
| ZnFe2O4/Ag3PO4/g-C3N4 (ZFO/AP/CN) photocatalyst | M. aeruginosa Microcystin-LR (MC-LR) | Physical | The photocatalytic removal of M. aeruginosa and MC-LR was 94.3% and 76.9%, respectively, under visible light. | NA | [11] |
| Floating BiOCl0.6I0.4/ZnO photocatalyst | Microcystis aeruginosa | Physical | The removal rate of chlorophyll a was 89.3% after 6 h of photocatalytic reaction under visible light. | 42 W/m2 | [50] |
| Chi/TiO2 (1 mL) | Alexandrium minutum | Physical | The removal of Alexandrium minutum was 76.1 ± 13.8% within 72 h. | 70 μmol photons m2s−1 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, N.H.; Iqbal, A.; Mohammad-Noor, N.; Razali, R.M.; Sreekantan, S.; Yanto, D.H.Y.; Mahadi, A.H.; Wilson, L.D. Photocatalytic Remediation of Harmful Alexandrium minutum Bloom Using Hybrid Chitosan-Modified TiO2 Films in Seawater: A Lab-Based Study. Catalysts 2022, 12, 707. https://doi.org/10.3390/catal12070707
Ibrahim NH, Iqbal A, Mohammad-Noor N, Razali RM, Sreekantan S, Yanto DHY, Mahadi AH, Wilson LD. Photocatalytic Remediation of Harmful Alexandrium minutum Bloom Using Hybrid Chitosan-Modified TiO2 Films in Seawater: A Lab-Based Study. Catalysts. 2022; 12(7):707. https://doi.org/10.3390/catal12070707
Chicago/Turabian StyleIbrahim, Nur Hanisah, Anwar Iqbal, Normawaty Mohammad-Noor, Roziawati Mohd Razali, Srimala Sreekantan, Dede Heri Yuli Yanto, Abdul Hanif Mahadi, and Lee D. Wilson. 2022. "Photocatalytic Remediation of Harmful Alexandrium minutum Bloom Using Hybrid Chitosan-Modified TiO2 Films in Seawater: A Lab-Based Study" Catalysts 12, no. 7: 707. https://doi.org/10.3390/catal12070707
APA StyleIbrahim, N. H., Iqbal, A., Mohammad-Noor, N., Razali, R. M., Sreekantan, S., Yanto, D. H. Y., Mahadi, A. H., & Wilson, L. D. (2022). Photocatalytic Remediation of Harmful Alexandrium minutum Bloom Using Hybrid Chitosan-Modified TiO2 Films in Seawater: A Lab-Based Study. Catalysts, 12(7), 707. https://doi.org/10.3390/catal12070707








