Ag-MnxOy on Graphene Oxide Derivatives as Oxygen Reduction Reaction Catalyst in Alkaline Direct Ethanol Fuel Cells
Abstract
:1. Introduction
2. Results and Discussion
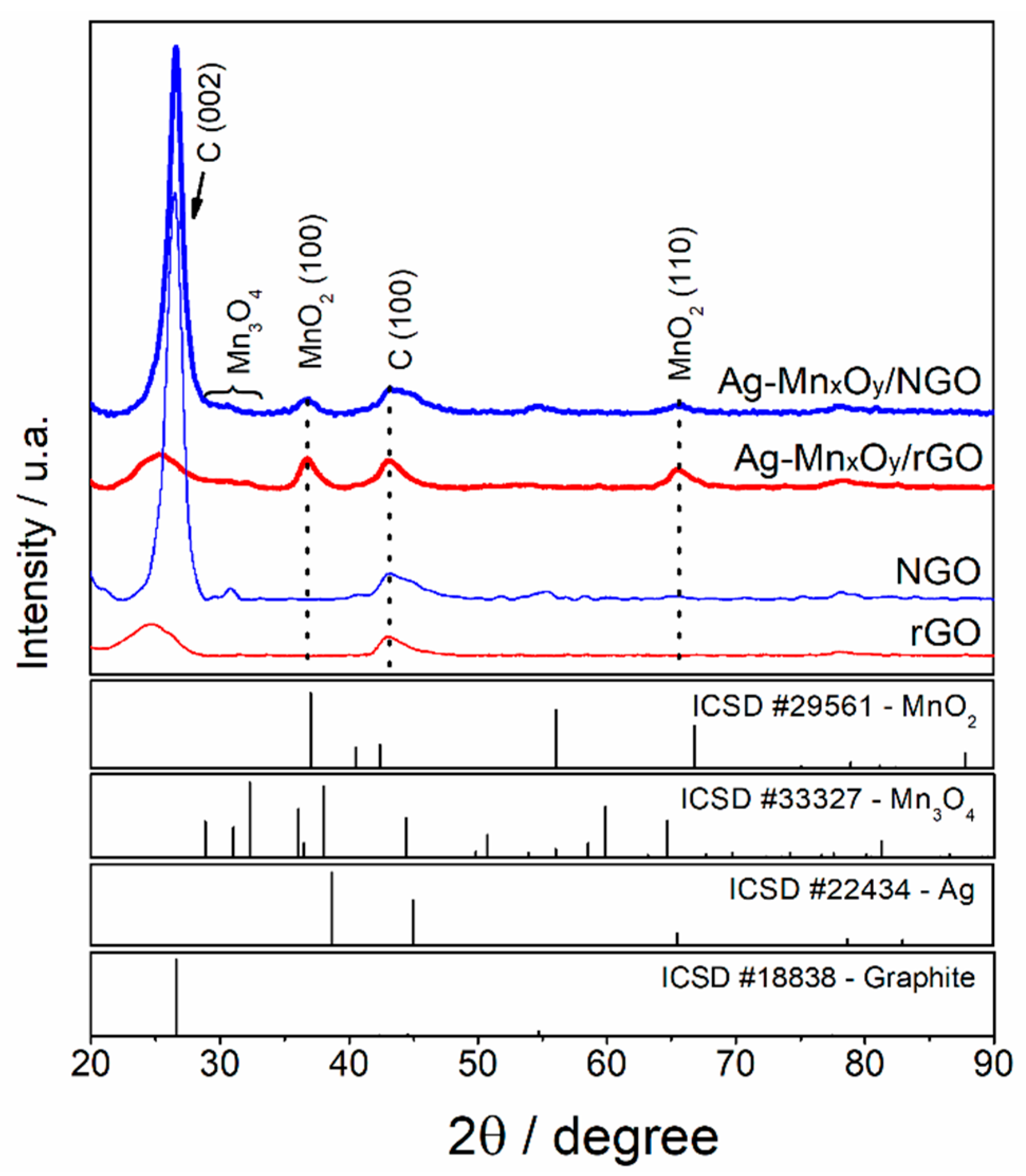
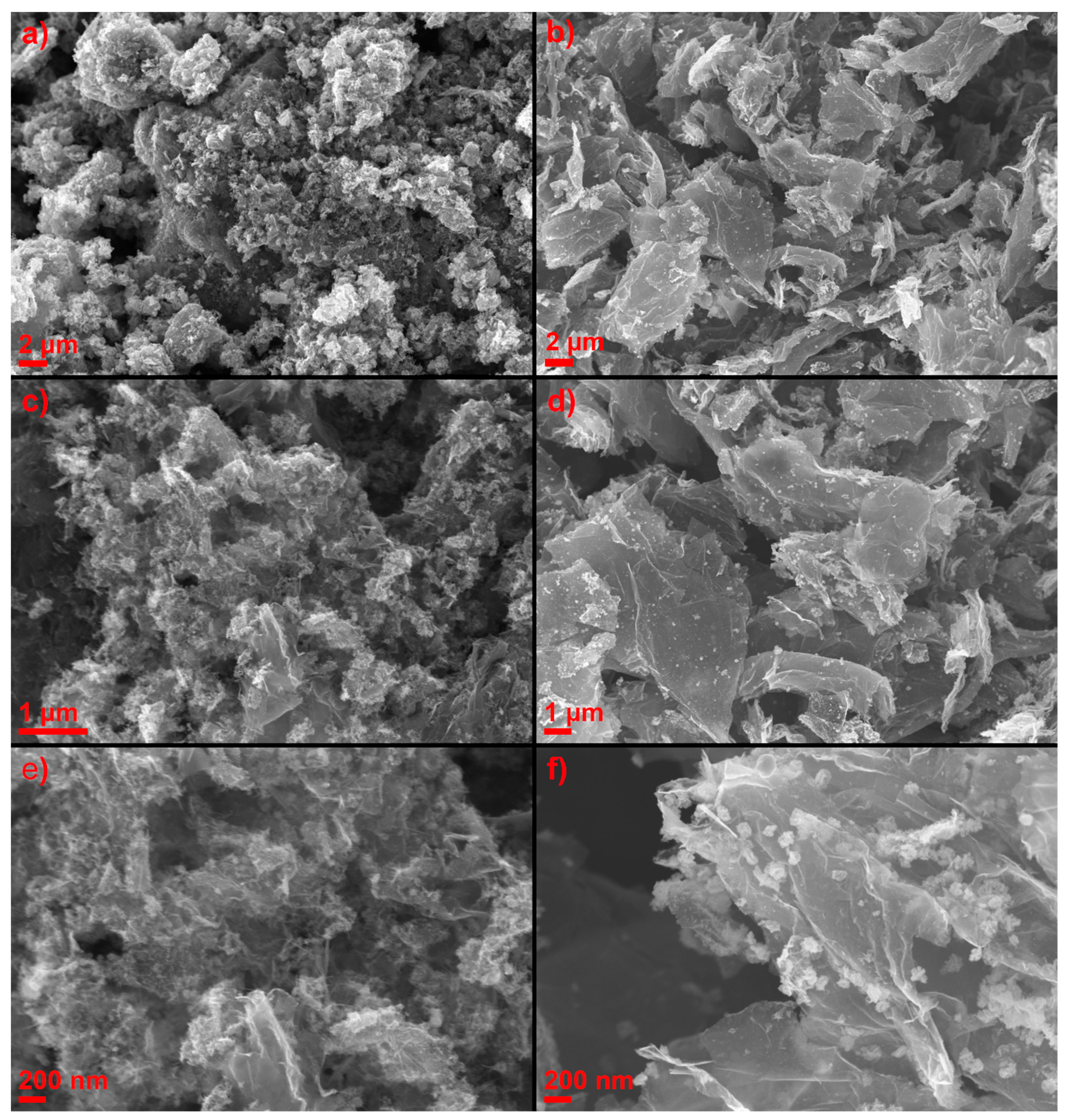
2.1. Physicochemical Characterization of ORR Catalysts
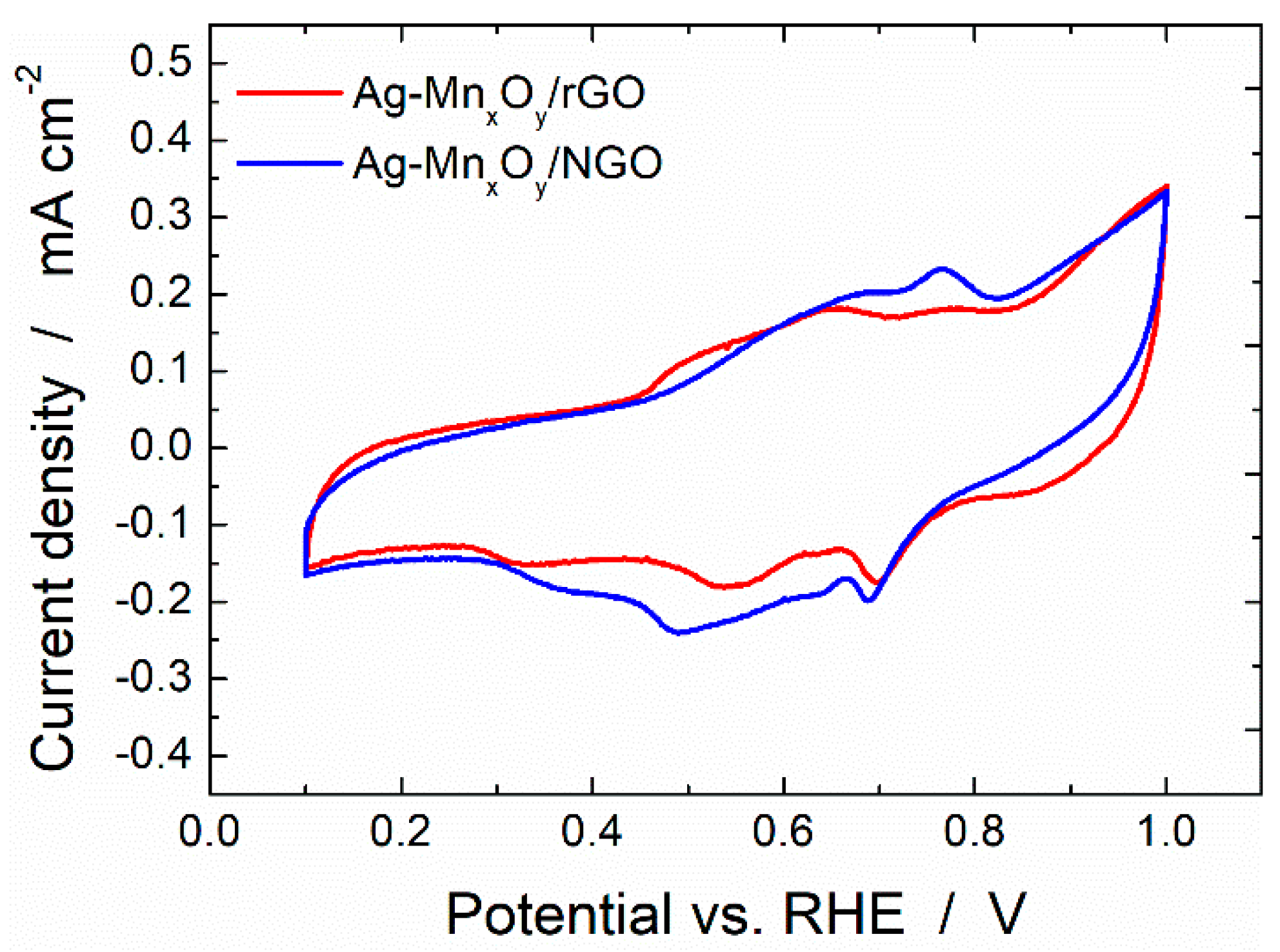
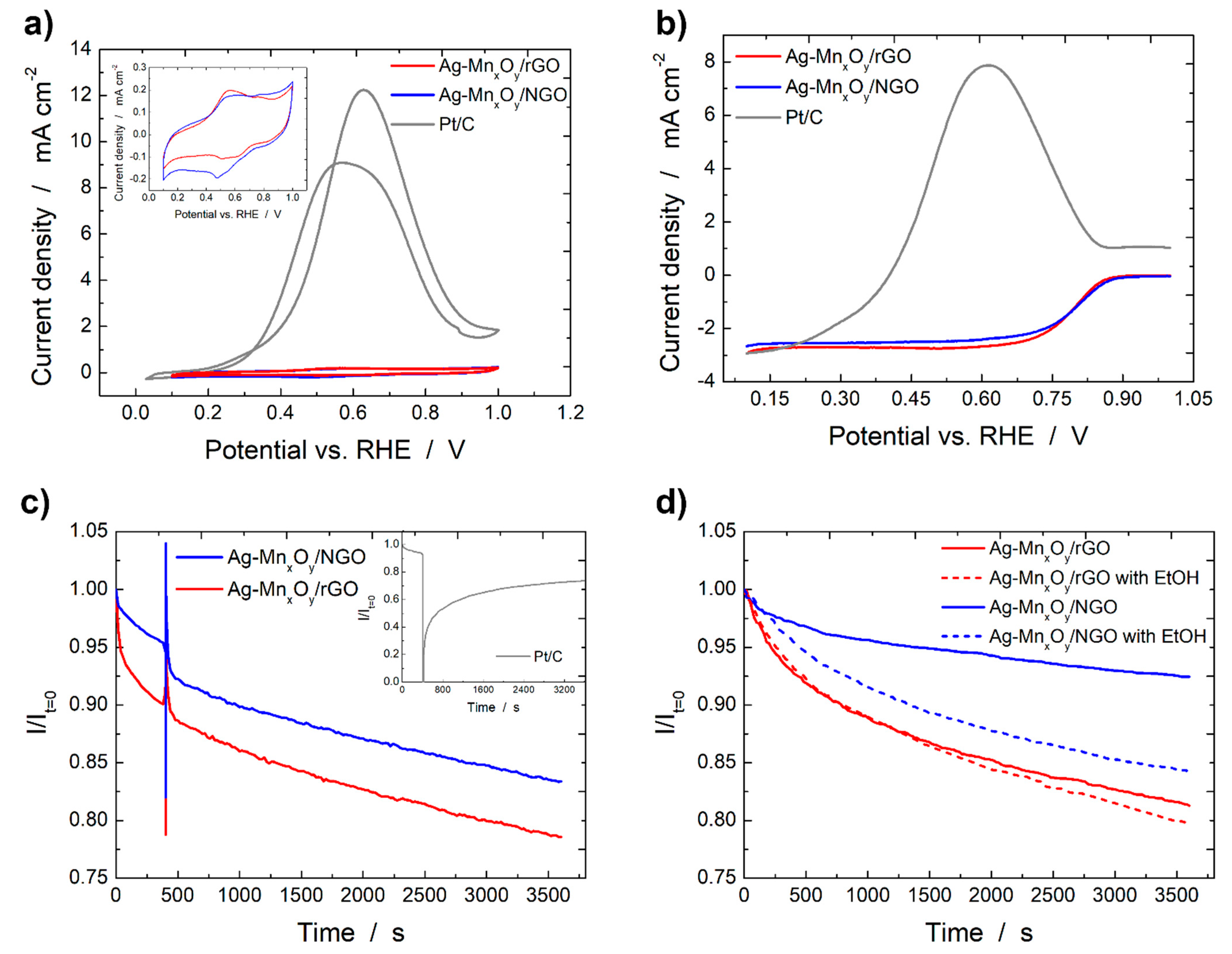
2.2. Base Cyclic Voltammograms of the Ag-MnxOy/C Catalysts
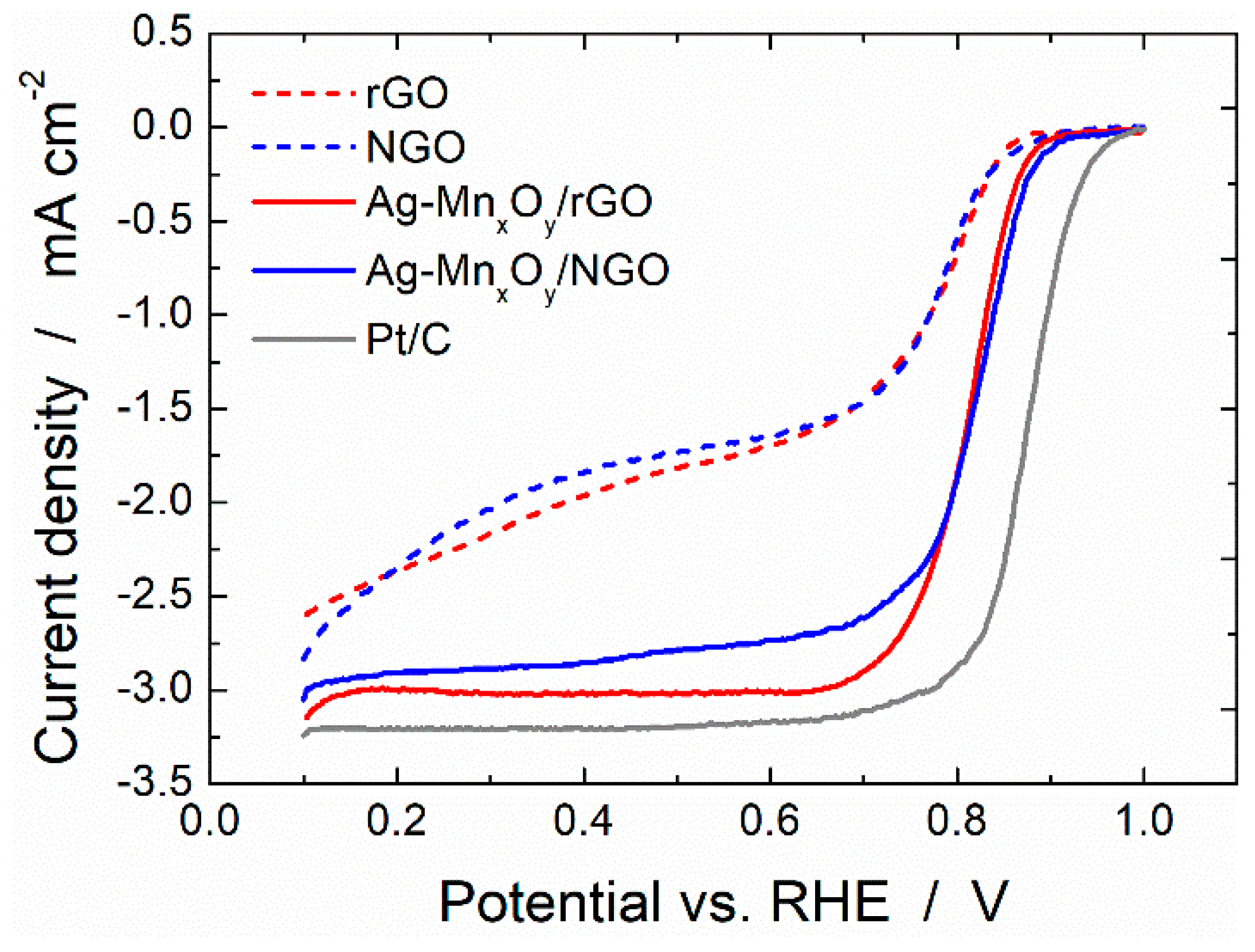
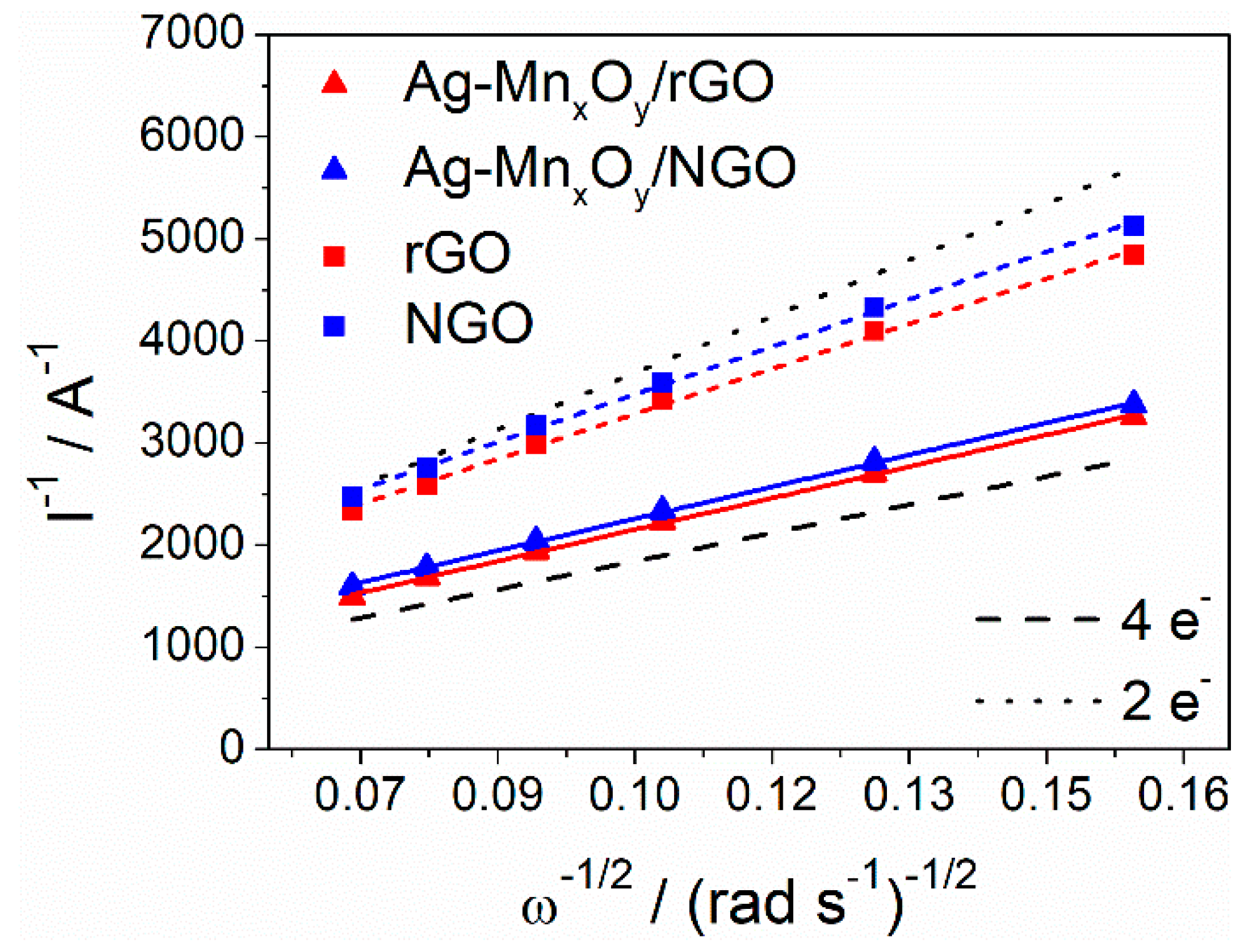
2.3. ORR Activity of the Graphene Derivative Supports and Ag-MnxOy/C Catalysts
2.4. Ethanol Tolerance and Catalyst Stability Tests
3. Materials and Methods
3.1. Materials
3.2. Preparation of Reduced Graphene Oxide (rGO) and N-Doped Graphene Oxide (NGO)
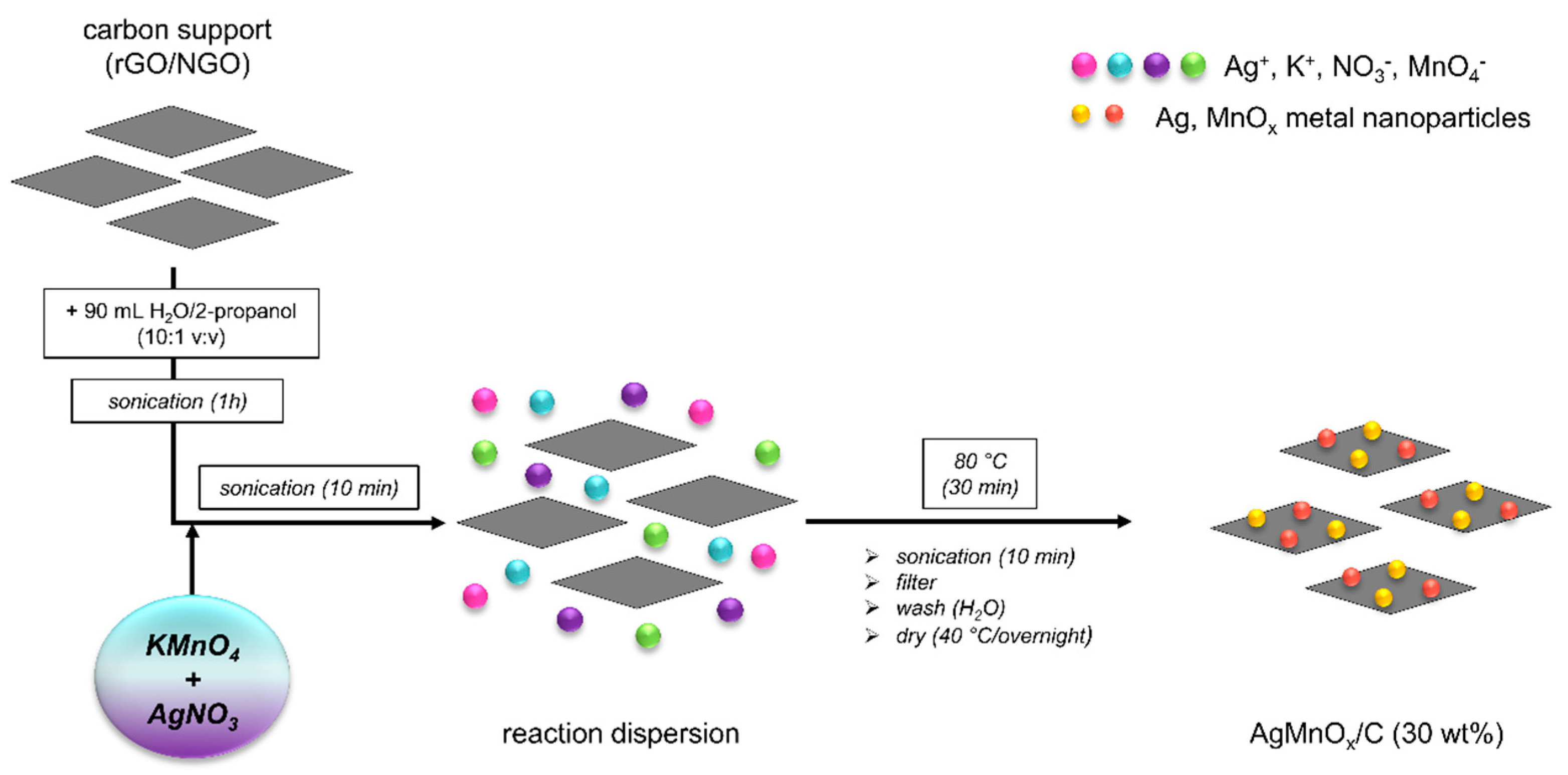
3.3. Preparation of rGO and NGO Supported Ag-MnxOy Catalysts
3.4. Physicochemical Characterization
3.5. Electrochemical Characterization
3.6. Ethanol Tolerance and Catalyst Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.-Y.; Lin, J.-S.; Pan, W.-H.; Shih, C.-M.; Liu, Y.-L.; Lue, S.J. Alkaline direct ethanol fuel cell performance using alkali-impregnated polyvinyl alcohol/functionalized carbon nano-tube solid electrolytes. J. Power Sources 2016, 303, 267–277. [Google Scholar] [CrossRef]
- Kamarudin, M.Z.F.; Kamarudin, S.K.; Masdar, M.S.; Daud, W.R.W. Review: Direct ethanol fuel cells. Int. J. Hydrogen Energy 2013, 38, 9438–9453. [Google Scholar] [CrossRef]
- An, L.; Zhao, T.S. Transport phenomena in alkaline direct ethanol fuel cells for sustainable energy production. J. Power Sources 2017, 341, 199–211. [Google Scholar] [CrossRef]
- Lee, K.; Ahmed, M.S.; Jeon, S. Electrochemical deposition of silver on manganese dioxide coated reduced graphene oxide for enhanced oxygen reduction reaction. J. Power Sources 2015, 288, 261–269. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Jeon, S. Highly Active Graphene-Supported NixPd100−x Binary Alloyed Catalysts for Electro-Oxidation of Ethanol in an Alkaline Media. ACS Catal. 2014, 4, 1830–1837. [Google Scholar] [CrossRef]
- Qaseem, A.; Chen, F.; Wu, X.; Johnston, R.L. Pt-free silver nanoalloy electrocatalysts for oxygen reduction reaction in alkaline media. Catal. Sci. Technol. 2016, 6, 3317–3340. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhang, H.; Zhong, H.; Zhang, S.; Chen, S. N-doped graphene/carbon composite as non-precious metal electrocatalyst for oxygen reduction reaction. Electrochim. Acta 2012, 81, 313–320. [Google Scholar] [CrossRef]
- Ramirez-Barria, C.S.; Fernandes, D.M.; Freire, C.; Villaro-Abalos, E.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Upgrading the properties of reduced graphene oxide and nitrogen-doped reduced graphene oxide produced by thermal reduction toward efficient ORR electrocatalysts. Nanomaterials 2019, 9, 1761. [Google Scholar] [CrossRef] [Green Version]
- Grimmer, I.; Zorn, P.; Weinberger, S.; Grimmer, C.; Pichler, B.; Cermenek, B.; Gebetsroither, F.; Schenk, A.; Mautner, F.-A.; Bitschnau, B.; et al. Ethanol tolerant precious metal free cathode catalyst for alkaline direct ethanol fuel cells. Electrochim. Acta 2017, 228, 325–331. [Google Scholar] [CrossRef]
- Valim, R.B.; Santos, M.C.; Lanza, M.R.V.; Machado, S.A.S.; Lima, F.H.B.; Calegaro, M.L. Oxygen reduction reaction catalyzed by ε-MnO2: Influence of the crystalline structure on the reaction mechanism. Electrochim. Acta 2012, 85, 423–431. [Google Scholar] [CrossRef]
- Amer, M.S.; Arunachalam, P.; Ghanem, M.A.; Al-Mayouf, A.M.; Shar, M.A. Enriched active surface structure in nanosized tungsten-cobalt oxides electrocatalysts for efficient oxygen redox reactions. Appl. Surf. Sci. 2020, 513, 145831. [Google Scholar] [CrossRef]
- Amer, M.S.; Ghanem, M.A.; Arunachalam, P.; Al-Mayouf, A.M.; Hadadi, S.M. Bifunctional electrocatalyst of low-symmetry mesoporous titanium dioxide modified with cobalt oxide for oxygen evolution and reduction reactions. Catalysts 2019, 9, 836. [Google Scholar] [CrossRef] [Green Version]
- Purwaningsih, H.; Suari, N.M.I.P.; Widiyastuti, W.; Setyawan, H. Preparation of rGO/MnO2 Composites through Simultaneous Graphene Oxide Reduction by Electrophoretic Deposition. ACS Omega 2022, 7, 6760–6767. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Jiang, L.; Qi, J.; Jiang, Q.; Wang, S.; Sun, G. One step synthesis of carbon-supported Ag/MnyOx composites for oxygen reduction reaction in alkaline media. Appl. Catal. B Environ. 2011, 104, 337–345. [Google Scholar] [CrossRef]
- Barsuk, D.; Zadick, A.; Chatenet, M.; Georgarakis, K.; Panagiotopoulos, N.T.; Champion, Y.; Jorge, A.M., Jr. Nanoporous silver for electrocatalysis application in alkaline fuel cells. Mater. Des. 2016, 111, 528–536. [Google Scholar] [CrossRef] [Green Version]
- Shypunov, I.; Kongi, N.; Kozlova, J.; Matisen, L.; Ritslaid, P.; Sammelselg, V.; Tammeveski, K. Enhanced Oxygen Reduction Reaction Activity with Electrodeposited Ag on Manganese Oxide–Graphene Supported Electrocatalyst. Electrocatalysis 2015, 6, 465–471. [Google Scholar] [CrossRef]
- Truong, V.M.; Yang, M.-K.; Yang, H. Functionalized Carbon Black Supported Silver (Ag/C) Catalysts in Cathode Electrode for Alkaline Anion Exchange Membrane Fuel Cells. Int. J. Precis. Eng. Manuf. Green Technol. 2019, 6, 711–721. [Google Scholar] [CrossRef]
- Selvakumar, K.; Kumar, S.M.S.; Thangamuthu, R.; Ganesan, K.; Murugan, P.; Rajput, P.; Jha, S.N.; Bhattacharyya, D. Physiochemical investigation of shape-designed MnO2 nanostructures and their influence on oxygen reduction reaction activity in alkaline solution. J. Phys. Chem. C 2015, 119, 6604–6618. [Google Scholar] [CrossRef]
- Sun, W.; Hsu, A.; Chen, R. Carbon-supported tetragonal MnOOH catalysts for oxygen reduction reaction in alkaline media. J. Power Sources 2011, 196, 627–635. [Google Scholar] [CrossRef]
- Calegaro, M.L.; Lima, F.H.B.; Ticianelli, E.A. Oxygen reduction reaction on nanosized manganese oxide particles dispersed on carbon in alkaline solutions. J. Power Sources 2006, 158, 735–739. [Google Scholar] [CrossRef]
- Sun, S.; Miao, H.; Xue, Y.; Wang, Q.; Li, S.; Liu, Z. Oxygen reduction reaction catalysts of manganese oxide decorated by silver nanoparticles for aluminum-air batteries. Electrochim. Acta 2016, 214, 49–55. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Song, W.; Wang, F.; Song, Y. The role of electronic interaction in the use of Ag and Mn3O4 hybrid nanocrystals covalently coupled with carbon as advanced oxygen reduction electrocatalysts. J. Mater. Chem. A 2014, 2, 17477–17488. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, L.; Qi, L.; Yuan, L.; Wang, E.; Sun, G. Electrocatalytic activity and stability of Ag-MnOx/C composites toward oxygen reduction reaction in alkaline solution. Electrochim. Acta 2014, 123, 167–175. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, M.; Pan, J.; Wang, P.; Li, W.; Wan, P. Manganese dioxide-supported silver bismuthate as an efficient electrocatalyst for oxygen reduction reaction in zinc-oxygen batteries. Electrochim. Acta 2016, 197, 68–76. [Google Scholar] [CrossRef]
- Marukawa, R.; Kiso, T.; Shimizu, T.; Katayama, Y.; Nakayama, M. Layered Manganese Dioxide Thin Films Intercalated with Ag+ Ions Reduceable In Situ for Oxygen Reduction Reaction. ACS Omega 2022, 7, 15854–15861. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qin, X. Preparation of a Ag—MnO2/graphene composite for the oxygen reduction reaction in alkaline solution. RSC Adv. 2015, 5, 15627–15633. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Naderi, H.R.; Sobhani-Nasab, A.; Rahimi-Nasrabadi, M.; Ganjali, M.R. Decoration of nitrogen-doped reduced graphene oxide with cobalt tungstate nanoparticles for use in high-performance supercapacitors. Appl. Surf. Sci. 2017, 423, 1025–1034. [Google Scholar] [CrossRef]
- Nosan, M.; Löffler, M.; Jerman, I.; Kolar, M.; Katsounaros, I.; Genorio, B. Understanding the Oxygen Reduction Reaction Activity of Quasi-1D and 2D N-Doped Heat-Treated Graphene Oxide Catalysts with Inherent Metal Impurities. ACS Appl. Energy Mater. 2021, 4, 3593–3603. [Google Scholar] [CrossRef]
- Tasdemir, A.; Kopuklu, B.B.; Kirlioglu, A.C.; Alkan Gursel, S.; Yurum, A. The influence of nitrogen doping on reduced graphene oxide as highly cyclable Li-ion battery anode with enhanced performance. Int. J. Hydrogen Energy 2021, 46, 11865–11877. [Google Scholar] [CrossRef]
- Lu, Z.-J.; Bao, S.-J.; Gou, Y.-T.; Cai, C.-J.; Ji, C.-C.; Xu, M.-W.; Song, J.; Wang, R. Nitrogen-doped reduced-graphene oxide as an efficient metal-free electrocatalyst for oxygen reduction in fuel cells. RSC Adv. 2013, 3, 3990–3995. [Google Scholar] [CrossRef]
- Dong, Y.; Deng, Y.; Zeng, J.; Song, H.; Liao, S. A high-performance composite ORR catalyst based on the synergy between binary transition metal nitride and nitrogen-doped reduced graphene oxide. J. Mater. Chem. A 2017, 5, 5829–5837. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Li, W.; Li, Y.; Chen, Q.; Zhan, F. Nitrogen-doped graphene aerogel-supported spinel CoMn2O4 nanoparticles as an efficient catalyst for oxygen reduction reaction. J. Power Sources 2015, 299, 492–500. [Google Scholar] [CrossRef]
- Linge, J.M.; Erikson, H.; Sarapuu, A.; Merisalu, M.; Rähn, M.; Matisen, L.; Sammelselg, V.; Tammeveski, K. Electroreduction of oxygen on nitrogen-doped graphene oxide supported silver nanoparticles. J. Electroanal. Chem. 2017, 794, 197–203. [Google Scholar] [CrossRef]
- Cao, Y.L.; Yang, H.X.; Ai, X.P.; Xiao, L.F. The mechanism of oxygen reduction on MnO2-catalyzed air cathode in alkaline solution. J. Electroanal. Chem. 2003, 557, 127–134. [Google Scholar] [CrossRef]
- Lima, F.H.B.; Calegaro, M.L.; Ticianelli, E.A. Electrocatalytic activity of manganese oxides prepared by thermal decomposition for oxygen reduction. Electrochim. Acta 2007, 52, 3732–3738. [Google Scholar] [CrossRef]
- Yasin, G.; Arif, M.; Shakeel, M.; Dun, Y.; Zuo, Y.; Khan, W.Q.; Tang, Y.; Khan, A.; Nadeem, M. Exploring the Nickel–Graphene Nanocomposite Coatings for Superior Corrosion Resistance: Manipulating the Effect of Deposition Current Density on its Morphology, Mechanical Properties, and Erosion-Corrosion Performance. Adv. Eng. Mater. 2018, 20, 1701166. [Google Scholar] [CrossRef]
- Gorgieva, S.; Osmić, A.; Hribernik, S.; Božič, M.; Svete, J.; Hacker, V.; Wolf, S.; Genorio, B. Efficient chitosan/nitrogen-doped reduced graphene oxide composite membranes for direct alkaline ethanol fuel cells. Int. J. Mol. Sci. 2021, 22, 1740. [Google Scholar] [CrossRef]
- Tian, K.; Su, Z.; Wang, H.; Tian, X.; Huang, W.; Xiao, C. N-doped reduced graphene oxide/waterborne polyurethane composites prepared by in situ chemical reduction of graphene oxide. Compos. Part A Appl. Sci. Manuf. 2017, 94, 41–49. [Google Scholar] [CrossRef]
- Li, P.-C.; Hu, C.-C.; Noda, H.; Habazaki, H. Synthesis and characterization of carbon black/manganese oxide air cathodes for zinc-air batteries: Effects of the crystalline structure of manganese oxides. J. Power Sources 2015, 298, 102–113. [Google Scholar] [CrossRef]
- Grimmer, C.; Zacharias, R.; Grandi, M.; Pichler, B.; Kaltenboeck, I.; Gebetsroither, F.; Wagner, J.; Cermenek, B.; Weinberger, S.; Schenk, A.; et al. A Membrane-Free and Practical Mixed Electrolyte Direct Borohydride Fuel Cell. J. Electrochem. Soc. 2016, 163, F278–F283. [Google Scholar] [CrossRef] [Green Version]
- Chaiburi, C.; Cermenek, B.; Pichler, B.E.; Grimmer, C.; Hacker, V. Ethanol: Tolerant Oxygen Reduction Reaction Catalysts in Alkaline Media. J. Electrochem. Energy Convers. Storage 2019, 16, 021004. [Google Scholar] [CrossRef]
- Huang, H.; Meng, Y.; Labonte, A.; Dobley, A.; Suib, S.L. Large-scale synthesis of silver manganese oxide nanofibers and their oxygen reduction properties. J. Phys. Chem. C 2013, 117, 25352–25359. [Google Scholar] [CrossRef]
- Du, C.; Tan, Q.; Yin, G.; Zhang, J. Rotating Disk Electrode Method. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Elsevier: Amsterdam, The Netherlands, 2014; pp. 171–198. [Google Scholar]
- Zakaria, Z.; Kamarudin, S.K.; Timmiati, S.N. Membranes for direct ethanol fuel cells: An overview. Appl. Energy 2016, 163, 334–342. [Google Scholar] [CrossRef]
- Erikson, H.; Sarapuu, A.; Tammeveski, K. Oxygen Reduction Reaction on Silver Catalysts in Alkaline Media: A Minireview. ChemElectroChem 2019, 6, 73–86. [Google Scholar] [CrossRef]
- Jalili, S.; Goliaei, E.M.; Schofield, J. Silver cluster supported on nitrogen-doped graphene as an electrocatalyst with high activity and stability for oxygen reduction reaction. Int. J. Hydrogen Energy 2017, 42, 14522–14533. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Rafati, A.A.; Bagheri, A.; Najafi, M. Experimental data and correlation of surface tensions of the binary and ternary systems of water + acetonitrile + 2-propanol at 298.15 K and atmospheric pressure. J. Chem. Eng. Data 2010, 55, 4039–4043. [Google Scholar] [CrossRef]
- Garsany, Y.; Singer, I.L.; Swider-Lyons, K.E. Impact of film drying procedures on RDE characterization of Pt/VC electrocatalysts. J. Electroanal. Chem. 2011, 662, 396–406. [Google Scholar] [CrossRef]
- Cheng, F.; Su, Y.; Liang, J.; Tao, Z.; Chen, J. MnO2-based nanostructures as catalysts for electrochemical oxygen reduction in alkaline media. Chem. Mater. 2010, 22, 898–905. [Google Scholar] [CrossRef]
- Qiao, J.; Xu, L.; Shi, P.; Zhang, L.; Baker, R.; Zhang, J. Effect of KOH concentration on the oxygen reduction kinetics catalyzed by heat-treated co-pyridine/C electrocatalysts. Int. J. Electrochem. Sci. 2013, 8, 1189–1208. [Google Scholar]
- Vincent, I.; Bessarabov, D. Electrochemical characterization and oxygen reduction kinetics of Cu-incorporated cobalt oxide catalyst. Int. J. Electrochem. Sci. 2016, 11, 8002–8015. [Google Scholar] [CrossRef]
- Masa, J.; Batchelor-McAuley, C.; Schuhmann, W.; Compton, R.G. Koutecky-Levich analysis applied to nanoparticle modified rotating disk electrodes: Electrocatalysis or misinterpretation? Nano Res. 2014, 7, 71–78. [Google Scholar] [CrossRef]


| Catalysts | BET Surface Area/m2 g−1 | External Area/m2 g−1 | Micro Pore/m2 g−1 | Pore Size/nm |
|---|---|---|---|---|
| Ag-MnxOy/rGO | 244.6 | 236.9 | 7.6 | 8.3 |
| Ag-MnxOy/NGO | 285.7 | 277.4 | 8.4 | 9.8 |
| Catalysts | ICP-MS | AAS | EDS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mn | Ag | Mn | Ag | Mn | Ag | C | O | N | |
| Ag-MnxOy/rGO | 13.81 | 9.51 | 14.99 | 7.85 | 20.31 | 11.96 | 30.91 | 36.82 | - |
| Ag-MnxOy/NGO | 11.64 | 9.05 | 12.63 | 7.45 | 12.80 | 9.52 | 59.28 | 17.93 | 0.47 |
| Catalysts | Eonseta,b/V vs. RHE | E1/2b/V vs. RHE | jDb,c/mA cm−2 | nc / | khd/cm s−1 |
|---|---|---|---|---|---|
| rGO | 0.856/0.838 | 0.772/0.748 | −1.96/−1.87 | 2.51 | 0.795 × 10−2 |
| NGO | 0.871/0.871 | 0.775/0.787 | −1.84/−1.69 | 2.38 | 0.598 × 10−2 |
| Ag-MnxOy/rGO | 0.889/0.877 | 0.814/0.791 | −3.01/−2.72 | 3.58 | 2.25 × 10−2 |
| Ag-MnxOy/NGO | 0.904/0.895 | 0.819/0.796 | −2.85/−2.52 | 3.54 | 1.24 × 10−2 |
| Pt/C | 0.957/- | 0.874/- | −3.21/- | 3.74 | 10.1 × 10−2 |
| Material | Electrolyte | Onset Potential/ V vs. Reference | Limiting Current Density/mA cm−2 | Electron Transfer Number/ | Tafel Slope/mV dec−1 | Reference |
|---|---|---|---|---|---|---|
| AgMnOx/C | 0.1 M NaOH | −0.045 (vs. Hg/HgO) | −0.92 * | 3.69 | - | [23] |
| rGO/MnO2/Ag | 0.1 M KOH | 0.9 (vs. RHE) | 3.4 | 3.90 | 120.2 | [4] |
| Ag-MnOx/G | 0.1 M KOH | 0.9 (vs. RHE) | −5.51 | ~4 | 122 and 57 | [16] |
| Ag–MnO2/graphene | 0.1 M KOH | 0.068 (vs. Hg/HgO) | −5.62 | 3.90 | 86 | [26] |
| 50%Ag-MnO2 | 0.1 M KOH | 0.83 (vs RHE) | −5.50 | 4.0 | 89 | [21] |
| Ag–Mn3O4/C | 1 M NaOH | −0.11 (vs. SCE) | approximately 2.6 | - | 120 and 60 | [22] |
| Ag/Mn3O4/C | 0.1 M NaOH | - | −5.40 | 3.9–4.0 | 110 and 55 | [14] |
| Ag-OMS-2 | 0.1 M KOH | −0.093 (vs. SCE) | −0.784 * | 3.94 | 124.3 and 55.3 | [43] |
| AgMnO2/C | 1 M KOH | - | approximately −3 | 2.18 | - | [42] |
| Ag-MnxOy/rGO | 1 M KOH | 0.88 (vs RHE) | −3.01 | 3.58 | 118.7 and 54.3 | This study |
| Ag-MnxOy/NGO | 1 M KOH | 0.90 (vs RHE) | −2.85 | 3.54 | 129.9 and 58.9 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolf, S.; Roschger, M.; Genorio, B.; Kolar, M.; Garstenauer, D.; Bitschnau, B.; Hacker, V. Ag-MnxOy on Graphene Oxide Derivatives as Oxygen Reduction Reaction Catalyst in Alkaline Direct Ethanol Fuel Cells. Catalysts 2022, 12, 780. https://doi.org/10.3390/catal12070780
Wolf S, Roschger M, Genorio B, Kolar M, Garstenauer D, Bitschnau B, Hacker V. Ag-MnxOy on Graphene Oxide Derivatives as Oxygen Reduction Reaction Catalyst in Alkaline Direct Ethanol Fuel Cells. Catalysts. 2022; 12(7):780. https://doi.org/10.3390/catal12070780
Chicago/Turabian StyleWolf, Sigrid, Michaela Roschger, Boštjan Genorio, Mitja Kolar, Daniel Garstenauer, Brigitte Bitschnau, and Viktor Hacker. 2022. "Ag-MnxOy on Graphene Oxide Derivatives as Oxygen Reduction Reaction Catalyst in Alkaline Direct Ethanol Fuel Cells" Catalysts 12, no. 7: 780. https://doi.org/10.3390/catal12070780
APA StyleWolf, S., Roschger, M., Genorio, B., Kolar, M., Garstenauer, D., Bitschnau, B., & Hacker, V. (2022). Ag-MnxOy on Graphene Oxide Derivatives as Oxygen Reduction Reaction Catalyst in Alkaline Direct Ethanol Fuel Cells. Catalysts, 12(7), 780. https://doi.org/10.3390/catal12070780










