London Disperse Interactions Assist Chiral Induction in the Soai Autoamplifying Reaction Provoked by 1- and 2-Aza[6]helicenes
Abstract
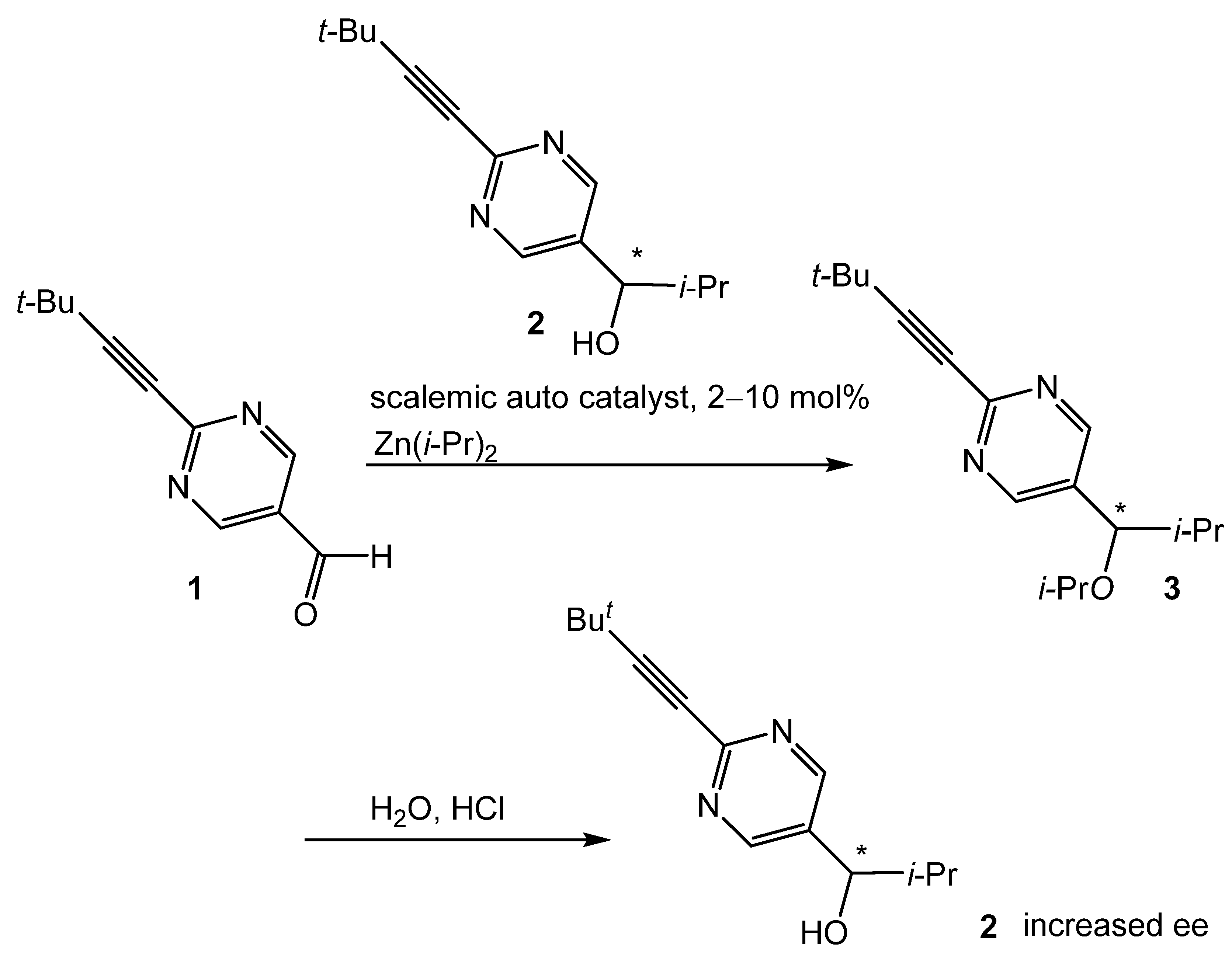
:1. Introduction
2. Results and Discussion
2.1. -N[6]helicene 4 as Inductor
2.2. -N[6]helicene 5 as Inductor
3. Conclusions
4. Computational Details
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Soai, K.; Shibata, T.; Morioka, H.; Shoji, K. Asymmetric autocatalysis and amplification of enantiomeric excess of a chiral molecule. Nature 1995, 378, 767–768. [Google Scholar] [CrossRef]
- Soai, K.; Kawasaki, T.; Matsumoto, A. The origins of homochirality examined by using asymmetric autocatalysis. Chem. Rec. 2014, 14, 70–83. [Google Scholar] [CrossRef]
- Soai, K.; Shibata, T.; Kowata, Y. Asymmetric Synthesis of Enantioenriched Alkanol by Spontaneous Asymmetric Synthesis. JP Patent 1997,9-268179, 18 April 1996. [Google Scholar]
- Soai, K.; Sato, I.; Shibata, T.; Komiya, S.; Hayashi, M.; Matsueda, Y.; Imamura, H.; Hayase, T.; Morioka, H.; Tabira, H.; et al. Asymmetric synthesis of pyrimidylalkanol without adding chiral substances by the addition of diisopropylzinc to pyrimidine-5-carbaldehyde in conjunction with asymmetric autocatalysis. Tetrahedron Asymm. 2003, 14, 185–188. [Google Scholar] [CrossRef]
- Mislow, K. Absolute asymmetric synthesis: A commentary. Collect. Czech. Chem. Commun. 2003, 68, 849–864. [Google Scholar] [CrossRef]
- Gridnev, I.D.; Serafimov, J.M.; Quiney, H.; Brown, J.M. Reflections on spontaneous asymmetric synthesis by amplifying autocatalysis. Org. Biomol. Chem. 2003, 1, 3811–3819. [Google Scholar] [CrossRef]
- Soai, K.; Kawasaki, T.; Matsumoto, A. Role of Asymmetric Autocatalysis in the Elucidation of Origins of Homochirality of Organic Compounds. Symmetry 2019, 11, 694. [Google Scholar] [CrossRef] [Green Version]
- Gridnev, I.D.; Serafimov, J.M.; Brown, J.M. Solution Structure and Reagent Binding of the Zinc Alkoxide Catalyst in the Soai Asymmetric Autocatalytic Reaction. Angew. Chem. Int. Ed. 2004, 43, 4884–4887. [Google Scholar] [CrossRef] [PubMed]
- Gridnev, I.D.; Brown, J.M. Asymmetric autocatalysis: Novel structures, novel mechanism? Proc. Natl. Acad. Sci. USA 2004, 101, 5727–5731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gridnev, I.D. Chiral Symmetry Breaking in Chiral Crystallization and Soai Autocatalytic Reaction. Chem. Lett. 2006, 35, 148–153. [Google Scholar] [CrossRef]
- Gridnev, I.D.; Vorobiev, A.K. Quantification of sophisticated equilibria in the reaction pool and amplifyingcatalytic cycle of the Soai reaction. ACS Catal. 2012, 2, 2137–2149. [Google Scholar] [CrossRef]
- Gridnev, I.D.; Vorobiev, A.K. On the origin and structure of the recently observed acetal in the Soai reaction. Bull. Chem. Soc. Jpn. 2015, 88, 333–340. [Google Scholar] [CrossRef]
- Matsumoto, A.; Yonemitsu, K.; Ozake, H.; Míšek, J.; Starý, I.; Stará, I.G.; Soai, K. Reversal of the sense of enantioselectivity between 1- and 2-aza[6]helicenes used as chiral inducers of asymmetric autocatalysis. Org. Biomol. Chem. 2017, 15, 1321–1324. [Google Scholar] [CrossRef] [Green Version]
- Gridnev, I.D. Birds of a Feather–Asymmetric Organocatalysis Meets Asymmetric Transition Metal Catalysis. Catalysts 2022, 12, 214. [Google Scholar] [CrossRef]
- Scholtes, F.; Trapp, O. Asymmetric induction and amplification in stereodynamic catalytic systems by noncovalent interactions. Synlett 2021, 32, 971–980. [Google Scholar]
- Noble-Terán, M.; Cruz, J.-M.; Micheau, J.-C.; Buhse, T. A Quantification of the Soai Reaction. ChemCatChem 2017, 10, 642–648. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.Y.; Schlegel, B. Combining Synchronous Transit and Quasi-Newton Methods to Find Transition States. Isr. J. Chem. 1994, 34, 449. [Google Scholar] [CrossRef]
- Barrett, C.; Wang, L.W. Double-charge model for classical force-field simulations. Phys. Rev. B 2015, 91, 235407. [Google Scholar] [CrossRef] [Green Version]




| Parameter | ΔE(ZVPE) Activation Energy | ΔH Activation Enthalpy | ΔG Gibbs Free Activation Energy | Imaginary Frequency, cm−1 | |
|---|---|---|---|---|---|
| From 6 | TS1(S) | 20.8 | 19.9 | 23.3 | i261.4 |
| From 7 | TS2(S) | 23.2 | 22.3 | 25.9 | i272.3 |
| TS2(R) | 22.7 | 21.7 | 24.8 | i286.4 | |
| From 8 | TS3(S) | 19.3 | 19.6 | 22.2 | i244.5 |
| TS3(R) | 17.3 | 16.4 | 19.9 | i254.5 | |
| No catalyst | 23.3 | 22.1 | 26.3 | i271.8 | |
| 3 as a catalyst | 17.0 a | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zonov, R.V.; Gridnev, I.D. London Disperse Interactions Assist Chiral Induction in the Soai Autoamplifying Reaction Provoked by 1- and 2-Aza[6]helicenes. Catalysts 2022, 12, 859. https://doi.org/10.3390/catal12080859
Zonov RV, Gridnev ID. London Disperse Interactions Assist Chiral Induction in the Soai Autoamplifying Reaction Provoked by 1- and 2-Aza[6]helicenes. Catalysts. 2022; 12(8):859. https://doi.org/10.3390/catal12080859
Chicago/Turabian StyleZonov, Roman V., and Ilya D. Gridnev. 2022. "London Disperse Interactions Assist Chiral Induction in the Soai Autoamplifying Reaction Provoked by 1- and 2-Aza[6]helicenes" Catalysts 12, no. 8: 859. https://doi.org/10.3390/catal12080859
APA StyleZonov, R. V., & Gridnev, I. D. (2022). London Disperse Interactions Assist Chiral Induction in the Soai Autoamplifying Reaction Provoked by 1- and 2-Aza[6]helicenes. Catalysts, 12(8), 859. https://doi.org/10.3390/catal12080859








