A Heterogeneous Bifunctional Carbon Nanocatalyst from Plastic Waste to Efficiently Catalyze Waste Cooking Oil into Biodiesel
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermal Degradation Analysis
2.2. Surface Area and Pore Size
2.3. Crystallinity Analysis
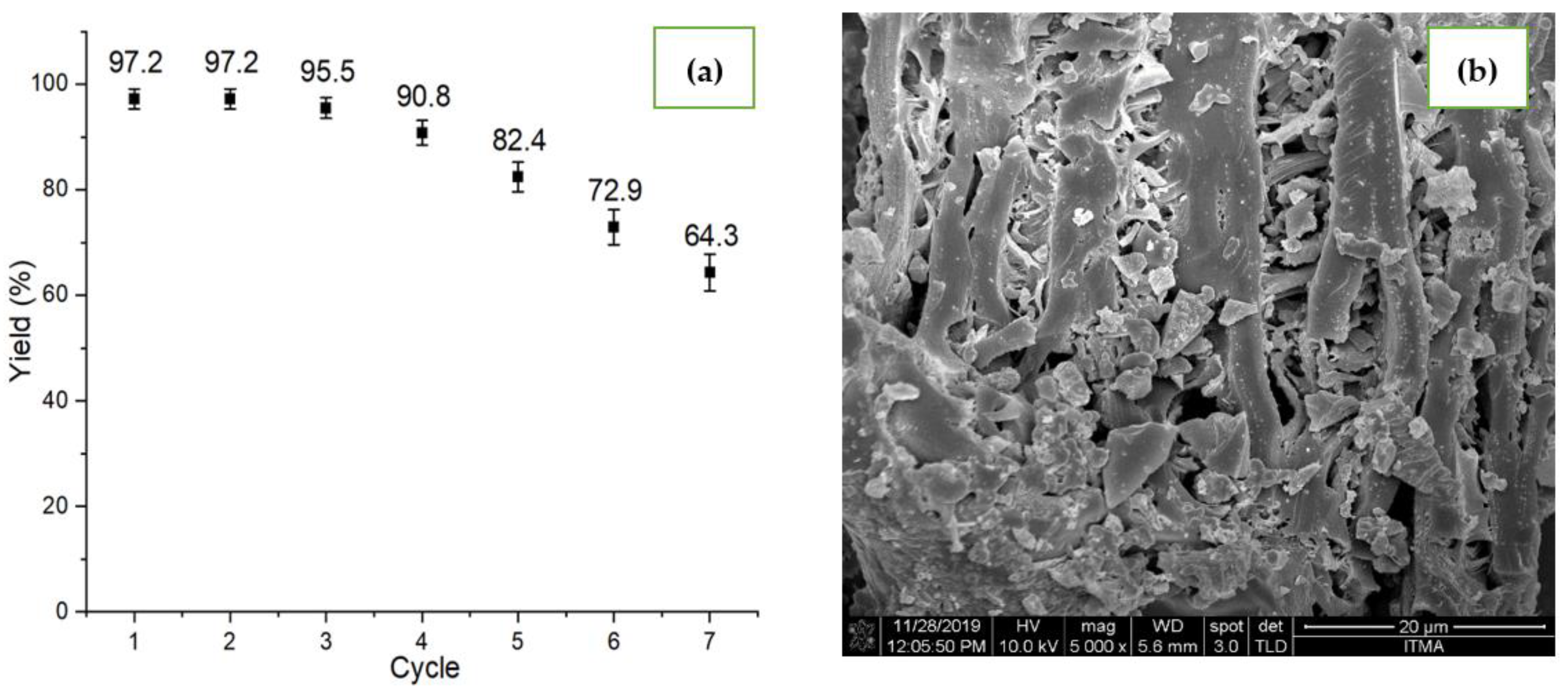
2.4. Structural Morphologies
2.5. Functional Group Analysis
2.6. Basicity and Acidity Strength
2.7. Catalytic Performance
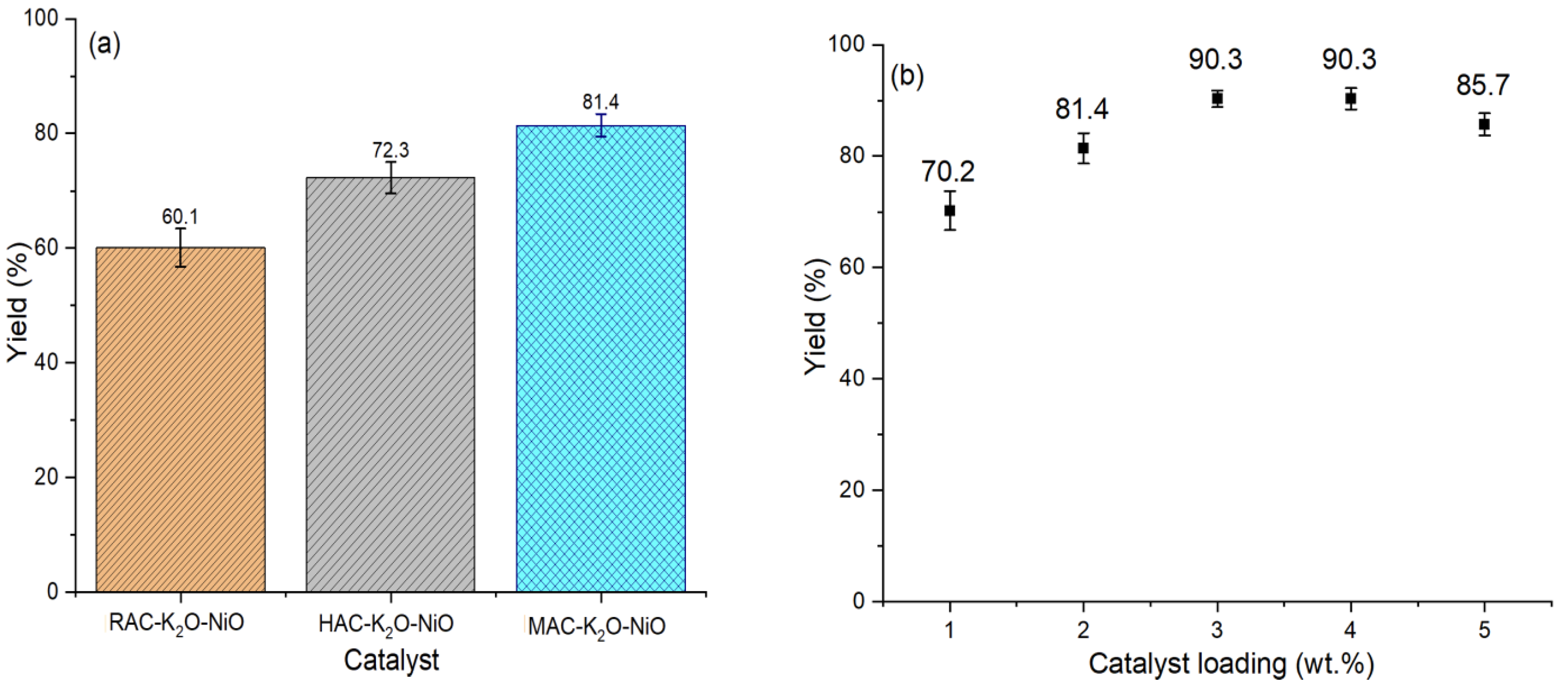
2.7.1. Catalyst Screening Evaluation
2.7.2. Catalyst Loading
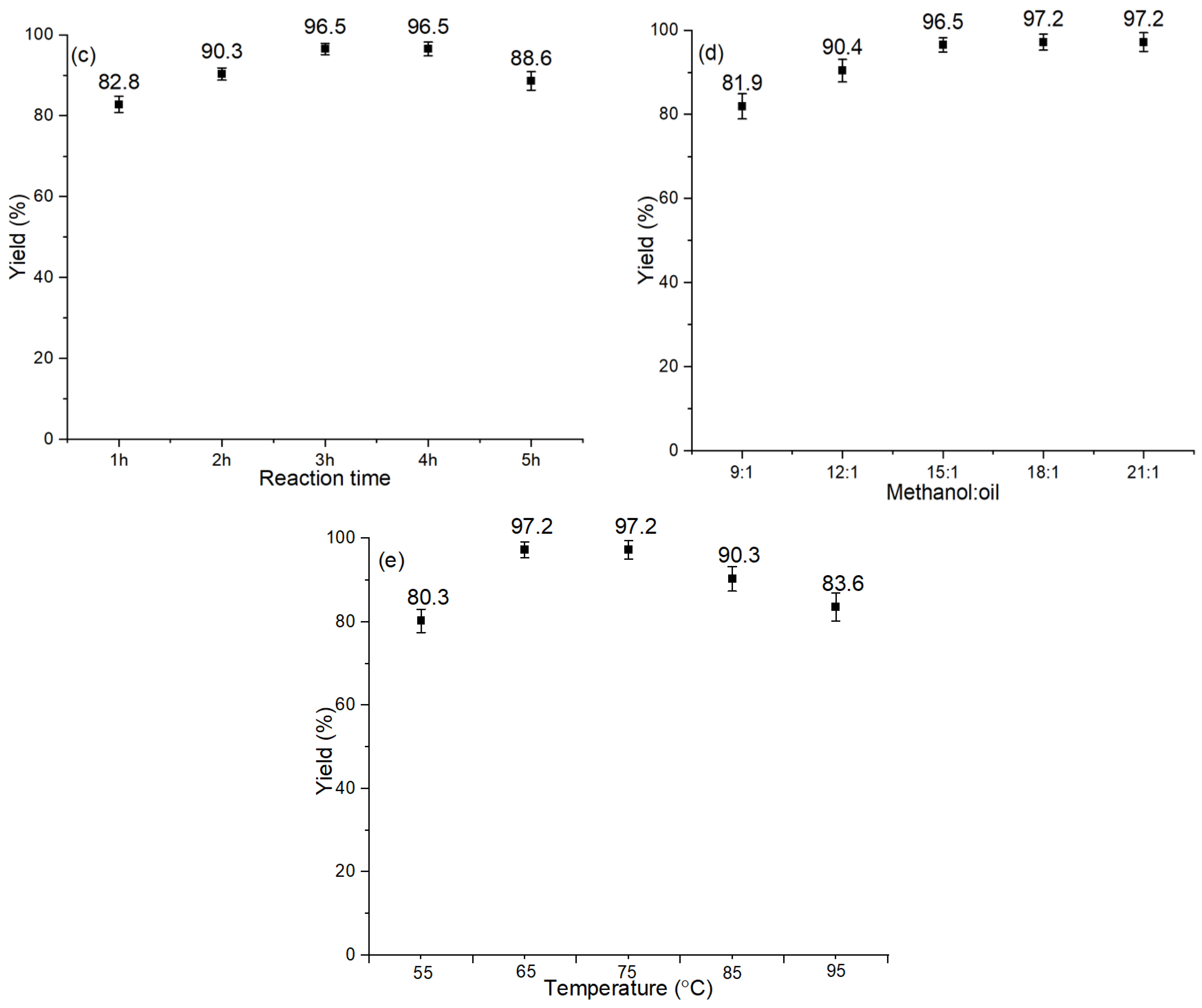
2.7.3. Reaction Time
2.7.4. Methanol: Oil Molar Ratio
2.7.5. Reaction Temperature
2.8. Reusability Study
2.9. Comparison of Produced Plastic Char Support Catalyst with the Commercial Activated Carbon Support-Based Catalysts
2.10. Biodiesel Physiochemical Properties
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of Activated Carbon Supported Bifunctional Catalyst
3.3. Catalyst Characterization
3.4. Transesterification Reaction
3.5. Biodiesel Analysis
3.6. Catalytic Reusability Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maheshwari, P.; Haider, M.B.; Yusuf, M.; Klemeš, J.J.; Bokhari, A.; Beg, M.; Al-Othman, A.; Kumar, R.; Jaiswal, A.K. A review on latest trends in cleaner biodiesel production: Role of feedstock, production methods, and catalysts. J. Clean. Prod. 2022, 355, 131588. [Google Scholar] [CrossRef]
- Soares de Carvalho Freitas, E.; Xavier, L.H.; Oliveira, L.B.; Guarieiro, L.L.N. System dynamics applied to second generation biofuel in Brazil: A circular economy approach. Sustain. Energy Technol. Assess. 2022, 52, 102288. [Google Scholar] [CrossRef]
- Abdullah, S.H.Y.S.; Hanapi, N.H.M.; Azid, A.; Umar, R.; Juahir, H.; Khatoon, H.; Endut, A. A review of biomass-derived heterogeneous catalyst for a sustainable biodiesel production. Renew. Sustain. Energy Rev. 2017, 70, 1040–1051. [Google Scholar] [CrossRef]
- Abdullah, A.; Zuhairi Abdullah, A.; Ahmed, M.; Khan, J.; Shahadat, M.; Umar, K.; Alim, M.A. A review on recent developments and progress in sustainable acrolein production through catalytic dehydration of bio-renewable glycerol. J. Clean. Prod. 2022, 341, 130876. [Google Scholar] [CrossRef]
- Ma, T.; Ding, J.; Liu, X.; Chen, G.; Zheng, J. Gas-phase dehydration of glycerol to acrolein over different metal phosphate catalysts. Korean J. Chem. Eng. 2020, 37, 955–960. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.L.; Tan, X.; Li, H.; Yang, S. Catalytic high-yield biodiesel production from fatty acids and non-food oils over a magnetically separable acid nanosphere. Ind. Crops Prod. 2021, 173, 114126. [Google Scholar] [CrossRef]
- Lokman, I.M.; Rashid, U.; Yunus, R.; Taufiq-Yap, Y.H. Carbohydrate-derived solid acid catalysts for biodiesel production from low-cost feedstocks: A review. Catal. Rev. Sci. Eng. 2014, 56, 187–219. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.A.H.S.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Chizoo, E.; Chimamkpam, A.S.; Gabriel, C.E.; Gerald, U. Adaptive neuro-fuzzy inference system-genetic algorithm versus response surface methodology-desirability function algorithm modelling and optimization of biodiesel synthesis from waste chicken fat. J. Taiwan Inst. Chem. Eng. 2022, 136, 104389. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Li, C.; Chen, X.; Peng, W.; Aghbashlo, M.; Lam, S.S.; Tabatabaei, M. Managing the hazardous waste cooking oil by conversion into bioenergy through the application of waste-derived green catalysts: A review. J. Hazard. Mater. 2022, 424, 127636. [Google Scholar] [CrossRef] [PubMed]
- Mansir, N.; Teo, S.H.; Rashid, U.; Saiman, M.I.; Tan, Y.P.; Alsultan, G.A.; Taufiq-Yap, Y.H. Modified waste egg shell derived bifunctional catalyst for biodiesel production from high FFA waste cooking oil. A review. Renew. Sustain. Energy Rev. 2018, 82, 3645–3655. [Google Scholar] [CrossRef]
- Ngaosuwan, K.; Lotero, E.; Suwannakarn, K.; Goodwin, J.G.; Praserthdam, P. Hydrolysis of triglycerides using solid acid catalysts. Ind. Eng. Chem. Res. 2009, 48, 4757–4767. [Google Scholar] [CrossRef]
- Islam, A.; Taufiq-Yap, Y.H.; Chan, E.S.; Moniruzzaman, M.; Islam, S.; Nabi, M.N. Advances in solid-catalytic and non-catalytic technologies for biodiesel production. Energy Convers. Manag. 2014, 88, 1200–1218. [Google Scholar] [CrossRef]
- Pinto, B.F.; Garcia, M.A.S.; Costa, J.C.S.; de Moura, C.V.R.; de Abreu, W.C.; de Moura, E.M. Effect of calcination temperature on the application of molybdenum trioxide acid catalyst: Screening of substrates for biodiesel production. Fuel 2019, 239, 290–296. [Google Scholar] [CrossRef]
- Li, H.; Niu, S.; Lu, C.; Li, J. Calcium oxide functionalized with strontium as heterogeneous transesterification catalyst for biodiesel production. Fuel 2016, 176, 63–71. [Google Scholar] [CrossRef]
- Pan, D.; Liu, T.; Yu, F.; Chen, S.; Yan, X.; Shi, X.; Fan, B.; Li, R. Synthesis of ordered mesoporous Mg–Al composite oxide-supported potassium catalysts for biodiesel production. Catal. Commun. 2018, 116, 76–80. [Google Scholar] [CrossRef]
- Rahimi, T.; Kahrizi, D.; Feyzi, M.; Ahmadvandi, H.R.; Mostafaei, M. Catalytic performance of MgO/Fe2O3-SiO2 core-shell magnetic nanocatalyst for biodiesel production of Camelina sativa seed oil: Optimization by RSM-CCD method. Ind. Crops Prod. 2021, 159, 113065. [Google Scholar] [CrossRef]
- Silva, M.; Peixoto, A.F.; Soliman, M.M.A.; Costa, P.; Alegria, E.C.B.A.; Freire, C. Biomass and bioenergy highly active organosulfonic aryl-silica nanoparticles as efficient catalysts for biomass derived biodiesel and fuel additives. Biomass Bioenerg. 2021, 145, 105936. [Google Scholar] [CrossRef]
- Ma, T.; Liu, D.; Liu, Z.; Xu, J.; Dong, Y.; Chen, G.; Yun, Z. 12-Tungstophosphoric acid-encapsulated metal-organic framework UiO-66: A promising catalyst for the esterification of acetic acid with n-butanol. J. Taiwan Inst. Chem. Eng. 2022, 133, 104277. [Google Scholar] [CrossRef]
- Munyentwali, A.; Li, H.; Yang, Q. Review of advances in bifunctional solid acid/base catalysts for sustainable biodiesel production. Appl. Catal. A Gen. 2022, 633, 118525. [Google Scholar] [CrossRef]
- Olatundun, E.A.; Borokini, O.O.; Betiku, E. Cocoa pod husk-plantain peel blend as a novel green heterogeneous catalyst for renewable and sustainable honne oil biodiesel synthesis: A case of biowastes-to-wealth. Renew. Energy 2020, 166, 163–175. [Google Scholar] [CrossRef]
- Al-Muhtaseb, A.H.; Jamil, F.; Al-Haj, L.; Zar Myint, M.T.; Mahmoud, E.; Ahmad, M.N.M.; Hasan, A.O.; Rafiq, S. Biodiesel production over a catalyst prepared from biomass-derived waste date pits. Biotechnol. Rep. 2018, 20, e00284. [Google Scholar] [CrossRef]
- Zeng, D.; Zhang, Q.; Chen, S.; Liu, S.; Wang, G. Synthesis porous carbon-based solid acid from rice husk for esterification of fatty acids. Microporous Mesoporous Mater. 2016, 219, 54–58. [Google Scholar] [CrossRef]
- Ahangar, F.A.; Rashid, U.; Ahmad, J.; Tsubota, T.; Alsalme, A. Conversion of waste polyethylene terephthalate (Pet) polymer into activated carbon and its feasibility to produce green fuel. Polymers 2021, 13, 3952. [Google Scholar] [CrossRef]
- Hazmi, B.; Rashid, U.; Kawi, S.; Nur, W.; Wan, A. Palm fatty acid distillate esterification using synthesized heterogeneous sulfonated carbon catalyst from plastic waste_ Characterization, catalytic efficacy and stability, and fuel properties. Process Saf. Environ. Prot. 2022, 162, 1139–1151. [Google Scholar] [CrossRef]
- Yashim, M.M.; Razali, N.; Saadon, N.; Rahman, N.A. Effect of activation temperature on properties of activated carbon prepared from oil palm kernel shell (OPKS). ARPN J. Eng. Appl. Sci. 2016, 11, 6389–6392. [Google Scholar]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Mohamad Yusop, M.F.; Mohd Johan Jaya, E.; Ahmad, M.A. Single-stage microwave assisted coconut shell based activated carbon for removal of Zn(II) ions from aqueous solution—Optimization and batch studies. Arab. J. Chem. 2022, 15, 104011. [Google Scholar] [CrossRef]
- Bora, A.P.; Dhawane, S.H.; Anupam, K.; Halder, G. Biodiesel synthesis from Mesua ferrea oil using waste shell derived carbon catalyst. Renew. Energy 2018, 121, 195–204. [Google Scholar] [CrossRef]
- Mapossa, A.B.; Dantas, J.; Silva, M.R.; Kiminami, R.H.G.A.; Costa, A.C.F.M.; Daramola, M.O. Catalytic performance of NiFe2O4 and Ni0.3Zn0.7Fe2O4 magnetic nanoparticles during biodiesel production. Arab. J. Chem. 2019, 13, 4462–4476. [Google Scholar] [CrossRef]
- Khandan, M.; Saffarzadeh-Matin, S.; Shalmashi, A. Green hydrophobization of fume silica: Tailoring of heterogeneous basic catalyst for biodiesel production. J. Clean. Prod. 2020, 260, 121066. [Google Scholar] [CrossRef]
- Witoon, T.; Bumrungsalee, S.; Vathavanichkul, P.; Palitsakun, S.; Saisriyoot, M.; Faungnawakij, K. Biodiesel production from transesterification of palm oil with methanol over CaO supported on bimodal meso-macroporous silica catalyst. Bioresour. Technol. 2014, 156, 329–334. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, Z.; Tait, W.R.T.; Pokhrel, M.; Mao, Y.; Liu, J.; Zhang, L.; Wang, W.; Sun, L. Synthesis of green phosphors from highly active amorphous silica derived from rice husks. J. Mater. Sci. 2018, 53, 1824–1832. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Xu, W.; Zhang, Y.; Li, X.; Ouyang, S.; Zhao, G.; Liu, F.; Wu, N. The effect of microwave on the crystallization behavior of CMAS system glass-ceramics. Materials 2020, 13, 4555. [Google Scholar] [CrossRef]
- Loy, A.C.M.; Quitain, A.T.; Lam, M.K.; Yusup, S.; Sasaki, M.; Kida, T. Development of high microwave-absorptive bifunctional graphene oxide-based catalyst for biodiesel production. Energy Convers. Manag. 2019, 180, 1013–1025. [Google Scholar] [CrossRef]
- Mohammadi-Mahani, H.; Badoei-dalfard, A.; Karami, Z. Synthesis and characterization of cross-linked lipase-metal hybrid nanoflowers on graphene oxide with increasing the enzymatic stability and reusability. Biochem. Eng. J. 2021, 172, 108038. [Google Scholar] [CrossRef]
- Zhao, C.; Lv, P.; Yang, L.; Xing, S.; Luo, W.; Wang, Z. Biodiesel synthesis over biochar-based catalyst from biomass waste pomelo peel. Energy Convers. Manag. 2018, 160, 477–485. [Google Scholar] [CrossRef]
- Inayat, A.; Ghenai, C.; Hammad, D.; Almarzooqi, S.; Tariq, R.; Jamil, F.; Bokhari, A.; Ayoub, M. Optimization of biodiesel production from neem oil using KOH supported with activated carbon. Chem. Eng. Trans. 2020, 81, 1051–1056. [Google Scholar] [CrossRef]
- Bahari Molla Mahaleh, Y.; Sadrnezhaad, S.K.; Hosseini, D. NiO nanoparticles synthesis by chemical precipitation and effect of applied surfactant on distribution of particle size. J. Nanomater. 2008, 2008, 470595. [Google Scholar] [CrossRef] [Green Version]
- Abu-Dief, A.M.; Abdel-Fatah, S.M. Development and functionalization of magnetic nanoparticles as powerful and green catalysts for organic synthesis. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 55–67. [Google Scholar] [CrossRef]
- Seffati, K.; Honarvar, B.; Esmaeili, H.; Esfandiari, N. Enhanced biodiesel production from chicken fat using CaO/CuFe2O4 nanocatalyst and its combination with diesel to improve fuel properties. Fuel 2019, 235, 1238–1244. [Google Scholar] [CrossRef]
- Li, L.; Yue, H.; Ji, T.; Li, W.; Zhao, X.; Wang, L.; She, J.; Gu, X.; Li, X. Novel mesoporous TiO2(B) whisker-supported sulfated solid superacid with unique acid characteristics and catalytic performances. Appl. Catal. A Gen. 2019, 574, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Leesing, R.; Siwina, S.; Fiala, K. Yeast-based biodiesel production using sulfonated carbon-based solid acid catalyst by an integrated biorefinery of durian peel waste. Renew. Energy 2021, 171, 647–657. [Google Scholar] [CrossRef]
- Iwanow, M.; Gärtner, T.; Sieber, V.; König, B. Activated carbon as catalyst support: Precursors, preparation, modification and characterization. Beilstein J. Org. Chem. 2020, 16, 1188–1202. [Google Scholar] [CrossRef]
- Bora, P.; Boro, J.; Konwar, L.J.; Deka, D. Formulation of microemulsion based hybrid biofuel from waste cooking oil—A comparative study with biodiesel. J. Energy Inst. 2016, 89, 560–568. [Google Scholar] [CrossRef]
- Santaraite, M.; Sendzikiene, E.; Makareviciene, V.; Kazancev, K. Biodiesel production by lipase-catalyzed in situ transesterification of rapeseed oil containing a high free fatty acid content with ethanol in diesel fuel media. Energies 2020, 13, 2588. [Google Scholar] [CrossRef]
- Adewale, P.; Dumont, M.J.; Ngadi, M. Enzyme-catalyzed synthesis and kinetics of ultrasonic assisted methanolysis of waste lard for biodiesel production. Chem. Eng. J. 2016, 284, 158–165. [Google Scholar] [CrossRef]
- Kumar, S.; Shamsuddin, M.R.; Farabi, M.A.; Saiman, M.I.; Zainal, Z.; Taufiq-Yap, Y.H. Production of methyl esters from waste cooking oil and chicken fat oil via simultaneous esterification and transesterification using acid catalyst. Energy Convers. Manag. 2020, 226, 113366. [Google Scholar] [CrossRef]
- Gohain, M.; Devi, A.; Deka, D. Musa balbisiana Colla peel as highly effective renewable heterogeneous base catalyst for biodiesel production. Ind. Crops Prod. 2017, 109, 8–18. [Google Scholar] [CrossRef]
- Essamlali, Y.; Amadine, O.; Fihri, A.; Zahouily, M. Sodium modified fluorapatite as a sustainable solid bi-functional catalyst for biodiesel production from rapeseed oil. Renew. Energy 2019, 133, 1295–1307. [Google Scholar] [CrossRef]
- Ashok, A.; Ratnaji, T.; John Kennedy, L.; Judith Vijaya, J. Magnetically separable Zn1-xCuxFe2O4 (0 ≤ x ≤ 0.5) nanocatalysts for the transesterification of waste cooking oil. Adv. Powder Technol. 2020, 31, 2573–2585. [Google Scholar] [CrossRef]
- Olutoye, M.A.; Wong, S.W.; Chin, L.H.; Amani, H.; Asif, M.; Hameed, B.H. Synthesis of fatty acid methyl esters via the transesterification of waste cooking oil by methanol with a barium-modified montmorillonite K10 catalyst. Renew. Energy 2016, 86, 392–398. [Google Scholar] [CrossRef]
- Malins, K.; Kampars, V.; Brinks, J.; Neibolte, I.; Murnieks, R. Synthesis of activated carbon based heterogenous acid catalyst for biodiesel preparation. Appl. Catal. B Environ. 2015, 176–177, 553–558. [Google Scholar] [CrossRef]
- Faria, D.N.; Cipriano, D.F.; Schettino, M.A.; Neto, Á.C.; Cunha, A.G.; Freitas, J.C.C. Na,Ca-based catalysts supported on activated carbon for synthesis of biodiesel from soybean oil. Mater. Chem. Phys. 2020, 249, 123173. [Google Scholar] [CrossRef]
- Guo, L.; Xie, W.; Gao, C. Heterogeneous H6PV3MoW8O40/AC-Ag catalyst for biodiesel production: Preparation, characterization and catalytic performance. Fuel 2022, 316, 123352. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Chuah, L.F.; Klemeš, J.J.; Yusup, S.; Bokhari, A.; Akbar, M.M. Influence of fatty acids in waste cooking oil for cleaner biodiesel. Clean Technol. Environ. Policy 2017, 19, 859–868. [Google Scholar] [CrossRef]
- Ahmad, S.; Chaudhary, S.; Pathak, V.V.; Kothari, R.; Tyagi, V.V. Optimization of direct transesterification of Chlorella pyrenoidosa catalyzed by waste egg shell based heterogenous nano—CaO catalyst. Renew. Energy 2020, 160, 86–97. [Google Scholar] [CrossRef]
- Saini, M.K.; Sharma, P.; Prasad, J.; Kothari, S.L.; Gour, V.S. Quality assessment of oil and biodiesel derived from Balanites aegyptiaca collected from different regions of Rajasthan. Biocatal. Agric. Biotechnol. 2019, 22, 101374. [Google Scholar] [CrossRef]
- Abomohra, A.E.F.; Elsayed, M.; Esakkimuthu, S.; El-Sheekh, M.; Hanelt, D. Potential of fat, oil and grease (FOG) for biodiesel production: A critical review on the recent progress and future perspectives. Prog. Energy Combust. Sci. 2020, 81, 100868. [Google Scholar] [CrossRef]
- Ruppel, T.; Huybrighs, T.; Shelton, C. Fatty Acid Methyl Esters in B100 Biodiesel by Gas Chromatography (Modified EN 14103). Perkin Elmer’s Application Note; PerkinElmer: Waltham, MA, USA, 2008; pp. 1–4. [Google Scholar]




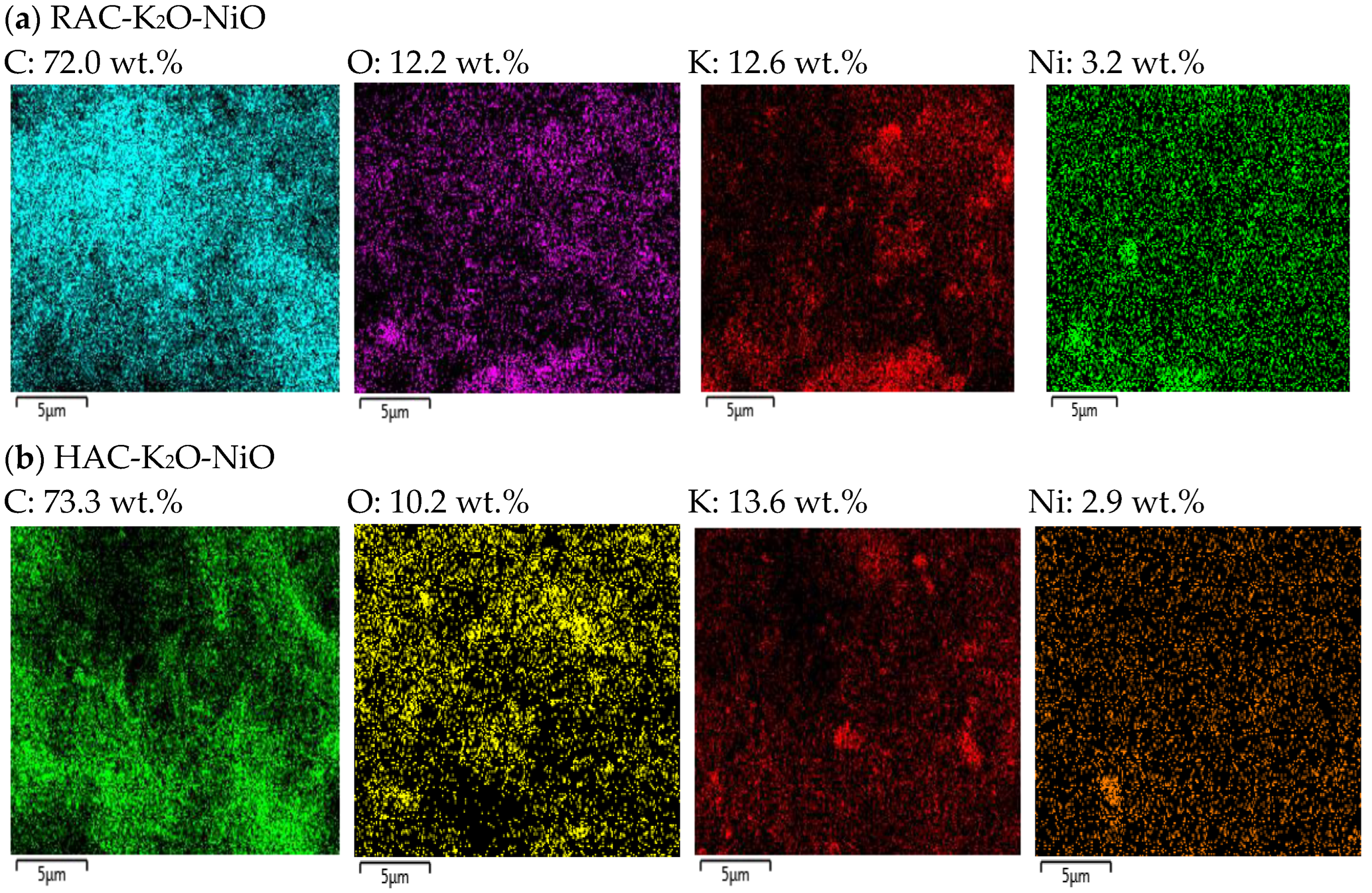
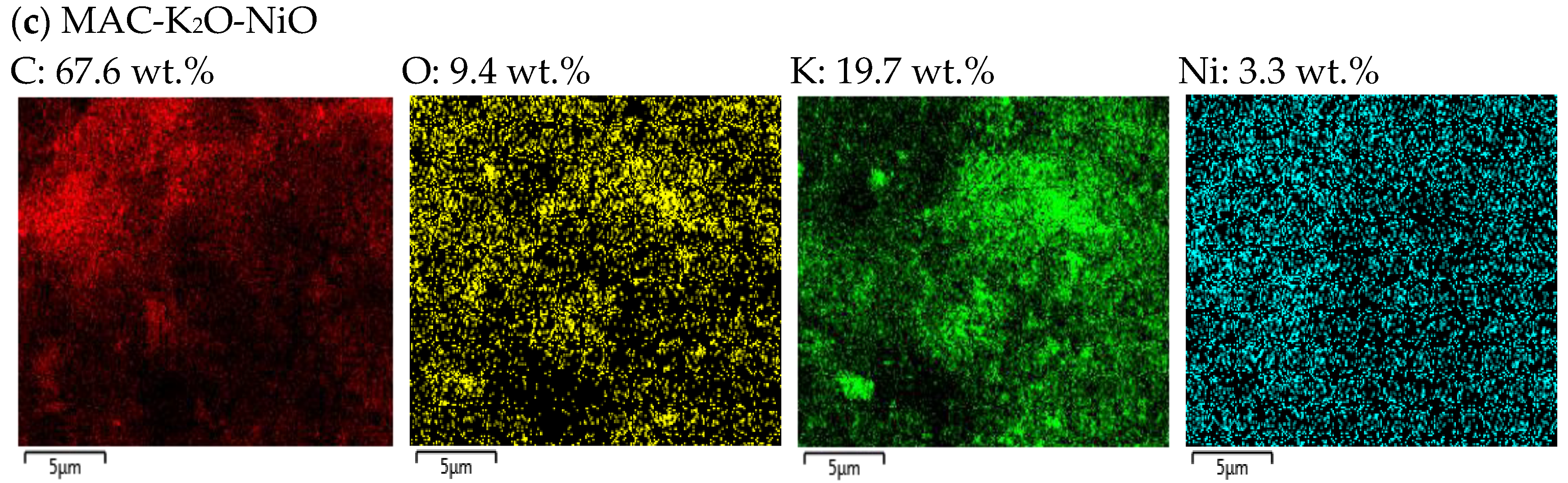

| Catalyst | Surface Area (m2/g) | Average Pore Size (nm) | Pore Volume (cm³/g) | Total Desorption (mmol/g) | Crystallite Size (nm) | |
|---|---|---|---|---|---|---|
| CO2 | NH3 | |||||
| RAC-K2O-NiO | 88 | 5.44 | 0.13 | 2.01 | 1.43 | 54.99 |
| HAC-K2O-NiO | 101 | 4.70 | 0.13 | 2.17 | 1.51 | 56.40 |
| MAC-K2O-NiO | 120 | 4.77 | 0.14 | 2.58 | 1.79 | 62.20 |
| Catalyst | Surface Area (m2/g) | Average Pore Size (nm) | Pore Volume (cm3/g) | TPD (mmol/g) | |
|---|---|---|---|---|---|
| CO2 | NH3 | ||||
| MAC-K2O-NiO | 120 | 4.77 | 0.14 | 2.58 | 1.79 |
| Spent MAC-K2O-NiO | 50 | 4.05 | 0.02 | 1.03 | 0.56 |
| Catalyst | Surface Area (m2/g) | Acidity/Basicity (mmol/g) | Biodiesel Feedstock | Reaction Parameters | Biodiesel Yield (%) | Reusability Cycle | Ref. |
|---|---|---|---|---|---|---|---|
| ACPhSO3H | 114 | 0.98 | Rapeseed oil | 10 wt.%, 7 h, 65 °C, 1:20 | 95.0 | 7 | [54] |
| Ca_AC_3_800 °C | 97 | - | Soybean oil | - | 91.0 | 4 | [55] |
| 35%H6PV3MoW8O4/AC-Ag | 310 | 0.11 | Soybean oil | 8 wt.%, 10 h, 140 °C, 1:30 | 91.3 | 5 | [56] |
| MAC-K2O-NiO | 120 | 1.79, 2.58 | WCO | 3 wt.%, 3 h, 65 °C, 10:1 | 97.2 | 7 | This study |
| Properties | ASTM D6751 | Biodiesel |
|---|---|---|
| Viscosity (mm2/g) | 1.9–6.0 | 3.95 |
| Moisture content (wt.%) | <0.05 | 0.01 |
| Flash point (°C) | >120 | 160 |
| Cloud point (°C) | −3–15 | 5 |
| Pour point (°C) | −5–10 | 3 |
| Cetane number | 47–65 | 60 |
| Density (g/cm−³) | 0.82–0.90 | 0.82 |
| Fatty Acid Methyl Ester (FAME) | Chemical Formula | Molecular Weight |
|---|---|---|
| Methyl myristate | C14:0 | 242.39 |
| Methyl palmitate | C16:0 | 270.45 |
| Methyl heptadecanoate (IS) | C17:0 | 284.48 |
| Methyl stearate | C18:0 | 298.51 |
| Methyl oleate | C18:1 n9c | 296.49 |
| Methyl linoleate | C18:2 n6c | 294.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazmi, B.; Rashid, U.; Kawi, S.; Mokhtar, W.N.A.W.; Ahangar, F.A.; Yaw, T.C.S.; Tsubota, T.; Alharthi, F.A.; Ngamcharussrivichai, C. A Heterogeneous Bifunctional Carbon Nanocatalyst from Plastic Waste to Efficiently Catalyze Waste Cooking Oil into Biodiesel. Catalysts 2022, 12, 874. https://doi.org/10.3390/catal12080874
Hazmi B, Rashid U, Kawi S, Mokhtar WNAW, Ahangar FA, Yaw TCS, Tsubota T, Alharthi FA, Ngamcharussrivichai C. A Heterogeneous Bifunctional Carbon Nanocatalyst from Plastic Waste to Efficiently Catalyze Waste Cooking Oil into Biodiesel. Catalysts. 2022; 12(8):874. https://doi.org/10.3390/catal12080874
Chicago/Turabian StyleHazmi, Balkis, Umer Rashid, Sibudjing Kawi, Wan Nur Aini Wan Mokhtar, Firdous Ahmad Ahangar, Thomas Choong Shean Yaw, Toshiki Tsubota, Fahad A. Alharthi, and Chawalit Ngamcharussrivichai. 2022. "A Heterogeneous Bifunctional Carbon Nanocatalyst from Plastic Waste to Efficiently Catalyze Waste Cooking Oil into Biodiesel" Catalysts 12, no. 8: 874. https://doi.org/10.3390/catal12080874
APA StyleHazmi, B., Rashid, U., Kawi, S., Mokhtar, W. N. A. W., Ahangar, F. A., Yaw, T. C. S., Tsubota, T., Alharthi, F. A., & Ngamcharussrivichai, C. (2022). A Heterogeneous Bifunctional Carbon Nanocatalyst from Plastic Waste to Efficiently Catalyze Waste Cooking Oil into Biodiesel. Catalysts, 12(8), 874. https://doi.org/10.3390/catal12080874












