The Novel Approach of Catalytic Interesterification, Hydrolysis and Transesterification of Pongamia pinnata Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oil Extraction and Vacuum Fractionation
2.3. Treatments of Oil
2.3.1. Interesterification
Preparation of Sodium Methoxide (CH3ONa)
Interesterification Reaction
2.3.2. Hydrolysis
Esterification
2.4. Production of Fatty Acid Methyl Esters (Biodiesel) Using Alkali Catalyzed Transesterification
2.5. Determination of Physiochemical Properties of Biodiesel
3. Results and Discussion
3.1. Mechanism of Interesterification, Hydrolysis and Alkali-Catalyzed Transesterification
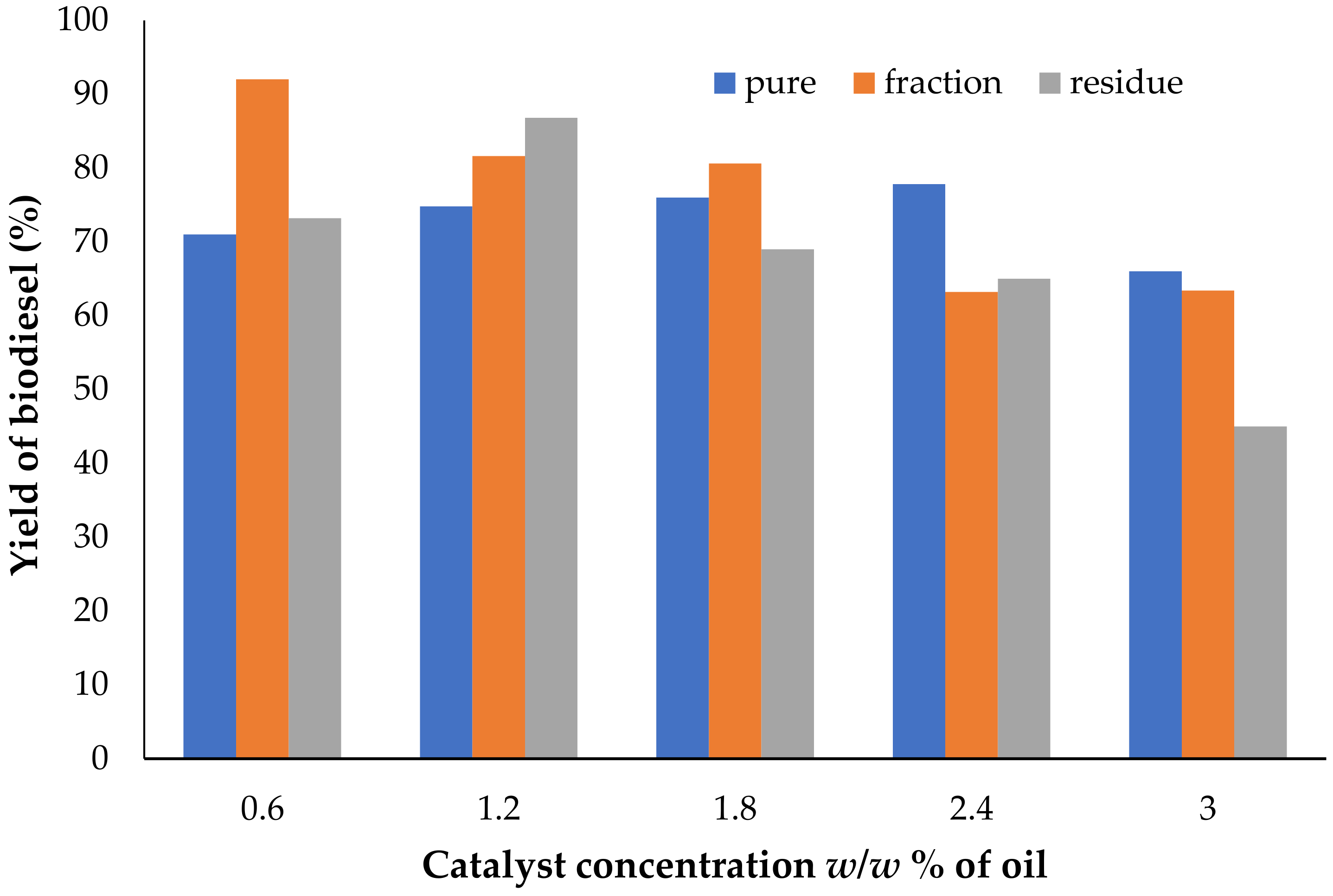
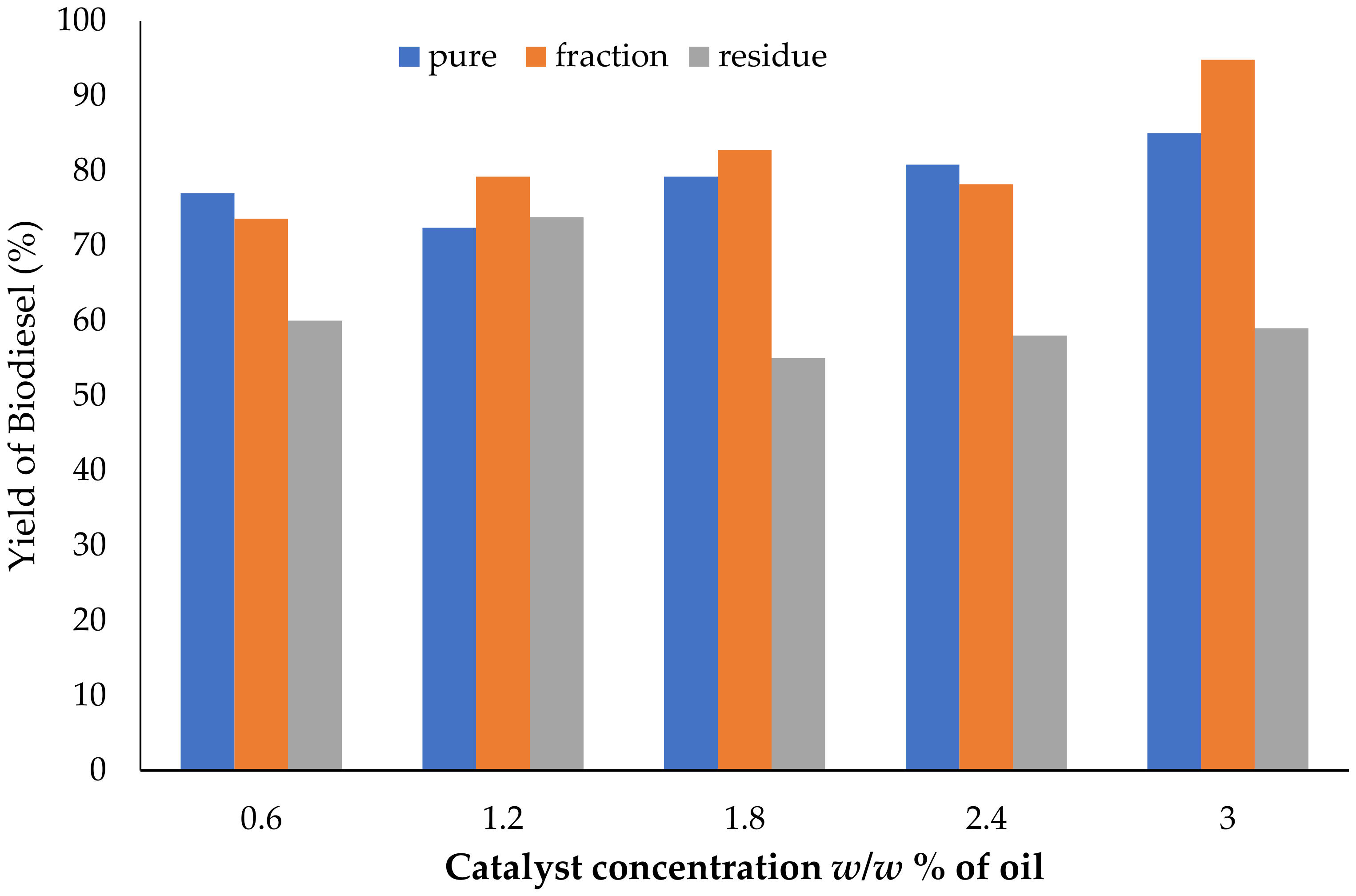
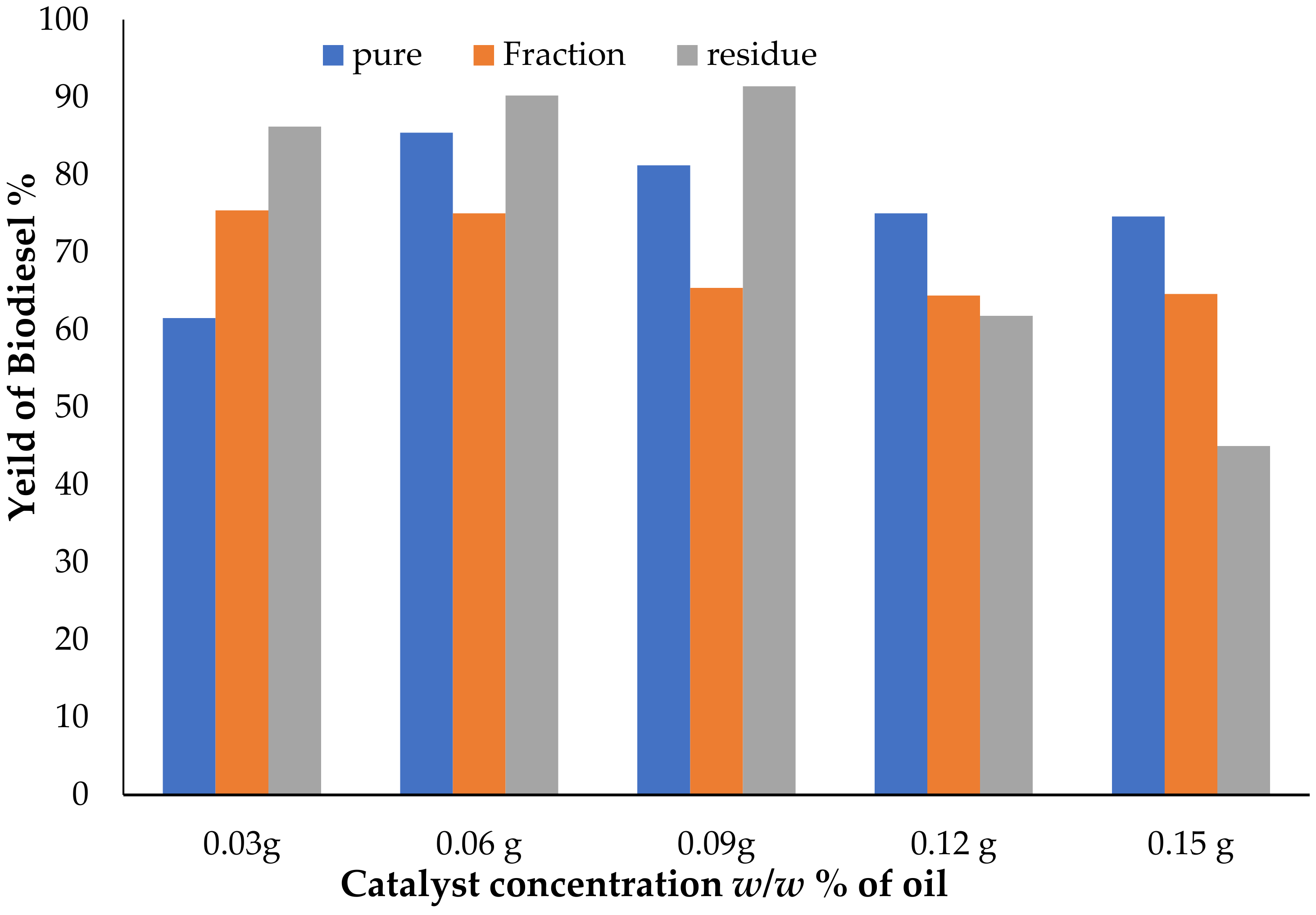
3.2. Effect of Catalyst Concentration on Biodiesel Yield
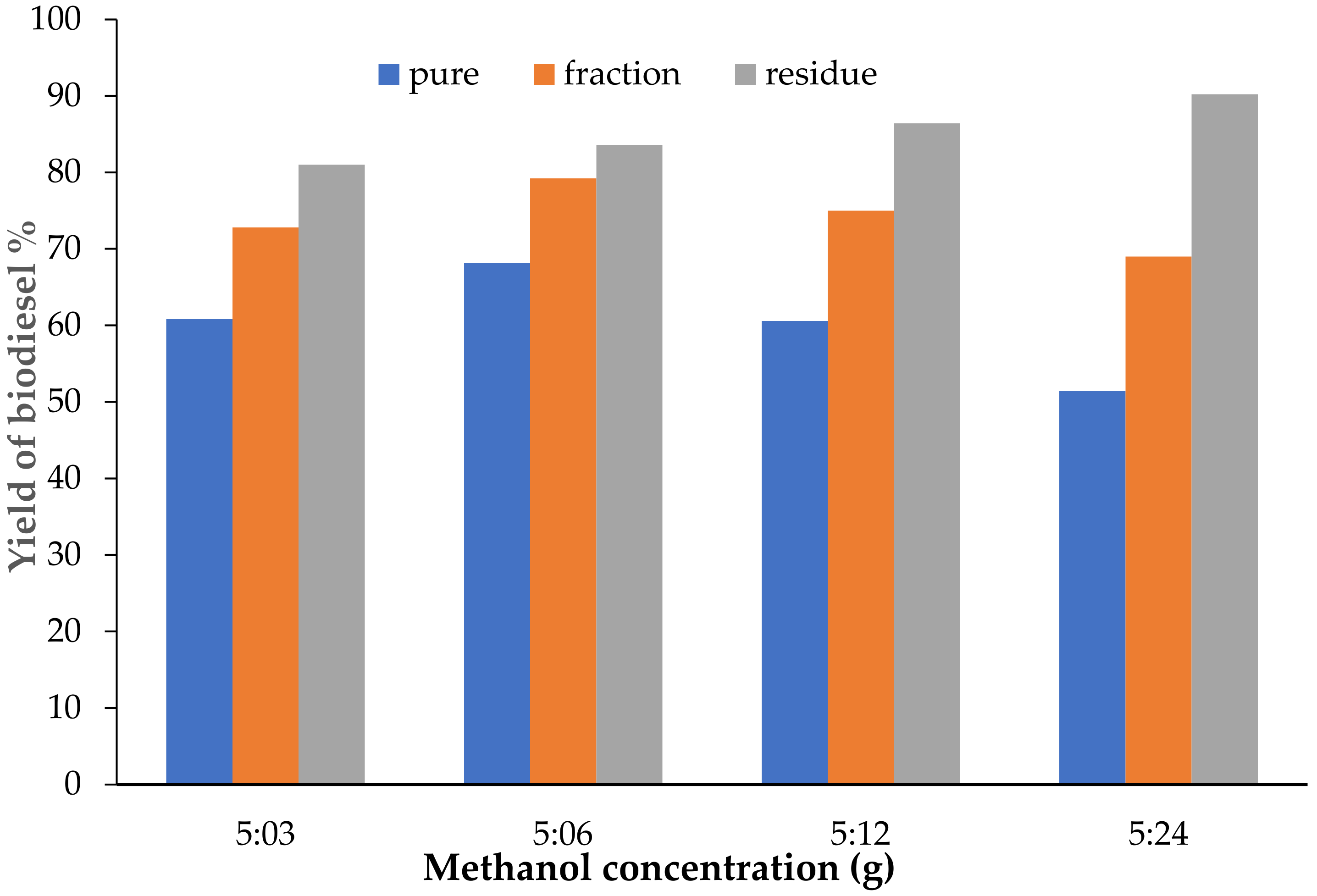
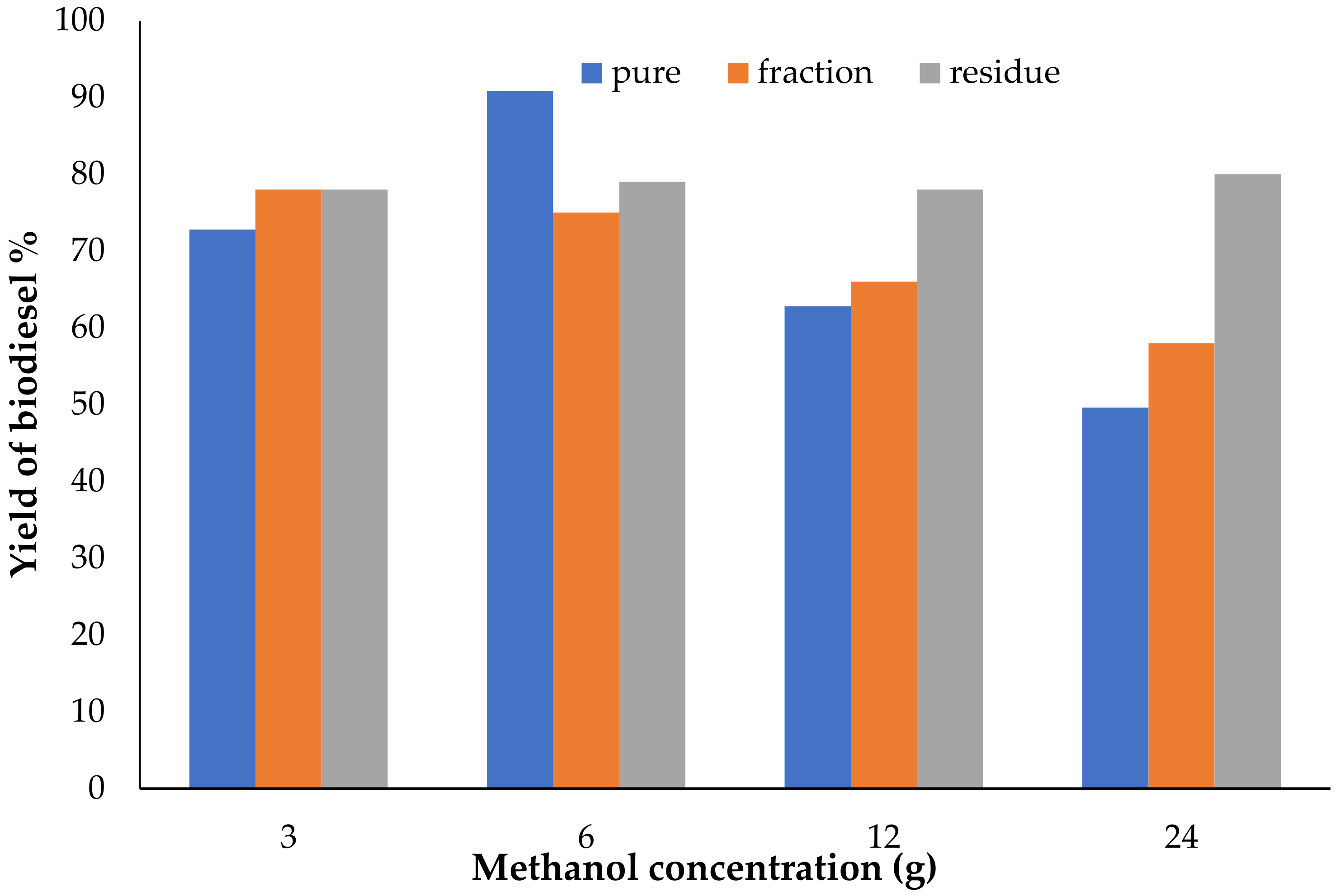
3.3. Effect of Methanol Concentration on Biodiesel Yield
3.4. Gas Chromatographic Analysis
3.5. Physiochemical Properties of Biodiesel
3.5.1. Density
3.5.2. Cold Flow Properties
3.5.3. Saponification Value
3.5.4. Iodine Value
3.5.5. Cetane Number
3.5.6. Acid Value
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jose, T.K.; Anand, K. Effects of biodiesel composition on its long term storage stability. Fuel 2016, 177, 190–196. [Google Scholar] [CrossRef]
- Balajii, M.; Niju, S. Banana peduncle—A green and renewable heterogeneous base catalyst for biodiesel production from Ceiba pentandra oil. Renew. Energy 2020, 146, 2255–2269. [Google Scholar] [CrossRef]
- Konur, O. Biodiesel and Petrodiesel Fuels: Science, Technology, Health, and the Environment. In Biodiesel Fuels; CRC Press: Boca Raton, FL, USA, 2021; pp. 3–36. [Google Scholar]
- Anwar, M. Biodiesel feedstocks selection strategies based on economic, technical, and sustainable aspects. Fuel 2021, 283, 119204. [Google Scholar] [CrossRef]
- Singh, R.; Arora, A.; Singh, V. Biodiesel from oil produced in vegetative tissues of biomass—A review. Bioresour. Technol. 2021, 326, 124772. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Joshi, R.; Park, Y.-K.; Jeon, J.-K. Biodiesel production from jatropha seeds with bead-type heterogeneous catalyst. Korean J. Chem. Eng. 2021, 38, 763–770. [Google Scholar] [CrossRef]
- Ahmed, R.; Huddersman, K. Review of biodiesel production by the esterification of wastewater containing fats oils and grease (FOGs). J. Ind. Eng. Chem. 2022, 110, 1–14. [Google Scholar] [CrossRef]
- Okolie, J.A.; Escobar, J.I.; Umenweke, G.; Khanday, W.; Okoye, P.U. Continuous biodiesel production: A review of advances in catalysis, microfluidic and cavitation reactors. Fuel 2022, 307, 121821. [Google Scholar] [CrossRef]
- Lokman, I.M.; Rashid, U.; Yunus, R.; Taufiq-Yap, Y.H. Carbohydrate-derived solid acid catalysts for biodiesel production from low-cost feedstocks: A review. Catal. Rev. Sci. Eng. 2014, 56, 187–219. [Google Scholar] [CrossRef]
- Jayakumar, M.; Karmegam, N.; Gundupalli, M.P.; Gebeyehu, K.B.; Asfaw, B.T.; Chang, S.W.; Balasubramani, R.; Awasthi, M.K. Heterogeneous base catalysts: Synthesis and application for biodiesel production—A review. Bioresour. Technol. 2021, 331, 125054. [Google Scholar] [CrossRef]
- Liu, X.; Xing, S.; Yang, L.; Fu, J.; Lv, P.; Zhang, X.; Li, M.; Wang, Z. Highly active and durable Ca-based solid base catalyst for biodiesel production. Fuel 2021, 302, 121094. [Google Scholar] [CrossRef]
- Mathew, G.M.; Raina, D.; Narisetty, V.; Kumar, V.; Saran, S.; Pugazhendi, A.; Sindhu, R.; Pandey, A.; Binod, P. Recent advances in biodiesel production: Challenges and solutions. Sci. Total Environ. 2021, 794, 148751. [Google Scholar] [CrossRef]
- Hoang, A.T.; Tabatabaei, M.; Aghbashlo, M.; Carlucci, A.P.; Ölçer, A.I.; Le, A.T.; Ghassemi, A. Rice bran oil-based biodiesel as a promising renewable fuel alternative to petrodiesel: A review. Renew. Sustain. Energy Rev. 2021, 135, 110204. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Sajjadi, B.; Raman, A.A.A.; Arandiyan, H. A comprehensive review on properties of edible and non-edible vegetable oil-based biodiesel: Composition, specifications and prediction models. Renew. Sustain. Energy Rev. 2016, 63, 62–92. [Google Scholar] [CrossRef]
- Caldeira, C.; Freire, F.; Olivetti, E.A.; Kirchain, R. Fatty acid based prediction models for biodiesel properties incorporating compositional uncertainty. Fuel 2017, 196, 13–20. [Google Scholar] [CrossRef]
- Gopinath, A.; Puhan, S.; Nagarajan, G. Effect of biodiesel structural configuration on its ignition quality. Energy Environ. 2010, 1, 295–306. [Google Scholar]
- Jacoby, M. Sowing the seeds of oil customization. Chem. Eng. News 2010, 88, 52–55. [Google Scholar] [CrossRef]
- Santoro, V.; Baiocchi, C.; Dal Bello, F.; Gastaldi, D.; Aigotti, R.; Zorzi, M.; Pellegrino, A.; Forte, E.; Romaniello, F.; Magni, M.; et al. Formation of by-products during chemical interesterification of lipids. Detection and characterization of dialkyl ketones by non-aqueous reversed-phase liquid chromatography-high resolution mass spectrometry and gas chromatography-mass spectrometry. J. Chromatogr. A 2018, 1581, 63–70. [Google Scholar] [CrossRef]
- Almashhadani, A.Q.; Leh, C.P.; Chan, S.-Y.; Lee, C.Y.; Goh, C.F. Nanocrystalline cellulose isolation via acid hydrolysis from non-woody biomass: Importance of hydrolysis parameters. Carbohydr. Polym. 2022, 286, 119285. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Oliveira, P.D.; Rodrigues, A.M.; Bezerra, C.V.; Silva, L.H. Chemical interesterification of blends with palm stearin and patawa oil. Food Chem. 2017, 215, 369–376. [Google Scholar] [CrossRef]
- dos Santos, L.K.; Hatanaka, R.R.; de Oliveira, J.E.; Flumignan, D.L. Production of biodiesel from crude palm oil by a sequential hydrolysis/esterification process using subcritical water. Renew. Energy 2019, 130, 633–640. [Google Scholar] [CrossRef]
- Azeem, M.W.; Hanif, M.A.; Al-Sabahi, J.N.; Khan, A.A.; Naz, S.; Ijaz, A. Production of biodiesel from low priced, renewable and abundant date seed oil. Renew. Energy 2016, 86, 124–132. [Google Scholar] [CrossRef]
- Efavi, J.; Kanbogtah, D.; Apalangya, V.; Nyankson, E.; Tiburu, E.; Dodoo-Arhin, D.; Onwona-Agyeman, B.; Yaya, A. The effect of NaOH catalyst concentration and extraction time on the yield and properties of Citrullus vulgaris seed oil as a potential biodiesel feed stock. S. Afr. J. Chem. Eng. 2018, 25, 98–102. [Google Scholar] [CrossRef]
- Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt. J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef]
- Zhang, L.; Sheng, B.; Xin, Z.; Liu, Q.; Sun, S. Kinetics of transesterification of palm oil and dimethyl carbonate for biodiesel production at the catalysis of heterogeneous base catalyst. Bioresour. Technol. 2010, 101, 8144–8150. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Folayan, A.J.; Anawe, P.A.L.; Aladejare, A.E.; Ayeni, A.O. Experimental investigation of the effect of fatty acids configuration, chain length, branching and degree of unsaturation on biodiesel fuel properties obtained from lauric oils, high-oleic and high-linoleic vegetable oil biomass. Energy Rep. 2019, 5, 793–806. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Oyekunle, J.A.O.; Obisesan, O.R.; Ojo, O.S. Effects of degumming on biodiesel properties of some non-conventional seedoils. Energy Rep. 2016, 2, 188–193. [Google Scholar] [CrossRef]
- Choi, H.L.; Sudiarto, S.I.; Renggaman, A. Prediction of livestock manure and mixture higher heating value based on fundamental analysis. Fuel 2014, 116, 772–780. [Google Scholar] [CrossRef]
- Giakoumis, E.G. A statistical investigation of biodiesel physical and chemical properties, and their correlation with the degree of unsaturation. Renew. Energy 2013, 50, 858–878. [Google Scholar] [CrossRef]
- Refaat, A. Correlation between the chemical structure of biodiesel and its physical properties. Int. J. Environ. Sci. Technol. 2009, 6, 677–694. [Google Scholar] [CrossRef]
- Anguebes-Franseschi, F.; Bassam, A.; Abatal, M.; May Tzuc, O.; Aguilar-Ucán, C.; Wakida-Kusunoki, A.; Diaz-Mendez, S.; San Pedro, L. Physical and Chemical Properties of Biodiesel Obtained from Amazon Sailfin Catfish (Pterygoplichthys pardalis) Biomass Oil. J. Chem. 2019, 2019, 7829630. [Google Scholar] [CrossRef]
- Karmee, S.K.; Chadha, A. Preparation of biodiesel from crude oil of Pongamia pinnata. Bioresour. Technol. 2005, 96, 1425–1429. [Google Scholar] [CrossRef]




| No. | Molecular Formula | Fatty Acids | Percentage Concentration (%) |
|---|---|---|---|
| 1 | C18H34O2 | Oleic acid | 54.32 |
| 2 | C18H32O2 | Linoleic acid | 12.01 |
| 3 | C16H32O2 | Palmitic acid | 9.83 |
| 4 | C20H40O2 | Eicosanoic acid | 7.43 |
| 5 | C22H44O2 | Behenic acid | 3.54 |
| 6 | C24H48O2 | Lignoceric acid | 3.23 |
| 7 | C18H30O2 | Linolenic acid | 3.39 |
| 8 | C18H36O2 | Stearic acid | 2.95 |
| 9 | C14H28O2 | Myristic acid | 0.88 |
| 10 | C20H40O2 | Arachidic acid | 0.72 |
| 11 | C12H24O2 | Lauric acid | 0.64 |
| 12 | C10H20O2 | Capric acid | 0.25 |
| 13 | C17H34O2 | Margaric acid | 0.12 |
| 14 | C18H34O3 | Ricinoleic acid | 0.06 |
| 15 | C22H42O2 | Erucic acid | 0.02 |
| Test Property | Non-Treated | Interesterified | Hydrolyzed | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pure Oil | Fraction | Residue | Pure Oil | Fraction | Residue | Pure Oil | Fraction | Residue | |
| Density (g/mL) | 0.85 | 0.86 | 1.08 | 0.86 | 0.86 | 0.88 | 0.85 | 0.86 | 0.89 |
| Cloud point (°C) | 12.55 | 13.37 | 16.03 | 6.7 | 8.1 | 9.58 | 2.96 | 2.03 | 3.63 |
| Pour point (°C) | −1.35 | −1.53 | 0.68 | −9.6 | −8.8 | −2.07 | −11.4 | −12.5 | −8.03 |
| Saponification value (mg KOH/g) | 171.41 | 269.63 | 295.43 | 289.2 | 222.9 | 209.8 | 267.3 | 297.9 | 171.85 |
| Iodine value | 50.8 | 73.6 | 71.1 | 63.08 | 62.05 | 119.9 | 67.7 | 64.0 | 103.0 |
| Cetane number | 66.71 | 50.54 | 49.1 | 50.92 | 53.61 | 45.37 | 51.75 | 50.15 | 39.4 |
| Acid value | 0.5 | 0.8 | 0.6 | 0.4 | 0.7 | 0.8 | 0.6 | 0.7 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inam, S.; Hanif, M.A.; Rashid, U.; Nadeem, F.; Alharthi, F.A.; Kazerooni, E.A. The Novel Approach of Catalytic Interesterification, Hydrolysis and Transesterification of Pongamia pinnata Oil. Catalysts 2022, 12, 896. https://doi.org/10.3390/catal12080896
Inam S, Hanif MA, Rashid U, Nadeem F, Alharthi FA, Kazerooni EA. The Novel Approach of Catalytic Interesterification, Hydrolysis and Transesterification of Pongamia pinnata Oil. Catalysts. 2022; 12(8):896. https://doi.org/10.3390/catal12080896
Chicago/Turabian StyleInam, Summayia, Muhammad Asif Hanif, Umer Rashid, Farwa Nadeem, Fahad A. Alharthi, and Elham Ahmed Kazerooni. 2022. "The Novel Approach of Catalytic Interesterification, Hydrolysis and Transesterification of Pongamia pinnata Oil" Catalysts 12, no. 8: 896. https://doi.org/10.3390/catal12080896
APA StyleInam, S., Hanif, M. A., Rashid, U., Nadeem, F., Alharthi, F. A., & Kazerooni, E. A. (2022). The Novel Approach of Catalytic Interesterification, Hydrolysis and Transesterification of Pongamia pinnata Oil. Catalysts, 12(8), 896. https://doi.org/10.3390/catal12080896










