Selective Hydrogenation of 5-Acetoxymethylfurfural over Cu-Based Catalysts in a Flow Reactor: Effect of Cu-Al Layered Double Hydroxides Synthesis Conditions on Catalytic Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Cu Layered Double Hydroxides and Mixed Oxides Based on the LDHs
2.2. Characterization of the Cu-Al Samples
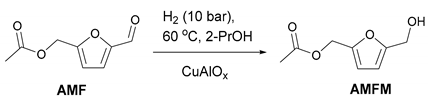
2.3. Catalytic Test of the Cu-Al Samples
3. Materials and Methods
3.1. Characterization Techniques
3.2. Catalytic Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S.; Liu, S. Recent Advances in Catalytic Transformation of Biomass-Derived 5-Hydroxymethylfurfural into the Innovative Fuels and Chemicals. Renew. Sust. Energ. Rev. 2017, 74, 230–257. [Google Scholar] [CrossRef]
- Gerardy, R.; Debecker, D.P.; Estager, J.; Luis, P.; Monbaliu, J.-C.M. Continuous Flow Upgrading of Selected C2−C6 Platform Chemicals Derived from Biomass. Chem. Rev. 2020, 120, 7219–7347. [Google Scholar] [CrossRef] [PubMed]
- Galkin, K.I.; Ananikov, V.P. When Will 5-Hydroxymethylfurfural, the “Sleeping Giant” of Sustainable Chemistry, Awaken? ChemSusChem 2019, 12, 2976–2982. [Google Scholar] [CrossRef]
- Galkin, K.I.; Krivodaeva, E.A.; Romashov, L.V.; Zalesskiy, S.S.; Kachala, V.V.; Burykina, J.V.; Ananikov, V.P. Critical Influence of 5-Hydroxymethylfurfural Aging and Decomposition on The Utility of Biomass Conversion in Organic Synthesis. Angew. Chem. Int. Ed. 2016, 55, 8338–8342. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.-S.; Hong, Y.-W.; Chae, D.W.; Kim, B.; Kim, B.; Kim, Y.J.; Cho, J.K.; Kim, Y.G. From Lignocellulosic Biomass to Furans Via 5-Acetoxymethylfurfural as an Alternative to 5-Hydroxymethylfurfural. ChemSusChem 2015, 8, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Gavilà, L.; Esposito, D. Cellulose Acetate as a Convenient Intermediate for the Preparation of 5-Acetoxymethylfurfural from Biomass. Green Chem. 2017, 19, 2496–2500. [Google Scholar] [CrossRef] [Green Version]
- Shinde, S.; Deval, K.; Chikate, R.; Rode, C. Cascade Synthesis of 5-(Acetoxymethyl) Furfural from Carbohydrates Over Sn-Mont Catalyst. ChemistrySelect 2018, 3, 8770–8778. [Google Scholar] [CrossRef]
- Rigo, D.; Polidoro, D.; Perosa, A.; Selva, M. Diversified upgrading of HMF via acetylation, aldol condensation, carboxymethylation, vinylation and reductive amination reactions. Mol. Catal. 2021, 514, 111838. [Google Scholar] [CrossRef]
- Mistri, R.; Kumar, B. Supported Transition Metal Catalysts for Organic Fine Chemical Synthesis: A Review. Asian J. Chem. 2021, 33, 489–498. [Google Scholar] [CrossRef]
- Kottappara, R.; Pillai, S.C.; Vijayan, B.K. Copper-Based Nanocatalysts for Nitroarene Reduction-A Review of Recent Advances. Inorg. Chem. Commun. 2020, 121, 1908181. [Google Scholar] [CrossRef]
- Stepanova, L.N.; Belskaya, O.B.; Leont’eva, N.N.; Kobzar, E.O.; Salanov, A.N.; Gulyaeva, T.I.; Trenikhin, M.V.; Likholobov, V.A. Study of the Properties of the Catalysts Based on Ni(Mg)Al-Layered Hydroxides for the Reaction of Furfural Hydrogenation. Mater. Chem. Phys. 2021, 263, 124091. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Yu, X.; Zhang, W.; Zhang, G.; Liu, M.; Jian Shen, J.; Yang, C.; Jin, X. Non-Noble Metal Catalysts for Transfer Hydrogenation of Levulinic Acid: The Role of Surface Morphology and Acid-Base Pairs. Mater. Today Energy. 2020, 18, 100501. [Google Scholar] [CrossRef]
- Shanta Dutta, S.; Yu, I.K.M.; Tsanga, D.C.W.; Ng, Y.H.; Ok, Y.S.; Sherwood, J.; Clark, J.H. Green Synthesis of Gamma-Valerolactone (GVL) Through Hydrogenation of Biomass-Derived Levulinic Acid Using Non-Noble Metal Catalysts: A Critical Review. Chem. Eng. J. 2019, 372, 992–1006. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Rodríguez-Padrón, D.; Len, C. Recent Advances in Catalytic Hydrogenation of Furfural. Catalysts. 2019, 9, 796. [Google Scholar] [CrossRef] [Green Version]
- Vishakha, G.; Ganesh, N.; Anand, N.; Kishore, N.; Jagadeesh, R.V. Recent Developments in Reductive N-Methylation with Base-Metal Catalysts. Tetrahedron. 2021, 98, 132414. [Google Scholar] [CrossRef]
- Bukhtiyarova, M.; Lunkenbein, T.; Kähler, K.; Schlögl, R. Methanol Synthesis from Industrial CO2 Sources: A Contribution to Chemical Energy Conversion. Catal. Lett. 2017, 147, 416–427. [Google Scholar] [CrossRef] [Green Version]
- Gogate, M.R. Methanol Synthesis Revisited: The Nature of The Active Site of Cu in Industrial Cu/Zno/Al2O3 Catalyst and Cu-Zn Synergy. Pet. Sci. Technol. 2019, 37, 671–678. [Google Scholar] [CrossRef]
- Sareen, S.; Mutreja, V.; Singh, S.; Pal, B. Fine CuO Anisotropic Nanoparticles Supported on Mesoporous SBA-15 For Selective Hydrogenation of Nitroaromatics. J. Colloid Interface Sci. 2016, 461, 203–210. [Google Scholar] [CrossRef]
- He, D.; Wang, T.; Li, T.; Wang, X.; Wang, H.; Dai, X.; Shi, F. Efficient Hydrogenation Catalyst Designing Via Preferential Adsorption Sites Construction Towards Active Copper. J. Catal. 2021, 400, 397–406. [Google Scholar] [CrossRef]
- Nuzhdin, A.L.; Artiukha, E.A.; Bukhtiyarova, G.A.; Derevyannikova, E.A.; Bukhtiyarov, V.I. Synthesis of Secondary Amines by Reductive Amination of Aldehydes with Nitroarenes Over Supported Copper Catalysts in a Flow Reactor. Catal. Commun. 2017, 102, 108–113. [Google Scholar] [CrossRef]
- Santoro, F.; Psaro, R.; Ravasio, N.; Zaccheria, F. Reductive Amination of Ketones or Amination of Alcohols over Heterogeneous Cu Catalysts: Matching the Catalyst Support with the N-Alkylating Agent. ChemCatChem. 2012, 4, 1249–1254. [Google Scholar] [CrossRef]
- Kim, J.; Bathula, H.B.; Yun, S.; Jo, Y.; Lee, S.; Baik, J.H.; Suh, Y.-W. Hydrogenation of 5-Hydroxymethylfurfural into 2,5-Bis (Hydroxymethyl)Furan Over Mesoporous Cu–Al2O3 Catalyst: From Batch to Continuous Processing. J. Ind. Eng. Chem. 2021, 102, 186–194. [Google Scholar] [CrossRef]
- Kumalaputri, A.J.; Bottari, G.; Erne, P.M.; Heeres, H.J.; Barta, K. Tunable and Selective Conversion of 5-HMF to 2,5-Furandimethanol And 2,5-Dimethylfuran Over Copper-Doped Porous Metal Oxides. ChemSusChem 2014, 7, 2266–2275. [Google Scholar] [CrossRef] [PubMed]
- Aldureid, A.; Francisco Medina, F.; Gregory, S.; Patience, G.S.; Montané, D. Ni-Cu/Al2O3 from Layered Double Hydroxides Hydrogenates Furfural to Alcohols. Catalysts 2022, 12, 390. [Google Scholar] [CrossRef]
- Nuzhdin, A.L.; Bukhtiyarova, M.V.; Bulavchenko, O.A.; Bukhtiyarova, G.A. Flow Hydrogenation of 5-Acetoxymethylfurfural over Cu-Based Catalysts. Mol. Catal. 2020, 494, 111132. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-Type Anionic Clays: Preparation, Properties and Applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Huang, L.; Megías-Sayago, C.; Bingre, R.; Zheng, Q.; Wang, Q.; Louis, B. Catalytic Performance of Layered Double Hydroxides (LDHs) Derived Materials in Gas-Solid and Liquid-Solid Phase Reactions. ChemCatChem 2019, 11, 3279–3286. [Google Scholar] [CrossRef]
- Haraketi, M.; Hosni, K.; Srasra, E. Intercalation Behavior of Salicylic Acid into Calcined Cu–Al-Layered Double Hydroxides for a Controlled Release Formulation. Surf. Eng. Appl. Electrochem. 2017, 53, 360–370. [Google Scholar] [CrossRef]
- Song, Q.; Liu, W.; Bohn, C.D.; Harper, R.N.; Sivaniah, E.; Scott, S.A.; Dennis, J.S. A High-Performance Oxygen Storage Material for Chemical Looping Processes with CO2 Capture. Energy Environ. Sci. 2013, 6, 288–298. [Google Scholar] [CrossRef]
- Bukhtiyarova, M.V.; Nuzhdin, A.L.; Kardash, T.Y.; Bukhtiyarov, A.V.; Gerasimov, E.Y.; Romanenko, A.V. N-Methylation of p-Anisidine on the Catalysts Based on Cu-Containing Layered Double Hydroxides. Kinet. Catal. 2019, 60, 343–354. [Google Scholar] [CrossRef]
- Bukhtiyarova, M.V.; Nuzhdin, A.L.; Bukhtiyarov, A.V.; Kardash, T.Y.; Romanenko, A.V. Cu Layered Double Hydroxides as Catalysts for N-Methylation of P-Anisidine: Influence of Synthesis Conditions. Catal. Commun. 2019, 127, 39–44. [Google Scholar] [CrossRef]
- Bukhtiyarova, M.V. A Review on Effect of Synthesis Conditions on the Formation of Layered Double Hydroxides. J. Solid State Chem. 2019, 269, 494–506. [Google Scholar] [CrossRef]
- Kühl, S.; Tarasov, A.; Zander, S.; Kasatkin, I.; Behrens, M. Cu-Based Catalyst Resulting from a Cu, Zn, Al Hydrotalcite-Like Compound: A Microstructural, Thermoanalytical, and in Situ XAS Study. Chem. Eur. J. 2014, 20, 3782–3792. [Google Scholar] [CrossRef]
- Nývlt, J.; Söhnel, O.; Matuchová, M.; Broul, M. The Kinetics of Industrial Crystallization. In Chemical Engineering Monographs; Elsevier: Amsterdam, The Netherlands, 1985; Volume 3. [Google Scholar]
- Gao, P.; Li, F.; Xiao, F.; Zhao, N.; Sun, N.; Wei, W.; Zhong, L.; Sun, Y. Preparation and Activity of Cu/Zn/Al/Zr Catalysts Via Hydrotalcite-Containing Precursors for Methanol Synthesis from CO2 Hydrogenation. Catal. Sci. Technol. 2012, 2, 1447–1454. [Google Scholar] [CrossRef]
- Kwak, B.K.; Park, D.S.; Yun, Y.S.; Yi, J. Preparation and Characterization of Nanocrystalline CuAl2O4 Spinel Catalysts by Sol–Gel Method for The Hydrogenolysis of Glycerol. Catal. Commun. 2012, 24, 90–95. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin—Elmer Corp.: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Nuzhdin, A.L.; Shchurova, I.A.; Bukhtiyarova, M.V.; Bulavchenko, O.A.; Alekseyeva, N.A.; Sysolyatin, S.V.; Bukhtiyarova, G.A. Flow Hydrogenation of 1,3,5-Trinitrobenzenes Over Cu-Based Catalysts as an Efficient Approach for the Preparation of Phloroglucinol Derivatives. Synthesis 2022, 54, 3605–3612. [Google Scholar] [CrossRef]

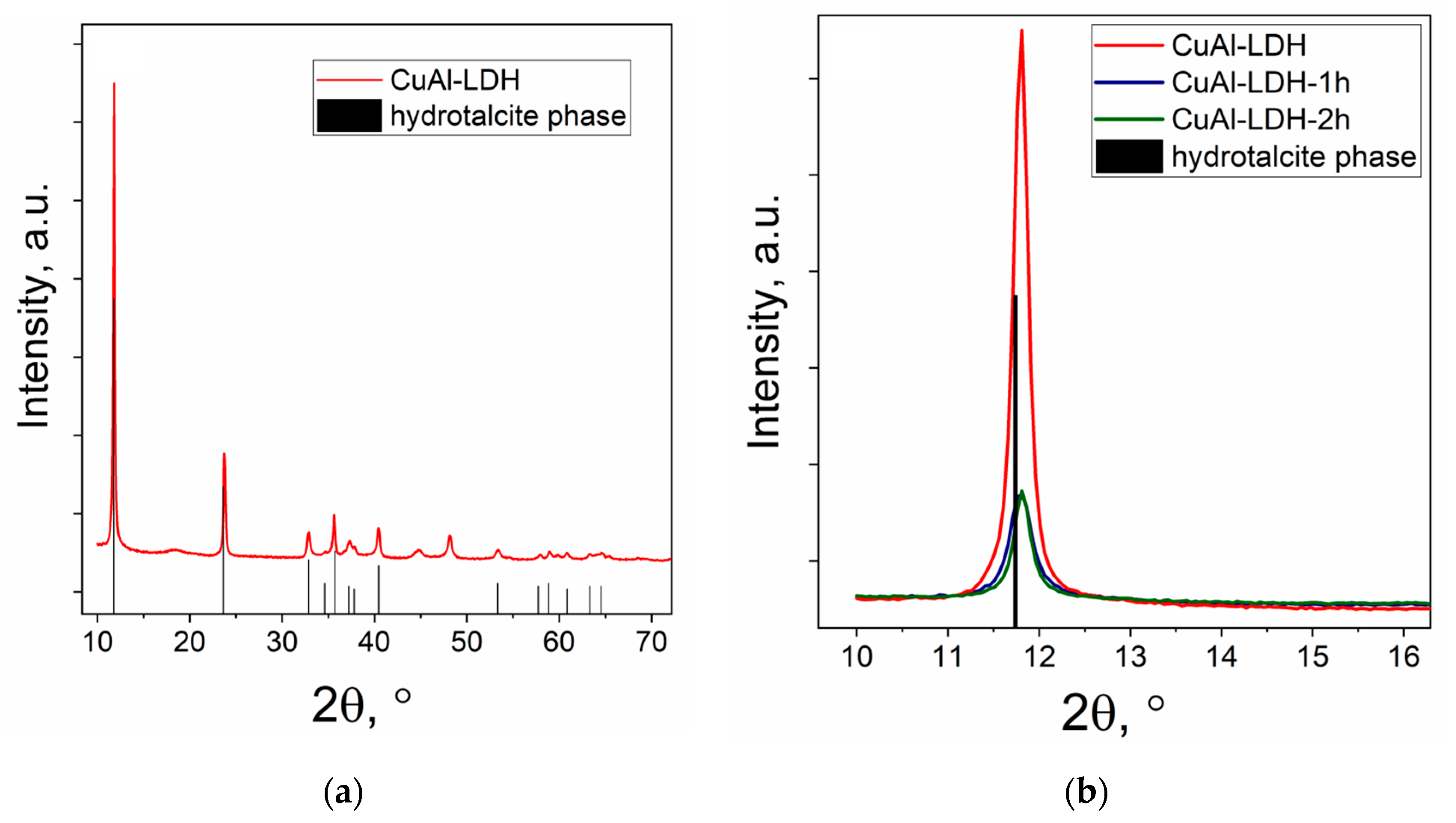
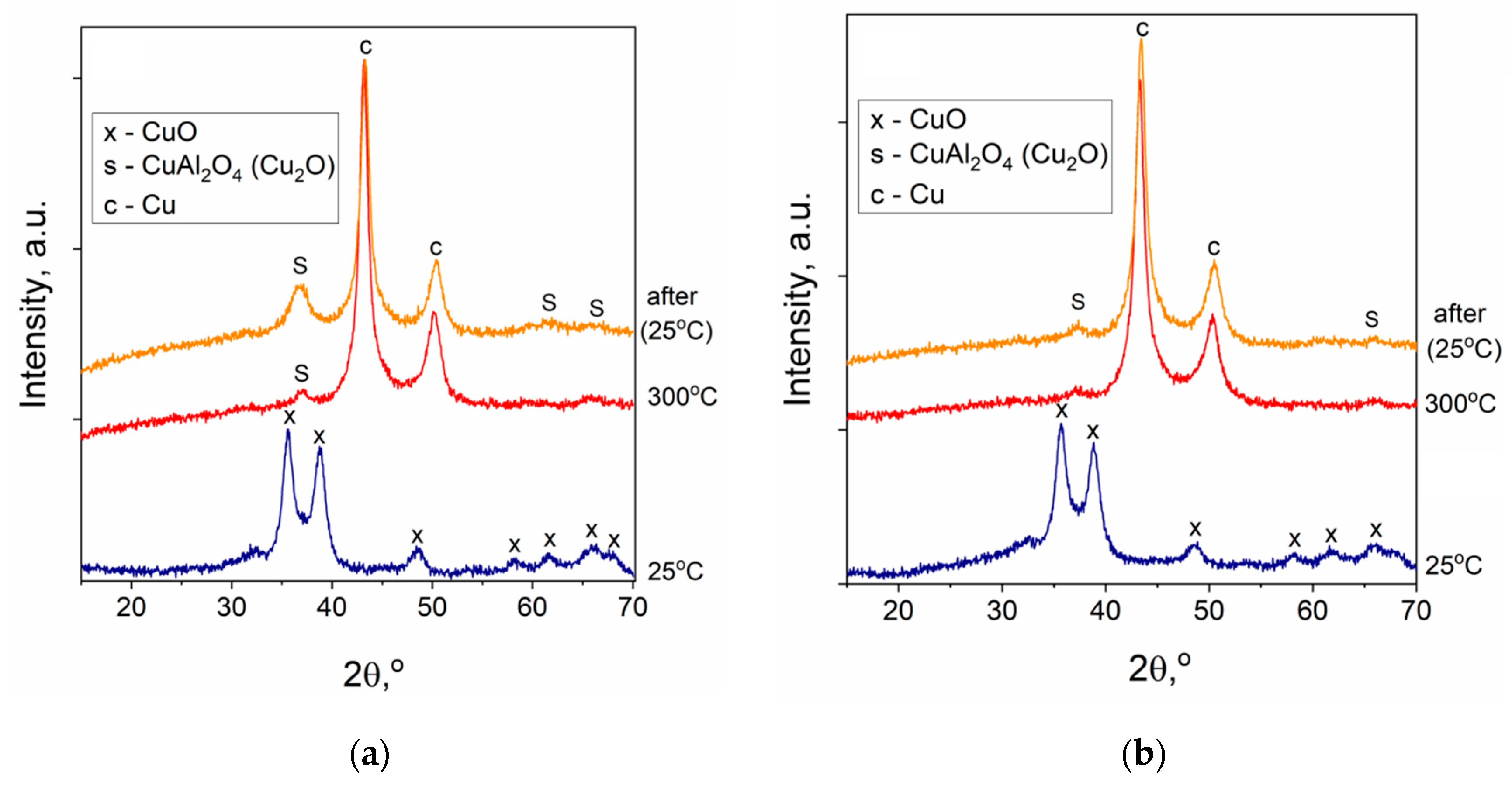
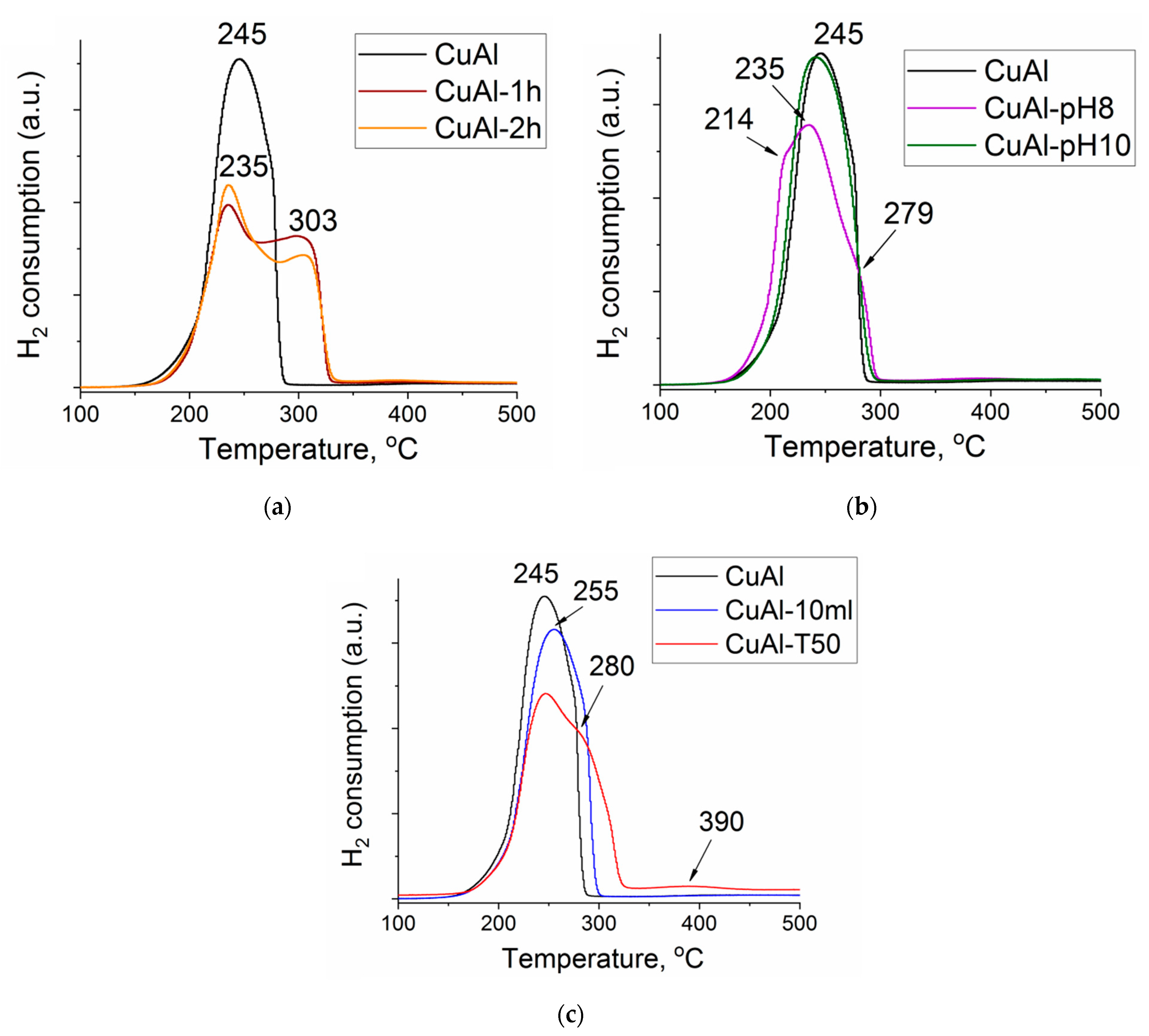

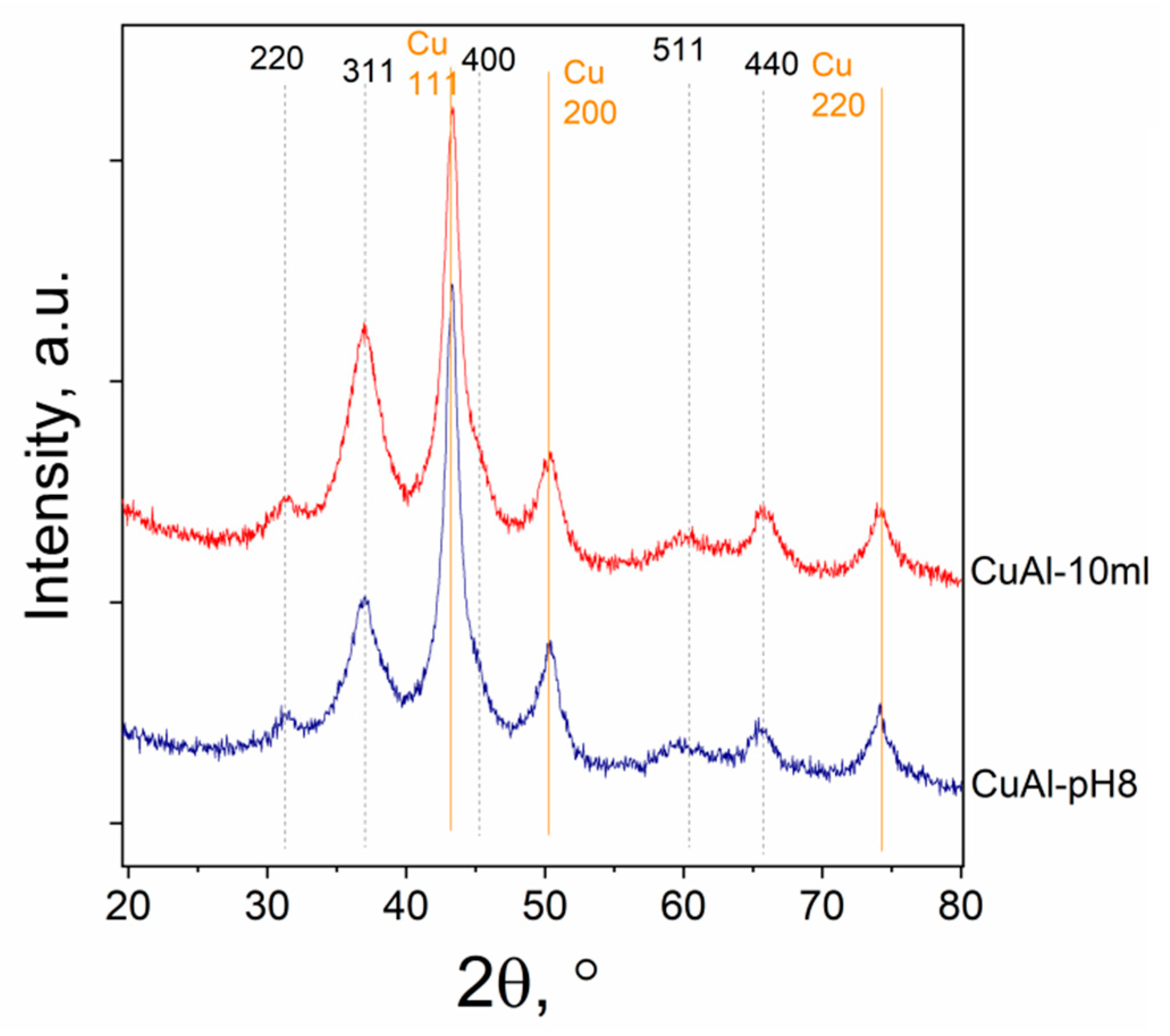
| Samples | Precursor | Synthesis Conditions | Chemical Composition 4, wt.% | ||||
|---|---|---|---|---|---|---|---|
| pH | T, °C 1 | v, mL/min 2 | τ, h 3 | Cu | Al | ||
| CuAl | CuAl-LDH | 9 | 70 | 5 | 4 | 47.2 | 17.3 |
| CuAl-10 mL | CuAl-LDH-10 mL | 9 | 70 | 10 | 4 | 47.7 | 17.4 |
| CuAl-pH8 | CuAl-LDH-pH8 | 8 | 70 | 5 | 4 | 47.3 | 17.2 |
| CuAl pH10 | CuAl-LDH-pH10 | 10 | 70 | 5 | 4 | 46.7 | 17.5 |
| CuAl-T50 | CuAl-LDH-T50 | 9 | 50 | 5 | 4 | 48.1 | 17.3 |
| CuAl-1 h | CuAl-LDH-1 h | 9 | 70 | 5 | 1 | 46.5 | 17.3 |
| CuAl-2 h | CuAl-LDH-2 h | 9 | 70 | 5 | 2 | 46.5 | 17.4 |
| Sample | Phase Composition | CSR, Å | Phase Composition | CSR, Å |
|---|---|---|---|---|
| 110 °C | 650 °C | |||
| CuAl-LDH | Hydrotalcite | 890 | CuO | 70 |
| CuAl-LDH-2 h | 590 | CuO | 70 | |
| CuAl-LDH-1 h | 420 | CuO | 75 | |
| CuAl-LDH-pH8 | 250 | CuO CuAl2O4 | 90 – | |
| CuAl-LDH-pH10 | 250 | CuO | 70 | |
| CuAl-LDH-10 mL | 680 | CuO | 60 | |
| CuAl-LDH-T50 | 130 | CuO CuAl2O4 | 80 – | |
| Sample | H2 Consumption, Mole × 104 | Cu/Al (XPS) | CSR of Cu Phase, Å | ||
|---|---|---|---|---|---|
| TPR Data | Calculated | 300 °C | 120 °C | ||
| CuAl | 7.6 | 7.5 | 0.22 | 0.33 | 70 |
| CuAl-10 mL | 7.5 | 7.5 | 0.22 | 0.35 | 70 |
| CuAl-pH8 | 7.6 | 7.5 | 0.22 | 0.34 | 70 |
| CuAl-pH10 | 7.5 | 7.4 | 0.20 | 0.30 | 75 |
| CuAl-T50 | 7.7 | 7.6 | 0.19 | 0.30 | 85 |
| CuAl-1 h | 7.0 | 7.3 | 0.19 | 0.33 | 80 |
| CuAl-2 h | 7.0 | 7.3 | 0.19 | 0.38 | 65 |
| Entry | Catalyst | Conversion, % | Selectivity, % |
| 1 2 | CuAl | 85.0 | 99 |
| 2 | CuAl | 93.8 | 98 |
| 3 3 | CuAl | 94.0 | 98 |
| 4 | CuAl-pH10 | 88.8 | 98 |
| 5 | CuAl-T50 | 86.8 | 98 |
| 6 | CuAl-pH8 | 97.2 | 98 |
| 7 | CuAl-10 mL | 93.9 | 98 |
| 8 | CuAl-1 h | 87.0 | 98 |
| 9 | CuAl-2 h | 98.3 | 98 |
| 10 4 | CuAl-2 h | >99.9 | 98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukhtiyarova, M.V.; Bulavchenko, O.A.; Bukhtiyarov, A.V.; Nuzhdin, A.L.; Bukhtiyarova, G.A. Selective Hydrogenation of 5-Acetoxymethylfurfural over Cu-Based Catalysts in a Flow Reactor: Effect of Cu-Al Layered Double Hydroxides Synthesis Conditions on Catalytic Properties. Catalysts 2022, 12, 878. https://doi.org/10.3390/catal12080878
Bukhtiyarova MV, Bulavchenko OA, Bukhtiyarov AV, Nuzhdin AL, Bukhtiyarova GA. Selective Hydrogenation of 5-Acetoxymethylfurfural over Cu-Based Catalysts in a Flow Reactor: Effect of Cu-Al Layered Double Hydroxides Synthesis Conditions on Catalytic Properties. Catalysts. 2022; 12(8):878. https://doi.org/10.3390/catal12080878
Chicago/Turabian StyleBukhtiyarova, Marina V., Olga A. Bulavchenko, Andrey V. Bukhtiyarov, Alexey L. Nuzhdin, and Galina A. Bukhtiyarova. 2022. "Selective Hydrogenation of 5-Acetoxymethylfurfural over Cu-Based Catalysts in a Flow Reactor: Effect of Cu-Al Layered Double Hydroxides Synthesis Conditions on Catalytic Properties" Catalysts 12, no. 8: 878. https://doi.org/10.3390/catal12080878







