Chemicals Production from Glycerol through Heterogeneous Catalysis: A Review
Abstract
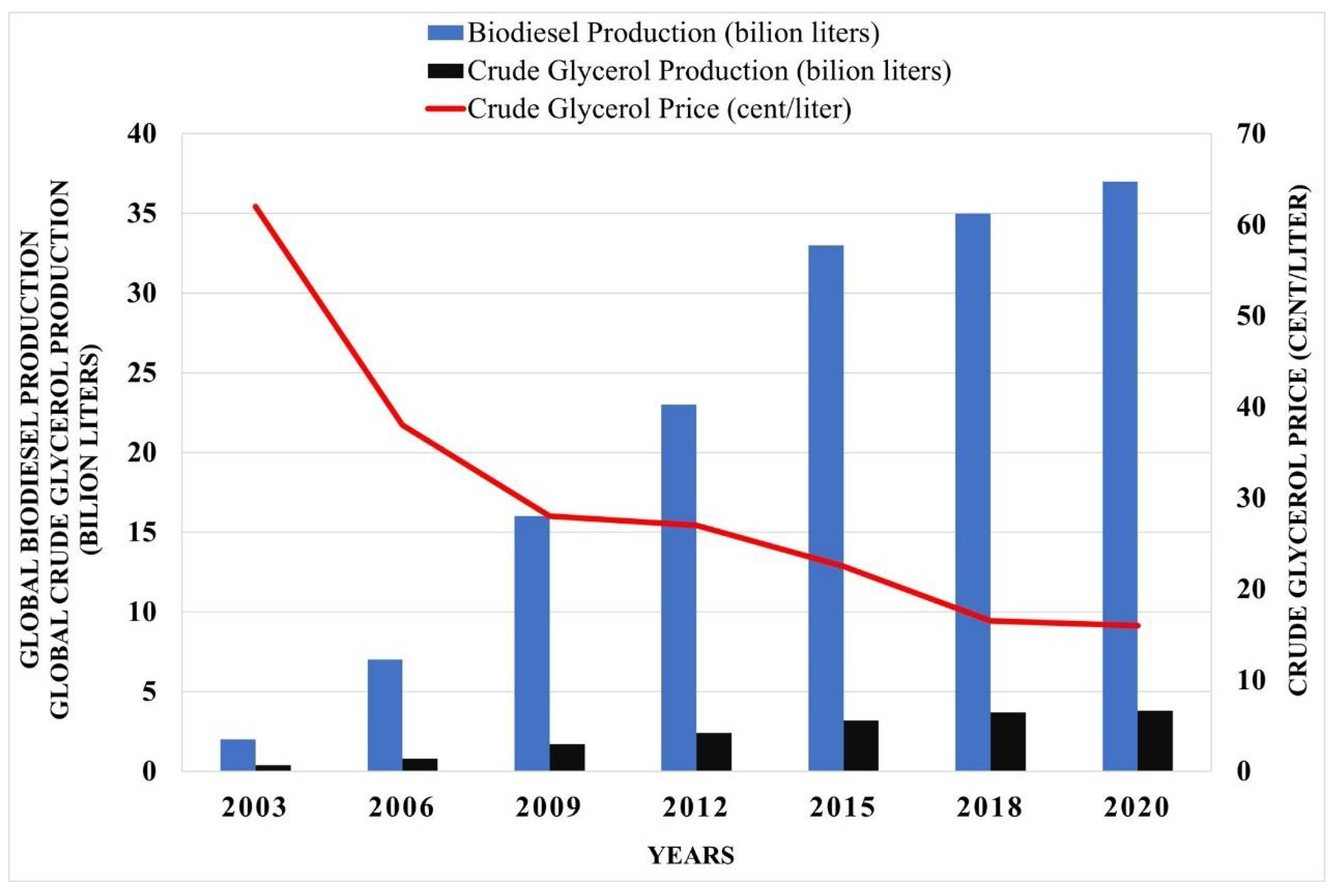
:1. Introduction
2. Glycerol Properties
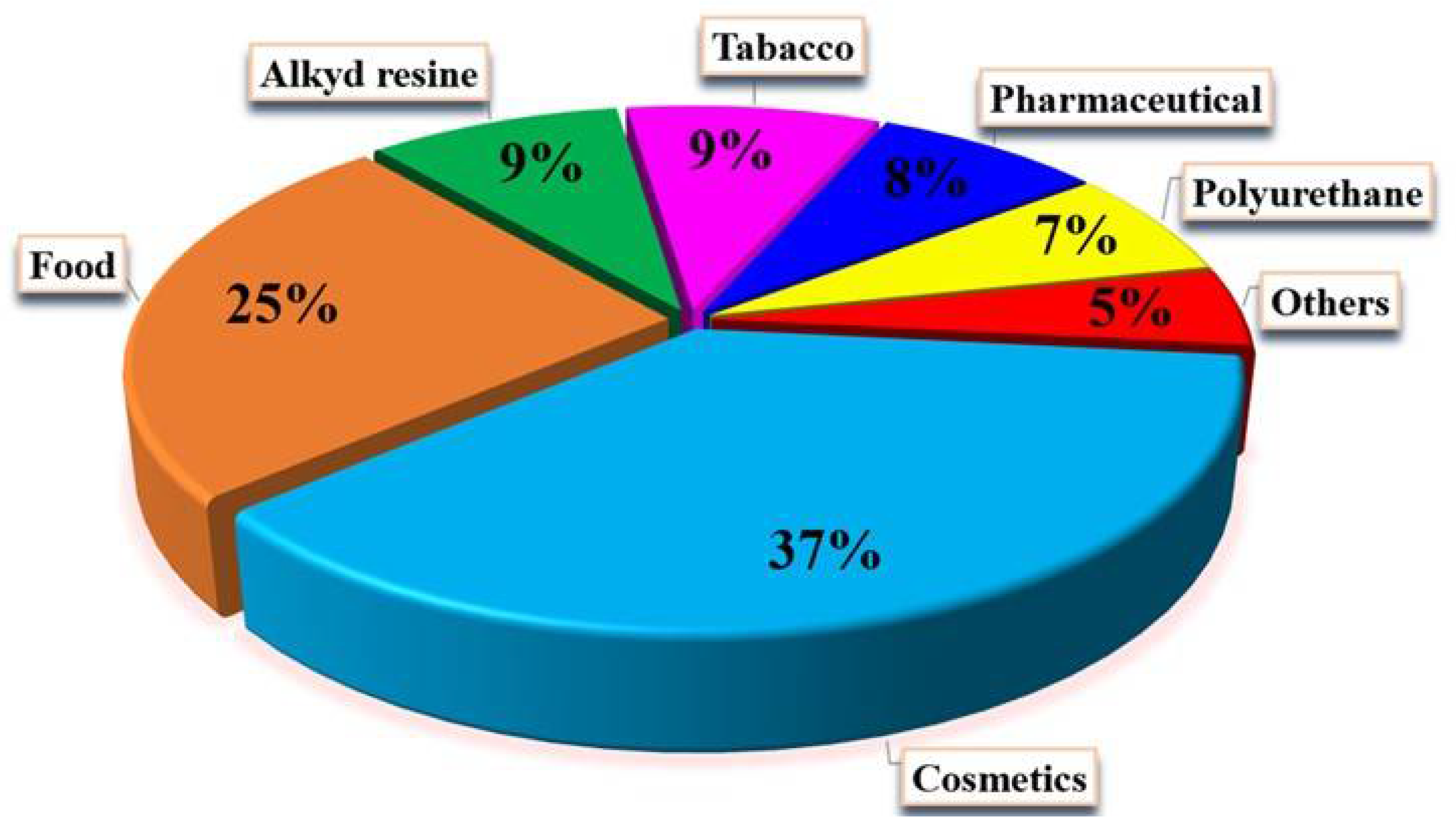
3. Applications of Glycerol
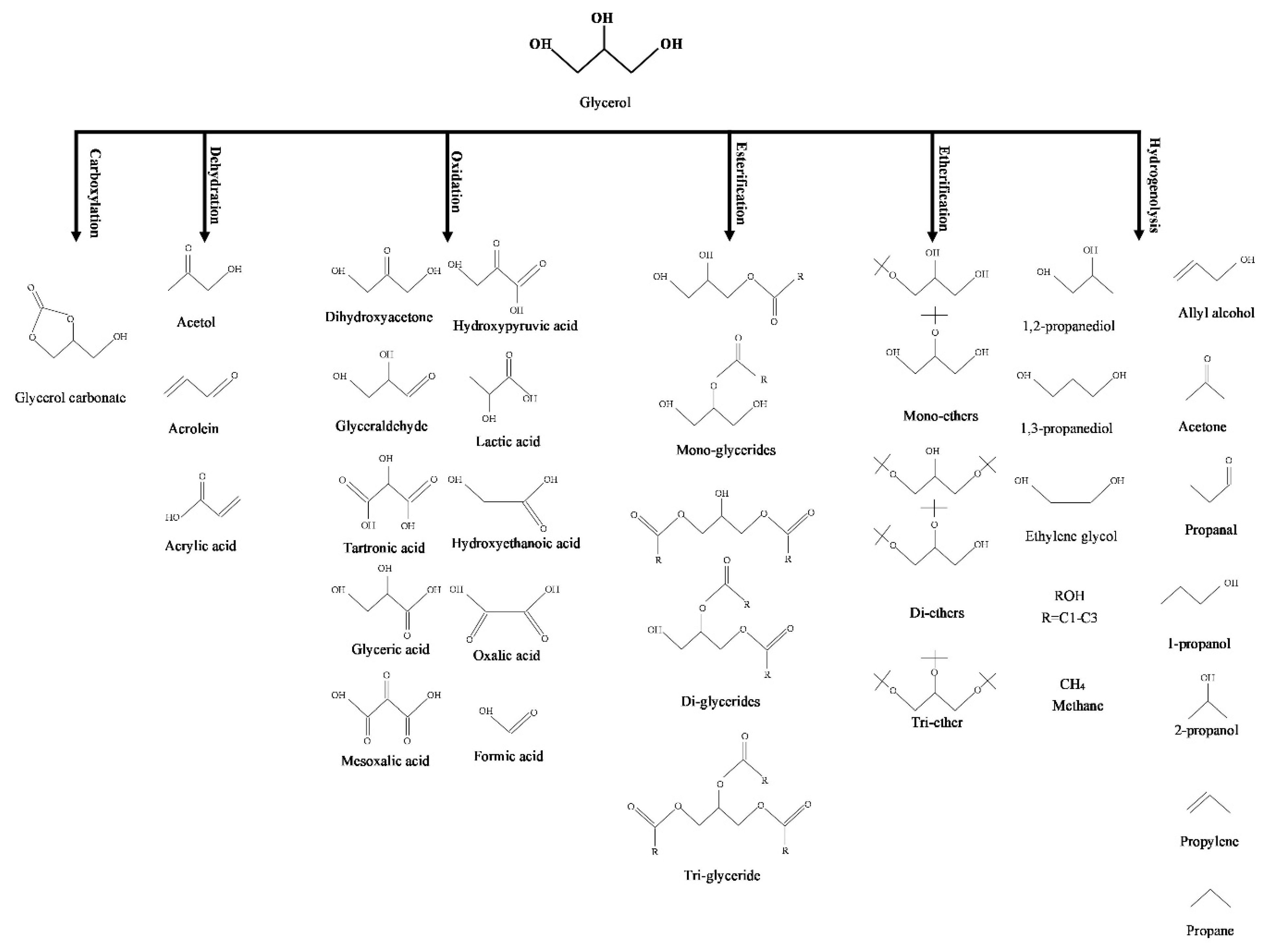
4. Methods of Conversion
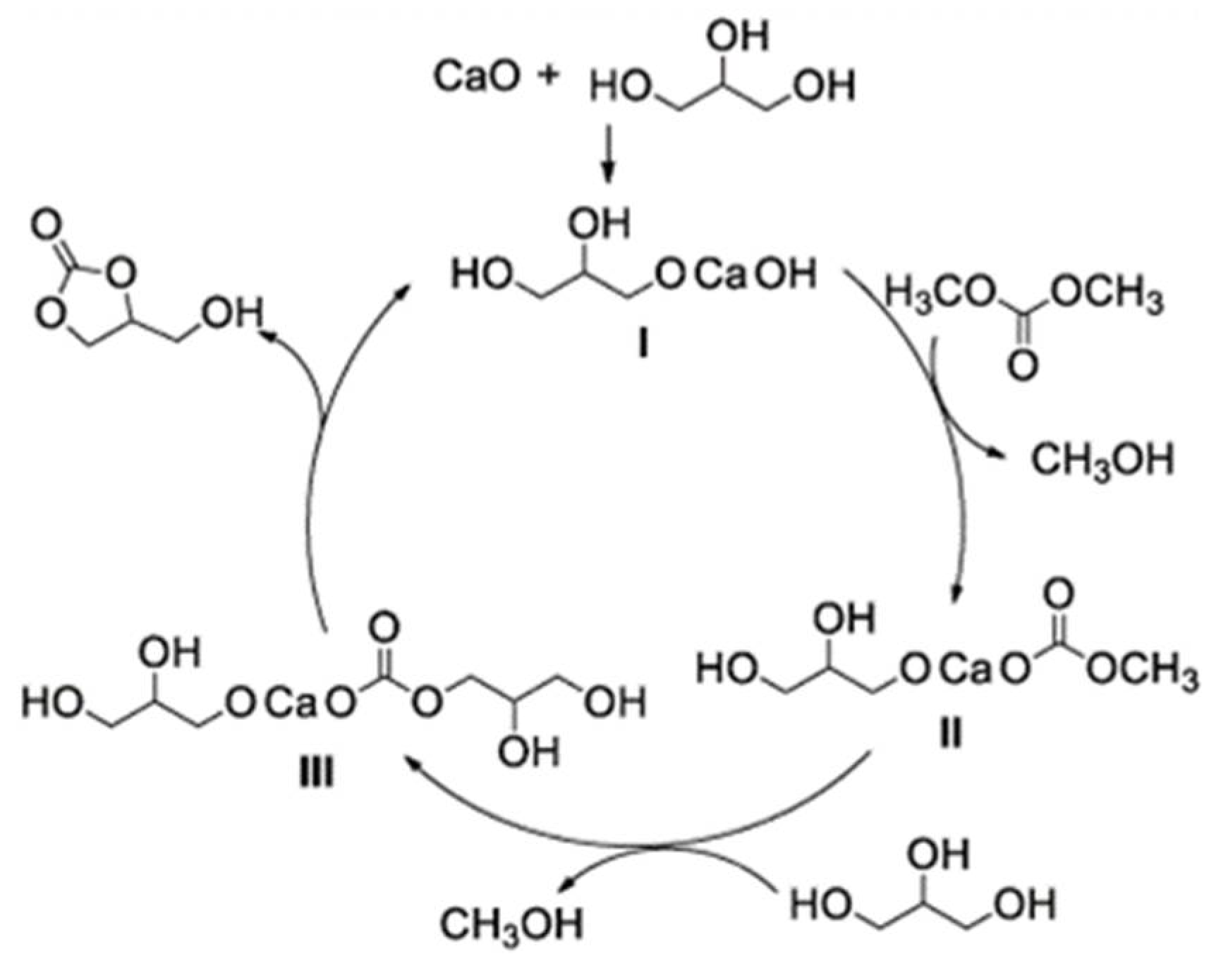
4.1. Carboxylation
4.2. Oxidation
| Catalyst | Molar Ratio | Temp. (°C) | Pressure (MPa) | Reaction Time (min) | Formic Acid Yield (wt%) | Ref. |
|---|---|---|---|---|---|---|
| Glycerol + oxygen | Molar Ratio of O2 to G | |||||
| H4PVMo11O40 | - | 150 | 2.0 | 180 | Around 50 | [104] |
| Ru(OH)4/ Reduced graphite oxide + FeCl3 | Excess O2 | 160 | 0.5 | 60 | Around 60 | [92] |
| Glycerol + hydrogen peroxide | Molar Ratio of H2O2 to G | |||||
| Au-phosphotungstic acid/Silica nanoparticles | 5:1 | 80 | - | 1440 | 26.6 | [106] |
| Aluminum (III) triflate | 10:1 | 70 | - | 720 | 72 | [109] |
| Fe(OTf)2 * | 11.2:1 | 21 | - | 36 | 98 | [108] |
| Fe(OTf)2 + BPA ** | 11.2:1 | 21 | - | 280 | 92 | [108] |
| Fe(OTf)2 + BPA | 2.8:1 | 21 | - | 90 | 6 | [108] |
| FeCl2 | 4.2:1 | 21 | - | 6 | 94 | [108] |
| FeCl3 | 4.2:1 | 21 | - | 6 | 96 | [108] |
| Glycerol + oxone | Molar Ratio of oxone to G | Glycerol conversion | ||||
| NHC–Pd complex | 1:1 | room | - | 360 | 10 | [109] |
4.3. Etherification
4.4. Hydrogenolysis
4.5. Esterification
4.6. Dehydration
5. Industrial Prospects and Barriers
6. Discussion on the Products
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ayoub, M.; Abdullah, A.Z. Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable energy industry. Renew. Sustain. Energy Rev. 2012, 16, 2671–2686. [Google Scholar] [CrossRef]
- Sadhukhan, S.; Sarkar, U. Production of purified glycerol using sequential desalination and extraction of crude glycerol obtained during trans-esterification of Crotalaria juncea oil. Energy Convers. Manag. 2016, 118, 450–458. [Google Scholar] [CrossRef]
- Hunsom, M.; Autthanit, C. Adsorptive purification of crude glycerol by sewage sludge-derived activated carbon prepared by chemical activation with H3PO4, K2CO3 and KOH. Chem. Eng. J. 2013, 229, 334–343. [Google Scholar] [CrossRef]
- Tan, H.W.; Aziz, A.R.A.; Aroua, M.K. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Garlapati, V.K.; Shankar, U.; Budhiraja, A. Bioconversion technologies of crude glycerol to value added industrial products. Biotechnol. Rep. 2016, 9, 9–14. [Google Scholar] [CrossRef]
- Anzar, E.; Yusi, S.; Bow, Y. Purification of crude glycerol from biodiesel by-product by adsorption using bentonite. Indones. J. Fundam. Appl. Chem. 2018, 3, 83–88. [Google Scholar] [CrossRef]
- Yang, F.; Hanna, M.A.; Sun, R. Value-added uses for crude glycerol--a byproduct of biodiesel production. Biotechnol. Biofuels 2012, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Dhabhai, R.; Ahmadifeijani, E.; Dalai, A.K.; Reaney, M. Purification of crude glycerol using a sequential physico-chemical treatment, membrane filtration, and activated charcoal adsorption. Sep. Purif. Technol. 2016, 168, 101–106. [Google Scholar] [CrossRef]
- Pradima, J.; Kulkarni, M.R. Archna Review on enzymatic synthesis of value added products of glycerol, a by-product derived from biodiesel production. Resour. Technol. 2017, 3, 394–405. [Google Scholar] [CrossRef]
- Guerrero-Urbaneja, P.; García-Sancho, C.; Moreno-Tost, R.; Mérida-Robles, J.; Santamaría-González, J.; Jiménez-López, A.; Maireles-Torres, P. Glycerol valorization by etherification to polyglycerols by using metal oxides derived from MgFe hydrotalcites. Appl. Catal. A Gen. 2014, 470, 199–207. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Hussein, R.; Ong, M.Y. Sustainability of biodiesel production in Malaysia by production of bio-oil from crude glycerol using microwave pyrolysis: A review. Green Chem. Lett. Rev. 2018, 11, 135–157. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Praveen Kumar, R.; Elavazhagan, S.; Barathiraja, B.; Jayakumar, M.; Varjani, S.J. Utilization of crude glycerol from biodiesel industry for the production of value-added bioproducts. In Waste to Wealth. Energy, Environment, and Sustainability; Agarwal, R.A., Kumar, R.P., Singhania, R.R., Sukumaran, R.K., Eds.; Springer: Singapore, 2018; pp. 65–82. [Google Scholar]
- Kosamia, N.M.; Samavi, M.; Uprety, B.K.; Rakshit, S.K. Valorization of biodiesel byproduct crude glycerol for the production of bioenergy and biochemicals. Catalysts 2020, 10, 609. [Google Scholar] [CrossRef]
- Fan, X.; Burton, R.; Zhou, Y. Glycerol (byproduct of biodiesel production) as a source for fuels and chemicals–Mini review. Open Fuels Energy Sci. J. 2010, 3, 17–22. [Google Scholar] [CrossRef]
- Naghshbandi, M.P.; Tabatabaei, M.; Aghbashlo, M.; Gupta, V.K.; Sulaiman, A.; Karimi, K.; Moghimi, H.; Maleki, M. Progress toward improving ethanol production through decreased glycerol generation in Saccharomyces cerevisiae by metabolic and genetic engineering approaches. Renew. Sustain. Energy Rev. 2019, 115, 109353. [Google Scholar] [CrossRef]
- Macedo, M.S.; Soria, M.A.; Madeira, L.M. Process intensification for hydrogen production through glycerol steam reforming. Renew. Sustain. Energy Rev. 2021, 146, 111151. [Google Scholar] [CrossRef]
- Goyal, S.; Hernández, N.B.; Cochran, E.W. An update on the future prospects of glycerol polymers. Polym. Int. 2021, 70, 911–917. [Google Scholar] [CrossRef]
- da Silva Ruy, A.D.; de Brito Alves, R.M.; Reis Hewer, T.L.; de Aguiar Pontes, D.; Gomes Teixeira, L.S.; Magalhães Pontes, L.A. Catalysts for glycerol hydrogenolysis to 1,3-propanediol: A review of chemical routes and market. Catal. Today 2020, 381, 243–253. [Google Scholar] [CrossRef]
- Procopio, D.; Di Gioia, M.L. An overview of the latest advances in the catalytic synthesis of glycerol carbonate. Catalysts 2022, 12, 50. [Google Scholar] [CrossRef]
- Restrepo, J.B.; Paternina-Arboleda, C.D.; Bula, A.J. 1, 2—Propanediol production from glycerol derived from biodiesel’s production: Technical and economic study. Energies 2021, 14, 5081. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Bellière-Baca, V.; Rey, P.; Dumeignil, F. Glycerol dehydration to acrolein in the context of new uses of glycerol. Green Chem. 2010, 12, 2079–2098. [Google Scholar] [CrossRef]
- Santacesaria, E.; Tesser, R.; Di Serio, M.; Casale, L.; Verde, D. New process for producing epichlorohydrin via glycerol chlorination. Ind. Eng. Chem. Res. 2010, 49, 964–970. [Google Scholar] [CrossRef]
- Mitrea, L.; Trif, M.; Cătoi, A.-F.; Vodnar, D.-C. Utilization of biodiesel derived-glycerol for 1, 3-PD and citric acid production. Microb. Cell Fact. 2017, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Razali, N.; Abdullah, A.Z. Production of lactic acid from glycerol via chemical conversion using solid catalyst: A review. Appl. Catal. A Gen. 2017, 543, 234–246. [Google Scholar] [CrossRef]
- Okoye, P.U.; Hameed, B.H. Review on recent progress in catalytic carboxylation and acetylation of glycerol as a byproduct of biodiesel production. Renew. Sustain. Energy Rev. 2016, 53, 558–574. [Google Scholar] [CrossRef]
- Kong, P.S.; Aroua, M.K.; Daud, W.M.A.W. Conversion of crude and pure glycerol into derivatives: A feasibility evaluation. Renew. Sustain. Energy Rev. 2016, 63, 533–555. [Google Scholar] [CrossRef]
- Rastegari, H.; Ghaziaskar, H.S. From glycerol as the by-product of biodiesel production to value-added monoacetin by continuous and selective esterification in acetic acid. J. Ind. Eng. Chem. 2015, 21, 856–861. [Google Scholar] [CrossRef]
- Quispe, C.A.G.; Coronado, C.J.R.; Carvalho, J.A., Jr. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Pagliaro, M.; Rossi, M. The Future of Glycerol; Green Chemistry Series; The Royal Society of Chemistry: London, UK, 2008; ISBN 978-0-85404-124-4. [Google Scholar]
- Wang, Z.; Zhuge, J.; Fang, H.; Prior, B.A. Glycerol production by microbial fermentation: A review. Biotechnol. Adv. 2001, 19, 201–223. [Google Scholar] [CrossRef]
- Mah, S.-K.; Leo, C.P.; Wu, T.Y.; Chai, S.-P. A feasibility investigation on ultrafiltration of palm oil and oleic acid removal from glycerin solutions: Flux decline, fouling pattern, rejection and membrane characterizations. J. Memb. Sci. 2012, 389, 245–256. [Google Scholar] [CrossRef]
- Isahak, W.; Jahim, J.M.; Ismail, M.; Nasir, N.; Ba-Abbad, M.; Yarmo, M. Purification of crude glycerol from industrial waste: Experimental and simulation studies. J. Eng. Sci. Technol. 2016, 11, 1056–1072. [Google Scholar]
- Bagheri, S.; Julkapli, N.M.; Yehye, W.A. Catalytic conversion of biodiesel derived raw glycerol to value added products. Renew. Sustain. Energy Rev. 2015, 41, 113–127. [Google Scholar] [CrossRef]
- Bagnato, G.; Iulianelli, A.; Sanna, A.; Basile, A. Glycerol production and transformation: A critical review with particular emphasis on glycerol reforming reaction for producing hydrogen in conventional and membrane reactors. Membranes 2017, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Q.; Yang, W.Z.; Huo, W.J.; Dong, K.H.; Huang, Y.X.; Yang, X.M.; He, D.C. Effects of glycerol on lactation performance, energy balance and metabolites in early lactation Holstein dairy cows. Anim. Feed Sci. Technol. 2009, 151, 12–20. [Google Scholar] [CrossRef]
- Thompson, J.C.; He, B.B. Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl. Eng. Agric. 2006, 22, 261–265. [Google Scholar] [CrossRef]
- Wang, Y.; Furukawa, S.; Song, S.; He, Q.; Asakura, H.; Yan, N. Catalytic production of alanine from waste glycerol. Angew. Chemie Int. Ed. 2020, 59, 2289–2293. [Google Scholar] [CrossRef]
- Sarchami, T.; Munch, G.; Johnson, E.; Kießlich, S.; Rehmann, L. A review of process-design challenges for industrial fermentation of butanol from crude glycerol by non-biphasic clostridium pasteurianum. Fermentation 2016, 2, 13. [Google Scholar] [CrossRef]
- Wolfson, A.; Litvak, G.; Dlugy, C.; Shotland, Y.; Tavor, D. Employing crude glycerol from biodiesel production as an alternative green reaction medium. Ind. Crops Prod. 2009, 30, 78–81. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A.; Wallace, C.W.; Wang, L.; Cheng, D. Enhanced bio-oil production from swine manure co-liquefaction with crude glycerol. Energy Convers. Manag. 2011, 52, 1004–1009. [Google Scholar] [CrossRef]
- Bewley, B.R.; Berkaliev, A.; Henriksen, H.; Ball, D.B.; Ott, L.S. Waste glycerol from biodiesel synthesis as a component in deep eutectic solvents. Fuel Process. Technol. 2015, 138, 419–423. [Google Scholar] [CrossRef]
- Robra, S.; Serpa da Cruz, R.; de Oliveira, A.M.; Neto, J.A.A.; Santos, J.V. Generation of biogas using crude glycerin from biodiesel production as a supplement to cattle slurry. Biomass Bioenergy 2010, 34, 1330–1335. [Google Scholar] [CrossRef]
- Wei, L.; Pordesimo, L.O.; Haryanto, A.; Wooten, J. Co-gasification of hardwood chips and crude glycerol in a pilot scale downdraft gasifier. Bioresour. Technol. 2011, 102, 6266–6272. [Google Scholar] [CrossRef]
- Siles, J.A.; Martín, M.A.; Chica, A.F.; Martín, A. Anaerobic co-digestion of glycerol and wastewater derived from biodiesel manufacturing. Bioresour. Technol. 2010, 101, 6315–6321. [Google Scholar] [CrossRef] [PubMed]
- Fountoulakis, M.S.; Manios, T. Enhanced methane and hydrogen production from municipal solid waste and agro-industrial by-products co-digested with crude glycerol. Bioresour. Technol. 2009, 100, 3043–3047. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Chaurasia, S.P.; Upadhyaya, S.; Agarwal, M.; Sridhar, S. Glycerol purification using membrane technology. In Membrane Processes; Wiley: New York, NY, USA, 2018; pp. 431–463. ISBN 9781119418399. [Google Scholar]
- Anuar, M.R.; Abdullah, A.Z. Challenges in biodiesel industry with regards to feedstock, environmental, social and sustainability issues: A critical review. Renew. Sustain. Energy Rev. 2016, 58, 208–223. [Google Scholar] [CrossRef]
- Morales-delaRosa, S.; Campos-Martin, J.M. Catalytic processes and catalyst development in biorefining. In Advances in Biorefineries; Elsevier: Amsterdam, The Netherlands, 2014; pp. 152–198. [Google Scholar]
- Dalai, A. Purification of Crude Glycerol and Its Conversion to Bio-Chemicals; A Report to Saskatchewan Ministry of Agriculture; SaskCanola: Regina, SK, Canada, 2017. [Google Scholar]
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Maestro-Madurga, B.; Pesquera-Rodríguez, A.; Ramírez-López, C.; Lorenzo-Ibarreta, L.; Torrecilla-Soria, J.; Villarán-Velasco, M.C. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate by transesterification: Catalyst screening and reaction optimization. Appl. Catal. A Gen. 2009, 366, 315–324. [Google Scholar] [CrossRef]
- Hu, J.; Li, J.; Gu, Y.; Guan, Z.; Mo, W.; Ni, Y.; Li, T.; Li, G. Oxidative carbonylation of glycerol to glycerol carbonate catalyzed by PdCl2 (phen)/KI. Appl. Catal. A Gen. 2010, 386, 188–193. [Google Scholar] [CrossRef]
- Mizuno, T.; Nakai, T.; Mihara, M. Facile synthesis of glycerol carbonate from glycerol using selenium-catalyzed carbonylation with carbon monoxide. Heteroat. Chem. 2010, 21, 541–545. [Google Scholar] [CrossRef]
- George, J.; Patel, Y.; Pillai, S.M.; Munshi, P. Methanol assisted selective formation of 1,2-glycerol carbonate from glycerol and carbon dioxide using nBu2SnO as a catalyst. J. Mol. Catal. A Chem. 2009, 304, 1–7. [Google Scholar] [CrossRef]
- Li, H.; Gao, D.; Gao, P.; Wang, F.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. The synthesis of glycerol carbonate from glycerol and CO2 over La2O2CO3–ZnO catalysts. Catal. Sci. Technol. 2013, 3, 2801–2809. [Google Scholar] [CrossRef]
- Zhang, J.; He, D. Surface properties of Cu/La2O3 and its catalytic performance in the synthesis of glycerol carbonate and monoacetin from glycerol and carbon dioxide. J. Colloid Interface Sci. 2014, 419, 31–38. [Google Scholar] [CrossRef]
- Li, H.; Jiao, X.; Li, L.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y.; Zhang, B. Synthesis of glycerol carbonate by direct carbonylation of glycerol with CO2 over solid. Catal. Sci. Technol. 2015, 5, 989–1005. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Zhang, J.; He, D. Glycerol carbonylation with CO2 to glycerol carbonate over CeO2 catalyst and the influence of CeO 2 preparation methods and reaction parameters. Appl. Catal. A Gen. 2016, 513, 9–18. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Liu, H.; He, D. Transformation of CO2 and glycerol to glycerol carbonate over CeO2 e ZrO2 solid solution—Effect of Zr doping. Biomass Bioenergy 2018, 118, 74–83. [Google Scholar] [CrossRef]
- Krisnandi, Y.K.; Eckelt, R.; Atia, H. ZnO/SiO2 Composite as catalyst for the transformation of glycerol to glycerol carbonate. Makara J. Sci. 2020, 24, 6. [Google Scholar] [CrossRef]
- Mallesham, B.; Rangaswamy, A.; Rao, B.G.; Rao, T.V.; Reddy, B.M. Solvent-free production of glycerol carbonate from bioglycerol with urea over nanostructured promoted SnO2 catalysts. Catal. Lett. 2020, 150, 3626–3641. [Google Scholar] [CrossRef]
- Kondawar, S.E.; Potdar, A.S.; Rode, C.V. Solvent-free carbonylation of glycerol with urea using metal loaded MCM-41 catalysts. RSC Adv. 2015, 5, 16452–16460. [Google Scholar] [CrossRef]
- Khayoon, M.S.; Hameed, B.H. Mg1+xCa1−xO2 as reusable and efficient heterogeneous catalyst for the synthesis of glycerol carbonate via the transesterification of glycerol with dimethyl carbonate. Appl. Catal. A Gen. 2013, 466, 272–281. [Google Scholar] [CrossRef]
- Singh, D.; Reddy, B.; Ganesh, A.; Mahajani, S. Zinc/lanthanum mixed-oxide catalyst for the synthesis of glycerol carbonate by transesterification of glycerol. Ind. Eng. Chem. Res. 2014, 53, 18786–18795. [Google Scholar] [CrossRef]
- Zheng, L.; Xia, S.; Hou, Z.; Zhang, M.; Hou, Z. Transesterification of glycerol with dimethyl carbonate over Mg-Al hydrotalcites. Chin. J. Catal. 2014, 35, 310–318. [Google Scholar] [CrossRef]
- Okoye, P.U.; Abdullah, A.Z.; Hameed, B.H. Glycerol carbonate synthesis from glycerol and dimethyl carbonate using trisodium phosphate. J. Taiwan Inst. Chem. Eng. 2016, 68, 51–58. [Google Scholar] [CrossRef]
- Wu, Y.; Song, X.; Cai, F.; Xiao, G. Synthesis of glycerol carbonate from glycerol and diethyl carbonate over Ce-NiO catalyst: The role of multiphase Ni. J. Alloys Compd. 2017, 720, 360–368. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Nocito, F.; Pastore, C. A study on the carboxylation of glycerol to glycerol carbonate with carbon dioxide: The role of the catalyst, solvent and reaction conditions. J. Mol. Catal. A Chem. 2006, 257, 149–153. [Google Scholar] [CrossRef]
- Hasbi Ab Rahim, M.; He, Q.; Lopez-Sanchez, J.A.; Hammond, C.; Dimitratos, N.; Sankar, M.; Carley, A.F.; Kiely, C.J.; Knight, D.W.; Hutchings, G.J. Gold, palladium and gold-palladium supported nanoparticles for the synthesis of glycerol carbonate from glycerol and urea. Catal. Sci. Technol. 2012, 2, 1914–1924. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, W.; Zhao, N.; Wei, W.; Sun, Y. Synthesis of cyclic carbonates from urea and diols over metal oxides. Catal. Today 2006, 115, 111–116. [Google Scholar] [CrossRef]
- Wang, L.; Ma, Y.; Wang, Y.; Liu, S.; Deng, Y. Efficient synthesis of glycerol carbonate from glycerol and urea with lanthanum oxide as a solid base catalyst. Catal. Commun. 2011, 12, 1458–1462. [Google Scholar] [CrossRef]
- Yoo, J.W.; Mouloungui, Z. Catalytic carbonylation of glycerin by urea in the presence of zinc mesoporous system for the synthesis of glycerol carbonate. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2003; Volume 146, pp. 757–760. ISBN 0167-2991. [Google Scholar]
- Jagadeeswaraiah, K.; Kumar, C.R.; Prasad, P.S.S.; Loridant, S.; Lingaiah, N. Synthesis of glycerol carbonate from glycerol and urea over tin-tungsten mixed oxide catalysts. Appl. Catal. A Gen. 2014, 469, 165–172. [Google Scholar] [CrossRef]
- Luo, W.; Sun, L.; Yang, Y.; Chen, Y.; Zhou, Z.; Liu, J.; Wang, F. Cu-Mn composite oxides: Highly efficient and reusable acid-base catalysts for the carbonylation reaction of glycerol with urea. Catal. Sci. Technol. 2018, 8, 6468–6477. [Google Scholar] [CrossRef]
- Manjunathan, P.; Ravishankar, R.; Shanbhag, G.V. Novel bifunctional Zn–Sn composite oxide catalyst for the selective synthesis of glycerol carbonate by carbonylation of glycerol with urea. ChemCatChem 2016, 8, 631–639. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Nocito, F.; Ferragina, C. Valorization of bio-glycerol: New catalytic materials for the synthesis of glycerol carbonate via glycerolysis of urea. J. Catal. 2009, 268, 106–114. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Kang, M.; Yin, N.; Wang, X.; Tan, Y.; Zhu, Y. Structure-activity correlations of LiNO3/Mg4AlO5. 5 catalysts for glycerol carbonate synthesis from glycerol and dimethyl carbonate. J. Ind. Eng. Chem. 2015, 21, 394–399. [Google Scholar] [CrossRef]
- Algoufi, Y.T.; Hameed, B.H. Synthesis of glycerol carbonate by transesterification of glycerol with dimethyl carbonate over K-zeolite derived from coal fly ash. Fuel Process. Technol. 2014, 126, 5–11. [Google Scholar] [CrossRef]
- Simanjuntak, F.S.H.; Kim, T.K.; Lee, S.D.; Ahn, B.S.; Kim, H.S.; Lee, H. CaO-catalyzed synthesis of glycerol carbonate from glycerol and dimethyl carbonate: Isolation and characterization of an active Ca species. Appl. Catal. A Gen. 2011, 401, 220–225. [Google Scholar] [CrossRef]
- Kumar, A.; Iwatani, K.; Nishimura, S.; Takagaki, A.; Ebitani, K. Promotion effect of coexistent hydromagnesite in a highly active solid base hydrotalcite catalyst for transesterifications of glycols into cyclic carbonates. Catal. Today 2012, 185, 241–246. [Google Scholar] [CrossRef]
- Malyaadri, M.; Jagadeeswaraiah, K.; Prasad, P.S.S.; Lingaiah, N. Synthesis of glycerol carbonate by transesterification of glycerol with dimethyl carbonate over Mg/Al/Zr catalysts. Appl. Catal. A Gen. 2011, 401, 153–157. [Google Scholar] [CrossRef]
- Takagaki, A.; Iwatani, K.; Nishimura, S.; Ebitani, K. Synthesis of glycerol carbonate from glycerol and dialkyl carbonates using hydrotalcite as a reusable heterogeneous base catalyst. Green Chem. 2010, 12, 578–581. [Google Scholar] [CrossRef]
- Rokicki, G.; Rakoczy, P.; Parzuchowski, P.; Sobiecki, M. Hyperbranched aliphatic polyethers obtained from environmentally benign monomer: Glycerol carbonate. Green Chem. 2005, 7, 529–539. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Sun, J.; Yang, R.; Dong, W. Ordered mesoporous BaCO3/C-catalyzed synthesis of glycerol carbonate from glycerol and dimethyl carbonate. Sci. China Chem. 2015, 58, 708–715. [Google Scholar] [CrossRef]
- Devi, P.; Das, U.; Dalai, A.K. Production of glycerol carbonate using a novel Ti-SBA-15 catalyst. Chem. Eng. J. 2018, 346, 477–488. [Google Scholar] [CrossRef]
- Pradhan, G.; Chandra Sharma, Y. Studies on green synthesis of glycerol carbonate from waste cooking oil derived glycerol over an economically viable NiMgOx heterogeneous solid base catalyst. J. Clean. Prod. 2020, 264, 121258. [Google Scholar] [CrossRef]
- Nguyen, N.; Demirel, Y. Economic analysis of biodiesel and glycerol carbonate production plant by glycerolysis. J. Sustain. Bioenergy Syst. 2013, 03, 209–216. [Google Scholar] [CrossRef]
- Ji, Y. Recent development of heterogeneous catalysis in the transesterification of glycerol to glycerol carbonate. Catalysts 2019, 9, 581. [Google Scholar] [CrossRef]
- Choi, J.S.; Simanjuntaka, F.S.H.; Oh, J.Y.; Lee, K.I.; Lee, S.D.; Cheong, M.; Kim, H.S.; Lee, H. Ionic-liquid-catalyzed decarboxylation of glycerol carbonate to glycidol. J. Catal. 2013, 297, 248–255. [Google Scholar] [CrossRef]
- Endah, Y.K.; Kim, M.S.; Choi, J.; Jae, J.; Lee, S.D.; Lee, H. Consecutive carbonylation and decarboxylation of glycerol with urea for the synthesis of glycidol via glycerol carbonate. Catal. Today 2017, 293–294, 136–141. [Google Scholar] [CrossRef]
- Bolívar-Diaz, C.L.; Calvino-Casilda, V.; Rubio-Marcos, F.; Fernández, J.F.; Bañares, M.A. New concepts for process intensification in the conversion of glycerol carbonate to glycidol. Appl. Catal. B Environ. 2013, 129, 575–579. [Google Scholar] [CrossRef]
- Coutanceau, C.; Baranton, S.; Kouamé, R.S.B. Selective electrooxidation of glycerol into value-added chemicals: A short overview. Front. Chem. 2019, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, Y.; Xu, H.; Zhang, H.; Yu, B.; Hao, L.; Liu, Z. Selective oxidation of glycerol to formic acid catalyzed by Ru(OH)4/r-GO in the presence of FeCl3. Appl. Catal. B Environ. 2014, 154–155, 267–273. [Google Scholar] [CrossRef]
- Armaroli, N.; Balzani, V. The future of energy supply: Challenges and opportunities. Angew. Chemie Int. Ed. 2007, 46, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Loges, B.; Boddien, A.; Gärtner, F.; Junge, H.; Beller, M. Catalytic generation of hydrogen from formic acid and its derivatives: Useful hydrogen storage materials. Top. Catal. 2010, 53, 902–914. [Google Scholar] [CrossRef]
- Berger, M.E.M.; Assenbaum, D.; Taccardi, N.; Spiecker, E.; Wasserscheid, P. Simple and recyclable ionic liquid based system for the selective decomposition of formic acid to hydrogen and carbon dioxide. Green Chem. 2011, 13, 1411–1415. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Q. Metal-nanoparticle-catalyzed hydrogen generation from formic acid. Acc. Chem. Res. 2017, 50, 1449–1458. [Google Scholar] [CrossRef]
- Song, F.; Zhu, Q.; Yang, X.; Zhan, W.; Pachfule, P.; Tsumori, N.; Xu, Q. Metal–organic framework templated porous carbon-metal oxide/reduced graphene oxide as superior support of bimetallic nanoparticles for efficient hydrogen generation from formic acid. Adv. Energy Mater. 2018, 8, 1701416. [Google Scholar] [CrossRef]
- Nie, W.; Luo, Y.; Yang, Q.; Feng, G.; Yao, Q.; Lu, Z.-H. An amine-functionalized mesoporous silica-supported PdIr catalyst: Boosting room-temperature hydrogen generation from formic acid. Inorg. Chem. Front. 2020, 7, 709–717. [Google Scholar] [CrossRef]
- Liu, J.; Lan, L.; Liu, X.; Yang, X.; Wu, X. Facile synthesis of agglomerated Ag–Pd bimetallic dendrites with performance for hydrogen generation from formic acid. Int. J. Hydrogen Energy 2021, 46, 6395–6403. [Google Scholar] [CrossRef]
- Kosider, A.; Blaumeiser, D.; Schötz, S.; Preuster, P.; Bösmann, A.; Wasserscheid, P.; Libuda, J.; Bauer, T. Enhancing the feasibility of Pd/C-catalyzed formic acid decomposition for hydrogen generation–catalyst pretreatment, deactivation, and regeneration. Catal. Sci. Technol. 2021, 11, 4259–4271. [Google Scholar] [CrossRef]
- Han, X.; Sheng, H.; Yu, C.; Walker, T.W.; Huber, G.W.; Qiu, J.; Jin, S. Electrocatalytic oxidation of glycerol to formic acid by CuCo2O4 Spinel oxide nanostructure catalysts. ACS Catal. 2020, 10, 6741–6752. [Google Scholar] [CrossRef]
- Eppinger, J.; Huang, K.-W. Formic acid as a hydrogen energy carrier. ACS Energy Lett. 2017, 2, 188–195. [Google Scholar] [CrossRef]
- van Putten, R.; Wissink, T.; Swinkels, T.; Pidko, E.A. Fuelling the hydrogen economy: Scale-up of an integrated formic acid-to-power system. Int. J. Hydrogen Energy 2019, 44, 28533–28541. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, M.; Han, Y. Selective oxidation of glycerol to formic acid in highly concentrated aqueous solutions with molecular oxygen using V-substituted phosphomolybdic acids. RSC Adv. 2014, 4, 35463–35466. [Google Scholar] [CrossRef]
- Ntho, T.; Aluha, J.; Gqogqa, P.; Raphulu, M.; Pattrick, G. Au/γ-Al2O3 catalysts for glycerol oxidation: The effect of support acidity and gold particle size. React. Kinet. Mech. Catal. 2013, 109, 133–148. [Google Scholar] [CrossRef]
- Lin, Y.T.; Yang, J.; Mou, C.Y. Highly selective conversion of glycerol to formic acid over a synergistic Au/phosphotungstic acid catalyst under nanoconfinement. ACS Sustain. Chem. Eng. 2021, 9, 3571–3579. [Google Scholar] [CrossRef]
- Kong, K.; Li, D.; Ma, W.; Zhou, Q.; Tang, G.; Hou, Z. Aluminum(III) triflate-catalyzed selective oxidation of glycerol to formic acid with hydrogen peroxide. Chin. J. Catal. 2019, 40, 534–542. [Google Scholar] [CrossRef]
- Farnetti, E.; Crotti, C. Selective oxidation of glycerol to formic acid catalyzed by iron salts. Catal. Commun. 2016, 84, 1–4. [Google Scholar] [CrossRef]
- Pullanikat, P.; Lee, J.H.; Yoo, K.S.; Jung, K.W. Direct conversion of glycerol into formic acid via water stable Pd(II) catalyzed oxidative carbon-carbon bond cleavage. Tetrahedron Lett. 2013, 54, 4463–4466. [Google Scholar] [CrossRef]
- Ruiz, C.P.T.; Dumeignil, F.; Capron, M. Catalytic production of glycolic acid from glycerol oxidation: An optimization using response surface methodology. Catalysts 2021, 11, 257. [Google Scholar] [CrossRef]
- Carrettin, S.; McMorn, P.; Johnston, P.; Griffin, K.; Kiely, C.J.; Hutchings, G.J. Oxidation of glycerol using supported Pt, Pd and Au catalysts. Phys. Chem. Chem. Phys. 2003, 5, 1329–1336. [Google Scholar] [CrossRef]
- Skrzyńska, E.; Zaid, S.; Girardon, J.S.; Capron, M.; Dumeignil, F. Catalytic behaviour of four different supported noble metals in the crude glycerol oxidation. Appl. Catal. A Gen. 2015, 499, 89–100. [Google Scholar] [CrossRef]
- Feng, S.; Yi, J.; Miura, H.; Miura, H.; Miura, H.; Nakatani, N.; Hada, M.; Shishido, T.; Shishido, T.; Shishido, T.; et al. Experimental and theoretical investigation of the role of bismuth in promoting the selective oxidation of glycerol over supported Pt-Bi catalyst under mild conditions. ACS Catal. 2020, 10, 6071–6083. [Google Scholar] [CrossRef]
- Zaid, S.; Skrzyńska, E.; Addad, A.; Nandi, S.; Jalowiecki-Duhamel, L.; Girardon, J.S.; Capron, M.; Dumeignil, F. Development of silver based catalysts promoted by noble metal M (M = Au, Pd or Pt) for glycerol oxidation in liquid phase. Top. Catal. 2017, 60, 1072–1081. [Google Scholar] [CrossRef]
- Liang, D.; Cui, S.; Gao, J.; Wang, J.; Chen, P.; Hou, Z. Glycerol oxidation with oxygen over bimetallic Pt-Bi catalysts under atmospheric pressure. Chin. J. Catal. 2011, 32, 1831–1837. [Google Scholar] [CrossRef]
- Xu, C.; Du, Y.; Li, C.; Yang, J.; Yang, G. Insight into effect of acid/base nature of supports on selectivity of glycerol oxidation over supported Au-Pt bimetallic catalysts. Appl. Catal. B Environ. 2015, 164, 334–343. [Google Scholar] [CrossRef]
- Sobczak, I.; Jagodzinska, K.; Ziolek, M. Glycerol oxidation on gold catalysts supported on group five metal oxides—A comparative study with other metal oxides and carbon based catalysts. Catal. Today 2010, 158, 121–129. [Google Scholar] [CrossRef]
- Augugliaro, V.; El Nazer, H.A.H.; Loddo, V.; Mele, A.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Partial photocatalytic oxidation of glycerol in TiO2 water suspensions. Catal. Today 2010, 151, 21–28. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Rodrigues, E.G.; Delgado, J.J.; Chen, X.; Pereira, M.F.R.; Órfão, J.J.M. Pd, Pt, and Pt-Cu catalysts supported on carbon nanotube (CNT) for the selective oxidation of glycerol in alkaline and base-free conditions. Ind. Eng. Chem. Res. 2016, 55, 8548–8556. [Google Scholar] [CrossRef]
- Ayoub, M.; Wei, W.J.; Ahmad, M.; Mathialagan, R.; Farrukh, S.; Danish, M.; Ullah, S.; Naqvi, S.R. Glycerol conversion to diglycerol via etherification under microwave irradiation. In Apolipoproteins, Triglycerides and Cholesterol; IntechOpen: Norderstedt, Germany, 2020; ISBN 1839625201. [Google Scholar]
- Frusteri, F.; Frusteri, L.; Cannilla, C.; Bonura, G. Catalytic etherification of glycerol to produce biofuels over novel spherical silica supported Hyflon® catalysts. Bioresour. Technol. 2012, 118, 350–358. [Google Scholar] [CrossRef]
- Olutoye, M.A.; Hameed, B.H. KyMg1 − xZn1 + xO3 as a heterogeneous catalyst in the transesterification of palm oil to fatty acid methyl esters. Appl. Catal. A Gen. 2009, 371, 191–198. [Google Scholar] [CrossRef]
- Clacens, J.-M.; Pouilloux, Y.; Barrault, J. Selective etherification of glycerol to polyglycerols over impregnated basic MCM-41 type mesoporous catalysts. Appl. Catal. A Gen. 2002, 227, 181–190. [Google Scholar] [CrossRef]
- Melero, J.A.; Vicente, G.; Paniagua, M.; Morales, G.; Muñoz, P. Etherification of biodiesel-derived glycerol with ethanol for fuel formulation over sulfonic modified catalysts. Bioresour. Technol. 2012, 103, 142–151. [Google Scholar] [CrossRef]
- Miranda, C.; Ramírez, A.; Sachse, A.; Pouilloux, Y.; Urresta, J.; Pinard, L. Sulfonated graphenes: Efficient solid acid catalyst for the glycerol valorization. Appl. Catal. A Gen. 2019, 580, 167–177. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Guo, X.; Mao, J.; Zhang, S. Etherification of glycerol with isobutene on sulfonated graphene: Reaction and separation. Green Chem. 2014, 16, 4669–4679. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Julio, A.A.; Ferreira, S.O.; Da Silva, R.C.; Chaves, D.M. Tin(II) phosphotungstate heteropoly salt: An efficient solid catalyst to synthesize bioadditives ethers from glycerol. Fuel 2019, 254, 115607. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Chaves, D.M.; Júlio, A.A.; Rodrigues, F.A.; Bruziquesi, C.G.O. Sn(II)-exchanged keggin silicotungstic acid-catalyzed etherification of glycerol and ethylene glycol with alkyl alcohols. Ind. Eng. Chem. Res. 2020, 59, 9858–9868. [Google Scholar] [CrossRef]
- da Silva, M.J.; Chaves, D.M.; ferreira, S.O.; da Silva, R.C.; Gabriel Filho, J.B.; Bruziquesi, C.G.O.; Al-Rabiah, A.A. Impacts of Sn(II) doping on the Keggin heteropolyacid-catalyzed etherification of glycerol with tert-butyl alcohol. Chem. Eng. Sci. 2022, 247, 116913. [Google Scholar] [CrossRef]
- Magar, S.; Kamble, S.; Mohanraj, G.T.; Jana, S.K.; Rode, C. Solid-acid-catalyzed etherification of glycerol to potential fuel additives. Energy Fuels 2017, 31, 1227–1277. [Google Scholar] [CrossRef]
- Pinto, B.P.; De Lyra, J.T.; Nascimento, J.A.C.; Mota, C.J.A. Ethers of glycerol and ethanol as bioadditives for biodiesel. Fuel 2016, 168, 76–80. [Google Scholar] [CrossRef]
- Veiga, P.M.; Gomes, A.C.L.; Veloso, C.O.; Henriques, C.A. Acid zeolites for glycerol etherification with ethyl alcohol: Catalytic activity and catalyst properties. Appl. Catal. A Gen. 2017, 548, 2–15. [Google Scholar] [CrossRef]
- Pariente, S.; Tanchoux, N.; Fajula, F. Etherification of glycerol with ethanol over solid acid catalysts. Green Chem. 2009, 11, 1256–1261. [Google Scholar] [CrossRef]
- Gholami, Z.; Abdullah, A.Z.; Lee, K.-T. Dealing with the surplus of glycerol production from biodiesel industry through catalytic upgrading to polyglycerols and other value-added products. Renew. Sustain. Energy Rev. 2014, 39, 327–341. [Google Scholar] [CrossRef]
- Chong, C.C.; Aqsha, A.; Ayoub, M.; Sajid, M.; Abdullah, A.Z.; Yusup, S.; Abdullah, B. A review over the role of catalysts for selective short-chain polyglycerol production from biodiesel derived waste glycerol. Environ. Technol. Innov. 2020, 19, 100859. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Y.; Xiao, G. Sustainable value-added C3 chemicals from glycerol transformations: A mini review for heterogeneous catalytic processes. Chin. J. Chem. Eng. 2019, 27, 1536–1542. [Google Scholar] [CrossRef]
- Martin, A.; Armbruster, U.; Gandarias, I.; Arias, P.L. Glycerol hydrogenolysis into propanediols using in situ generated hydrogen—A critical review. Eur. J. Lipid Sci. Technol. 2013, 115, 9–27. [Google Scholar] [CrossRef]
- Zhao, H.; Zheng, L.; Li, X.; Chen, P.; Hou, Z. Hydrogenolysis of glycerol to 1,2-propanediol over Cu-based catalysts: A short review. Catal. Today 2020, 355, 84–95. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Guo, X. Catalytic hydrogenolysis of glycerol to propanediols: A review. RSC Adv. 2015, 5, 74611–74628. [Google Scholar] [CrossRef]
- Shan, J.; Liu, H.; Lu, K.; Zhu, S.; Li, J.; Wang, J.; Fan, W. Identification of the dehydration active sites in glycerol hydrogenolysis to 1,2-propanediol over Cu/SiO2 catalysts. J. Catal. 2020, 383, 13–23. [Google Scholar] [CrossRef]
- de Andrade, T.S.; Souza, M.M.V.M.; Manfro, R.L. Hydrogenolysis of glycerol to 1,2-propanediol without external H2 addition in alkaline medium using Ni-Cu catalysts supported on Y zeolite. Renew. Energy 2020, 160, 919–930. [Google Scholar] [CrossRef]
- Pandey, D.K.; Biswas, P. Continuous production of propylene glycol (1,2-propanediol) by the hydrogenolysis of glycerol over a bi-functional Cu-Ru/MgO catalyst. React. Chem. Eng. 2020, 5, 2221–2235. [Google Scholar] [CrossRef]
- Sharma, R.V.; Kumar, P.; Dalai, A.K. Selective hydrogenolysis of glycerol to propylene glycol by using Cu:Zn:Cr:Zr mixed metal oxides catalyst. Appl. Catal. A Gen. 2014, 477, 147–156. [Google Scholar] [CrossRef]
- Gatti, M.N.; Cerioni, J.L.; Pompeo, F.; Santori, G.F.; Nichio, N.N. High yield to 1-propanol from crude glycerol using two reaction steps with ni catalysts. Catalysts 2020, 10, 615. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Guo, X.; Mao, J.; Zhang, S. Ag/Al2O3 for glycerol hydrogenolysis to 1,2-propanediol: Activity, selectivity and deactivation. Green Chem. 2012, 14, 156–163. [Google Scholar] [CrossRef]
- Gandarias, I.; Arias, P.L.; Requies, J.; Güemez, M.B.; Fierro, J.L.G. Hydrogenolysis of glycerol to propanediols over a Pt/ASA catalyst: The role of acid and metal sites on product selectivity and the reaction mechanism. Appl. Catal. B Environ. 2010, 97, 248–256. [Google Scholar] [CrossRef]
- Feng, J.; Xiong, W.; Xu, B.; Jiang, W.; Wang, J.; Chen, H. Basic oxide-supported Ru catalysts for liquid phase glycerol hydrogenolysis in an additive-free system. Catal. Commun. 2014, 46, 98–102. [Google Scholar] [CrossRef]
- Balaraju, M.; Rekha, V.; Prasad, P.S.S.; Prasad, R.B.N.; Lingaiah, N. Selective hydrogenolysis of glycerol to 1,2 propanediol over Cu–ZnO catalysts. Catal. Lett. 2008, 126, 119–124. [Google Scholar] [CrossRef]
- Hirunsit, P.; Luadthong, C.; Faungnawakij, K. Effect of alumina hydroxylation on glycerol hydrogenolysis to 1,2-propanediol over Cu/Al2O3: Combined experiment and DFT investigation. RSC Adv. 2015, 5, 11188–11197. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, J.; Wang, L.; Xie, W.; Chen, P.; Hou, Z.; Zheng, X. Biodiesel derived glycerol hydrogenolysis to 1,2-propanediol on Cu/MgO catalysts. Bioresour. Technol. 2010, 101, 7088–7092. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wang, L.; Wang, J.; Xia, S.; Chen, P.; Hou, Z.; Zheng, X. Hydrogenolysis of glycerol over homogenously dispersed copper on solid base catalysts. Appl. Catal. B Environ. 2011, 101, 431–440. [Google Scholar] [CrossRef]
- Xia, S.; Yuan, Z.; Wang, L.; Chen, P.; Hou, Z. Hydrogenolysis of glycerol on bimetallic Pd-Cu/solid-base catalysts prepared via layered double hydroxides precursors. Appl. Catal. A Gen. 2011, 403, 173–182. [Google Scholar] [CrossRef]
- Liu, S.; Tamura, M.; Shen, Z.; Zhang, Y.; Nakagawa, Y.; Tomishige, K. Hydrogenolysis of glycerol with in-situ produced H2 by aqueous-phase reforming of glycerol using Pt-modified Ir-ReOx/SiO2 catalyst. Catal. Today 2018, 303, 106–116. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Shinmi, Y.; Koso, S.; Tomishige, K. Direct hydrogenolysis of glycerol into 1,3-propanediol over rhenium-modified iridium catalyst. J. Catal. 2010, 272, 191–194. [Google Scholar] [CrossRef]
- Kurosaka, T.; Maruyama, H.; Naribayashi, I.; Sasaki, Y. Production of 1,3-propanediol by hydrogenolysis of glycerol catalyzed by Pt/WO3/ZrO2. Catal. Commun. 2008, 9, 1360–1363. [Google Scholar] [CrossRef]
- Gong, L.; Lu, Y.; Ding, Y.; Lin, R.; Li, J.; Dong, W.; Wang, T.; Chen, W. Selective hydrogenolysis of glycerol to 1,3-propanediol over a Pt/WO3/TiO2/SiO2 catalyst in aqueous media. Appl. Catal. A Gen. 2010, 390, 119–126. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.-C.; Wang, Y.; Zhou, L.; Zhang, J.; Wang, J.; Wang, A.; Zhang, T. Mesoporous Ti–W oxide: Synthesis, characterization, and performance in selective hydrogenolysis of glycerol. J. Mater. Chem. A 2013, 1, 3724–3732. [Google Scholar] [CrossRef]
- Yu, L.; Yuan, J.; Zhang, Q.; Liu, Y.-M.; He, H.-Y.; Fan, K.-N.; Cao, Y. Propylene from renewable resources: Catalytic conversion of glycerol into propylene. ChemSusChem 2014, 7, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Lei, N.; Zhao, X.; Hou, B.; Yang, M.; Zhou, M.; Liu, F.; Wang, A.; Zhang, T. Effective hydrogenolysis of glycerol to 1,3-propanediol over metal-acid concerted Pt/WOx/Al2O3 catalysts. ChemCatChem 2019, 11, 3903–3912. [Google Scholar] [CrossRef]
- Liu, L.; Asano, T.; Nakagawa, Y.; Tamura, M.; Okumura, K.; Tomishige, K. Selective hydrogenolysis of glycerol to 1,3-propanediol over rhenium-oxide-modified iridium nanoparticles coating rutile titania support. ACS Catal. 2019, 9, 10913–10930. [Google Scholar] [CrossRef]
- Gandarias, I.; Requies, J.; Arias, P.L.; Armbruster, U.; Martin, A. Liquid-phase glycerol hydrogenolysis by formic acid over Ni–Cu/Al2O3 catalysts. J. Catal. 2012, 290, 79–89. [Google Scholar] [CrossRef]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol hydrogenolysis into useful C3 chemicals. Appl. Catal. B Environ. 2016, 193, 75–92. [Google Scholar] [CrossRef]
- Arceo, E.; Marsden, P.; Bergman, R.G.; Ellman, J.A. An efficient didehydroxylation method for the biomass-derived polyols glycerol and erythritol. Mechanistic studies of a formic acid-mediated deoxygenation. Chem. Commun. 2009, 23, 3357–3359. [Google Scholar]
- Mota, C.J.A.; Gonçalves, V.L.C.; Mellizo, J.E.; Rocco, A.M.; Fadigas, J.C.; Gambetta, R. Green propene through the selective hydrogenolysis of glycerol over supported iron-molybdenum catalyst: The original history. J. Mol. Catal. A Chem. 2016, 422, 158–164. [Google Scholar] [CrossRef]
- Singh, D.; Patidar, P.; Ganesh, A.; Mahajani, S. Esterification of oleic acid with glycerol in the presence of supported zinc oxide as catalyst. Ind. Eng. Chem. Res. 2013, 52, 14776–14786. [Google Scholar] [CrossRef]
- Mostafa, N.A.; Maher, A.; Abdelmoez, W. Production of mono-, di-, and triglycerides from waste fatty acids through esterification with glycerol. Adv. Biosci. Biotechnol. 2013, 04, 900–907. [Google Scholar] [CrossRef]
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Renew. Sustain. Energy Rev. 2010, 14, 987–1000. [Google Scholar] [CrossRef]
- Sakthivel, A.; Nakamura, R.; Komura, K.; Sugi, Y. Esterification of glycerol by lauric acid over aluminium and zirconium containing mesoporous molecular sieves in supercritical carbon dioxide medium. J. Supercrit. Fluids 2007, 42, 219–225. [Google Scholar] [CrossRef]
- Kong, P.S.; Aroua, M.K.; Wan Daud, W.M.A. Catalytic esterification of bioglycerol to value-added products. Rev. Chem. Eng. 2015, 31, 437–451. [Google Scholar] [CrossRef]
- Corma, A.; Abd Hamid, S.B.; Iborra, S.; Velty, A. Lewis and Brönsted basic active sites on solid catalysts and their role in the synthesis of monoglycerides. J. Catal. 2005, 234, 340–347. [Google Scholar] [CrossRef]
- Okoye, P.U.; Abdullah, A.Z.; Hameed, B.H. Synthesis of oxygenated fuel additives via glycerol esterification with acetic acid over bio-derived carbon catalyst. Fuel 2017, 209, 538–544. [Google Scholar] [CrossRef]
- Hamerski, F.; Prado, M.A.; da Silva, V.R.; Voll, F.A.P.; Corazza, M.L. Kinetics of layered double hydroxide catalyzed esterification of fatty acids with glycerol. React. Kinet. Mech. Catal. 2016, 117, 253–268. [Google Scholar] [CrossRef]
- Hamerski, F.; Corazza, M.L. LDH-catalyzed esterification of lauric acid with glycerol in solvent-free system. Appl. Catal. A Gen. 2014, 475, 242–248. [Google Scholar] [CrossRef]
- Hoo, P.-Y.; Abdullah, A.Z. Direct synthesis of mesoporous 12-tungstophosphoric acid SBA-15 catalyst for selective esterification of glycerol and lauric acid to monolaurate. Chem. Eng. J. 2014, 250, 274–287. [Google Scholar] [CrossRef]
- Wee, L.H.; Lescouet, T.; Fritsch, J.; Bonino, F.; Rose, M.; Sui, Z.; Garrier, E.; Packet, D.; Bordiga, S.; Kaskel, S. Synthesis of monoglycerides by esterification of oleic acid with glycerol in heterogeneous catalytic process using tin–organic framework catalyst. Catal. Lett. 2013, 143, 356–363. [Google Scholar] [CrossRef]
- Bombos, D.; Bombos, M.; Bolocan, I.; Vasilievici, G.; Zaharia, E. Esterification of glycerol with technical olein in heterogeneous catalysis. Rev. Chim. 2010, 61, 784. [Google Scholar]
- Singh, D.; Bhoi, R.; Ganesh, A.; Mahajani, S. Synthesis of biodiesel from vegetable oil using supported metal oxide catalysts. Energy fuels 2014, 28, 2743–2753. [Google Scholar] [CrossRef]
- Li, Z.; Miao, Z.; Wang, X.; Zhao, J.; Zhou, J.; Si, W.; Zhuo, S. One-pot synthesis of ZrMo-KIT-6 solid acid catalyst for solvent-free conversion of glycerol to solketal. Fuel 2018, 233, 377–387. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Ab Rahim, M.H.; Alqahtani, T.M.; Witoon, T.; Lim, J.W.; Cheng, C.K. A review on advances in green treatment of glycerol waste with a focus on electro-oxidation pathway. Chemosphere 2021, 276, 130128. [Google Scholar] [CrossRef] [PubMed]
- Mane, R.B.; Yamaguchi, A.; Malawadkar, A.; Shirai, M.; Rode, C.V. Active sites in modified copper catalysts for selective liquid phase dehydration of aqueous glycerol to acetol. RSC Adv. 2013, 3, 16499–16508. [Google Scholar] [CrossRef]
- Rosas, I.P.; Contreras, J.L.; Salmones, J.; Tapia, C.; Zeifert, B.; Navarrete, J.; Vázquez, T.; García, D.C. Catalytic dehydration of glycerol to acrolein over a catalyst of Pd/LaY zeolite and comparison with the chemical equilibrium. Catalysts 2017, 7, 73. [Google Scholar] [CrossRef]
- Stošić, D.; Bennici, S.; Sirotin, S.; Calais, C.; Couturier, J.L.; Dubois, J.L.; Travert, A.; Auroux, A. Glycerol dehydration over calcium phosphate catalysts: Effect of acidic-basic features on catalytic performance. Appl. Catal. A Gen. 2012, 447–448, 124–134. [Google Scholar] [CrossRef]
- García-Sancho, C.; Cecilia, J.A.; Mérida-Robles, J.M.; Santamaría González, J.; Moreno-Tost, R.; Infantes-Molina, A.; Maireles-Torres, P. Effect of the treatment with H3PO4 on the catalytic activity of Nb2O5 supported on Zr-doped mesoporous silica catalyst. Case study: Glycerol dehydration. Appl. Catal. B Environ. 2018, 221, 158–168. [Google Scholar] [CrossRef]
- Suprun, W.; Lutecki, M.; Haber, T.; Papp, H. Acidic catalysts for the dehydration of glycerol: Activity and deactivation. J. Mol. Catal. A Chem. 2009, 309, 71–78. [Google Scholar] [CrossRef]
- Xie, Q.; Li, S.; Gong, R.; Zheng, G.; Wang, Y.; Xu, P.; Duan, Y.; Yu, S.; Lu, M.; Ji, W. Microwave-assisted catalytic dehydration of glycerol for sustainable production of acrolein over a microwave absorbing catalyst. Appl. Catal. B Environ. 2019, 243, 455–462. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L. Mesoporous silica supported phosphotungstic acid catalyst for glycerol dehydration to acrolein. Catal. Today 2021, 376, 55–64. [Google Scholar] [CrossRef]
- Shan, J.; Li, Z.; Zhu, S.; Liu, H.; Li, J.; Wang, J.; Fan, W. Nanosheet MFI Zeolites for gas phase glycerol dehydration to acrolein. Catalysts 2019, 9, 121. [Google Scholar] [CrossRef]
- Basu, S.; Shree, V.; Sen, A.K. Role of cerium as a promoter and process optimization studies for dehydration of glycerol to acetol over copper chromite catalyst. J. Rare Earths 2022, 40, 63–72. [Google Scholar] [CrossRef]
- Mazarío, J.; Concepción, P.; Ventura, M.; Domine, M.E. Continuous catalytic process for the selective dehydration of glycerol over Cu-based mixed oxide. J. Catal. 2020, 385, 160–175. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Hezaveh, H. Glycerol for renewable acrolein production by catalytic dehydration. Renew. Sustain. Energy Rev. 2014, 40, 28–59. [Google Scholar] [CrossRef]
- Pathak, K.; Reddy, K.M.; Bakhshi, N.N.; Dalai, A.K. Catalytic conversion of glycerol to value added liquid products. Appl. Catal. A Gen. 2010, 372, 224–238. [Google Scholar] [CrossRef]
- Kaur, J.; Sarma, A.K.; Jha, M.K.; Gera, P. Valorisation of crude glycerol to value-added products: Perspectives of process technology, economics and environmental issues. Biotechnol. Rep. 2020, 27, e00487. [Google Scholar] [CrossRef]
- Singhabhandhu, A.; Tezuka, T. A perspective on incorporation of glycerin purification process in biodiesel plants using waste cooking oil as feedstock. Energy 2010, 35, 2493–2504. [Google Scholar] [CrossRef]
- Chol, C.G.; Dhabhai, R.; Dalai, A.K.; Reaney, M. Purification of crude glycerol derived from biodiesel production process: Experimental studies and techno-economic analyses. Fuel Process. Technol. 2018, 178, 78–87. [Google Scholar] [CrossRef]
- Liao, X.; Zhu, Y.; Wang, S.-G.; Chen, H.; Li, Y. Theoretical elucidation of acetylating glycerol with acetic acid and acetic anhydride. Appl. Catal. B Environ. 2010, 94, 64–70. [Google Scholar] [CrossRef]
- Chiu, C.-W.; Dasari, M.A.; Sutterlin, W.R.; Suppes, G.J. Removal of Residual catalyst from simulated biodiesel’s crude glycerol for glycerol hydrogenolysis to propylene glycol. Ind. Eng. Chem. Res. 2006, 45, 791–795. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, L.; Ye, X.P. Acrolein Production from Crude Glycerol in Sub- and Super-Critical Water. J. Am. Oil Chem. Soc. 2013, 90, 601–610. [Google Scholar] [CrossRef]
- Skrzyńska, E.; Wondołowska-Grabowska, A.; Capron, M.; Dumeignil, F. Crude glycerol as a raw material for the liquid phase oxidation reaction. Appl. Catal. A Gen. 2014, 482, 245–257. [Google Scholar] [CrossRef]
| Catalyst | Molar Ratio | Solvent | Temp. (°C) | Pressure (MPa) | Reaction Time (h) | GC Yield (wt%) | Ref. |
|---|---|---|---|---|---|---|---|
| Glycerol + CO2 | Molar Ratio G to CO2 | ||||||
| 1 mol% n-Bu2SnO | Excess CO2 | MeOH | 80 | 3.5 | 4 | 35 | [53] |
| Bu2SnO | _ | Methanol/Zeolite | 120 | 13.8 | 4 | 35 | [53] |
| 6 mol% n-Bu2Sn(OMe)2 | Excess CO2 | free | 180 | 5 | 15 | 7 | [67] |
| Zn/Al/La/M (M = Li, Mg, Zr) | Zn:La:Al = 4:1:1 | free | 170 | 4 | 12 | 15.1 | [56] |
| CeO2 | Excess CO2 | DMF | 150 | 4 | 5 | 78.9 | [57] |
| Cu/La2O3 | Excess CO2 | Acetonitrile | 150 | 7 | 12 | 15.2 | [55] |
| La2O2CO3/ZnO | La:Zn = 1:4 | Acetonitrile | 170 | 4 | 12 | 14.3 | [54] |
| Zr-Ce Oxide | Excess CO2 | DMF | 150 | 3 | 5 | 36.3 | [58] |
| Glycerol + urea | Molar Ratio G to Urea | ||||||
| ZnO | 1:1 | free | 140 | - | 6 | 57.86 | [59] |
| ZnO/SiO2 | 1:1 | free | 140 | - | 6 | 64.3 | [59] |
| MoO3 + SnO2 | 1:3 | free | 150 | - | 4 | 67 | [60] |
| Au/Fe2O3 | 1:1.5 | free | 150 | - | 4 | 38 | [68] |
| Co3O4/ZnO | 1:3 | free | 145 | - | 4 | 60 | [60] |
| Zn/MCM-41 | 1:1 | free | 145 | - | 3 | 73 | [61] |
| Ni/MCM-41 | 1:1 | free | 145 | - | 3 | 53 | [61] |
| Cu/MCM-41 | 1:1 | free | 145 | - | 3 | 45 | [61] |
| CaO, La2O3, MgO, ZrO2, Al2O3 | 3:2 | free | 150 | - | 3 | 28–93 | [69] |
| 0.5 wt% calcined La2O3 | 3:1 | free | 140 | - | 1 | 91 | [70] |
| manganese sulfate | 1:1 | free | 150 | - | 2 | 61 | [71] |
| Zinc sulfate | 1:1 | free | 140 | - | 2 | 86 | [71] |
| Sn/W mixed oxide | 2:1 | free | 140 | - | 4 | 49.7 | [72] |
| 1wt% Au/MgO | 1:1.5 | free | 110 | - | 4 | 46 | [68] |
| 1wt% Pd/MgO | 1:1.5 | free | 110 | - | 4 | 62 | [68] |
| 1wt% Au-Pd/MgO | 1:1.5 | free | 110 | - | 4 | 67 | [68] |
| Cu-Mn | 1:1.5 | free | 140 | - | 6 | 90.2 | [73] |
| Zn-Sn | 1:1 | free | 155 | - | 4 | 97.6 | [74] |
| Zr-Phosfate | 1:1 | free | 145 | - | 3 | 80 | [75] |
| Glycerol + dimethyl carbonate | Molar Ratio G to DMC | ||||||
| CaO | 1:3.5 | free | 95 | 1.5 | 95 | [50] | |
| CaO | 1:2 | free | 70 | 1.5 | 68 | [62] | |
| MgO | 1:2 | free | 70 | 1.5 | 23 | [62] | |
| Mg0.9Ca1.1O2 | 1:2 | free | 70 | 1.5 | 75.4 | [62] | |
| Mg1.2Ca0.8O2 | 1:2 | free | 70 | 1.5 | 100 | [62] | |
| Mg-Al hydrotalcite (Mg/Al = 2) | 1:3 | Methanol | 70 | 3 | 65 | [64] | |
| LiNO3/Mg4AlO5.5 | 1:3 | free | 80 | 1.5 | 96 | [76] | |
| ZnO/La2O3 | 1:4 | free | 150 | 2 | 97 | [63] | |
| K-zeolite | 1:3 | Methanol | 75 | 1.5 | 96 | [77] | |
| Na2O | 1:2 | free | 75 | 0.5 | 92 | [78] | |
| trisodium phosphate (TSP) | 2:1 | free | 70 | 1 | 99.5 | [65] | |
| hydrotalcite-hydromagnesite | 1:5 | DMF | 100 | 0.5 | 79 | [79] | |
| Mg/Al/Zr | 1:5 | free | 75 | 1.5 | 95 | [80] | |
| Mg–Al hydrotalcite | 1:5 | DMF | 100 | 2 | 75 | [81] | |
| K2CO3 | 1:3 | free | 75 | 3 | 97 | [82] | |
| BaCO3 | 1:5 | DMF | 140 | 2 | 96.4 | [83] | |
| Ti/Si | 1:3 | free | 65 | 7 | 52 | [84] | |
| TiO2 | 1:3 | free | 65 | 7 | 4 | [84] | |
| Ni-Mg Oxide | 1:4 | free | 90 | 1.5 | 82 | [85] | |
| Glycerol + carbon monoxide | Molar Ratio G to CO | ||||||
| K2CO3/Se | Excess CO and O2 | DMF | 20 | 0.1 | 4 | 84 | [52] |
| Glycerol + diethyl carbonate | Molar Ratio G to DEC | ||||||
| Ce-NiO | 1:3 | free | 85 | - | 8 | 85.6 | [66] |
| Catalyst | Glycerol Conv. (%) | Selectivity (%) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Glycolic Acid | Oxalic Acid | Glyceral Dehyde | Dehydroxy Acetone | Glyceric Acid | Tartronic Acid | Formic Acid | |||
| Pt-Bi/SBA-15 (0.4 wt.% Bi) | 63.2 | - | - | - | 63.3 | 5.4 | - | - | [113] |
| Ag–Pd/CeO2 | 37.1 | 44.9 | 0.0 | - | - | 25.8 | 4.8 | 24.5 | [114] |
| Ag–Au/CeO2 | 43.8 | 46.2 | 0.0 | - | - | 23.3 | 1.8 | 25.2 | [114] |
| Ag–Pt/CeO2 | 54.2 | 51.0 | 0.0 | - | - | 18.9 | 2.7 | 27.4 | [114] |
| Ag/Al2O3 | 30.0 | 44.8 | 0.0 | - | - | 27.2 | 0.0 | 28.0 | [112] |
| Au/Al2O3 | 30.0 | 20.7 | 0.2 | - | - | 60.4 | 0.9 | 12.5 | [112] |
| Pd/Al2O3 | 30.0 | 2.6 | 0.3 | - | - | 85.8 | 5.7 | 1.0 | [112] |
| Pt/Al2O3 | 30.0 | 9.9 | 2.6 | - | - | 74.0 | 5.1 | 8.1 | [112] |
| Pt/C | 70.3 | 8.0 | 0.0 | 1.3 | 9.2 | 66.4 | - | - | [115] |
| 5% Pt-3% Bi/C | 74.4 | 6.6 | 19.5 | 0.0 | 46.5 | 10.2 | - | - | [115] |
| 5% Pt-5% Bi/C | 91.5 | 0.0 | 39.9 | 0.0 | 49.0 | 5.4 | - | - | [115] |
| 5% Pt-7% Bi/C | 72.0 | 14.1 | 18.6 | 0.0 | 38.8 | 15.6 | - | - | [115] |
| Au-Pt (1:3)/hydrotalcite | 57 | 10 | 0.5 | 12 | - | 72 | 5 | - | [116] |
| V2O5 | 5 | 1 | 0.0 | - | - | 1 | - | 22 | [117] |
| Au/V2O5 | 20 | 4 | 0.0 | - | - | 29 | 1 | 3 | [117] |
| Ta2O5 | 2 | 6 | 0.0 | - | - | 3 | - | 2 | [117] |
| Au/Ta2O5 | 13 | 6 | 0.0 | - | - | 6 | 3 | 3 | [117] |
| TiO2 (P25) | ~36 | - | - | 13 | 7.5 | - | - | 8 | [118] |
| Catalysts | Reactant | Time (h) | Glycerol Conv. (%) | Selectivity (%) | Ref. | ||
|---|---|---|---|---|---|---|---|
| Mono Glycerol Ether | Di Glycerol Ether | Tri Glycerol Ether | |||||
| SnSO4 | tert-butyl alcohol | 4 | ~30 | ~15 | 0 | 0 | [127] |
| Sn1.5PW12O40 | tert-butyl alcohol | 4 | ~90 | ~72 | ~24 | ~1 | [127] |
| Sn2SiW12O40 | tert-butyl alcohol | 4 | ~57 | ~53 | ~22 | ~1 | [128] |
| Sn3PMo12O40 | tert-butyl alcohol | 8 | ~71 | ~71 | ~28 | ~1 | [129] |
| Montmorillonite-Al-Pillared | tert-butyl alcohol | 6 | ~86 | ~75 | ~0 | ~25 | [130] |
| Montmorillonite-K-10 | tert-butyl alcohol | 6 | ~92 | ~87 | ~4 | ~4 | [130] |
| Montmorillonite-KSF/O | tert-butyl alcohol | 6 | ~95 | ~83 | ~10 | ~2 | [130] |
| Amberlyst-15 | Ethanol | 4 | 90 | 87 | 5 | 8 | [131] |
| Amberlyst-15 | Ethanol | 8 | 96 | 65 | 19 | 16 | [131] |
| H-Beta zeolite | Ethanol | 8 | 92 | 71 | 17 | 12 | [131] |
| H-ZSM-5 zeolite | Ethanol | 8 | 61 | 94 | 4 | 2 | [131] |
| Sulfonate graphene | Isobutene | 7 | 99.7 | 7.9 | 56.4 | 35.7 | [126] |
| sulfonated reduced graphene oxide | Tert-butyl Ether | 10 | 77 | 73 | 27 | - | [125] |
| Cs/ZSM-5 | - | 8 | 13 | 100 | 0 | 0 | [123] |
| Cs/ZSM-5 | - | 24 | 42 | 80 | 20 | 0 | [123] |
| Catalyst | Temperature (°C) | Pressure (MPa) | Time (h) | Conversion of Glycerol (%) | Selectivity | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| 1,2-PDO | 1,3-PDO | 1-Propanol | ||||||
| Ni/γ-Al2O3 | 220 | - | 5 | 100 | 87.0 | - | 0.1 | [144] |
| Ni/CS-P * | 260 | - | 5 | 100 | 0.0 | - | 71.0 | [144] |
| Ni/CS-P * | 260 | - | 2 | 100 | 0.0 | - | 91.0 | [144] |
| Ni/Y-Zeolite | 260 | 4.5 | 23–30 | 95.6 | 42.9 | - | - | [141] |
| Cu/Y-Zeolite | 260 | 4.5 | 23–30 | 71.1 | 12.6 | - | - | [141] |
| Ni-Cu/Y-Zeolite | 260 | 4.5 | 23–30 | 96.4 | 43.9 | - | - | [141] |
| Cu/SBA-15 | 230 | 4 | 1.5 | 90.3 | 97.3 | - | - | [140] |
| Ag/Al2O3 | 220 | 1.5 | 10 | 46.0 | 96 | - | - | [145] |
| Pt/SiO2-Al2O3 | 220 | 4.5 | 24 | 19.8 | 32 | - | - | [146] |
| Ru/CeO2 | 180 | 5 | 10 | 85.2 | 62.7 | - | - | [147] |
| Cu/ZnO | 200 | 2 | 16 | 37.0 | 92 | - | - | [148] |
| Cu/Al2O3 | 220 | 5 | 6 | 61 | 93.3 | - | - | [149] |
| Cu/MgO | 180 | 3 | 20 | 72 | 97.6 | - | - | [150] |
| Cu/MgAlO | 180 | 3 | 20 | 80 | 98.2 | - | - | [151] |
| Cu-Pd/MgAlO | 180 | 2 | 10 | 77 | 97.2 | - | - | [152] |
| Pt(0.5)-Ir-ReOx/SiO2 | 190 | 2 | 17 | 30 | 19 | - | 4.1 | [153] |
| Ir-ReOx/SiO2 | 120 | 8 | 36 | 81 | - | 46 | - | [154] |
| Pt-WO3/ZrO2 | 170 | 8 | 18 | 86 | - | 28 | - | [155] |
| Pt/WO3-TiO2/SiO2 | 180 | 5.6 | 12 | 15.3 | - | 50 | - | [156] |
| Pt/WOx-TiOx | 180 | 5.6 | 12 | 18.4 | - | 40.3 | - | [157] |
| Ir/ZrO2 with HZSM-5 ** | 250 | 1 | 2 | 100 | - | - | - | [158] |
| Catalyst | Reactant | Acid/Glycerol Ratio | Temp. (°C) | Time (h) | Glycerol Conv. (%) | Selectivity (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Mono | Di | Tri | |||||||
| Glycerol-based carbon catalyst | Acetic acid | 1:3 | 110 | 3 | 99 | - | 88 | [171] | |
| Layered double hydroxide of MgAlCO3 | Oleic acid | 2:1 | 180 | 2 | ≃63 | ≃51 mono-olein | ≃44 di-olein | ≃5 tri-olein | [172] |
| Layered double hydroxide of MgAlCO3 | Oleic acid | 3:1 | 140 | 2 | ≃56 | ≃88 mono-olein | ≃10 di-olein | ≃0 tri-olein | [172] |
| MgAlCO3 | Lauric acid | 3:1 | 180 | 2 | 99 | 90 mono- and di-laurine | - | [173] | |
| HPW/SBA-15 | Lauric acid | 1:4 | 160 | 6 | 70 | 50 | - | - | [174] |
| ZnO/Zeolite | Oleic acid | 1:4 | 150 | 6 | 85 | 70 | - | - | [165] |
| Sn-EOF (Organic framework) | Oleic acid | 1:1 | 150 | 20 | 40 | 98 | - | - | [175] |
| Catalyst | Temp. (°C) | Reaction Time (h) | Glycerol Conv. (%) | Selectivity (%) | Ref. | |
|---|---|---|---|---|---|---|
| Acrolein | Acetol | |||||
| Al2O3–PO4 | 280 | 10 | 100 | 42 | 23 | [184] |
| TiO2–PO4 | 280 | 10 | 98 | 37 | 30 | [184] |
| SAPO-34 | 280 | 10 | 59 | 72 | 6.8 | [184] |
| Ca/Ca10(PO4)6(OH)2 | 350 | 1.5 | 85 | 9 | 22 | [182] |
| P/Ca10(PO4)6(OH)2 | 350 | 1.5 | 97 | 35 | 19 | [182] |
| Zr | 325 | 2 | 91 | 25 | 5 | [183] |
| ZrNb | 325 | 2 | 80 | 36 | 8 | [183] |
| ZrNbP0.2 | 325 | 2 | 100 | 56 | 10 | [183] |
| WO3/ZrO2 at SiC | 250 | - | 100 | 71.1 | 8.1 | [185] |
| 30 wt% HPW/MSU-x | 300 | 4 | 100 | 69.5 | 7.5 | [186] |
| 30 wt% HPW/SBA-15 | 300 | 4 | 100 | 61 | 4.5 | [186] |
| nanosheet MFI zeolite (Si/Al = 30) | 320 | 8 | 98.3 | 82.8 | 8.4 | [187] |
| nanosheet MFI zeolite (Si/Al = 50) | 320 | 4 | 99.8 | 85.4 | 4.9 | [187] |
| SiCuCr40-Ce5 | 200 | 3 | 98.6 | - | 60.35 | [188] |
| Hydrotalcite type-M2+/M3+ = 4 | 240 | 1–9 | 6.9 | - | 39 | [189] |
| 5.0% Cu-Hydrotalcite type-M2+/M3+ = 4 | 240 | 1–9 | 64.1 | - | 52.2 | [189] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koranian, P.; Huang, Q.; Dalai, A.K.; Sammynaiken, R. Chemicals Production from Glycerol through Heterogeneous Catalysis: A Review. Catalysts 2022, 12, 897. https://doi.org/10.3390/catal12080897
Koranian P, Huang Q, Dalai AK, Sammynaiken R. Chemicals Production from Glycerol through Heterogeneous Catalysis: A Review. Catalysts. 2022; 12(8):897. https://doi.org/10.3390/catal12080897
Chicago/Turabian StyleKoranian, Parvaneh, Qian Huang, Ajay Kumar Dalai, and Ramaswami Sammynaiken. 2022. "Chemicals Production from Glycerol through Heterogeneous Catalysis: A Review" Catalysts 12, no. 8: 897. https://doi.org/10.3390/catal12080897






