Abstract
Acid catalysts including Amberlyst 15 and sulfuric acid were used for heterogeneous and homogeneous catalyst reactions respectively, to reduce high free fatty acid (FFA) in sludge palm oil (SPO) using an esterification process. The goal of this research was to reduce high FFA content in SPO to less than 1 wt.% FFA so that it can be employed as a raw material in a transesterification process to produce biodiesel. Amberlyst 15 is an eco-friendly catalyst with many benefits, such as being reusable and generating non-toxic waste after reactions, compared to homogeneous catalysts, although the reaction time of the homogeneous catalyst was faster than the heterogeneous catalytic reaction. Therefore, esterification reactions with a heterogeneous and homogeneous catalytic reaction were carried out to examine conversion of FFA. The heterogeneous catalytic reaction decreased the FFA content from 89.16 wt.% to 1.26 wt.% under the recommended conditions of 44.7 wt.% methanol, 38.6 wt.% Amberlyst 15 catalyst loading, and 360 min reaction time. For homogeneous catalytic reaction, the FFA content of 1.03 wt.% was achieved under the recommended conditions of 58.4 wt.% methanol, 16.8 wt.% sulfuric acid, and 79.7 min reaction time. Furthermore, the results of the reusability research demonstrate that the heterogeneous catalyst may be reused for at least nine cycles. This research showed the promising potential of using SPO non-edible oil for biodiesel production by employing an eco-friendly heterogeneous catalyst for cost-effective environmental remediation.
1. Introduction
One of the primary factors of economic development is energy derived from fossil fuels that have been deposited a long time ago and have been used so extensively that fuel shortages have emerged. As a result, there is a critical need to discover renewable energy sources to supplement the limited supply of fossil fuels [1,2]. Diesel is the most commonly used fuel in the transport sector. As a result, the fuel which contains diesel fuel components such as biodiesel, bioethanol, etc., is the key significant fuel in the automotive sector. These fuels were effectively deployed in many countries throughout the world several years ago [3]. Alternative energy sources must be investigated in order to reduce the impact of petroleum diesel fuel consumption [4]. One of the most attractive energy sources is methyl ester. In general, biodiesel is produced from edible and non-edible oils, as well as vegetable and animal fats, used cooking oil, and waste oil from the milling of crude palm oil (CPO) [5]. Biodiesel is extensively employed in the United States of America, Europe, Latin, Canada, Australia, Japan, and other Asian countries. As a result, global CPO consumption increased by around 19.6% between the years 2021 and 2015, from 73.86 million tons to 59.38 million tons. Thailand is presently the world’s fifth biggest producer of biodiesel. It is usually made from CPO [6]. However, the cost of raw materials is one of the issues in biodiesel production. The global price of CPO is increasing year by year. In 2020, the global price of CPO was 910.65 USD per metric ton. In 2021, the global price was 1279.64 USD per metric ton, which is an increase of 28.8% compared to 2020 [7]. Therefore, the waste from palm oil mill plants, the low-cost raw material of sludge palm oil (SPO) from the CPO milling process, is a promising raw material for biodiesel production [8,9]. Sterilization, double screw pressing, and the utilization of separator sludge are used to produce SPO with a high free fatty acid (FFA) concentration [10]. SPO is a byproduct of CPO production, emerging from several stages of the CPO milling process that requires steam sterilization and water dilution, which is eventually discharged into a large volume of water discharged into open ponds of effluent from palm oil mill effluent (POME) [11]. When it is time for the POME pond to settle, SPO floats on the top layer and cleans the wastewater for about 7 d before it is released into the environment [12]. POME is a kind of liquid wastewater that contains oily phases from three basic processes: condensate decomposition, water hydrocyclones, and mud separators [13]. POME is a non-toxic resource; nonetheless, incorrect management may result in unpleasant odors surrounding the industrial area [14]. The liquid waste drainage POME will then harm the water and soil surrounding the industrial area while also spreading a large amount of methane and other harmful gases, influencing greenhouse gas emissions [15,16]. As a consequence of these issues, POME must be transformed to energy to be mitigated. Using POME as a raw material in biodiesel manufacturing would lower the cost of the production process, which is a major problem in biodiesel production. POME has not been widely used and it has been discharged into the initial stage of the pond to separate the contaminated waste and reduce biological oxygen demand (BOD), chemical oxygen demand (COD), the color, and smell of POME before discharge into the environment [17,18]. Moreover, Tabassum et al. reported that POME contained BOD that has ranged from 10,250 to 43,750 mg/L and COD that has ranged from 15,000 to 100,000 mg/L [19]. To remove SPO, some CPO producers used a drying method and generated wastewater in open POME ponds [20]. To assist the wastewater treatment, POME could be provided to livestock, in which case it could function as a partial solution to the problem [21]. CPO producers in Thailand are commonly fertilized to produce biogas to solve this issue. Currently, most wastewater treatment methods from palm oil mills are comprised of an anaerobic pond followed by either an aerobic or anaerobic pond to produce raw material for the process of producing biogas. Anaerobic and aerobic biological wastewater treatment relies on using various microorganisms for decomposition and turning organic matter into carbon dioxide and ammonia, a system for treating wastewater that is quite suitable for reducing the amount of organic matter in wastewater resources [22,23]. However, in treating such wastewater, it is necessary to select conditions ideal for the functioning of microorganisms, which is related to the number of microorganisms and the decomposition time. Conditions such as temperature, alkalinity, volatile fatty acid, hydraulic retention time, and harmful substances must be suitable and controlled for both aerobic and anaerobic microorganisms [24]. Normally, the density of the oil is lower than the water, so the highly oily phase floats on the upper layer of the POME pond where the aerobic biological treatment is occurring. Oxygen maintains aerobic conditions in the upper layer of the pond. Algae have been found in the upper layer, which perform photosynthetic activities and discharge oxygen that causes the aerobic conditions with the bacteria in the upper layer [25]. According to studies, the SPO layer thickness should not exceed 1.5 m because when the sludge palm oil content surpasses 1.5 m the oxygen content at the surface of the pond is blocked [26]. As a result, aerobic microorganisms used in wastewater treatment are unable to operate properly. Using sludge palm oil as a biodiesel source is another option to get rid of unnecessary sludge palm oil, which might prevent aerobic microorganisms from functioning [27]. Therefore, the high residual oil content in SPO should be transformed into biofuel, with biodiesel being one of the most viable alternatives for turning low-cost POME into energy. This technique is an effort to promote second-generation biofuel while simultaneously producing biodiesel without compromising food availability [28]. In addition, utilizing SPO to produce biodiesel may help minimize the harmful impacts of POME waste on the environment around the factory [29,30].
However, SPO oil with a high concentration of FFA should be converted in two steps, beginning with an acid-catalyzed esterification process and finishing with a base-catalyzed transesterification reaction. For FFA reduction of SPO, Hayyan et al. investigated biodiesel production from feedstock SPO utilizing a standard approach that included esterification to decrease high FFA content using a sulfuric acid catalyst followed by transesterification to produce biodiesel [31]. In recent years, homogeneous acid catalysts have mostly been used to decrease FFA in oil. The use of sulfuric acid as a homogeneous reaction has some difficulties in recovery after the reaction process, producing toxic wastewater, and equipment, or reactor corrosion [32]. Furthermore, wastewater generated during the esterification process was found to significantly reduce the conversion of ester due to dilution from wastewater as described by Kusdiana and Saka. These authors described the side effect of water formation during the esterification process using sulfuric acid, which caused a reduction in the generation of a high methyl ester yield. They reported that the conversion rate decreased when the amount of water produced in the reaction increased [33].
High concentrations of acid catalyst are currently used in esterification reactions for several reasons, such as relatively fast catalytic performance, a relatively short reaction time, and a wide functional range, while the heterogeneous acid catalyst might have high cost and slow catalytic performance when compared with the homogeneous acid catalyst [34]. However, these compounds from the homogeneous catalytic reaction might harm the environment. Therefore, they must be pretreated before being discharged. Otherwise, the separation of the catalyst from the product is problematic, leading to equipment corrosion and sensitivity to water content in the oil. Conversely, heterogeneous acid catalysts are potentially effective catalysts due to their many benefits. Many kinds of studies have examined many advantages of heterogeneous catalysts, such as nontoxicity, nonvolatility, temperature stability, uniformity, reliability, and simplified separation, minimizing the corrosion problem, regeneration, and reusability [35,36,37].
For using heterogeneous acid catalysts in the esterification reaction, Boz et al. studied biodiesel production from waste cooking oil (WCO) using esterification and transesterification processes with Amberlyst 15 and altered Amberlyst 15 catalysts. The Amberlyst 15 was modified by heating it at 220 °C for a maximum duration time of 48 h, known as the thermal treatment. The operation parameters were performed under a molar ratio of oil to alcohol ratio of 1:6 to 1:15, reaction temperature between 25 and 65 °C, catalyst loading (1–9 wt.%), and reaction duration of 1 to 48 h. Their results showed that the FFA level of 1.04 wt.% WCO with the optimized conditions of oil to methanol ratio of 1:12, reaction temperature of 65 °C, unmodified catalyst loading of 3 wt.%, and duration of 9 h distributed 78 wt.% of the highest biodiesel yield. Furthermore, Amberlyst 15 has better hydrogen ion-exchange capabilities than altered Amberlyst 15 catalysts. This is because heat treatment reduces exchange capabilities, reducing the methyl ester purity of biodiesel that can be produced [38]. Another study by Park et al. demonstrated biodiesel synthesis from high FFA oil by esterification using heterogeneous acid catalysts. Two types of solid catalysts, such as Amberlyst 15 and Amberlyst BD20, were used to reduce FFA from the 99.8 wt.% FFA content of oleic acid. A pressurized stainless-steel reactor was used for the reaction process under the operating conditions: methanol to oil 6:1, catalysis loading of 20 wt.%, reacted temperature of 80 °C, and a reaction period of 6 h. The reusability of catalysts was tested for five cycles. The FFA content of less than 2 wt.% was obtained using both catalysts. However, the catalytic activity of Amberlyst 15 decreased gradually after each recycled process, while the catalytic action of Amberlyst BD20 was not significantly different with recycling. Using a scanning electron microscope (SEM), many pores were found on the surface of Amberlyst 15, but only a few were observed in Amberlyst BD20 [39]. The Summary of reviews for the biodiesel production process using heterogeneous acid catalysts is tabulated in Table 1.

Table 1.
Summary of reviews for the biodiesel production process using heterogeneous acid catalysts.
According to the many benefits of applying heterogeneous catalysts in a sustainable biofuel, the aim of this investigation was to reduce FFA from a high FFA content of 89.16 wt.% SPO, which is the second generation of biodiesel production using the Amberlyst 15 as a heterogeneous acid catalyst. To clarify and compare the gap between the heterogeneous and homogeneous acid catalysts, sulfuric acid was utilized as an acid catalyst for the esterification process. FFA reduction using a heterogeneous catalyst relies on essential considerations such as methanol concentration (26.5–63.5 wt.%), Amberlyst 15 loading (35.0–45.0 wt.%), and reaction time (180–540 min). For the homogeneous catalyst, methanol concentration (9.5–110.5 wt.%), sulfuric acid loading (3.0–33.0 wt.%), and reaction time (12.0–147.0 min) were tested. The important parameters were varied to find out the optimum condition using a response surface methodology (RSM). The interesting outcomes, such as the composition of esterified oil, the yield of purified esterified oil, and residual methanol analysis, were determined. The BET surface area of the solid catalyst was determined using a surface area and porosity analyzer. The investigation of the reusability of the Amberlyst 15 was also completed. Furthermore, the effect of the adsorption average pore diameter of Amberlyst 15 after the first and last recycling processes on FFA reduction was also analyzed.
2. Results and Discussion
2.1. Response Surface Methodology and Statistical Analyses
In this research, 18 experimental operations arranging independent variables such as the methanol concentration, reaction time, and catalyst concentration were tested in the design of the experiment. Table 2 shows the results of the esterification procedure using the batch reactor for minimizing the FFA content. To effectively convert the SPO to biodiesel using this two-step method, the pretreated oil should contain less than 1 wt.% FFA [44,45]. The RSM was applied to decrease FFA content via the batch process by model fitting using multiple regression. The predictive models were shown in Equation (1) for the heterogeneous catalytic reaction and Equation (2) for the homogeneous catalytic reaction. The FFA reduction in SPO was influenced by three independent variables: methanol (M), reaction time (T), and catalyst concentration (C). The statistical significance of every regression coefficient in the models depended on the p-value. The results were considered statistically significant if the p-value was less than 0.05 at a confidence level of 95%. Table 3 and Table 4 show the coefficients and ANOVA of the predictive models.
where FFA is a free fatty acid (wt.%), M is methanol (wt.%), C is the amount of catalyst (wt.%), T is reaction time (min), and β is the coefficient value.

Table 2.
Design of experiment and results of the free fatty acid content from the batch process.

Table 3.
Coefficients of the predictive model.

Table 4.
ANOVA of the predictive model.
2.2. Response Surface Plots
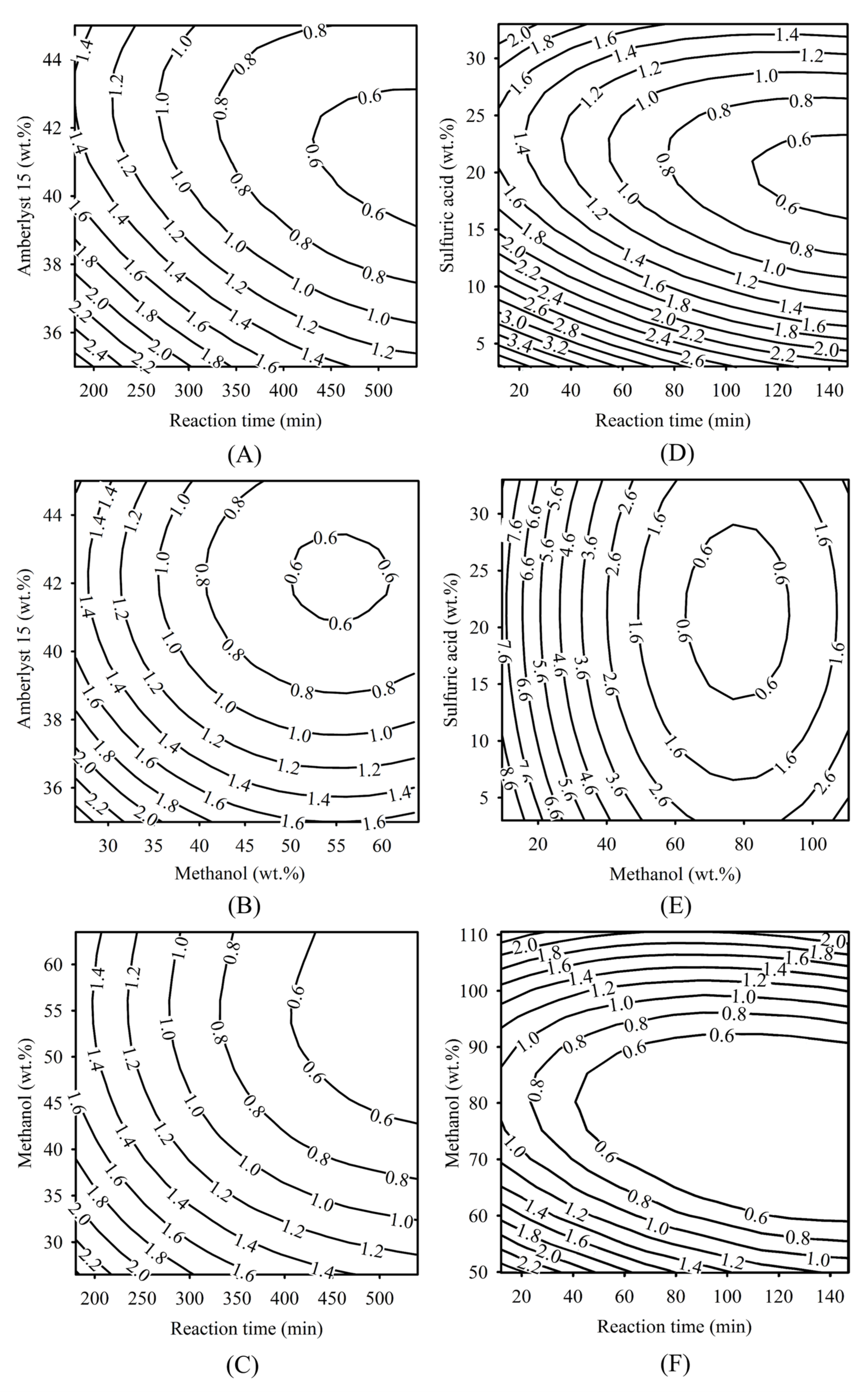
The FFA reduction contour plots related to the dependent variable of FFA (wt.%) and independent variables of methanol (M, wt.%), reaction time (T, min), acid catalyst (C, wt.%) of the batch esterification of the heterogeneous and homogeneous catalytic processes are shown in Figure 1. In Table 3, the most significant coefficient was found in the term of β3C1, followed by the term of β7C12, which had the lowest p-value. Therefore, Amberlyst 15 concentration is the most important priority parameter for the heterogeneous catalytic process. The lowest significant term was found in the term β6T1C1, having the highest p-value. Therefore, the relationship between reaction time and catalyst concentration is the lowest priority important term for using the heterogeneous catalytic reaction process. In the contour plot of Figure 1A, the effect of the reaction time and Amberlyst 15 concentration was investigated. The result showed that the regional areas covered by the reaction time of 450 to 530 min, and the solid acid catalyst is in the range of 40 to 43 wt.%, can reduce the FFA to less than 0.6 wt.%. In the contour plot of Figure 1B, the effect of FFA on methanol concentration and Amberlyst 15 concentration was demonstrated. When the methanol concentration is in the range of 52 to 60 wt.%, low FFA content of around 0.6 wt.% was obtained in the range of Amberlyst 15 loading between 41 to 43 wt.%. The effect of the relationships between the reaction time and methanol is shown in Figure 1C. The result showed that the reaction time range from 450 to 530 min and methanol range between 45 and 63 wt.%, can reduce the FFA to less than 0.6 wt.%. In a similar research report, the investigation of FFA reduction in the esterification process of propionic acid with isopropyl alcohol using ion-exchange resins as a catalyst was presented by Chandane et al. [46]. The analysis was executed by arranging independent variables such as the methanol to acid molar ratio (1–3), catalyst concentration (7–11 wt.%), and reacted temperature (60–80 °C). The highest conversion yield of 83.26% was obtained under the 2.44:1 molar ratio of isopropyl alcohol to propionic acid, 72 °C reaction temperature, and 9.18 wt.% catalyst concentration. These authors found that the catalyst concentration is the most significant parameter for acid conversion.
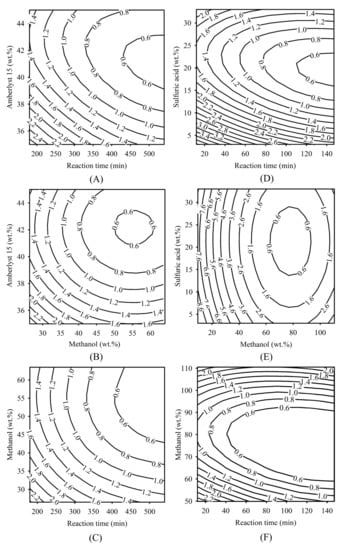
Figure 1.
Contour plots of optimized conditions for FFA reduction in SPO using esterification. (A–C) Effect of reaction time, methanol, and Amberlyst 15 on FFA level using a heterogeneous catalytic reaction. (D–F) Effect of reaction time, methanol, and sulfuric acid on FFA level using homogeneous catalytic reaction.
When using the homogeneous catalytic reaction, the most significant coefficient was found in the term of β1M2, followed by the term of β4M22, and the lowest significant term was β7T2C2. Therefore, the methanol concentration is the strongest significant independent variable. In Figure 1D, low FFA content values around 0.6 wt.% were obtained when the range of the reaction time was between 120 and 147 min, and sulfuric acid concentration was from 18 to 23 wt.%. In the contour plot of Figure 1E, a FFA content of around 0.6 wt.% was obtained at a methanol concentration between 62 and 86 wt.%, and the sulfuric acid concentration was between 15 and 27 wt.%. In the contour plot of Figure 1F, the effect of FFA content on the reaction time and methanol concentration was investigated. When the reaction time was in the range of 60 to 147 min and methanol was in the range of 70 to 90 wt.%, FFA can be reduced to less than 0.6 wt.%. Similar research was reported on biodiesel synthesis from Buriti oil soapstock using RSM by Pantoja et al., [47]. The biodiesel production process was executed by having the methanol to oil molar ratio at 9:1–27:1, sulfuric acid catalyst loading at 2–6%, and the reaction time for 1–14 h. The highest FAME yield of 99.9% was attained under the conditions of 18:1 methanol to oil molar ratio, 14 h reaction time, and 4% catalyst concentration. The authors found that the most important parameter for the ester conversion factor in the production of biodiesel was methanol concentration [47].
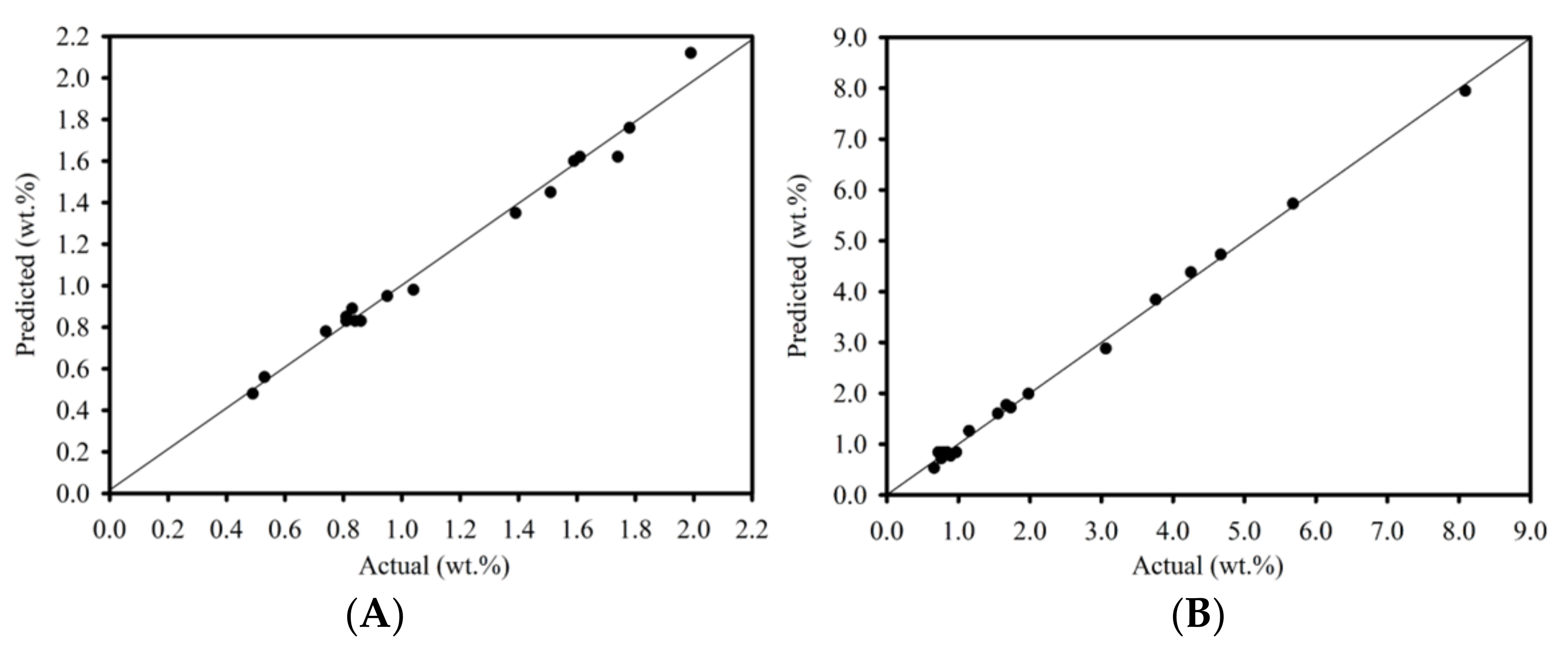
The collected data were analyzed using ANOVA to fit a quadratic response surface model using the least squares method, and to assess the quality of the fit. The model’s fit to the experimental data was evaluated at the 95% confidence level, as shown in Table 4. When employing the F-test to reject the null hypothesis of each model, the calculated F-value from the model (F0) must be greater than the critical value (Fcritical). Noticeably, a test statistic was computed with ν1 and ν2 degrees of freedom, while the result was compared to the 0.05 alpha value of the F-distribution table. The results showed that the F0 values of 104.4 and 491.4 for the heterogeneous and homogeneous catalytic reaction were higher than the Fcritical values. However, the number of experiments was sufficient to study the effect of the variable factors on FFA reduction. Therefore, all the fitted models plausibly predicted that the heterogeneous and homogeneous catalytic reactions using a batch process would be able to reduce the FFA content in SPO. Figure 2 shows the relationship between predicted FFA content and experimental FFA content for heterogeneous and homogeneous catalytic reactions to the data predicted by an empirical model. The model’s suitability was assessed using the adjusted determination coefficient (R2adj) and the determination coefficient (R2). The adjusted determination coefficient (R2adj) was 0.977 and 0.996, while the determination coefficient (R2) was 0.987 and 0.998 for the heterogeneous and homogeneous catalytic reactions, respectively. The high value of both coefficients indicates an excellent correlation between the independent variables and confirms the model’s high significance. Table 2 shows the reasonable agreement between the actual results and the predicted outcomes. As a result, these statistical tests showed that the chosen model accurately predicts FFA content across the entire range of experiment variable studies.
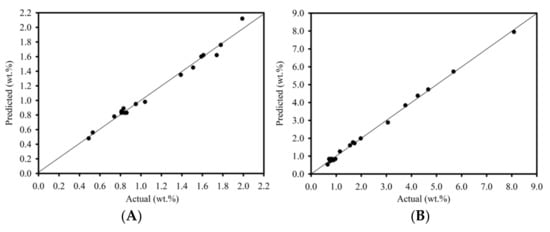
Figure 2.
Predicted FFA content versus experimental FFA content (A) heterogeneous catalytic reaction and (B) homogeneous catalytic reaction.
2.3. Optimal Conditions for Reducing FFA Content in SPO
To optimize the esterification process using a heterogeneous catalytic reaction, the optimal conditions for reducing the FFA content were considered by utilizing the model in Equation (1), which was analyzed by using FFA results in the 18 experimental operations arranging independent variables. The minimum FFA content of 0.59 wt.% from actual experimentation was achieved with conditions of 55.5 wt.% methanol concentration, 527.3 min of reaction time, and 41.2 wt.% of Amberlyst 15. Nonetheless, the methanol consumption was high, resulting in increasing costs from chemical demands, and the reaction time was longer. Thus, the dependent variable (FFA1) affecting the independent variables such as the methanol concentration (M1), reaction time (T1), and Amberlyst 15 concentration (C1), were solved by the Excel Solver tool by changing the various variables of the FFA content in Equation (1). The predicted dependent variable (FFA1) of 1 wt.% was fixed in Equation (1) to solve by the add-in Excel Solver tool. Therefore, the new recommended conditions of the heterogeneous catalytic reaction were 44.7 wt.% methanol concentration, 360 min reaction time, and 38.6 wt.% Amberlyst 15 concentration. The actual experimental result of heterogeneous catalytic reaction gave 1.26 wt.% of the FFA content. Considering the optimal esterification using the homogeneous catalytic reaction, the FFA content of 0.31 wt.% from the actual experiment was obtained with 76.0 wt.% methanol concentration, 120.9 min reaction time, and 20.4 wt.% sulfuric acid concentration. However, the methanol usage, in this case, was high, resulting in an increased cost from the chemical demands. To set the value of the dependent variable (FFA2) and search for the suggested condition, an estimated FFA content of 1 wt.% was entered into Equation (2). Hence, the recommended conditions for the homogeneous catalytic reaction, were 58.4 wt.% methanol concentration, 79.7 min reaction time, and 16.8 wt.% sulfuric acid concentration. As a result, the actual experiment delivered a FFA content of 1.03 wt.% which was suitable for use in the next step of the base-catalyzed transesterification process.
Considering the comparison between the optimal conditions and the recommended conditions for methanol consumption for the heterogeneous catalytic reaction, the results showed that the methanol consumption under recommended conditions for reducing the FFA content, was decreased by approximately 20% when compared with optimal conditions. Furthermore, at the recommended conditions, the reaction time and catalyst were lowered by approximately 32% and 6%, respectively. However, the FFA content under optimal conditions was 0.59 wt.%, while the recommended conditions were only able to reduce the FFA value to 1.26 wt.%. However, it was a suitable value for biodiesel production by transesterification in the next step. For the homogeneous catalytic reaction, the methanol consumption of the recommended condition was lower than the optimal condition by approximately 23%. The reaction time and the sulfuric acid were reduced by up to 34% and 17%, respectively. Although the recommended condition can only reduce the FFA content by approximately 1.03 wt.%, which was the acceptable value for the next transesterification process, the optimal condition can reduce the amount of FFA by up to 0.31 wt.%.
The results of residual methanol were investigated with a GC analyzer under the BS EN 14110 standard test strategy, as listed in Table 5. For the heterogeneous catalytic reaction, approximately 0.35 wt.% generated water was found in the esterified oil. For the homogeneous catalytic reaction, the methanol contamination in generated wastewater was high at 63.33 and 61.05 wt.% in the optimal and recommend conditions, respectively. As a result, the industrial-scale operation required the removal of excess methanol from the generated wastewater from the homogeneous catalytic reaction. Nonetheless, the residual methanol in the esterified oil for the optimal and recommended conditions was 13.9 wt.% and 10.8 wt.% of the heterogeneous catalytic reaction, and 5.17 wt.% and 4.15 wt.% for the homogeneous catalytic reaction, respectively. The recovery of this residual methanol in both reactions was unnecessary because this methanol amount would be applied as a forward reaction in the next step of transesterification.

Table 5.
Condition, physical property, compositions, density, yield, and residual methanol.
2.4. Reusability of Solid Acid Catalyst
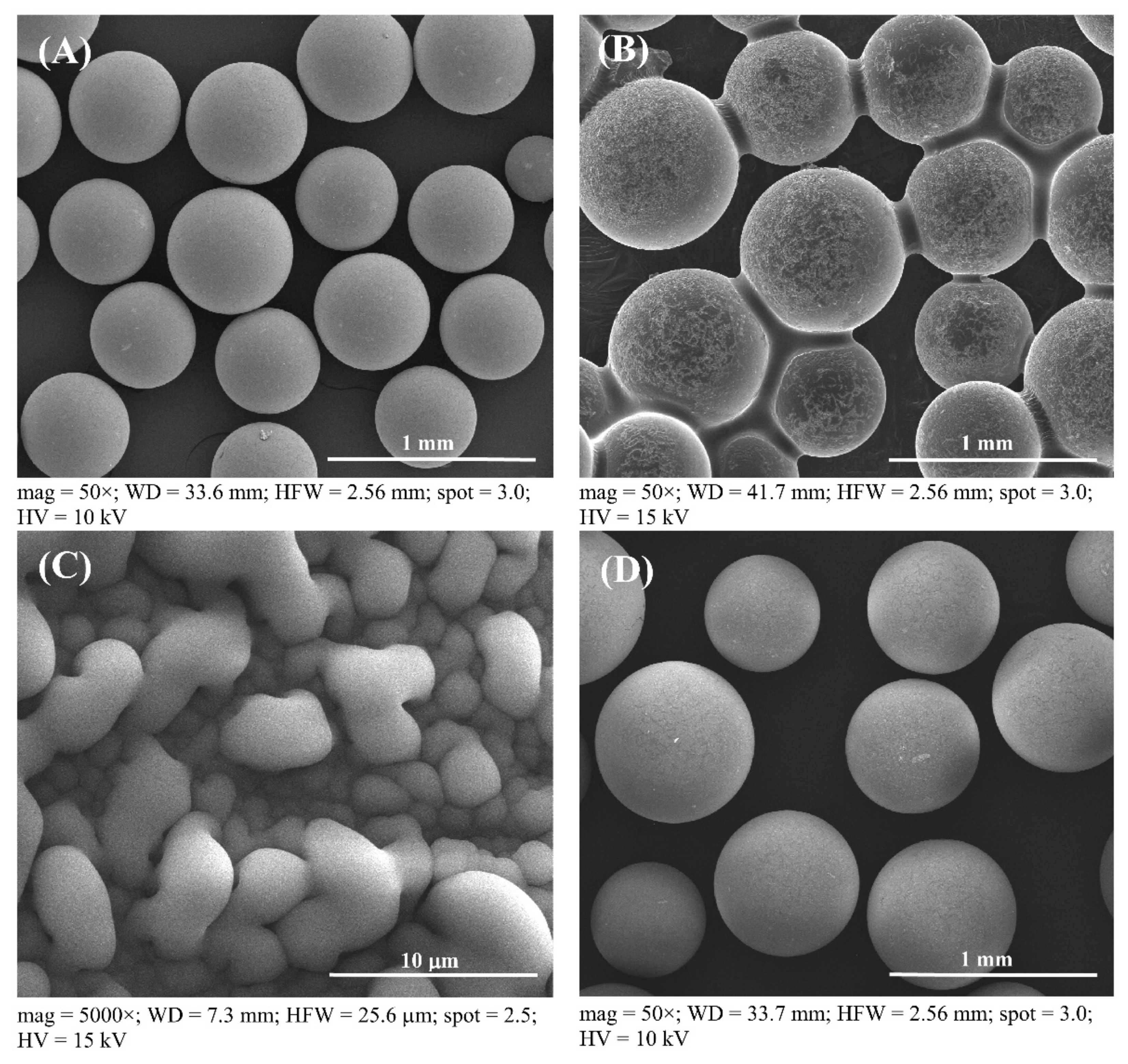
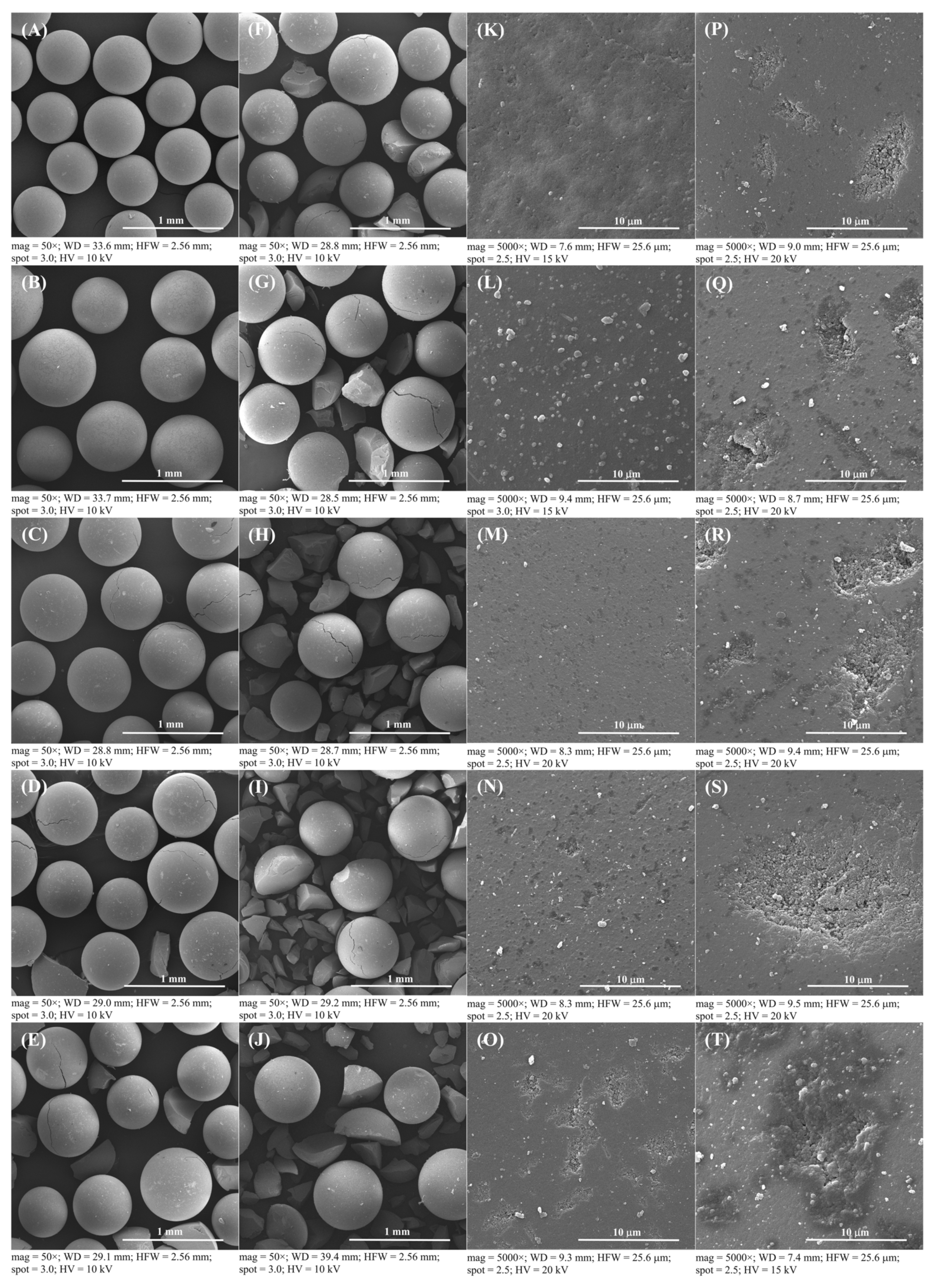
For the consideration of Amberlyst 15 reusability in the esterification process, Amberlyst 15 could be reused to produce esterified oil from fresh SPO in subsequent esterification batch processes. Therefore, the cost of the treatment process, waste generation, toxic chemical usage, and the cost of catalyst can be decreased when the recycled solid catalyst is used in the esterification. The process of employing recycled solid catalysts for each new reaction, the reusability effect of solid catalysts in multiple esterification procedures, called here a repeated catalyst after treatment, was investigated. The catalysts were utilized repeatedly to minimize the FFA level in subsequent batches of fresh SPO. Catalysts were repeatedly cycled up to nine cycles. Figure 3 shows SEM images of a fresh catalyst, a used catalyst without further treatment after the first run, and a recovered catalyst at 50 and 5000× magnifications. Fresh Amberlyst 15 appears to have a smooth spherical surface before FFA reduction with esterification, as shown in Figure 3A (at 50× magnification), and used catalyst after the first run has larger oil droplets than a fresh catalyst, as shown in Figure 3B (at 50× magnification) and Figure 3C (at 5000× magnification), respectively. The used catalyst was required to extract the oil droplets from the Amberlyst 15’s surface using a pretreatment process of solid catalysts, because the oil droplets were drawn out of the recovered catalyst by solvent extraction. Moreover, there seems to be no difference in the outer surface of the fresh catalyst and the reused catalyst the first time after recycling, as shown in Figure 3D. This conclusion was further supported by the SEM images in Figure 3A for the fresh catalyst and Figure 3D for the recovered catalyst, which exhibited no cracks on the surface of the recovered catalyst after the first cycle at 50× magnification. The recovered catalyst was analyzed using SEM images (Figure 4) to confirm the reusability of the Amberlyst 15, which can be cycled up to nine cycles for a new batch of FFA reduction in SPO by the esterification reaction. The results showed that there was no difference in the surface of the fresh catalyst until the catalyst was recovered after the first cycle. However, some minor cracks were found on the surface of the catalyst after the second time because the used catalyst was recovered using violent stirring. In a supporting result, Zare et al. reported that a stirrer could help uniform mixing, but it could also cause depth cracks on the heterogeneous acid catalyst [48]. This important point of solid catalyst cracking was further confirmed by the SEM image at 50× magnification in Figure 4, in which obvious cracks started to form and then were broken into small pieces in the used catalyst after six cycles of reaction. This issue is ascribed to catalyst fragmentation from the violent stirring caused by the rotation of a six-bladed disk turbine. Therefore, a commercial solid catalyst would not be sufficient to endure the violent stirring during the reaction. Moreover, the SEM image with 5000× magnification of the Amberlyst 15 surface to analyze the morphology, is shown in Figure 4K–T. It was found that the outermost surface of the catalyst has many pores. Consequently, some fluid content, such as water, might be absorbed on the inner surface of the catalyst. The hydrophobic oil produced several issues in the catalytic process, reducing the activity of the catalyst [39].
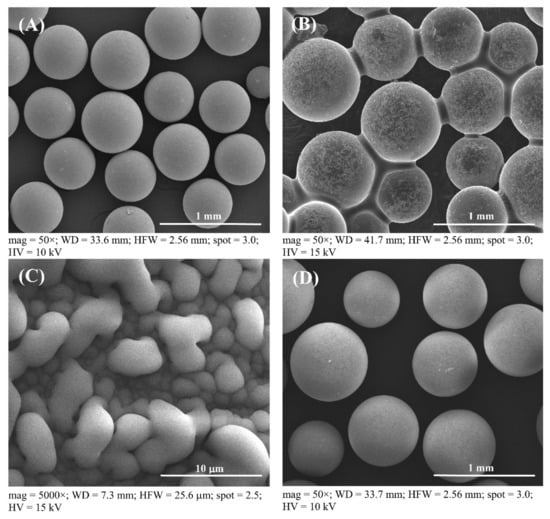
Figure 3.
SEM images of Amberlyst 15: (A) the fresh catalyst at 50× magnification, (B) no cleaning after first run at 50× magnification, (C) no cleaning after the first run at 5000× magnification, and (D) the recovered catalyst after the first run at 50× magnification.
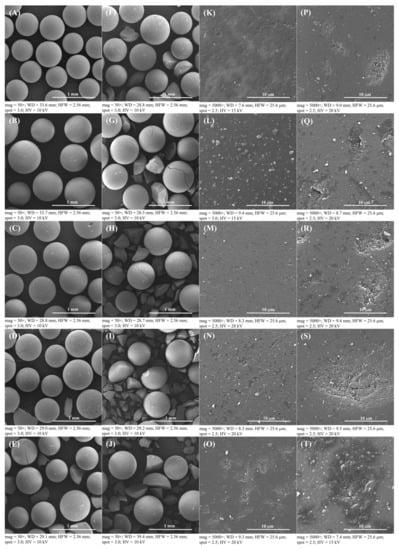
Figure 4.
SEM images of the Amberlyst 15 catalyst: (A), (K) fresh catalyst at 50 and 5000× magnifications, respectively; (B–J), (K–T) recovered catalysts after first to ninth cycles at 50 and 5000× magnifications, respectively.
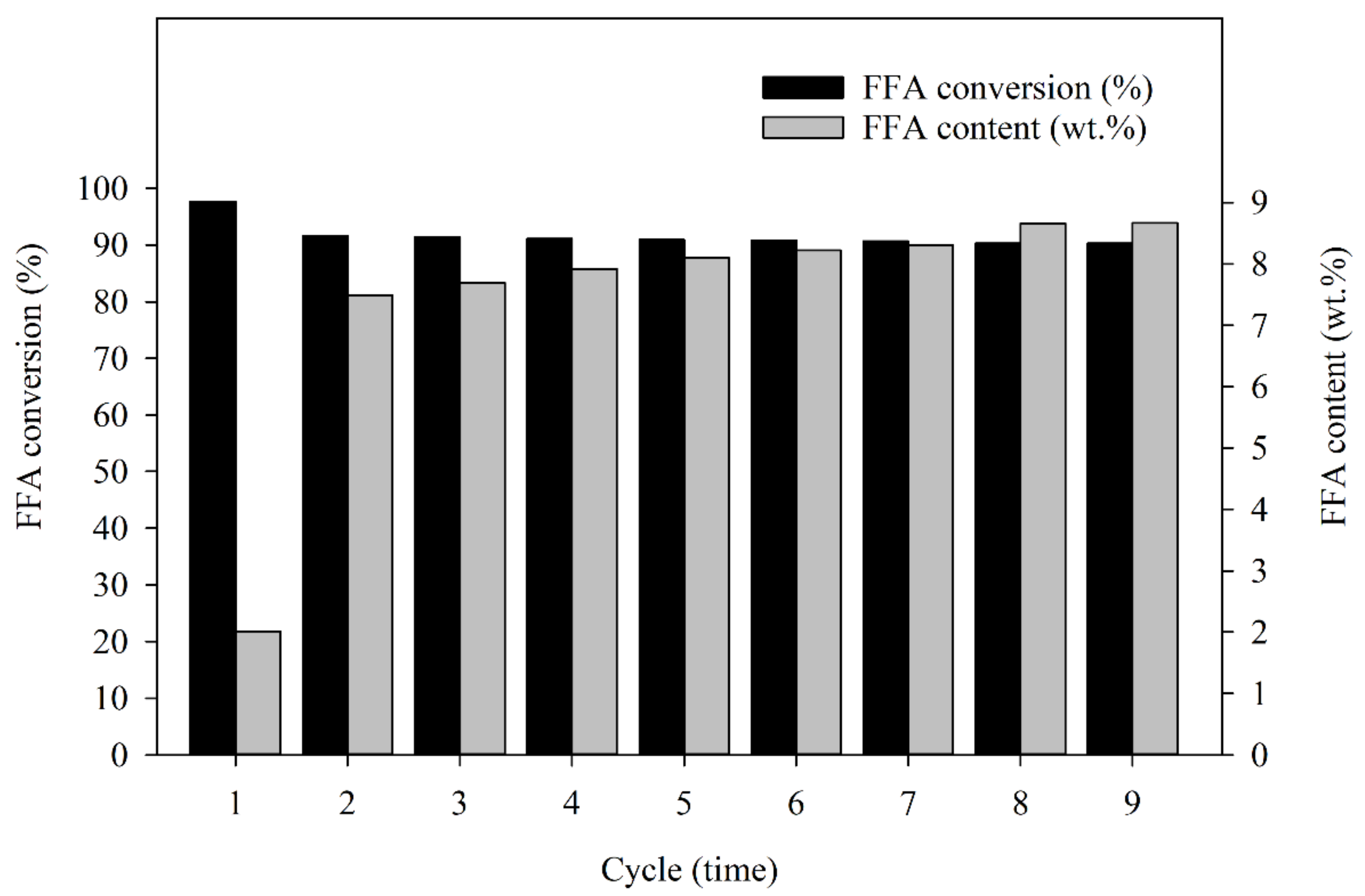
In terms of the level of catalytic activity, the BET surface area was measured by the surface area and porosity analyzer. The absorption rate of the BET surface area was estimated at approximately 42.87 m2/g and 325.1 Å absorption average pore diameter of the fresh Amberlyst 15. For the recovered catalyst after nine cycles of reaction, the BET surface area absorption was estimated at approximately 37.29 m2/g and 339.95 Å absorption average pore diameter. The BET surface area decreased after the last cycle compared to the first cycle, which affected the reduction in FFA content conversion because the higher BET surface area distributes a higher absorption capacity of reactants from the medium resulting in a higher reaction rate, and catalytic activity [49]. Therefore, the reduction in FFA conversion percentage after the last cycle was lower than after the first cycle. Nonetheless, the catalyst began to crack after three cycles of reaction, as shown in Figure 4D (at 50× magnification). The cracking of the solid catalyst was due to the violence of the stirring, mentioned above, and more cracking was found in the next cycle after the third round, but the capacity for FFA reduction by reused Amberlyst 15 catalysts remained. Thus, the catalyst can minimize FFA content even if it loses morphological condition. Considering the SEM photographs which are present in Figure 4A (at 50× magnification), the low magnification of the fresh catalyst shows a smooth surface compared with the recovered catalyst, as shown in Figure 4J (at 50× magnification). The results showed that when the Amberlyst 15 is reused, it has a low mechanical strength, which may not be quite enough to resist the vigorous stirring after the third cycle of the reaction. However, the Amberlyst 15 is compatible with a stirring speed of 300 rpm, but the number of cycles of the reaction must be considered. For surface cracking of the catalyst, Fan et al. [50] also studied the synthesis of cellulose acetate using Amberlyst 15. The results showed that Amberlyst 15 does have a lower mechanical strength when vigorous stirring was performed, which causes surface cracking during the reaction. According to the reusability study, the results show that the catalyst can be reused to catalyze the reaction for up to four cycles with no significant loss of catalytic activity. In this study, the recovered catalysts were used after a treatment process; the performance in terms of FFA conversions after each cycle of esterification using recycled solid catalysts was studied. The results showed that the FFA conversion percentage in esterified oil from the first cycle through to the ninth cycle was: 97.7%, 91.6%, 91.4%, 91.1%, 90.9%, 90.8%, 90.7%, 90.3%, and 90.3% and the FFA contents in esterified oil were: 2.0, 7.5, 7.7, 7.9, 8.1, 8.2, 8.3, 8.7, and 8.7 wt.% as shown in Figure 5. Clearly, the fragmentation of a solid catalyst could also be used to reduce FFA in an SPO, but the FFA reduction efficiency may be less than approximately 25% compared to reaction with a fresh catalyst. The benefits of reusing the Amberlyst 15 catalyst were proven worthwhile: reducing the cost of the treatment process using sulfuric acid in esterification, minimizing waste generation, and saving the chemical cost to reduce FFA in SPO.
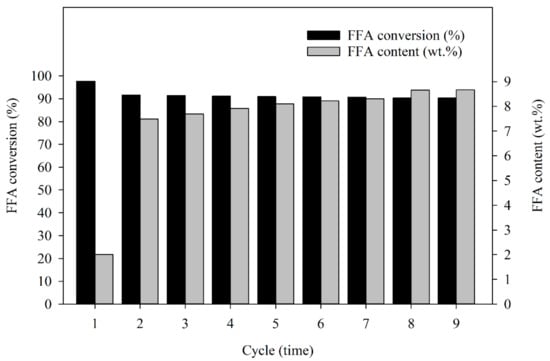
Figure 5.
The FFA conversion (%) and FFA content (wt.%) in esterified oil from the first cycle through to the ninth cycle.
3. Material and Methods
3.1. Materials
SPO was obtained from a commercial scale palm oil milling process in southern Thailand, and had a free fatty acid content of 89.16 wt.%. It was employed as a raw material in the acid-catalyzed esterification process, which used heterogeneous and homogeneous catalysts to reduce FFA in SPO, as shown in Figure 6A. The other compositions of SPO are triglyceride (TG) of 9.79 wt.%, diglyceride (DG) of 0.80 wt.%, monoglyceride (MG) of 0.24 wt.%, 306.99 g/mole mean molecular weight, and 0.865 kg/L density at 60 °C. The physical properties and compositions of SPO are shown in Table 5.

Figure 6.
Production steps of biodiesel using batch process esterification (A) sludge palm oil, (B) heterogeneous catalytic reaction esterified oil, and (C) homogeneous catalytic reaction esterified oil (top layer) and generated wastewater (bottom layer).
In the esterification process, three chemical reactants with commercial standards were used: 99.7% methanol, 98% sulfuric acid as a homogeneous catalyst, and Amberlyst 15 as a heterogeneous acid catalyst. Amberlyst 15 was a black sphere-shaped bead purchased from Sigma Aldrich, United States. The porosity, hydrogen ion-exchange capacity, thermal stability, and average pore diameter of Amberlyst 15 are 0.32 ± 0.01, 5.1 meqH+/g, 120 °C, and 300 Å. The detailed properties of the Amberlyst 15 are listed in Table 6 [51]. Analytical chemicals were: 98.5% purity n-hexane (Avantor Performance, Rednor, PA, USA), formic acid (Fisher Scientific, Loughborough, UK), diethyl ether (Avantor Performance, Rednor, PA, USA), and benzene (Loba Chemie Pvt Ltd., Mumbai, India). In order to analyze the methyl ester percentages, TG, DG, MG, and FFA in the esterified oils, the chemical mixtures of hexane-diethyl ether-formic acid (80:20:0.2 vol.%) in the first tank and hexane-benzene (1:1 vol.%) in the second tank were prepared to be used as the mobile phases in a thin layer chromatography with flame ionization detection (TLC/FID, model: IATROSCAN MK-65; Mitsubishi Kagaku latron Inc., Tokyo, Japan).

Table 6.
The properties of Amberlyst 15.
3.2. Procedure
3.2.1. Experimental Setup for Esterification
In preparation for the esterification using the heterogeneous acid catalyst, 300 g SPO was put into a five-neck round bottom flask which performed as a batch reactor. The SPO was heated to 60 °C, and the temperature was kept constant. Subsequently, the methanol was poured into the reactor. SPO and methanol mixing was continuously stirred at 300 rpm until it reached a homogenous phase using a six-bladed disk turbine and mechanical stirrer (model: RW 20, IKA, Staufen, Germany). Amberlyst 15 and sulfuric acid were slowly poured into the reactor when heterogeneous and homogeneous catalytic reactions were employed, respectively, to enhance the esterification reaction and begin the counting of reaction time. Therefore, the parameters of methanol, acid catalysts, and reaction time were examined in the required ranges to minimize FFA reduction in SPO of both heterogeneous and homogeneous catalysts, as described in the experimental design section. For the technical pouring method of sulfuric acid, the reaction temperature was accurately monitored while adding sulfuric acid into the reactor, as this mixing generated heat. Therefore, the reaction temperature of the mixture was precisely controlled below 64.7 °C regarding the boiling point of methanol, particularly to prevent the exothermic reaction that occurs when sulfuric acid is added to a reactor, leading the mixtures to rapidly rise over the boiling point of methanol. Conversely, adding an Amberlyst 15 catalyst to the process had no impact on increasing the reaction temperature. All samples were cooled with cold water to inhibit any forward or reverse reaction for the sampling process.
The residuals of sulfuric acid and methanol were eliminated in the esterified oil using a washing technique for purifying the products from homogenous reactions. After the heterogeneous catalytic reaction, the used solid catalyst was carefully removed, as provided in the section recovery method for heterogeneous catalysts. A recovered solid catalyst was reused in a new cycle of the esterification production process. The details of the recovery process have been provided in the section on the heterogeneous catalyst recovery procedure. Analysis of the compositions of the esterified oils from both homogenous and heterogeneous catalytic reactions in the presence of FFA, ME, TG, DG, and MG were performed. Finally, actual experiments, including TLC/FID analysis, were used to validate the model-based optimal conditions. The compositions of the free fatty acid fraction in SPO and residual methanol in the products were analyzed with gas chromatography (GC) according to EN 14103 and BS EN 14110 test standards, respectively.
3.2.2. Recovery Method for Heterogeneous Catalysts
The recovery of the heterogeneous solid catalyst was performed after the reaction of the esterification process had been completed. The solid catalysts in the reactant mixture were filtered using an Erlenmeyer flask coupled with filtering through filter paper, which was attached to a ceramic funnel for separating solid catalyst and esterified oil. The remaining esterified oil on the surface of the Amberlyst 15 was then removed by a vacuum pump (model: Rocker 300, Taipeh, Taiwan), which was connected to the flask through the vacuum pipeline. In the following step, the oil in the heterogeneous catalyst was extracted with hexane solvent to leach residual oil from the catalyst surface. Finally, recovered Amberlyst 15 catalysts were dried at 105 °C for 5 h in a hot air oven to remove residual hexane in the solid catalyst before use in the next cycle. The recycled solid catalysts were balanced for the next cycle of the esterification production process, to ensure that the required loading of catalyst was added into the reactor.
3.2.3. Surface Analysis of Amberlyst 15
In this study, Amberlyst 15 was recovered and reused after the esterification process to investigate the stable catalytic performance of the recycled catalyst. Therefore, the surface morphology method was used to observe the surface of the catalyst using a SEM (model: Quanta 400, Thermo Fisher Scientific, Brno, Czech Republic). SEM was used to analyze the surface morphologies of the fresh and recovered heterogeneous acid catalysts at 50 and 5000× magnifications. The specific Brunauer–Emmett–Teller (BET) method was carried out by an ASAP 2460 analyzer (Micromeritics, Norcross, GA, USA) to determine the surface area of the catalysts, and pore sizes were examined after each run of the esterification process. For analyzing the surface area and pore size of Amberlyst 15, a surface area analyzer was used to calculate the surface area of Amberlyst 15 with degassing at 100 °C. The surface area and pore size distribution (PSD) of fresh and recovered catalysts were accurately determined with the static volumetric N2 absorption method on a catalyst surface at a constant temperature of −196.15 °C using a surface area and porosity analyzer [52]. Therefore, fresh and recovered catalysts were analyzed after each cycle of esterification. For analyzing the quality of esterified oils, TLC/FID was used.
3.3. Experimental Design
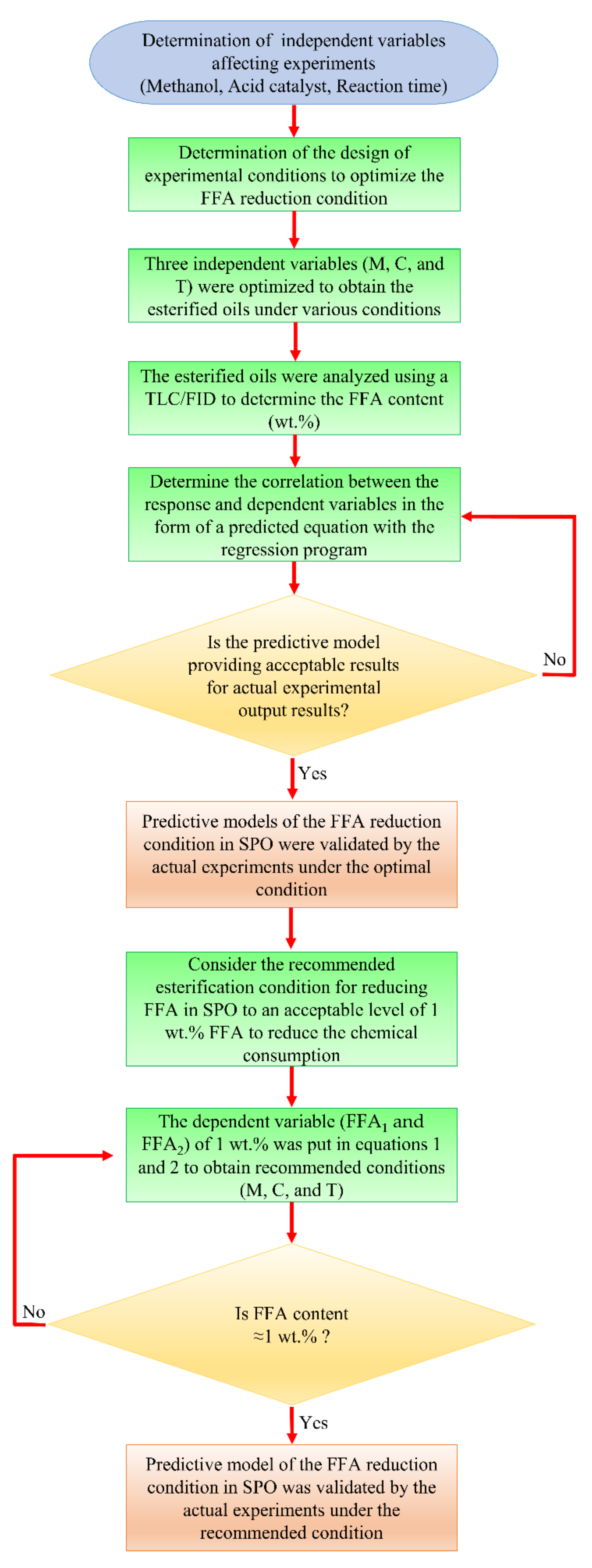
The experimental results from the heterogeneous and homogeneous catalytic reactions were implemented utilizing the RSM method to optimize the conditions for FFA reduction. For the central composite design (CCD), the RSM was run with five levels and three factors. Coding factors for the five-levels such as −αx, −1, 0, +1, and +αx were used to apply in the spinning of the independent variable values in CCD. The number of variables (k) was used to calculate the axial point (αx) of a rotatable CCD. The experiments were operated by arranging three independent variables. For this experiment, Equation (3) was applied to generate three independent variables, within the coded level of −1.682, −1, 0, +1, and +1.682. Table 7 shows the values of independent variables in the coded factor level ranges for each independent variable. The experimental design and FFA results from heterogeneous and homogeneous catalytic reactions are described in Table 2. To calculate the FFA content from each batch process operation, the second-order polynomial model, Equation (3) for multiple regression analysis was used. In this experiment, three independent variables were methanol (M), catalyst concentration (C), and reaction time (T). These three independent variables affected the achievement of low FFA content (FFA1 and FFA2, for the heterogeneous catalytic and homogeneous catalytic reactions, respectively), which was the dependent variable in this optimization. There are two processes: heterogeneous and homogeneous catalytic reactions; thus, the variables M1, C1, and T1 represent heterogeneous catalytic reactions, and the variables M2, C2, and T2 represent homogeneous catalytic reactions. The FFA contents were determined using an experimental analysis with a TLC/FID. The experimental outcomes after checking with TLC/FID were analyzed with Microsoft Excel Solver, which can be obtained in the add-in tools. The flowchart of methodology of this study using RSM optimization method was shown in Figure 7.
where Y is the dependent variable, xi and xj are independent variables, β0, βi, βii, and βij are the independent constant, the linear independent coefficient, the quadratic independent coefficient, and the interaction independent coefficient, respectively, k is the number of variables, and ε is the error.

Table 7.
Range of independent variables.
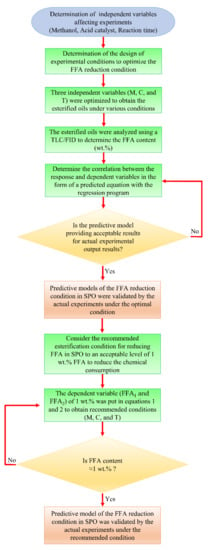
Figure 7.
Flowchart methodology of this research.
4. Conclusions
In this study, the acid catalysed esterification process for high FFA feedstock SPO was investigated using heterogeneous and homogeneous catalytic reactions. The optimum conditions of heterogeneous and homogeneous catalytic reactions such as methanol, reaction time, and catalyst concentrations were considered using RSM. For the heterogeneous catalytic reaction, a value of FFA of 1.26 wt.% was achieved under the recommended conditions: methanol at 44.7 wt.%, a reaction time of 360 min, and Amberlyst 15 at 38.6 wt.%. The recommended conditions of homogeneous catalytic reaction were 58.4 wt.% methanol concentration, 79.7 min reaction time, and 16.8 wt.% sulfuric acids, which can decrease the FFA contamination from 89.16 wt.% to 1.03 wt.%. Considering the comparative analysis between two different catalytic reactions under the recommended conditions, the chemical consumption, especially for the methanol consumption from the heterogeneous catalytic reaction was significantly lower than that of the homogeneous catalytic reaction by 23.5%, although the reaction time for the heterogeneous catalytic reaction took longer than homogeneous catalytic reaction time. For the analysis of the catalyst’s reusability, Amberlyst 15 can be reused at least nine cycles with the FFA conversion not less than 90% in every cycle. Moreover, Amberlyst 15 can be capable of FFA reduction even though its mechanical strength properties have been lost, which is an essential issue for decreasing the cost of chemical consumption. In addition, applying the heterogeneous catalytic reaction can minimize the generated wastewater, which can reduce the quality and yield of the biodiesel. For further perspectives on research development, the heterogeneous catalyst has been suggested to synthesize biodiesel as a renewable fuel for limiting environmental impact by using advanced reactors to increase the performance of the reaction beyond conventional methods for catalytic reactions.
Author Contributions
Conceptualization, P.J.-O., K.P., Y.M.O. and K.S.; methodology, P.J.-O. and K.P.; validation, P.J.-O., K.P. and K.S.; carried out the SEM analyses, P.J.-O. and K.S.; investigation, P.J.-O. and K.P.; data curation, P.J.-O. and K.P.; writing—original draft preparation, P.J.-O.; writing—review and editing, P.J.-O., K.S. and Y.M.O.; project administration, K.S.; funding acquisition, K.S. All authors contributed to the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science, Research and Innovation Fund (NSRF) and Prince of Songkla University (Grant No. ENG6505020S), and National Research Council of Thailand (NRCT), grant no. NRCT5-RSA63022-04.
Data Availability Statement
Not applicable.
Acknowledgments
This research was also supported by the National Science, Research and Innovation Fund (NSRF) and Prince of Songkla University (Grant No ENG6505020S), and National Research Council of Thailand (NRCT), grant no. NRCT5-RSA63022-04.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Karacan, R.; Mukhtarov, S.; Barış, İ.; İşleyen, A.; Yardımcı, M.E. The impact of oil price on transition toward renewable energy consumption? Evidence from Russia. Energies 2021, 14, 2947. [Google Scholar] [CrossRef]
- Sgouridis, S.; Csala, D.; Bardi, U. The sower’s way: Quantifying the narrowing net-energy pathways to a global energy transition. Environ. Res. Lett. 2016, 11, 094009. [Google Scholar] [CrossRef]
- Rivera-González, L.; Bolonio, D.; Mazadiego, L.F.; Naranjo-Silva, S.; Escobar-Segovia, K. Long-term forecast of energy and fuels demand towards a sustainable road transport sector in Ecuador (2016–2035): A leap model application. Sustainability 2020, 12, 472. [Google Scholar] [CrossRef]
- Noor, C.W.M.; Noor, M.M.; Mamat, R. Biodiesel as alternative fuel for marine diesel engine applications: A review. Renew. Sustain. Energy Rev. 2018, 94, 127–142. [Google Scholar] [CrossRef]
- Shaah, M.A.H.; Hossain, M.S.; Allafi, F.A.S.; Alsaedi, A.; Ismail, N.; Ab Kadir, M.O.; Ahmad, M.I. A review on non-edible oil as a potential feedstock for biodiesel: Physicochemical properties and production technologies. RSC Adv. 2021, 11, 25018–25037. [Google Scholar] [CrossRef] [PubMed]
- Thailand Industry Outlook 2021–2023: Biodiesel. Available online: https://www.krungsri.com/getmedia/61faa668-cd22-4f7c-a923-11f60562864f/IO_Biodiesel_210520_EN_EX.pdf.aspx (accessed on 21 May 2022).
- Global Price of Palm Oil. 2022. Available online: https://fred.stlouisfed.org/series/PPOILUSDM/ (accessed on 17 May 2022).
- Muanruksa, P.; Kaewkannetra, P. Combination of fatty acids extraction and enzymatic esterification for biodiesel production using sludge palm oil as a low-cost substrate. Renew. Energy 2020, 146, 901–906. [Google Scholar] [CrossRef]
- Rachmadona, N.; Amoah, J.; Quayson, E.; Hama, S.; Yoshida, A.; Kondo, A.; Ogino, C. Lipase-catalyzed ethanolysis for biodiesel production of untreated palm oil mill effluent. Sustain. Energy Fuels 2020, 4, 1105–1111. [Google Scholar] [CrossRef]
- Poh, P.E.; Yong, W.J.; Chong, M.F. Palm oil mill effluent (POME) characteristic in high crop season and the applicability of high-rate anaerobic bioreactors for the treatment of POME. Ind. Eng. Chem. Res. 2010, 49, 11732–11740. [Google Scholar] [CrossRef]
- Igwe, J.C.; Onyegbado, C.C. A review of palm oil mill effluent (POME) water treatment. Glob. J. Environ. Res. 2007, 1, 54–62. [Google Scholar]
- Laohaprapanon, T.; Prasertsan, P. Screening of thermotolerant microorganisms and application for oil separation from palm oil mill wastewater. Songklanakarin J. Sci. Technol. 2007, 29, 801–808. [Google Scholar]
- Liew, W.L.; Kassim, M.A.; Muda, K.; Loh, S.K.; Affam, A.C. Conventional methods and emerging wastewater polishing technologies for palm oil mill effluent treatment: A review. J. Environ. Manag. 2015, 149, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Esa, N.; Ibrahim, M.H.; Rupani, P.F.; Singh, R.P. Review of current palm oil mill effluent (POME) treatment methods: Vermicomposting as a sustainable practice. World Appl. Sci. J. 2010, 10, 1190–1201. [Google Scholar]
- Choi, W.H.; Shin, C.H.; Son, S.M.; Ghorpade, P.A.; Kim, J.J.; Park, J.Y. Anaerobic treatment of palm oil mill effluent using combined high-rate anaerobic reactors. Bioresour. Technol. 2013, 141, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Low, S.S.; Bong, K.X.; Mubashir, M.; Cheng, C.K.; Lam, M.K.; Lim, J.W.; Ho, Y.C.; Lee, K.T.; Munawaroh, H.S.H.; Show, P.L. Microalgae cultivation in palm oil mill effluent (POME) treatment and biofuel production. Sustainability 2021, 13, 3247. [Google Scholar] [CrossRef]
- Igwe, J.C.; Onyegbado, C.O.; Abia, A.A. Adsorption isotherm studies of BOD, TSS and colour reduction from palm oil mill effluent (POME) using boiler fly ash. Eclet. Quím. 2010, 35, 195–208. [Google Scholar] [CrossRef]
- Poh, P.E.; Chong, M.F. Development of anaerobic digestion methods for palm oil mill effluent (POME) treatment. Bioresour. Technol. 2009, 100, 1–9. [Google Scholar] [CrossRef]
- Tabassum, S.; Zhang, Y.; Zhang, Z. An integrated method for palm oil mill effluent (POME) treatment for achieving zero liquid discharge—a pilot study. J. Clean. Prod. 2015, 95, 148–155. [Google Scholar] [CrossRef]
- Ohimain, E.I.; Izah, S.C. A review of biogas production from palm oil mill effluents using different configurations of bioreactors. Renew. Sustain. Energy Rev. 2017, 70, 242–253. [Google Scholar] [CrossRef]
- Kamarudin, K.F.; Yaakob, Z.; Sobri Takriff, M. A review on wastewater treatment and microalgal by-product production with a prospect of palm oil mill effluent (POME) utilization for algae. Pharma Chem. 2015, 7, 73–89. [Google Scholar]
- Demirbas, A.; Coban, V.; Taylan, O.; Kabli, M. Aerobic digestion of sewage sludge for waste treatment. Energy Sources A Recovery Util. Environ. Eff. 2017, 39, 1056–1062. [Google Scholar] [CrossRef]
- Adane, T.; Adugna, A.T.; Alemayehu, E. Textile industry effluent treatment techniques. J. Chem. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Shete, B.S.; Shinkar, N.P. Anaerobic digestion of dairy industry waste water-biogas evolution-a review. Int. J. Appl. Environ. Sci. 2017, 12, 1117–1130. [Google Scholar]
- Al-Amshawee, S.K.; Yunus, M.Y.; Azoddein, A.A. A review on aerobic biological processes for palm oil mill effluent: Possible approaches. Mater. Sci. Eng. 2020, 736, 022035. [Google Scholar] [CrossRef]
- Okereke, J.N.; Ginikanwa, R.C. Environmental impact of palm oil mill effluent and its management through biotechnological approaches. Int. J. Adv. Res. Biol. Sci. 2020, 7, 117–127. [Google Scholar]
- Anwar, Z.; Irshad, M.; Fareed, I.; Saleem, A. Characterization and recycling of organic waste after co-composting—A review. J. Agric. Sci. 2015, 7, 68–79. [Google Scholar] [CrossRef]
- Kuchler, M. Sweet dreams (are made of cellulose): Sociotechnical imaginaries of second-generation bioenergy in the global debate. Ecol. Econ. 2014, 107, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Nasaruddin, R.R.; Alam, M.Z.; Jami, M.S. Evaluation of solvent system for the enzymatic synthesis of ethanol-based biodiesel from sludge palm oil (SPO). Bioresour. Technol. 2014, 154, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Ricca, R.N.; Md, Z.A.; Mohammed, S.J. Enzymatic biodiesel production from sludge palm oil (SPO) using locally produced Candida cylindracea lipase. Afr. J. Biotechnol. 2013, 12, 4966–4974. [Google Scholar] [CrossRef]
- Hayyan, A.; Alam, M.Z.; Mirghani, M.E.S.; Kabbashi, N.A.; Hakimi, N.I.N.M.; Siran, Y.M.; Tahiruddin, S. Reduction of high content of free fatty acid in sludge palm oil via acid catalyst for biodiesel production. Fuel Process. Technol. 2011, 92, 920–924. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef]
- Kusdiana, D.; Saka, S. Effects of water on biodiesel fuel production by supercritical methanol treatment. Bioresour. Technol. 2004, 91, 289–295. [Google Scholar] [CrossRef]
- Su, C.-H. Recoverable and reusable hydrochloric acid used as a homogeneous catalyst for biodiesel production. Appl. Energy 2013, 104, 503–509. [Google Scholar] [CrossRef]
- Helwani, Z.; Othman, M.R.; Aziz, N.; Kim, J.; Fernando, W.J.N. Solid heterogeneous catalysts for transesterification of triglycerides with methanol: A review. Appl. Catal. A Gen. 2009, 363, 1–10. [Google Scholar] [CrossRef]
- Helwani, Z.; Othman, M.R.; Aziz, N.; Fernando, W.J.N.; Kim, J. Technologies for production of biodiesel focusing on green catalytic techniques: A review. Fuel Process. Technol. 2009, 90, 1502–1514. [Google Scholar] [CrossRef]
- Talha, N.S.; Sulaiman, S. Overview of catalysts in biodiesel production. ARPN J. Eng. Appl. Sci. 2016, 11, 439–448. [Google Scholar]
- Boz, N.; Degirmenbasi, N.; Kalyon, D.M. Esterification and transesterification of waste cooking oil over Amberlyst 15 and modified Amberlyst 15 catalysts. Appl. Catal. B Environ. 2015, 165, 723–730. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, D.-K.; Lee, J.-S. Esterification of free fatty acids using water-tolerable Amberlyst as a heterogeneous catalyst. Bioresour. Technol. 2010, 101, 62–65. [Google Scholar] [CrossRef]
- Shu, Q.; Gao, J.; Nawaz, Z.; Liao, Y.; Wang, D.; Wang, J. Synthesis of biodiesel from waste vegetable oil with large amounts of free fatty acids using a carbon-based solid acid catalyst. Appl. Energy 2010, 87, 2589–2596. [Google Scholar] [CrossRef]
- Calgaroto, C.; Calgaroto, S.; Mazutti, M.A.; de Oliveira, D.; Pergher, S.; De Oliveira, J.V. Production of biodiesel from soybean and Jatropha Curcas oils with KSF and amberlyst 15 catalysts in the presence of co-solvents. Sustain. Chem. Process. 2013, 1, 1–6. [Google Scholar] [CrossRef]
- Lawer-Yolar, G.; Dawson-Andoh, B.; Atta-Obeng, E. Synthesis of biodiesel from tall oil fatty acids by homogeneous and heterogeneous catalysis. Sustain. Chem. 2021, 2, 12. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, F.; Xu, L.; Peng, R.; Li, Y.; Deng, J. Batch and continuous esterification for the direct synthesis of high qualified biodiesel from waste cooking oils (WCO) with Amberlyst-15/Poly (vinyl alcohol) membrane as a bifunctional catalyst. Chem. Eng. J. 2020, 388, 124214. [Google Scholar] [CrossRef]
- Somnuk, K.; Smithmaitrie, P.; Prateepchaikul, G. Optimization of continuous acid-catalyzed esterification for free fatty acids reduction in mixed crude palm oil using static mixer coupled with high-intensity ultrasonic irradiation. Energy Conv. Manag. 2013, 68, 193–199. [Google Scholar] [CrossRef]
- Oo, Y.M.; Prateepchaikul, G.; Somnuk, K. Continuous acid-catalyzed esterification using a 3D printed rotor-stator hydrodynamic cavitation reactor reduces free fatty acid content in mixed crude palm oil. Ultrason. Sonochem. 2021, 72, 105419. [Google Scholar]
- Chandane, V.S.; Rathod, A.P.; Wasewar, K.L.; Sonawane, S.S. Esterification of propionic acid with isopropyl alcohol over ion exchange resins: Optimization and kinetics. Korean J. Chem. Eng. 2017, 34, 249–258. [Google Scholar] [CrossRef]
- Pantoja, S.S.; Mescouto, V.A.D.; Costa, C.E.F.D.; Zamian, J.R.; Rocha Filho, G.N.D.; Nascimento, L.A.S.D. High-quality biodiesel production from buriti (Mauritia flexuosa) oil soapstock. Molecules 2019, 24, 94. [Google Scholar] [CrossRef]
- Zare, M.; Golmakani, M.T.; Sardarian, A. Green synthesis of banana flavor using different catalysts: A comparative study of different methods. Green Chem. Lett. Rev. 2020, 13, 82–91. [Google Scholar] [CrossRef]
- Abdullah, R.F.; Rashid, U.; Taufiq-Yap, Y.H.; Ibrahim, M.L.; Ngamcharussrivichai, C.; Azam, M. Synthesis of bifunctional nanocatalyst from waste palm kernel shell and its application for biodiesel production. RSC Adv. 2020, 10, 27183–27193. [Google Scholar] [CrossRef]
- Fan, G.; Liao, C.; Fang, T.; Luo, S.; Song, G. Amberlyst 15 as a new and reusable catalyst for the conversion of cellulose into cellulose acetate. Carbohydr. Polym. 2014, 112, 203–209. [Google Scholar] [CrossRef]
- Amberlyst 15 Hydrogen Form. Available online: https://www.sigmaaldrich.com/TH/en/product/sial/216380 (accessed on 3 January 2011).
- Siril, P.F.; Cross, H.E.; Brown, D.R. New polystyrene sulfonic acid resin catalysts with enhanced acidic and catalytic properties. J. Mol. Catal. A Chem. 2008, 279, 63–68. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).