Recent Advancements in Photocatalysis Coupling by External Physical Fields
Abstract
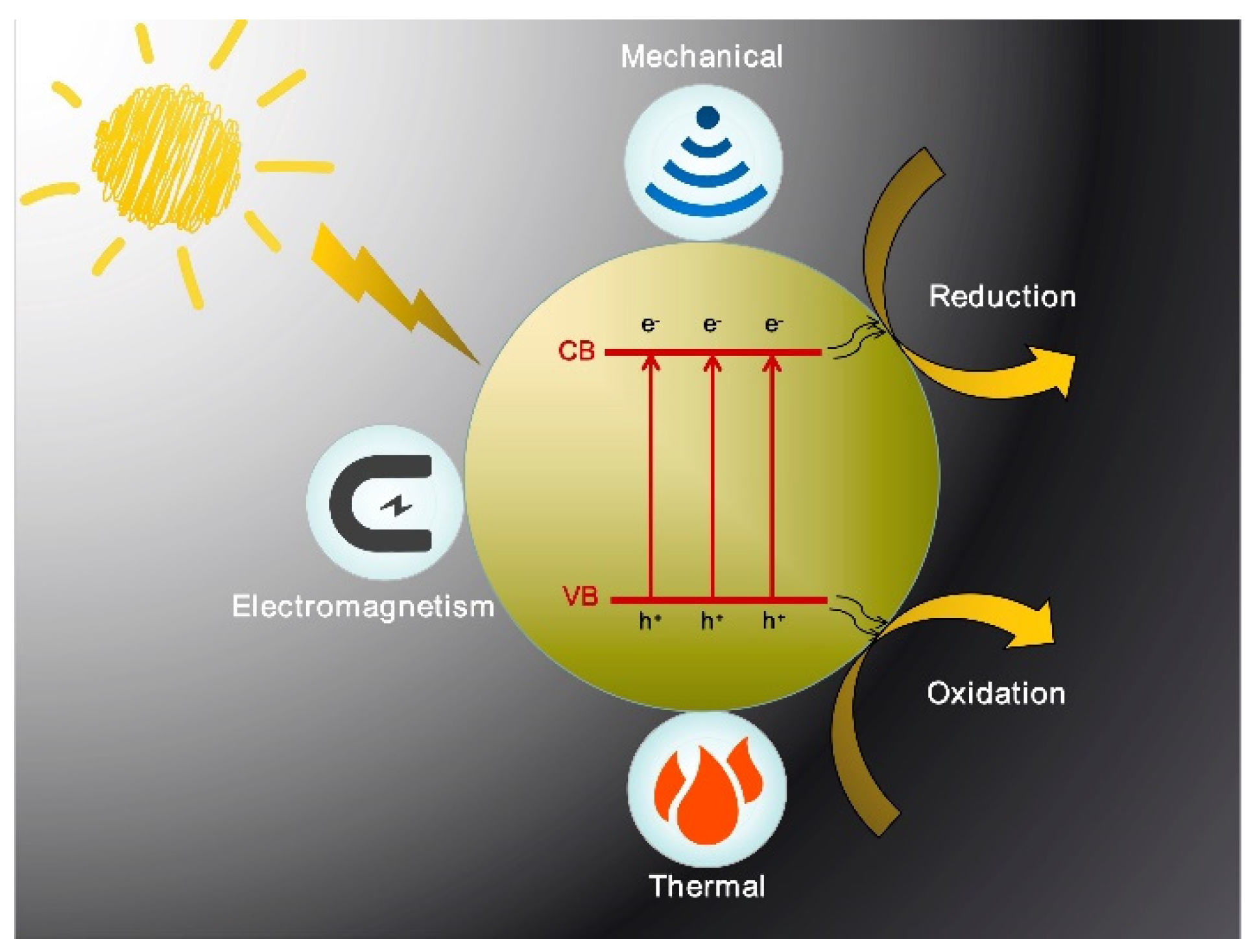
:1. Introduction
2. Thermal-Coupled Photocatalysis (TCP)
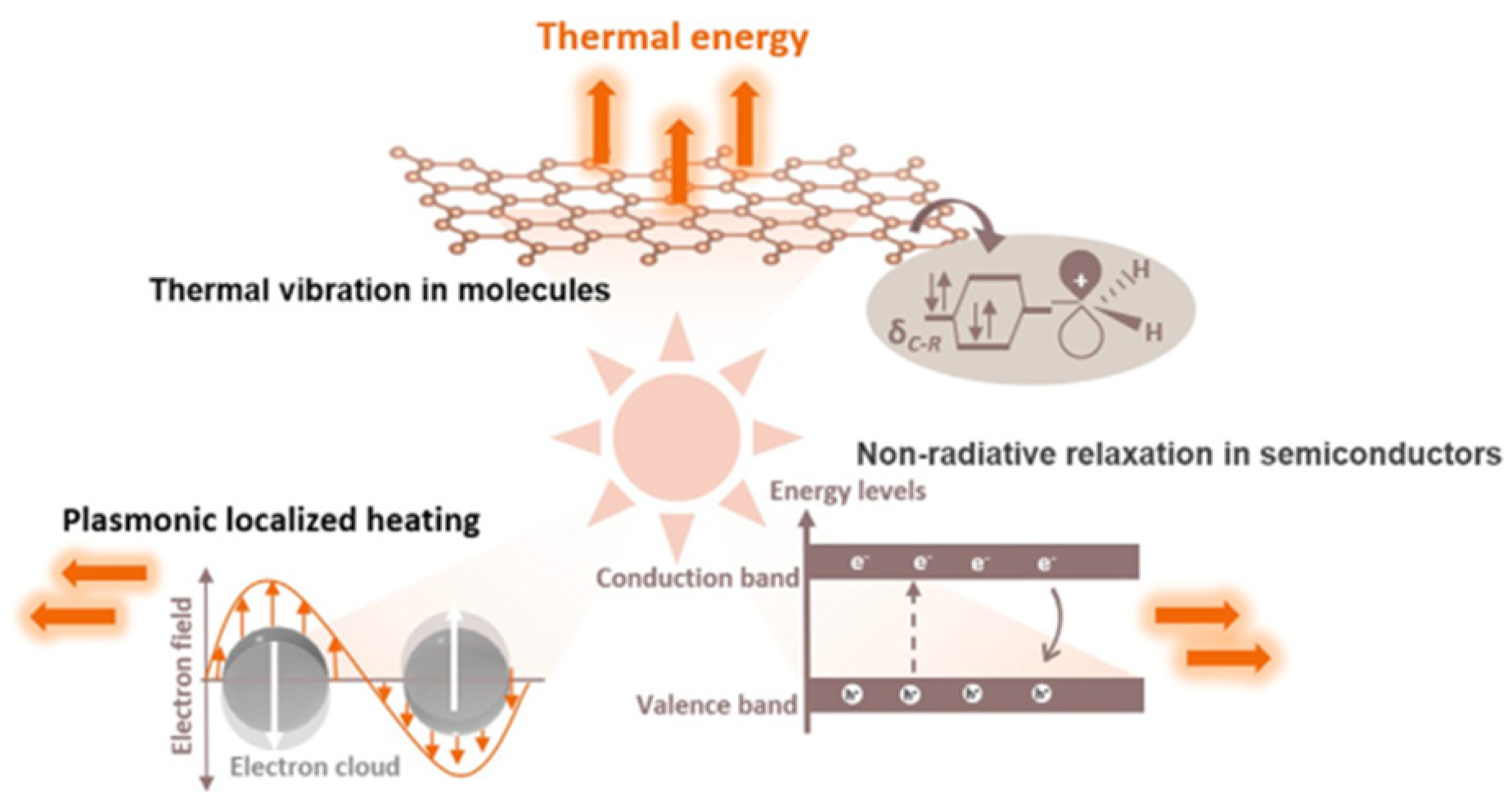
2.1. The Primary Source of Thermal Energy
2.1.1. External Direct Heating
2.1.2. Near-Infrared Indirect Heating
2.1.3. Microwave Indirect Heating
2.2. Materials of Thermal-Coupled Photocatalysis
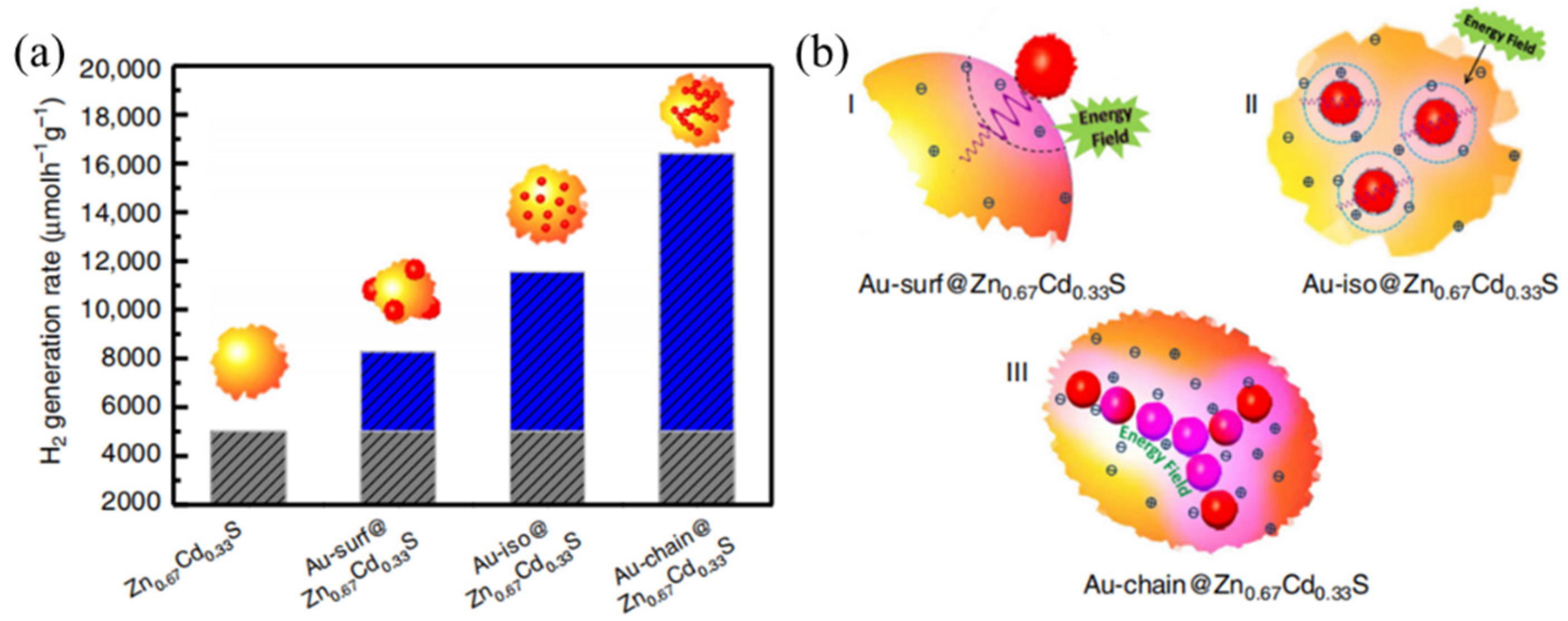
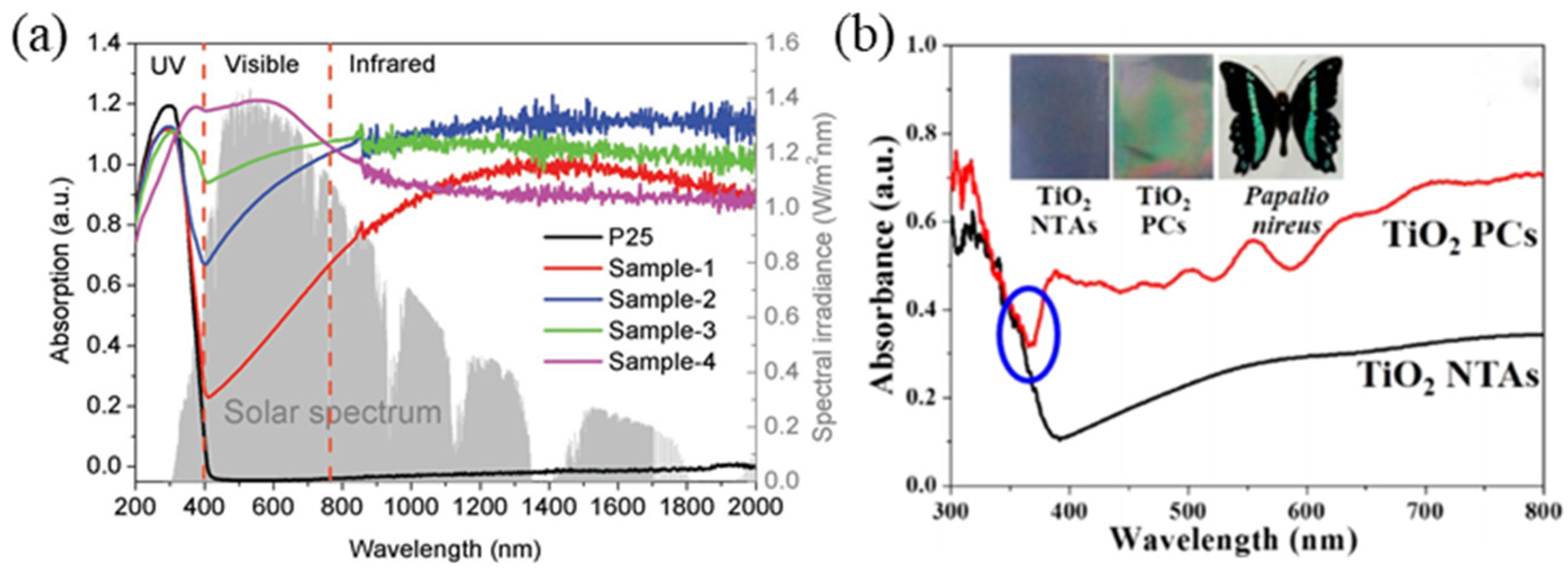
2.2.1. Metallic Materials with Localized Surface Plasmon Effect
2.2.2. Narrow-Band Semiconductors with Non-Radiative Relaxation
2.2.3. Carbon-Based Materials with Thermal Vibration
2.3. Applications of Thermal-Coupled Photocatalysis
2.3.1. Artificial Photosynthesis
2.3.2. Water Splitting
2.3.3. Pollutants Degradation
3. Mechanical-Coupled Photocatalysis (MCP)
3.1. The Primary Source of Mechanical Energy
3.1.1. Ultrasound
3.1.2. Stir
3.2. Materials for Mechanical-Coupled Photocatalysis
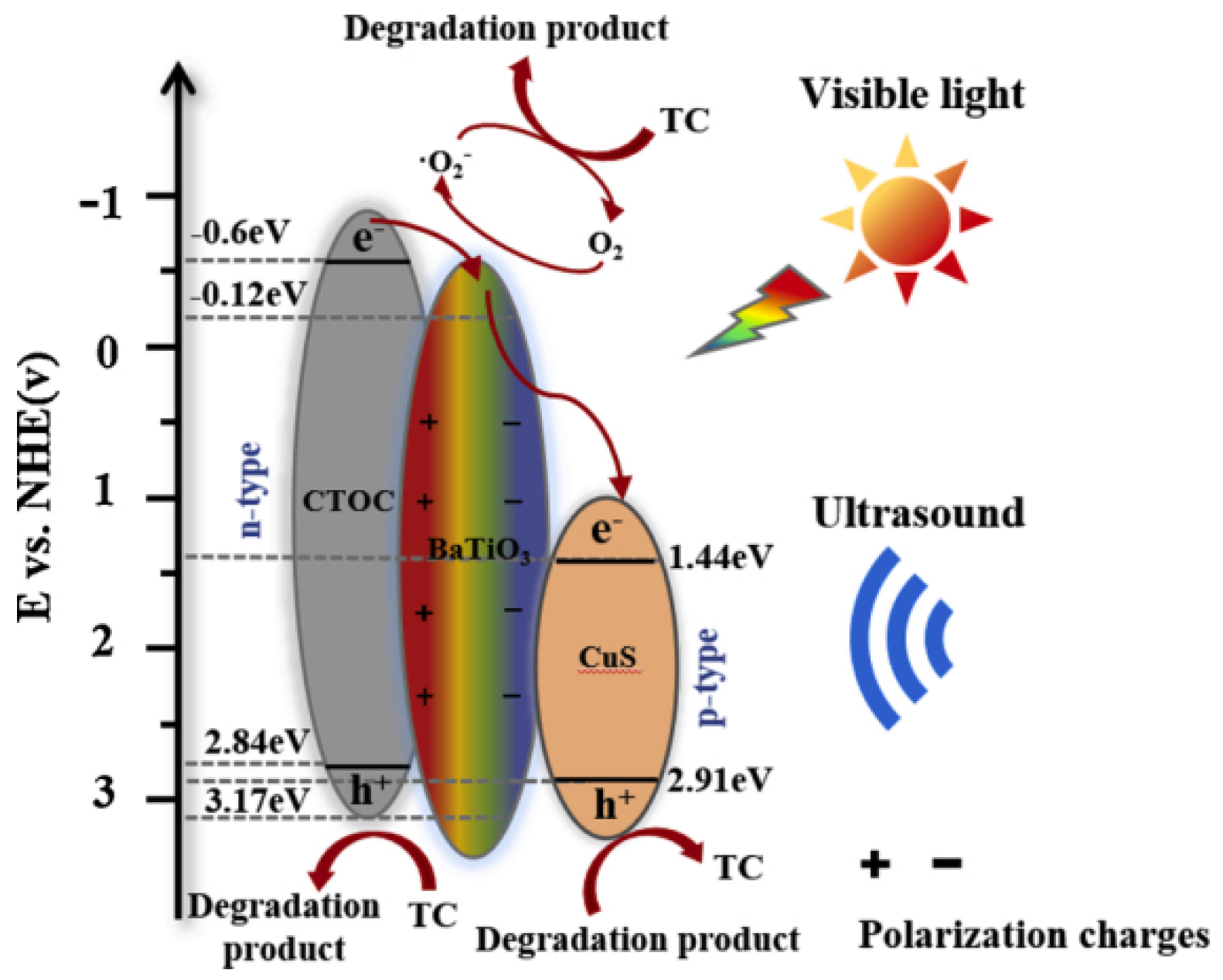
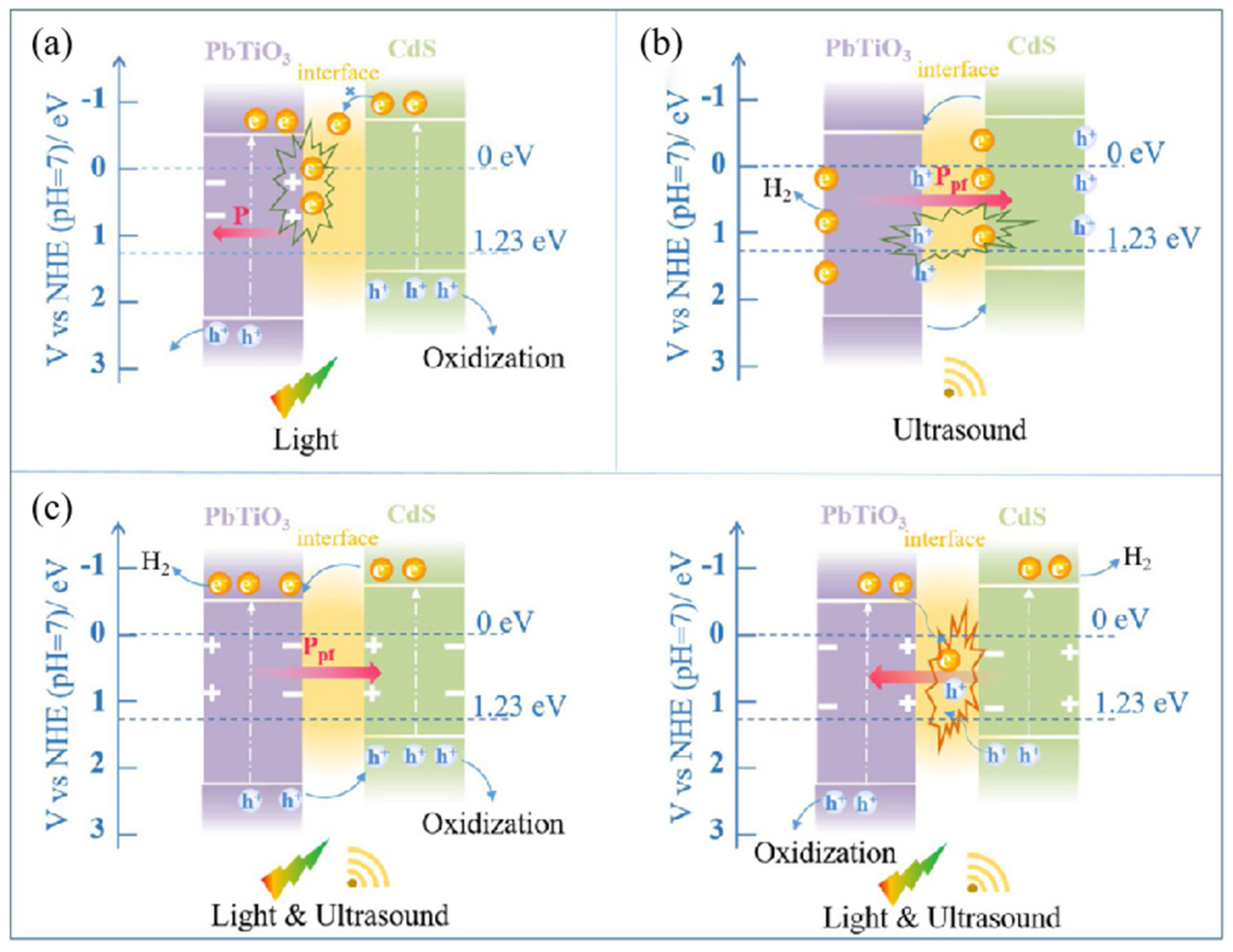
3.2.1. Titanate-Based Materials
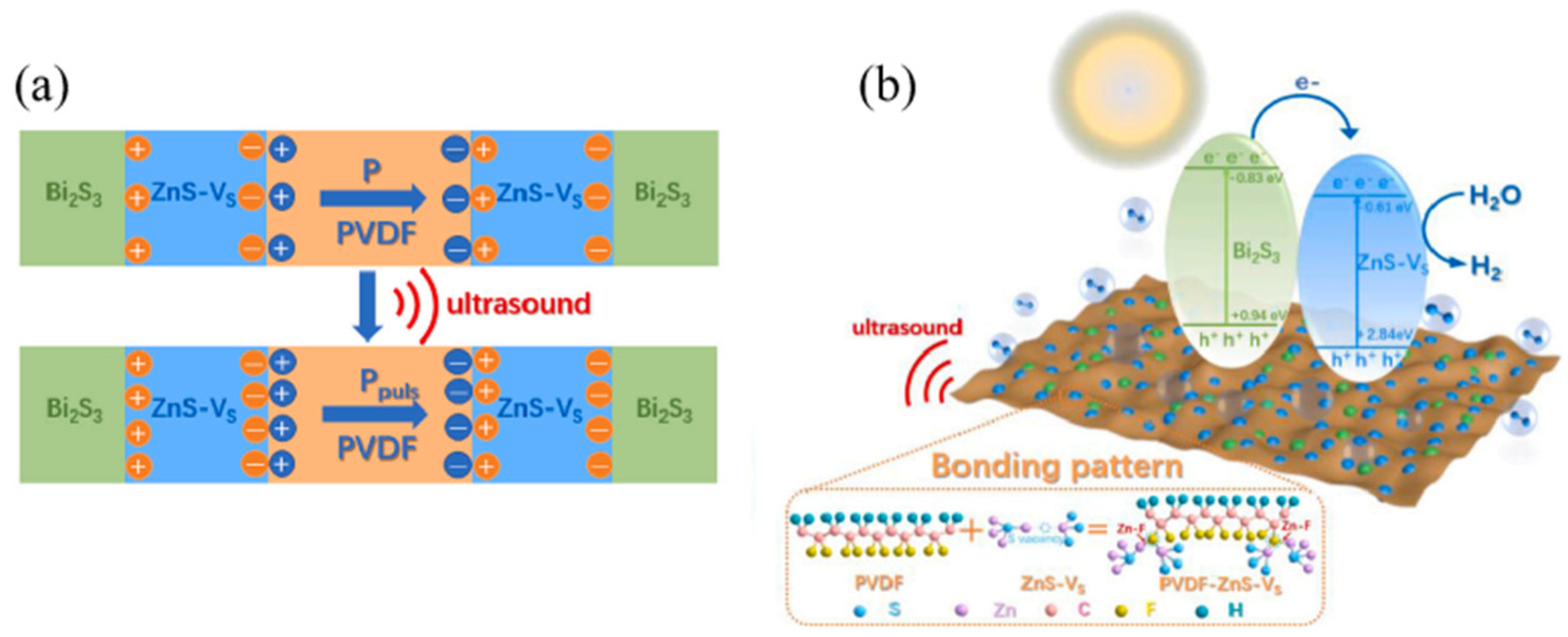
3.2.2. Sulfide-Based Materials
3.2.3. Polyvinylidene Fluoride
4. Electromagnetism-Coupled Photocatalysis (ECP)
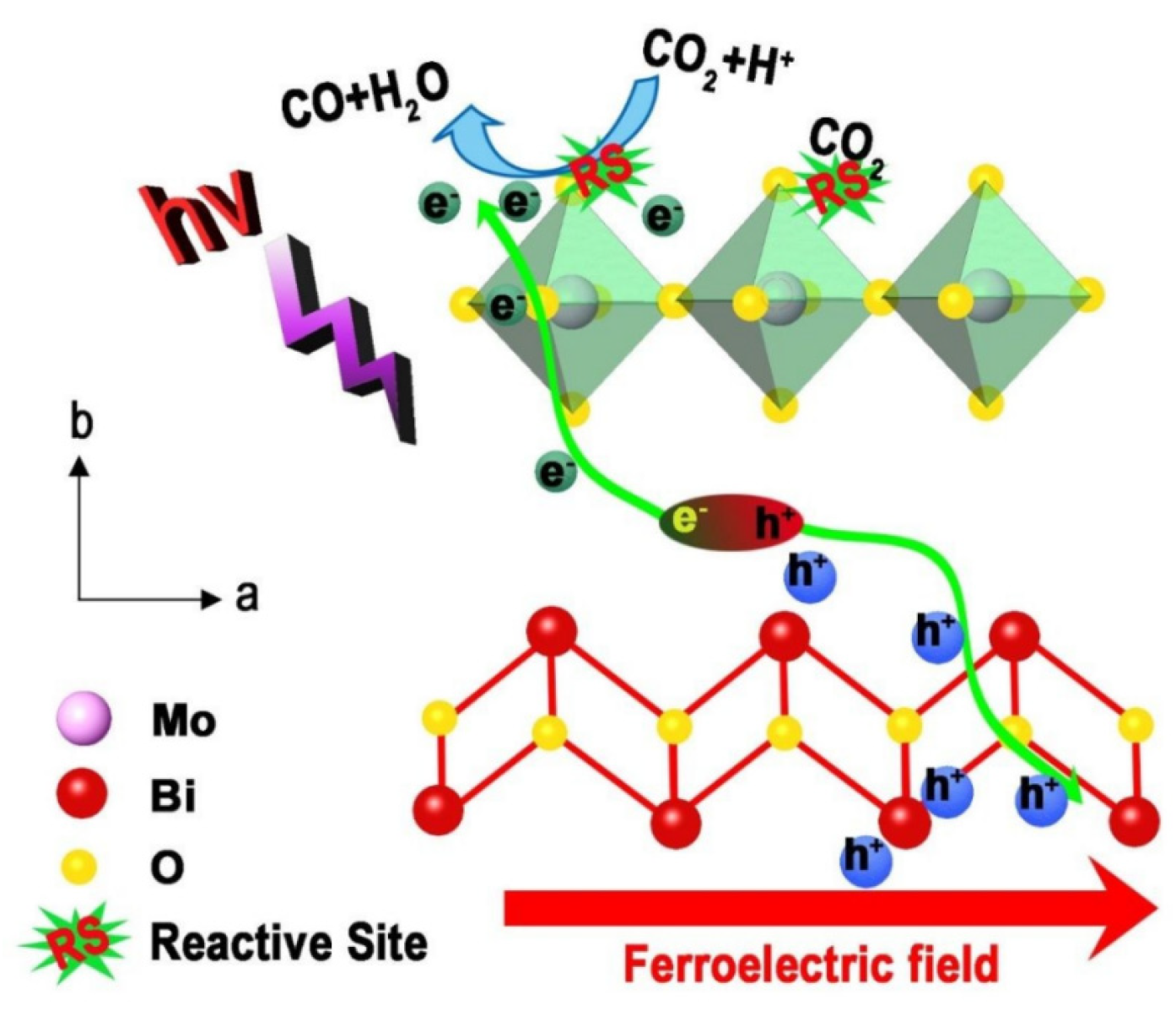
4.1. Electro-Coupled Photocatalysis
4.2. Magnetism-Coupled Photocatalysis
5. Conclusions
- (i)
- Evaluation of energy conversion efficiency. When external physical fields are applied to the photocatalytic reaction process, additional energy will inevitably be input into the system. Therefore, the solar energy conversion efficiency cannot only be considered when calculating energy conversion efficiency. The extra energy generated by external physical fields should be taken into account.
- (ii)
- Ubiquitous thermal effect. Thermal energy is low-quality energy. The external physical fields will eventually dissipate into heat energy. The special thermal effects can be generated by microwaves, ultrasonic waves, and electromagnetism waves. Thus, it is important to distinguish thermal effects and non-thermal effects on photocatalysis coupling by external physical fields.
- (iii)
- A single material response for multiple-physical fields. To realize photocatalysis coupling by external physical fields, composite materials combining photocatalysts and external field absorption materials are usually used. However, the composite materials are complicated both in the synthesis and photocatalytic reaction process. So, it is interesting to explore a single material that can respond to multiple-physical fields. For example, the spontaneous symmetry-breaking semiconductors can absorb multiple-physical fields at the same time, which might be used in photocatalysis coupling by external physical fields.
- (iv)
- Mechanisms for potential barrier formation by external physical fields. When external physical fields are applied to photocatalysts, a built-in electric field with different directions will be generated to separate the photogenerated carriers. However, mechanisms for potential barrier formation by external physical fields need to be uncovered. Thus, this built-in electric field can be controlled and optimized in favor of photocatalysis coupling by external physical fields.
- (v)
- Reactor design for photocatalysis coupling by external physical fields. The traditional photocatalytic reactor is not satisfied with photocatalysis coupling by external physical fields. The design principles of the reactor must be high-efficiency and well-adapted for different external physical fields.
- (vi)
- Horizontal comparison is neglected in the study of photocatalysis coupling by external physical fields. Different external physical fields should make different contributions to photocatalysis. Keeping a balance between photocatalytic efficiency and economic efficiency, the best assisted physical field for photocatalysis needs to be further studied.
Author Contributions
Funding
Conflicts of Interest
References
- Gong, E.; Ali, S.; Hiragond, C.B.; Kim, H.S.; Powar, N.S.; Kim, D.; Kim, H.; In, S.-I. Solar fuels: Research and development strategies to accelerate photocatalytic CO2 conversion into hydrocarbon fuels. Energ Environ. Sci. 2022, 15, 880–937. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Najeeb, J.; Hussain, Z. Fundamentals and photocatalysis of methylene blue dye using various nanocatalytic assemblies- a critical review. J. Clean Prod. 2021, 298, 126567. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, J.; Shangguan, W. A review on photocatalysis in antibiotic wastewater: Pollutant degradation and hydrogen production. Chin. J. Catal. 2020, 41, 1440–1450. [Google Scholar] [CrossRef]
- Shafiq, I.; Shafique, S.; Akhter, P.; Abbas, G.; Qurashi, A.; Hussain, M. Efficient catalyst development for deep aerobic photocatalytic oxidative desulfurization: Recent advances, confines, and outlooks. Catal. Rev. 2021, 1–46. [Google Scholar] [CrossRef]
- Shafiq, I.; Hussain, M.; Rashid, R.; Shafique, S.; Akhter, P.; Yang, W.; Ahmed, A.; Nawaz, Z.; Park, Y.-K. Development of hierarchically porous LaVO4 for efficient visible-light-driven photocatalytic desulfurization of diesel. Chem. Eng. J. 2021, 420, 130529. [Google Scholar] [CrossRef]
- Mahboob, I.; Shafiq, I.; Shafique, S.; Akhter, P.; Amjad, U.-e.-S.; Hussain, M.; Park, Y.-K. Effect of active species scavengers in photocatalytic desulfurization of hydrocracker diesel using mesoporous Ag3VO4. Chem. Eng. J. 2022, 441, 136063. [Google Scholar] [CrossRef]
- Lei, W.; Suzuki, N.; Terashima, C.; Fujishima, A. Hydrogel photocatalysts for efficient energy conversion and environmental treatment. Front. Energy 2021, 15, 577–595. [Google Scholar] [CrossRef]
- Bedia, J.; Muelas-Ramos, V.; Penas-Garzon, M.; Gomez-Aviles, A.; Rodriguez, J.J.; Belver, C. A review on the synthesis and characterization of metal organic frameworks for photocatalytic water purification. Catalysts 2019, 9, 52. [Google Scholar] [CrossRef]
- Chen, Y.L.; Bai, X. A review on quantum dots modified g-C3N4-based photocatalysts with improved photocatalytic activity. Catalysts 2020, 10, 142. [Google Scholar] [CrossRef] [Green Version]
- Kumaravel, V.; Imam, M.D.; Badreldin, A.; Chava, R.K.; Do, J.Y.; Kang, M.; Abdel-Wahab, A. Photocatalytic hydrogen production: Role of sacrificial reagents on the activity of oxide, carbon, and sulfide catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Tao, Y.; Shen, L.; Xu, Z.; Bian, Z.; Li, H. Challenges of photocatalysis and their coping strategies. Chem. Catal. 2022, 2, 1315–1345. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, H.; Wang, S.; Li, X. How to apply metal halide perovskites to photocatalysis: Challenges and development. Nanoscale 2021, 13, 10281–10304. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Chen, S.; Yin, H.; Liu, P.; Wang, Y.; Zhao, H. Stabilisation and Performance Enhancement Strategies for Halide Perovskite Photocatalysts. Adv. Mater. 2022, e2203836. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.; Chen, D.; Tachibana, Y.; Suzuki, H.; Abe, R.; Caruso, R.A. Developing sustainable, high-performance perovskites in photocatalysis: Design strategies and applications. Chem. Soc. Rev. 2021, 50, 13692–13729. [Google Scholar] [CrossRef]
- Tasleem, S.; Tahir, M. Current trends in strategies to improve photocatalytic performance of perovskites materials for solar to hydrogen production. Renew. Sustain. Energ Rev. 2020, 132, 110073. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, Q.; Xi, X.; Xing, M.; Zhang, J. Z-scheme CdS/WO3 on a carbon cloth enabling effective hydrogen evolution. Front. Energy 2021, 15, 678–686. [Google Scholar] [CrossRef]
- Li, X.; Wang, W.; Dong, F.; Zhang, Z.; Han, L.; Luo, X.; Huang, J.; Feng, Z.; Chen, Z.; Jia, G.; et al. Recent Advances in Noncontact External-Field-Assisted Photocatalysis: From Fundamentals to Applications. ACS Catal. 2021, 11, 4739–4769. [Google Scholar] [CrossRef]
- Hu, C.; Tu, S.; Tian, N.; Ma, T.; Zhang, Y.; Huang, H. Photocatalysis Enhanced by External Fields. Angew. Chem. Int. Ed. 2021, 60, 16309–16328. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, B.; Chen, F.; Liu, C.; Lu, S.; Yu, Y.; Zhang, B. Thermally-assisted photocatalytic CO2 reduction to fuels. Chem. Eng. J. 2021, 408, 127280. [Google Scholar] [CrossRef]
- Song, C.; Wang, Z.; Yin, Z.; Xiao, D.; Ma, D. Principles and applications of photothermal catalysis. Chem. Catal. 2022, 2, 52–83. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, H.; Fan, D.; Chen, Z.; Yang, X. Coupling solar-driven photothermal effect into photocatalysis for sustainable water treatment. J. Hazard. Mater. 2022, 423, 127128. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Sun, J.; Li, D.H.; Wei, J.J. Review of synergistic photo-thermo-catalysis: Mechanisms, materials and applications. Int. J. Hydrogen Energ. 2020, 45, 30288–30324. [Google Scholar] [CrossRef]
- Wang, Z.J.; Song, H.; Liu, H.; Ye, J. Coupling of Solar Energy and Thermal Energy for Carbon Dioxide Reduction: Status and Prospects. Angew. Chem. Int. Ed. 2020, 59, 8016–8035. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Bian, X.; Zhao, Y.; Shi, R.; Zhang, T. Progress and Prospect of Photothermal Catalysis. Chem. Res. Chin. Univ. 2022, 38, 723–734. [Google Scholar] [CrossRef]
- Keller, N.; Ivanez, J.; Highfield, J.; Ruppert, A.M. Photo-/thermal synergies in heterogeneous catalysis: Towards low-temperature (solar-driven) processing for sustainable energy and chemicals. Appl. Catal. B Environ. 2021, 296, 120320. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, J.; Guo, X.; Xing, F.; Huang, C.; Song, M. Thermal-assisted photocatalytic H2 production over sulfur vacancy-rich Co0.85Se/Mn0.3Cd0.7S nanorods under visible light. Appl. Surf. Sci. 2021, 557, 149812. [Google Scholar] [CrossRef]
- Hu, S.; Shi, J.; Luo, B.; Ai, C.; Jing, D. Significantly enhanced photothermal catalytic hydrogen evolution over Cu2O-rGO/TiO2 composite with full spectrum solar light. J. Colloid Interface Sci. 2022, 608, 2058–2065. [Google Scholar] [CrossRef]
- Li, P.; Liu, L.; An, W.; Wang, H.; Cui, W. Efficient photothermal catalytic CO2 reduction to CH3CH2OH over Cu2O/g-C3N4 assisted by ionic liquids. Appl. Surf. Sci. 2021, 565, 150448. [Google Scholar] [CrossRef]
- Du, C.; Yan, B.; Yang, G. Promoting photocatalytic hydrogen evolution by introducing hot islands: SnSe nanoparticles on ZnIn2S4 monolayer. Chem. Eng. J. 2021, 404, 126477. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Wang, Y.; Zhang, J. Two-photon induced NIR active core-shell structured WO3/CdS for enhanced solar light photocatalytic performance. Appl. Catal. B Environ. 2020, 272, 118979. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, W.; Shang, Q.; Guo, Y.; Hu, H.; Li, Z.; Zhang, Y.; Liu, L.; Wang, H.; Tan, X.; et al. Discerning the mechanism of expedited interfacial electron transformation boosting photocatalytic hydrogen evolution by metallic 1T-WS2-induced photothermal effect. Appl. Catal. B Environ. 2022, 310, 121295. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, M.; Kang, Z.; Wang, B.; Wang, B.; Jiang, F.; Wang, X.; Yang, D.-P.; Luque, R. NIR-triggered photocatalytic/photothermal/photodynamic water remediation using eggshell-derived CaCO3/CuS nanocomposites. Chem. Eng. J. 2020, 388, 124304. [Google Scholar] [CrossRef]
- Ai, Z.; Yang, P.; Lu, X. Degradation of 4-chlorophenol by a microwave assisted photocatalysis method. J. Hazard. Mater. 2005, 124, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Nüchter, M.; Müller, U.; Ondruschka, B.; Tied, A.; Lautenschläger, W. Microwave-Assisted Chemical Reactions. Chem. Eng. Technol. 2003, 26, 1207–1216. [Google Scholar] [CrossRef]
- Ling, L.; Feng, Y.; Li, H.; Chen, Y.; Wen, J.; Zhu, J.; Bian, Z. Microwave induced surface enhanced pollutant adsorption and photocatalytic degradation on Ag/TiO2. Appl. Surf. Sci. 2019, 483, 772–778. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, L.; Li, K.; Wang, J.; Fang, D.; Zhang, Y.; Tian, D.; Zhang, Z.; Dionysiou, D.D. Fabrication of novel Z-scheme SrTiO3/MnFe2O4 system with double-response activity for simultaneous microwave-induced and photocatalytic degradation of tetracycline and mechanism insight. Chem. Eng. J. 2020, 400, 125981. [Google Scholar] [CrossRef]
- Gayathri, P.V.; Yesodharan, S.; Yesodharan, E.P. Microwave/Persulphate assisted ZnO mediated photocatalysis (MW/PS/UV/ZnO) as an efficient advanced oxidation process for the removal of RhB dye pollutant from water. J. Environ. Chem. Eng. 2019, 7, 103122. [Google Scholar] [CrossRef]
- Mateo, D.; Cerrillo, J.L.; Durini, S.; Gascon, J. Fundamentals and applications of photo-thermal catalysis. Chem. Soc. Rev. 2021, 50, 2173–2210. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef]
- Manthiram, K.; Alivisatos, A.P. Tunable localized surface plasmon resonances in tungsten oxide nanocrystals. J. Am. Chem. Soc. 2012, 134, 3995–3998. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Aslam, U.; Boerigter, C.; Morabito, M. Photochemical transformations on plasmonic metal nanoparticles. Nat. Mater. 2015, 14, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.H.; Zhao, J.; Hicks, E.M.; Schatz, G.C.; Van Duyne, R.P. Plasmonic Properties of Copper Nanoparticles Fabricated by Nanosphere Lithography. Nano Lett. 2007, 7, 1947–1952. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Chen, H.; Jiang, R.; Sun, L.D.; Li, Q.; Wang, J.; Yu, J.C.; Yan, C.H. Plasmonic harvesting of light energy for Suzuki coupling reactions. J. Am. Chem. Soc. 2013, 135, 5588–5601. [Google Scholar] [CrossRef]
- Yu, G.; Qian, J.; Zhang, P.; Zhang, B.; Zhang, W.; Yan, W.; Liu, G. Collective excitation of plasmon-coupled Au-nanochain boosts photocatalytic hydrogen evolution of semiconductor. Nat. Commun. 2019, 10, 4912. [Google Scholar] [CrossRef]
- Jian, C.-c.; Zhang, J.; He, W.; Ma, X. Au-Al intermetallic compounds: A series of more efficient LSPR materials for hot carriers-based applications than noble metal Au. Nano Energy 2021, 82, 105763. [Google Scholar] [CrossRef]
- Han, B.; Chen, L.; Jin, S.; Guo, S.; Park, J.; Yoo, H.S.; Park, J.H.; Zhao, B.; Jung, Y.M. Modulating Mechanism of the LSPR and SERS in Ag/ITO Film: Carrier Density Effect. J. Phys. Chem. Lett. 2021, 12, 7612–7618. [Google Scholar] [CrossRef]
- Li, P.; Liu, L.; An, W.; Wang, H.; Guo, H.; Liang, Y.; Cui, W. Ultrathin porous g-C3N4 nanosheets modified with AuCu alloy nanoparticles and C-C coupling photothermal catalytic reduction of CO to ethanol. Appl. Catal. B Environ. 2020, 266, 118618. [Google Scholar] [CrossRef]
- Yang, Y.; Cong, Y.; Lin, X.; Cao, B.; Dong, D.; Liu, K.; Xiao, Y.; Shang, J.; Bao, Y.; Liu, Y.; et al. Dual LSPR of Au/W18O49 heterostructures for upconversion enhancement and application of molecular detection. J. Mater. Chem. A 2020, 8, 4040–4048. [Google Scholar] [CrossRef]
- Kong, W.; Xing, Z.; Fang, B.; Cui, Y.; Li, Z.; Zhou, W. Plasmon Ag/Na-doped defective graphite carbon nitride/NiFe layered double hydroxides Z-scheme heterojunctions toward optimized photothermal-photocatalytic-Fenton performance. Appl. Catal. B Environ. 2022, 304, 120969. [Google Scholar] [CrossRef]
- Liao, J.; Xu, Y.; Zhao, Y.; Wang, C.-C.; Ge, C. Ag and Fe3O4 Comodified WO3-x Nanocomposites for Catalytic Photothermal Degradation of Pharmaceuticals and Personal Care Products. ACS Appl. Nano Mater. 2021, 4, 1898–1905. [Google Scholar] [CrossRef]
- Wei, T.; Liu, Y.; Dong, W.; Zhang, Y.; Huang, C.; Sun, Y.; Chen, X.; Dai, N. Surface-dependent localized surface plasmon resonances in CuS nanodisks. ACS Appl. Mater. Interfaces 2013, 5, 10473–10477. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Swihart, M.T. Reversible Crystal Phase Interconversion between Covellite CuS and High Chalcocite Cu2S Nanocrystals. Chem. Mater. 2017, 29, 4783–4791. [Google Scholar] [CrossRef]
- Gao, H.; Chen, Y.; Li, H.; Zhang, F.; Tian, G. Hierarchical Cu7S4-Cu9S8 heterostructure hollow cubes for photothermal aerobic oxidation of amines. Chem. Eng. J. 2019, 363, 247–258. [Google Scholar] [CrossRef]
- Lin, H.; Wang, X.; Yu, L.; Chen, Y.; Shi, J. Two-Dimensional Ultrathin MXene Ceramic Nanosheets for Photothermal Conversion. Nano Lett. 2017, 17, 384–391. [Google Scholar] [CrossRef]
- Naik, G.V.; Schroeder, J.L.; Ni, X.; Kildishev, A.V.; Sands, T.D.; Boltasseva, A. Titanium nitride as a plasmonic material for visible and near-infrared wavelengths [erratum]. Opt. Mater. Express 2013, 3, 1658. [Google Scholar] [CrossRef]
- Lou, Z.; Gu, Q.; Liao, Y.; Yu, S.; Xue, C. Promoting Pd-catalyzed Suzuki coupling reactions through near-infrared plasmon excitation of WO3-x nanowires. Appl. Catal. B Environ. 2016, 184, 258–263. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Wu, C.; Gao, J.; Li, M.; Xing, Z.; Li, Z.; Zhou, W. Hollow Nanoboxes Cu2-xS@ZnIn2S4 Core-Shell S-Scheme Heterojunction with Broad-Spectrum Response and Enhanced Photothermal-Photocatalytic Performance. Small 2022, 18, e2202544. [Google Scholar] [CrossRef]
- Xie, Z.; Duo, Y.; Lin, Z.; Fan, T.; Xing, C.; Yu, L.; Wang, R.; Qiu, M.; Zhang, Y.; Zhao, Y.; et al. The Rise of 2D Photothermal Materials beyond Graphene for Clean Water Production. Adv. Sci. 2020, 7, 1902236. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, Y.; Deng, L.; Wei, N.; Weng, Y.; Dong, S.; Qi, D.; Qiu, J.; Chen, X.; Wu, T. High-Performance Photothermal Conversion of Narrow-Bandgap Ti2O3 Nanoparticles. Adv. Mater. 2017, 29, 1603730. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. Visible-light photocatalytic, solar thermal and photoelectrochemical properties of aluminium-reduced black titania. Energy Environ. Sci. 2013, 6, 3007–3014. [Google Scholar] [CrossRef]
- Ye, M.; Jia, J.; Wu, Z.; Qian, C.; Chen, R.; O’Brien, P.G.; Sun, W.; Dong, Y.; Ozin, G.A. Synthesis of Black TiOx Nanoparticles by Mg Reduction of TiO2 Nanocrystals and their Application for Solar Water Evaporation. Adv. Energy Mater. 2017, 7, 1601811. [Google Scholar] [CrossRef]
- Low, J.; Zhang, L.; Zhu, B.; Liu, Z.; Yu, J. TiO2 Photonic Crystals with Localized Surface Photothermal Effect and Enhanced Photocatalytic CO2 Reduction Activity. ACS Sustain. Chem. Eng. 2018, 6, 15653–15661. [Google Scholar] [CrossRef]
- Pan, J.; Yu, X.; Dong, J.; Zhao, L.; Liu, L.; Liu, J.; Zhao, X.; Liu, L. Diatom-Inspired TiO2-PANi-Decorated Bilayer Photothermal Foam for Solar-Driven Clean Water Generation. ACS Appl. Mater. Interfaces 2021, 13, 58124–58133. [Google Scholar] [CrossRef]
- Chou, S.S.; Kaehr, B.; Kim, J.; Foley, B.M.; De, M.; Hopkins, P.E.; Huang, J.; Brinker, C.J.; Dravid, V.P. Chemically exfoliated MoS2 as near-infrared photothermal agents. Angew. Chem. Int. Ed. 2013, 52, 4160–4164. [Google Scholar] [CrossRef] [PubMed]
- Ghim, D.; Jiang, Q.; Cao, S.; Singamaneni, S.; Jun, Y.-S. Mechanically interlocked 1T/2H phases of MoS2 nanosheets for solar thermal water purification. Nano Energy 2018, 53, 949–957. [Google Scholar] [CrossRef]
- Ding, D.; Huang, W.; Song, C.; Yan, M.; Guo, C.; Liu, S. Non-stoichiometric MoO3-x quantum dots as a light-harvesting material for interfacial water evaporation. Chem. Commun. 2017, 53, 6744–6747. [Google Scholar] [CrossRef]
- Qi, Y.; Song, L.; Ouyang, S.; Liang, X.; Ning, S.; Zhang, Q.; Ye, J. Photoinduced Defect Engineering: Enhanced Photothermal Catalytic Performance of 2D Black In2O3-x Nanosheets with Bifunctional Oxygen Vacancies. Adv. Mater. 2020, 32, e1903915. [Google Scholar] [CrossRef]
- Zhang, Y.; Heo, Y.-J.; Son, Y.-R.; In, I.; An, K.-H.; Kim, B.-J.; Park, S.-J. Recent advanced thermal interfacial materials: A review of conducting mechanisms and parameters of carbon materials. Carbon 2019, 142, 445–460. [Google Scholar] [CrossRef]
- Li, J.; Ma, L.; Fu, C.; Huang, Y.; Luo, B.; Cao, J.; Geng, J.; Jing, D. Urchinlike Carbon-Coated TiO2 Microspheres with Enhanced Photothermal–Photocatalytic Hydrogen Evolution Performance for Full-Spectrum Solar Energy Conversion. Ind. Eng. Chem. Res. 2022, 61, 6436–6447. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, W.; Wang, X. Carbon nanocomposites with high photothermal conversion efficiency. Sci. China Mater. 2018, 61, 905–914. [Google Scholar] [CrossRef]
- Tong, X.; Li, N.; Zeng, M.; Wang, Q. Organic phase change materials confined in carbon-based materials for thermal properties enhancement: Recent advancement and challenges. Renew. Sustain. Energy Rev. 2019, 108, 398–422. [Google Scholar] [CrossRef]
- Guo, X.; Cheng, S.; Cai, W.; Zhang, Y.; Zhang, X.-a. A review of carbon-based thermal interface materials: Mechanism, thermal measurements and thermal properties. Mater. Design 2021, 209, 109936. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.; Song, J.; Yang, Z.; Kuang, Y.; Hitz, E.; Jia, C.; Gong, A.; Jiang, F.; Zhu, J.Y.; et al. Highly Flexible and Efficient Solar Steam Generation Device. Adv. Mater. 2017, 29, 1701756. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, S.; Jia, Y.; Qi, T.; Xiao, L.; Cui, X.; Zhuang, D.; Wei, J. Ultra-black and self-cleaning all carbon nanotube hybrid films for efficient water desalination and purification. Carbon 2020, 169, 134–141. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, D.; Zhang, D.; Ma, L.; Wang, J.; Huang, Y.; Chen, M.; Qian, H.; Li, X. A durable and photothermal superhydrophobic coating with entwinned CNTs-SiO2 hybrids for anti-icing applications. Chem. Eng. J. 2021, 423, 130238. [Google Scholar] [CrossRef]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.; Wang, H.; Casalongue, H.S.; Vinh, D.; Dai, H. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Song, X.; Megarajan, S.K.; Jiang, H. A facile nanocomposite strategy to fabricate a rGO–MWCNT photothermal layer for efficient water evaporation. J. Mater. Chem. A 2018, 6, 963–971. [Google Scholar] [CrossRef]
- Zhang, C.-R.; Cui, W.-R.; Niu, C.-P.; Yi, S.-M.; Liang, R.-P.; Qi, J.-X.; Chen, X.-J.; Jiang, W.; Zhang, L.; Qiu, J.-D. rGO-based covalent organic framework hydrogel for synergistically enhance uranium capture capacity through photothermal desalination. Chem. Eng. J. 2022, 428, 131178. [Google Scholar] [CrossRef]
- Zha, Z.; Yue, X.; Ren, Q.; Dai, Z. Uniform polypyrrole nanoparticles with high photothermal conversion efficiency for photothermal ablation of cancer cells. Adv. Mater. 2013, 25, 777–782. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, Q.; Ghim, D.; Singamaneni, S.; Jun, Y.-S. Localized heating with a photothermal polydopamine coating facilitates a novel membrane distillation process. J. Mater. Chem. A 2018, 6, 18799–18807. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Wu, S.; Xu, B.; Xu, H. Multilayer Polypyrrole Nanosheets with Self-Organized Surface Structures for Flexible and Efficient Solar-Thermal Energy Conversion. Adv. Mater. 2019, 31, e1807716. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Luo, Y.; Li, Q.; Chen, X. Microgroove-Structured PDA/PEI/PPy@PI-MS Photothermal Aerogel with a Multilevel Water Transport Network for Highly Salt-Rejecting Solar-Driven Interfacial Evaporation. ACS Appl. Mater. Interfaces 2021, 13, 40531–40542. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Sun, C.; Xuan, Y.; Zhang, K.; Chang, K. Full solar spectrum driven plasmonic-assisted efficient photocatalytic CO2 reduction to ethanol. Chem. Eng. J. 2022, 430, 132940. [Google Scholar] [CrossRef]
- Lu, C.; Li, X.; Li, J.; Mao, L.; Zhu, M.; Chen, Q.; Wen, L.; Li, B.; Guo, T.; Lou, Z. Nonmetallic surface plasmon resonance coupling with pyroelectric effect for enhanced near-infrared-driven CO2 reduction. Chem. Eng. J. 2022, 445, 136739. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, L.; Li, Z.; Zhang, X.; Dong, X.; Zhang, Y. Efficient CO2 reduction with H2O via photothermal chemical reaction based on Au-MgO dual catalytic site on TiO2. J. CO2 Util. 2022, 55, 101801. [Google Scholar] [CrossRef]
- Han, R.; Chen, L.; Xing, B.; Guo, Q.; Tian, J.; Sha, N.; Zhao, Z. Pr3+-doped La1-xPrxMn0.6Ni0.4O3-δ as efficient artificial photosynthesis catalysts for solar methanol. Catal. Commun. 2022, 165, 106440. [Google Scholar] [CrossRef]
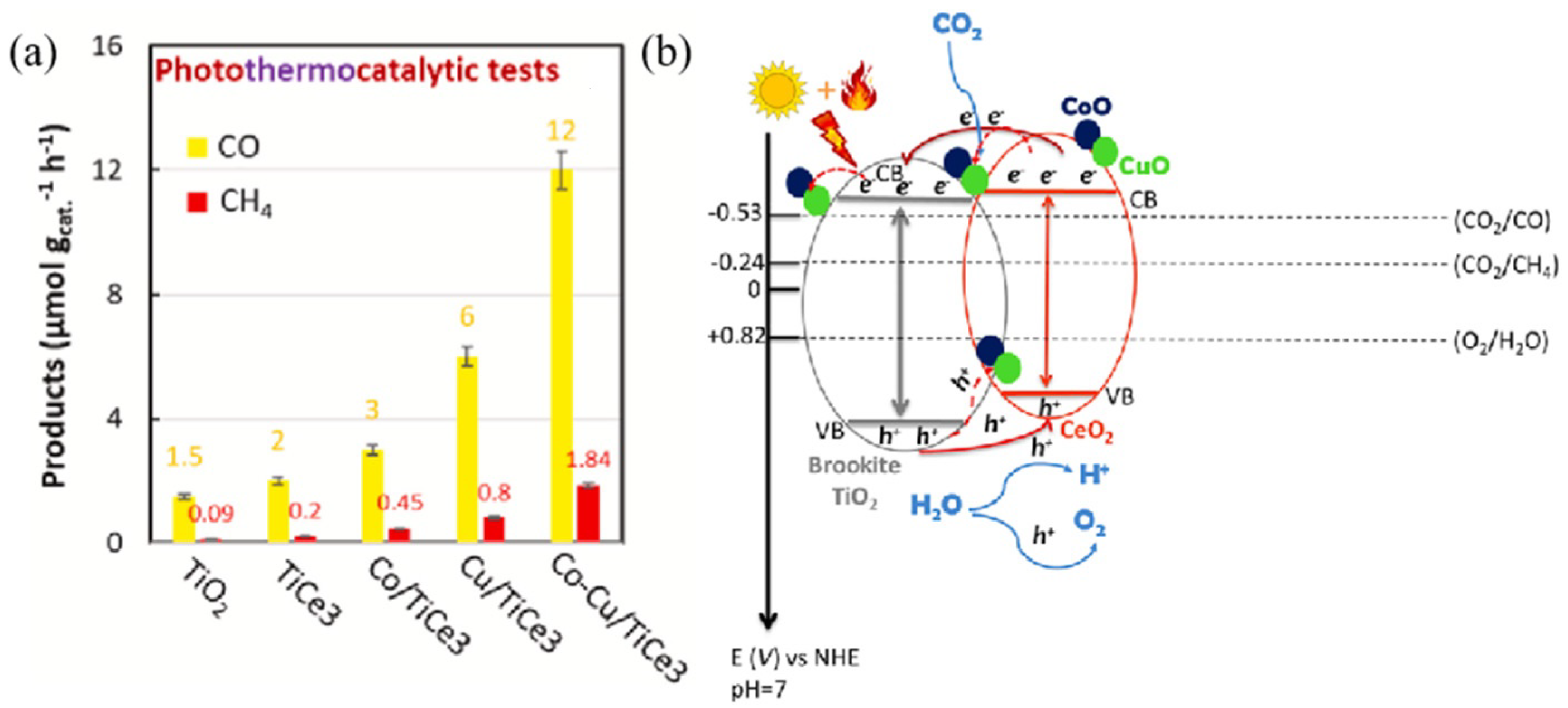
- Fiorenza, R.; Bellardita, M.; Balsamo, S.A.; Spitaleri, L.; Gulino, A.; Condorelli, M.; D’Urso, L.; Scirè, S.; Palmisano, L. A solar photothermocatalytic approach for the CO2 conversion: Investigation of different synergisms on CoO-CuO/brookite TiO2-CeO2 catalysts. Chem. Eng. J. 2022, 428, 131249. [Google Scholar] [CrossRef]
- Bian, H.; Liu, T.; Li, D.; Xu, Z.; Lian, J.; Chen, M.; Yan, J.; Frank Liu, S. Unveiling the effect of interstitial dopants on CO2 activation over CsPbBr3 catalyst for efficient photothermal CO2 reduction. Chem. Eng. J. 2022, 435, 135071. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, L.; Guan, J.; Qian, S.; Zhang, Z.; Ngaw, C.K.; Wan, S.; Wang, S.; Lin, J.; Wang, Y. Controlled Synthesis of Cu0/Cu2O for Efficient Photothermal Catalytic Conversion of CO2 and H2O. Acs Sustain. Chem. Eng. 2021, 9, 1754–1761. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Q.; Zhu, Y.; Xu, D. High efficiency reduction of CO2 to CO and CH4 via photothermal synergistic catalysis of lead-free perovskite Cs3Sb2I9. Appl. Catal. B Environ. 2021, 294, 120236. [Google Scholar] [CrossRef]
- Li, Y.; Wen, M.; Wang, Y.; Tian, G.; Wang, C.; Zhao, J. Plasmonic Hot Electrons from Oxygen Vacancies for Infrared Light-Driven Catalytic CO2 Reduction on Bi2O3-x. Angew. Chem. Int. Ed. 2021, 60, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Guo, L.; Zhang, X.; Ribeiro, C.; He, T. Solar-heating boosted catalytic reduction of CO2 under full-solar spectrum. Chin. J. Catal. 2020, 41, 131–139. [Google Scholar] [CrossRef]
- Yu, F.; Wang, C.; Li, Y.; Ma, H.; Wang, R.; Liu, Y.; Suzuki, N.; Terashima, C.; Ohtani, B.; Ochiai, T.; et al. Enhanced Solar Photothermal Catalysis over Solution Plasma Activated TiO2. Adv. Sci. 2020, 7, 2000204. [Google Scholar] [CrossRef]
- Yang, F.; Li, Z.; Xie, Y.; Wang, S.; Li, M.; Liao, L.; Zhou, W. Ag/polydopamine nanoparticles co-decorated defective mesoporous carbon nitride nanosheets assemblies for wide spectrum response and robust photothermal-photocatalytic performance. Appl. Surf. Sci. 2022, 598, 153895. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Li, Q.; Guan, Z.; Zhang, M.; Yang, J. The photothermal effect enhance visible light-driven hydrogen evolution using urchin-like hollow RuO2/TiO2/Pt/C nanomaterial. J. Alloys Compd. 2022, 890, 161722. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Wang, Y.; Rui, Z. Photothermocatalytic water splitting over Pt/ZnIn2S4 for hydrogen production without external heat. Catal. Today 2022, 402, 210–219. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, L.; Xie, L.; Sun, C.; Zhao, W.; Liu, X.; Zhuang, Z.; Liu, S.; Zhao, Q. Defect-driven selective oxidation of MoS2 nanosheets with photothermal effect for Photo-Catalytic hydrogen evolution reaction. Chem. Eng. J. 2022, 439, 135757. [Google Scholar] [CrossRef]
- Li, Y.; Xue, J.; Shen, Q.; Jia, S.; Li, Q.; Li, Y.; Liu, X.; Jia, H. Construction of a ternary spatial junction in yolk–shell nanoreactor for efficient photo-thermal catalytic hydrogen generation. Chem. Eng. J. 2021, 423, 130188. [Google Scholar] [CrossRef]
- Li, X.; Zhao, S.; Duan, X.; Zhang, H.; Yang, S.-z.; Zhang, P.; Jiang, S.P.; Liu, S.; Sun, H.; Wang, S. Coupling hydrothermal and photothermal single-atom catalysis toward excellent water splitting to hydrogen. Appl. Catal. B Environ. 2021, 283, 119660. [Google Scholar] [CrossRef]
- Li, M.; Sun, J.; Chen, G.; Yao, S.; Cong, B.; Liu, P. Construction photothermal/pyroelectric property of hollow FeS2/Bi2S3 nanostructure with enhanced full spectrum photocatalytic activity. Appl. Catal. B Environ. 2021, 298, 120573. [Google Scholar] [CrossRef]
- Guo, S.; Li, X.; Li, J.; Wei, B. Boosting photocatalytic hydrogen production from water by photothermally induced biphase systems. Nat. Commun. 2021, 12, 1343. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.W.L.; Gao, M.; Lu, W.; Hong, M.; Ho, G.W. Selective Wavelength Enhanced Photochemical and Photothermal H2 Generation of Classical Oxide Supported Metal Catalyst. Adv. Funct. Mater. 2021, 31, 2104750. [Google Scholar] [CrossRef]
- Luo, X.; Li, R.; Homewood, K.P.; Chen, X.; Gao, Y. Hybrid 0D/2D Ni2P quantum dot loaded TiO2(B) nanosheet photothermal catalysts for enhanced hydrogen evolution. Appl. Surf. Sci. 2020, 505, 144099. [Google Scholar] [CrossRef]
- Ren, H.; Yang, J.-L.; Yang, W.-M.; Zhong, H.-L.; Lin, J.-S.; Radjenovic, P.M.; Sun, L.; Zhang, H.; Xu, J.; Tian, Z.-Q.; et al. Core–Shell–Satellite Plasmonic Photocatalyst for Broad-Spectrum Photocatalytic Water Splitting. ACS Mater. Lett. 2020, 3, 69–76. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, Q.; Ma, K.; Ding, Y.; Li, C. Pyroelectric effect in CdS nanorods decorated with a molecular Co-catalyst for hydrogen evolution. Nano Energy 2020, 73, 104810. [Google Scholar] [CrossRef]
- Dai, B.; Fang, J.; Yu, Y.; Sun, M.; Huang, H.; Lu, C.; Kou, J.; Zhao, Y.; Xu, Z. Construction of Infrared-Light-Responsive Photoinduced Carriers Driver for Enhanced Photocatalytic Hydrogen Evolution. Adv. Mater. 2020, 32, e1906361. [Google Scholar] [CrossRef]
- Yang, F.; Wang, S.; Li, Z.; Xu, Y.; Yang, W.; Yv, C.; Yang, D.; Xie, Y.; Zhou, W. Polydopamine/defective ultrathin mesoporous graphitic carbon nitride nanosheets as Z-scheme organic assembly for robust photothermal-photocatalytic performance. J. Colloid Interface Sci. 2022, 613, 775–785. [Google Scholar] [CrossRef]
- Wang, R.; Shan, G.; Wang, T.; Yin, D.; Chen, Y. Photothermal enhanced photocatalytic activity based on Ag-doped CuS nanocomposites. J. Alloys Compd. 2021, 864, 158591. [Google Scholar] [CrossRef]
- Shi, S.; Han, X.; Liu, J.; Lan, X.; Feng, J.; Li, Y.; Zhang, W.; Wang, J. Photothermal-boosted effect of binary CuFe bimetallic magnetic MOF heterojunction for high-performance photo-Fenton degradation of organic pollutants. Sci. Total Environ. 2021, 795, 148883. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, W.; Lin, H.; Shen, Y.; Fang, Q.; Liu, F. Construction of hierarchical Prussian blue microcrystal with high sunlight absorption for efficient photo-thermal degradation of organic pollutants. Sep. Purif. Technol. 2021, 269, 118724. [Google Scholar] [CrossRef]
- Li, Y.; Chang, H.; Wang, Z.; Shen, Q.; Liu, X.; Xue, J.; Jia, H. A 3D C@TiO2 multishell nanoframe for simultaneous photothermal catalytic hydrogen generation and organic pollutant degradation. J. Colloid Interface Sci. 2022, 609, 535–546. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Jiao, X.; Shi, J.; Ye, J.; Song, K.; Bao, J.; Li, G.; Lei, K. NIR-driven PtCu-alloy nanocages via photothermal enhanced fenton catalytic degradation of pollutant dyes under neutral pH. J. Alloys Compd. 2022, 895, 162624. [Google Scholar] [CrossRef]
- Lin, E.; Huang, R.; Wu, J.; Kang, Z.; Ke, K.; Qin, N.; Bao, D. Recyclable CoFe2O4 modified BiOCl hierarchical microspheres utilizing photo, photothermal and mechanical energy for organic pollutant degradation. Nano Energy 2021, 89, 106403. [Google Scholar] [CrossRef]
- Chen, J.; Luo, W.; Yu, S.; Yang, X.; Wu, Z.; Zhang, H.; Gao, J.; Mai, Y.-W.; Li, Y.; Jia, Y. Synergistic effect of photocatalysis and pyrocatalysis of pyroelectric ZnSnO3 nanoparticles for dye degradation. Ceram. Int. 2020, 46, 9786–9793. [Google Scholar] [CrossRef]
- Tian, J.; Wu, S.; Liu, S.; Zhang, W. Photothermal enhancement of highly efficient photocatalysis with bioinspired thermal radiation balance characteristics. Appl. Surf. Sci. 2022, 592, 153304. [Google Scholar] [CrossRef]
- Okeil, S.; Yadav, S.; Bruns, M.; Zintler, A.; Molina-Luna, L.; Schneider, J.J. Photothermal catalytic properties of layered titanium chalcogenide nanomaterials. Dalton Trans. 2020, 49, 1032–1047. [Google Scholar] [CrossRef]
- Zhu, Q.; Song, J.; Liu, Z.; Wu, K.; Li, X.; Chen, Z.; Pang, H. Photothermal catalytic degradation of textile dyes by laccase immobilized on Fe3O4@SiO2 nanoparticles. J. Colloid. Interf. Sci. 2022, 623, 992–1001. [Google Scholar] [CrossRef]
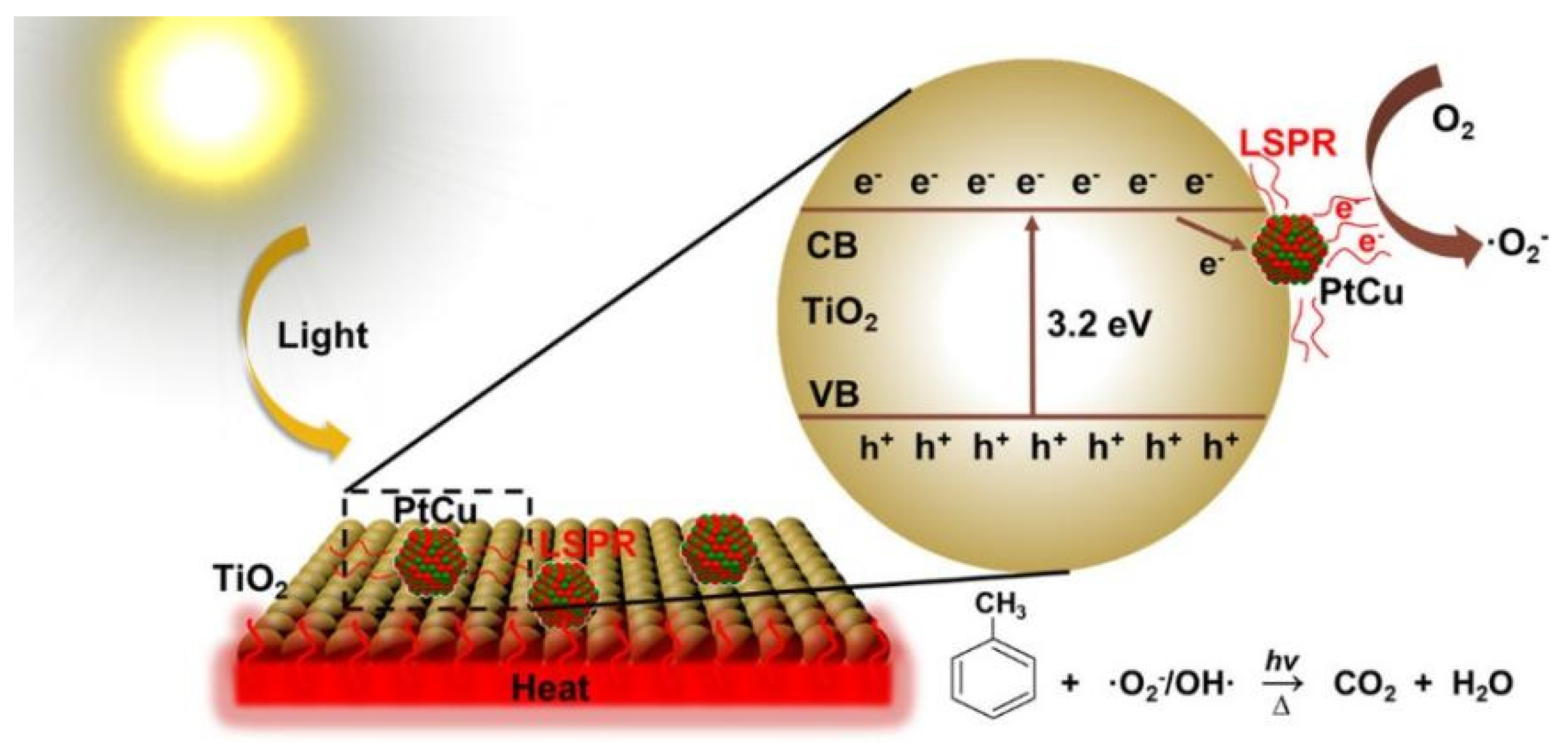
- Qi, Y.; Yang, Z.; Jiang, Y.; Han, H.; Wu, T.; Wu, L.; Liu, J.; Wang, Z.; Wang, F. Platinum−Copper Bimetallic Nanoparticles Supported on TiO2 as Catalysts for Photo−thermal Catalytic Toluene Combustion. ACS Appl. Nano Mater. 2022, 5, 1845–1854. [Google Scholar] [CrossRef]
- Li, J.; Feng, J.; Guo, X.; Fang, H.; Chen, J.; Ma, C.; Li, R.; Wang, Y.; Rui, Z. Defect-band bridge photothermally activates Type III heterojunction for CO2 reduction and typical VOCs oxidation. Appl. Catal. B Environ. 2022, 309, 121248. [Google Scholar] [CrossRef]
- Li, A.; Zhang, Q.; Zhao, S.; Chong, Y.; Wu, P.; Li, Y.; Jin, X.; Chen, G.; Qiu, Y.; Yang, S.; et al. A dual plasmonic core—shell Pt/[TiN@TiO2] catalyst for enhanced photothermal synergistic catalytic activity of VOCs abatement. Nano Res. 2022, 15, 7071–7080. [Google Scholar] [CrossRef]
- Xie, A.; Wang, H.; Qi, S.; Li, X.; Zhu, Z.; Zhang, W.; Wang, Q.; Tang, Y.; Luo, S. Mesoporous SmMnO3/CuMnO catalyst for photothermal synergistic degradation of gaseous toluene. Ceram. Int. 2021, 47, 31485–31496. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Elimian, E.A.; Lv, X.; Chen, J.; Jia, H. Convergent ambient sunlight-powered multifunctional catalysis for toluene abatement over in situ exsolution of Mn3O4 on perovskite parent. Chem. Eng. J. 2021, 412, 128560. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, K.; Yang, Z.; Xing, Z.; Li, Z.; Pan, K.; Zhou, W. Plasma Cu-decorated TiO2-x/CoP particle-level hierarchical heterojunctions with enhanced photocatalytic-photothermal performance. J. Hazard. Mater. 2021, 414, 125487. [Google Scholar] [CrossRef]
- Jiang, H.; Xing, Z.; Zhao, T.; Yang, Z.; Wang, K.; Li, Z.; Yang, S.; Xie, L.; Zhou, W. Plasmon Ag nanoparticle/Bi2S3 ultrathin nanobelt/oxygen-doped flower-like MoS2 nanosphere ternary heterojunctions for promoting charge separation and enhancing solar-driven photothermal and photocatalytic performances. Appl. Catal. B Environ. 2020, 274, 118947. [Google Scholar] [CrossRef]
- Guo, M.; Zhao, T.; Xing, Z.; Qiu, Y.; Pan, K.; Li, Z.; Yang, S.; Zhou, W. Hollow Octahedral Cu2-xS/CdS/Bi2S3 p-n-p Type Tandem Heterojunctions for Efficient Photothermal Effect and Robust Visible-Light-Driven Photocatalytic Performance. ACS Appl. Mater. Interfaces 2020, 12, 40328–40338. [Google Scholar] [CrossRef]
- Guo, M.; Xing, Z.; Zhao, T.; Qiu, Y.; Tao, B.; Li, Z.; Zhou, W. Hollow flower-like polyhedral α-Fe2O3/Defective MoS2/Ag Z-scheme heterojunctions with enhanced photocatalytic-Fenton performance via surface plasmon resonance and photothermal effects. Appl. Catal. B Environ. 2020, 272, 118978. [Google Scholar] [CrossRef]
- Lee, H.; Park, S.H.; Park, Y.-K.; Kim, S.-J.; Seo, S.-G.; Ki, S.J.; Jung, S.-C. Photocatalytic reactions of 2,4-dichlorophenoxyacetic acid using a microwave-assisted photocatalysis system. Chem. Eng. J. 2015, 278, 259–264. [Google Scholar] [CrossRef]
- Li, M.; Xing, Z.; Zhang, Z.; Wang, Y.; Liu, M.; Li, Z.; Wang, N.; Zhou, W. Hollow core-shell Z-scheme heterojunction on self-floating carbon fiber cloth with robust photocatalytic-photothermal performance. J. Clean. Prod. 2022, 360, 132166. [Google Scholar] [CrossRef]
- Qiu, Y.; Xing, Z.; Guo, M.; Li, Z.; Wang, N.; Zhou, W. Hollow cubic Cu2-xS/Fe-POMs/AgVO3 dual Z-scheme heterojunctions with wide-spectrum response and enhanced photothermal and photocatalytic-fenton performance. Appl. Catal. B Environ. 2021, 298, 120628. [Google Scholar] [CrossRef]
- Yuan, Y.; Pan, W.-g.; Guo, R.-t.; Hong, L.-f.; Lin, Z.-d.; Ji, X.-y. Flower spherical-like Bi7O9I3/AgI S-scheme heterojunction for phenol photodegradation: The synergetic effect of dual surface plasmon resonance and photothermal property. Sep. Purif. Technol. 2022, 297, 121538. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Zhang, Y.; Sun, X.; Lou, Y.; Zhang, Y.; Dong, Y.; Pan, C.; Zhu, Y. Efficient photothermal degradation on Bi12CoO20 sillenite with a strong internal electric field induced by the thermal effect. Appl. Catal. B Environ. 2022, 313, 121452. [Google Scholar] [CrossRef]
- Zheng, Y.; Tan, J.; Zhang, G.; Ma, Y.; Liu, F.; Liu, M.; Wang, Y.; Zou, H.; Huang, H. Yolk-Shell AuAgPt Alloy Nanostructures with Tunable Morphologies: Plasmon-Enhanced Photothermal and Catalytic Properties. Adv. Energy Sustain. Res. 2022, 3, 2100222. [Google Scholar] [CrossRef]
- Wang, R.; Hao, Q.; Feng, J.; Wang, G.-C.; Ding, H.; Chen, D.; Ni, B. Enhanced separation of photogenerated charge carriers and catalytic properties of ZnO-MnO2 composites by microwave and photothermal effect. J. Alloys Compd. 2019, 786, 418–427. [Google Scholar] [CrossRef]
- Borjigin, B.; Ding, L.; Li, H.; Wang, X. A solar light-induced photo-thermal catalytic decontamination of gaseous benzene by using Ag/Ag3PO4/CeO2 heterojunction. Chem. Eng. J. 2020, 402, 126070. [Google Scholar] [CrossRef]
- Zeng, X.; Shan, C.; Sun, M.; Ding, D.; Rong, S. Graphene enhanced α-MnO2 for photothermal catalytic decomposition of carcinogen formaldehyde. Chin. Chem. Lett. 2022, 33, 4771–4775. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhong, J.; Wang, H.; Fu, M.; Ye, D.; Hu, Y. Synergistic effect of tunable oxygen-vacancy defects and graphene on accelerating the photothermal degradation of methanol over Co3O4/rGO nanocomposites. Chem. Eng. J. 2021, 425, 131658. [Google Scholar] [CrossRef]
- Pang, Y.; Kong, L.; Lei, H.; Chen, D.; Yuvaraja, G. Combined microwave-induced and photocatalytic oxidation using zinc ferrite catalyst for efficient degradation of tetracycline hydrochloride in aqueous solution. J. Taiwan Inst. Chem. E 2018, 93, 397–404. [Google Scholar] [CrossRef]
- Barik, A.J.; Kulkarni, S.V.; Gogate, P.R. Degradation of 4-chloro 2-aminophenol using combined approaches based on microwave and photocatalysis. Sep. Purif. Technol. 2016, 168, 152–160. [Google Scholar] [CrossRef]
- Liu, X.; Chen, T.; Xue, Y.; Fan, J.; Shen, S.; Hossain, M.S.A.; Amin, M.A.; Pan, L.; Xu, X.; Yamauchi, Y. Nanoarchitectonics of MXene/semiconductor heterojunctions toward artificial photosynthesis via photocatalytic CO2 reduction. Coordin. Chem. Rev. 2022, 459, 214440. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Huang, L.; Zaman, S.; Lei, K.; Yue, T.; Li, Z.A.; You, B.; Xia, B.Y. Engineering 2D Photocatalysts toward Carbon Dioxide Reduction. Adv. Energy Mater. 2021, 11, 2003159. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Kudo, A. Visible light responsive photocatalysts developed by substitution with metal cations aiming at artificial photosynthesis. Front. Energy 2021, 15, 568–576. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Tahir, M.; Tasleem, S.; Tahir, B. Recent development in band engineering of binary semiconductor materials for solar driven photocatalytic hydrogen production. Int. J. Hydrogen Energy 2020, 45, 15985–16038. [Google Scholar] [CrossRef]
- Gopinath, C.S.; Nalajala, N. A scalable and thin film approach for solar hydrogen generation: A review on enhanced photocatalytic water splitting. J. Mater. Chem. A 2021, 9, 1353–1371. [Google Scholar] [CrossRef]
- Zheng, H.; Li, X.; Zhu, K.; Liang, P.; Wu, M.; Rao, Y.; Jian, R.; Shi, F.; Wang, J.; Yan, K.; et al. Semiconducting BaTiO3@C core-shell structure for improving piezo-photocatalytic performance. Nano Energy 2022, 93, 106831. [Google Scholar] [CrossRef]
- Shi, J.; Xie, Z.; Tang, X.; Wang, Y.; Yuan, G.; Liu, J.-M. Enhanced piezo-photocatalytic performance of Ag@Na0.5Bi0.5TiO3 composites. J. Alloys Compd. 2022, 911, 164885. [Google Scholar] [CrossRef]
- Shen, W.; Li, N.; Zuo, S.; Wu, M.; Sun, G.; Li, Q.; Shi, M.; Ma, J. Remarkably enhanced piezo-photocatalytic performance of Z-scheme Bi2WO6/Black TiO2 heterojunction via piezoelectric effect. Ceram. Int. 2022, 48, 15899–15907. [Google Scholar] [CrossRef]
- Kang, Z.; Ke, K.; Lin, E.; Qin, N.; Wu, J.; Huang, R.; Bao, D. Piezoelectric polarization modulated novel Bi2WO6/g-C3N4/ZnO Z-scheme heterojunctions with g-C3N4 intermediate layer for efficient piezo-photocatalytic decomposition of harmful organic pollutants. J. Colloid Interface Sci. 2022, 607, 1589–1602. [Google Scholar] [CrossRef]
- Gotipamul, P.P.; Vattikondala, G.; Rajan, K.D.; Khanna, S.; Rathinam, M.; Chidambaram, S. Impact of piezoelectric effect on the heterogeneous visible photocatalysis of g-C3N4/Ag/ZnO tricomponent. Chemosphere 2022, 287, 132298. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Li, Z.; Yu, S.; Yang, B.; Yin, Y.; Zan, L.; Myung, N.V. Piezo-photocatalytic flexible PAN/TiO2 composite nanofibers for environmental remediation. Sci. Total Environ. 2022, 824, 153790. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Niu, P.; Wang, S.; Li, L. High visible light photocatalytic activities obtained by integrating g-C3N4 with ferroelectric PbTiO3. J. Mater. Sci. Technol. 2021, 74, 128–135. [Google Scholar] [CrossRef]
- Sun, H.; Xu, Z.; Xie, X.; Niu, J.; Wang, M.; Zhang, X.; Chen, X.; Han, J. Enhanced photocatalytic activity of ferroelectric-based Ag2O/Bi4Ti3O12 hybrids by piezoelectric effect. J. Alloys Compd. 2021, 882, 160609. [Google Scholar] [CrossRef]
- Liu, X.; Shen, X.; Sa, B.; Zhang, Y.; Li, X.; Xue, H. Piezotronic-enhanced photocatalytic performance of heterostructured BaTiO3/SrTiO3 nanofibers. Nano Energy 2021, 89, 106391. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Wu, Z.; Zhu, K.; Wang, J.; Li, Z.; Tai, G.; Liu, X.; Lu, S. Enhanced visible-light photocatalytic performances of ZnO through loading AgI and coupling piezo-photocatalysis. J. Alloys Compd. 2021, 852, 156848. [Google Scholar] [CrossRef]
- Lei, H.; Wu, M.; Liu, Y.; Mo, F.; Chen, J.; Ji, S.; Zou, Y.; Dong, X. Built-in piezoelectric field improved photocatalytic performance of nanoflower-like Bi2WO6 using low-power white LEDs. Chin. Chem. Lett. 2021, 32, 2317–2321. [Google Scholar]
- Jiang, X.; Wang, H.; Wang, X.; Yuan, G. Synergetic effect of piezoelectricity and Ag deposition on photocatalytic performance of barium titanate perovskite. Sol. Energy 2021, 224, 455–461. [Google Scholar] [CrossRef]
- Dilly Rajan, K.; Gotipamul, P.P.; Khanna, S.; Chidambaram, S.; Rathinam, M. Piezo-photocatalytic effect of NaNbO3 interconnected nanoparticles decorated CuBi2O4 nanocuboids. Mater. Lett. 2021, 296, 129902. [Google Scholar] [CrossRef]
- Wu, J.; Wang, W.; Tian, Y.; Song, C.; Qiu, H.; Xue, H. Piezotronic effect boosted photocatalytic performance of heterostructured BaTiO3/TiO2 nanofibers for degradation of organic pollutants. Nano Energy 2020, 77, 105122. [Google Scholar]
- Wu, W.; Yin, X.; Dai, B.; Kou, J.; Ni, Y.; Lu, C. Water flow drived piezo-photocatalytic flexible films: Bi-piezoelectric integration of ZnO nanorods and PVDF. Appl. Surf. Sci. 2020, 517, 146119. [Google Scholar] [CrossRef]
- Ren, Z.; Li, X.; Guo, L.; Wu, J.; Li, Y.; Liu, W.; Li, P.; Fu, Y.; Ma, J. Facile synthesis of ZnO/ZnS heterojunction nanoarrays for enhanced piezo-photocatalytic performance. Mater. Lett. 2021, 292, 129635. [Google Scholar] [CrossRef]
- Dursun, S.; Akyildiz, H.; Kalem, V. PMN-PT nanoparticle/SnO2 nanofiber heterostructures: Enhanced photocatalytic degradation performance by ultrasonic wave induced piezoelectric field. J. Alloys Compd. 2021, 889, 161769. [Google Scholar] [CrossRef]
- Cheng, T.; Gao, H.; Li, R.; Wang, S.; Yi, Z.; Yang, H. Flexoelectricity-induced enhancement in carrier separation and photocatalytic activity of a photocatalyst. Appl. Surf. Sci. 2021, 566, 150669. [Google Scholar] [CrossRef]
- Yu, Y.; Yao, B.; He, Y.; Cao, B.; Ren, Y.; Sun, Q. Piezo-enhanced photodegradation of organic pollutants on Ag3PO4/ZnO nanowires using visible light and ultrasonic. Appl. Surf. Sci. 2020, 528, 146819. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Zhao, H.; Zhang, Z.; An, B.; Bai, C.; Ren, Z.; Wu, J.; Li, Y.; Liu, W.; et al. Synthesis of ternary ZnO/ZnS/MoS2 piezoelectric nanoarrays for enhanced photocatalytic performance by conversion of dual heterojunctions. Appl. Surf. Sci. 2021, 556, 149695. [Google Scholar] [CrossRef]
- Yu, C.; Tan, M.; Tao, C.; Hou, Y.; Liu, C.; Meng, H.; Su, Y.; Qiao, L.; Bai, Y. Remarkably enhanced piezo-photocatalytic performance in BaTiO3/CuO heterostructures for organic pollutant degradation. J. Adv. Ceram. 2022, 11, 414–426. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, F.; Wu, S.; Shen, B.; Zeng, H.; Zhai, J. Remarkable Piezophoto Coupling Catalysis Behavior of BiOX/BaTiO3 (X = Cl, Br, Cl0.166 Br0.834) Piezoelectric Composites. Small 2020, 16, e2001573. [Google Scholar] [CrossRef]
- Wang, R.; Xie, X.; Xu, C.; Lin, Y.; You, D.; Chen, J.; Li, Z.; Shi, Z.; Cui, Q.; Wang, M. Bi-piezoelectric effect assisted ZnO nanorods/PVDF-HFP spongy photocatalyst for enhanced performance on degrading organic pollutant. Chem. Eng. J. 2022, 439, 135787. [Google Scholar] [CrossRef]
- Fu, Y.; Ren, Z.; Wu, J.; Li, Y.; Liu, W.; Li, P.; Xing, L.; Ma, J.; Wang, H.; Xue, X. Direct Z-scheme heterojunction of ZnO/MoS2 nanoarrays realized by flowing-induced piezoelectric field for enhanced sunlight photocatalytic performances. Appl. Catal. B Environ. 2021, 285, 119785. [Google Scholar] [CrossRef]
- Tong, W.; Zhang, Y.; Huang, H.; Xiao, K.; Yu, S.; Zhou, Y.; Liu, L.; Li, H.; Liu, L.; Huang, T.; et al. A highly sensitive hybridized soft piezophotocatalyst driven by gentle mechanical disturbances in water. Nano Energy 2018, 53, 513–523. [Google Scholar] [CrossRef]
- Yao, Z.; Sun, H.; Xiao, S.; Hu, Y.; Liu, X.; Zhang, Y. Synergetic piezo-photocatalytic effect in a Bi2MoO6/BiOBr composite for decomposing organic pollutants. Appl. Surf. Sci. 2021, 560, 150037. [Google Scholar] [CrossRef]
- Zhu, Q.; Dar, A.A.; Zhou, Y.; Zhang, K.; Qin, J.; Pan, B.; Lin, J.; Patrocinio, A.O.T.; Wang, C. Oxygen Vacancies Promoted Piezoelectricity toward Piezo-Photocatalytic Decomposition of Tetracycline over SrBi4Ti4O15. ACS EST Eng. 2022, 2, 1365–1375. [Google Scholar] [CrossRef]
- Zhou, L.; Dai, S.; Xu, S.; She, Y.; Li, Y.; Leveneur, S.; Qin, Y. Piezoelectric effect synergistically enhances the performance of Ti32-oxo-cluster/BaTiO3/CuS p-n heterojunction photocatalytic degradation of pollutants. Appl. Catal. B Environ. 2021, 291, 120019. [Google Scholar] [CrossRef]
- Chen, J.; Liao, B.; Liao, X.; Xie, H.; Yu, Y.; Hou, S.; Wang, C.; Fan, X. Strain-Driven Polarized Electric Field-Promoted Photocatalytic Activity in Borate-Based CsCdBO3 Bulk Materials. ACS Appl. Mater. Interfaces 2021, 13, 34202–34212. [Google Scholar] [CrossRef]
- Zhang, C.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. The ultrasonic-induced-piezoelectric enhanced photocatalytic performance of ZnO/CdS nanofibers for degradation of bisphenol A. J. Alloys Compd. 2021, 885, 160987. [Google Scholar] [CrossRef]
- Zhang, C.; Fei, W.; Wang, H.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. p-n Heterojunction of BiOI/ZnO nanorod arrays for piezo-photocatalytic degradation of bisphenol A in water. J. Hazard. Mater. 2020, 399, 123109. [Google Scholar] [CrossRef]
- Feng, Y.; Li, H.; Ling, L.; Yan, S.; Pan, D.; Ge, H.; Li, H.; Bian, Z. Enhanced Photocatalytic Degradation Performance by Fluid-Induced Piezoelectric Field. Environ. Sci. Technol. 2018, 52, 7842–7848. [Google Scholar] [CrossRef]
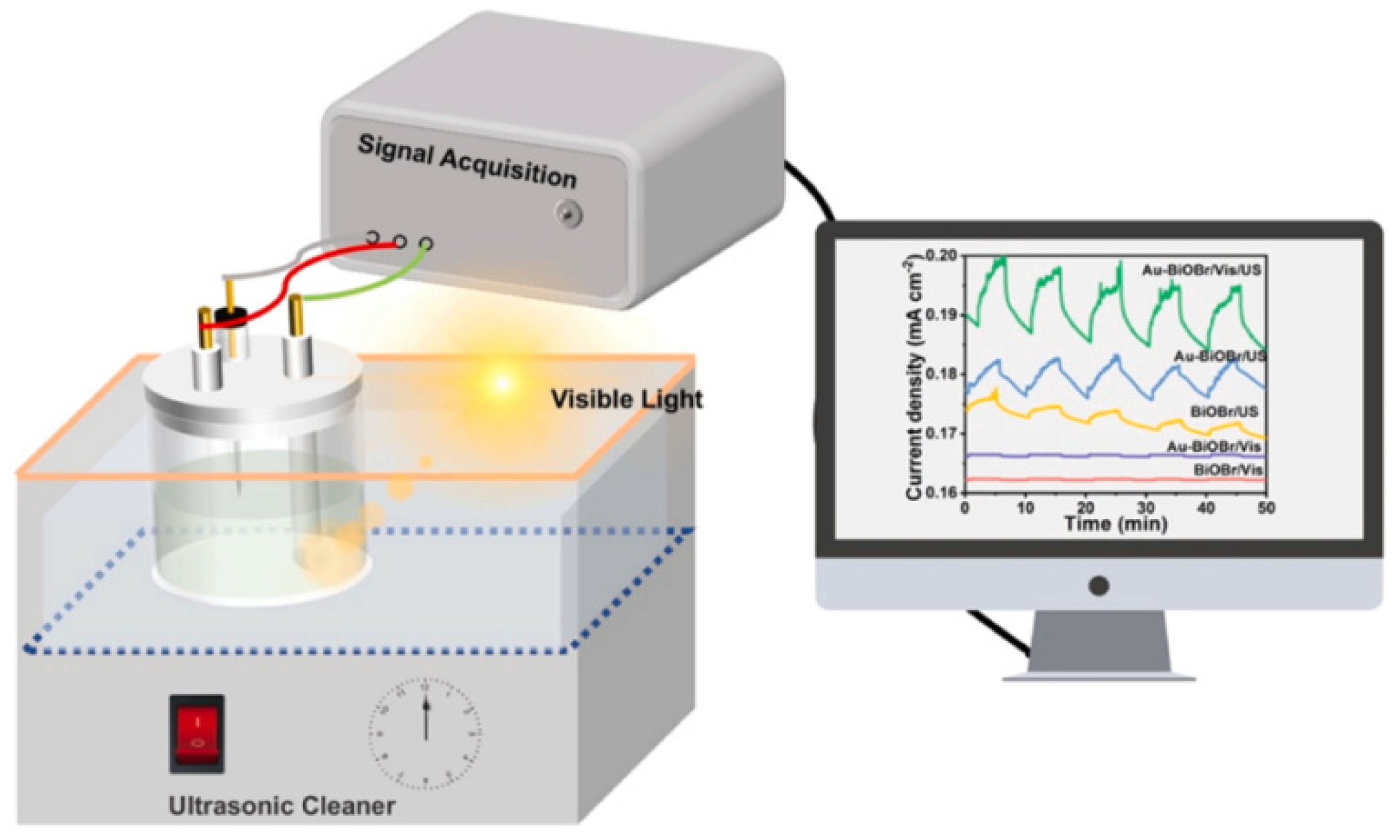
- Hu, J.; Chen, Y.; Zhou, Y.; Zeng, L.; Huang, Y.; Lan, S.; Zhu, M. Piezo-enhanced charge carrier separation over plasmonic Au-BiOBr for piezo-photocatalytic carbamazepine removal. Appl. Catal. B Environ. 2022, 311, 121369. [Google Scholar] [CrossRef]
- Li, Y.; Li, R.; Zhai, Y.; Huang, Y.; Lee, S.; Cao, J. Improved photocatalytic activity of BaTiO3/La2Ti2O7 heterojunction composites via piezoelectric-enhanced charge transfer. Appl. Surf. Sci. 2021, 570, 151146. [Google Scholar] [CrossRef]
- Li, M.; Sun, J.; Chen, G.; Yao, S.; Cong, B. Construction double electric field of sulphur vacancies as medium ZnS/Bi2S3-PVDF self-supported recoverable piezoelectric film photocatalyst for enhanced photocatalytic performance. Appl. Catal. B Environ. 2022, 301, 120792. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, J.; Lu, Z.; Hu, J.; Hao, A.; Cao, Y. Insight into the effect of OH modification on the piezo-photocatalytic hydrogen production activity of SrTiO3. J. Colloid Interface Sci. 2022, 612, 111–120. [Google Scholar] [CrossRef]
- Huang, X.; Lei, R.; Yuan, J.; Gao, F.; Jiang, C.; Feng, W.; Zhuang, J.; Liu, P. Insight into the piezo-photo coupling effect of PbTiO3/CdS composites for piezo-photocatalytic hydrogen production. Appl. Catal. B Environ. 2021, 282, 119586. [Google Scholar] [CrossRef]
- Hu, C.; Chen, F.; Wang, Y.; Tian, N.; Ma, T.; Zhang, Y.; Huang, H. Exceptional Cocatalyst-Free Photo-Enhanced Piezocatalytic Hydrogen Evolution of Carbon Nitride Nanosheets from Strong In-Plane Polarization. Adv. Mater. 2021, 33, e2101751. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Z.; Li, B.; Yan, Y.; Xu, R.; Meng, M.; Yan, Y. Fluid-induced piezoelectric field enhancing photocatalytic hydrogen evolution reaction on g-C3N4/LiNbO3/PVDF membrane. Nano Energy 2022, 99, 107429. [Google Scholar] [CrossRef]
- Tang, R.; Gong, D.; Zhou, Y.; Deng, Y.; Feng, C.; Xiong, S.; Huang, Y.; Peng, G.; Li, L.; Zhou, Z. Unique g-C3N4/PDI-g-C3N4 homojunction with synergistic piezo-photocatalytic effect for aquatic contaminant control and H2O2 generation under visible light. Appl. Catal. B Environ. 2022, 303, 120929. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, B.; Zhai, J.; Conesa, J.C. High Performance Generation of H2O2 under Piezophototronic Effect with Multi-Layer In2S3 Nanosheets Modified by Spherical ZnS and BaTiO3 Nanopiezoelectrics. Small Methods 2021, 5, e2100269. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, R.; Mansingh, S.; Parida, K.; Parida, K. Boosting sluggish photocatalytic hydrogen evolution through piezo-stimulated polarization: A critical review. Mater. Horiz. 2022, 9, 1332–1355. [Google Scholar] [CrossRef]
- Yan, X.; Li, G.; Wang, Z.; Yu, Z.; Wang, K.; Wu, Y. Recent progress on piezoelectric materials for renewable energy conversion. Nano Energy 2020, 77, 105180. [Google Scholar] [CrossRef]
- Pan, L.; Sun, S.; Chen, Y.; Wang, P.; Wang, J.; Zhang, X.; Zou, J.J.; Wang, Z.L. Advances in Piezo-Phototronic Effect Enhanced Photocatalysis and Photoelectrocatalysis. Adv. Energy Mater. 2020, 10, 2000214. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, X.; Li, L. Piezopotential augmented photo- and photoelectro-catalysis with a built-in electric field. Chin. J. Catal. 2020, 41, 534–549. [Google Scholar] [CrossRef]
- Lops, C.; Ancona, A.; Di Cesare, K.; Dumontel, B.; Garino, N.; Canavese, G.; Hernandez, S.; Cauda, V. Sonophotocatalytic degradation mechanisms of Rhodamine B dye via radicals generation by micro- and nano-particles of ZnO. Appl. Catal. B Environ. 2019, 243, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sang, Y.; Chang, S.; Huang, X.; Zhang, Y.; Yang, R.; Jiang, H.; Liu, H.; Wang, Z.L. Enhanced ferroelectric-nanocrystal-based hybrid photocatalysis by ultrasonic-wave-generated piezophototronic effect. Nano Lett. 2015, 15, 2372–2379. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Guo, L.; Sun, Q.; Wang, Z.L. Piezotronic Effect Enhanced Plasmonic Photocatalysis by AuNPs/BaTiO3 Heterostructures. Adv. Funct. Mater. 2019, 29, 1808737. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, W.; Ao, Z.; An, T. Photo-piezoelectric synergistic degradation of typical volatile organic compounds on BaTiO3. Chin. Chem. Lett. 2022, 33, 410–414. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, M.; Mi, Y.; Guo, L.; Fang, W.; Zeng, X.; Zhou, T.; Liu, Y. The influence of piezoelectric effect on the heterogeneous photocatalytic hydrogen production of strontium titanate nanoparticles. Nano Energy 2021, 85, 105949. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, H.; Li, Y.; Lu, K. Photocatalytic activity of CdS nanoparticles enhanced by the interaction between piezotronic effect and phase junction. J. Alloys Compd. 2020, 815, 152494. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, X.; Gao, F.; Zhang, L.; Tian, Q.; Fang, Z.B.; Liu, P. Study on water splitting characteristics of CdS nanosheets driven by the coupling effect between photocatalysis and piezoelectricity. Nanoscale 2019, 11, 9085–9090. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Wang, H.; Tian, J.; Cui, H. Novel Ag2O nanoparticles modified MoS2 nanoflowers for piezoelectric-assisted full solar spectrum photocatalysis. J. Colloid Interface Sci. 2019, 537, 206–214. [Google Scholar] [CrossRef]
- Chou, T.-M.; Chan, S.-W.; Lin, Y.-J.; Yang, P.-K.; Liu, C.-C.; Lin, Y.-J.; Wu, J.-M.; Lee, J.-T.; Lin, Z.-H. A highly efficient Au-MoS2 nanocatalyst for tunable piezocatalytic and photocatalytic water disinfection. Nano Energy 2019, 57, 14–21. [Google Scholar] [CrossRef]
- Venkatesan, M.; Chen, W.-C.; Cho, C.-J.; Veeramuthu, L.; Chen, L.-G.; Li, K.-Y.; Tsai, M.-L.; Lai, Y.-C.; Lee, W.-Y.; Chen, W.-C.; et al. Enhanced piezoelectric and photocatalytic performance of flexible energy harvester based on CsZn0.75Pb0.25I3/CNC–PVDF composite nanofibers. Chem. Eng. J. 2022, 433, 133620. [Google Scholar] [CrossRef]
- Pan, J.; Li, Y.; Guo, G.; Zhao, X.; Yu, J.; Li, Z.; Xu, S.; Man, B.; Wei, D.; Zhang, C. Synergizing piezoelectric and plasmonic modulation of PVDF/MoS2 cavity/Au for enhanced photocatalysis. Appl. Surf. Sci. 2022, 577, 151811. [Google Scholar] [CrossRef]
- Sittipol, W.; Sronsri, C.; U-yen, K. Effect of magnetic fields on the efficiency of the photocatalytic degradation of methylene blue in a dynamic fluid system. J. Clean Prod. 2021, 325, 129284. [Google Scholar] [CrossRef]
- Silva, E.C.; Bonacin, J.A.; Passos, R.R.; Pocrifka, L.A. The effect of an external magnetic field on the photocatalytic activity of CoFe2O4 particles anchored in carbon cloth. J. Photoch. Photobio. A 2021, 416, 113317. [Google Scholar] [CrossRef]
- Tank, C.M.; Sakhare, Y.S.; Kanhe, N.S.; Nawale, A.B.; Das, A.K.; Bhoraskar, S.V.; Mathe, V.L. Electric field enhanced photocatalytic properties of TiO2 nanoparticles immobilized in porous silicon template. Solid State Sci. 2011, 13, 1500–1504. [Google Scholar] [CrossRef]
- Gao, W.; Zhao, X.; Cui, C.; Su, X.; Zhang, S.; Wang, X.; Zhang, X.L.; Sang, Y.; Liu, H. High-efficiency separation and transfer of photo-induced charge carrier in graphene/TiO2 via heterostructure in magnetic field. J. Alloys Compd. 2021, 862, 158283. [Google Scholar] [CrossRef]
- Bian, Y.; Zheng, G.; Ding, W.; Hu, L.; Sheng, Z. Magnetic field effect on the photocatalytic degradation of methyl orange by commercial TiO2 powder. RSC Adv. 2021, 11, 6284–6291. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, L.; Zhang, Z.; Gao, Z.; Weng, H.; Zhang, W.; Li, L.; Song, Y.Y. Insight of the Influence of Magnetic-Field Direction on Magneto-Plasmonic Interfaces for Tuning Photocatalytical Performance of Semiconductors. J. Phys. Chem. Lett. 2020, 11, 9931–9937. [Google Scholar] [CrossRef]
- Pan, L.; Ai, M.; Huang, C.; Yin, L.; Liu, X.; Zhang, R.; Wang, S.; Jiang, Z.; Zhang, X.; Zou, J.J.; et al. Manipulating spin polarization of titanium dioxide for efficient photocatalysis. Nat. Commun. 2020, 11, 418. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, G.; Gao, Z.; Cao, J.; Li, D.; Yun, H.; Zeng, T. Enhanced visible-light-driven photocatalytic activity of BiFeO3 via electric-field control of spontaneous polarization. J. Alloys Compd. 2019, 783, 943–951. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Wu, Y.; Wang, Z.; Zhang, H.; Cao, Z.; He, J.; Li, W.; Yang, Z.; Zheng, L.; et al. A α-Fe2O3/rGO magnetic photocatalyst: Enhanced photocatalytic performance regulated by magnetic field. J. Alloys Compd. 2021, 851, 156733. [Google Scholar] [CrossRef]
- Lu, Y.; Ren, B.; Chang, S.; Mi, W.; He, J.; Wang, W. Achieving effective control of the photocatalytic performance for CoFe2O4/MoS2 heterojunction via exerting external magnetic fields. Mater. Lett. 2020, 260, 126979. [Google Scholar] [CrossRef]
- Huang, H.; Chen, Y.; Shi, H. Boosting Separation of Charge Carriers in 2D/0D BiOBr Nanoflower Sheets/BN Quantum Dots with the Lorentz Force via Magnetic Field. Energ. Fuel 2022. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Z.; Chang, W.; Wei, C.; Feng, X.; Ma, L.; Qi, X.; Li, Z. Microwave-assisted solvothermal synthesis of hierarchical TiO2 microspheres for efficient electro-field-assisted-photocatalytic removal of tributyltin in tannery wastewater. Chemosphere 2017, 179, 75–83. [Google Scholar] [CrossRef]
- Li, N.; He, M.; Lu, X.; Liang, L.; Li, R.; Yan, B.; Chen, G. Enhanced norfloxacin degradation by visible-light-driven Mn3O4/gamma-MnOOH photocatalysis under weak magnetic field. Sci. Total Environ. 2021, 761, 143268. [Google Scholar] [CrossRef]
- Wu, Y.; Li, T.; Ren, X.; Fu, Y.; Zhang, H.; Feng, X.; Huang, H.; Xie, R. Magnetic field assisted alpha-Fe2O3/Zn1-xFexO heterojunctions for accelerating antiviral agents degradation under visible-light. J. Environ. Chem. Eng. 2022, 10, 106990. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Wang, Y.; Kovács, A.; Foo, C.; Dunin-Borkowski, R.E.; Lu, Y.; Taylor, R.A.; Wu, C.; Tsang, S.C.E. Local magnetic spin mismatch promoting photocatalytic overall water splitting with exceptional solar-to-hydrogen efficiency. Energy Environ. Sci. 2022, 15, 265–277. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, W.; Liu, Q.; Cui, C.; Zhou, W.; Wang, X.; Zhang, X.L.; Zhao, L.; Sang, Y.; Liu, H. Enhanced photo-induced carrier separation of CdS/MoS2 via micro-potential of Mo microsheet derived from electromagnetic induction. Chem. Eng. J. 2021, 404, 126972. [Google Scholar] [CrossRef]
- Gao, W.; Liu, Q.; Zhang, S.; Yang, Y.; Zhang, X.; Zhao, H.; Qin, W.; Zhou, W.; Wang, X.; Liu, H.; et al. Electromagnetic induction derived micro-electric potential in metal-semiconductor core-shell hybrid nanostructure enhancing charge separation for high performance photocatalysis. Nano Energy 2020, 71, 104624. [Google Scholar] [CrossRef]
- Pan, H.; Sun, M.; Wang, X.; Zhang, M.; Murugananthan, M.; Zhang, Y. A novel electric-assisted photocatalytic technique using self-doped TiO2 nanotube films. Appl. Catal. B Environ. 2022, 307, 121174. [Google Scholar] [CrossRef]
- Park, S.; Lee, C.W.; Kang, M.-G.; Kim, S.; Kim, H.J.; Kwon, J.E.; Park, S.Y.; Kang, C.-Y.; Hong, K.S.; Nam, K.T. A ferroelectric photocatalyst for enhancing hydrogen evolution: Polarized particulate suspension. Phys. Chem. Chem. Phys. 2014, 16, 10408–10413. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.C.; Ding, H.L.; Pan, S.S.; Luo, Y.Y.; Li, G.H. Electric-Field-Enhanced Photocatalytic Removal of Cr(VI) under Sunlight of TiO2 Nanograss Mesh with Nondestructive Regeneration and Feasible Collection for Cr(III). Acs Sustain. Chem. Eng. 2016, 4, 6887–6893. [Google Scholar] [CrossRef]
- Li, S.; Bai, L.; Ji, N.; Yu, S.; Lin, S.; Tian, N.; Huang, H. Ferroelectric polarization and thin-layered structure synergistically promoting CO2 photoreduction of Bi2MoO6. J. Mater. Chem. A 2020, 8, 9268–9277. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, D.; Gao, R.; Wen, G.; Feng, M.; Song, G.; Zhu, J.; Luo, D.; Tan, H.; Ge, X.; et al. Magnetic-Field-Stimulated Efficient Photocatalytic N2 Fixation over Defective BaTiO3 Perovskites. Angew. Chem. Int. Ed. 2021, 60, 11910–11918. [Google Scholar] [CrossRef]
- Gao, W.; Lu, J.; Zhang, S.; Zhang, X.; Wang, Z.; Qin, W.; Wang, J.; Zhou, W.; Liu, H.; Sang, Y. Suppressing Photoinduced Charge Recombination via the Lorentz Force in a Photocatalytic System. Adv. Sci. 2019, 6, 1901244. [Google Scholar] [CrossRef]
- Zhao, J.; Li, N.; Yu, R.; Zhao, Z.; Nan, J. Magnetic field enhanced denitrification in nitrate and ammonia contaminated water under 3D/2D Mn2O3/g-C3N4 photocatalysis. Chem. Eng. J. 2018, 349, 530–538. [Google Scholar] [CrossRef]
- Peng, C.; Fan, W.; Li, Q.; Han, W.; Chen, X.; Zhang, G.; Yan, Y.; Gu, Q.; Wang, C.; Zhang, H.; et al. Boosting photocatalytic activity through tuning electron spin states and external magnetic fields. J. Mater. Sci. Technol. 2022, 115, 208–220. [Google Scholar] [CrossRef]









| Material | Condition | Application | Activity | Blank Control | Ref. |
|---|---|---|---|---|---|
| SrTiO3/Cu@Ni/TiN | Xe lamp (600 mW/cm2) | artificial photosynthesis | 21.33 μmol g−1 h−1 (C2H6O) | 380 °C: 1.44 μmol g−1 h−1 (C2H6O) | [85] |
| BP/WO | 300 W Xe lamp (UV-vis-NIR) | artificial photosynthesis | 26.1 μmol g−1 h−1 (CO) | BP: 1.47 μmol g−1 h−1 (CO) | [86] |
| Au-MgO/TiO2 | 300 W Xe lamp (UV-vis-NIR) | artificial photosynthesis | 6.624 μmol g−1 h−1 (CH4) | P25: 2.07 μmol g−1 h−1 (CH4) | [87] |
| La0.4Pr0.6Mn0.6 Ni0.4O3-δ | 300 W Xe lamp ET: 300 °C | artificial photosynthesis | 3970 μmol g−1 h−1 (CH4O) | PC: 0.24 μmol g−1 h−1 (CH4O) | [88] |
| CoO-CuO/TiO2-CeO2 | 300 W Xe lamp | artificial photosynthesis | 1.84 μmol g−1 h−1 (CH4) | PC: 0.5 μmol g−1 h−1 (CH4) | [89] |
| Cu:CsPbBr3 | 300 W Xe lamp | artificial photosynthesis | 14.72 μmol g−1 h−1 (CH4) | CsPbBr3: 3.62 μmol g−1 h−1 (CH4) | [90] |
| Cu0/Cu2O | 300 W Xe lamp (λ > 400 nm) | artificial photosynthesis | 2.6 μmol g−1 h−1 (CH4O) | Cu2O: 0 μmol g−1 h−1 (CH4O) | [91] |
| Cs3Sb2I9 | Xe lamp (200 mW/cm2) ET: 235 °C | artificial photosynthesis | 95.7 μmol g−1 h−1 (CO) | PC: 1.1 μmol g−1 h−1 (CO) | [92] |
| Bi2O3−x | LED light (365–940 nm) | artificial photosynthesis | AQY = 0.113% (940 nm) | AOY = 0.028% (450 nm) | [93] |
| Au/rutile | 300 W Xe lamp (UV-vis-NIR) | artificial photosynthesis | ∼5 μmol g−1 h−1 (CO) | rutile: ∼2.25 μmol g−1 h−1 (CO) | [94] |
| TiO2(AB) | 150 W Xe lamp (UV-vis-NIR) | artificial photosynthesis | 11.93 μmol g−1 h−1 (CH4) | PC: 0.09 μmol g−1 h−1 (CH4) | [95] |
| AuCu/g-C3N4 | 300 W Xe lamp ET: 120 °C | artificial photosynthesis | 0.89 mmol g−1 h−1 (C2H6O) | PC: 0.21 mmol g−1 h−1 (C2H6O) | [49] |
| Cu2O/g-C3N4 | 300 W Xe lamp (λ > 420 nm) ET: 100 °C | artificial photosynthesis | 0.71 mmol g−1 h−1 (C2H6O) | PC: 0.37 mmol g−1 h−1 (C2H6O) | [30] |
| Ag-PDA/DCN | 300 W Xe lamp (λ > 420 nm) | H2 production | 3840 μmol g−1 h−1 | PDA/DCN: 548 μmol g−1 h−1 | [96] |
| 1T-WS2/CdS | 300 W Xe lamp (λ > 420 nm) | H2 production | 70.9 mmol g−1 h−1 | Pt0.02-CdS: 20.2 mmol g−1 h−1 | [33] |
| TiO2@CS | 300 W Xe lamp ET: 120 °C | H2 production | 22772.6 μmol g−1 h−1 | 120 °C: ∼1100 μmol g−1 h−1 | [71] |
| RuO2/TiO2/Pt/C | 300 W Xe lamp (λ > 420 nm) | H2 production | 81.62 μmol g−1 h−1 | RuO2-Pt/TiO2/C: 37.45 μmol g−1 h−1 | [97] |
| Cu2O-rGO/TiO2 | 300 W Xe lamp ET: 90 °C | H2 production | 17.8 mmol g−1 h−1 | PC: 3.8 mmol g−1 h−1 | [29] |
| Pt/ZnIn2S4 | 300 W Xe lamp (200 mW/cm2) | H2 production | 19.4 mmol g−1 h−1 | Co9S8 @ZnIn2S4: 6.25 mmol g−1 h−1 | [98] |
| oxide-MoS2 | Xe lamps | H2 production | 7.85 mmol g−1 h−1 | 2H-MoS2: 2.52 mmol g−1 h−1 | [99] |
| C@TiO2/TiO2-x | 300 W Xe lamp ET: 80 °C | H2 production | 3667 μmol g−1 h−1 | TiO2-x: 262 μmol g−1 h−1 | [100] |
| SAAg-g-CN | 300 W Xe lamp ET: 55 °C | H2 production | 498 μmol g−1 h−1 | 25 °C: 248 μmol g−1 h−1 | [101] |
| FeS2/Bi2S3 | 300 W Xe lamp | H2 production | 16.8 mmol g−1 h−1 | FeS2: 0.52 mmol g−1 h−1 | [102] |
| wood/CuS–MoS2 | AM 1.5 G (100 mW/cm2) | H2 production | 85604 μmol g−1 h−1 | MoSx-TiO2: 11090 μmol g−1 h−1 | [103] |
| Ag@SiO2@TiO2/Au | UV LED (365 nm) (150 mW/cm2) | H2 production | 30.2 mmol g−1 h−1 | SiO2@TiO2: 0.41 mmol g−1 h−1 | [104] |
| SnSe/ZnIn2S4 | 300 W Xe lamp (UV-vis-NIR) | H2 production | 5058 μmol g−1 h−1 | ZnIn2S4: 1691 μmol g−1 h−1 | [31] |
| Co0.85Se/Mn0.3Cd0.7S | 300 W Xe lamp (λ > 420 nm) ET: 25 °C | H2 production | 79.7 mmol g−1 h−1 | 5 °C: 46.7 mmol g−1 h−1 | [28] |
| Ni2P/TiO2(B) | 300 W Xe lamp ET: 90 °C | H2 production | 20.129 mmol g−1 h−1 | 50 °C: 6.752 mmol g−1 h−1 | [105] |
| Au/SiO2/CdS/Ag | LEDs (400–800 nm) | H2 production | 130 mmol g−1 h−1 | SiO2/CdS/Ag: 37.53 mmol g−1 h−1 | [106] |
| WO3/CdS | 300 W Xe lamp (UV-vis-NIR) | H2 production | 65.98 mmol g−1 h−1 | 10 °C: 20.82 mmol g−1 h−1 | [32] |
| Zn NPs | halogen lamp (4.67 W/cm2) | H2 production | 200 μmol g−1 h−1 | Zn powder: 20 μmol g−1 h−1 | [107] |
| PVDF-HFP/CdS/CNT | 280 W Xe lamp | H2 production | 451 μmol g−1 h−1 | PVDF-CTFE/CdS: 136 μmol g−1 h−1 | [108] |
| PDA/DCN | Xe lamp (λ > 420 nm) | MB degradation | DE = 98% (70 min) | DCN: DE = 48% | [109] |
| Cu0.75Ag0.5S | 300 W Xe lamp | MB degradation | DE = 93.8% (30 min) | CuS/rGO: DE = 80% (140 min) | [110] |
| CuFe2O4@MIL-100(Fe, Cu) | 300 W Xe lamp (λ > 400 nm) | MB degradation | k = 0.075 min−1 | CuFe2O4: k = 0.023 min−1 | [111] |
| Prussian blue (PB) microcrystals | solar simulator (100 mW/cm2) | MB degradation | k = 0.0430 min−1 | PC: k = 0.0231 min−1 | [112] |
| C@TiO2 | 300 W Xe lamp | RhB degradation | k = 0.045 min−1 | TiO2 MNF: k = 0.011 min−1 | [113] |
| OPtCu-NCs | 808 nm NIR laser | RhB degradation | DE = 91.87% (120 min) | – | [114] |
| CoFe2O4-BiOCl | 300 W Xe lamp | RhB degradation | k = 1.16 min−1 | Vis: k = 0.74 min−1 | [115] |
| ZnSnO3 | UV light (293–338 K) | RhB degradation | DE = 98.1% (80 min) | UV: DE = 76.8% | [116] |
| ZnO | 6 W mercury arc lamp | RhB degradation | DE ≈ 75% (30 min) | PC: DE ≈ 50% | [39] |
| Au/TiO2_PW | 300 W Xe lamp (UV–vis light) | MO degradation | DE = 74% (30 min) | Au-TiO2: DE = 10% | [117] |
| TiXn | 150 W Xe lamp | MO degradation | DE = 90% (120 min) | TiS3: DE = 34% (180 min) | [118] |
| Ag/TiO2 | 300 W Hg lamp | MO degradation | DE = 100% (60 min) | - | [37] |
| Fe3O4@SiO2-laccase | 500 W Xe lamp (λ > 400 nm) | MG degradation | DE = 99.6% (60 min) | alizarin red: DE = 79.3% | [119] |
| Pt−Cu/TiO2 | 300 W Xe lamp | toluene degradation | DE = ∼100% (120 min) | 110 °C: DE = ∼1% | [120] |
| WO3-x-R/GdCrO3 | Xe lamp (400 mW/cm2) | toluene degradation | k = 0.029 min−1 | SrTiO3/TiO2: k = 0.0054 min−1 | [121] |
| Pt/[TiN@TiO2] | Xe lamp (500 mW/cm2) | toluene degradation | DE = 100% (24 min) | Pt/TiO2: DE = ∼45% | [122] |
| SmMnO3/CuMnOx | 400 W Xe lamp (λ > 420 nm) | toluene degradation | DE = 100% (275 °C) | SmMnO3: DE = 100% (300 °C) | [123] |
| LaMn1.3O3 | 300 W Xe lamp (94 mW/cm2) | toluene degradation | DE = 98% (120 min) | - | [124] |
| Cu/TiO2-x/CoP | 300 W Xe lamp | 2,4-D degradation | DE = 99.2% (180 min) | TiO2-x/CoP: DE = 75.8% | [125] |
| Ag/Bi2S3/MoS2 | 500 W Xe lamp (λ > 420 nm) | 2,4-D degradation | k = 0.02334 min−1 | Bi2S3/MoS2: k = 0.00638 min−1 | [126] |
| Cu2-xS/CdS/Bi2S3 | 300 W Xe lamp (λ > 420 nm) | 2,4-D degradation | k = 0.03193 min−1 | Cu2-xS/Bi2S3: k = 0.00422 min−1 | [127] |
| α-Fe2O3/Defective MoS2/Ag | Xe lamp (λ > 420 nm) | 2,4-D degradation | k = 0.043 min−1 | α-Fe2O3: k = 0.0013 min−1 | [128] |
| TiO2 | 200 W mercury lamp | 2,4-D degradation | k ≈ 0.007 min−1 (O3) | PC: k ≈ 0.001 min−1 | [129] |
| W18O49@ZnIn2S4/CC | 300 W Xe lamp (λ > 420 nm) | BPA degradation | DE = 95% (150 min) | CC: DE = 15% | [130] |
| Ag/NaCNN/NiFe-LDH | 500 W Xe lamp (λ > 420 nm) | BPA degradation | k = 0.0432 min−1 | NiFe-LDH: k=0.00292 min−1 | [51] |
| Cu2-xS/Fe-POMs/AgVO3 | 300 W Xe lamp (λ > 420 nm) | BPA degradation | DE = 98.6% (150 min) | AgVO3: DE = 19.6% | [131] |
| Bi7O9I3/AgI | Xe lamp (λ > 420 nm) | phenol degradation | DE = 95.38% (80 min) | Bi7O9I3: DE = 30.1% | [132] |
| Bi12CoO20 | 300 W Xe lamp ET: 90 °C | phenol degradation | k = 0.113 min−1 | 15 °C: k = 0.009 min−1 | [133] |
| AuAgPt-12 YSNSs | 500 W Xe lamp | 4-NP degradation | k = 0.155 min−1 | AuAgPt-6 YSNSs: k=0.023 min−1 | [134] |
| CaCO3/CuS | NIR laser (2.5 W/cm2) | 4-NP degradation | DE = 98% (15 min) | CuS NPs: DE = 0% | [34] |
| SrTiO3/MnFe2O4 | 200 W low-pressure Hg lamp | TC degradation | DE = 100% (20 min) | PC: DE = 38.2% (25 min) | [38] |
| ZnO-MnO2 | 300 W Xe lamp | TC degradation | k ≈ 0.23 min−1 | MnO2: k ≈ 0.2 min−1 | [135] |
| Ag/Ag3PO4/CeO2 | 300 W Xe lamp | benzene degradation | DE = 90.18% (180 min) | CeO2: DE ≈ 70% | [136] |
| α-MnO2/GO | 300 W Xe lamp | formaldehyde degradation | DE = 100% (60 min) | - | [137] |
| Co3O4/rGO | 500 W Xe lamp (UV-vis-NIR) | fethanol degradation | DE = 96% (90 min) | UV-vis: DE = 41% | [138] |
| ZnFe2O4 | MEDL MW oven (100 W) | TCH degradation | DE = 91.6% (250 s) | PC: DE ≈ 20% | [139] |
| TiO2 | UV lamps | 4C2AP degradation | DE = 93.23% (30 min) | PC: DE = 85.28% | [140] |
| Material | Condition | Application | Activity | Blank Control | Ref. |
|---|---|---|---|---|---|
| BaTiO3@C | 300 W Xe lamp 40 kHz US cleaner | RhB degradation | k = 0.03585 min−1 | PZC: k = 0.00085 min−1 | [147] |
| Ag@Na0.5Bi0.5TiO3 | 300 W Xe lamp 40 kHz US cleaner | RhB degradation | k = 0.146 min−1 | Na0.5Bi0.5TiO3(PC): k = 0.019 min−1 | [148] |
| Bi2WO6/Black TiO2 | 220 W Xe lamp US cleaner | RhB degradation | DE = 98.43% (60 min) | PC: DE = 54.23% (60 min) | [149] |
| Bi2WO6/g-C3N4/ZnO | 300 W Xe lamp 40 kHz US cleaner | RhB degradation | k = 0.231 min−1 | PC: k = 0.097 min−1 | [150] |
| g-C3N4/Ag/ZnO | 50 W LED US cleaner | RhB degradation | DE = 89% (180 min) | PC: DE = 70% | [151] |
| PAN/TiO2 | 350 W Xe lamp 100 kHz EPR spectrometer | RhB degradation | k = 0.036 min−1 | PC: k = 0.015 min−1 | [152] |
| PbTiO3/g-C3N4 | 300 W Xe lamp (λ > 420 nm) US vibration | RhB degradation | k = 0.1357 min−1 | PC: k = 0.1044 min−1 | [153] |
| Ag2O/Bi4Ti3O12 | 400 W metal halide lamp US vibration | RhB degradation | k = 0.1557 min−1 | PC: k = 0.0363 min−1 | [154] |
| BaTiO3/SrTiO3 | LED UV lamp (30 W, 365 nm) 40 kHz US cleaner | RhB degradation | DE = 97.4% (30 min) | SrTiO3: DE = 44.3% | [155] |
| AgI/ZnO | 250 W Xe lamp (λ > 400 nm) US vibration | RhB degradation | k = 0.037 min−1 | ZnO: k = 0.002 min−1 | [156] |
| Bi2WO6 | 9 W LED 120 W US cleaner | RhB degradation | k = 0.141 min−1 | PC: k = 0.008 min−1 | [157] |
| Ag/BaTiO3 | 500 W Xe lamp 150 W US vibration | RhB degradation | DE = 70% (120 min) | PC: DE = 25% | [158] |
| NaNbO3/CuBi2O4 | 50 W LED 35 W US vibration | RhB degradation | DE = 75% (90 min) | NaNbO3: DE = 40% | [159] |
| BaTiO3/TiO2 | 250 W Xe lamp 40 kHz US cleaner | RhB degradation | k = 0.0967 min−1 | TiO2: k = 0.0275 min−1 | [160] |
| ZnO@PVDF | 300 W Xe lamp magnetic stirrer | RhB degradation | DE ≈ 95% (100 min) | PC: DE ≈ 55% | [161] |
| ZnO/ZnS | 300 W Xe lamp 180 W US vibration | MB degradation | DE = 53.8% (50 min) | PC: DE = 19.1% | [162] |
| PMN-PT@SnO2 | 250 W metal halide lamp 45 kHz US cleaner | MB degradation | DE = 97% (120 min) | SnO2: DE = 87% | [163] |
| Ag2MoO4 | 300 W Xe lamp 40 kHz US vibration | MB degradation | DE = 96.2% (40 min) | PC: DE = 82% | [164] |
| Ag3PO4/ZnO | 300 W Xe lamp (λ > 420 nm) 40 kHz US cleaner | MB degradation | DE = 98.16% (30 min) | PC: DE = 90.18% | [165] |
| ZnO/ZnS/MoS2 | 300 W Xe lamp magnetic stirrer (1000 rpm) | MB degradation | k = 0.0411 min−1 | PC: k = 0.0089 min−1 | [166] |
| BaTiO3/CuO | 200 W Xe lamp US cleaner | MO degradation | k = 0.05 min−1 | PZC: k = 0.007 min−1 | [167] |
| BiOBr/BaTiO3 | Xe lamp (100 mW cm–2) 40 kHz US vibration | MO degradation | k = 0.1123 min−1 | BaTiO3: k = 0.001 min−1 | [168] |
| ZnO NR/PVDF-HFP | Xe lamp (180 mW/cm2) magnetic stirrer (1000 rpm) | MO degradation | k = 0.0399 min−1 | PC(200 rpm): k = 0.0101 min−1 | [169] |
| ZnO/MoS2 | 300 W Xe lamp magnetic stirrer (1000 rpm) | MO degradation | DE = 92.7% (50 min) | PC: DE = 50.6% | [170] |
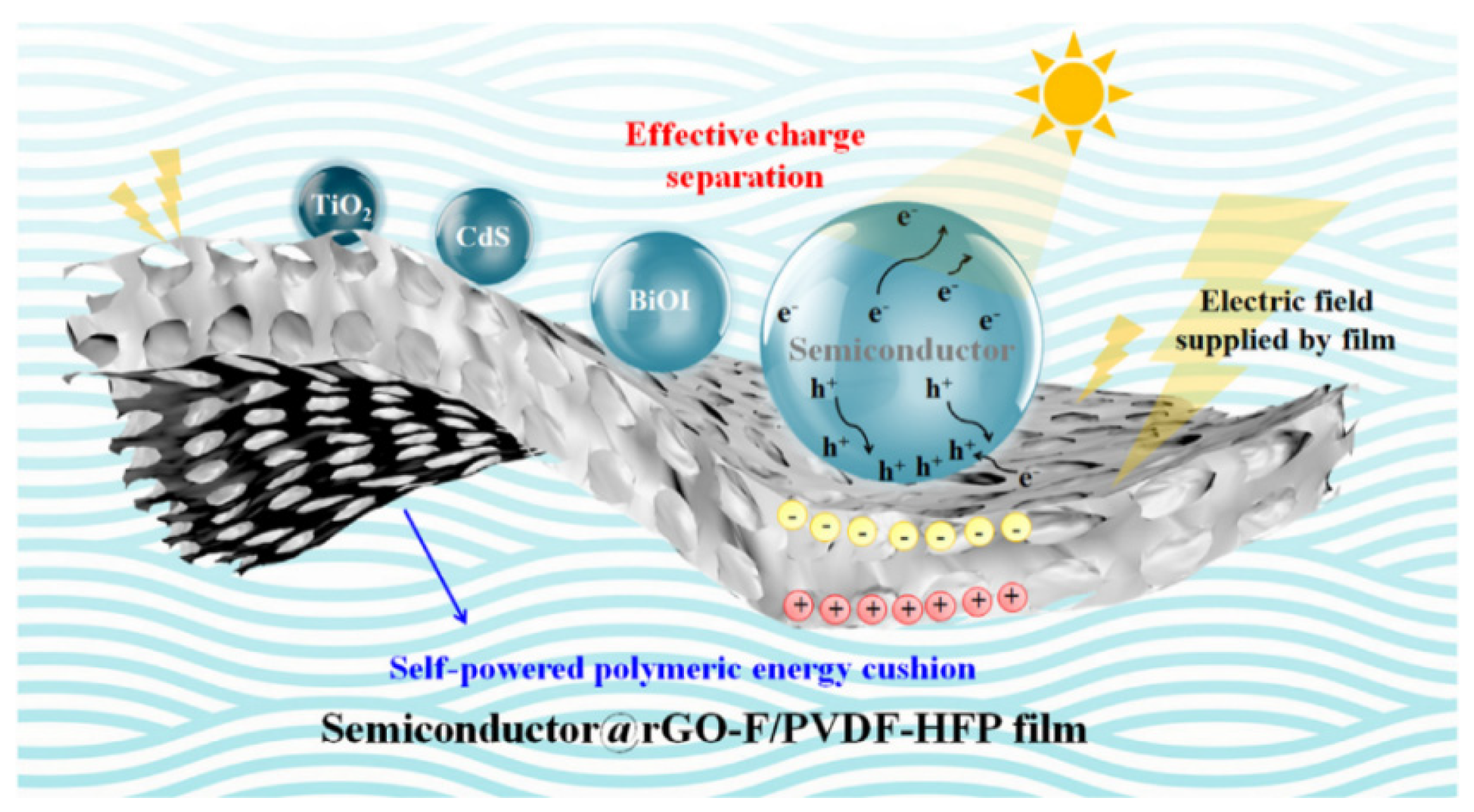
| TiO2@rGO-F/PVDF-HFP | 300 W Xe lamp (UV light) magnetic stirrer | MO degradation | DE = 99% (100 min) | - | [171] |
| Bi2MoO6/BiOBr | 400 W metal halide lamp US cleaner | MV degradation | k = 0.0284 min−1 | Bi2MoO6: k = 0.0082 min−1 | [172] |
| SrBi4Ti4O15 | 300 W Xe lamp 40 kHz US cleaner | TC degradation | k = 0.058 min−1 | PC: k = 0.004 min−1 | [173] |
| Ti32-oxo-cluster/BaTiO3/CuS | 300 W Xe lamp 120 W US vibration | TC degradation | DE = 100% (60 min) | PC: DE = 55.67% | [174] |
| CsCdBO3 | 300 W Xe lamp (λ > 420 nm) 40 kHz US cleaner | TC degradation | DE = 92% (30 min) | - | [175] |
| ZnO/CdS | 300 W Xe lamp 150 W US cleaner | BPA degradation | k = 0.1557 min−1 | PC: k = 0.0135 min−1 | [176] |
| BiOI/ZnO | 300 W Xe lamp 40 kHz US cleaner | BPA degradation | DE = 100% (30 min) | PC: DE = 25% | [177] |
| PZT/TiO2 | LED light (15 mW/cm2) magnetic stirrer (800 rpm) | BPA degradation | DE ≈ 90% (40 min) | PC(200 rpm): DE ≈ 70% | [178] |
| Au-BiOBr | 300 W Xe lamp 40 kHz US cleaner | CBZ degradation | k = 0.091 min−1 | k = 0.00516 min−1 | [179] |
| BaTiO3/La2Ti2O7 | 300 W Xe lamp (λ > 420 nm) 40 kHz US cleaner | CIP degradation | k = 0.0844 min−1 | BaTiO3: k = 0.0469 min−1 | [180] |
| ZnS/ Bi2S3-PVDF | 300 W Xe lamp (λ > 420 nm) US cleaner | H2 production | 10.07 mmol g−1 h−1 | ZnS/Bi2S3: 1.77 mmol g−1 h−1 | [181] |
| OH-modified SrTiO3 | 300 W Xe lamp 40 kHz US cleaner | H2 production | 701.2 μmol g−1 h−1 | PC: 295.4 μmol g−1 h−1 | [182] |
| PbTiO3/CdS | 300 W Xe lamp US vibration | H2 production | 849 μmol g−1 h−1 | PC: 98.9 μmol g−1 h−1 | [183] |
| g-C3N4 | 300 W Xe lamp (λ > 420 nm) US cleaner | H2 production | 12.16 mmol g−1 h−1 | PZC: 8.35 mmol g−1 h−1 | [184] |
| g-C3N4/LiNbO3/PVDF | 300 W Xe lamp magnetic stirrer | H2 production | 136.02 μmol g−1 h−1 | PC: 87.71 μmol g−1 h−1 | [185] |
| g-C3N4/PDI-g-C3N4 | 300 W Xe lamp 40 kHz US cleaner | H2O2 production | 625.54 μmol g−1 h−1 | PC: 149.85 μmol g−1 h−1 | [186] |
| ZnS/In2S3/BaTiO3 | 300 W Xe lamp (λ > 400 nm) 40 kHz US horn | H2O2 production | 228 μmol g−1 h−1 | 72 μmol g−1 h−1 | [187] |
| Material | Condition | Application | Activity | Blank Control | Ref. |
|---|---|---|---|---|---|
| ZnO | UV light magnet (600 mT) | MB degradation | DE = 60% (3 min) | PC: DE = 24% | [203] |
| CoFe2O4 | UV light (100 mW/cm2) magnet (200 mT) | MB degradation | DE = 80% (60 min) | PC: DE = 28% | [204] |
| n-TiO2/PS | 400 W UV light power supply (3 V) | MB degradation | DE = 38% (30 min) | PC: DE = 20% | [205] |
| rGO/TiO2 | 40 W UV lamp Nd2Fe14B magnet | MO degradation | DE = 91% (120 min) | TiO2: DE = 68% | [206] |
| TiO2 | 4 W mercury lamp magnet (280 mT) | MO degradation | DE ≈ 94% (75 min) | PC: DE ≈ 70% | [207] |
| α-Fe2O3/TiO2 | Diode Green Laser magnet (400 mT) | RhB degradation | DE ≈ 70% (60 min) | PC: DE ≈ 45% | [208] |
| Ti0.936O2 | 300 W Xe lamp electromagnet (80 mT) | RhB degradation | k ≈ 0.075 min−1 | PC: k ≈ 0.05 min−1 | [209] |
| BiFeO3 | 500 W Xe lamp (λ > 420 nm) electrical poling | RhB degradation | k = 0.035 min−1 | PC: k = 0.016 min−1 | [210] |
| α-Fe2O3/rGO | 300 W Xe lamp magnet | CR degradation | DE = 87% (30 min) | PC: DE = 60% | [211] |
| CoFe2O4/MoS2 | 300 W Xe lamp electromagnet (150 mT) | CR degradation | DE = 96.6% (60 min) | PC (50 mT): DE = 82% | [212] |
| BiOBr/BNQDs | 300 W Xe lamp (λ > 420 nm) magnet | TC degradation | DE = 81% (60 min) | BiOBr: DE = 60% | [213] |
| hierarchical TiO2 microspheres | 500 W Xe lamp power supply (2 V) | TBT degradation | k = 0.0488 min−1 | PC: k = 0.0052 min−1 | [214] |
| Mn3O4/γ-MnOOH | 300 W Xe lamp magnet (60 mT) | NOR degradation | DE = 98.8% (160 min) | PC: DE = 90.3% | [215] |
| α-Fe2O3/Zn1-xFexO | Xe lamp (λ > 420 nm) ten magnets (20 mT) | RIB degradation | k = 0.0125 min−1 | PC: k = 0.0072 min−1 | [216] |
| Au/Fe3O4/N-TiO2 | 70 W tungsten light magnet (180 mT) | H2 production | 21230 μmol g−1 h−1 | PC: 7600 μmol g−1 h−1 | [217] |
| CdS/MoS2/Mo | 300 W Xe lamp (100 mW/cm2) rotating magnet | H2 production | 1.97 mmol g−1 h−1 | PC: 1.04 mmol g−1 h−1 | [218] |
| Au-CdS | 300 W Xe lamp rotating magnet | H2 production | 105 μmol g−1 h−1 | PC: 223 μmol g−1 h−1 | [219] |
| Pt/TiO2 | 3 W LED lamp DC power (1 V) | H2 production | 3242.6 μmol g−1 h−1 | PC: 1102.8 μmol g−1 h−1 | [220] |
| K0.5Na0.5NbO3 | 300 W Xe lamp corona poling (690 kV/cm) | H2 production | 470 μmol g−1 h−1 | PC: 63 μmol g−1 h−1 | [221] |
| Rutile TiO2 nanograss | sunlight power supply (2 V) | Cr ion removal | 143.8 mg/g | - | [222] |
| Bi2MoO6 | 300 W Xe lamp corona poling (20 kV/cm) | CO2 reduction | 14.38 μmol g−1 h−1 (CO) | PC: 4.08 μmol g−1 h−1 | [223] |
| BaTiO3 | 300 W Xe lamp magnet | nitrogen fixation | 1.93 mgL−1 h−1 | PC: 1.35 mgL−1 h−1 | [224] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, Y.; Fang, W.; Jiang, Y.; Yang, Y.; Liu, Y.; Shangguan, W. Recent Advancements in Photocatalysis Coupling by External Physical Fields. Catalysts 2022, 12, 1042. https://doi.org/10.3390/catal12091042
Mi Y, Fang W, Jiang Y, Yang Y, Liu Y, Shangguan W. Recent Advancements in Photocatalysis Coupling by External Physical Fields. Catalysts. 2022; 12(9):1042. https://doi.org/10.3390/catal12091042
Chicago/Turabian StyleMi, Yan, Wenjian Fang, Yawei Jiang, Yang Yang, Yongsheng Liu, and Wenfeng Shangguan. 2022. "Recent Advancements in Photocatalysis Coupling by External Physical Fields" Catalysts 12, no. 9: 1042. https://doi.org/10.3390/catal12091042
APA StyleMi, Y., Fang, W., Jiang, Y., Yang, Y., Liu, Y., & Shangguan, W. (2022). Recent Advancements in Photocatalysis Coupling by External Physical Fields. Catalysts, 12(9), 1042. https://doi.org/10.3390/catal12091042













