Development of Adsorptive Materials for Selective Removal of Toxic Metals in Wastewater: A Review
Abstract
1. Introduction
2. Types of Adsorption
3. Adsorption Isotherm and Kinetic
3.1. Adsorption Isotherm Model
3.2. Adsorption Kinetic Model
3.2.1. Pseudo-First-order Model
3.2.2. Pseudo-Second-Order Model
3.2.3. Intraparticle Diffusion Model
3.2.4. Elovich Model
4. Materials for Adsorption of Toxic Materials in Wastewater
4.1. Activated Carbons
4.1.1. Chemical Activation Method
4.1.2. Physical Activation Method
4.2. Zeolites
4.3. Clay Minerals
4.3.1. Thermal Treatment of Clay
4.3.2. Acid Activation of Clay
4.3.3. Polymerization of Clay
5. Nanotechnology
5.1. Metal Oxide Nanoparticle
5.1.1. Iron Oxide-Based Nanoparticles
Magnetite (Fe3O4)
Hematite (α-Fe2O3)
Maghemite (γ-Fe2O3)
5.2. Titanium Oxide-Based Nanoparticles
5.3. Zinc Oxide-Based Nanoparticles
6. Carbon Nanomaterials
Graphene Nanomaterials
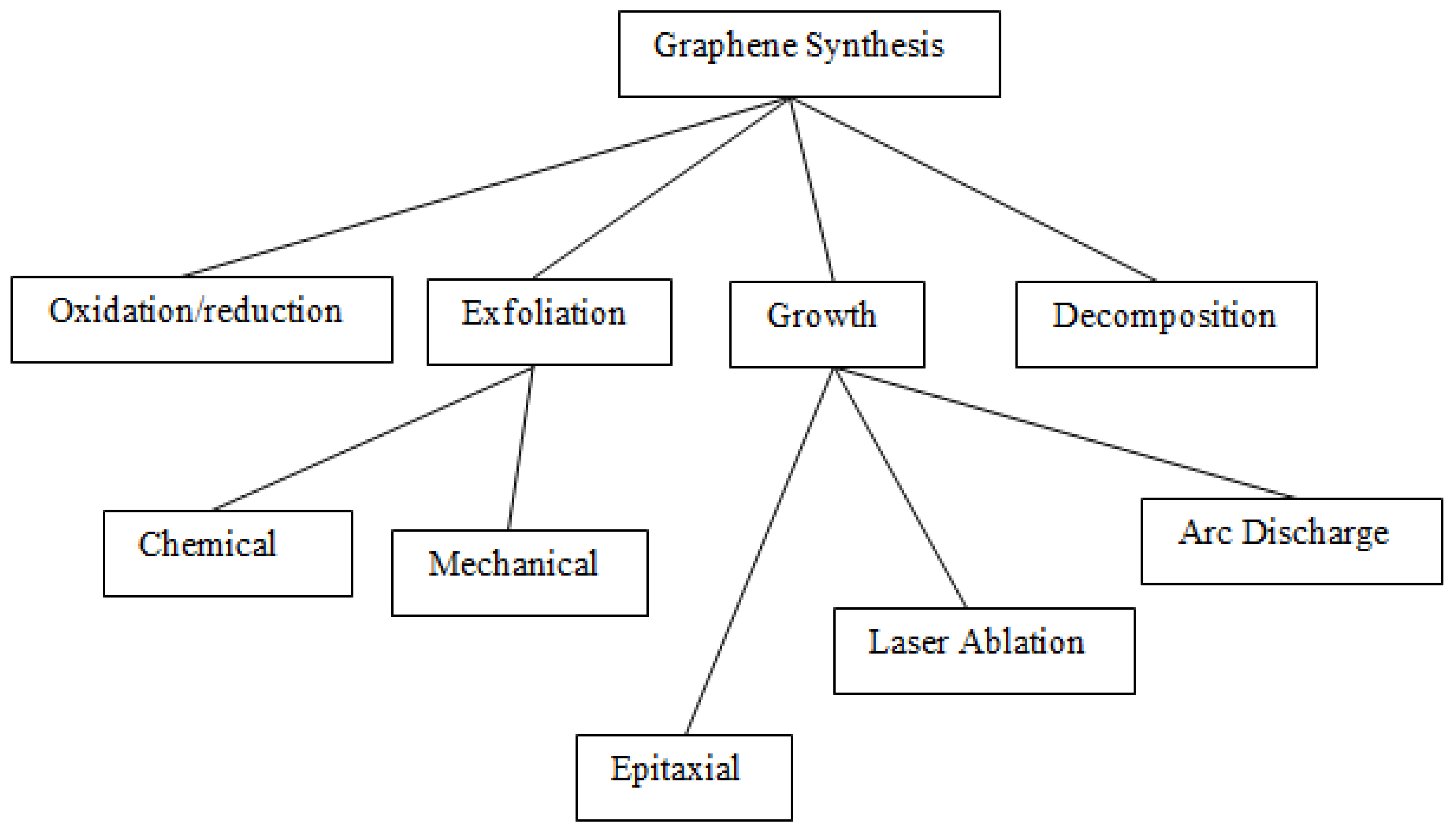
7. Synthesis of Metal Oxide Nanoparticles
7.1. Direct methods
7.1.1. Arc Discharge
7.1.2. Laser Procedures
7.1.3. Pulsed Laser Method
7.1.4. Reduction of Graphene Oxide
7.1.5. Mechanical Exfoliation
7.1.6. Chemical and Electrochemical Exfoliation
7.2. Indirect Methods
7.2.1. Epitaxial Growth
7.2.2. Chemical Vapour Deposition (CVD)
7.2.3. Carbon Nanotube (CNT)
8. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, M.; Kalita, P.; Senapati, K. Study on Magnetic Materials for Removal of Water Pollutants. In Emerging Pollutants-Some Strategies for the Quality Preservation of Our Environment; BoD—Books on Demand: Norderstedt, Germany, 2018; pp. 221–244. [Google Scholar]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, S.; Kumar, I.A.; Viswanathan, N. Activated Carbon from Different Waste Materials for the Removal of Toxic Metals. Green Mater. Wastewater Treat. 2020, 38, 47–68. [Google Scholar] [CrossRef]
- Alloway, B.J. Sources of Heavy Metals and Metalloids in Soils. In Heavy Metals in Soils; Springer: Dordrecht, The Netherlands, 2013; pp. 11–50. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Sankaran, R.; Show, P.L.; Ooi, C.-W.; Ling, T.C.; Shu-Jen, C.; Chen, S.-Y.; Chang, Y.-K. Feasibility assessment of removal of heavy metals and soluble microbial products from aqueous solutions using eggshell wastes. Clean Technol. Environ. Policy 2020, 22, 773–786. [Google Scholar] [CrossRef]
- Sachan, D.; Ramesh, A.; Das, G. Green synthesis of silica nanoparticles from leaf biomass and its application to remove heavy metals from synthetic wastewater: A comparative analysis. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100467. [Google Scholar] [CrossRef]
- Sharma, R.K.; Solanki, K.; Dixit, R.; Sharma, S.; Dutta, S. Nanoengineered iron oxide-based sorbents for separation of various water pollutants: Current status, opportunities and future outlook. Environ. Sci. Water Res. Technol. 2021, 7, 818–860. [Google Scholar] [CrossRef]
- Abdullah, N.; Yusof, N.; Lau, W.J.; Jaafar, J.; Ismail, A.F. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Qu, J. Research progress of novel adsorption processes in water purification: A review. J. Environ. Sci. 2008, 20, 1–13. [Google Scholar] [CrossRef]
- Jawed, A.; Saxena, V.; Pandey, L.M. Engineered nanomaterials and their surface functionalization for the removal of heavy metals: A review. J. Water Process. Eng. 2020, 33, 101009. [Google Scholar] [CrossRef]
- Fato, F.P.; Li, D.-W.; Zhao, L.-J.; Qiu, K.; Long, Y.-T. Simultaneous Removal of Multiple Heavy Metal Ions from River Water Using Ultrafine Mesoporous Magnetite Nanoparticles. ACS Omega 2019, 4, 7543–7549. [Google Scholar] [CrossRef]
- Tripathi, A.; Rawat Ranjan, M. Heavy Metal Removal from Wastewater Using Low Cost Adsorbents. J. Bioremediat. Biodegrad. 2015, 6, 315. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Agboola, O.D.; Benson, N.U. Physisorption and Chemisorption Mechanisms Influencing Micro (Nano) Plastics-Organic Chemical Contaminants Interactions: A Review. Front. Environ. Sci. 2021, 167. [Google Scholar] [CrossRef]
- Tamiru, M.; Bekele, G. International Journal of Water and Wastewater Treatment Various Absorbents and Parameters Affecting Removal of Water Hardness from Wastewater: Review. Int. J. Water Wastewater Treat. 2020, 6, 173. [Google Scholar] [CrossRef]
- De Freitas, G.R.; da Silva, M.G.C.; Vieira, M.G.A. Biosorption technology for removal of toxic metals: A review of commercial biosorbents and patents. Environ. Sci. Pollut. Res. 2019, 26, 19097–19118. [Google Scholar] [CrossRef]
- Ugwu, E.I.; Tursunov, O.; Kodirov, D.; Shaker, L.M.; Al-Amiery, A.A.; Yangibaeva, I.; Shavkarov, F. Adsorption mechanisms for heavy metal removal using low cost adsorbents: A review. IOP Conf. Ser. Earth Environ. Sci. 2020, 614, 012166. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A Review of Adsorbents for Heavy Metal Decontamination: Growing Approach to Wastewater Treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.S.; Hossain, M.M.; Jeong, H.M.; Kim, K. Heavy metal removal applications using adsorptive membranes. Nano Converg. 2020, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Zand, A.D.; Abyaneh, M.R. Adsorption of Lead, manganese, and copper onto biochar in landfill leachate: Implication of non-linear regression analysis. Sustain. Environ. Res. 2020, 30, 18. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Bushra, R.; Shahadat, M.; Ahmad, A.; Nabi, S.A.; Umar, K.; Oves, M.; Raeissi, A.S.; Muneer, M. Synthesis, characterization, antimicrobial activity and applications of polyanilineTi(IV)arsenophosphate adsorbent for the analysis of organic and inorganic pollutants. J. Hazard. Mater. 2014, 264, 481–489. [Google Scholar] [CrossRef]
- Ayranci, E.; Duman, O. In-Situ UV-Visible Spectroscopic Study on the Adsorption of some Dyes onto Activated Carbon Cloth. Sep. Sci. Technol. 2009, 44, 3735–3752. [Google Scholar] [CrossRef]
- Deliyanni, E.A.; Kyzas, G.Z.; Triantafyllidis, K.S.; Matis, K.A. Activated carbons for the removal of heavy metalions: A systematic review of recent literaturefocused on lead and arsenic ion. Open Chem. 2015, 13, 699–708. [Google Scholar]
- Shehzad, A.; Bashir, M.J.K.; Sethupathi, S.; Lim, J.W. An overview of heavily polluted landfill leachate treatment using food waste as an alternative and renewable source of activated carbon. Process. Saf. Environ. Prot. 2015, 98, 309–318. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Nabais, J.V.; Carrott, P.; Ribeiro Carrott, M.M.L.; Luz, V.; Ortiz, A.L. Influence of preparation conditions in the textural and chemical properties of activated carbons from a novel biomass precursor: The coffee endocarp. Bioresour. Technol. 2008, 99, 7224–7231. [Google Scholar] [CrossRef]
- Deng, H.; Yang, L.; Tao, G.; Dai, J. Preparation and characterization of activated carbon from cotton stalk by microwave assisted chemical activation—Application in methylene blue adsorption from aqueous solution. J. Hazard. Mater. 2009, 166, 1514–1521. [Google Scholar] [CrossRef]
- Tran, H.N.; Chao, H.P.; You, S.J. Activated carbons from golden shower upon different chemical activation methods: Synthesis and characterizations. Adsorpt. Sci. Technol. 2017, 36, 95–113. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B.; Li, A. Insight into activated carbon from different kinds of chemical activating agents: A review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef]
- Salman, S.; Rasheed, I.; Mohammed, A. IOP Conference Series: Earth and Environmental Science. IOP Conf. Ser. Earth Environ. Sci. 2021, 779, 012074. [Google Scholar] [CrossRef]
- Gebretsadik, H.; Gebrekidan, A.; Demlie, L. Removal of heavy metals from aqueous solutions using Eucalyptus Camaldulensis: An alternate low cost adsorbent. Cogent Chem. 2020, 6, 1720892. [Google Scholar] [CrossRef]
- Rashed, M.; Soltan, M.; Ahmed, M.; Abdou, A. Heavy Metals Removal from Wastewater by Adsorption on Modified Physically Activated Sewage Sludge. Arch. Org. Inorg. Chem. Sci. 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Ibrahim, W.M.; Hassan, A.F.; Azab, Y.A. Biosorption of toxic heavy metals from aqueous solution by Ulva lactuca activated carbon. Egypt. J. Basic Appl. Sci. 2019, 3, 241–249. [Google Scholar] [CrossRef]
- Hernández-Montoya, V.; García-Servin, J.; Bueno-López, J.I. Thermal Treatments and Activation Procedures Used in the Preparation of Activated Carbons. In Lignocellulosic Precursors Used in the Synthesis of Activated Carbon-Characterization Techniques and Applications in the Wastewater Treatment; BoD—Books on Demand: Norderstedt, Germany, 2012; pp. 19–36. [Google Scholar]
- Tasić, Ž.Z.; Bogdanović, G.D.; Antonijević, M.M. Application of natural zeolite in wastewater treatment: A review. J. Min. Metall. A Min. 2019, 55, 67–79. [Google Scholar] [CrossRef]
- Salih, A.M. The Purification of Industrial Wastewater to Remove Heavy Metals and Investigation into the Use of Zeolite as a Remediation Tool; University of Wolverhampton: Wolverhampton, UK, 2017. [Google Scholar]
- Turksoy, R.; Terzioglu, G.; Ertugrul Yalcin, I.; Turksoy Terzioglu, O.; Demir, G. Removal of heavy metals from textile industry wastewater. Front. Life Sci. Relat. Technol. 2021, 2, 44–50. [Google Scholar] [CrossRef]
- Taamneh, Y.; Sharadqah, S. The removal of heavy metals from aqueous solution using natural Jordanian zeolite. Appl. Water Sci. 2017, 7, 2021–2028. [Google Scholar] [CrossRef]
- Gülen, J.; Zorbay, F.; Arslan, S. Zeolites and their uses. Karaelmas J. Sci. Eng. 2012, 2, 63–68. [Google Scholar]
- Yuna, Z. Review of the natural, modified, and synthetic zeolites for heavy metals removal from wastewater. Environ. Eng. Sci. 2016, 33, 443–454. [Google Scholar] [CrossRef]
- La Vega, D.P.D.; González, C.; Escalante, C.A.; Gallego, J.; Salamanca, M.; Manrique-Losada, L. Use of faujasite-type zeolite for ion adsorption in municipal wastewater. Tecnol. Cienc. Agua 2018, 9, 184–208. [Google Scholar] [CrossRef]
- Iqra, J.; Faryal, M.; Uzaira, R.; Noshaba, T. Preparation of zeolite from incinerator ash and its application for the remediation of selected inorganic pollutants: A greener approach. IOP Conf. Ser. Mater. Sci. Eng. 2014, 60, 012060. [Google Scholar] [CrossRef]
- Hanane, B.; Seyd Abdelkader, H.; Kromia, M.; Salah, A.M. Removal of Heavy Metals from an Industrial Effluent by Synthesized Zeolite: Case of Bounoura Industrial Zone. Leban. Sci. J. 2020, 21, 80–94. [Google Scholar] [CrossRef]
- Ismail, A.I.M.; El-Shafey, O.I.; Amr, M.H.A.; El-Maghraby, M.S. Pumice Characteristics and Their Utilization on the Synthesis of Mesoporous Minerals and on the Removal of Heavy Metals. Int. Sch. Res. Not. 2014, 2014, 259379. [Google Scholar] [CrossRef]
- Wołowicz, A.; Wawrzkiewicz, M. Screening of Ion Exchange Resins for Hazardous Ni(II) Removal from Aqueous Solutions: Kinetic and Equilibrium Batch Adsorption Method. Processes 2021, 9, 285. [Google Scholar] [CrossRef]
- Gu, S.; Kang, X.; Wang, L.; Lichtfouse, E.; Wang, C. Clay mineral adsorbents for heavy metal removal from wastewater: A review. Environ. Chem. Lett. 2018, 17, 629–654. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Upadhyay, S.; Bhuiyan, M.; Bhattacharya, P.R. A review on prospects of essential oils as biopesticide in insect-pest management. J. Pharmacogn. Phyther. 2009, 1, 52–63. Available online: http://www.academicjournals.org/jpp (accessed on 10 July 2022).
- Nakato, T.; Miyamoto, N. Liquid Crystalline Behavior and Related Properties of Colloidal Systems of Inorganic Oxide Nanosheets. Materials 2009, 2, 1734–1761. [Google Scholar] [CrossRef]
- Ayalew, A.A. A critical review on clay-based nanocomposite particles for application of wastewater treatment. Water Sci. Technol. 2022, 85, 3002–3022. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S. Sen Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef]
- Heller-Kallai, L. Thermally Modified Clay Minerals. Dev. Clay Sci. 2006, 1, 289–308. [Google Scholar] [CrossRef]
- Barakan, S.; Aghazadeh, V. The advantages of clay mineral modification methods for enhancing adsorption efficiency in wastewater treatment: A review. Environ. Sci. Pollut. Res. 2020, 28, 2572–2599. [Google Scholar] [CrossRef]
- El Mouzdahir, Y.; Elmchaouri, A.; Mahboub, R.; Gil, A.; Korili, S.A. Equilibrium modeling for the adsorption of methylene blue from aqueous solutions on activated clay minerals. Desalination 2010, 250, 335–338. [Google Scholar] [CrossRef]
- Akar, S.T.; Yetimoglu, Y.; Gedikbey, T. Removal of chromium (VI) ions from aqueous solutions by using Turkish montmorillonite clay: Effect of activation and modification. Desalination 2009, 244, 97–108. [Google Scholar] [CrossRef]
- Shawabkeh, A.; Al-Khashman, O.; Al-Omari, H.; Shawabkeh, A. Cobalt and zinc removal from aqueous solution by chemically treated bentonite. Environmentalist 2007, 27, 357. [Google Scholar] [CrossRef][Green Version]
- Komadel, P.; Madejová, J. Acid Activation of Clay Minerals. Dev. Clay Sci. 2013, 5, 385–409. [Google Scholar] [CrossRef]
- Borji, H.; Ayoub, G.M.; Bilbeisi, R.; Nassar, N.; Malaeb, L. How Effective Are Nanomaterials for the Removal of Heavy Metals from Water and Wastewater? Water Air Soil Pollut. 2020, 231, 330. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Bhati, M.; Rai, R. Nanotechnology and water purification: Indian know-how and challenges. Environ. Sci. Pollut. Res. 2017, 24, 23423–23435. [Google Scholar] [CrossRef]
- Kolluru, S.S.; Agarwal, S.; Sireesha, S.; Sreedhar, I.; Kale, S.R. Heavy metal removal from wastewater using nanomaterials-process and engineering aspects. Process. Saf. Environ. Prot. 2021, 150, 323–355. [Google Scholar] [CrossRef]
- Liosis, C.; Papadopoulou, A.; Karvelas, E.; Karakasidis, T.E.; Sarris, I.E. Heavy Metal Adsorption Using Magnetic Nanoparticles for Water Purification: A Critical Review. Materials 2021, 14, 7500. [Google Scholar] [CrossRef]
- Saharan, P.; Chaudhary, G.R.; Mehta, S.K.; Umar, A. Removal of water contaminants by iron oxide nanomaterials. J. Nanosci. Nanotechnol. 2014, 14, 627–643. [Google Scholar] [CrossRef]
- Tsedenbal, B.; Lee, J.E.; Huh, S.H.; Koo, B.H.; Lee, C.G. Removal of Heavy Metals from Wastewater using α-Fe2O3 Nanocrystals. Korean J. Mater. Res. 2020, 30, 447–452. [Google Scholar] [CrossRef]
- Jiang, W.; Pelaez, M.; Dionysiou, D.D.; Entezari, M.H.; Tsoutsou, D.; O’Shea, K. Chromium(VI) removal by maghemite nanoparticles. Chem. Eng. J. 2013, 222, 527–533. [Google Scholar] [CrossRef]
- Parvin, F.; Rikta, S.Y.; Tareq, S.M. Application of Nanomaterials for the Removal of Heavy Metal from Wastewater. In Nanotechnology in Water and Wastewater Treatment: Theory and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 137–157. [Google Scholar]
- Tamjidi, S.; Esmaeili, H.; Moghadas, B.K. Application of magnetic adsorbents for removal of heavy metals from wastewater: A review study. Mater. Res. Express 2019, 6, 102004. [Google Scholar] [CrossRef]
- Vojoudi, H.; Badiei, A.; Bahar, S.; Mohammadi Ziarani, G.; Faridbod, F.; Ganjali, M.R. A new nano-sorbent for fast and efficient removal of heavy metals from aqueous solutions based on modification of magnetic mesoporous silica nanospheres. J. Magn. Magn. Mater. 2017, 441, 193–203. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Chang, Y.Y.; Yang, J.K.; Singh, J.; Choi, E.H.; Shiratani, M.; Koduru, J.R.; Attri, P. Biogenic reductive preparation of magnetic inverse spinel iron oxide nanoparticles for the adsorption removal of heavy metals. Chem. Eng. J. 2017, 307, 74–84. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Abdelwahab, M.S.; Abdou, A.E.H. Enhanced removal of lead and cadmium from water by Fe3O4-cross linked-O-phenylenediamine nano-composite. Sep. Sci. Technol. 2015, 51, 237–247. [Google Scholar] [CrossRef]
- Giraldo, L.; Erto, A.; Moreno-Piraján, J.C. Magnetite nanoparticles for removal of heavy metals from aqueous solutions: Synthesis and characterization. Adsorption 2013, 19, 465–474. [Google Scholar] [CrossRef]
- Shahzad, A.; Miran, W.; Rasool, K.; Nawaz, M.; Jang, J.; Lim, S.-R.; Lee, D.S. Heavy metals removal by EDTA-functionalized chitosan graphene oxide nanocomposites. RSC Adv. 2017, 7, 9764. [Google Scholar] [CrossRef]
- Zawrah, M.F.; El Shereefy, E.S.E.; Khudir, A.Y. Reverse Precipitation Synthesis of ≤10 nm Magnetite Nanoparticles and Their Application for Removal of Heavy Metals from Water. Silicon 2018, 11, 85–104. [Google Scholar] [CrossRef]
- Muhajir, M.; Puspitasari, P.; Razak, J.A. Synthesis and Applications of Hematite α-Fe2O3: A Review. J. Mech. Eng. Sci. Technol. 2020, 3, 51–58. [Google Scholar] [CrossRef]
- Basavegowda, N.; Mishra, K.; Lee, Y.R. Synthesis, characterization, and catalytic applications of hematite (α-Fe2O3) nanoparticles as reusable nanocatalyst. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 025017. [Google Scholar] [CrossRef]
- Davarnejad, R.; Nikandam, K. Zinc Oxide Nanoparticles Preparation for Pd2+ Ions Adsorption from Aqueous Wastewaters: A Green Technique; Research Square: Durham, NC, USA, 2021. [Google Scholar] [CrossRef]
- Yu, G.; Lu, Y.; Guo, J.; Patel, M.; Bafana, A.; Wang, X.; Qiu, B.; Jeffryes, C.; Wei, S.; Guo, Z.; et al. Carbon nanotubes, graphene, and their derivatives for heavy metal removal. Mater. Sci. 2017, 1, 56–78. [Google Scholar] [CrossRef]
- Grover, V.A.; Hu, J.; Engates, K.E.; Shipley, H.J. Adsorption and desorption of bivalent metals to hematite nanoparticles. Environ. Toxicol. Chem. 2012, 31, 86–92. [Google Scholar] [CrossRef]
- Marcus, M.; Karni, M.; Baranes, K.; Levy, I.; Alon, N.; Margel, S.; Shefi, O. Iron oxide nanoparticles for neuronal cell applications: Uptake study and magnetic manipulations. J. Nanobiotechnol. 2016, 14, 37. [Google Scholar] [CrossRef]
- Badmus, K.O.; Irakoze, N.; Adeniyi, O.R.; Petrik, L. Synergistic advance Fenton oxidation and hydrodynamic cavitation treatment of persistent organic dyes in textile wastewater. J. Environ. Chem. Eng. 2020, 8, 103521. [Google Scholar] [CrossRef]
- Blanchart, P. Extraction, Properties and Applications of Titania. In Industrial Chemistry of Oxides for Emerging Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 255–309. [Google Scholar]
- Rodriguez, A.; Segura, X.; Ferrer, A. Removal of Cadmium(II), Lead(II) and Chromiun(VI) in Water with Nanomaterials; Universitat Autonoma de Barcelona: Barcelona, Spain, 2015. [Google Scholar]
- Wang, X.; Cai, W.; Lin, Y.; Wang, G.; Liang, C. Mass production of micro/nanostructured porous ZnO plates and their strong structurally enhanced and selective adsorption performance for environmental remediation. J. Mater. Chem. 2010, 20, 8582–8590. [Google Scholar] [CrossRef]
- Máynez-Navarro, O.D.; Sánchez-Salas, J.L. Focus on Zinc Oxide as a Photocatalytic Material for Water Treatment. Int. J. Bioremediat. Biodegrad. 2017, 2018, 106. [Google Scholar] [CrossRef]
- Mohammadi, F.M.; Ghasemi, N. Influence of temperature and concentration on biosynthesis and characterization of zinc oxide nanoparticles using cherry extract. J. Nanostruct. Chem. 2018, 8, 93–102. [Google Scholar] [CrossRef]
- Shaba, E.Y.; Jacob, J.O.; Tijani, J.O.; Suleiman, M.A.T. A critical review of synthesis parameters affecting the properties of zinc oxide nanoparticle and its application in wastewater treatment. Appl. Water Sci. 2021, 11, 48. [Google Scholar] [CrossRef]
- Azizi, S.; Shahri, M.M.; Mohamad, R. Green synthesis of zinc oxide nanoparticles for enhanced adsorption of lead Ions from aqueous solutions: Equilibrium, kinetic and thermodynamic studies. Molecules 2017, 22, 831. [Google Scholar] [CrossRef] [PubMed]
- Çavuş, S.; Gürdaǧ, G. Noncompetitive Removal of Heavy Metal Ions from Aqueous Solutions by Poly[2-(acrylamido)-2-methyl-1-propanesulfonic acid-co-itaconic acid] Hydrogel. Ind. Eng. Chem. Res. 2009, 48, 2652–2658. [Google Scholar] [CrossRef]
- Thines, R.K.; Mubarak, N.M.; Nizamuddin, S.; Sahu, J.N.; Abdullah, E.C.; Ganesan, P. Application potential of carbon nanomaterials in water and wastewater treatment: A review. J. Taiwan Inst. Chem. Eng. 2017, 72, 116–133. [Google Scholar] [CrossRef]
- Yoo, M.J.; Park, H.B. Effect of hydrogen peroxide on properties of graphene oxide in Hummers method. Carbon N.Y. 2019, 141, 515–522. [Google Scholar] [CrossRef]
- Khan, S.; Achazhiyath Edathil, A.; Banat, F. Sustainable synthesis of graphene-based adsorbent using date syrup. Sci. Rep. 2019, 9, 18106. [Google Scholar] [CrossRef]
- Ahmad, S.Z.N.; Wan Salleh, W.N.; Ismail, A.F.; Yusof, N.; Mohd Yusop, M.Z.; Aziz, F. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: Toxicity, roles of functional groups and mechanisms. Chemosphere 2020, 248, 126008. [Google Scholar] [CrossRef]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 59–81. [Google Scholar] [CrossRef]
- Ali, I.; Peng, C.; Naz, I.; Amjed, M.A. Water Purification Using Magnetic Nanomaterials: An Overview. In Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2019; pp. 161–179. [Google Scholar]
- Arole, V.M.; Munde, S.V. Fabrication of nanomaterials by top-down and bottom-up approaches—An overview. JAAST Mater. Sci. 2014, 1, 89–93. [Google Scholar]
- Yazid, N.A.; Joon, Y.C. Co-precipitation synthesis of magnetic nanoparticles for efficient removal of heavy metal from synthetic wastewater. AIP Conf. Proc. 2019, 2124, 020019. [Google Scholar] [CrossRef]
- Singh, S.; Barick, K.C.; Bahadur, D. Functional Oxide Nanomaterials and Nanocomposites for the Removal of Heavy Metals and Dyes. Nanomater. Nanotechnol. 2013, 3, 3–20. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Morales, M.D.P.; Mazarío, E. Effect of the Surface Charge on the Adsorption Capacity of Chromium(VI) of Iron Oxide Magnetic Nanoparticles Prepared by Microwave-Assisted Synthesis. Water 2019, 11, 2372. [Google Scholar] [CrossRef]
- Müller, B.R. Effect of particle size and surface area on the adsorption of albumin-bonded bilirubin on activated carbon. Carbon 2010, 48, 3607–3615. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R. Graphene synthesis, characterization and its applications: A review. Results Chem. 2021, 3, 100163. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211, 317–331. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Komvopoulos, K. A review of graphene synthesis by indirect and direct deposition methods. J. Mater. Res. 2020, 35, 76–89. [Google Scholar] [CrossRef]
- Lee, X.J.; Hiew, B.Y.Z.; Lai, K.C.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Rigby, S. Review on graphene and its derivatives: Synthesis methods and potential industrial implementation. J. Taiwan Inst. Chem. Eng. 2019, 98, 163–180. [Google Scholar] [CrossRef]
- Karim, M.R.; Hayami, S. Chemical, Thermal, and Light-Driven Reduction of Graphene Oxide: Approach to Obtain Graphene and its Functional Hybrids. In Graphene Materials—Advanced Applications; IntechOpen: London, UK, 2017; p. 89. [Google Scholar]
- Ali, M.; Urgen, M. Morphology and Structure of Carbon Films Deposited at Varying Chamber Pressure; COMSATS University: Islamabad, Pakistan, 2018. [Google Scholar]
- Ferreira, F.V.; Franceschi, W.; Menezes, B.R.C.; Biagioni, A.F.; Coutinho, A.R.; Cividanes, L.S. Synthesis, characterization, and applications of carbon nanotubes. In Carbon-Based Nanofillers and Their Rubber Nanocomposites: Carbon Nano-Objects; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–45. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, H.; Sheng, L.; Yu, L.; An, K.; Xu, J.; Ando, Y.; Zhao, X. Mass-production of highly-crystalline few-layer graphene sheets by arc discharge in various H2–inert gas mixtures. Chem. Phys. Lett. 2012, 538, 72–76. [Google Scholar] [CrossRef]
- Shoukat, R.; Khan, M.I. Carbon nanotubes: A review on properties, synthesis methods and applications in micro and nanotechnology. Microsyst. Technol. 2021, 27, 4183–4192. [Google Scholar] [CrossRef]
- Qian, M.; Zhou, Y.S.; Gao, Y.; Park, J.B.; Feng, T.; Huang, S.M.; Sun, Z.; Jiang, L.; Lu, Y.F. Formation of graphene sheets through laser exfoliation of highly ordered pyrolytic graphite. Appl. Phys. Lett. 2011, 98, 173108. [Google Scholar] [CrossRef]
- Wang, J.; Fan, L.; Wang, X.; Xiao, T.; Peng, L.; Wang, X.; Yu, J.; Cao, L.; Xiong, Z.; Fu, Y.; et al. Pulsed laser deposition of monolayer and bilayer graphene. Appl. Surf. Sci. 2019, 494, 651–658. [Google Scholar] [CrossRef]
- Jiang, N.; Li, X.; Guo, H.; Li, J.; Shang, K.; Lu, N.; Wu, Y. Plasma-assisted catalysis decomposition of BPA over graphene-CdS nanocomposites in pulsed gas-liquid hybrid discharge: Photocorrosion inhibition and synergistic mechanism analysis. Chem. Eng. J. 2021, 412, 128627. [Google Scholar] [CrossRef]
- Sadeghi, H.; Solati, E.; Dorranian, D. Producing graphene nanosheets by pulsed laser ablation: Effects of liquid environment. J. Laser Appl. 2019, 31, 042003. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Tan, W.K.; Kar, K.K.; Matsuda, A. Recent progress in the synthesis of graphene and derived materials for next generation electrodes of high performance lithium ion batteries. Prog. Energy Combust. Sci. 2019, 75, 100786. [Google Scholar] [CrossRef]
- Bhuyan, M.S.A.; Uddin, M.N.; Islam, M.M.; Bipasha, F.A.; Hossain, S.S. Synthesis of graphene. Int. Nano Lett. 2016, 6, 65–83. [Google Scholar] [CrossRef]
- Yan, Y.; Nashath, F.Z.; Chen, S.; Manickam, S.; Lim, S.S.; Zhao, H.; Lester, E.; Wu, T.; Pang, C.H. Synthesis of graphene: Potential carbon precursors and approaches. Nanotechnol. Rev. 2020, 9, 1284–1314. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, L.; Wang, X.; Diankov, G.; Dai, H. Narrow graphene nanoribbons from carbon nanotubes. Nature 2009, 458, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Bhoria, R.S. Enhancing liquid phase exfoliation of graphene in organic solvents with additives. In Graphene and Its Derivatives-Synthesis and Applications; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Suslick, K.S.; Crum, L.A. Sonochemistry and Sonoluminescence; Springer: Berlin, Germany, 2007; pp. 271–281. [Google Scholar] [CrossRef]
- Wei, Y.; Sun, Z. Liquid-phase exfoliation of graphite for mass production of pristine few-layer graphene. Curr. Opin. Colloid Interface Sci. 2015, 20, 311–321. [Google Scholar] [CrossRef]
- Tyurnina, A.V.; Tzanakis, I.; Morton, J.; Mi, J.; Porfyrakis, K.; Maciejewska, B.M.; Grobert, N.; Eskin, D.G. Ultrasonic exfoliation of graphene in water: A key parameter study. Carbon N.Y. 2020, 168, 737–747. [Google Scholar] [CrossRef]
- Bleu, Y.; Bourquard, F.; Tite, T.; Loir, A.S.; Maddi, C.; Donnet, C.; Garrelie, F. Review of graphene growth from a solid carbon source by pulsed laser deposition (PLD). Front. Chem. 2018, 6, 572. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef]
- Narayan, R.; Kim, S.O. Surfactant mediated liquid phase exfoliation of graphene. Nano Converg. 2015, 2, 20. [Google Scholar] [CrossRef]
- Noroozi, M.; Zakaria, A.; Radiman, S.; Wahab, Z.A. Environmental Synthesis of Few Layers Graphene Sheets Using Ultrasonic Exfoliation with Enhanced Electrical and Thermal Properties. PLoS ONE 2016, 11, e152699. [Google Scholar] [CrossRef]
- Shan, X.; Wang, Q.; Bian, X.; Li, W.; Chen, G.; Zhu, H. Graphene layers on Si-face and C-face surfaces and interaction with Si and C atoms in layer controlled graphene growth on SiC substrates. RSC Adv. 2015, 5, 78625–78633. [Google Scholar] [CrossRef]
- Brisebois, P.P.; Siaj, M. Harvesting graphene oxide—Years 1859 to 2019: A review of its structure, synthesis, properties and exfoliation. J. Mater. Chem. C 2020, 8, 1517–1547. [Google Scholar] [CrossRef]
- Wang, C.; Vinodgopal, K.; Dai, G.-P. Large-Area Synthesis and Growth Mechanism of Graphene by Chemical Vapor Deposition; IntechOpen: London, UK, 2018. [Google Scholar]
- Saeed, M.; Alshammari, Y.; Majeed, S.A.; Al-Nasrallah, E. Chemical Vapour Deposition of Graphene—Synthesis, Characterisation, and Applications: A Review. Molecules 2020, 25, 3856. [Google Scholar] [CrossRef]
- Bhandari, H.; Garg, S.; Gaba, R. Advanced Nanocomposites for Removal of Heavy Metals from Wastewater. Macromol. Symp. 2021, 397, 2000337. [Google Scholar] [CrossRef]
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon nanotube—A review on Synthesis, Properties and plethora of applications in the field of biomedical science. Sens. Int. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Ates, M.; Eker, A.A.; Eker, B. Carbon nanotube-based nanocomposites and their applications. J. Adhes. Sci. Technol. 2017, 31, 1977–1997. [Google Scholar] [CrossRef]
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A review on carbon nanotube: An overview of synthesis, properties, functionalization, characterization, and the application. Mater. Sci. Eng. B 2021, 268, 115095. [Google Scholar] [CrossRef]
- Hamzah, N.; Yasin, M.F.M.; Yusop, M.Z.M.; Saat, A.; Subha, N.A.M. Rapid production of carbon nanotubes: A review on advancement in growth control and morphology manipulations of flame synthesis. J. Mater. Chem. A 2017, 5, 25144–25170. [Google Scholar] [CrossRef]
- Arora, B.; Attri, P. Carbon Nanotubes (CNTs): A Potential Nanomaterial for Water Purification. J. Compos. Sci. 2020, 4, 135. [Google Scholar] [CrossRef]
- Li, D.; Tong, L. Direct Growth of Carbon Nanotubes on Aluminum Foil by Atmospheric Pressure Microwave Plasma Chemical Vapor Deposition. Processes 2020, 9, 36. [Google Scholar] [CrossRef]
- Szymanski, L.; Kolacinski, Z.; Wiak, S.; Raniszewski, G.; Pietrzak, L. Synthesis of Carbon Nanotubes in Thermal Plasma Reactor at Atmospheric Pressure. Nanomaterials 2017, 7, 45. [Google Scholar] [CrossRef]
| Chemisorption | Physisorption |
|---|---|
| Ideal for single-layer adsorption | Suitable for multilayer adsorption |
| Irreversible sorption | Reversible sorption |
| Best fit for monolayer (homogeneous system) | Suitable for non-ideal (heterogeneous system) |
| Highly specific bonding | Non-specific bonding |
| Strong process | Weak process |
| Effective for chemical bonding | Effective for physical bonding |
| Slow adsorption at low temperature | Fast adsorption at low temperature |
| Not suitable for multilayer adsorption | Suitable for multilayer adsorption |
| Costly due to the irreversibility | Inexpensive due to desorption |
| Large adsorption enthalpy | Low activation energy |
| Not reusable | Reusable |
| Adsorption Isotherm Model | Assumption | Linear Expression | Parameter |
|---|---|---|---|
| Langmuir Model | All the active adsorptive sites possess equivalent binding energy and a single adsorbate can only bind with one active site at a time. Suitable for monolayer adsorbent | qe is the adsorption capacity (mg/g) at equilibrium; Ce is the adsorbate’s equilibrium concentration (mg/L); qm is the monolayer adsorption capacity (mg/g), KL is the adsorption equilibrium constant (L/mg). | |
| Freundlich Model | This model better describes the non-ideal adsorption systems and provides insights on the exponential distribution on active surface sites. Suitable for multilayer adsorption | ) | qe is the adsorption equilibrium capacity of the adsorbent (mg/g); n is the Freundlich constant; Kf is the Freundlich constant and Ce is the adsorbate equilibrium concentration (mg/L |
| Sips Model | An hybrid of the Freundlich and the Langmuir isotherms. It adequately reduces to the Freundlich adsorption behaviour and effectively predicts monolayer adsorption system by Langmuir mode at high concentrations of the adsorbate | qe is the adsorption equilibrium capacity (mg/g); Ce is the adsorption equilibrium concentration (mg/L); qm is the Sips adsorption capacity (mg/g); KS is the Langmuir adsorption equilibrium constant (L/mg); nS is related to Freundlich heterogeneity factor (nF) [nS = 1nF]. | |
| Dubinin-Radushkevich Model | The model account for the porous structure effects of theadsorbents. It is grounded on the adsorption potential theory and supposes that the adsorption was achieved through micropore space-filling, instead of layer-by-layer adsorption on pore surfaces. | qe is the equilibrium adsorption capacity (mg/g); qmax is the maximum adsorption capacity, β is the constant related to the adsorption energy (mol2/kJ-2), ε—adsorption potential (kJ/mol) | |
| Temkin Model | The adsorption is non-uniform and active sites possess non-uniform adsorptive energies. The adsorption heat depreciates with the coverage due to adsorbate/adsorbent interaction. | KT is the Temkin isotherm constant (L/g); R is the ideal gas constant (8.314 J/mol K), T is the temperature (K), 𝑏T is a constant related to the adsorption heat (J/mol), Ce is the equilibrium concentration of adsorbate in solution (mg/L) | |
| Redlich-Peterson Model | An hybrid of Langmuir and Freundlich isotherm model | (L/mg) are R-P isotherm constants; g is the exponent lying between 0 and 1. The limiting cases are Henry’s law for g = 0 and Langmuir’s form for g = 1. |
| Material | Property | Magnitude | Material Comparison |
|---|---|---|---|
| Graphene | Thermal conductivity | 5 × 103 W/Mk | 10 × greater than Cu |
| Young’s modulus | 1.1 TPa | - | |
| Electron mobility | 2 × 105 cm2/Vs | 140 × greater than Si | |
| Radiation transmittance | 97.7% | Alternative to fluorine-doped tin oxide (FTO) and indium-tin oxide (ITO) | |
| Tensile strength | 125 GPa | Specific strength 100 × higher than steel | |
| Permeability | Impermeable towards gas/liquids; permeable to protons | Pore size is smaller than H2, and He diameter | |
| Surface area | 2630 m2/g | 2 × larger than carbon nanotubes (CNTs) |
| Method | Highlight | Influencing Factor | Merit | Demerit |
|---|---|---|---|---|
| Hydrothermal | The reaction is performed in an autoclave or reactor in an aqueous media at a pressure beyond the solvent boiling point and high temperature | Incubation time, temperature, precursor concentration, reactants ratio | Structural shape and size are tunable. Capable of producing impurity-free nanomaterial since it is a closed system reaction | High energy consumption. High-pressure use |
| Co-precipitation | The reaction of precursor salt in an aqueous solution to a base with a mild oxidant. | Highly dependent on reagent ratio use, stirring rate, pH, and temperature | Highly scalable. Relatively narrow size distribution. Less reaction period. Simplicity and can be performed under ambient conditions | Possible agglomeration. May result in impurity formation. High pH use during the synthesis and purification. Not suitable for precise stoichiometric phase fabrication. |
| Micro-emulsion | Water immersed in an oil medium, then stabilized molecular surface medium followed by elimination of surfactant and particle purification | Salt type, pH, type of surfactant consumed | Fairly low-temperature use (20–80 °C). Time-consuming method (roughly to hours). Better shape control. | Processability may be complex—low yield of colloids. |
| Biosynthesis | Biomaterials are used as reducing agents to yield particles from the appropriate precursors | Precursor-biomaterial mole ratio, temperature, incubation time, pH, agitation rate | Achievable under room temperature. Reduced toxic chemical use. | May be complex to support reproducibility. Low production yield. Time-consuming. Inadequate nanoparticle shape control. |
| Sonochemical | Applying high-power ultrasound radiation initiates a chemical reaction by generating acoustic cavitation. | Temperature, pressure, heating and cooling rates, frequency | Low-temperature use (18–50 °C). Simple and can be performed under ambient conditions. Less time consumption | Bad shape tuning. Non-trivial for large-scale nanoparticle production. |
| Vapor methods | The vapor composition is made unstable relative to the generation of resultant particles | Temperature, atmosphere, precursor, synthesis time, evaporation-condensation rate | Reasonable synthesis time (minutes–hours). Highly scalable. Fair shape control. Good material purity | Not simple to perform. High-temperature consumption. |
| Electrochemical deposition | The occurrence of deposition is at the electrolyte interface housing the metal to be deposited along with the conductive metal substrate | Applied potential, electrode nature, residence electrolyte chemical nature, metal substrate | Eco-friendly (less chemical consumption). Readily performed under room temperature. Fair shape control. | Expensive instrumentation. May suffer yield control—limited electroactive electrode use. |
| Thermal decomposition | The disintegration of bulk material into nano/finer particles via the high-temperature application | The atmosphere used, temperature, heating rate | Highly scalable. Good shape tuning. The capability of producing contaminant-free particles. | May be complex to use. High-temperature use. May induce structural disintegration |
| Sol-gel | It comprised of hydroxylation and precursor condensation followed by solvent removal or gelling by reaction | Precursor concentration, temperature, gel nature, pH, stirring rate, reaction kinetics | Wide reaction temperature use including room temperature (25–200 °C). | Medium yield. Better shape tuning. The reaction may span hours for quality colloid production |
| Adsorbing Material | Preparation Method | Surface Area (m2/g) | Size (nm) | Adsorbed Metal | Conditions | Kinetic Model | Isotherm Model | Removal Capacity (mg/g) Or Efficiency (%) |
|---|---|---|---|---|---|---|---|---|
| α-Fe2O3 | Co-precipitation | 24.82 | 75 | Cu | 25 °C, pH = 5.2, 225 min | pseudo-second-order model | Langmuir | 84.46 mg/g |
| γ-Fe2O3 | Sol-gel | 198 | 10 | Cr, Cu | 25 °C, pH = 2.5 (Cr), 6.5 (Cu), 10 min | pseudo-second-order model | Langmuir isotherm | 17.0 mg/g (Cr), 26.8 mg/g (Cu) |
| Co-precipitation | NA | 14 | Cr, Cu, Cd | 70 °C, pH = 10 (Cd), 6.5 (Cu), 2.6(Cr), 10 min | NA | Langmuir model | 8.4% (Cd), 84.4% (Cr), 88.2% (Cu) | |
| α-FeOOH | Co-precipitation | 71.49 | 15 | Cu | 25 °C, pH = 5.2, 225 min. | pseudo-second-order | Langmuir model | 149.25 mg/g |
| Fe3O4 | Co-precipitation | - | 8 | Pb, Cu, Zn | 25 °C, pH = 5.5, 24 hr. | pseudo-second-order model | Langmuir model | 41.76 mg/g (Zn), 43.21 mg/g (Cu), 149.18 mg/g (Pb) |
| Fe3O4 | Co-precipitation | - | <10 | Pb, Cu, Zn | 25 °C, pH = 5.5, 6 & 6.5, 120 min | pseudo-second-order, Elovich model | Langmuir model | Pb—(90%), Cu—(40%), Zn—(30%) |
| Fe3O4 | Solvothermal | 11.3 | 45 | Pb, Cr | 25 °C, pH = 5 & 6, 48 h | pseudo-second-order | Langmuir | 19 mg/g (Pb), 9 mg/g (Cr) |
| TiO2 | Degassing and thermal treatment | 185.5 | 8.3 | Pb, Cd | 25 °C, pH = 8, 120 min | - | Langmuir | 401.14 mg/g (Pb), 135.14 mg/g (Cd) |
| TiO2 | Co-precipitation | 208 | 50 | Zn, Cd | 25 °C, pH = 9, 30 min. | - | - | 15.3 mg/g-(Zn), 7.9 mg/g-(Cd) |
| TiO2 | Co-precipitation | - | 15 | Cu | 25 °C, pH = 9, 30 min. | - | Langmuir | Cu—(97.72%) |
| ZnO | Green synthesis | - | 10 ± 2.6 | Pb | 70 °C, pH = 5, 60 min. | pseudo-second-order model | Langmuir model | 19.65 mg/g, 93% (Pb) |
| ZnO | Co-precipitation | 15.75 | 25 | Cr | 50 °C, pH = 2, 120 min | pseudo-second-order | Freundlich | 95% |
| ZnO | Green synthesis | 701.88 | 10 | Cd, Pb | 30 °C, pH = 7, 30 min | pseudo-second-order | Langmuir | Cd—(156.74), Pb—(194.93), ~ 90% |
| ZnO | Sol-gel | 8.25 | 46.5 | Cd | 25 °C, pH = 7, 12 hr. | pseudo-second-order | Langmuir | 214.4 mg/g |
| ZnO | Co-precipitation | - | 24.7 | Cu | 69.85 °C, pH = 4, 120 min. | pseudo-first-order | Freundlich | 226 mg/g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motitswe, M.G.; Badmus, K.O.; Khotseng, L. Development of Adsorptive Materials for Selective Removal of Toxic Metals in Wastewater: A Review. Catalysts 2022, 12, 1057. https://doi.org/10.3390/catal12091057
Motitswe MG, Badmus KO, Khotseng L. Development of Adsorptive Materials for Selective Removal of Toxic Metals in Wastewater: A Review. Catalysts. 2022; 12(9):1057. https://doi.org/10.3390/catal12091057
Chicago/Turabian StyleMotitswe, Moeng Geluk, Kassim Olasunkanmi Badmus, and Lindiwe Khotseng. 2022. "Development of Adsorptive Materials for Selective Removal of Toxic Metals in Wastewater: A Review" Catalysts 12, no. 9: 1057. https://doi.org/10.3390/catal12091057
APA StyleMotitswe, M. G., Badmus, K. O., & Khotseng, L. (2022). Development of Adsorptive Materials for Selective Removal of Toxic Metals in Wastewater: A Review. Catalysts, 12(9), 1057. https://doi.org/10.3390/catal12091057








