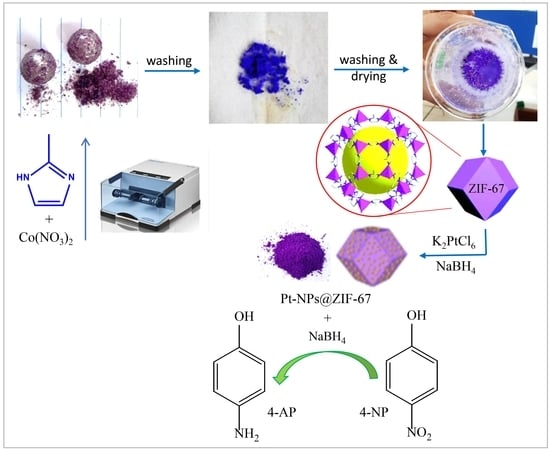
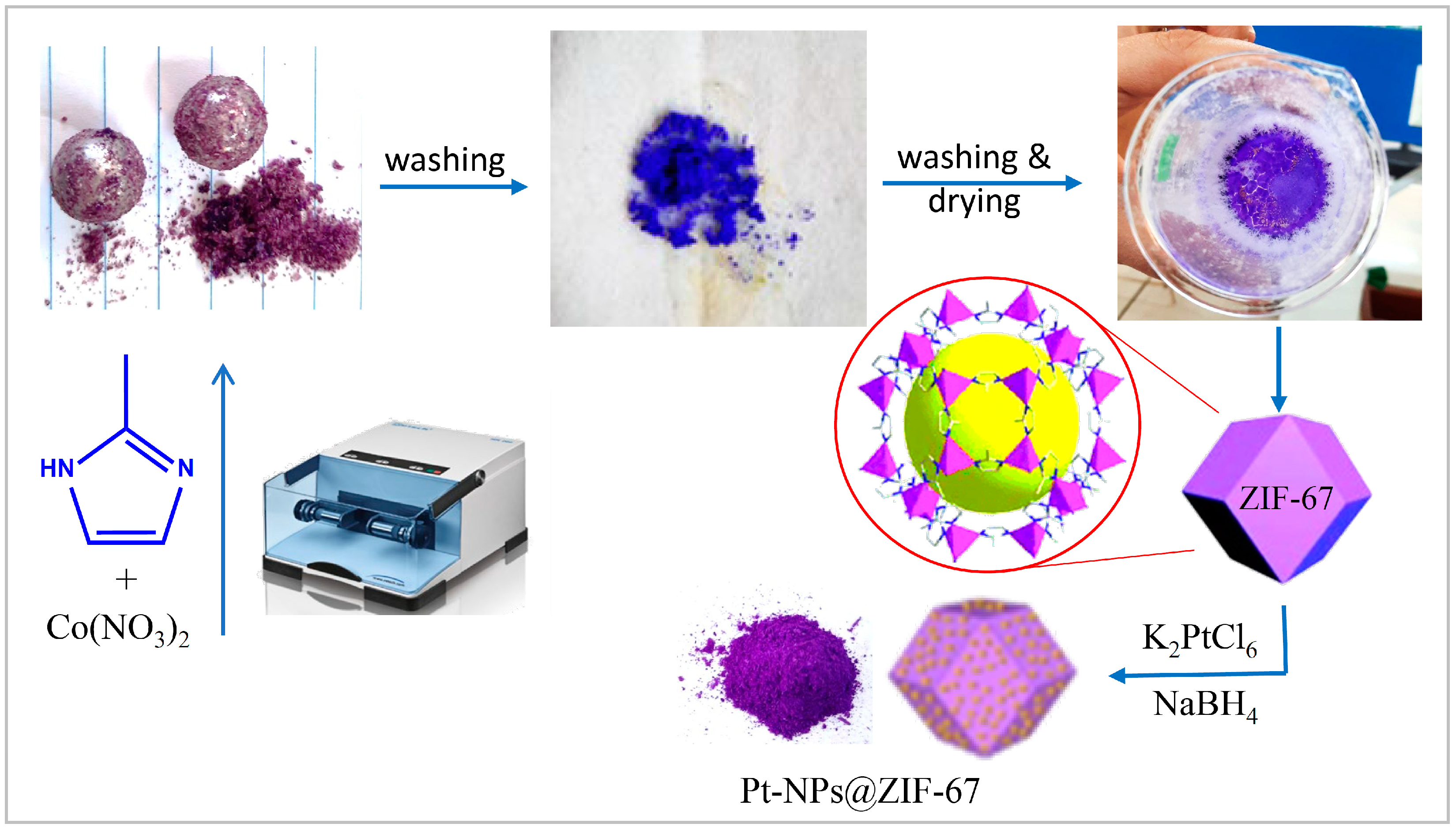
Solvent-Free Mechanochemical Preparation of Metal-Organic Framework ZIF-67 Impregnated by Pt Nanoparticles for Water Purification
Abstract
1. Introduction
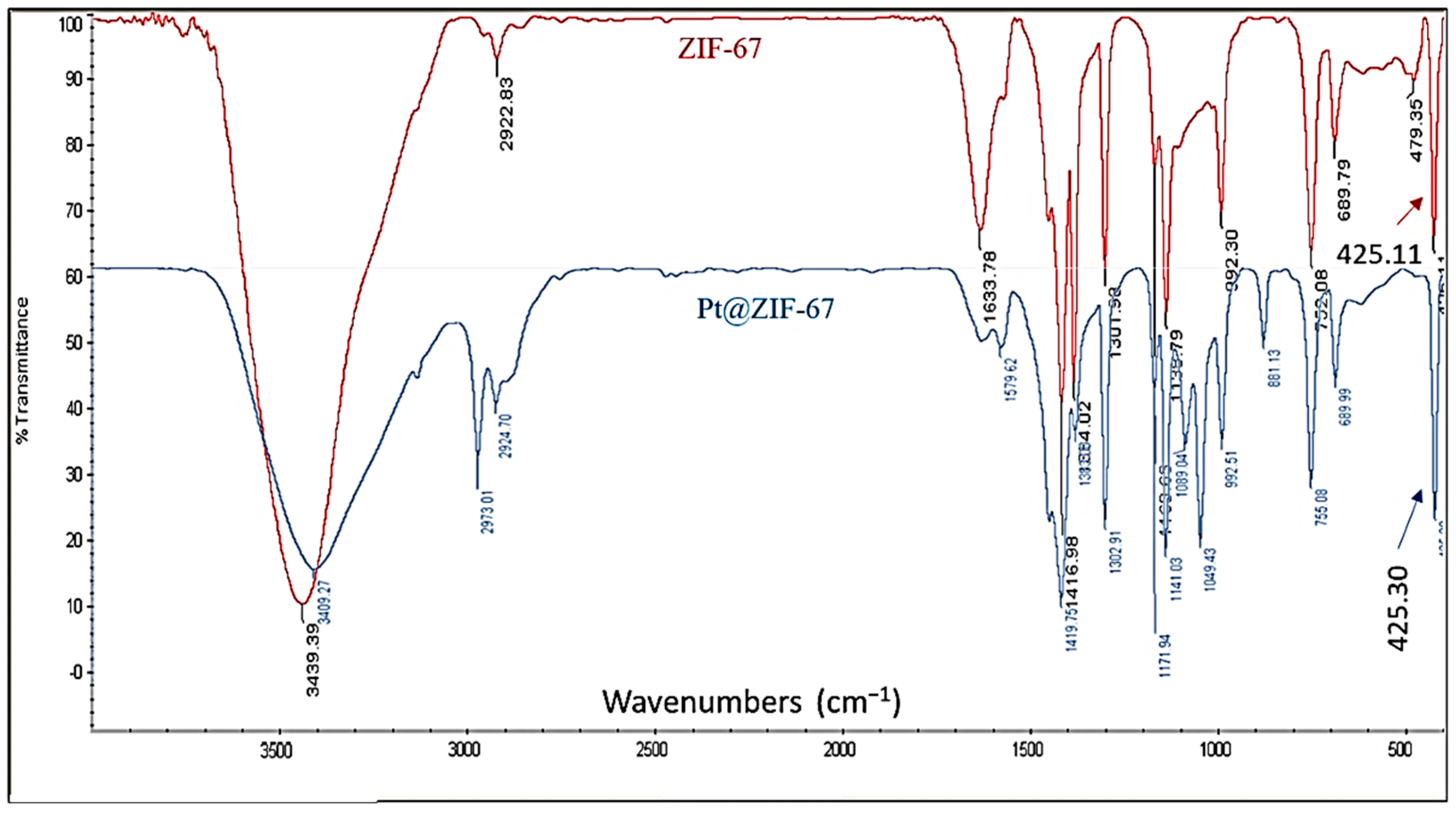
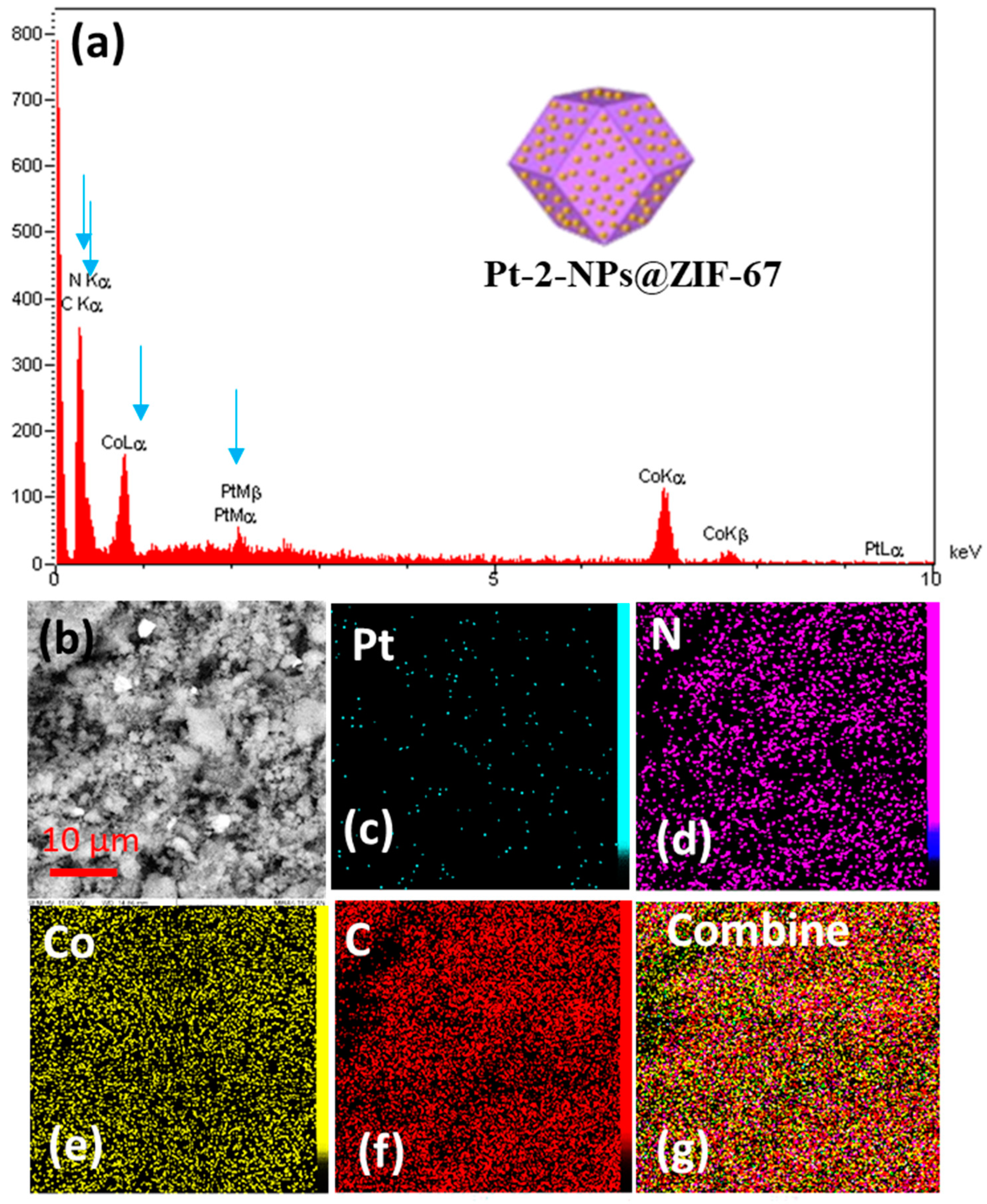
2. Results and Discussion
3. Materials and Methods
3.1. Ball Mill Mediated Preparation of ZIF-67
3.2. Preparation of Pt@ZIF-67
3.3. Catalytic Activity of NaBH4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tao, C.-A.; Wang, J.-F. Synthesis of metal organic frameworks by ball-milling. Crystals 2020, 11, 15. [Google Scholar] [CrossRef]
- Stolle, A.; Szuppa, T.; Leonhardt, S.E.; Ondruschka, B. Ball milling in organic synthesis: Solutions and challenges. Chem. Soc. Rev. 2011, 40, 2317–2329. [Google Scholar] [CrossRef] [PubMed]
- Szczęśniak, B.; Borysiuk, S.; Choma, J.; Jaroniec, M. Mechanochemical synthesis of highly porous materials. Mater. Horiz. 2020, 7, 1457–1473. [Google Scholar] [CrossRef]
- Serrà, A.; Artal, R.; Pozo, M.; Garcia-Amorós, J.; Gómez, E. Simple environmentally-friendly reduction of 4-nitrophenol. Catalysts 2020, 10, 458. [Google Scholar] [CrossRef]
- Ji, W.; Xu, Z.; Liu, P.; Zhang, S.; Zhou, W.; Li, H.; Zhang, T.; Li, L.; Lu, X.; Wu, J.; et al. Metal–organic framework derivatives for improving the catalytic activity of the co oxidation reaction. ACS Appl. Mater. Interfaces 2017, 9, 15394–15398. [Google Scholar] [CrossRef]
- Amirnejad, M.; Naimi-Jamal, M.R.; Tourani, H.; Ghafuri, H. A facile solvent-free one-pot three-component method for the synthesis of 2-amino-4H-pyrans and tetrahydro-4H-chromenes at ambient temperature. Mon. Chem. Chem. Mon. 2013, 144, 1219–1225. [Google Scholar] [CrossRef]
- Chen, D.-D.; Yi, X.-H.; Wang, C.-C. Preparation of metal-organic frameworks and their composites using mechanochemical methods. Chin. J. Inorg. Chem. 2020, 36, 1805–1821. [Google Scholar]
- Taghavi, R.; Rostamnia, S. Four-Component Synthesis of Polyhydroquinolines via Unsymmetrical Hantzsch Reaction Employing Cu-IRMOF-3 as a Robust Heterogeneous Catalyst. Chem. Methodol. 2022, 6, 639–648. [Google Scholar]
- Hoseini Rad, M.; Ghasemzadeh, M.A.; Sharif, M.S. Multi-component synthesis of spiro [indoline-3, 4′-pyrrolo [3, 4-c] pyrazoles] using Zn (BDC) metal-organic frameworks as a green and efficient catalyst. Eurasian Chem. Commun. 2019, 1, 344–351. [Google Scholar]
- Khosravi, A.; Mokhtari, J.; Naimi-Jamal, M.R.; Tahmasebi, S.; Panahi, L. Cu2(BDC)2(BPY)–MOF: An efficient and reusable heterogeneous catalyst for the aerobic Chan–Lam coupling prepared via ball-milling strategy. RSC Adv. 2017, 7, 46022–46027. [Google Scholar] [CrossRef]
- Hu, Z.; Tao, C.-A.; Wang, F.; Zou, X.; Wang, J. Flexible metal–organic framework-based one-dimensional photonic crystals. J. Mater. Chem. C 2015, 3, 211–216. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, P.-F.; Yang, G.-P.; Hou, L.; Zhang, W.-Y.; Han, Y.-F.; Liu, P.; Wang, Y.-Y. Supramolecular control of MOF pore properties for the tailored guest adsorption/separation applications. Coord. Chem. Rev. 2021, 434, 213709. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Liu, P.; Yang, Z.; Feng, X.; Huo, F.; Lu, X. A template-free method for stable CuO hollow microspheres fabricated from a metal organic framework (HKUST-1). Nanoscale 2015, 7, 9411–9415. [Google Scholar] [CrossRef] [PubMed]
- Panahi, L.; Naimi-Jamal, M.R.; Mokhtari, J.; Morsali, A. Mechanochemically synthesized nanoporous metal-organic framework Cu2 (BDC) 2 (DABCO): An efficient heterogeneous catalyst for preparation of carbamates. Microporous Mesoporous Mater. 2017, 244, 208–217. [Google Scholar] [CrossRef]
- Zhao, M.; Deng, K.; He, L.; Liu, Y.; Li, G.; Zhao, H.; Tang, Z. Core–shell palladium nanoparticle@ metal–organic frameworks as multifunctional catalysts for cascade reactions. J. Am. Chem. Soc. 2014, 136, 1738–1741. [Google Scholar] [CrossRef]
- Hu, Z.; Tao, C.-A.; Liu, H.; Zou, X.; Zhu, H.; Wang, J. Fabrication of an NH2-MIL-88B photonic film for naked-eye sensing of organic vapors. J. Mater. Chem. A 2014, 2, 14222–14227. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Liu, J.; Xiong, Y.; Zheng, J.; Liu, Y.; Tang, Z. Core–shell noble-metal@ metal-organic-framework nanoparticles with highly selective sensing property. Angew. Chem. 2013, 125, 3829–3833. [Google Scholar] [CrossRef]
- Shu, J.C.; Cao, W.Q.; Cao, M.S. Diverse metal–organic framework architectures for electromagnetic absorbers and shielding. Adv. Funct. Mater. 2021, 31, 2100470. [Google Scholar] [CrossRef]
- Nadizadeh, Z.; Naimi-Jamal, M.R.; Panahi, L. Mechanochemical solvent-free in situ synthesis of drug-loaded {Cu2(1,4-bdc)2(dabco)} n MOFs for controlled drug delivery. J. Solid State Chem. 2018, 259, 35–42. [Google Scholar] [CrossRef]
- Zhang, Z.; Tao, C.-A.; Zhao, J.; Wang, F.; Huang, J.; Wang, J. Microwave-assisted solvothermal synthesis of UiO-66-NH2 and its catalytic performance toward the hydrolysis of a nerve agent simulant. Catalysts 2020, 10, 1086. [Google Scholar] [CrossRef]
- Nazir, M.A.; Najam, T.; Shahzad, K.; Wattoo, M.A.; Hussain, T.; Tufail, M.K.; Shah, S.S.A.; Rehman, A.U. Heterointerface engineering of water stable ZIF-8@ ZIF-67: Adsorption of rhodamine B from water. Surf. Interfaces 2022, 34, 102324. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Lu, G.; Wang, Y.; Li, S.; Cui, C.; Wu, J.; Xu, Z.; Tian, D.; Huang, W.; et al. Mesoporous metal–organic frameworks with size-, shape-, and space-distribution-controlled pore structure. Adv. Mater. 2015, 27, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lu, G.; Cui, C.; Liu, Y.; Li, S.; Yan, W.; Xing, C.; Chi, Y.R.; Yang, Y.; Huo, F. A family of metal-organic frameworks exhibiting size-selective catalysis with encapsulated noble-metal nanoparticles. Adv. Mater. 2014, 26, 4056–4060. [Google Scholar] [CrossRef]
- Zhao, S.; Yin, H.; Du, L.; He, L.; Zhao, K.; Chang, L.; Yin, G.; Zhao, H.; Liu, S.; Tang, Z. Carbonized nanoscale metal–organic frameworks as high performance electrocatalyst for oxygen reduction reaction. ACS Nano 2014, 8, 12660–12668. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Wang, C.; Wu, Y.-Z.; Xiong, Y.; Xu, Q.; Yu, S.-H.; Jiang, H.-Z. From bimetallic metal-organic framework to porous carbon: High surface area and multicomponent active dopants for excellent electrocatalysis. Adv. Mater. 2015, 27, 5010–5016. [Google Scholar] [CrossRef]
- Luo, Y.; Calvillo, L.; Daiguebonne, C.; Daletou, M.K.; Granozzi, G.; Alonso-Vante, N. A highly efficient and stable oxygen reduction reaction on Pt/CeOx/C electrocatalyst obtained via a sacrificial precursor based on a metal-organic framework. Appl. Catal. B Environ. 2016, 189, 39–50. [Google Scholar] [CrossRef]
- Koo, W.-T.; Choi, S.-J.; Kim, S.-J.; Jang, J.-S.; Tuller, H.L.; Kim, I.-D. Heterogeneous sensitization of metal–organic framework driven metal@ metal oxide complex catalysts on an oxide nanofiber scaffold toward superior gas sensors. J. Am. Chem. Soc. 2016, 138, 13431–13437. [Google Scholar] [CrossRef]
- Lu, G.; Li, S.; Guo, Z.; Farha, O.K.; Hauser, B.G.; Qi, X.; Wang, Y.; Wang, X.; Han, S.; Liu, X.; et al. Imparting functionality to a metal–organic framework material by controlled nanoparticle encapsulation. Nat. Chem. 2012, 4, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, R.; Rostamnia, S. Schiff-base Post-Synthetic Modification of IRMOF-3 to Encapsulate Pd Nanoparticles: It’s Application in C-C Bond Formation Cross-Coupling Suzuki Reaction. Chem. Methodol. 2022, 6, 629–638. [Google Scholar] [CrossRef]
- Li, Z.; Yang, L.; Cao, H.; Chang, Y.; Tang, K.; Cao, Z.; Chang, J.; Cao, Y.; Wang, W.; Gao, M.; et al. Carbon materials derived from chitosan/cellulose cryogel-supported zeolite imidazole frameworks for potential supercapacitor application. Carbohydr. Polym. 2017, 175, 223–230. [Google Scholar] [CrossRef]
- Nazir, M.A.; Khan, N.A.; Cheng, C.; Shah, S.S.A.; Najam, T.; Arshad, M.; Sharif, A.; Akhtar, S.; Rehman, A.U. Surface induced growth of ZIF-67 at Co-layered double hydroxide: Removal of methylene blue and methyl orange from water. Appl. Clay Sci. 2020, 190, 105564. [Google Scholar] [CrossRef]
- Chu, C.; Rao, S.; Ma, Z.; Han, H. Copper and cobalt nanoparticles doped nitrogen-containing carbon frameworks derived from CuO-encapsulated ZIF-67 as high-efficiency catalyst for hydrogenation of 4-nitrophenol. Appl. Catal. B Environ. 2019, 256, 117792. [Google Scholar] [CrossRef]
- Dhandole, L.K.; Kim, S.-G.; Seo, Y.-S.; Mahadik, M.A.; Chung, H.S.; Lee, S.Y.; Choi, S.H.; Cho, M.; Ryu, J.; Jang, J.S. Enhanced photocatalytic degradation of organic pollutants and inactivation of Listeria monocytogenes by visible light active Rh–Sb codoped TiO2 nanorods. ACS Sustain. Chem. Eng. 2018, 6, 4302–4315. [Google Scholar] [CrossRef]
- Wauchope, R.D. The pesticide content of surface water draining from agricultural fields—A review. J. Environ. Qual. 1978, 7, 459–472. [Google Scholar] [CrossRef]
- Badmus, K.O.; Tijani, J.O.; Massima, E.; Petrik, L. Treatment of persistent organic pollutants in wastewater using hydrodynamic cavitation in synergy with advanced oxidation process. Environ. Sci. Pollut. Res. 2018, 25, 7299–7314. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, X.; Huang, J.; Wang, G.; Zhao, Y.; Chen, F.; Wei, J.; Song, Z.; Zhao, Y. Polydopamine@ gold nanowaxberry enabling improved SERS sensing of pesticides, pollutants, and explosives in complex samples. Anal. Chem. 2018, 90, 9048–9054. [Google Scholar] [CrossRef]
- Hu, L.; Peng, F.; Xia, D.; He, H.; He, C.; Fang, Z.; Yang, J.; Tian, S.; Sharma, V.K.; Shu, D. Carbohydrates-derived nitrogen-doped hierarchical porous carbon for ultrasensitive detection of 4-nitrophenol. ACS Sustain. Chem. Eng. 2018, 6, 17391–17401. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, X.; An, T.; Song, Z.; Fu, J.; Sheng, G.; Cui, M. Kinetics, degradation pathway and reaction mechanism of advanced oxidation of 4-nitrophenol in water by a UV/H2O2 process. J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. Clean Technol. 2003, 78, 788–794. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Huang, W.; Tan, X.; Dong, H.; Goodman, B.A.; Du, H.; Lei, F.; Diao, K. Adsorption of phenolic compounds from water by a novel ethylenediamine rosin-based resin: Interaction models and adsorption mechanisms. Chemosphere 2019, 214, 821–829. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Zheng, T.; Wang, P.; Jiang, J.-P.; Li, N. Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem. Eng. J. 2010, 157, 348–356. [Google Scholar] [CrossRef]
- Ahn, W.-Y.; Sheeley, S.A.; Rajh, T.; Cropek, D.M. Photocatalytic reduction of 4-nitrophenol with arginine-modified titanium dioxide nanoparticles. Appl. Catal. B Environ. 2007, 74, 103–110. [Google Scholar] [CrossRef]
- Gazi, S.; Ananthakrishnan, R. Metal-free-photocatalytic reduction of 4-nitrophenol by resin-supported dye under the visible irradiation. Appl. Catal. B Environ. 2011, 105, 317–325. [Google Scholar] [CrossRef]
- Wang, C.; Ciganda, R.; Salmon, L.; Gregurec, D.; Irigoyen, J.; Moya, S.; Ruiz, J.; Astruc, D. Highly efficient transition metal nanoparticle catalysts in aqueous solutions. Angew. Chem. 2016, 128, 3143–3147. [Google Scholar] [CrossRef]
- Liu, R.; Mahurin, S.M.; Li, C.; Unocic, R.R.; Idrobo, J.C.; Gao, H.; Pennycook, S.J.; Dai, S. Dopamine as a carbon source: The controlled synthesis of hollow carbon spheres and yolk-structured carbon nanocomposites. Angew. Chem. Int. Ed. 2011, 50, 6799–6802. [Google Scholar] [CrossRef]
- Martin-Martinez, M.; Barreiro, M.F.F.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Lignin-based activated carbons as metal-free catalysts for the oxidative degradation of 4-nitrophenol in aqueous solution. Appl. Catal. B Environ. 2017, 219, 372–378. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.-Y.; Liu, Y. Au/graphene hydrogel: Synthesis, characterization and its use for catalytic reduction of 4-nitrophenol. J. Mater. Chem. 2012, 22, 8426–8430. [Google Scholar] [CrossRef]
- Kubendhiran, S.; Sakthivel, R.; Chen, S.-M.; Mutharani, B.; Chen, T.-W. Innovative strategy based on a novel carbon-black− β-cyclodextrin nanocomposite for the simultaneous determination of the anticancer drug flutamide and the environmental pollutant 4-nitrophenol. Anal. Chem. 2018, 90, 6283–6291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, M.; Tang, K.; Verpoort, F.; Sun, T. Polymer-based stimuli-responsive recyclable catalytic systems for organic synthesis. Small 2014, 10, 32–46. [Google Scholar] [CrossRef]
- Tedsree, K.; Li, T.; Jones, S.; Chan, C.W.A.; Yu, K.M.K.; Bagot, P.A.J.; Marquis, E.A.; Smith, G.D.W.; Tsang, S.C.E. Hydrogen production from formic acid decomposition at room temperature using a Ag–Pd core–shell nanocatalyst. Nat. Nanotechnol. 2011, 6, 302–307. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Wang, D.; Turhong, M.; Zhang, M. Recyclable and effective Pd/poly (N-isopropylacrylamide) catalyst for hydrodechlorination of 4-chlorophenol in aqueous solution. Chem. Eng. J. 2015, 280, 158–164. [Google Scholar] [CrossRef]
- Yan, Z.; Fu, L.; Zuo, X.; Yang, H. Green assembly of stable and uniform silver nanoparticles on 2D silica nanosheets for catalytic reduction of 4-nitrophenol. Appl. Catal. B Environ. 2018, 226, 23–30. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, Y.; Huang, J.; Zhu, Y.; Zhu, J.; Yang, X.; Chen, W.; Yao, Y.; Qian, S.; Jiang, H.; et al. In-situ SERS monitoring of reaction catalyzed by multifunctional Fe3O4@ TiO2@ Ag-Au microspheres. Appl. Catal. B Environ. 2017, 205, 11–18. [Google Scholar] [CrossRef]
- Mostafazadeh, N.; Ghoreyshi, A.A.; Pirzadeh, K. Optimization of solvothermally synthesized ZIF-67 metal organic framework and its application for Cr (VI) adsorption from aqueous solution. Iran. J. Chem. Eng. (IJChE) 2018, 15, 27–47. [Google Scholar]
- Roosta, M.; Ghaedi, M.; Daneshfar, A.; Sahraei, R.; Asghari, A. Optimization of the ultrasonic assisted removal of methylene blue by gold nanoparticles loaded on activated carbon using experimental design methodology. Ultrason. Sonochemistry 2014, 21, 242–252. [Google Scholar] [CrossRef]
- Hayati, P.; Mehrabadi, Z.; Karimi, M.; Janczak, J.; Mohammadi, K.; Mahmoudi, G.; Dadi, F.; Fard, M.J.S.; Hasanzadeh, A.; Rostamnia, S. Photocatalytic activity of new nanostructures of an Ag (i) metal–organic framework (Ag-MOF) for the efficient degradation of MCPA and 2, 4-D herbicides under sunlight irradiation. New J. Chem. 2021, 45, 3408–3417. [Google Scholar] [CrossRef]
- Jia, W.-G.; Gao, L.-L.; Wang, Z.-B.; Sun, L.-Y.; Han, Y.-F. Synthesis, characterization, and catalytic activities of palladium complexes with phenylene-bridged bis (thione) ligands. Organometallics 2019, 38, 1946–1954. [Google Scholar] [CrossRef]
- Sravanthi, K.; Ayodhya, D.; Swamy, P.Y. Green synthesis, characterization and catalytic activity of 4-nitrophenol reduction and formation of benzimidazoles using bentonite supported zero valent iron nanoparticles. Mater. Sci. Energy Technol. 2019, 2, 298–307. [Google Scholar] [CrossRef]
- Xie, X.; Shi, J.; Pu, Y.; Wang, Z.; Zhang, L.-L.; Wang, J.-X.; Wang, D. Cellulose derived nitrogen and phosphorus co-doped carbon-based catalysts for catalytic reduction of p-nitrophenol. J. Colloid Interface Sci. 2020, 571, 100–108. [Google Scholar] [CrossRef]


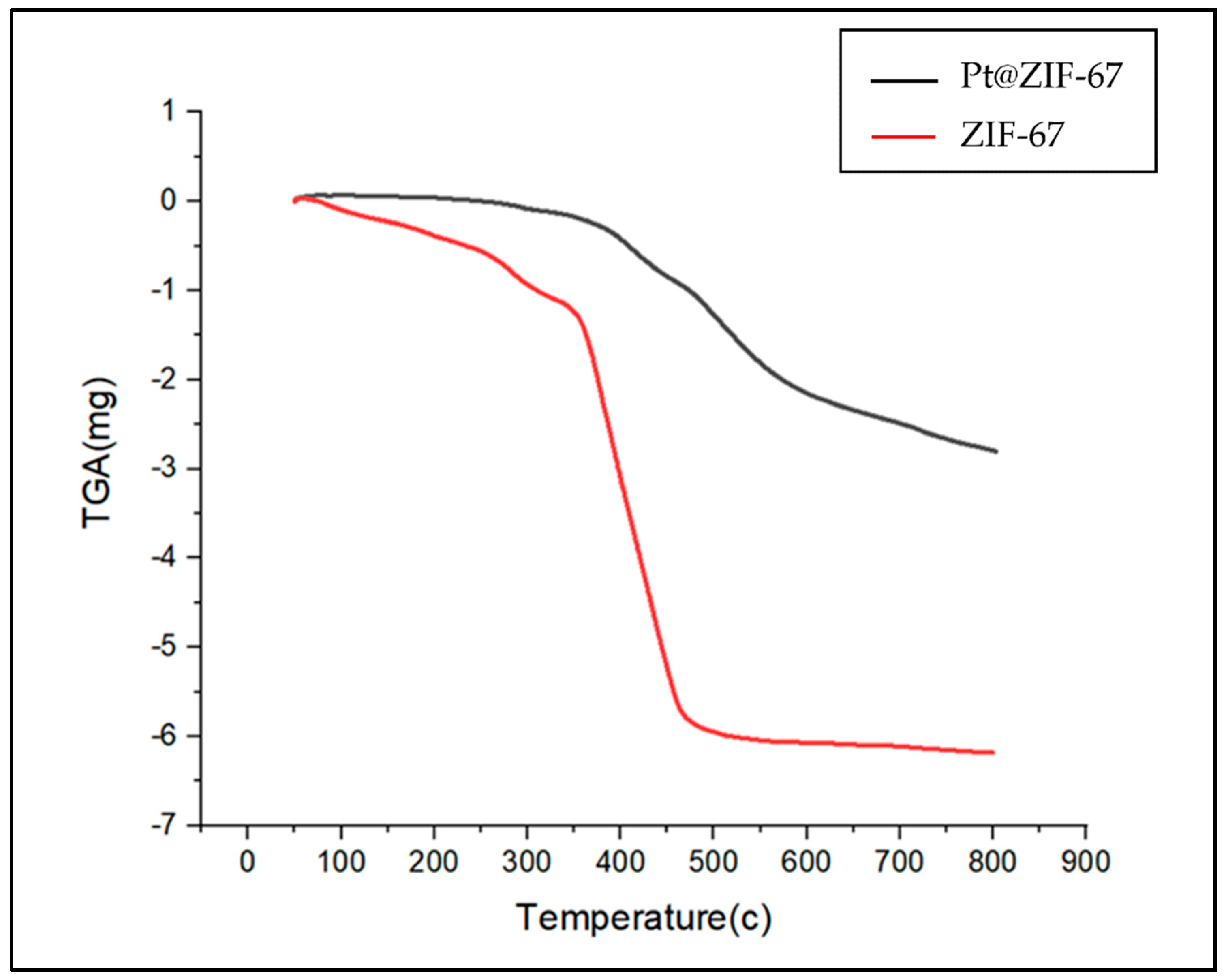
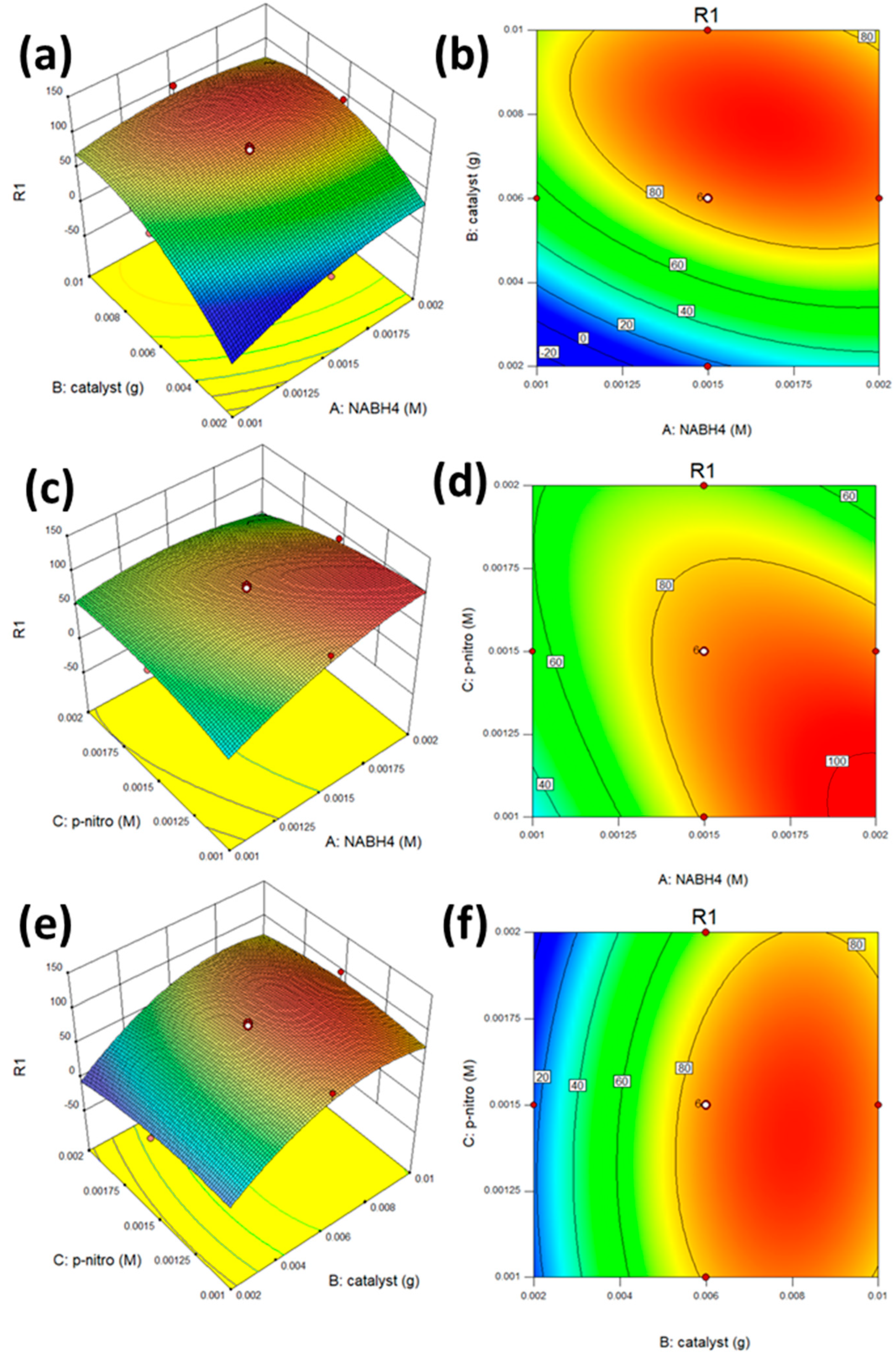
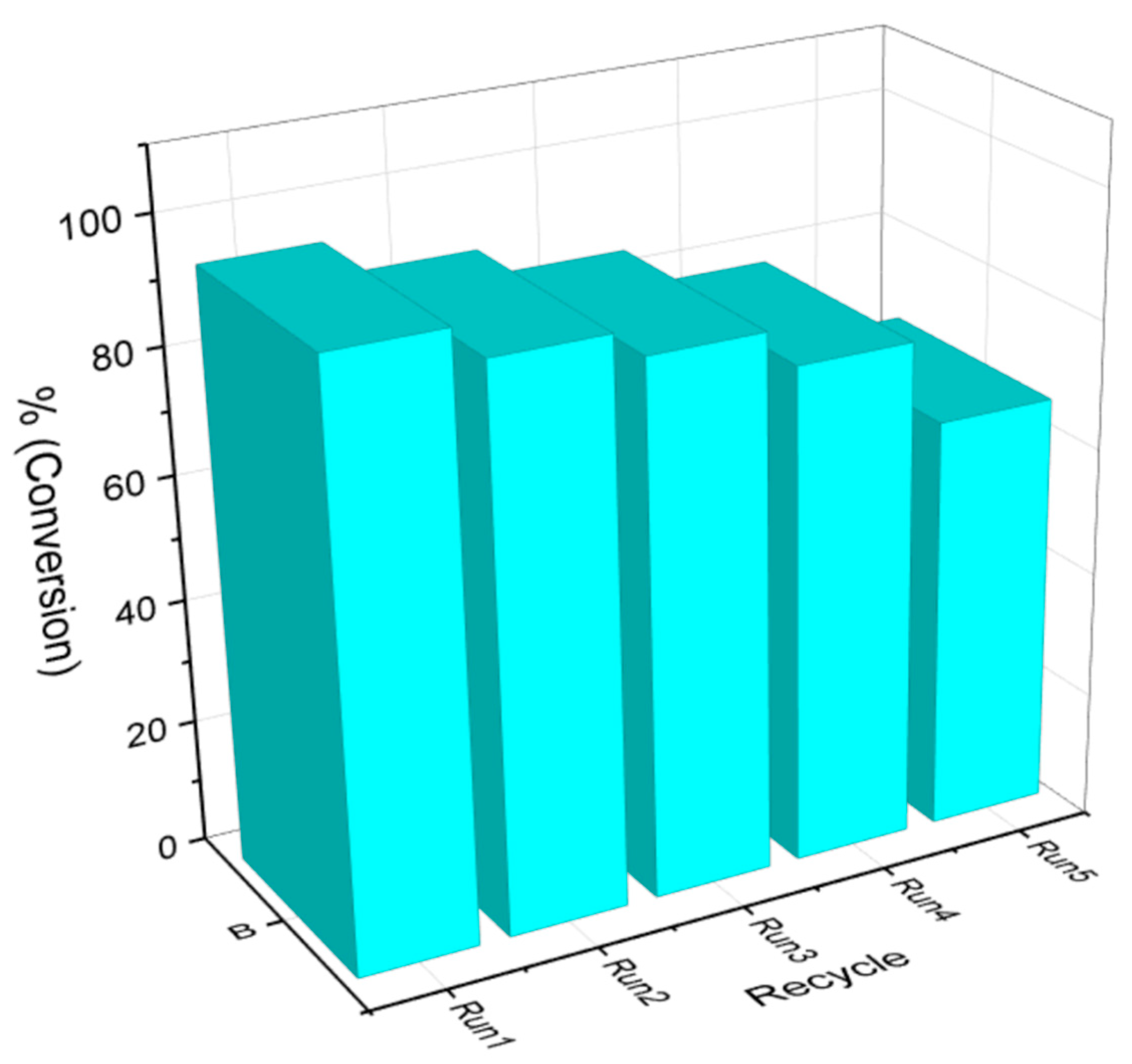
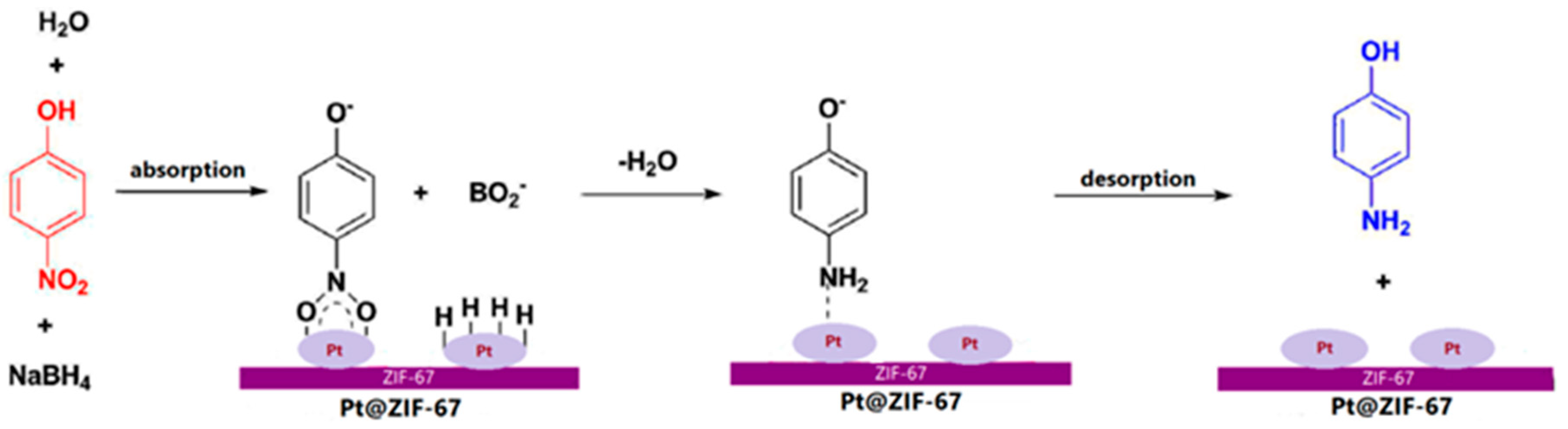
| Sample | BET Surface Area (m2 g−1) | Average Diameter (nm) | Total Pore Volume (cm3 g−1) |
|---|---|---|---|
| ZIF-67 | 1138 | 1.84 | 0.5235 |
| Pt@ZIF-67 | 648.89 | 1.86 | 0.302 |
| Element | Line | Int | Error | K | Kr | W% | A% | ZAF | Ox% | Pk/Bg |
|---|---|---|---|---|---|---|---|---|---|---|
| C | Ka | 345.6 | 187.3370 | 0.4099 | 0.1759 | 38.51 | 51.78 | 0.4569 | 0.00 | 67.97 |
| N | Ka | 91.6 | 187.3370 | 0.1468 | 0.0630 | 36.32 | 41.87 | 0.1735 | 0.00 | 43.69 |
| Cl | Ka | 20.6 | 1.8728 | 0.0063 | 0.0027 | 0.32 | 0.15 | 0.8383 | 0.00 | 2.63 |
| Co | Ka | 313.0 | 0.6091 | 0.3972 | 0.1705 | 21.67 | 5.94 | 0.7868 | 0.00 | 25.37 |
| Pt | La | 2.2 | 0.6091 | 0.0398 | 0.0171 | 3.18 | 0.26 | 0.5364 | 0.00 | 2.30 |
| 1.0000 | 0.4292 | 100.00 | 100.00 | 0.00 |
| Std. | Run | Response (R) | Time (min) |
|---|---|---|---|
| 1 | 9 | 26.48 | 10 |
| 2 | 11 | 69.48 | 8 |
| 3 | 13 | 70.56 | 8 |
| 4 | 2 | 89.78 | 6 |
| 5 | 17 | 39.27 | 7 |
| 6 | 20 | 50.58 | 14 |
| 7 | 10 | 85.56 | 5 |
| 8 | 15 | 79.30 | 7 |
| 9 | 4 | 53.54 | 4 |
| 10 | 18 | 93.07 | 6 |
| 11 | 1 | 8.37 | 10 |
| 12 | 12 | 98.58 | 4 |
| 13 | 6 | 90.80 | 11 |
| 14 | 8 | 62.81 | 17 |
| 15 | 14 | 82.11 | 5 |
| 16 | 16 | 96.92 | 9 |
| 17 | 19 | 79.71 | 6 |
| 18 | 5 | 82.50 | 5 |
| 19 | 7 | 83.97 | 11 |
| 20 | 3 | 92.87 | 6 |
| Number | NaBH4 (M) | Catalyst (g) | 4-NP (M) | R (Accuracy) | Desirability |
|---|---|---|---|---|---|
| 1 | 0.0015 | 0.006 | 0.0015 | 86.44 | 1.000 |
| Catalyst | Reduction Condition | Efficiency (%) | Time (min) | Refs. |
|---|---|---|---|---|
| Pd-PBL | Cat. (1 mol %) 4-NP (10−3 mmol) NaBH4 (10−4 mmol) | 98.3 | 32 | [56] |
| Bentonite clay supported Fe NPs | Cat. (10 mg/L) 4-NP (0.2 mM) NaBH4 (0.2 M) | 96.8 | 20 | [57] |
| NPC | Cat (30 mg) 4-NP (30 mL, 0.015 mmol) NaBH4 (1.5 mmol) | 100 | 20 | [58] |
| Pt@ZIF-67 | Cat (6 mg) 4-NP (1.5 mM) NaBH4 (1.5 mM) | 96.5 | 5 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afkhami-Ardekani, M.; Naimi-Jamal, M.R.; Doaee, S.; Rostamnia, S. Solvent-Free Mechanochemical Preparation of Metal-Organic Framework ZIF-67 Impregnated by Pt Nanoparticles for Water Purification. Catalysts 2023, 13, 9. https://doi.org/10.3390/catal13010009
Afkhami-Ardekani M, Naimi-Jamal MR, Doaee S, Rostamnia S. Solvent-Free Mechanochemical Preparation of Metal-Organic Framework ZIF-67 Impregnated by Pt Nanoparticles for Water Purification. Catalysts. 2023; 13(1):9. https://doi.org/10.3390/catal13010009
Chicago/Turabian StyleAfkhami-Ardekani, Mahya, Mohammad Reza Naimi-Jamal, Samira Doaee, and Sadegh Rostamnia. 2023. "Solvent-Free Mechanochemical Preparation of Metal-Organic Framework ZIF-67 Impregnated by Pt Nanoparticles for Water Purification" Catalysts 13, no. 1: 9. https://doi.org/10.3390/catal13010009
APA StyleAfkhami-Ardekani, M., Naimi-Jamal, M. R., Doaee, S., & Rostamnia, S. (2023). Solvent-Free Mechanochemical Preparation of Metal-Organic Framework ZIF-67 Impregnated by Pt Nanoparticles for Water Purification. Catalysts, 13(1), 9. https://doi.org/10.3390/catal13010009