Ring Opening Polymerization of Lactides and Lactones by Multimetallic Titanium Complexes Derived from the Acids Ph2C(X)CO2H (X = OH, NH2)
Abstract
:1. Introduction
2. Results and Discussion
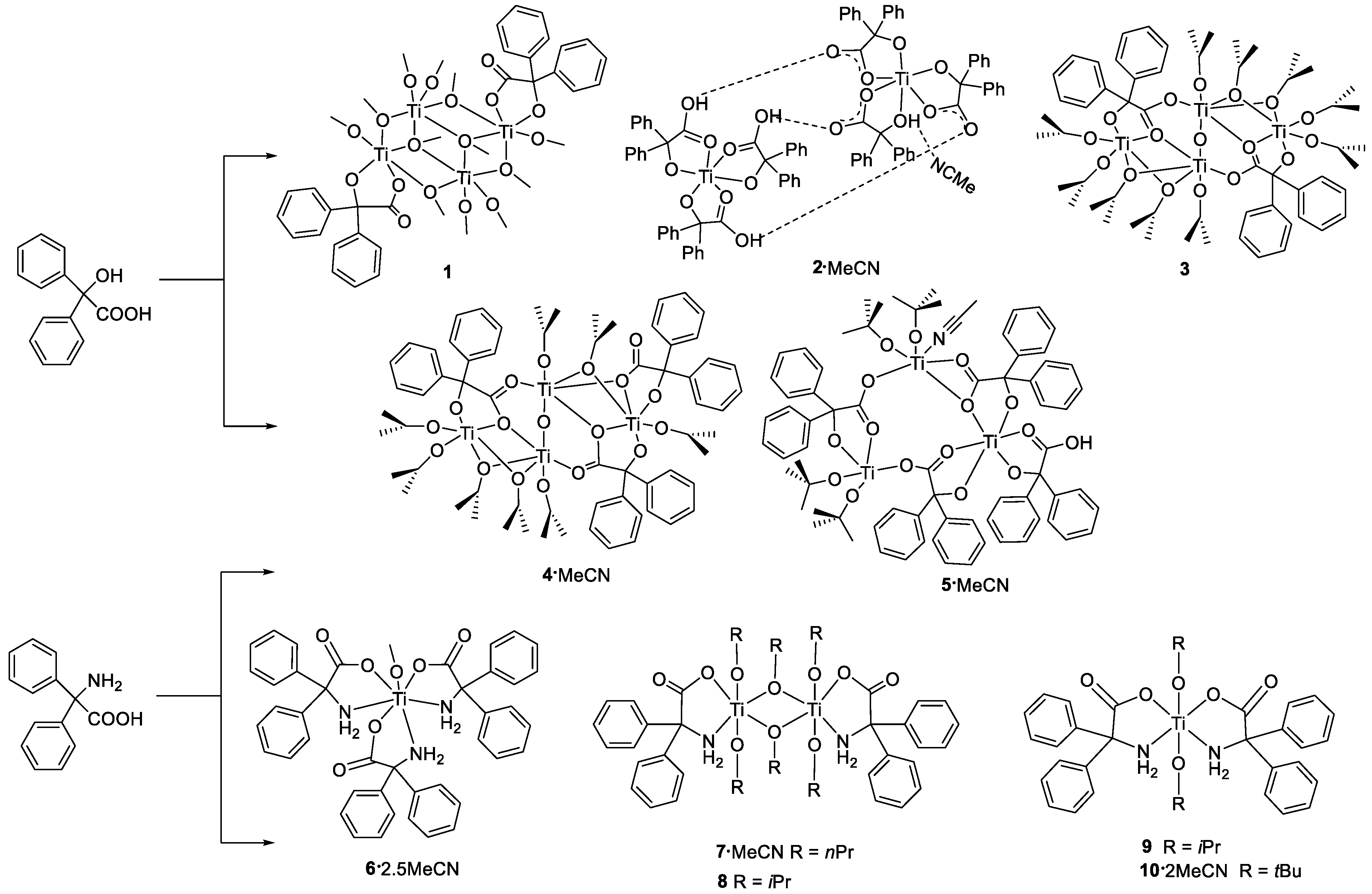
2.1. Synthesis and Characterization of Ti Complexes
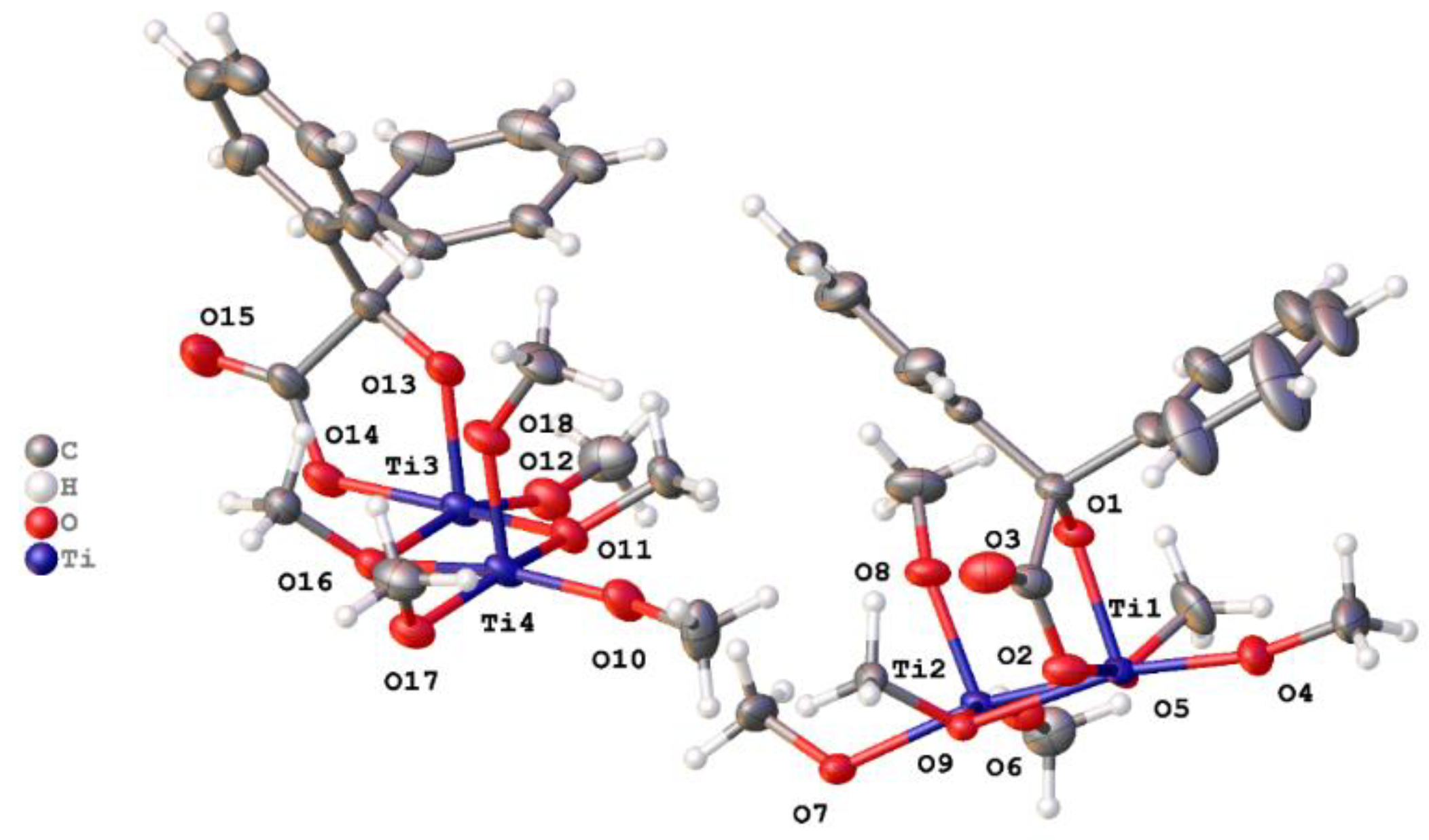
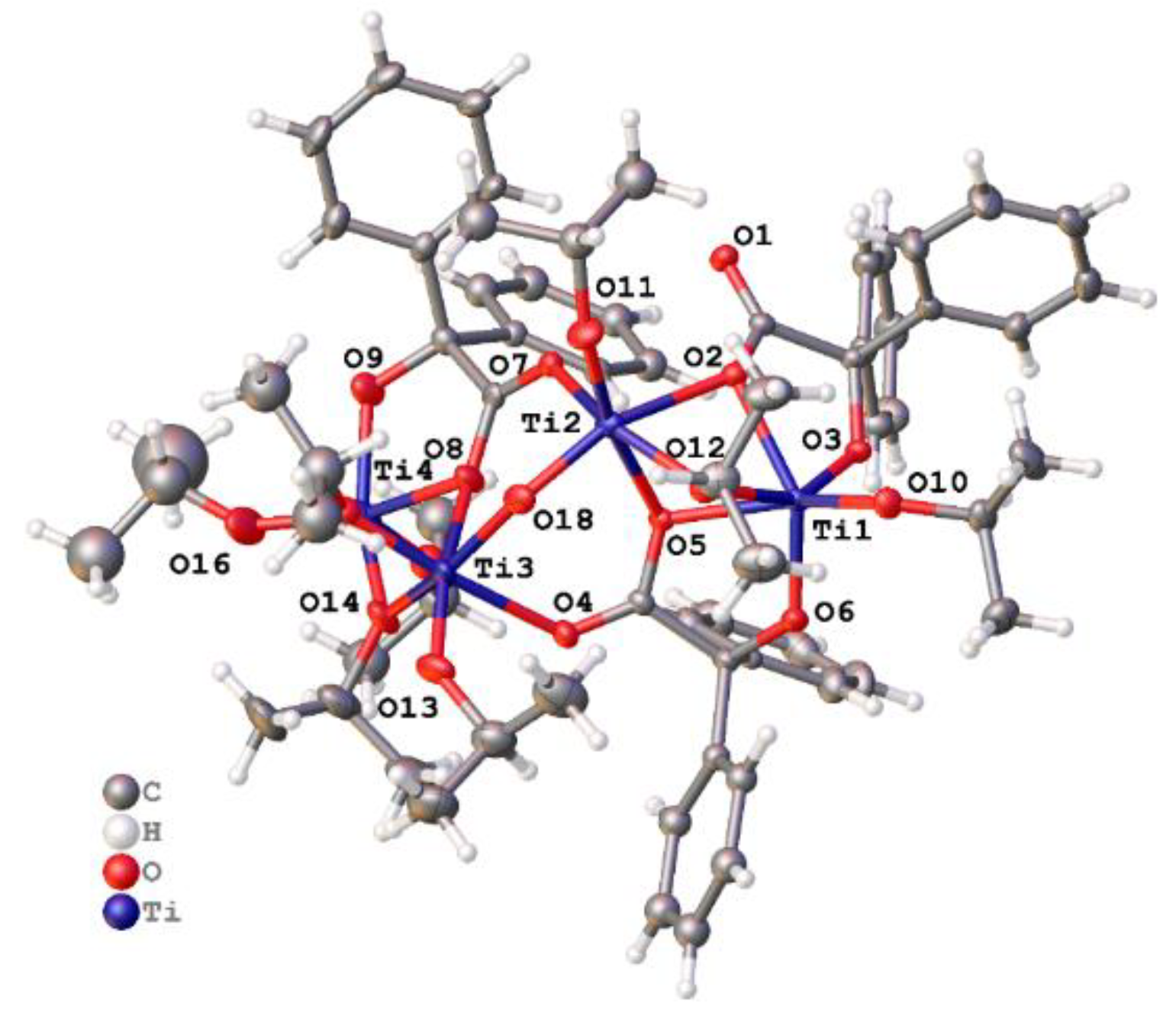
2.1.1. Benzilic Acid (L1H2)-Derived Complexes
2.1.2. Infrared and 1H NMR Spectra of Benzilic-Derived Complexes
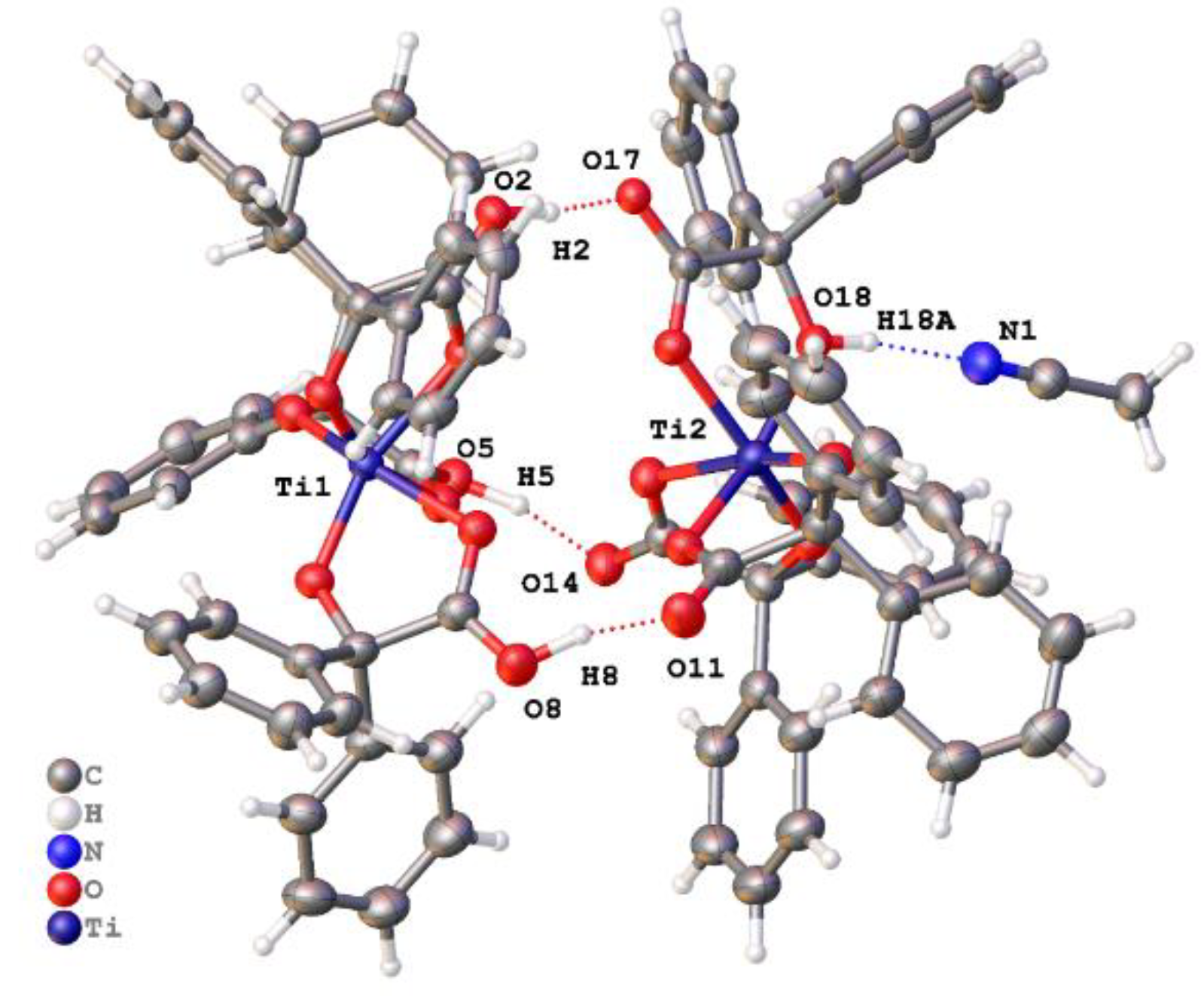
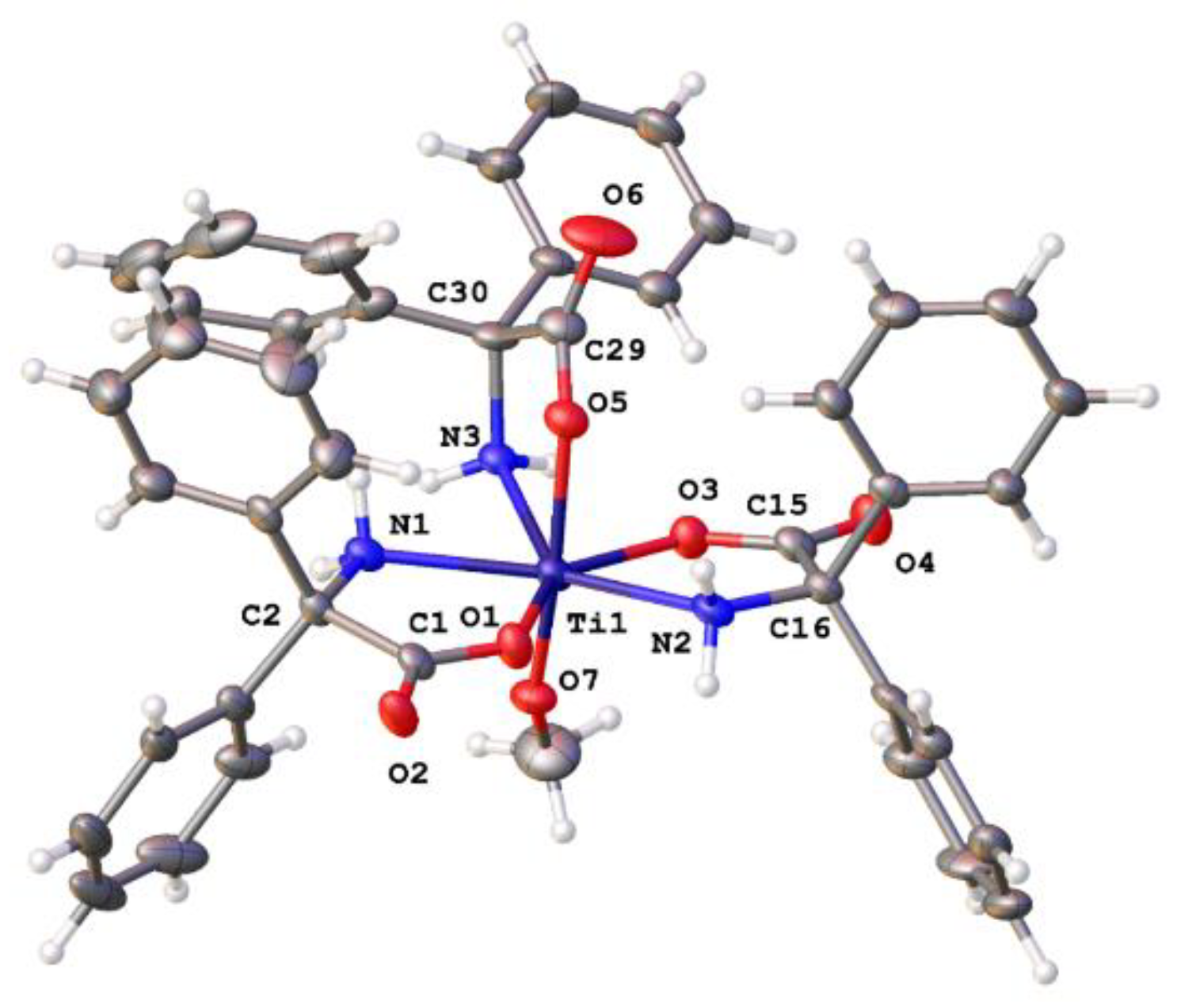
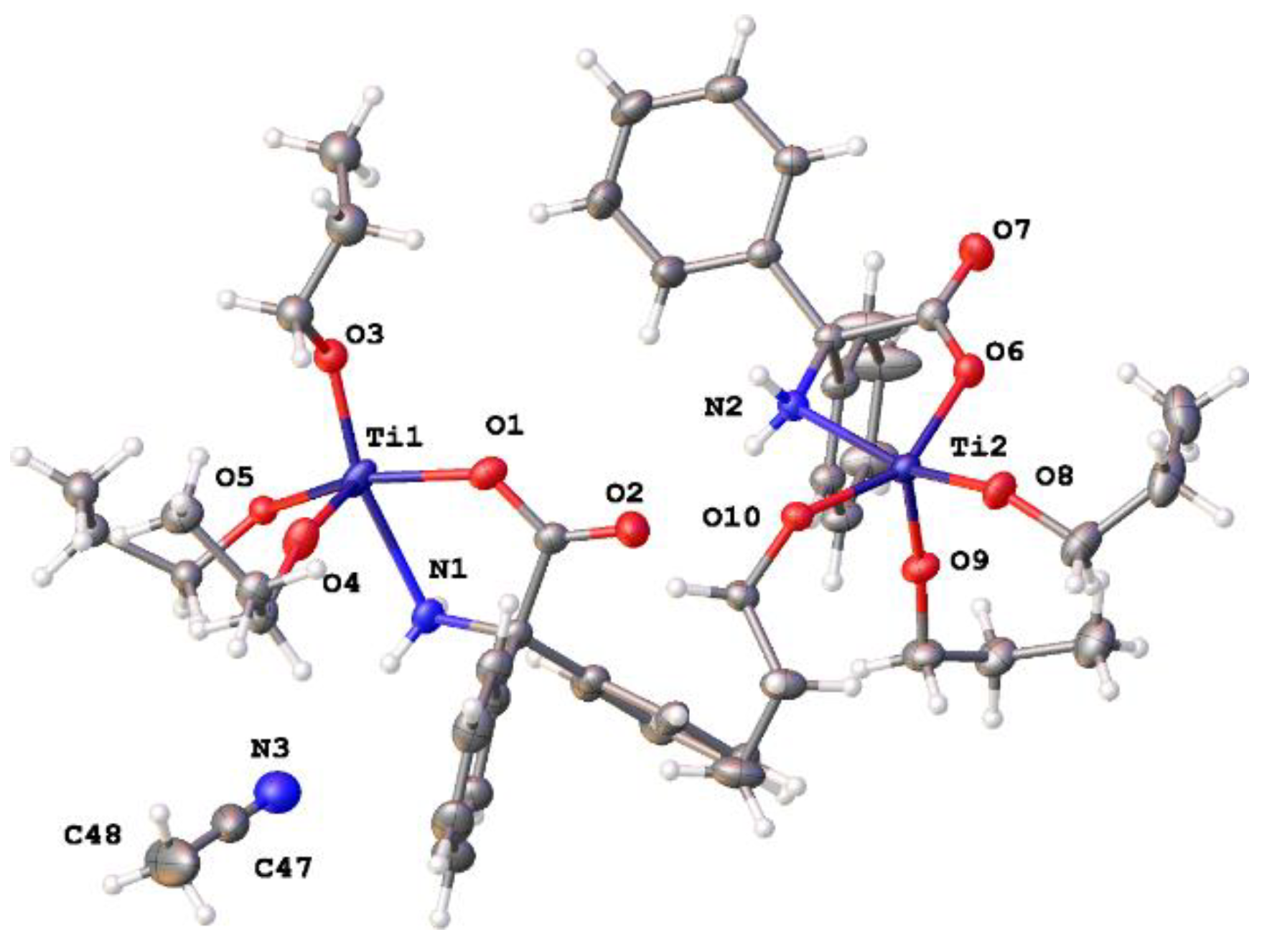
2.1.3. Diphenylglycine (L2H3)-Derived Complexes
2.1.4. Infrared and 1H NMR Spectra of the L2H3-Derived Complexes
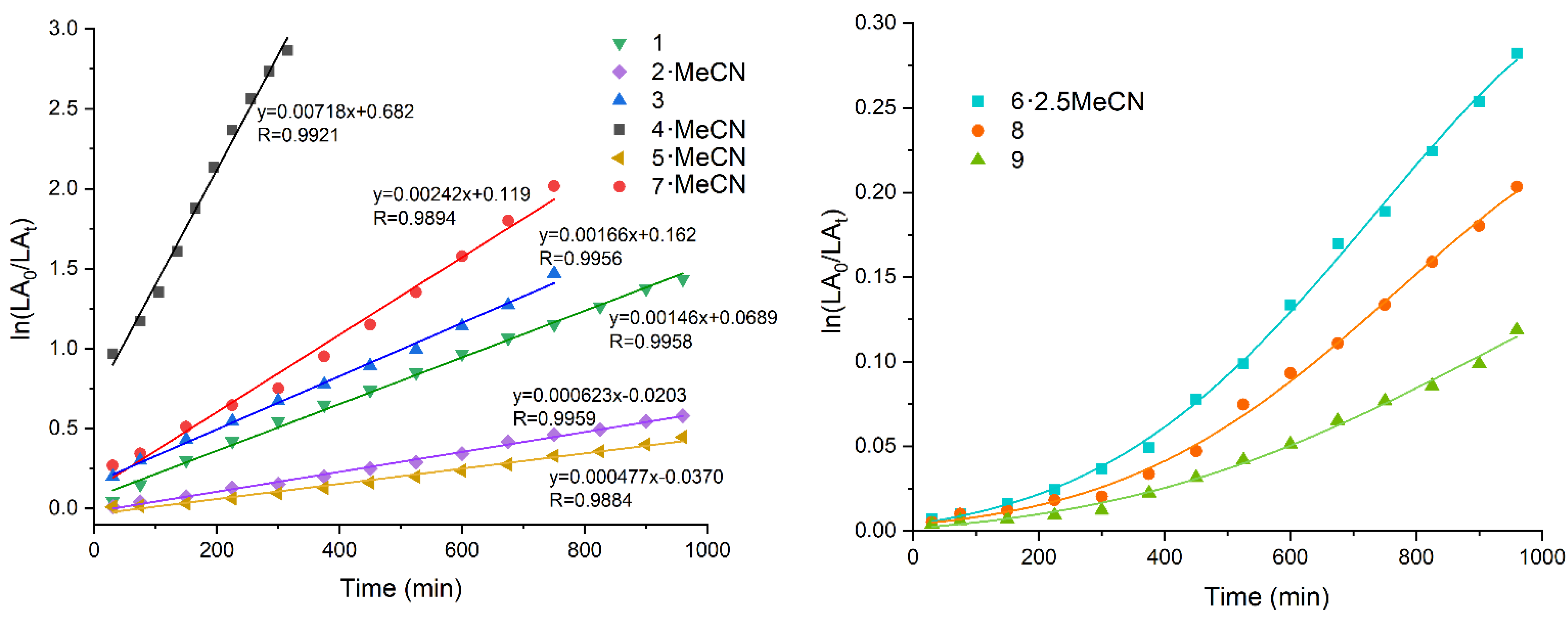
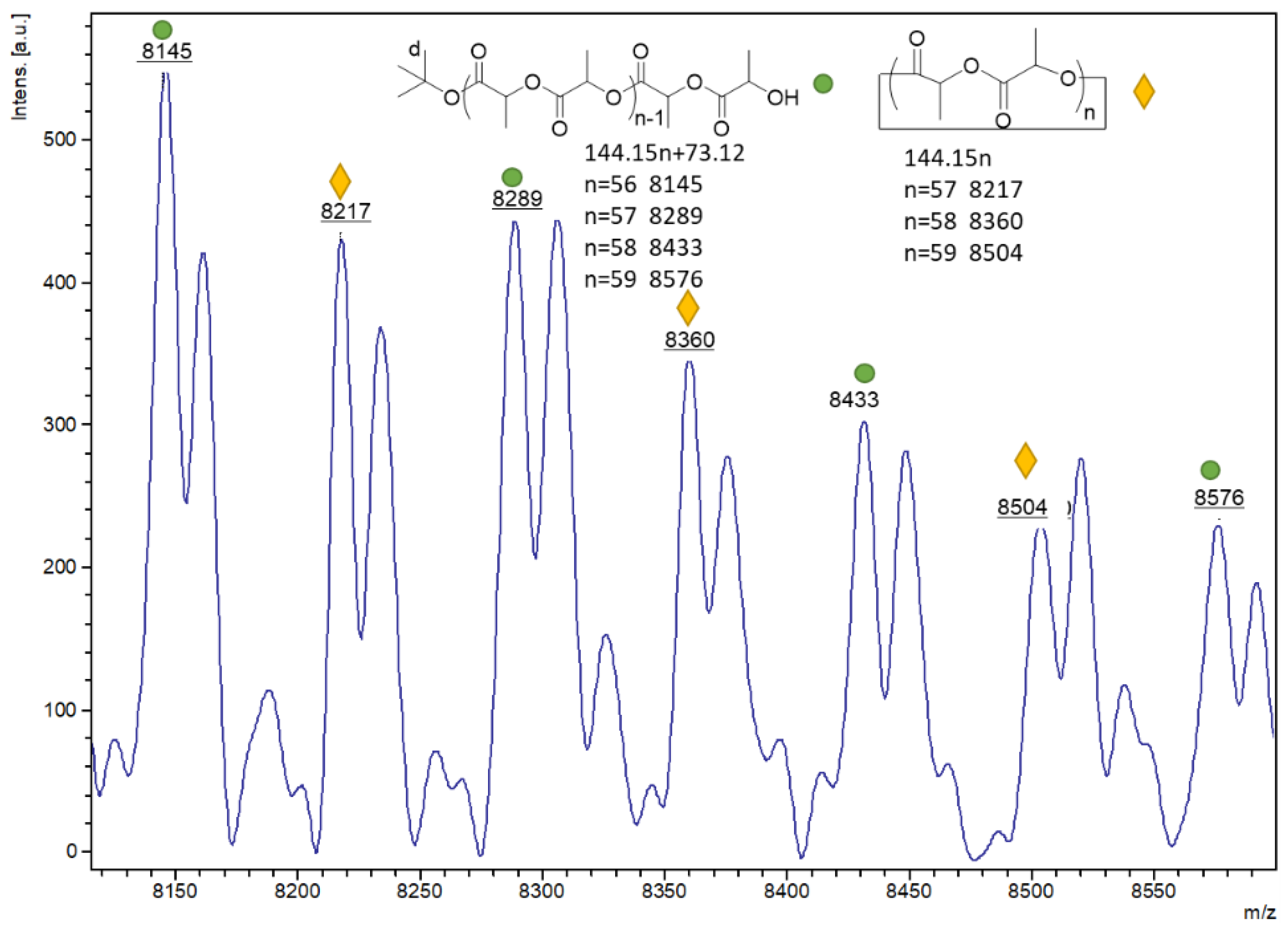
2.2. Ring Opening Polymerization Studies of ε-Caprolactone (ε-CL)
2.3. Ring Opening Polymerization Studies of Rac-Lactide (r-LA)
3. Materials and Methods
3.1. General
3.2. Preparation of [Ti4(L1)2(OMe)12] (1)
3.3. Preparation of [Ti(L1H)3][Ti(L1H)(L1H)2] MeCN (2·MeCN)
3.4. Preparation of [Ti4O(L1)2(OiPr)10] (3)
3.5. Preparation of [Ti4O(L1)3(OiPr)8] (4·MeCN)
3.6. Preparation of [Ti3(L1)4(OtBu)4(CH3CN)] (5·CH3CN)
3.7. Preparation of [Ti(L2H2)3OMe]·2.5CH3CN (6·2.5CH3CN)
3.8. Preparation of [Ti(L2H2)2(OnPr)6]·CH3CN (7·CH3CN)
3.9. Preparation of [Ti2(L2H2)2(OiPr)6] (8)
3.10. Preparation of [(L2H2)2Ti(OiPr)2] (9)
3.11. Preparation of [(L2H2)Ti(OtBu)2]·2MeCN (10·2MeCN)
3.12. ROP of ε-Caprolactone or Rac-Lactide
3.13. Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kambar, N.E.; Jeong, W.; Waymouth, R.M.; Pratt, R.C.; Lohmeijer, B.G.G.; Hedrick, J.L. Organocatalytic Ring-Opening Polymerization. Chem. Rev. 2007, 107, 5813–5840. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.K.; Hillmyer, M.A. Polymers from Renewable Resources: A Perspective for a Special Issue of Polymer Reviews. Polym. Rev. 2008, 48, 1–10. [Google Scholar] [CrossRef]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.H. Synthetic Polymer Scaffolds for Tissue Engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Labet, M.; Thielemans, W. Synthesis of Polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Arbaoui, A.; Redshaw, C. Metal Catalysts for ε-caprolactone polymerisation. Polym. Chem. 2010, 1, 801–826. [Google Scholar] [CrossRef]
- Lecomte, P.; Jérȏme, C. Synthetic Biodegradable Polymers; Rieger, B., Künkel, A., Coates, G., Reichardt, R., Dinjus, E., Zevaco, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 245, pp. 173–211. ISBN -13:978-3642271533. [Google Scholar]
- Redshaw, C. Use of Metal Catalysts Bearing Schiff-Base Macrocycles for the Ring Opening Polymerization (ROP) of Cyclic Esters. Catalysts 2017, 7, 165. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Ivchenko, P. Coordination Ring Opening Polymerization of Cyclic Esters: A Critical Overview of DFT Modeling and Visualization of the Reaction Mechanisms. Molecules 2019, 24, 4117. [Google Scholar] [CrossRef]
- Lynbov, D.M.; Tolpygin, A.O.; Trifoner, A.A. Rare earth metal complexes as catalysts for ring opening polymerization of cyclic esters. Coord. Chem. Rev. 2019, 392, 83–145. [Google Scholar] [CrossRef]
- Santoro, O.; Zhang, X.; Redshaw, C. Synthesis of Biodegradable Polymers: A Review on the use of Schiff-Base Metal Complexes as Catalysts for the Ring Opening Polymerization (ROP) of Cyclic Esters. Catalysts 2020, 10, 800. [Google Scholar] [CrossRef]
- Gruszka, W.; Garden, J.A. Advances in heterometallic (co)polymerization catalysis. Nat. Commun. 2021, 12, 3252. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Varma, I.K. Recent Developments in Ring Opening Polymerization of Lactones for Biomedical Applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable Synthetic Polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Zhang, X.; Fevre, M.; Jones, G.O.; Waymouth, R.M. Catalysis as an Enabling Science for Sustainable Polymers. Chem. Rev. 2018, 118, 839–885. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, C. Metallocalixarene catalysts: α-olefin polymerization and ROP of cyclic esters. Dalton Trans. 2016, 45, 9018–9030. [Google Scholar] [CrossRef]
- Jianming, R.; Anguo, X.; Hongwei, W.; Hailin, Y. Review—Recent development of ring-opening polymerization of cyclic esters using aluminum complexes. Des. Monomers Polym. 2014, 17, 345–355. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, D.; Zhang, W.; Solan, G.A.; Ma, Y.; Sun, W.-H. Recent progress in the application of group 1, 2 & 13 metal complexes as catalysts for the ring opening polymerization of cyclic esters. Inorg. Chem. Front. 2019, 6, 2619–2652. [Google Scholar] [CrossRef]
- Santoro, O.; Redshaw, C. Use of Titanium Complexes Bearing Diphenolate or Calix[n]arene Ligands in α-Olefin Polymerization and the ROP of Cyclic Esters. Catalysts 2020, 10, 210. [Google Scholar] [CrossRef]
- Stevels, W.M.; Ankoné, M.J.K.; Dijkstra, P.J.; Feijen, J. Kinetics and Mechanism of L-Lactide Polymerization Using Two Different Yttrium Alkoxides as Initiators. Macromolecules 1996, 29, 6132–6138. [Google Scholar] [CrossRef]
- Simic, V.; Spassky, N.; Hubert-Pfalzgraf, L.G. Ring-Opening Polymerization of D,L-Lactide Using Rare-Earth µ-Oxo Isopropoxides as Initiator Systems. Macromolecules 1997, 30, 7338–7340. [Google Scholar] [CrossRef]
- O’Keefe, B.J.; Monnier, S.M.; Hillmyer, M.A.; Tolman, W.B. Rapid and Controlled Polymerization of Lactide by Structurally Characterized Ferric Alkoxides. J. Am. Chem. Soc. 2001, 123, 339–340. [Google Scholar] [CrossRef]
- Kim, Y.; Verkade, J.G. A Tetrameric Titanium Alkoxide as a Lactide Polymerization Catalyst. Macromol. Rapid Commun. 2002, 23, 917–921. [Google Scholar] [CrossRef]
- Egevardt, C.; Giese, S.O.K.; Santos, A.D.d.C.; Barison, A.; de Sá, E.L.; Filho, A.Z.; da Silva, T.A.; Zawadzki, S.F.; Soares, J.F.; Nunes, G.G. ɛ-caprolactone and lactide polymerization promoted by an ionic titanium(IV)/iron(III) polynuclear halo-alkoxide. J. Polym. Sci. A Polym. Chem. 2014, 52, 2509–2517. [Google Scholar] [CrossRef]
- Ryan, J.D.; Gagnon, K.J.; Teat, S.J.; McIntosh, R.D. Flexible macrocycles as versatile supports for catalytically active metal clusters. Chem. Commun. 2016, 52, 9071–9073. [Google Scholar] [CrossRef] [PubMed]
- Cols, J.-M.E.P.; Taylor, C.E.; Gagnon, K.J.; Teat, S.J.; Mcintosh, R.D. Well-defined Ti4 pre-catalysts for the ring-opening polymerisation of lactide. Dalton Trans. 2016, 45, 17729–17738. [Google Scholar] [CrossRef]
- Cols, J.M.E.P.; Hill, V.G.; Williams, S.K.; Mcintosh, R.D. Aggregated initiators: Defining their role in the ROP of rac-lactide. Dalton Trans. 2018, 47, 10626–10635. [Google Scholar] [CrossRef]
- Jenkins, D.T.; Fazekas, E.; Patterson, S.B.H.; Rosair, G.M.; Vilela, F.; McIntosh, R.D. Polymetallic Group 4 Complexes: Catalysts for the Ring Opening Polymerisation of rac-Lactide. Catalysts 2021, 11, 551. [Google Scholar] [CrossRef]
- Braun, M. The “Magic” Diarylhydroxymethyl group. Angew. Chem. Int. Ed. 1996, 35, 519–522. [Google Scholar] [CrossRef]
- Braun, M. The diaryl (oxy) methyl group: More than an innocent bystander in chiral auxiliaries, catalysts, and dopants. Angew. Chem. Int. Ed. 2012, 51, 2550–2562. [Google Scholar] [CrossRef]
- Al-Khafaji, Y.; Elsegood, M.R.J.; Frese, J.W.A.; Redshaw, C. Ring opening polymerization of lactides and lactones by multimetallic alkyl zinc complexes derived from the acids Ph2C(X)CO2H (X = OH, NH2). RSC Adv. 2017, 7, 4510–4517. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, K.-Q.; Mo, S.; Al-Khafaji, Y.; Prior, T.J.; Elsegood, M.R.J.; Redshaw, C. Organoaluminium Complexes Derived from Anilines or Schiff Bases for the Ring-Opening Polymerization of ε-Caprolactone, δ-Valerolactone and rac-Lactide. Eur. J. Inorg. Chem. 2017, 2017, 1951–1965. [Google Scholar] [CrossRef] [Green Version]
- Al-Khafaji, Y.F.; Prior, T.J.; Horsburgh, L.; Elsegood, M.R.J.; Redshaw, C. Multimetallic Lithium Complexes Derived from the Acids Ph2C(X)CO2H (X = OH, NH2): Synthesis, Structure and Ring Opening Polymerization of Lactides and Lactones. Chem. Select 2017, 2, 759–768. [Google Scholar] [CrossRef]
- Collins, J.; Santoro, O.; Prior, T.J.; Chen, K.; Redshaw, C. Rare-earth metal complexes derived from the acids Ph2C(X)CO2H (X = OH, NH2): Structural and ring opening polymerization (ROP) studies. J. Mol. Struct. 2021, 1224, 129083. [Google Scholar] [CrossRef]
- Zhang, X.; Prior, T.J.; Redshaw, C. Niobium and Tantalum complexes derived from the acids Ph2C(X)CO2H (X = OH, NH2): Synthesis, structure and ROP capability. New J. Chem. 2022, 46, 14146–14154. [Google Scholar] [CrossRef]
- Alshamrani, A.F.A.; Santoro, O.; Ounsworth, S.; Prior, T.J.; Stasiuk, G.J.; Redshaw, C. Synthesis, characterisation and ROP catalytic evaluation of Cu(II) complexes bearing 2,2′-diphenylglycine-derived moieties. Polyhedron 2021, 195, 114977. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; Clegg, W.; Elsegood, M.R.J. Synthesis and characterisation of molybdenum complexes bearing highly functionalised imido substituents. Dalton Trans. 1997, 18, 3207–3212. [Google Scholar] [CrossRef]
- Redshaw, C.; Gibson, V.C.; Clegg, W.; Edwards, A.J.; Miles, B. Pentamethylcyclopentadienyl tungsten complexes containing imido, hydrazido and amino acid derived N-O chelate ligands. Dalton Trans. 1997, 18, 3343–3347. [Google Scholar] [CrossRef]
- Redshaw, C.; Elsegood, M.R.J.; Holmes, K.E. Synthesis of hexa- and dodecanuclear organoaluminum ring structures incorporating the “magic” Ph2C(X) Group (X = O−, NH−). Angew. Chem. Int. Ed. 2005, 44, 1850–1853. [Google Scholar] [CrossRef]
- Redshaw, C.; Elsegood, M.R.J. Synthesis of Tetra-, Hexa-, and Octanuclear Organozinc Ring Systems. Angew. Chem. Int. Ed. 2007, 119, 7453–7457. [Google Scholar] [CrossRef]
- Arbaoui, A.; Redshaw, C.; Hughes, D.L.; Elsegood, M.R.J. Arylboron complexes of the acids Ph2C(XH)CO2H (X = O, NH). Inorg. Chim. Acta 2009, 362, 509–514. [Google Scholar] [CrossRef]
- Thielemann, D.T.; Wagner, A.T.; Lan, Y.; Anson, C.E.; Gamer, M.T.; Powell, A.K.; Roesky, P.W. Slow magnetic relaxation in four square-based pyramidal dysprosium hydroxo clusters ligated by chiral amino acid anions—A comparative study. Dalton Trans. 2013, 42, 14794–14800. [Google Scholar] [CrossRef]
- Alshamrani, A.F.; Prior, T.J.; Burke, B.P.; Roberts, D.P.; Archibald, S.J.; Stasiuk, G.; Higham, L.J.; Redshaw, C. Water-soluble rhenium phosphine complexes incorporating the Ph2C(X) motif (X = O−, NH−): Structural and Cytotoxicity studies. Inorg. Chem. 2020, 59, 2367–2378. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, E. Recent advances on tailor-made titanium catalysts for biopolymer synthesis. Coord. Chem. Rev. 2016, 306, 65–85. [Google Scholar] [CrossRef]
- Webster, R.L. Random copolymerisations catalysed by simple titanium α-amino acid complexes. RSC Adv. 2014, 4, 5254–5260. [Google Scholar] [CrossRef]
- Bermejo, E.; Carballo, R.; Castiñeiras, A.; Lago, A.B. Coordination of α-hydroxycarboxylic acids with first-row transition ions. Coord. Chem. Rev. 2013, 257, 2639–2651. [Google Scholar] [CrossRef]
- At the Time of Writing (18.6.22), the Price of 100 g of Benzilic acid Costs £17.90 + VAT (from Merck/Sigma Aldrich). Available online: https://www.sigmaaldrich.com/GB/en/substance/benzilicacid2282476937 (accessed on 18 June 2022).
- Coxall, R.A.; Harris, S.G.; Henderson, D.K.; Parsons, S.; Tasker, P.A.; Winpenny, R.E.P. Inter-ligand reactions: In situ formation of new polydentate ligands. J. Chem. Soc. Dalton Trans. 2000, 14, 2349–2356. [Google Scholar] [CrossRef]
- Kemmitt, T.; Gainsford, G.J.; Al-Salim, N.I. An oxo-bridged centrosymmetric tetranuclear titanium compound. Acta Cryst. Sect. C 2004, 60, m42–m43. [Google Scholar] [CrossRef]
- At the Time of Writing (18.6.22), the Price of 5 g of 2,2′-Diphenylglycine Costs £104.00 + VAT (from Merck/Sigma Aldrich). Available online: https://www.sigmaaldrich.com/GB/en/product/aldrich/161918 (accessed on 18 June 2022).
- Baśko, M.; Kubisa, P. Cationic copolymerization of ε-caprolactone and L,L-lactide by an activated monomer mechanism. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 7071–7081. [Google Scholar] [CrossRef]
- Delcroix, D.; Couffin, A.; Susperregui, N.; Navarro, C.; Maron, L.; Martin-Vaca, B.; Bourissou, D. Phosphoric and phosphoramidic acids as bifunctional catalysts for the ring-opening polymerization of ε-caprolactone: A combined experimental and theoretical study. Polym. Chem. 2011, 2, 2249–2256. [Google Scholar] [CrossRef]
- Wright, D.A.; Williams, D.A. The Crystal and Molecular Structure of Titanium Methoxide. Acta Cryst. 1968, B24, 1107–1114. [Google Scholar] [CrossRef]
- Martin, R.L.; Winter, G. Structure of the Trinuclear Titanium(IV) Alkoxides. Nature 1960, 188, 313–315. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, C.; Sun, Y.; Wu, J.; Pan, X. Isoselective mechanism of the ring-opening polymerization of rac-lactide catalyzed by chiral potassium binolates. Inorg. Chem. Front. 2017, 4, 261–269. [Google Scholar] [CrossRef]
- Zhong, Z.; Dijkstra, P.J.; Feijen, J. Controlled and stereoselective polymerization of lactide: Kinetics, selectivity, and microstructures. J. Am. Chem. Soc. 2003, 125, 11291–11298. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, C.; Bai, F.; Yue, J.; Woo, H.-G. Living ring-opening polymerization of L-lactide catalyzed by red-Al. Organometallics 2004, 23, 1411–1415. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]









| Run | Catalyst | L1/L2 | OR Group | CL:Ti | T (°C) | Time (min) | Conv. a (%) | Mn (calc.) b (kDa) | Mn (obs.) c,d (kDa) | Ð c |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | L1 | OMe | 250:1 | 100 | 195 | 99 | 28.3 | 7.2 | 1.87 |
| 2 | 2·MeCN | - | 250:1 | 100 | 1440 | 20 | 5.7 | 4.6 | 1.25 | |
| 3 | 3 | OiPr | 250:1 | 100 | 150 | 99 | 28.3 | 30.0 | 1.93 | |
| 4 | 4·MeCN | OiPr | 250:1 | 100 | 1440 | 31 | 8.9 | 6.0 | 1.53 | |
| 5 | 5·MeCN | OtBu | 250:1 | 100 | 1440 | 53 | 15.2 | 4.1 | 1.25 | |
| 6 | 6·2.5MeCN | L2 | OMe | 250:1 | 100 | 480 | 95 | 27.1 | 6.0 | 1.22 |
| 7 | 7·MeCN | OnPr | 250:1 | 100 | 12 | 99 | 28.3 | 8.4 | 1.49 | |
| 8 | 8 | OiPr | 250:1 | 100 | 210 | 99 | 28.3 | 6.7 | 1.80 | |
| 9 | 9 | OiPr | 250:1 | 100 | 345 | 99 | 28.3 | 12.0 | 1.97 | |
| 10 | 10·2MeCN | OtBu | 250:1 | 100 | 255 | 99 | 28.3 | 39.0 | 2.41 | |
| 11 | 7·MeCN | OnPr | 250:1 | 50 | 150 | 99 | 28.3 | 4.8 | 1.20 | |
| 12 | 7·MeCN | OnPr | 500:1 | 100 | 6 | 99 | 56.6 | 13.0 | 2.35 | |
| 13 | 7·MeCN | OnPr | 1000:1 | 100 | 9 | 99 | 113 | 8.7 | 1.45 | |
| 14 | 7·MeCN | OnPr | 2000:1 | 100 | 12 | 99 | 226 | 8.6 | 1.84 | |
| 15 | 7·MeCN | OnPr | 250:1 | 25 | 1440 | 14 | - | - | - | |
| 16 e | 7·MeCN | OnPr | 250:1 | 100 | 1440 | 6 | - | - | - | |
| 17 f | 7·MeCN | OnPr | 250:1 | 100 | 6 | 98 | 28.0 | 10.5 | 1.29 | |
| 18 | [Ti(OR)4] | - | OMe | 250:1 | 100 | 195 | 3 | 0.9 | 1.0 | 1.22 |
| 19 | OnPr | 250:1 | 100 | 2 | 92 | 26.3 | 6.7 | 2.79 | ||
| 20 | OiPr | 250:1 | 100 | 195 | 94 | 26.8 | 7.8 | 3.01 | ||
| 21 | OtBu | 250:1 | 100 | 195 | 43 | 12.3 | 5.4 | 2.94 | ||
| 22 e | OnPr | 250:1 | 100 | 2 | 96 | 27.4 | 5.6 | 3.21 |
| Run | Complex | kobs (×104) | Induction Period (min) |
|---|---|---|---|
| 1 | 1 | 302 (R2 = 0.992) | 60 |
| 2 | 2·MeCN | 2.15 (R2 = 0.999) | 275 |
| 3 | 3 | 416 (R2 = 0.969) | 60 |
| 4 | 4·MeCN | 2.57 (R2 = 0.994) | 215 |
| 5 | 5·MeCN | 2.87 (R2 = 0.977) | 300 |
| 6 | 6·2.5MeCN | 159 (R2 = 0.977) | 270 |
| 7 | 7·MeCN | 4743 (R2 = 0.978) | 0 |
| 8 | 8 | 358 (R2 = 0.968) | 105 |
| 9 | 9 | 253 (R2 = 0.986) | 210 |
| 10 | 10·2.5MeCN | 246 (R2 = 0.981) | 105 |
| Run | Catalyst | L1/L2 | OR Group | LA:Ti | T (°C) | Conv. a (%) | Mn (calc.) b (kDa) | Mn (obs.) c,d (kDa) | Ð c | Pi e |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | L1 | OMe | 250:1 | 130 | 90 | 32.0 | 22.0 | 1.34 | 0.39 |
| 2 | 2·MeCN | - | 250:1 | 130 | 78 | 28.0 | 14.0 | 1.22 | 0.47 | |
| 3 | 3 | OiPr | 250:1 | 130 | 92 | 33.0 | 13.0 | 1.15 | 0.46 | |
| 4 | 4·MeCN | OiPr | 250:1 | 130 | 98 | 35.0 | 16.0 | 1.19 | 0.49 | |
| 5 | 5·MeCN | OtBu | 250:1 | 130 | 72 | 26.0 | 13.0 | 1.72 | 0.48 | |
| 6 | 6·2.5MeCN | L2 | OMe | 250:1 | 130 | 65 | 23.0 | 11.0 | 1.21 | 0.26 |
| 7 | 7·MeCN | OnPr | 250:1 | 130 | 98 | 35.0 | 30.0 | 1.29 | 0.49 | |
| 8 | 8 | OiPr | 250:1 | 130 | 52 | 19.0 | 14.0 | 1.19 | 0.46 | |
| 9 | 9 | OiPr | 250:1 | 130 | 44 | 16.0 | 8.0 | 1.15 | 0.51 | |
| 10 | 10·2MeCN | OtBu | 250:1 | 130 | 28 | 10.0 | 12.0 | 1.37 | 0.16 | |
| 11 f | 4·MeCN | OiPr | 250:1 | 130 | 20 | 7.3 | 4.6 | 1.18 | - | |
| 12 f | 7·MeCN | OnPr | 250:1 | 130 | 16 | 5.8 | 3.6 | 1.10 | - | |
| 15 | [Ti(OR)4] | - | OMe | 250:1 | 130 | 99 | 35.7 | 0.5 | 1.24 | - |
| 16 | OnPr | 250:1 | 130 | 99 | 35.7 | 0.7 | 3.03 | - | ||
| 17 | OiPr | 250:1 | 130 | 86 | 31.0 | 0.5 | 2.75 | - | ||
| 18 | OtBu | 250:1 | 130 | 98 | 35.4 | 1.7 | 2.60 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Prior, T.J.; Chen, K.; Santoro, O.; Redshaw, C. Ring Opening Polymerization of Lactides and Lactones by Multimetallic Titanium Complexes Derived from the Acids Ph2C(X)CO2H (X = OH, NH2). Catalysts 2022, 12, 935. https://doi.org/10.3390/catal12090935
Zhang X, Prior TJ, Chen K, Santoro O, Redshaw C. Ring Opening Polymerization of Lactides and Lactones by Multimetallic Titanium Complexes Derived from the Acids Ph2C(X)CO2H (X = OH, NH2). Catalysts. 2022; 12(9):935. https://doi.org/10.3390/catal12090935
Chicago/Turabian StyleZhang, Xin, Timothy J. Prior, Kai Chen, Orlando Santoro, and Carl Redshaw. 2022. "Ring Opening Polymerization of Lactides and Lactones by Multimetallic Titanium Complexes Derived from the Acids Ph2C(X)CO2H (X = OH, NH2)" Catalysts 12, no. 9: 935. https://doi.org/10.3390/catal12090935
APA StyleZhang, X., Prior, T. J., Chen, K., Santoro, O., & Redshaw, C. (2022). Ring Opening Polymerization of Lactides and Lactones by Multimetallic Titanium Complexes Derived from the Acids Ph2C(X)CO2H (X = OH, NH2). Catalysts, 12(9), 935. https://doi.org/10.3390/catal12090935