Photocatalytic H2O2 Generation Using Au-Ag Bimetallic Alloy Nanoparticles loaded on ZnO
Abstract
:1. Introduction
2. Results and Discussion
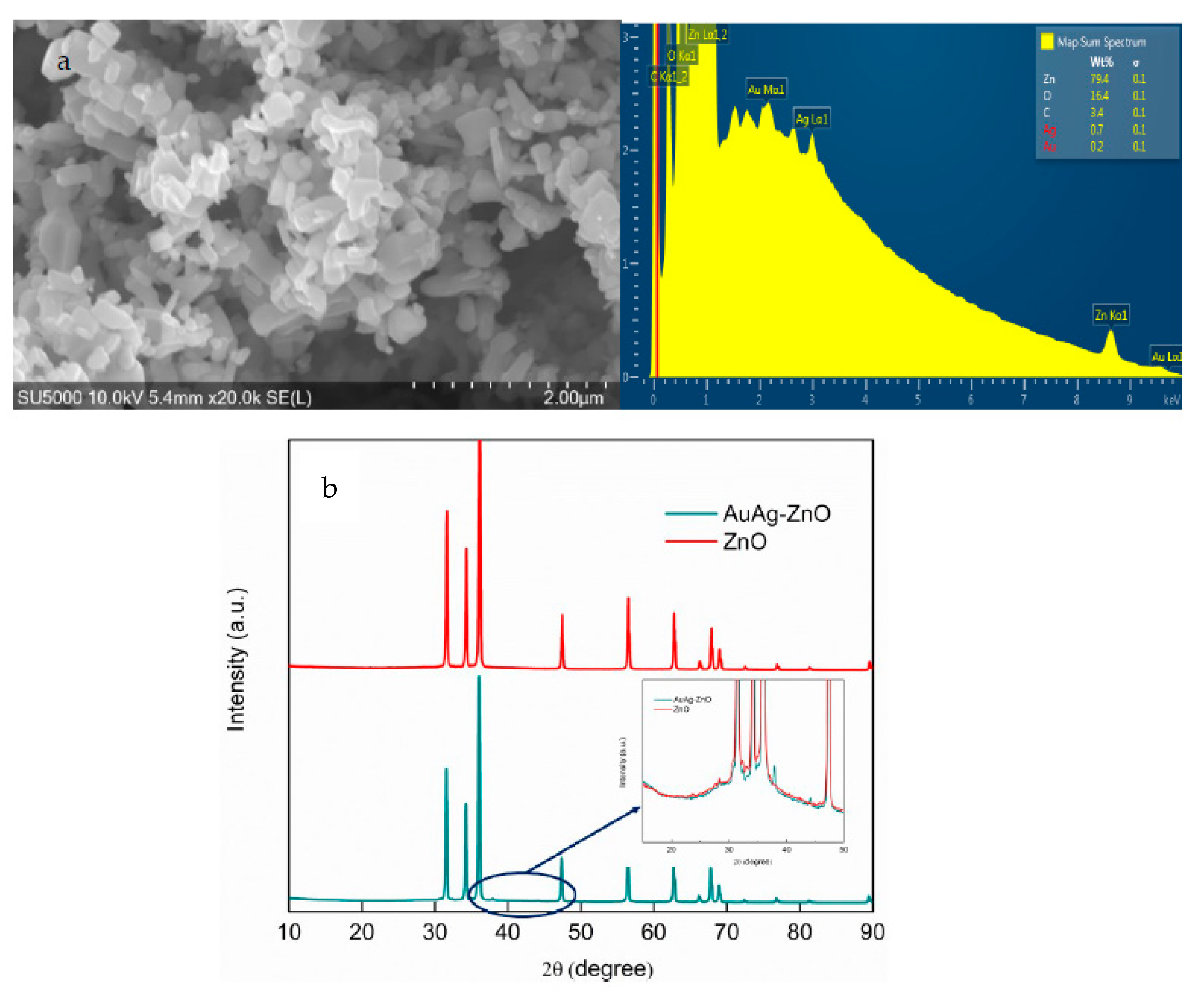
2.1. Characterization of Catalysts
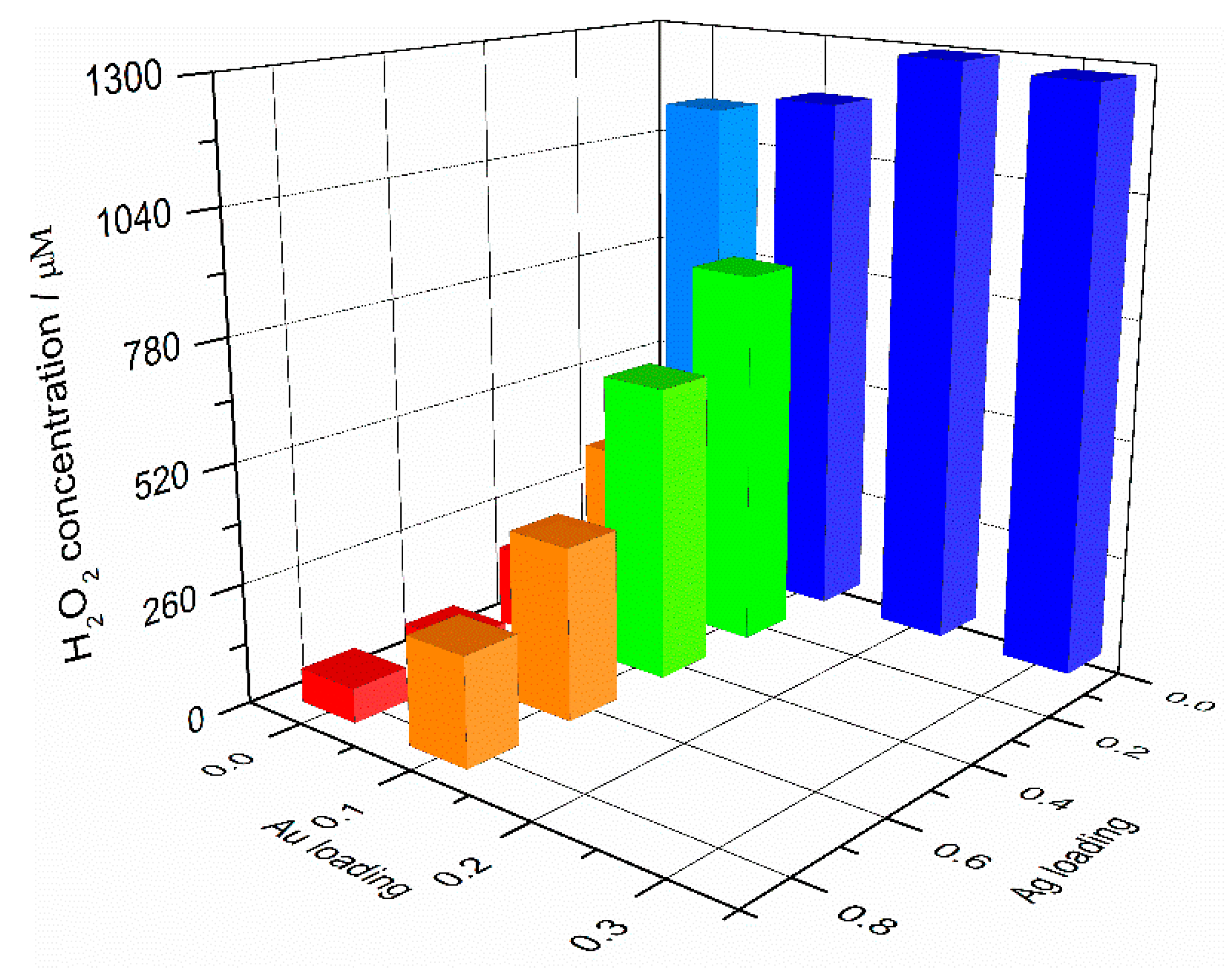
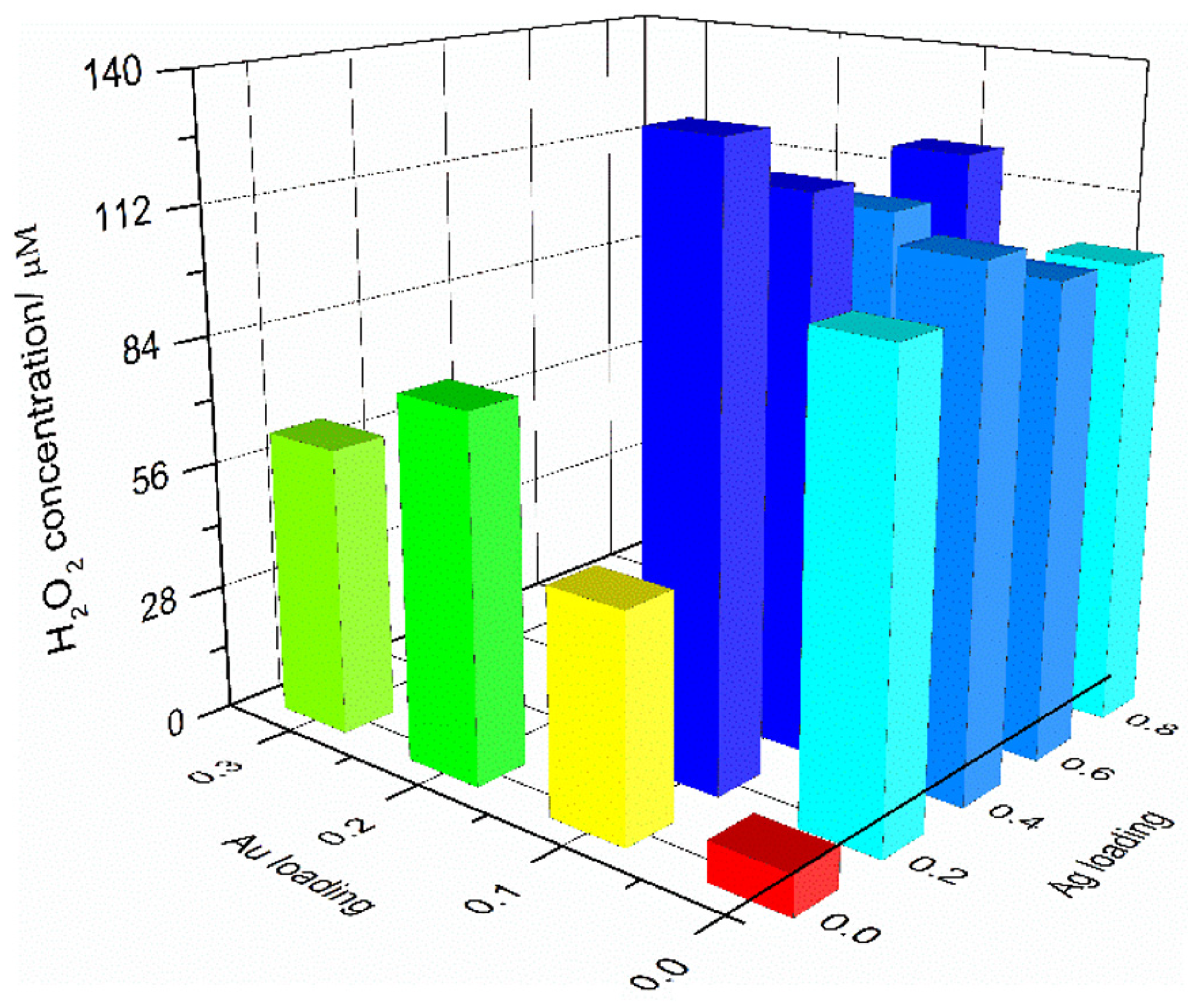
2.2. Photocatalytic Generation of H2O2
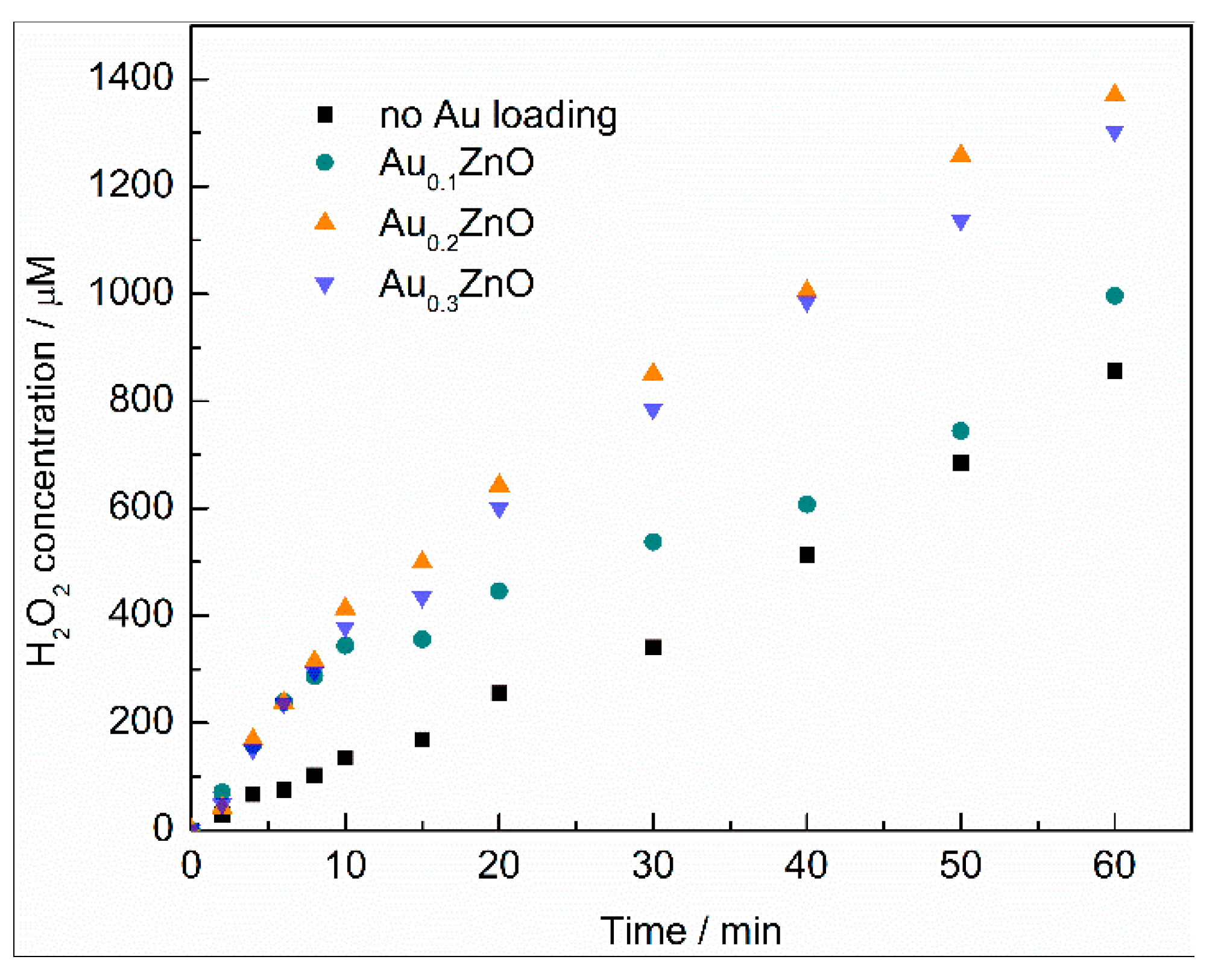
2.3. Kinetics of H2O2 Formation on Modified ZnO
3. Materials and Methods
3.1. Materials
3.2. Preparation of Photocatalyst
3.3. Photocatalytic Generation of H2O2
3.4. Adsorption of H2O2 on Catalysts
3.5. Characterization of Photocatalyst
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sato, K.; Aoki, M.; Noyori, R. A “green” route to adipic acid: Direct oxidation of cyclohexenes with 30 percent hydrogen peroxide. J. Sci. 1998, 281, 1646–1647. [Google Scholar] [CrossRef]
- Kofuji, Y.; Isobe, Y.; Shiraishi, Y.; Sakamoto, H.; Tanaka, S.; Ichikawa, S.; Hirai, T. Carbon Nitride-Aromatic Diimide-Graphene Nanohybrids: Metal-Free Photocatalysts for Solar-to-Hydrogen Peroxide Energy Conversion with 0.2% Efficiency. J. Am. Chem. Soc. 2016, 138, 10019–10025. [Google Scholar] [CrossRef] [PubMed]
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L.G. Hydrogen peroxide synthesis: An outlook beyond the anthraquinone process. Angew. Chem. Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef] [PubMed]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photocatalytic production of H2O2 and organic peroxides in aqueous suspensions of TiO2, ZnO, and desert sand. Environ. Sci. Technol. 1988, 22, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zeng, X.; Zhang, X. Production of Hydrogen Peroxide by Photocatalytic Processes. Angew. Chem. Int. Ed. 2020, 59, 17356–17376. [Google Scholar] [CrossRef]
- Burek, B.O.; Bahnemann, D.W.; Bloh, J.Z. Modeling and Optimization of the Photocatalytic Reduction of Molecular Oxygen to Hydrogen Peroxide over Titanium Dioxide. ACS Catal. 2019, 9, 25–37. [Google Scholar] [CrossRef]
- Hasnat, M.A.; Uddin, M.M.; Samed, A.J.F.; Alam, S.S.; Hossain, S. Adsorption and photocatalytic decolorization of a synthetic dye erythrosine on anatase TiO2 and ZnO surfaces. J. Hazard. Mater. 2007, 147, 471–477. [Google Scholar] [CrossRef]
- Teranishi, M.; Naya, S.I.; Tada, H. Temperature- and pH-Dependence of Hydrogen Peroxide Formation from Molecular Oxygen by Gold Nanoparticle-Loaded Titanium(IV) Oxide Photocatalyst. J. Phys. Chem. C 2016, 120, 1083–1088. [Google Scholar] [CrossRef]
- Zuo, G.; Li, B.; Guo, Z.; Wang, L.; Yang, F.; Hou, W.; Zhang, S.; Zong, P.; Liu, S.; Meng, X.; et al. Efficient Photocatalytic Hydrogen Peroxide Production over TiO2 Passivated by SnO2. Catalysts 2019, 9, 623. [Google Scholar] [CrossRef]
- Teranishi, M.; Naya, S.I.; Tada, H. In situ liquid phase synthesis of hydrogen peroxide from molecular oxygen using gold nanoparticle-loaded titanium(IV) dioxide photocatalyst. J. Am. Chem. Soc. 2010, 132, 7850–7851. [Google Scholar] [CrossRef]
- Tsukamoto, D.; Shiro, A.; Shiraishi, Y.; Sugano, Y.; Ichikawa, S.; Tanaka, S.; Hirai, T. Photocatalytic H2O2 production from ethanol/O2 system using TiO2 loaded with Au-Ag bimetallic alloy nanoparticles. ACS Catal. 2012, 2, 599–603. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Kofuji, Y.; Sakamoto, H.; Tanaka, S.; Ichikawa, S.; Hirai, T. Effects of Surface Defects on Photocatalytic H2O2 Production by Mesoporous Graphitic Carbon Nitride under Visible Light Irradiation. ACS Catal. 2015, 5, 3058–3066. [Google Scholar] [CrossRef]
- Li, S.; Dong, G.; Hailili, R.; Yang, L.; Li, Y.; Wang, F.; Zeng, Y.; Wang, C. Effective photocatalytic H2O2 production under visible light irradiation at g-C3N4 modulated by carbon vacancies. Appl. Catal. B Environ. 2016, 190, 26–35. [Google Scholar] [CrossRef]
- Hirakawa, H.; Shiota, S.; Shiraishi, Y.; Sakamoto, H.; Ichikawa, S.; Hirai, T. Au Nanoparticles Supported on BiVO4: Effective Inorganic Photocatalysts for H2O2 Production from Water and O2 under Visible Light. ACS Catal. 2016, 6, 4976–4982. [Google Scholar] [CrossRef]
- Meng, X.; Zong, P.; Wang, L.; Yang, F.; Hou, W.; Zhang, S.; Li, B.; Guo, Z.; Liu, S.; Zuo, G.; et al. Au-nanoparticle-supported ZnO as highly efficient photocatalyst for H2O2 production. Catal. Commun. 2020, 134, 105860. [Google Scholar] [CrossRef]
- Kawano, S.; Fujishima, M.; Tada, H. Size effect of zinc oxide-supported gold nanoparticles on the photocatalytic activity for two-electron oxygen reduction reaction. Catal. Commun. 2020, 144, 106076. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, X.; Liu, S.; Zhao, J.; Xu, Y. Sustained production of H2O2 in alkaline water solution using borate and phosphate-modified Au/TiO2 photocatalysts. Photochem. Photobiol. Sci. 2018, 17, 1018–1022. [Google Scholar] [CrossRef]
- Miah, M.R.; Ohsaka, T. Cathodic detection of H2O2 using iodide-modified gold electrode in alkaline media. Anal. Chem. 2006, 78, 1200–1205. [Google Scholar] [CrossRef]
- Hirakawa, T.; Yawata, K.; Nosaka, Y. Photocatalytic reactivity for O2·- and OH· radical formation in anatase and rutile TiO2 suspension as the effect of H2O2 addition. Appl. Catal. A Gen. 2007, 325, 105–111. [Google Scholar] [CrossRef]
- Ma, X.; Li, H.; Liu, T.; Du, S.; Qiang, Q.; Wang, Y.; Yin, S.; Sato, T. Comparison of photocatalytic reaction-induced selective corrosion with photocorrosion: Impact on morphology and stability of Ag-ZnO. Appl. Catal. B Environ. 2017, 201, 348–358. [Google Scholar] [CrossRef] [Green Version]
- Sahel, K.; Elsellami, L.; Mirali, I.; Dappozze, F.; Bouhent, M.; Guillard, C. Hydrogen peroxide and photocatalysis. Appl. Catal. B Environ. 2016, 188, 106–112. [Google Scholar] [CrossRef]
- Skillen, N.; Ralphs, K.; Craig, D.; McCalmont, S.; Muzio, A.F.V.; O’Rourke, C.; Manyar, H.; Robertson, P. Photocatalytic Reforming of Glycerol to H2 in a Thin Film Pt-TiO2 Recirculating Photo Reactor. J. Chem. Technol. Biotechnol. 2020, 95, 2619–2627. [Google Scholar] [CrossRef]

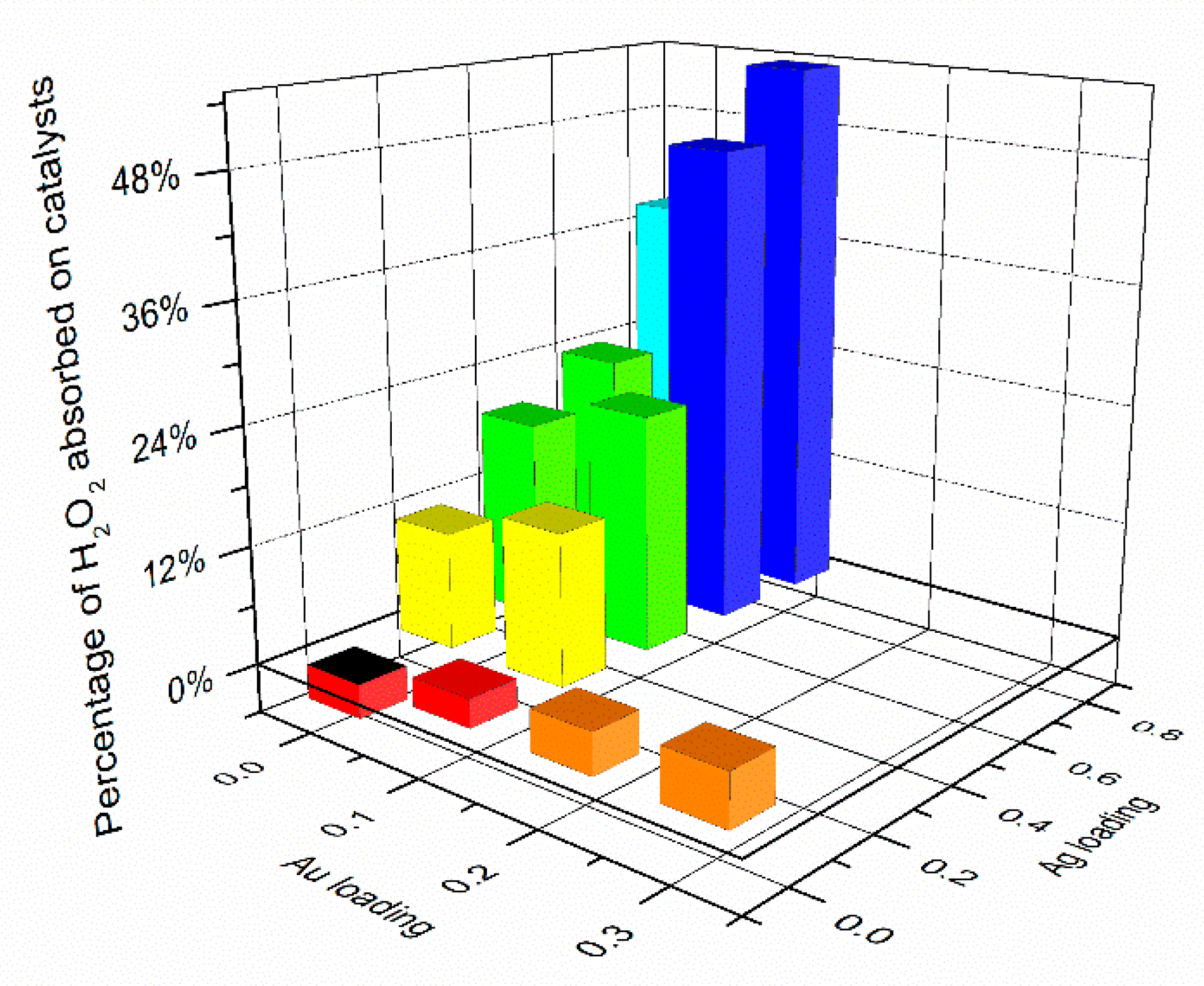
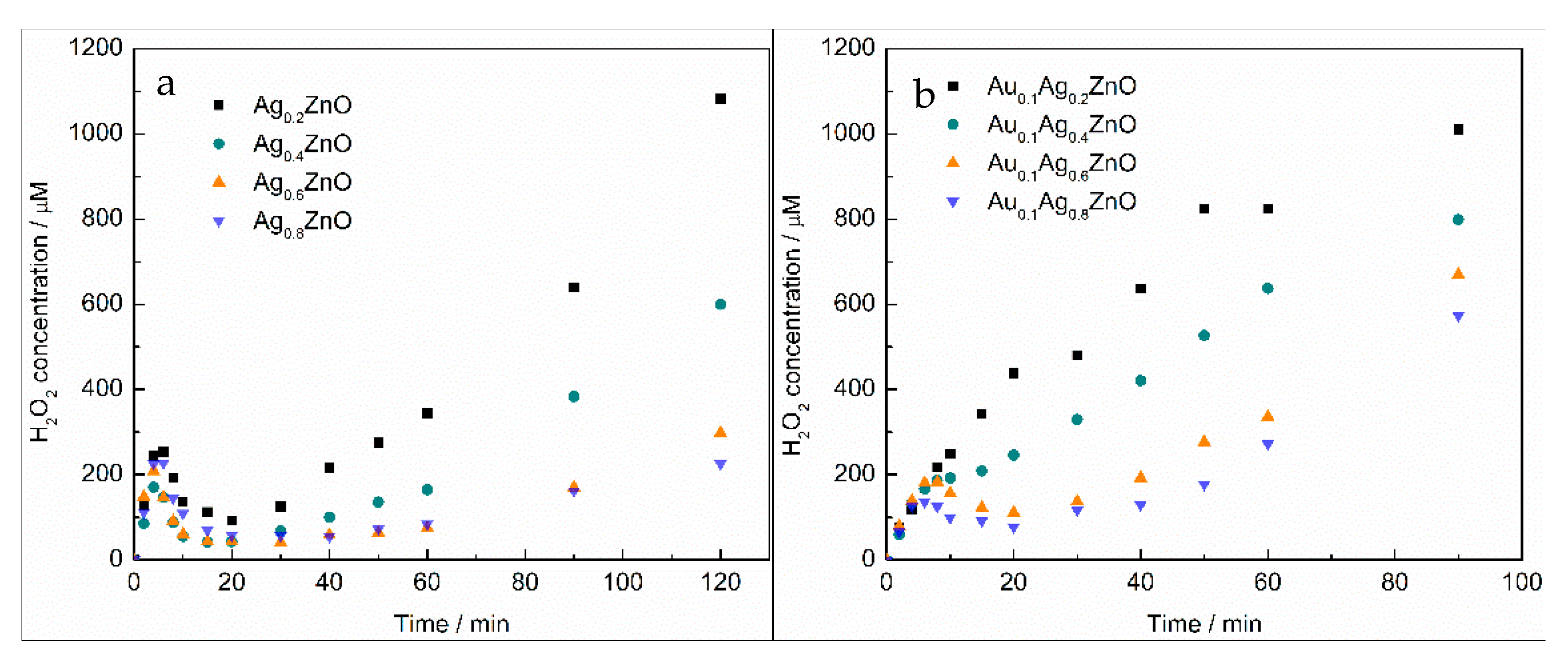
| Au Content/mol% a | Ag Content/mol% a | Particle Size/nm | |
|---|---|---|---|
| Au0.1Ag0.4/ZnO | 0.14 | 0.68 | 309 |
| Au0.1Ag0.4/TiO2 | 0.14 | 0.73 | 65 |
| Au0.1/ZnO | 0.15 | - | 411 |
| Au0.2/ZnO | 0.26 | - | |
| Au0.3/ZnO | 0.37 | - | |
| Ag0.2/ZnO | - | 0.27 | 235 |
| Ag0.2/ZnO b | 0.14 |
| ZnO | Au0.2/ZnO | Ag0.2/ZnO | Au0.1Ag0.2/ZnO | |
|---|---|---|---|---|
| Zn2+ after reaction/ppm | 3.9 | 3.0 | 6.5 | 3.3 |
| ZnO loss/% | 0.97 | 0.74 | 1.63 | 0.82 |
| Au Loading | 0 | 0.1 mol% | 0.2 mol% | 0.3 mol% |
|---|---|---|---|---|
| Yield after 1 h illumination/µM | 1117.97 | 1160.00 | 1281.93 | 1266.59 |
| kF/µM min−1 | 18.633 | 23.496 | 33.213 | 32.586 |
| kD/min−1 | 0 | 0.0137 | 0.0118 | 0.0134 |
| ξ/% | 3.41 | 4.30 | 6.08 | 5.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, X.; Skillen, N.; Bahnemann, D.W.; Rooney, D.W.; Robertson, P.K.J. Photocatalytic H2O2 Generation Using Au-Ag Bimetallic Alloy Nanoparticles loaded on ZnO. Catalysts 2022, 12, 939. https://doi.org/10.3390/catal12090939
Pang X, Skillen N, Bahnemann DW, Rooney DW, Robertson PKJ. Photocatalytic H2O2 Generation Using Au-Ag Bimetallic Alloy Nanoparticles loaded on ZnO. Catalysts. 2022; 12(9):939. https://doi.org/10.3390/catal12090939
Chicago/Turabian StylePang, Xinzhu, Nathan Skillen, Detlef W. Bahnemann, David W. Rooney, and Peter K. J. Robertson. 2022. "Photocatalytic H2O2 Generation Using Au-Ag Bimetallic Alloy Nanoparticles loaded on ZnO" Catalysts 12, no. 9: 939. https://doi.org/10.3390/catal12090939
APA StylePang, X., Skillen, N., Bahnemann, D. W., Rooney, D. W., & Robertson, P. K. J. (2022). Photocatalytic H2O2 Generation Using Au-Ag Bimetallic Alloy Nanoparticles loaded on ZnO. Catalysts, 12(9), 939. https://doi.org/10.3390/catal12090939








