Highlighting the Implantation of Metal Particles into Hollow Cavity Yeast-Based Carbon for Improved Electrochemical Performance of Lithium–Sulfur Batteries
Abstract
:1. Introduction
2. Results and Discussion
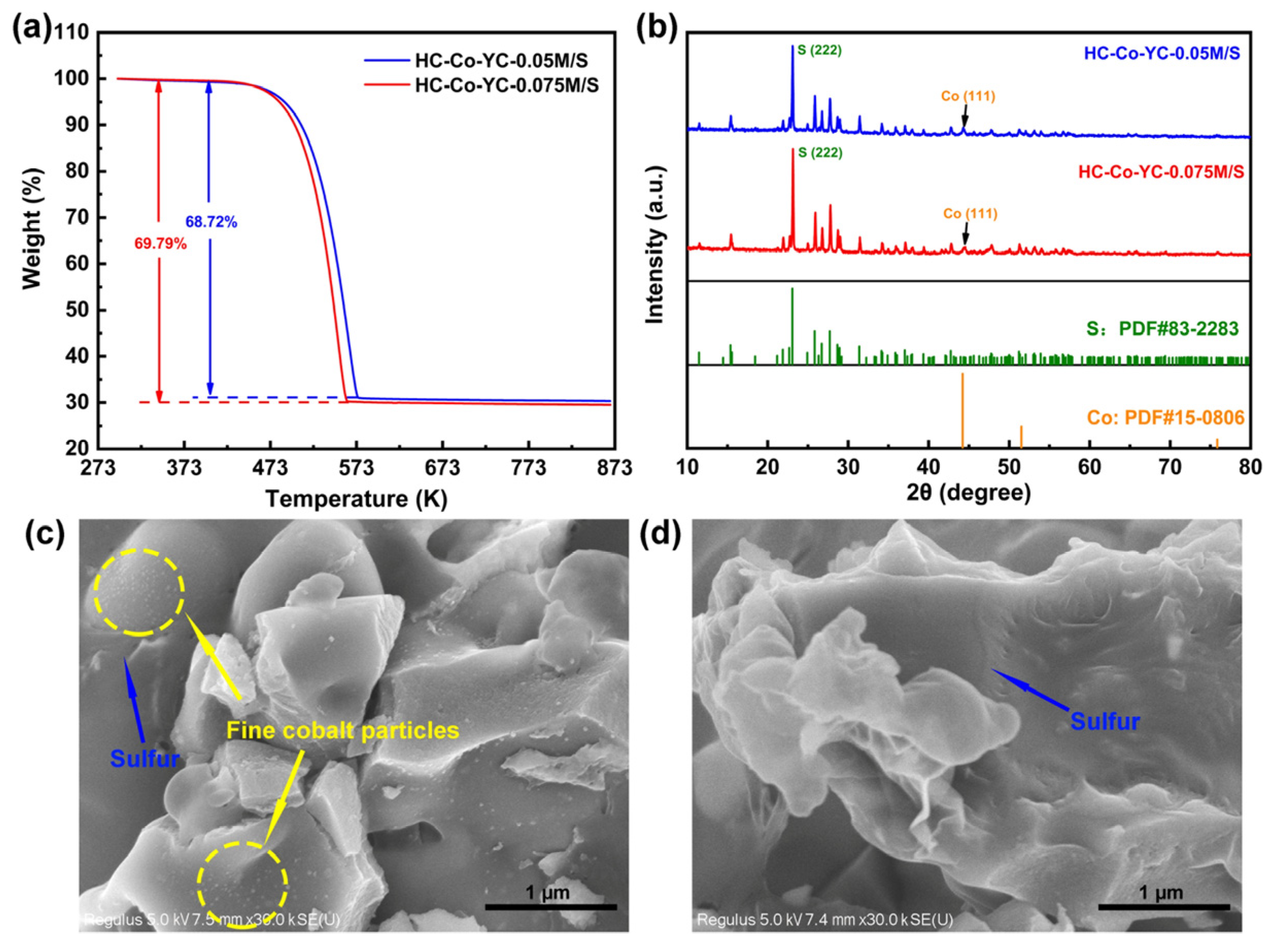
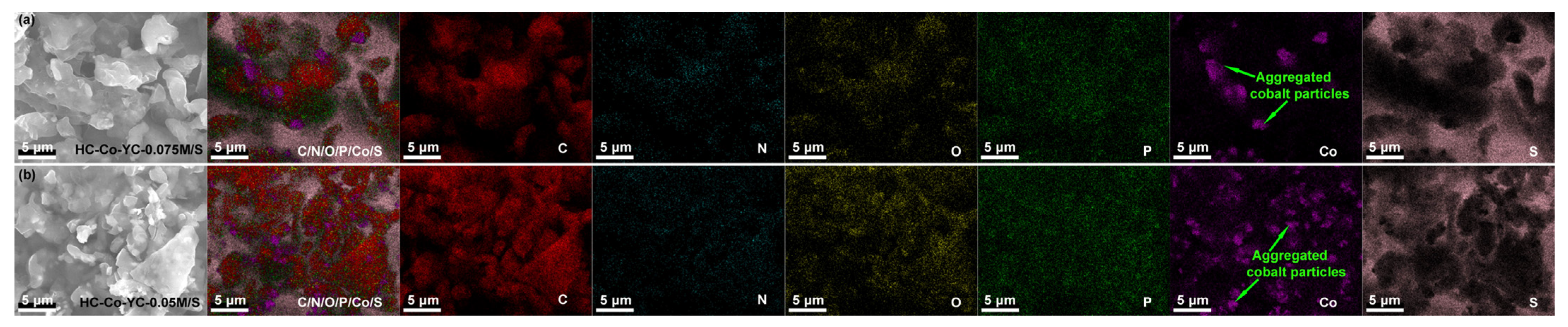
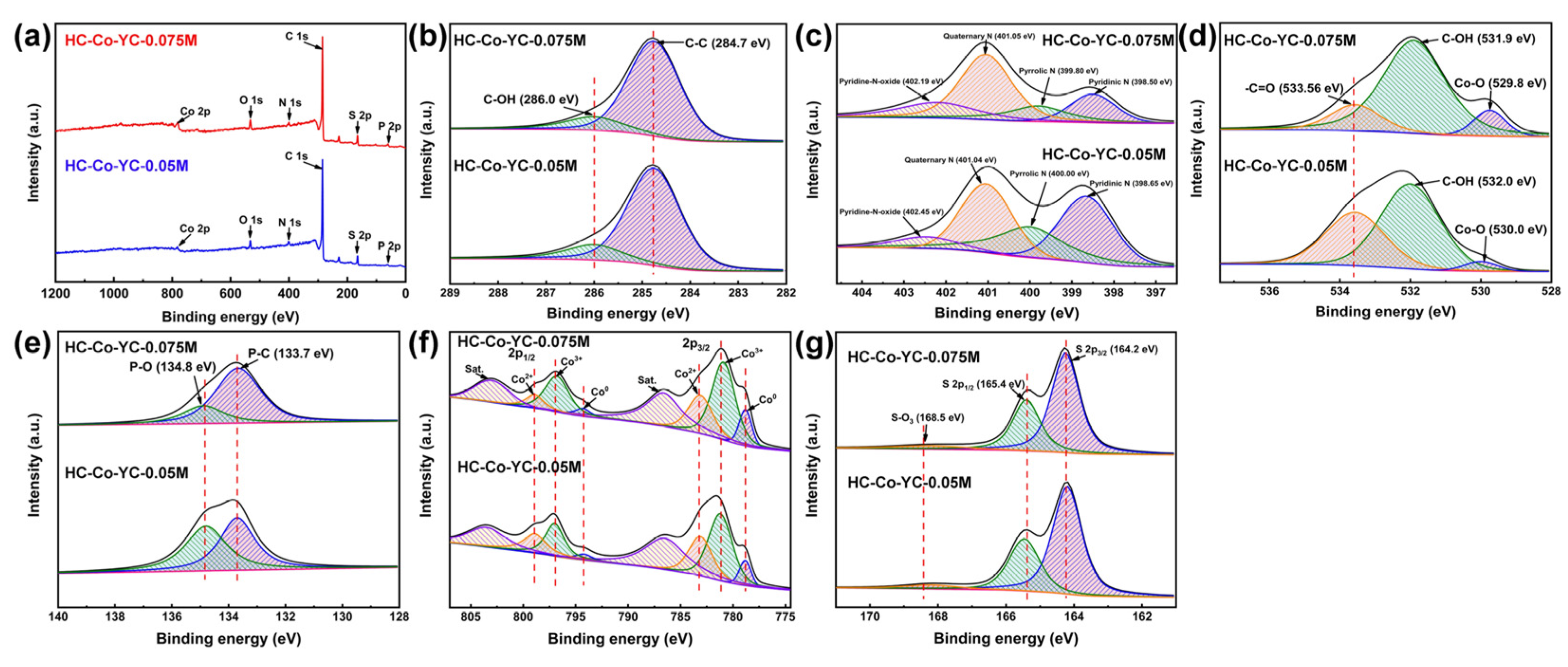
2.1. Microstructure Characterizations
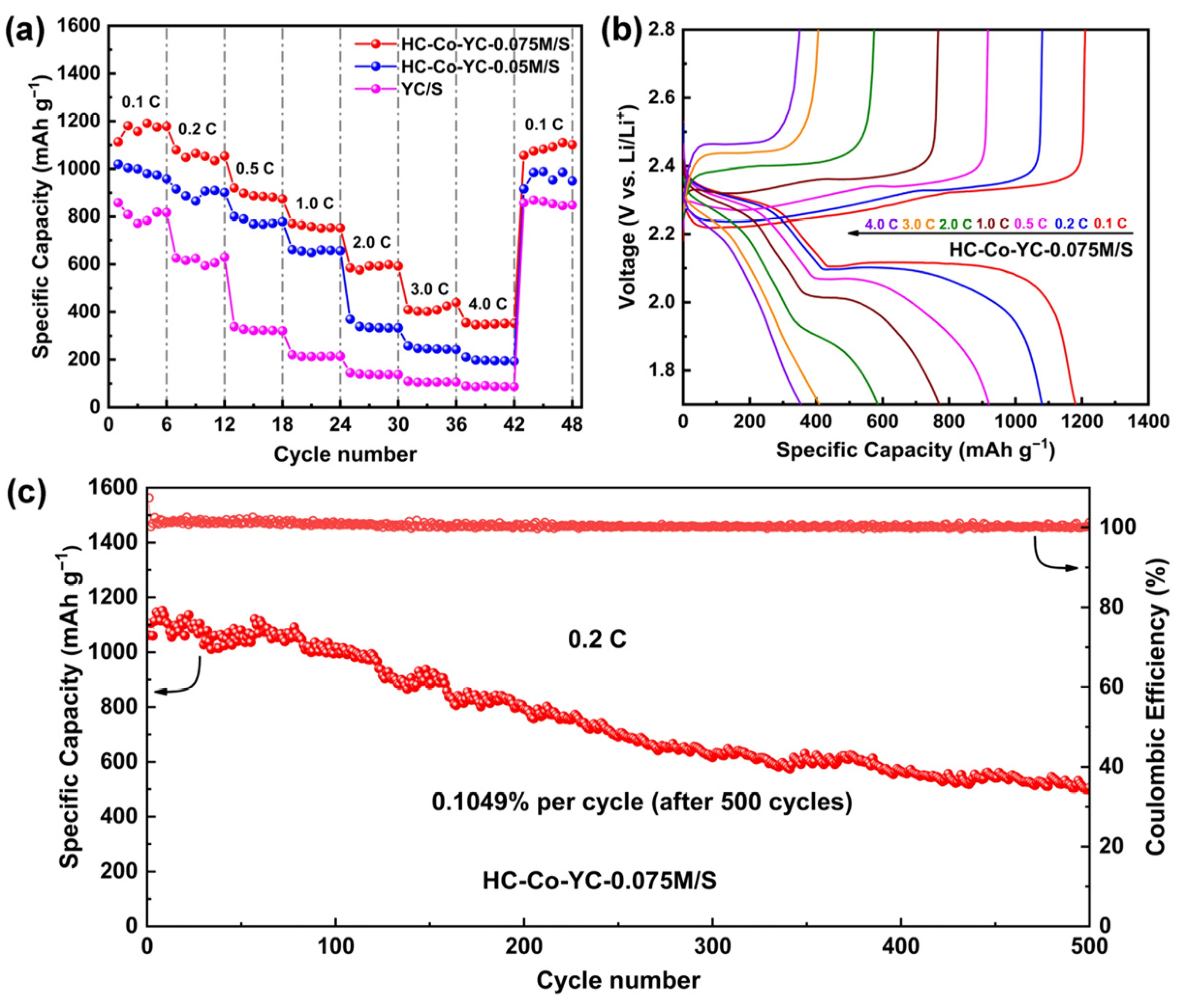
2.2. Electrochemical Performance
3. Experiment Section
3.1. Preparation of HC–Co–YC
3.2. Preparation of Three HC–Co–YC/S Composites
3.3. Material Characterization
3.4. Electrochemical Characterization
3.5. Experiment of the Li2S Nucleation
3.6. Catalytic Studies of Polysulfide Conversion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fang, R.; Zhao, S.; Sun, Z.; Wang, D.W.; Cheng, H.M.; Li, F. More reliable lithium-sulfur batteries: Status, solutions and prospects. Adv. Mater. 2017, 29, 1606823. [Google Scholar] [CrossRef] [PubMed]
- Service, R.F. A boost for lithium-sulfur batteries. Science 2013, 339, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2011, 11, 19–29. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. A progress report on metal-sulfur batteries. Adv. Funct. Mater. 2020, 30, 2004084. [Google Scholar] [CrossRef]
- Jana, M.; Xu, R.; Cheng, X.B.; Yeon, J.S.; Park, J.M.; Huang, J.Q.; Zhang, Q.; Park, H.S. Rational design of two-dimensional nanomaterials for lithium-sulfur batteries. Energy Environ. Sci. 2020, 13, 1049–1075. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. Electrode-electrolyte interfaces in lithium-sulfur batteries with liquid or inorganic solid electrolytes. Acc. Chem. Res. 2017, 50, 2653–2660. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.Y.; Xiao, R.J.; Gu, L.; Guo, Y.G.; Wen, R.; Wan, L.J. Interfacial mechanism in lithium-sulfur batteries: How salts mediate the structure evolution and dynamics. J. Am. Chem. Soc. 2018, 140, 8147–8155. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhou, G.; Shi, F.; Zhou, D.; Zhao, J.; Liu, K.; Liu, Y.; Zu, C.; Chen, W.; Zhang, R.; et al. Reactivation of dead sulfide species in lithium polysulfide flow battery for grid scale energy storage. Nat. Commun. 2017, 8, 462. [Google Scholar] [CrossRef]
- Deng, R.; Wang, M.; Yu, H.; Luo, S.; Li, J.; Chu, F.; Liu, B.; Wu, F. Recent advances and applications toward emerging lithium-sulfur batteries: Working principles and opportunities. Energy Environ. Mater. 2022, 5, 777–799. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Q.; Hao, S.M.; Deng, S.; He, Q.; Lin, Z.; Yang, Y. Polymers in lithium-sulfur batteries. Adv. Sci. 2022, 9, 2103798. [Google Scholar] [CrossRef]
- Chen, J.; Feng, W.; Zhao, W.; Shi, Z. Transition metal phosphide composite with metal-organic framework and carbon nanotubes for high-performance lithium-sulfur batteries. J. Alloys Compd. 2022, 890, 161794. [Google Scholar] [CrossRef]
- Feng, W.; Chen, J.; Niu, Y.; Zhao, W.; Zhang, L. CeO2 composite metal organic framework is used to construct high-performance lithium-sulfur batteries. J. Alloys Compd. 2022, 906, 164341. [Google Scholar] [CrossRef]
- Feng, W.; Yang, H.; Pu, Z.; Zhang, L. Study of CNTs-MoS2/CeO2 composites for lithium-sulfur battery performance. Ionics 2022, 28, 2781–2791. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Na, S.; Chen, C.; Yu, T.; Wang, H.; Zang, H. Porous hollow carbon nanospheres embedded with well-dispersed cobalt monoxide nanocrystals as effective polysulfide reservoirs for high-rate and long-cycle lithium-sulfur batteries. J. Mater. Chem. A 2017, 5, 17352–17359. [Google Scholar] [CrossRef]
- Barghamadi, M.; Best, A.S.; Bhatt, A.I.; Hollenkamp, A.F.; Musameh, M.; Rees, R.J.; Rüther, T. Lithium-sulfur batteries-the solution is in the electrolyte, but is the electrolyte a solution? Energy Environ. Sci. 2014, 7, 3902–3920. [Google Scholar] [CrossRef]
- Peng, H.J.; Huang, J.Q.; Cheng, X.B.; Zhang, Q. Review on high-loading and high-energy lithium-sulfur batteries. Adv. Energy Mater. 2017, 7, 1700260. [Google Scholar] [CrossRef]
- Zhao, M.; Li, B.Q.; Zhang, X.Q.; Huang, J.Q.; Zhang, Q. A perspective toward practical lithium-sulfur batteries. ACS Cent. Sci. 2020, 26, 1095–1104. [Google Scholar] [CrossRef]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Review-The importance of chemical interactions between sulfur host materials and lithium polysulfides for advanced lithium-sulfur batteries. J. Electrochem. Soc. 2015, 162, A2567–A2576. [Google Scholar] [CrossRef]
- Tsao, Y.; Gong, H.; Chen, S.; Chen, G.; Liu, Y.; Gao, T.Z.; Cui, Y.; Bao, Z. A nickel-decorated carbon flower/sulfur cathode for lean-electrolyte lithium-sulfur batteries. Adv. Energy Mater. 2021, 11, 2101449. [Google Scholar] [CrossRef]
- Wang, J.; Han, W.Q. A review of heteroatom doped materials for advanced lithium-sulfur batteries. Adv. Funct. Mater. 2022, 32, 2107166. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, T.; Liu, Y.; Nai, J.; Wang, Y.; Zhang, W.; Tao, X. A review of biomass materials for advanced lithium sulfur batteries. Chem. Sci. 2019, 10, 7484–7495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, B.; Zhang, L.; Li, W.; Li, S.; Zhang, J.; Jiang, G.; Cui, Z.; Song, H.; Grundish, N.; et al. Co4N-decorated 3D wood-derived carbon host enables enhanced cathodic electrocatalysis and homogeneous lithium deposition for lithium-sulfur full cells. Small 2022, 18, 2105664. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.V.; Jo, K.; Kim, C.H.; Kim, J.H.; Lee, H.J.; Lee, S.M. Activated carbon textile via chemistry of metal extraction for supercapacitors. ACS Nano 2016, 10, 11351–11359. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xia, X.; Deng, S.; Xie, D.; Shen, S.; Zhang, K.; Guo, W.; Wang, X.; Tu, J. Spore carbon from aspergillus oryzae for advanced electrochemical energy storage. Adv. Mater. 2018, 30, 1805165. [Google Scholar] [CrossRef]
- Huang, L.; Shen, S.; Zhong, Y.; Zhang, Y.; Zhang, L.; Wang, X.; Xia, X.; Tong, X.; Zhou, J.; Tu, J. Multifunctional hyphae carbon powering lithium-sulfur batteries. Adv. Mater. 2022, 34, 2107415. [Google Scholar] [CrossRef]
- Zhuang, Y.; Ma, J.; Chen, J.; Shi, Z.; Leng, F.; Feng, W. Fabrication and electrochemical applications of the Co-embedded N&P-codoped hierarchical porous carbon host from yeast for Li-S batteries. Appl. Surf. Sci. 2021, 545, 148936. [Google Scholar]
- Xie, Y.P.; Cheng, H.W.; Chai, W.; Yue, H.; Zhang, X.; Fang, J.H.; Zhao, H.B.; Xu, J.Q. Natural nitrogen-doped multiporous carbon from biological cells as sulfur stabilizers for lithium-sulfur batteries. Chin. Chem. Lett. 2017, 28, 738–742. [Google Scholar] [CrossRef]
- Xia, Y.; Zhong, H.; Fang, R.; Liang, C.; Xiao, Z.; Huang, H.; Gan, Y.; Zhang, J.; Tao, X.; Zhang, W. Biomass derived Ni(OH)2 @porous carbon/sulfur composites synthesized by a novel sulfur impregnation strategy based on supercritical CO2 technology for advanced Li-S batteries. J. Power Sources 2018, 378, 73–80. [Google Scholar] [CrossRef]
- Liang, S.; Chen, J.; He, X.; Liu, L.; Zhou, N.; Hu, L.; Wang, L.; Liang, D.; Yu, T.; Tian, C.; et al. N-doped porous carbon microspheres derived from yeast as lithium sulfide hosts for advanced lithium-ion batteries. Processes 2021, 9, 1822. [Google Scholar] [CrossRef]
- Li, Y.; Cai, Y.; Cai, Z.; Xu, J.; Sonamuthu, J.; Zhu, G.; Militky, J.; Jin, W.; Yao, J. Sulfur-infiltrated yeast-derived nitrogen-rich porous carbon microspheres @ reduced graphene cathode for high-performance lithium-sulfur batteries. Electrochim. Acta. 2018, 285, 317–325. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Shen, S.; Xia, X.; Zhong, Y.; Deng, S.; Xie, D.; Liu, B.; Zhang, Y.; Pan, G.; Wang, X.; Tu, J. Implanting niobium carbide into Trichoderma spore carbon: A new advanced host for sulfur cathodes. Adv. Mater. 2019, 31, 1900009. [Google Scholar] [CrossRef] [PubMed]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Nie, B.; Zhou, C.; Gao, M.; He, D.; Yao, Z.; Liu, Y.; Shen, X.; Wang, X.; Pan, H. Bi-structural fibers of carbon nanotube coated with nitrogen/oxygen dual-doped porous carbon layer as superior sulfur host for lithium-sulfur batteries. J. Alloys Compd. 2019, 707, 1205–1215. [Google Scholar] [CrossRef]
- Li, X.; Cheng, X.; Gao, M.; Ren, D.; Liu, Y.; Guo, Z.; Shang, C.; Sun, L.; Pan, H. Amylose derived macro-hollow core and micro-porous shell carbon spheres as sulfur host for superior lithium-sulfur battery cathodes. ACS Appl. Mater. Interfaces 2017, 9, 10717–10729. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Zhang, Y.; Wang, Y.; Zhao, Y. Synthesis of CoO nanocrystals decorated porous carbon nanotube microspheres as sulfur host for high performance Li/S batteries. Nanotechnology 2020, 31, 025403. [Google Scholar] [CrossRef]
- Wu, W.; Pu, J.; Wang, J.; Shen, Z.; Tang, H.; Deng, Z.; Tao, X.; Pan, F.; Zhang, H. Biomimetic bipolar microcapsules derived from staphylococcus aureus for enhanced properties of lithium-sulfur battery cathodes. Adv. Energy Mater. 2018, 8, 1702373. [Google Scholar] [CrossRef]
- Kim, J.; Young, C.; Lee, J.; Heo, Y.U.; Park, M.S.; Hossain, M.S.A.; Yamauchi, Y.; Kim, J.H. Nanoarchitecture of MOF-derived nanoporous functional composites for hybrid supercapacitors. J. Mater. Chem. A 2017, 5, 15065–15072. [Google Scholar] [CrossRef]
- Pang, Q.; Tang, J.; Huang, H.; Liang, X.; Hart, C.; Tam, K.C.; Nazar, L.F. A nitrogen and sulfur dual-doped carbon derived from polyrhodanine@cellulose for advanced lithium-sulfur batteries. Adv. Mater. 2015, 27, 6021–6028. [Google Scholar] [CrossRef]
- Xing, L.B.; Xi, K.; Li, Q.; Su, Z.; Lai, C.; Zhao, X.; Kumar, R.V. Nitrogen, sulfur-codoped graphene sponge as electroactive carbon interlayer for high-energy and -power lithium-sulfur batteries. J. Power Sources 2016, 303, 22–28. [Google Scholar] [CrossRef]
- Zhang, J.; Li, G.; Zhang, Y.; Zhang, W.; Wang, X.; Zhao, Y.; Li, J.; Chen, Z. Vertically rooting multifunctional tentacles on carbon scaffold as efficient polysulfide barrier toward superior lithium-sulfur batteries. Nano Energy 2019, 64, 103905. [Google Scholar] [CrossRef]
- Chen, Y.; Elangovan, A.; Zeng, D.; Zhang, Y.; Ke, H.; Li, J.; Sun, Y.; Cheng, H. Vertically aligned carbon nanofibers on Cu foil as a 3D current collector for reversible Li plating/stripping toward high-performance Li-S batteries. Adv. Funct. Mater. 2019, 29, 1906444. [Google Scholar] [CrossRef]
- Su, L.; Zhang, J.; Chen, Y.; Yang, W.; Wang, J.; Ma, Z.; Shao, G.; Wang, G. Cobalt-embedded hierarchically-porous hollow carbon microspheres as multifunctional confined reactors for high-loading Li-S batteries. Nano Energy 2021, 85, 105981. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Uchaker, E.; Zhang, M.; Tian, J.; Liu, X.; Cao, G. Sn-doped V2O5 film with enhanced lithium-ion storage performance. J. Phys. Chem. C 2013, 117, 23507–23514. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Z.; Tian, W.; Chen, W.; Xi, B.; Li, H.; Feng, J.; Xiong, S. Ni12P5 nanoparticles bound on graphene sheets for advanced lithium-sulfur batteries. Nanoscale 2020, 12, 10760–10770. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Zhang, B.; Wang, W.; Zhang, G.; Seh, Z.W.; Zhang, N.; Sun, J.; Huang, L.; Jiang, J.; et al. Fast conversion and controlled deposition of lithium (poly)sulfides in lithium-sulfur batteries using high-loading cobalt single atoms. Energy Storage Mater. 2020, 30, 250–259. [Google Scholar] [CrossRef]
- He, J.; Hartmann, G.; Lee, M.; Hwang, G.S.; Chen, Y.; Manthiram, A. Freestanding 1T MoS2/graphene heterostructures as a highly efficient electrocatalyst for lithium polysulfides in Li-S batteries. Energy Environ. Sci. 2019, 12, 344–350. [Google Scholar] [CrossRef]
- Yu, M.; Zhou, S.; Wang, Z.; Wang, Y.; Zhang, N.; Wang, S.; Zhao, J.; Qiu, J. Accelerating polysulfide redox conversion on bifunctional electrocatalytic electrode for stable Li-S batteries. Energy Storage Mater. 2019, 20, 98–107. [Google Scholar] [CrossRef]
- Li, Y.; Lei, X.; Yuan, Y.; Wu, S.; Han, B.; Liu, X.; Liu, W.; Hu, J.; Yang, C.; Lin, Z.; et al. Fe2P-decorated N,P codoped carbon synthesized via direct biological recycling for endurable sulfur encapsulation. ACS Cent. Sci. 2020, 6, 1827–1834. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, J.; Wei, Z.; Yang, H.; Ma, Z.; Ma, R.; Zhou, J.; Yang, Y.; Peng, L.; Fei, H.; et al. Bacteria-derived biological carbon building robust Li-S batteries. Nano Lett. 2019, 19, 4384–4390. [Google Scholar] [CrossRef]
- Tao, X.; Wang, J.; Liu, C.; Wang, H.; Yao, H.; Zheng, G.; She, Z.W.; Cai, Q.; Li, W.; Zhou, G.; et al. Balancing surface adsorption and diffusion of lithium-polysulfides on nonconductive oxides for lithium-sulfur battery design. Nat. Commun. 2016, 7, 11203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhao, Z.; Hou, Y.N.; Tang, Y.; Liang, J.; Liu, X.; Zhang, Z.; Wang, X.; Qiu, J. Highly stable lithium-sulfur batteries based on p-n heterojunctions embedded on hollow sheath carbon propelling polysulfides conversion. J. Mater. Chem. A 2019, 7, 9230–9240. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Zheng, Y.; Huang, R.; Yao, J. Facile and efficient synthesis of α-Fe2O3 nanocrystals by glucose-assisted thermal decomposition method and its application in lithium ion batteries. J. Power Sources 2019, 416, 62–71. [Google Scholar] [CrossRef]
- Liang, J.; Sun, Z.H.; Li, F.; Cheng, H.M. Carbon materials for Li-S batteries: Functional evolution and performance improvement. Energy Storage Mater. 2016, 2, 76–106. [Google Scholar] [CrossRef]
- Fan, F.Y.; Carter, W.C.; Chiang, Y.-M. Mechanism and kinetics of Li2S precipitation in lithium-sulfur batteries. Adv. Mater. 2015, 27, 5203–5209. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Zhu, L.; Zhou, J.; Guan, Y.; Chen, L.; Niu, S.; Cai, J.; Sun, D.; Zhu, Y.; et al. Deciphering the modulation essence of p bands in Co-based compounds on Li-S chemistry. Joule 2018, 2, 2681–2693. [Google Scholar] [CrossRef] [Green Version]



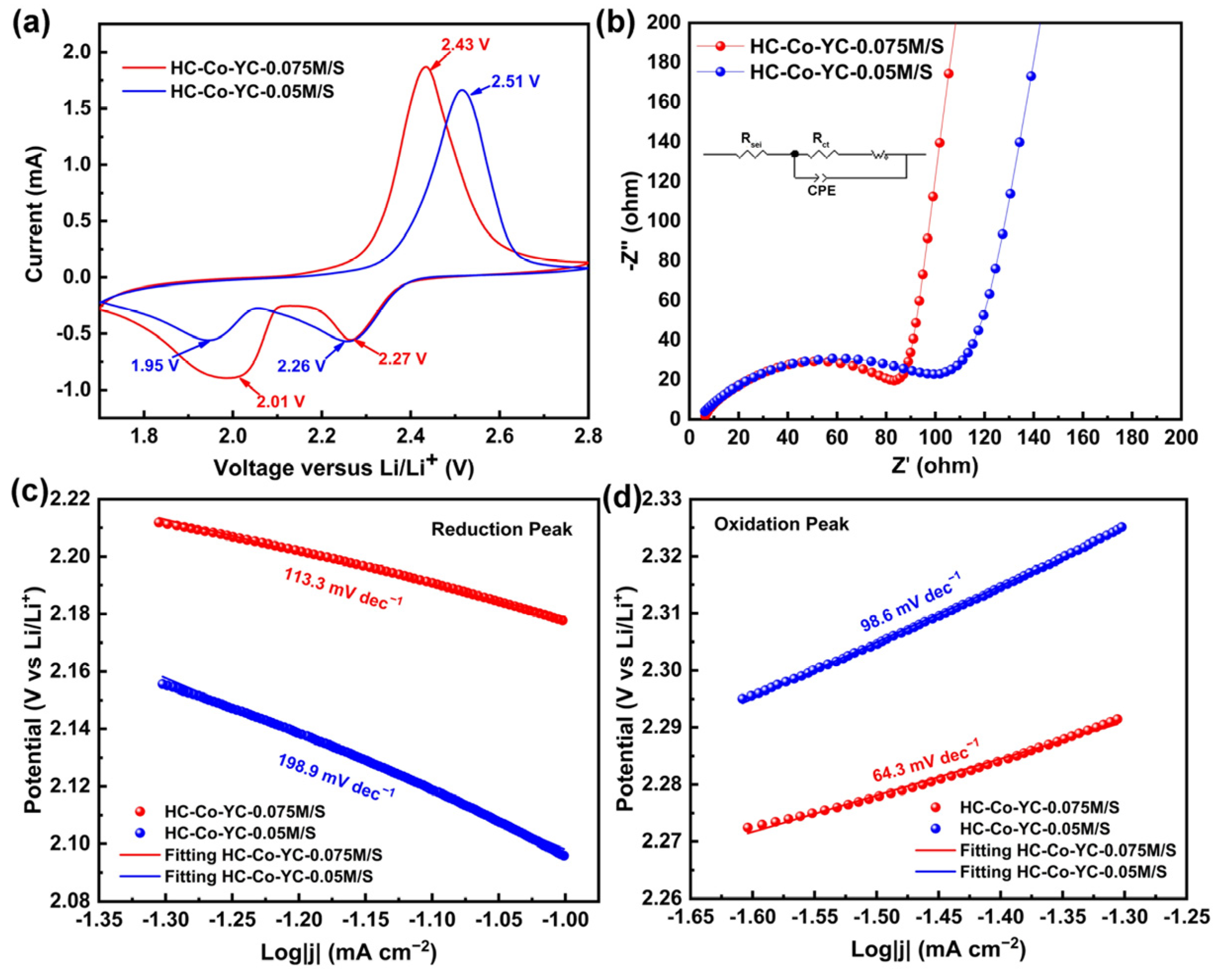

| Electrodes | Sulfur Loading (mg cm−2) | Rsei (Ω) | Rct (Ω) | Ref. |
|---|---|---|---|---|
| LSB–D | 2 | 24.48 | 161.6 | [25] |
| HYC/S | 1.4–2.0 | 6.525 | 456.4 | [30] |
| rGO@HYC/S | 1.4–2.0 | 5.733 | 115.5 | [30] |
| S/NPC | 2.6 | 8.758 | 123.1 | [49] |
| GOC@NPBCS | 2–3 | 3.2 | 128 | [50] |
| YC/S | 1–1.5 | − | 229.5 | [26] |
| HC–Co–YC–0.075M/S | 1–1.5 | 5.231 | 88.2 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, Y.; Ma, J.-L.; Feng, W.-J. Highlighting the Implantation of Metal Particles into Hollow Cavity Yeast-Based Carbon for Improved Electrochemical Performance of Lithium–Sulfur Batteries. Catalysts 2022, 12, 951. https://doi.org/10.3390/catal12090951
Zhuang Y, Ma J-L, Feng W-J. Highlighting the Implantation of Metal Particles into Hollow Cavity Yeast-Based Carbon for Improved Electrochemical Performance of Lithium–Sulfur Batteries. Catalysts. 2022; 12(9):951. https://doi.org/10.3390/catal12090951
Chicago/Turabian StyleZhuang, Yan, Jing-Lin Ma, and Wang-Jun Feng. 2022. "Highlighting the Implantation of Metal Particles into Hollow Cavity Yeast-Based Carbon for Improved Electrochemical Performance of Lithium–Sulfur Batteries" Catalysts 12, no. 9: 951. https://doi.org/10.3390/catal12090951





