Heterostructured Co2P Nanocomposite Embedded in a N, P Co-Doped Carbon Layer as a High Performance Electrocatalyst for Overall Water Splitting
Abstract
:1. Introduction
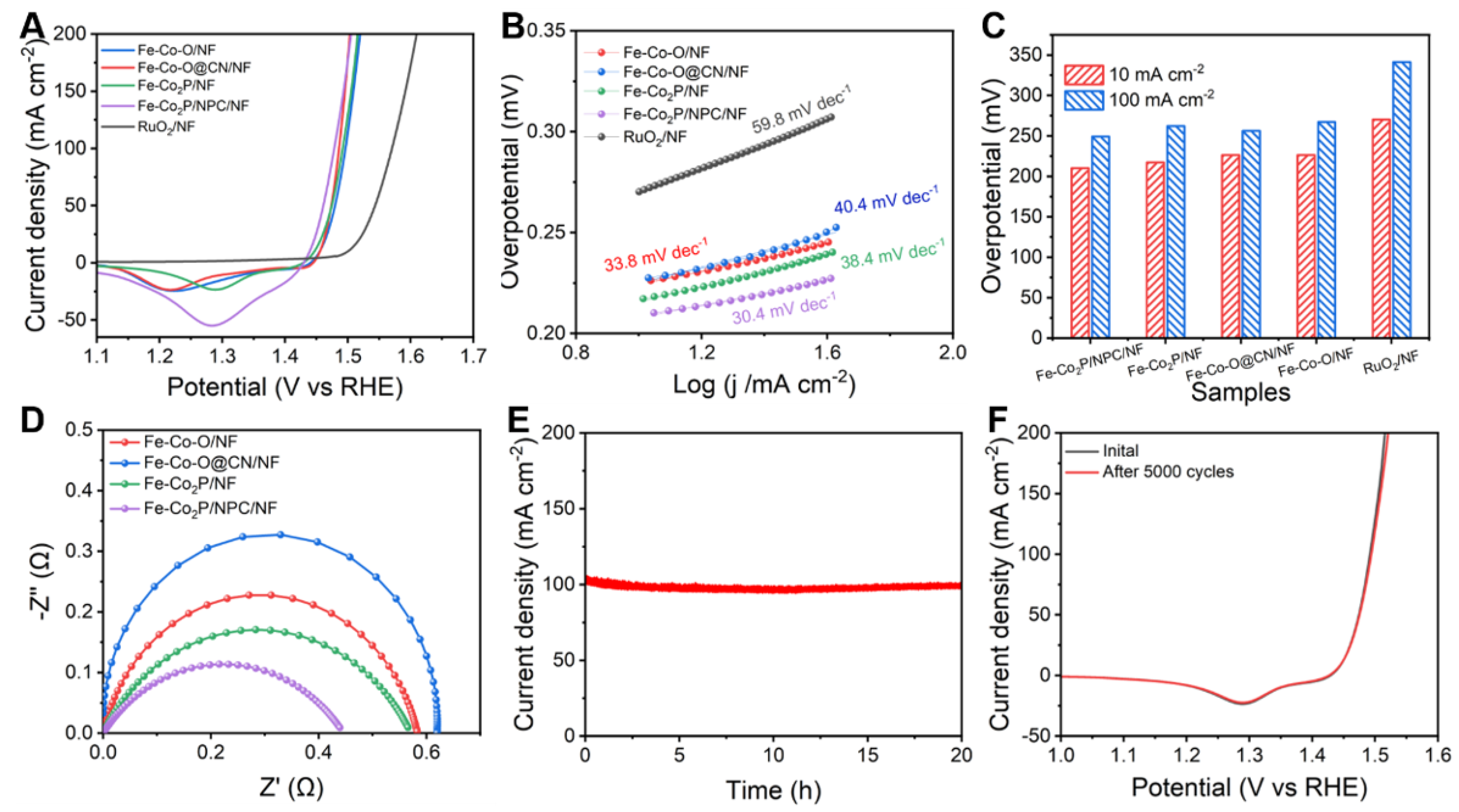
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Characterization Methods
3.2. Materials Preparation
3.3. HER and OER Electrochemical Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Chang, C.; Teng, F.; Zhao, Y.F.; Chen, G.B.; Shi, R.; Waterhouse, G.I.N.; Huang, W.F.; Zhang, T.R. Defect-engineered ultrathin delta-MnO2 nanosheet arrays as bifunctional electrodes for efficient overall water splitting. Adv. Energy Mater. 2017, 7, 1700005. [Google Scholar] [CrossRef]
- Wu, D.; Wei, Y.C.; Ren, X.; Ji, X.Q.; Liu, Y.W.; Guo, X.D.; Liu, Z.; Asiri, A.M.; Wei, Q.; Sun, X.P. Co(OH)2 nanoparticle-encapsulating conductive nanowires array: Room-temperature electrochemical preparation for high-performance water oxidation electrocatalysis. Adv. Mater. 2018, 30, 1705366. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, X.; Zhou, C.; Du, S.; Zhen, D.; Chen, B.; Li, J.; Wu, Q.; Iru, Y.; Chen, D. A modulated electronic state strategy designed to integrate active HER and OER components as hybrid heterostructures for efficient overall water splitting. Appl. Catal. B Environ. 2020, 260, 118197. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, Y.; Wu, H.; Gao, Y.; Chen, Z.; Jin, W.; Wang, J.; Ma, T.; Wang, L. Corrosion engineering on iron foam toward efficiently electrocatalytic overall water splitting powered by sustainable energy. Adv. Funct. Mater. 2021, 31, 2010437. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Jia, X.D.; Chen, G.B.; Shang, L.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; O’Hare, D.; Zhang, T.R. Ultrafine NiO nanosheets stabilized by TiO2 from monolayer NiTi-LDH precursors: An active water oxidation electrocatalyst. J. Am. Chem. Soc. 2016, 138, 6517–6524. [Google Scholar] [CrossRef]
- Lu, Z.; Zhu, W.; Yu, X.; Zhang, H.; Li, Y.; Sun, X.; Wang, X.; Wang, H.; Wang, J.; Luo, J.; et al. Ultrahigh hydrogen evolution performance of under-water “superaerophobic” MoS2 nanostructured electrodes. Adv. Mater. 2014, 26, 2683–2687. [Google Scholar] [CrossRef]
- Cao, B.; Cheng, Y.; Hu, M.; Jing, P.; Ma, Z.; Liu, B.; Gao, R.; Zhang, J. Efficient and durable 3D self-supported nitrogen-doped carbon-coupled nickel/cobalt phosphide electrodes: Stoichiometric ratio regulated phase- and morphology-dependent overall water splitting performance. Adv. Funct. Mater. 2019, 29, 29. [Google Scholar] [CrossRef]
- Liu, X.; Gong, M.; Deng, S.; Zhao, T.; Shen, T.; Zhang, J.; Wang, D. Transforming damage into benefit: Corrosion engineering enabled electrocatalysts for water splitting. Adv. Funct. Mater. 2021, 31, 2009032. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Liu, P.; Liao, Z.; Liu, S.; Zhuang, X.; Chen, M.; Zschech, E.; Feng, X. Efficient hydrogen production on MoNi4 electrocatalysts with fast water dissociation kinetics. Nat. Commun. 2017, 8, 15437. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Chang, C.; Hsu, C.W.; Chang, C.W.; Lu, S.Y. Hollow nanocubes composed of well-dispersed mixed metal-rich phosphides in N-doped carbon as highly efficient and durable electrocatalysts for the oxygen evolution reaction at high current densities. J. Mater. Chem. A 2017, 5, 19656–19663. [Google Scholar] [CrossRef]
- Yan, G.; Wu, C.X.; Tan, H.Q.; Feng, X.J.; Yan, L.K.; Zang, H.Y.; Li, Y.G. N-Carbon coated P-W2C composite as efficient electrocatalyst for hydrogen evolution reactions over the whole pH range. J. Mater. Chem. A 2017, 5, 765–772. [Google Scholar] [CrossRef]
- Yu, F.; Yu, L.; Mishra, I.; Yu, Y.; Ren, Z.; Zhou, H. Recent developments in earth abundant and non-noble electrocatalysts for water electrolysis. Mater. Today Phys. 2018, 7, 121–138. [Google Scholar] [CrossRef]
- Li, D.; Baydoun, H.; Kulikowski, B.; Brock, S.L. Boosting the catalytic performance of iron phosphide nanorods for the oxygen evolution reaction by incorporation of manganese. Chem. Mater. 2017, 29, 3048–3054. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, L.; Sun, L.; Chen, G.; Luo, Q.; Xin, H.; Peng, J.; Li, Y.; Ma, F. Sulfur doping enhanced desorption of intermediates on NiCoP for efficient alkaline hydrogen evolution. Nanoscale 2020, 12, 1985–1993. [Google Scholar] [CrossRef]
- Zhang, S.S.; Guo, M.J.; Song, S.Y.; Zhan, K.; Yan, Y.; Yang, J.H.; Zhao, B. Hierarchical Mo-doped CoP3 interconnected nanosheet arrays on carbon cloth as an efficient bifunctional electrocatalyst for water splitting in an alkaline electrolyte. Dalton Trans. 2020, 49, 5563. [Google Scholar] [CrossRef]
- Guo, M.J.; Song, S.Y.; Zhang, S.S.; Yan, Y.; Zhan, K.; Yang, J.H.; Zhao, B. Fe-Doped Ni−Co Phosphide Nanoplates with Planar Defects as an Efficient Bifunctional Electrocatalyst for Overall Water Splitting. ACS Sustain. Chem. Eng. 2020, 8, 7436. [Google Scholar] [CrossRef]
- Xu, Y.L.; Wang, R.; Wang, J.Y.; Zhang, Y.R.; Jiao, T.F. Encapsulation of Fe-CoP with P, N-co-doped porous carbon matrix as a multifunctional catalyst for wide electrochemical applications. J. Energy Chem. 2022, 71, 36. [Google Scholar] [CrossRef]
- Wang, X.; Kolen’ko, Y.V.; Bao, X.Q.; Kovnir, K.; Liu, L. One-step synthesis of self-supported nickel phosphide nanosheet array cathodes for efficient electrocatalytic hydrogen generation. Angew. Chem. Int. Ed. 2015, 127, 8306–8310. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Z.; Jiao, L. Multifunctional transition metal-based phosphides in energy-related electrocatalysis. Adv. Energy Mater. 2020, 10, 1902104. [Google Scholar] [CrossRef]
- Menezes, P.W.; Indra, A.; Das, C.; Walter, C.; Göbel, C.; Gutkin, V.; Schmeiβer, D.; Driess, M. Uncovering the nature of active species of nickel phosphide catalysts in high-performance electrochemical overall water splitting. ACS Catal. 2017, 7, 103–109. [Google Scholar] [CrossRef]
- Lv, Z.; Tahir, M.; Lang, X.W.; Yuan, G.; Pan, L.; Zhang, X.W.; Zou, J.J. Well-dispersed molybdenum nitrides on a nitrogen-doped carbon matrix for highly efficient hydrogen evolution in alkaline media. J. Mater. Chem. A 2017, 5, 20932–20937. [Google Scholar] [CrossRef]
- Li, W.; Xiong, D.H.; Gao, X.F.; Liu, L.F. The oxygen evolution reaction enabled by transition metal phosphide and chalcogenide pre-catalysts with dynamic changes. Chem. Commun. 2019, 55, 8744–8763. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Y.; Li, J.J.; Xiong, D.H.; Zhang, B.S.; Liu, Y.F.; Wu, K.H.; Amorim, I.; Li, W.; Liu, L.F. Trends in activity for the oxygen evolution reaction on transition metal (M = Fe, Co, Ni) phosphide pre-catalysts. Chem. Sci. 2018, 9, 3470–3476. [Google Scholar] [CrossRef]
- Li, W.; Gao, X.F.; Wang, X.G.; Xiong, D.H.; Huang, P.P.; Song, W.G.; Bao, X.Q.; Liu, L.F. From water reduction to oxidation: Janus Co-Ni-P nanowires as high-efficiency and ultrastable electrocatalysts for over 3000h water splitting. J. Power Sources 2016, 330, 156–166. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Li, A.; Zheng, X.; Peng, L.; Huang, J.; Deng, Z.; Chen, H.; Wei, Z. Rational construction of macroporous CoFeP triangular plate arrays from bimetaleorganic frameworks as high-performance overall water-splitting catalysts. J. Mater. Chem. 2019, 7, 17529–17535. [Google Scholar] [CrossRef]
- Amorim, I.; Xu, J.; Zhang, N.; Xiong, D.; Thalluri, S.M.; Thomas, R.; Sousa, J.P.S.; Araújo, A.; Li, H.; Liu, L. Bi-metallic cobalt-nickel phosphide nanowires for electrocatalysis of the oxygen and hydrogen evolution reactions. Catal. Today 2020, 358, 196–202. [Google Scholar] [CrossRef]
- Wang, R.; Cheng, Q.; Mao, C.; Su, W.; Yang, L.; Wang, G.; Zou, L.; Shi, Y.; Yan, C.; Zou, Z.; et al. Regulation of oxygen vacancy within oxide pyrochlores by F-doping to boost oxygen-evolution activity. J. Power Sources 2021, 502, 229903. [Google Scholar] [CrossRef]
- Wu, C.; Yang, Y.; Dong, D.; Zhang, Y.; Li, J. In Situ Coupling of CoP Polyhedrons and Carbon Nanotubes as Highly Efficient Hydrogen Evolution Reaction Electrocatalyst. Small 2017, 13, 1602873. [Google Scholar] [CrossRef]
- Wang, R.; Dong, X.Y.; Du, J.; Zhao, J.Y.; Zang, S.Q. MOF-Derived Bifunctional Cu3P Nanoparticles Coated by a N, P-Codoped Carbon Shell for Hydrogen Evolution and Oxygen Reduction. Adv. Mater. 2018, 30, 1703711. [Google Scholar] [CrossRef]
- Chung, D.Y.; Jun, S.W.; Yoon, G.; Kim, H.; Yoo, J.M.; Lee, K.S.; Kim, T.; Shin, H.; Sinha, A.K.; Kwon, S.G.; et al. Large-Scale Synthesis of Carbon-Shell-Coated FeP Nanoparticles for Robust Hydrogen Evolution Reaction Electrocatalyst. J. Am. Chem. Soc. 2017, 139, 6669–6674. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, H.; Cao, B.; Jiang, J.; Gao, R.; Zhang, J. Few Layered N, P Dual-Doped Carbon-Encapsulated Ultrafine MoP Nanocrystal/MoP Cluster Hybrids on Carbon Cloth: An Ultrahigh Active and Durable 3D Self-Supported Integrated Electrode for Hydrogen Evolution Reaction in a Wide pH Range. Adv. Funct. Mater. 2018, 28, 1801527. [Google Scholar] [CrossRef]
- Sun, Y.; Hang, L.; Shen, Q.; Zhang, T.; Li, H.; Zhang, X.; Lyu, X.; Li, Y. Mo doped Ni2P nanowire arrays: An efficient electrocatalyst for the hydrogen evolution reaction with enhanced activity at all pH values. Nanoscale 2017, 9, 16674–16679. [Google Scholar] [CrossRef] [PubMed]
- Babar, P.; Lokhande, A.; Karade, V.; Lee, I.J.; Lee, D.; Pawar, S.; Kim, J.H. Trifunctional layered electrodeposited nickel iron hydroxide electrocatalyst with enhanced performance towards the oxidation of water, urea and hydrazine. J. Colloid Interface Sci. 2019, 557, 10–17. [Google Scholar] [CrossRef]
- Liu, H.; Ma, X.; Hu, H.; Pan, Y.Y.; Zhao, W.N.; Liu, J.L.; Zhao, X.Y.; Wang, J.L.; Yang, Z.Y.; Zhao, Q.S.; et al. Robust NiCoP/CoP heterostructures for highly efficient hydrogen evolution electrocatalysis in alkaline solution. ACS Appl. Mater. Interfaces 2019, 11, 15528–15536. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, B.; Yu, Z.P.; Bondarchuk, O.; Araaujo, A.; Amorim, I.; Zhang, N.; Xu, J.Y.; Neves, I.C.; Liu, L.F. Bifunctional porous cobalt phosphide foam for high-current-density alkaline water electrolysis with 4000 h long stability. ACS Sustain. Chem. Eng. 2020, 8, 10193–10200. [Google Scholar] [CrossRef]
- Wang, X.; Tong, R.; Wang, Y.; Tao, H.; Zhang, Z.; Wang, H. Surface roughening of nickel cobalt phosphide nanowire arrays/Ni foam for enhanced hydrogen evolution activity. ACS Appl. Mater. Interfaces 2016, 8, 34270–34279. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, Y.; Yang, R.; Li, D.; Meng, S.; Chen, M. CoP3/CoMoP heterogeneous nanosheet arrays as robust electrocatalyst for pH-universal hydrogen evolution reaction. ACS Sustain. Chem. Eng. 2019, 7, 9309–9317. [Google Scholar]
- Zhang, W.G.; Liu, Y.H.; Zhou, H.B.; Li, J.; Yao, S.W.; Wang, H.Z. A high-performance electrocatalyst of CoMoP@NF nanosheet arrays for hydrogen evolution in alkaline solution. J. Mater. Sci. 2019, 54, 11585–11595. [Google Scholar] [CrossRef]
- Liu, X.Y.; Yao, Y.D.; Zhang, H.; Pan, L.; Shi, C.X.; Zhang, X.W.; Huang, Z.F.; Zou, J.J. In situ-grown cobalt-iron phosphide-based integrated electrode for long-term water splitting under a large current density at the industrial electrolysis temperature. ACS Sustain. Chem. Eng. 2020, 8, 17828–17838. [Google Scholar] [CrossRef]
- Rezaee, S.; Shahrokhian, S. 3D ternary NixCo2−xP/C nanoflower/nanourchin arrays grown on HCNs: A highly efficient bi-functional electrocatalyst for boosting hydrogen production via the urea electro-oxidation reaction. Nanoscale 2020, 12, 16123–16135. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Ryu, S.Y.; Yoon, J.Y.; Lee, H.K.; Choi, N.G.; Park, I.H.; Choi, H.Y. Three-dimensional dendritic Cu-Co-P electrode by one-step electrodeposition on a hydrogen bubble template for hydrogen evolution reaction. ACS Sustain. Chem. Eng. 2019, 7, 10734–10741. [Google Scholar]
- Duan, J.; Chen, S.; Vasileff, A.; Qiao, S.Z. Anion and cation modulation in metal compounds for bifunctional overall water splitting. ACS Nano 2016, 10, 8738–8745. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xiong, D.; Amorim, I.; Liu, L. Template-free synthesis of hollow iron phosphide-phosphate composite nanotubes for use as active and stable oxygen evolution electrocatalysts. ACS Appl. Nano Mater. 2018, 1, 617–624. [Google Scholar] [CrossRef]
- Huang, X.K.; Xu, X.P.; Li, C.; Wu, D.F.; Cheng, D.J.; Cao, D.P. Vertical CoP Nanoarray Wrapped by N, P-Doped Carbon for Hydrogen Evolution Reaction in Both Acidic and Alkaline Conditions. Adv. Energy Mater. 2019, 9, 1803970. [Google Scholar] [CrossRef]
- Wu, J.; Wang, D.; Wan, S.; Liu, H.; Wang, C.; Wang, X. An Efficient Cobalt Phosphide Electrocatalyst Derived from Cobalt Phosphonate Complex for All-pH Hydrogen Evolution Reaction and Overall Water Splitting in Alkaline Solution. Small 2020, 16, e1900550. [Google Scholar] [CrossRef]
- Li, J.S.; Kong, L.X.; Wu, Z.; Zhang, S.; Yang, X.-Y.; Sha, J.-Q.; Liu, G.-D. Polydopamine-assisted construction of cobalt phosphide encapsulated in N-doped carbon porous polyhedrons for enhanced overall water splitting. Carbon 2019, 145, 694–700. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, K.; Liu, S.; Cao, X.; Wu, K.; Cheong, W.C.; Chen, Z.; Wang, Y.; Li, Y.; Liu, Y.; et al. Core-Shell ZIF-8@ZIF-67-Derived CoP Nanoparticle-Embedded N-Doped Carbon Nanotube Hollow Polyhedron for Efficient Overall Water Splitting. J. Am. Chem. Soc. 2018, 140, 2610–2618. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, S.; Li, C.; Zhu, C.; Chen, Y.; Gao, P.; Qi, L.; Zhang, X. Hollow CoP nanopaticle/N-doped graphene hybrids as highly active and stable bifunctional catalysts for full water splitting. Nanoscale 2016, 8, 10902–10907. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Y.; He, H.; Peng, L.; Xiao, S.; Yao, S.; Xiao, P. Carbon-incorporated porous honeycomb NiCoFe phosphide nanospheres derived from a MOF precursor for overall water splitting. Chem. Commun. (Camb) 2019, 55, 10896–10899. [Google Scholar] [CrossRef]
- Yang, D.; Hou, W.; Lu, Y.; Wang, X.; Zhang, W.; Chen, Y. Scalable Synthesis of Bimetallic Phosphide Decorated in Carbon Nanotube Network as Multifunctional Electrocatalyst for Water Splitting. ACS Sustain. Chem. Eng. 2019, 7, 13031–13040. [Google Scholar] [CrossRef]
- Huang, C.; Ouyang, T.; Zou, Y.; Li, N.; Liu, Z.-Q. Ultrathin NiCo2Px nanosheets strongly coupled with CNTs as efficient and robust electrocatalysts for overall water splitting. J. Mater. Chem. A 2018, 6, 7420–7427. [Google Scholar] [CrossRef]
- Yuan, C.Z.; Zhong, S.L.; Jiang, Y.F.; Yang, Z.K.; Zhao, Z.W.; Zhao, S.J.; Jiang, N.; Xu, A.W. Direct growth of cobalt-rich cobalt phosphide catalysts on cobalt foil: An efficient and self-supported bifunctional electrode for overall water splitting in alkaline media. J. Mater. Chem. A 2017, 5, 10561–10566. [Google Scholar] [CrossRef]
- Guo, B.; Sun, J.; Hu, X.; Wang, Y.; Sun, Y.; Hu, R.; Yu, L.; Zhao, H.; Zhu, J. Fe3O4-CoPx Nanoflowers Vertically Grown on TiN Nanoarrays as Efficient and Stable Electrocatalysts for Overall Water Splitting. ACS Appl. Nano Mater. 2018, 2, 40–47. [Google Scholar] [CrossRef]
- Ai, L.; Niu, Z.; Jiang, J. Mechanistic insight into oxygen evolution electrocatalysis of surface phosphate modified cobalt phosphide nanorod bundles and their superior performance for overall water splitting. Electrochim. Acta 2017, 242, 355–363. [Google Scholar] [CrossRef]
- Lu, M.; Li, L.; Chen, D.; Li, J.; Klyui, N.I.; Han, W. MOF-derived nitrogen-doped CoO@CoP arrays as bifunctional electrocatalysts for efficient overall water splitting. Electrochim. Acta 2020, 330, 135210. [Google Scholar] [CrossRef]
- Niu, Z.; Qiu, C.; Jiang, J.; Ai, L. Hierarchical CoP–FeP Branched Heterostructures for Highly Efficient Electrocatalytic Water Splitting. ACS Sustain. Chem. Eng. 2018, 7, 2335–2342. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Fan, Q.; Zhang, F.; Xu, S. Hierarchically scaffolded CoP/CoP2 nanoparticles: Controllable synthesis and their application as a well-matched bifunctional electrocatalyst for overall water splitting. Nanoscale 2017, 9, 5677–5685. [Google Scholar] [CrossRef]
- Dutta, S.; Indra, A.; Han, H.; Song, T. An Intriguing Pea-Like Nanostructure of Cobalt Phosphide on Molybdenum Carbide Incorporated Nitrogen-Doped Carbon Nanosheets for Efficient Electrochemical Water Splitting. Chem. Sus. Chem. 2018, 11, 3956–3964. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhao, S.; Zhao, Y.; Zhou, W.; Dai, R.; Zhao, X.; Chen, Z.; Zhang, H.; Chen, A. Heterostructured Co2P Nanocomposite Embedded in a N, P Co-Doped Carbon Layer as a High Performance Electrocatalyst for Overall Water Splitting. Catalysts 2022, 12, 957. https://doi.org/10.3390/catal12090957
Chen J, Zhao S, Zhao Y, Zhou W, Dai R, Zhao X, Chen Z, Zhang H, Chen A. Heterostructured Co2P Nanocomposite Embedded in a N, P Co-Doped Carbon Layer as a High Performance Electrocatalyst for Overall Water Splitting. Catalysts. 2022; 12(9):957. https://doi.org/10.3390/catal12090957
Chicago/Turabian StyleChen, Ji, Shuwen Zhao, Yifan Zhao, Weijie Zhou, Ruijie Dai, Xuan Zhao, Zhengang Chen, Hua Zhang, and Anran Chen. 2022. "Heterostructured Co2P Nanocomposite Embedded in a N, P Co-Doped Carbon Layer as a High Performance Electrocatalyst for Overall Water Splitting" Catalysts 12, no. 9: 957. https://doi.org/10.3390/catal12090957
APA StyleChen, J., Zhao, S., Zhao, Y., Zhou, W., Dai, R., Zhao, X., Chen, Z., Zhang, H., & Chen, A. (2022). Heterostructured Co2P Nanocomposite Embedded in a N, P Co-Doped Carbon Layer as a High Performance Electrocatalyst for Overall Water Splitting. Catalysts, 12(9), 957. https://doi.org/10.3390/catal12090957




