The Application of Mineral Kaolinite for Environment Decontamination: A Review
Abstract
1. Introduction
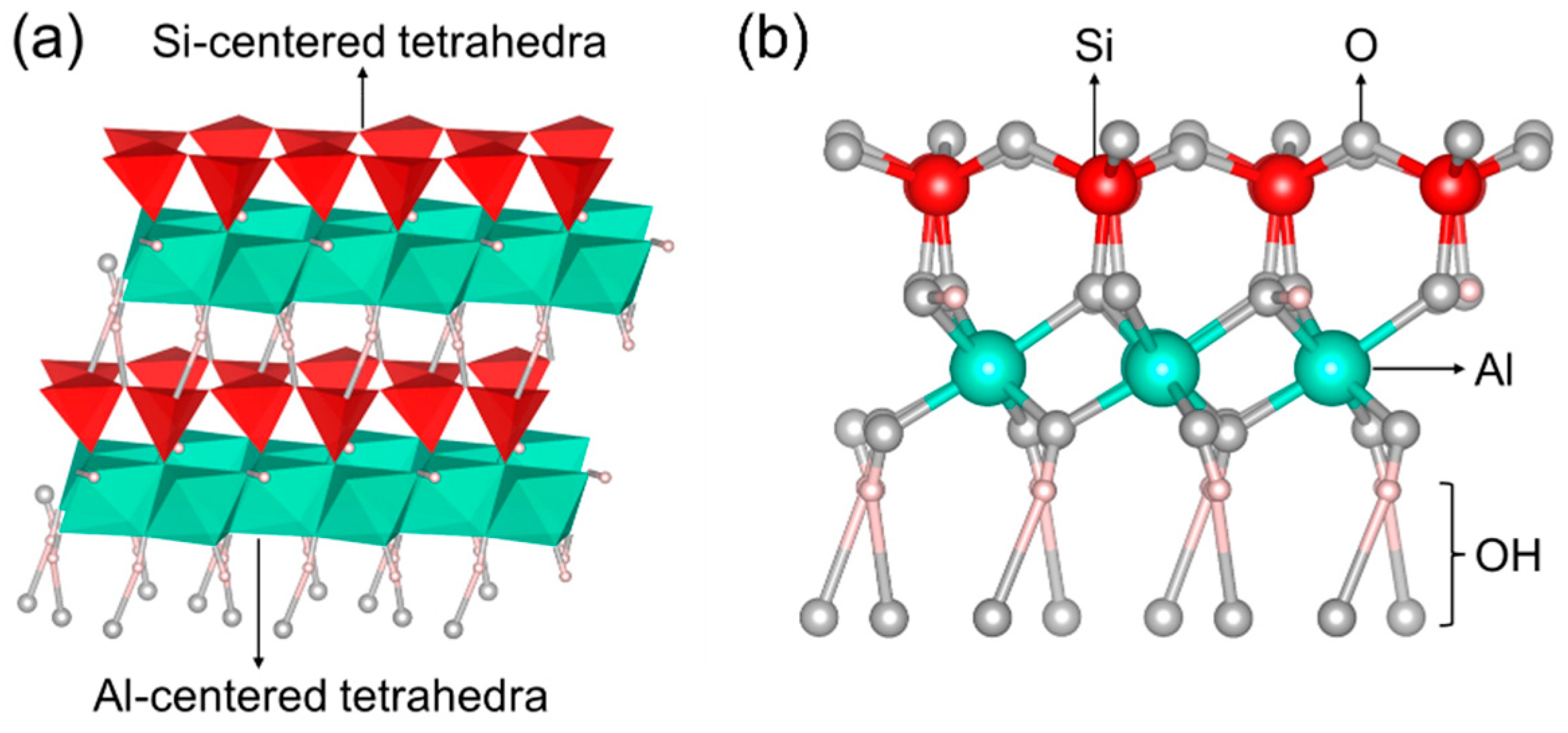
2. The Properties of Kaolinite
3. Photocatalytic Degradation of Organic Pollutions
3.1. Photocatalysis Mechanism
3.2. TiO2/Kaolinite Photocatalysis
3.3. Other Semiconductor/Kaolinite Photocatalysis
3.4. Dual Semiconductor-Based Kaolinite Photocatalysis
4. Disinfection with Ag Materials and Photocatalysis
4.1. Disinfection with Ag Materials
4.2. Disinfection with Photocatalysis
5. Adsorption of Heavy Metals
5.1. Natural Kaolinites
5.2. Thermal-Modified Kaolinite
5.3. Acid-Modified Kaolinite
5.4. Co-Modified Kaolinite with Thermal Treatment and Acid Treatment
5.5. Transition Metal-Modified Kaolinite
5.6. Organic-Modified Kaolinite
6. Removal of Gas Phase Pollutants
6.1. CO2 Reduction and Adsorption
6.2. Removal of VOCs by Adsorption, Photocatalysis, and Catalytic Combustion
7. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Akkari, M.; Aranda, P.; Ben Rhaiem, H.; Ben Haj Amara, A.; Ruiz-Hitzky, E. ZnO/clay nanoarchitectures: Synthesis, characterization and evaluation as photocatalysts. Appl. Clay Sci. 2016, 131, 131–139. [Google Scholar] [CrossRef]
- Ma, J.; Huang, D.; Zhang, W.; Zou, J.; Kong, Y.; Zhu, J.; Komarneni, S. Nanocomposite of exfoliated bentonite/g-C3N4/Ag3PO4 for enhanced visible-light photocatalytic decomposition of Rhodamine B. Chemosphere 2016, 162, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yao, G.; Zhang, X.; Zheng, S.; Frost, R.L. Enhanced visible-light photocatalytic activity of kaolinite/g-C3N4 composite synthesized via mechanochemical treatment. Appl. Clay Sci. 2016, 129, 7–14. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, W.; Chen, H.; Ni, B.J.E.-E. Recent advances in waste-derived functional materials for wastewater remediation. Eco-Environ. Health 2022, 1, 86–104. [Google Scholar] [CrossRef]
- Dědková, K.; Matějová, K.; Lang, J.; Peikertová, P.; Kutláková, K.M.; Neuwirthová, L.; Frydrýšek, K.; Kukutschová, J. Antibacterial activity of kaolinite/nanoTiO2 composites in relation to irradiation time. J. Photochem. Photobiol. B 2014, 135, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, I.; Sahroni, I.; Putra, H.P.; Rifky Nugraha, M.; Hasanah, U.A. Ceramic membrane based on TiO2-modified kaolinite as a low cost material for water filtration. Appl. Clay Sci. 2015, 118, 207–211. [Google Scholar] [CrossRef]
- Hai, Y.; Li, X.; Wu, H.; Zhao, S.; Deligeer, W.; Asuha, S. Modification of acid-activated kaolinite with TiO2 and its use for the removal of azo dyes. Appl. Clay Sci. 2015, 114, 558–567. [Google Scholar] [CrossRef]
- García-Giménez, R.; de la Villa, R.V.; Martínez-Ramírez, S.; Fernández-Carrasco, L.; Frías, M. Influence of ZnO on the activation of kaolinite-based coal waste: Pozzolanic activity and mineralogy in the pozzolan/lime system. Appl. Clay Sci. 2018, 156, 202–212. [Google Scholar] [CrossRef]
- Sun, Z.; Yuan, F.; Li, X.; Li, C.; Xu, J.; Wang, B. Fabrication of Novel Cyanuric Acid Modified g-C3N4/Kaolinite Composite with Enhanced Visible Light-Driven Photocatalytic Activity. Minerals 2018, 8, 437. [Google Scholar] [CrossRef]
- Dong, X.; Sun, Z.; Zhang, X.; Li, C.; Zheng, S. Construction of BiOCl/g-C3N4/kaolinite composite and its enhanced photocatalysis performance under visible-light irradiation. J. Taiwan Inst. Chem. Eng. 2018, 84, 203–211. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Shuai, D.; Shen, Y.; Xiong, W.; Wang, L. Graphitic carbon nitride (g-C3N4)-based photocatalysts for water disinfection and microbial control: A review. Chemosphere 2019, 214, 462–479. [Google Scholar] [CrossRef]
- Reli, M.; Huo, P.; Šihor, M.; Ambrožová, N.; Troppová, I.; Matějová, L.; Lang, J.; Svoboda, L.; Kuśtrowski, P.; Ritz, M.; et al. Novel TiO2/C3N4 Photocatalysts for Photocatalytic Reduction of CO2 and for Photocatalytic Decomposition of N2O. J. Phys. Chem. A 2016, 120, 8564–8573. [Google Scholar] [CrossRef] [PubMed]
- Svarovsky, L. 1—Introduction to solid-liquid separation. In Solid-Liquid Separation, 4th ed.; Svarovsky, L., Ed.; Butterworth-Heinemann: Oxford, UK, 2001; pp. 1–29. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Unuabonah, I.E.; Olu-Owolabi, B.I. The effect of some operating variables on the adsorption of lead and cadmium ions on kaolinite clay. J. Hazard. Mater. 2006, 134, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, K.G.; Gupta, S.S. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, K.G.; Gupta, S.S. Kaolinite and montmorillonite as adsorbents for Fe(III), Co(II) and Ni(II) in aqueous medium. Appl. Clay Sci. 2008, 41, 1–9. [Google Scholar] [CrossRef]
- Zheng, R.; Gao, H.; Ren, Z.; Cen, D.; Chen, Z. Preparation of activated bentonite and its adsorption behavior on oil-soluble green pigment. Physicochem. Probl. Miner. Process. 2017, 53, 829–845. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, Q.; Cheng, H. Recent advances in kaolinite-based material for photocatalysts. Chin. Chem. Lett. 2021, 32, 2617–2628. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Alfred, M.O.; Omorogie, M.O.; Bodede, O.; Moodley, R.; Ogunlaja, A.; Adeyemi, O.G.; Günter, C.; Taubert, A.; Iermak, I.; Eckert, H. Solar-active clay-TiO2 nanocomposites prepared via biomass assisted synthesis: Efficient removal of ampicillin, sulfamethoxazole and artemether from water. Chem. Eng. J. 2020, 398, 125544. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Dai, W.; Mu, J.; Chen, Z.; Zhang, J.; Pei, X.; Luo, W.; Ni, B.-J.J. Design of few-layer carbon nitride/BiFeO3 composites for efficient organic pollutant photodegradation. Environ. Res. 2022, 215, 114190. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, W.; Ni, B.-J.; Chen, H. Plastic wastes derived carbon materials for green energy and sustainable environmental applications. Environ. Funct. Mater. 2022, 1, 34–48. [Google Scholar] [CrossRef]
- Li, C.; Zhu, N.; Yang, S.; He, X.; Zheng, S.; Sun, Z.; Dionysiou, D.D. A review of clay based photocatalysts: Role of phyllosilicate mineral in interfacial assembly, microstructure control and performance regulation. Chemosphere 2021, 273, 129723. [Google Scholar] [CrossRef]
- Li, C.; Zhu, N.; Dong, X.; Zhang, X.; Chen, T.; Zheng, S.; Sun, Z. Tuning and controlling photocatalytic performance of TiO2/kaolinite composite towards ciprofloxacin: Role of 0D/2D structural assembly. Adv. Powder Technol. 2020, 31, 1241–1252. [Google Scholar] [CrossRef]
- Azeez, S.O.; Saheed, I.O.; Adekola, F.A.; Salau, S.S. Preparation of TiO2-activated kaolinite composite for photocatalytic degradation of rhodamine B dye. Appl. Clay Sci. 2011, 53, 646–649. [Google Scholar] [CrossRef]
- Bockstaller, M.R.; Mickiewicz, R.A.; Thomas, E.L. Block copolymer nanocomposites: Perspectives for tailored functional materials. Adv. Mater. 2005, 17, 1331–1349. [Google Scholar] [CrossRef]
- Li, X.; Peng, K.; Chen, H.; Wang, Z. TiO2 nanoparticles assembled on kaolinites with different morphologies for efficient photocatalytic performance. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Zhang, P.; Li, Y. Synthesis and characterization of kaolinite/TiO2 nano-photocatalysts. Appl. Clay Sci. 2011, 53, 646–649. [Google Scholar] [CrossRef]
- Shao, P.; Tian, J.; Yang, F.; Duan, X.; Gao, S.; Shi, W.; Luo, X.; Cui, F.; Luo, S.; Wang, S. Identification and Regulation of Active Sites on Nanodiamonds: Establishing a Highly Efficient Catalytic System for Oxidation of Organic Contaminants. Adv. Funct. Mater. 2018, 28, 1705295. [Google Scholar] [CrossRef]
- Kutláková, K.M.; Tokarský, J.; Peikertová, P. Functional and eco-friendly nanocomposite kaolinite/ZnO with high photocatalytic activity. Appl. Catal. B 2015, 162, 392–400. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Helmy, A.; Sharaf, M.F.; El-Meligy, M.A.; Soliman, A.T.A. Instantaneous oxidation of levofloxacin as toxic pharmaceutical residuals in water using clay nanotubes decorated by ZnO (ZnO/KNTs) as a novel photocatalyst under visible light source. J. Environ. Manag. 2020, 271, 111019. [Google Scholar] [CrossRef]
- Roy, P.; Jahromi, H.; Adhikari, S.; Finfrock, Y.Z.; Rahman, T.; Ahmadi, Z.; Mahjouri-Samani, M.; Feyzbar-Khalkhali-Nejad, F.; Oh, T.-S. Performance of biochar assisted catalysts during hydroprocessing of non-edible vegetable oil: Effect of transition metal source on catalytic activity. Energy Convers. Manag. 2022, 252, 115131. [Google Scholar] [CrossRef]
- Tong, Z.; Yang, D.; Xiao, T.; Tian, Y.; Jiang, Z. Biomimetic fabrication of g-C3N4/TiO2 nanosheets with enhanced photocatalytic activity toward organic pollutant degradation. Chem. Eng. J. 2015, 260, 117–125. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Zhang, W.; Yu, C.; Zheng, S. Highly efficient g-C3N4/TiO2/kaolinite composite with novel three-dimensional structure and enhanced visible light responding ability towards ciprofloxacin and S. aureus. Appl. Catal. B 2018, 220, 272–282. [Google Scholar] [CrossRef]
- Huang, Z.; Li, L.; Li, Z.; Li, H.; Wu, J. Synthesis of novel kaolin-supported g-C3N4/CeO2 composites with enhanced photocatalytic removal of ciprofloxacin. Materials 2020, 13, 3811. [Google Scholar] [CrossRef]
- Dhandole, L.K.; Kim, S.-G.; Seo, Y.-S.; Mahadik, M.A.; Chung, H.S.; Lee, S.Y.; Choi, S.H.; Cho, M.; Ryu, J.; Jang, J.S. Enhanced Photocatalytic Degradation of Organic Pollutants and Inactivation of Listeria monocytogenes by Visible Light Active Rh-Sb Codoped TiO2 Nanorods. ACS Sustain. Chem. Eng. 2018, 6, 4302–4315. [Google Scholar] [CrossRef]
- Awad, M.E.; López-Galindo, A.; Medarević, D.; Milenković, M.; Ibrić, S.; El-Rahmany, M.M.; Iborra, C.V. Enhanced antimicrobial activity and physicochemical stability of rapid pyro-fabricated silver-kaolinite nanocomposite. Int. J. Pharm. 2021, 598, 120372. [Google Scholar] [CrossRef] [PubMed]
- Woldegebreal, T.; Teju, E.; Kebede, A.; Taddesse, A.; Bezu, Z. Water disinfection using Kaolinite supported magnetic silver nanoparticle. Chem. Data Collect. 2022, 39, 100857. [Google Scholar] [CrossRef]
- Jou, S.K.; Malek, N.A.N.N. Characterization and antibacterial activity of chlorhexidine loaded silver-kaolinite. Appl. Clay Sci. 2016, 127–128, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Qiu, L.; Lan, X.; Zhu, C.; Duan, J.; Liu, Y.; Li, H.; Yu, Y.; Yang, W. In-situ synthesis of dual Z-scheme heterojunctions of cuprous oxide/layered double hydroxides/nitrogen-rich graphitic carbon nitride for photocatalytic sterilization. J. Colloid Interface Sci. 2022, 620, 313–321. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, C.; Zhang, G.; Hu, L.; Wang, P. Photocatalytic Fe-doped TiO2/PSF composite UF membranes: Characterization and performance on BPA removal under visible-light irradiation. Chem. Eng. J. 2017, 319, 39–47. [Google Scholar] [CrossRef]
- Li, R.; Cui, L.; Chen, M.; Huang, Y. Nanomaterials for airborne virus inactivation: A short review. Aerosol Sci. Eng. 2021, 5, 1–11. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Saint, C.P. Bacterial inactivation kinetics of a photo-disinfection system using novel titania-impregnated kaolinite photocatalyst. Chem. Eng. J. 2011, 171, 16–23. [Google Scholar] [CrossRef]
- Fatimah, I.; Hasanah, U.A.; Putra, H.P. Preparation of bifunctional ceramic membrane based on TiO2/kaolinite for water disinfection. J. Mater. Environ. Sci. 2014, 5, 1976–1981. [Google Scholar]
- B.Aritonang, A.; Sapar, A.; Furqonita, A. Photocatalytic Bacterial Inactivation Using Bi-doped TiO2/Kaolinite Under Visible Light Irradiation. Int. Conf. Sci. Eng. 2021, 105–111. [Google Scholar] [CrossRef]
- Misra, A.J.; Das, S.; Rahman, A.P.H.; Das, B.; Jayabalan, R.; Behera, S.K.; Suar, M.; Tamhankar, A.J.; Mishra, A.; Lundborg, C.S.; et al. Doped ZnO nanoparticles impregnated on Kaolinite (Clay): A reusable nanocomposite for photocatalytic disinfection of multidrug resistant Enterobacter sp under visible light. J. Colloid Interface Sci. 2018, 530, 610–623. [Google Scholar] [CrossRef]
- Pratap Reddy, M.; Venugopal, A.; Subrahmanyam, M. Hydroxyapatite-supported Ag–TiO2 as Escherichia coli disinfection photocatalyst. Water Res. 2007, 41, 379–386. [Google Scholar] [CrossRef]
- Ugwuja, C.G.; Adelowo, O.O.; Ogunlaja, A.; Omorogie, M.O.; Olukanni, O.D.; Ikhimiukor, O.O.; Iermak, I.; Kolawole, G.A.; Guenter, C.; Taubert, A.; et al. Visible-Light-Mediated Photodynamic Water Disinfection @ Bimetallic-Doped Hybrid Clay Nanocomposites. ACS Appl. Mater. Interfaces 2019, 11, 25483–25494. [Google Scholar] [CrossRef]
- Kozera-Sucharda, B.; Gworek, B.; Kondzielski, I.; Chojnicki, J. The Comparison of the Efficacy of Natural and Synthetic Aluminosilicates, Including Zeolites, in Concurrent Elimination of Lead and Copper from Multi-Component Aqueous Solutions. Processes 2021, 9, 812. [Google Scholar] [CrossRef]
- Hutchison, J.M.; Mayer, B.K.; Vega, M.; Chacha, W.E.; Zilles, J.L. Making Waves: Biocatalysis and Biosorption: Opportunities and Challenges Associated with a New Protein-Based Toolbox for Water and Wastewater Treatment. Water Res. X 2021, 12, 100112. [Google Scholar] [CrossRef]
- Li, D.; Liu, S.-A.; Li, S. Copper isotope fractionation during adsorption onto kaolinite: Experimental approach and applications. Chem. Geol. 2015, 396, 74–82. [Google Scholar] [CrossRef]
- Turan, P.; Doğan, M.; Alkan, M. Uptake of trivalent chromium ions from aqueous solutions using kaolinite. J. Hazard. Mater. 2007, 148, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, W.; Zhao, Y.; Bai, H.; Wen, T.; Kang, S.; Song, G.; Song, S.; Komarneni, S. Removal of heavy metals and dyes by clay-based adsorbents: From natural clays to 1D and 2D nano-composites. Chem. Eng. J. 2021, 420, 127574. [Google Scholar] [CrossRef]
- Yavuz, Ö.; Altunkaynak, Y.; Güzel, F. Removal of copper, nickel, cobalt and manganese from aqueous solution by kaolinite. Water Res. 2003, 37, 948–952. [Google Scholar] [CrossRef]
- Et, A.; Shahmohammadi-Kalalagh, S. Isotherm and kinetic studies on adsorption of Pb, Zn and Cu by kaolinite. Casp. J. Environ. Sci. 2011, 9, 243–255. [Google Scholar]
- Chai, W.; Huang, Y.; Su, S.; Han, G.; Liu, J.; Cao, Y. Adsorption behavior of Zn (II) onto natural minerals in wastewater. A comparative study of bentonite and kaolinite. Physicochem. Probl. Miner. Process. 2017, 53, 264–278. [Google Scholar] [CrossRef]
- Farsi, A.; Aghasi, M.; Esmaeili, A.; Eslami, H. Efficient removal of Cu (II) and Zn (II) from aqueous solution and real acid mine drainage by natural vermiculite and kaolinite. Desalin. Water Treat. 2020, 204, 224–237. [Google Scholar] [CrossRef]
- Novikau, R.; Lujaniene, G. Adsorption behaviour of pollutants: Heavy metals, radionuclides, organic pollutants, on clays and their minerals (raw, modified and treated): A review. J. Environ. Manag. 2022, 309, 114685. [Google Scholar] [CrossRef]
- Yin, H.; Han, M.; Tang, W. Phosphorus sorption and supply from eutrophic lake sediment amended with thermally-treated calcium-rich attapulgite and a safety evaluation. Chem. Eng. J. 2016, 285, 671–678. [Google Scholar] [CrossRef]
- Davidovits, J.; Sawyer, J.L. Early High-Strength Mineral Polymer; U.S. Patent and Trademark Office: Washington, DC, USA, 1985. [Google Scholar]
- Yan, Z.; Fu, L.; Yang, H. Functionalized 2D clay derivative: Hybrid nanosheets with unique lead sorption behaviors and interface structure. Adv. Mater. Interfaces 2018, 5, 1700934. [Google Scholar] [CrossRef]
- Krupskaya, V.; Novikova, L.; Tyupina, E.; Belousov, P.; Dorzhieva, O.; Zakusin, S.; Kim, K.; Roessner, F.; Badetti, E.; Brunelli, A. The influence of acid modification on the structure of montmorillonites and surface properties of bentonites. Appl. Clay Sci. 2019, 172, 1–10. [Google Scholar] [CrossRef]
- España, V.A.A.; Sarkar, B.; Biswas, B.; Rusmin, R.; Naidu, R. Environmental applications of thermally modified and acid activated clay minerals: Current status of the art. Environ. Technol. Innov. 2019, 13, 383–397. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Removal of Cu (II) by natural and acid-activated clays: An insight of adsorption isotherm, kinetic and thermodynamics. Desalination 2011, 272, 66–75. [Google Scholar] [CrossRef]
- Chai, J.-B.; Au, P.-I.; Mubarak, N.M.; Khalid, M.; Ng, W.P.-Q.; Jagadish, P.; Walvekar, R.; Abdullah, E.C. Adsorption of heavy metal from industrial wastewater onto low-cost Malaysian kaolin clay–based adsorbent. Environ. Sci. Pollut. Res. 2020, 27, 13949–13962. [Google Scholar] [CrossRef]
- Unuabonah, E.; Olu-Owolabi, B.; Adebowale, K. Competitive adsorption of metal ions onto goethite–humic acid-modified kaolinite clay. Int. J. Environ. Sci. Technol. 2016, 13, 1043–1054. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Panchenko, V.N.; Volcho, K.P.; Zakusin, S.V.; Krupskaya, V.V.; Gil, A.; Mikhalchenko, O.S.; Vicente, M.A. Effect of acid modification of kaolin and metakaolin on Brønsted acidity and catalytic properties in the synthesis of octahydro-2H-chromen-4-ol from vanillin and isopulegol. J. Mol. Catal. A Chem. 2016, 414, 160–166. [Google Scholar] [CrossRef]
- Abdallah, S. Remediation of Copper and Zinc from wastewater by modified clay in Asir region southwest of Saudi Arabia. Open Geosci. 2019, 11, 505–512. [Google Scholar] [CrossRef]
- Zhang, M.; Xia, M.; Li, D.; Sun, Z.; You, Y.; Dou, J. The effects of transitional metal element doping on the Cs (I) adsorption of kaolinite (001): A density functional theory study. Appl. Surf. Sci. 2021, 547, 149210. [Google Scholar] [CrossRef]
- Üzüm, Ç.; Shahwan, T.; Eroğlu, A.E.; Hallam, K.R.; Scott, T.B.; Lieberwirth, I. Synthesis and characterization of kaolinite-supported zero-valent iron nanoparticles and their application for the removal of aqueous Cu2+ and Co2+ ions. Appl. Clay Sci. 2009, 43, 172–181. [Google Scholar] [CrossRef]
- Jiang, M.-Q.; Wang, Q.-P.; Jin, X.-Y.; Chen, Z.-L. Removal of Pb (II) from aqueous solution using modified and unmodified kaolinite clay. J. Hazard. Mater. 2009, 170, 332–339. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M. Cd (II) adsorption from aqueous solution by raw and modified kaolinite. Appl. Clay Sci. 2014, 88, 63–72. [Google Scholar] [CrossRef]
- Egirani, D.; Latif, M.T.; Wessey, N.; Poyi, N.; Acharjee, S. Synthesis and characterization of kaolinite coated with copper oxide and its effect on the removal of aqueous Lead (II) ions. Appl. Water Sci. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Abou-El-Sherbini, K.S.; Wahba, M.A.; Drweesh, E.A.; Akarish, A.I.; Shaban, S.A.; Elzahany, E.A. Zirconia-Intercalated Kaolinite: Synthesis, Characterization, and Evaluation of Metal-Ion Removal Activity. Clays Clay Miner. 2021, 69, 463–476. [Google Scholar] [CrossRef]
- Tarasevich, Y.I.; Klimova, G. Complex-forming adsorbents based on kaolinite, aluminium oxide and polyphosphates for the extraction and concentration of heavy metal ions from water solutions. Appl. Clay Sci. 2001, 19, 95–101. [Google Scholar] [CrossRef]
- Mao, S.; Gao, M. Functional organoclays for removal of heavy metal ions from water: A review. J. Mol. Liq. 2021, 334, 116143. [Google Scholar] [CrossRef]
- Liu, P. Polymer modified clay minerals: A review. Appl. Clay Sci. 2007, 38, 64–76. [Google Scholar] [CrossRef]
- Irandoost, M.; Pezeshki-Modaress, M.; Javanbakht, V. Removal of lead from aqueous solution with nanofibrous nanocomposite of polycaprolactone adsorbent modified by nanoclay and nanozeolite. J. Water Process. Eng. 2019, 32, 100981. [Google Scholar] [CrossRef]
- Wang, A.; Chu, Y.; Muhmood, T.; Xia, M.; Xu, Y.; Yang, L.; Lei, W.; Wang, F. Adsorption properties of Pb2+ by amino group’s functionalized montmorillonite from aqueous solutions. J. Chem. Eng. Data 2018, 63, 2940–2949. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Kaolinite, montmorillonite, and their modified derivatives as adsorbents for removal of Cu (II) from aqueous solution. Sep. Purif. Technol. 2006, 50, 388–397. [Google Scholar] [CrossRef]
- Unuabonah, E.; Adebowale, K.; Olu-Owolabi, B.; Yang, L. Comparison of sorption of Pb2+ and Cd2+ on kaolinite clay and polyvinyl alcohol-modified kaolinite clay. Adsorption 2008, 14, 791–803. [Google Scholar] [CrossRef]
- Koteja, A.; Matusik, J. Di-and triethanolamine grafted kaolinites of different structural order as adsorbents of heavy metals. J. Colloid Interface Sci. 2015, 455, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Kočí, K.; Matějka, V.; Kovář, P.; Lacný, Z.; Obalová, L. Comparison of the pure TiO2 and kaolinite/TiO2 composite as catalyst for CO2 photocatalytic reduction. Catal. Today 2011, 161, 105–109. [Google Scholar] [CrossRef]
- Kang, S.; Hwang, J. CoMn2O4 embedded hollow activated carbon nanofibers as a novel peroxymonosulfate activator. Chem. Eng. J. 2021, 406, 127158. [Google Scholar] [CrossRef]
- Knížek, A.; Kubelík, P.; Bouša, M.; Ferus, M.; Civiš, S. Acidic Hydrogen Enhanced Photocatalytic Reduction of CO2 on Planetary Surfaces. ACS Earth Space Chem. 2020, 4, 1001–1009. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, L.; Chen, J.; Luo, H.A.; Liu, J. In situ growth of g-C3N4 on clay minerals of kaolinite, sepiolite, and talc for enhanced solar photocatalytic energy conversion. Appl. Clay Sci. 2022, 216, 106337. [Google Scholar] [CrossRef]
- Schaef, H.T.; Glezakou, V.A.; Owen, A.T.; Ramprasad, S.; Martin, P.F.; McGrail, B.P. Surface Condensation of CO2 onto Kaolinite. Environ. Sci. Technol. Lett. 2013, 1, 142–145. [Google Scholar] [CrossRef]
- Kadoura, A.; Narayanan Nair, A.K.; Sun, S. Adsorption of carbon dioxide, methane, and their mixture by montmorillonite in the presence of water. Microporous Mesoporous Mater. 2016, 225, 331–341. [Google Scholar] [CrossRef]
- Hou, J.; Chen, M.; Zhou, Y.; Bian, L.; Dong, F.; Tang, Y.; Ni, Y.; Zhang, H. Regulating the effect of element doping on the CO2 capture performance of kaolinite: A density functional theory study. Appl. Surf. Sci. 2020, 512, 145642. [Google Scholar] [CrossRef]
- Li, R.; Rao, Y.; Huang, Y. Advances in catalytic elimination of atmospheric pollutants by two-dimensional transition metal oxides. Chin. Chem. Lett. 2022, 12, 108000. [Google Scholar] [CrossRef]
- Liu, D.; Yuan, P.; Liu, H.; Li, T.; Tan, D.; Yuan, W.; He, H. High-pressure adsorption of methane on montmorillonite, kaolinite and illite. Appl. Clay Sci. 2013, 85, 25–30. [Google Scholar] [CrossRef]
- Heller, R.; Zoback, M. Adsorption of methane and carbon dioxide on gas shale and pure mineral samples. J. Unconv. Oil Gas Resour. 2014, 8, 14–24. [Google Scholar] [CrossRef]
- Deng, L.; Yuan, P.; Liu, D.; Annabi-Bergaya, F.; Zhou, J.; Chen, F.; Liu, Z. Effects of microstructure of clay minerals, montmorillonite, kaolinite and halloysite, on their benzene adsorption behaviors. Appl. Clay Sci. 2017, 143, 184–191. [Google Scholar] [CrossRef]
- Lainé, J.; Foucaud, Y.; Bonilla-Petriciolet, A.; Badawi, M. Molecular picture of the adsorption of phenol, toluene, carbon dioxide and water on kaolinite basal surfaces. Appl. Surf. Sci. 2022, 585, 152699. [Google Scholar] [CrossRef]
- Swasy, M.I.; Campbell, M.L.; Brummel, B.R.; Guerra, F.D.; Attia, M.F.; Smith, G.D.; Alexis, F.; Whitehead, D.C. Poly(amine) modified kaolinite clay for VOC capture. Chemosphere 2018, 213, 19–24. [Google Scholar] [CrossRef]
- Mora, L.D.; Bonfim, L.F.; Barbosa, L.V.; da Silva, T.H.; Nassar, E.J.; Ciuffi, K.J.; Gonzalez, B.; Vicente, M.A.; Trujillano, R.; Rives, V.; et al. White and Red Brazilian Sao Simao’s Kaolinite-TiO2 Nanocomposites as Catalysts for Toluene Photodegradation from Aqueous Solutions. Materials 2019, 12, 3943. [Google Scholar] [CrossRef] [PubMed]
- Kibanova, D.; Trejo, M.; Destaillats, H.; Cervinisilva, J. Synthesis of hectorite–TiO2 and kaolinite–TiO2 nanocomposites with photocatalytic activity for the degradation of model air pollutants. Appl. Clay Sci. 2009, 42, 563–568. [Google Scholar] [CrossRef]
- Yang, P.; Li, J.; Zuo, S. Promoting oxidative activity and stability of CeO2 addition on the MnOx modified kaolin-based catalysts for catalytic combustion of benzene. Chem. Eng. Sci. 2017, 162, 218–226. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Yang, T.; Han, J.; Zhang, Y.; Zhao, L.; Zhao, J.; Li, R.; Huang, Y.; Gu, Z.; Wu, J. The Application of Mineral Kaolinite for Environment Decontamination: A Review. Catalysts 2023, 13, 123. https://doi.org/10.3390/catal13010123
Chen M, Yang T, Han J, Zhang Y, Zhao L, Zhao J, Li R, Huang Y, Gu Z, Wu J. The Application of Mineral Kaolinite for Environment Decontamination: A Review. Catalysts. 2023; 13(1):123. https://doi.org/10.3390/catal13010123
Chicago/Turabian StyleChen, Meijuan, Tongxi Yang, Jichang Han, Yang Zhang, Liyun Zhao, Jinghan Zhao, Rong Li, Yu Huang, Zhaolin Gu, and Jixian Wu. 2023. "The Application of Mineral Kaolinite for Environment Decontamination: A Review" Catalysts 13, no. 1: 123. https://doi.org/10.3390/catal13010123
APA StyleChen, M., Yang, T., Han, J., Zhang, Y., Zhao, L., Zhao, J., Li, R., Huang, Y., Gu, Z., & Wu, J. (2023). The Application of Mineral Kaolinite for Environment Decontamination: A Review. Catalysts, 13(1), 123. https://doi.org/10.3390/catal13010123







