Photocatalytic NOx Removal in Bismuth-Oxyhalide (BiOX, X = I, Cl) Cement-Based Materials Exposed to Outdoor Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of BiOX Photocatalysts
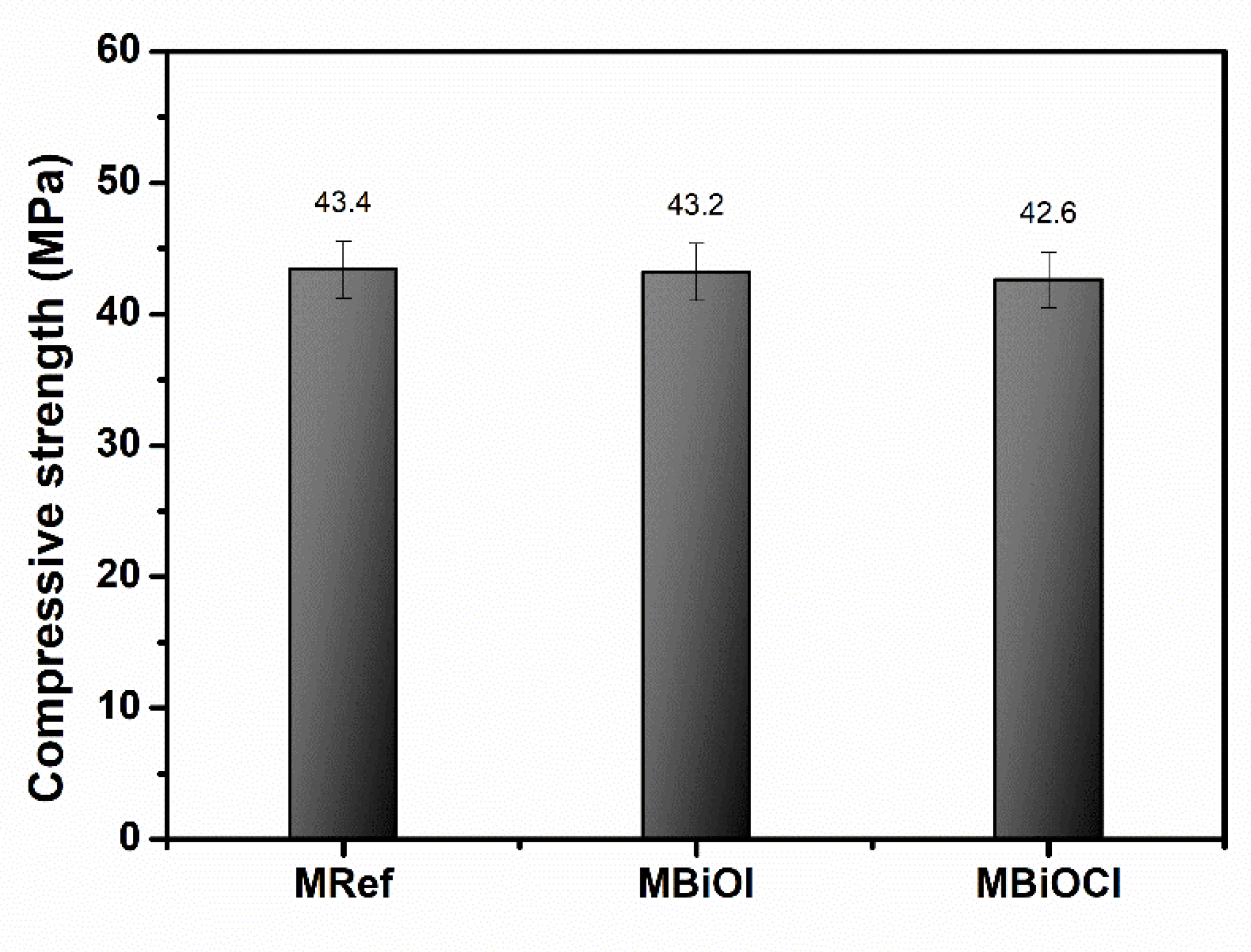
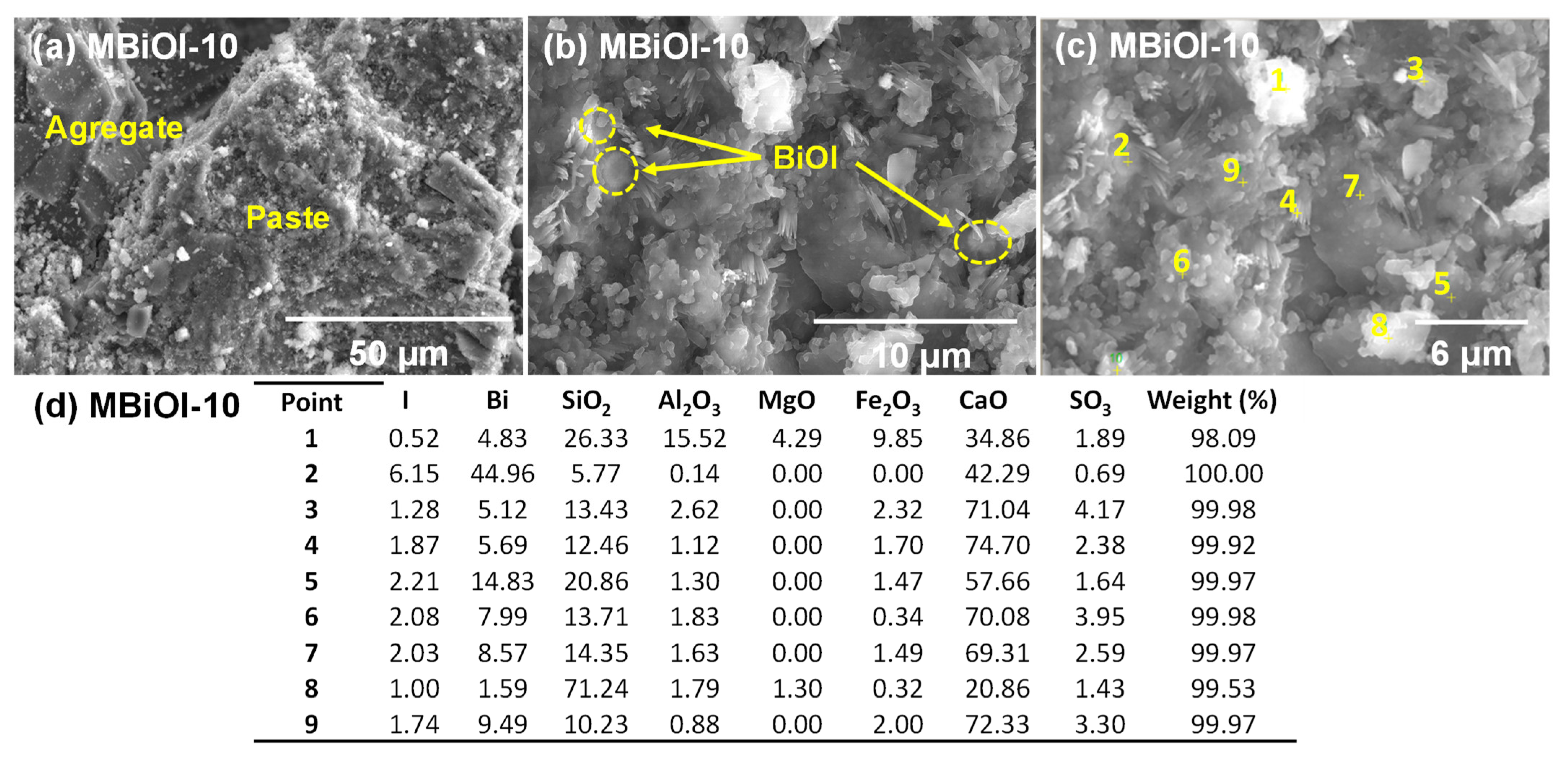
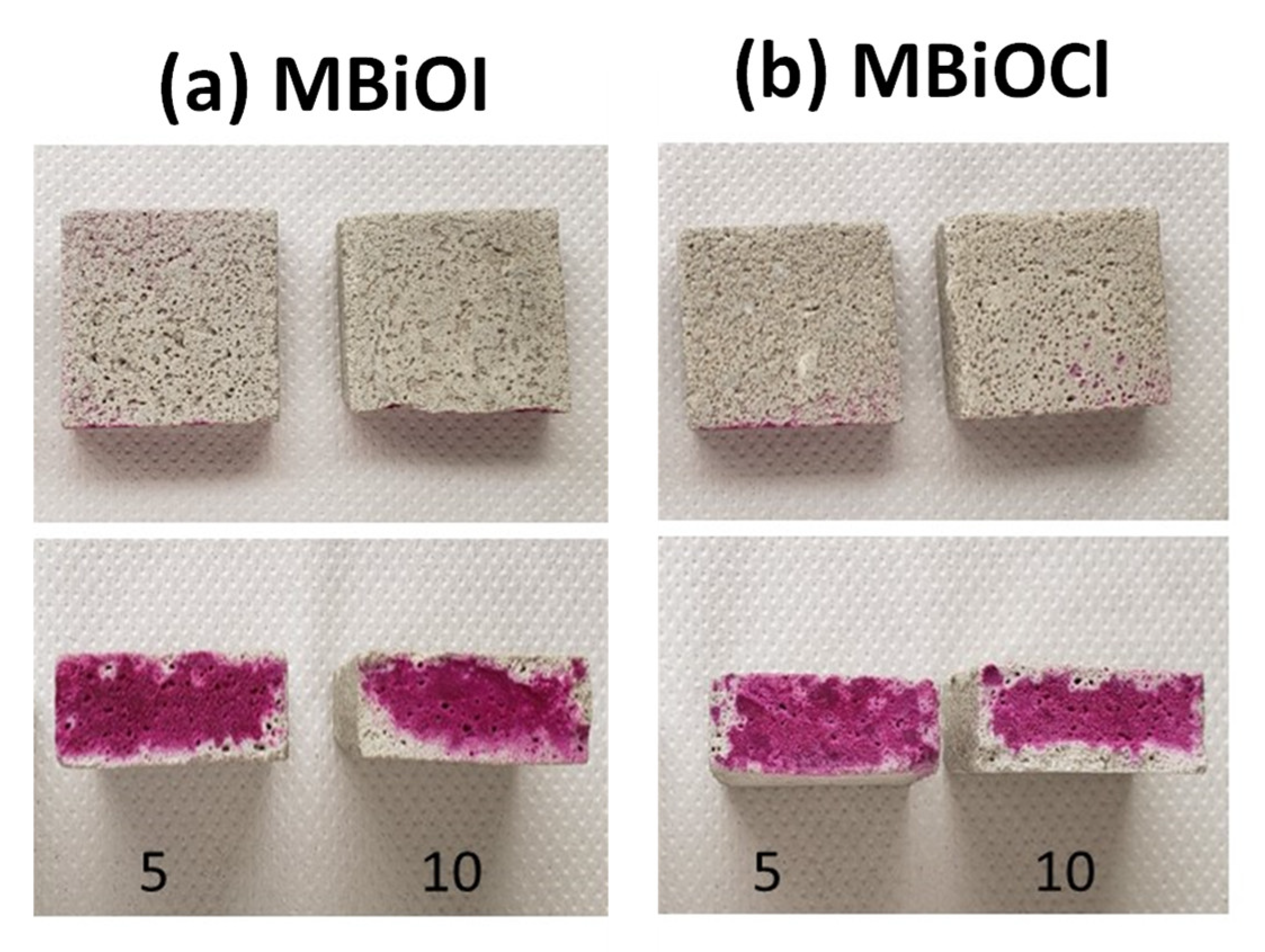
2.2. Characterization of Photocatalytic Mortar Samples
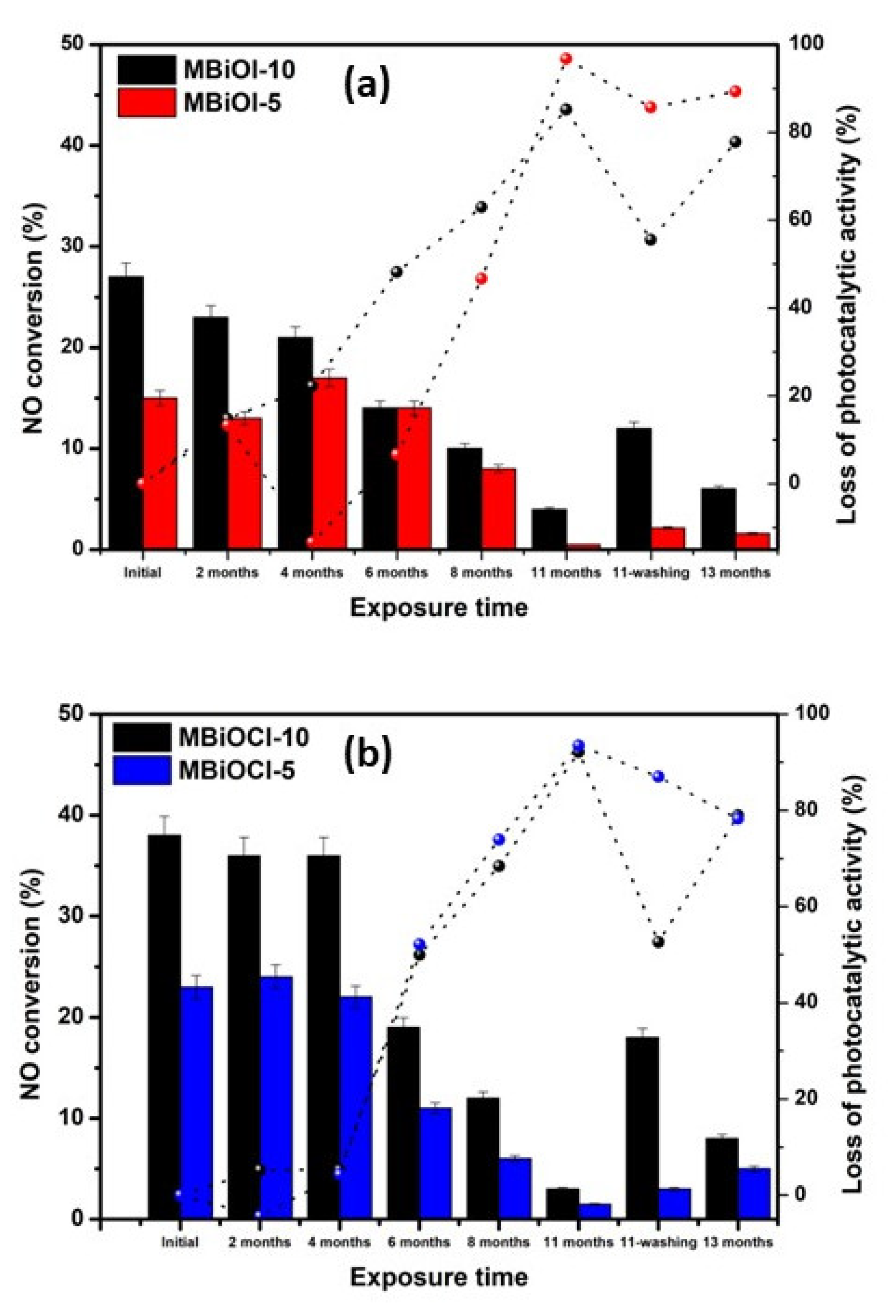
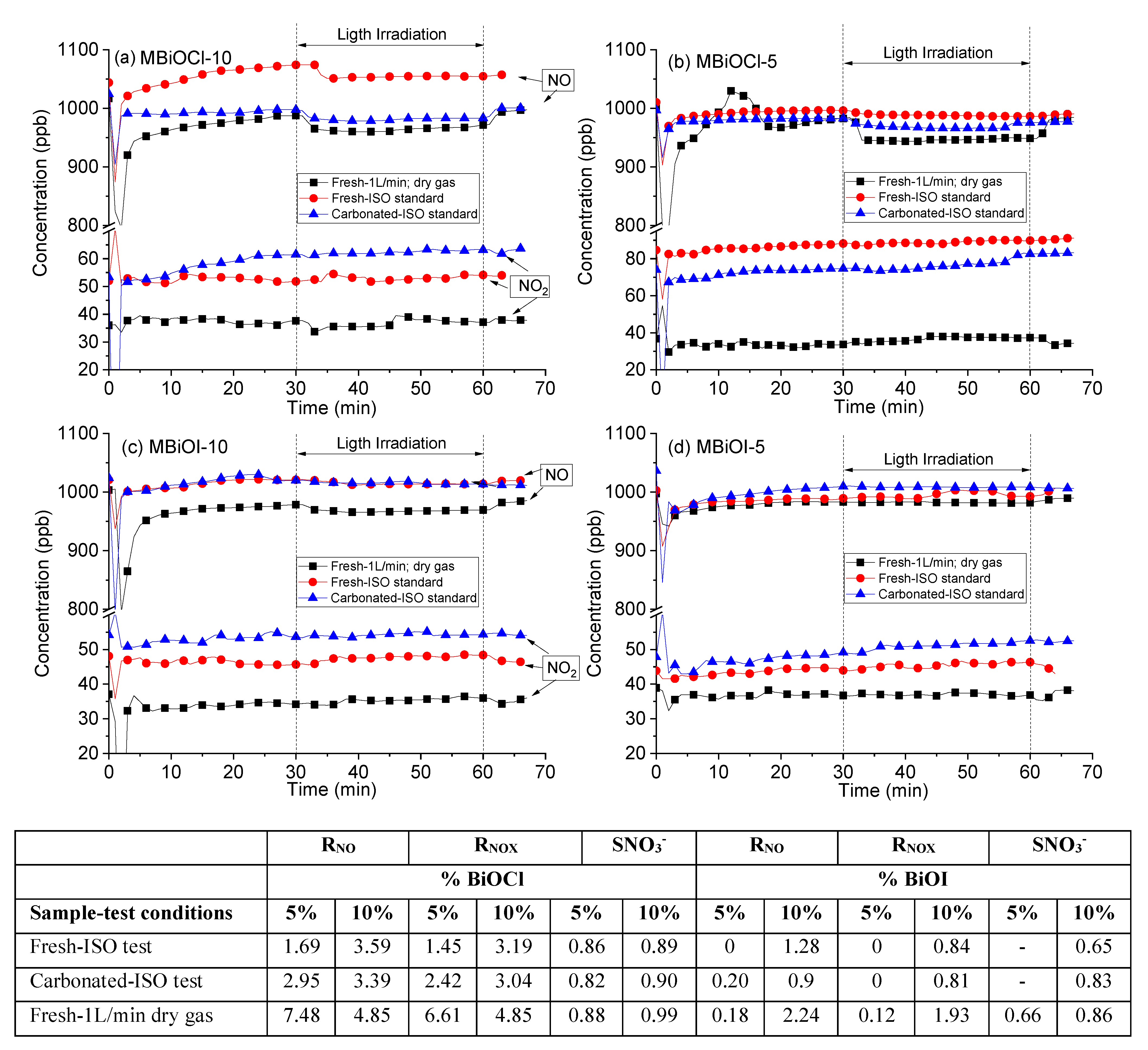
2.3. Photocatalytic Activity
3. Materials and Methods
3.1. Synthesis of BiOX (X = I and Cl)
3.2. Preparation of Photocatalytic Cement-Based Materials
3.3. Characterization of the Photocatalysts
3.4. Characterization of Photocatalytic Mortars
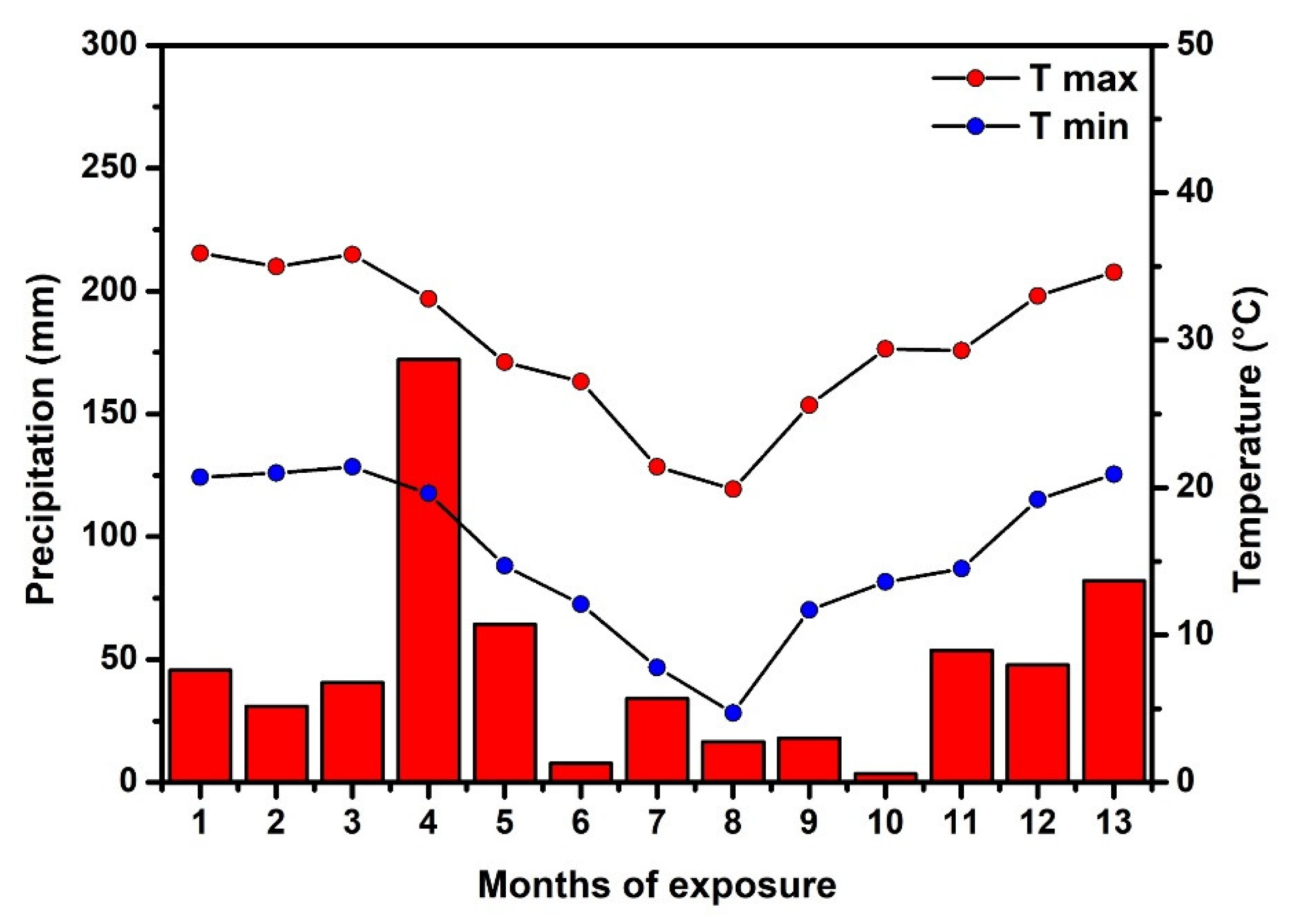
3.5. Exposure of Photocatalytic Mortars to Real Weathering
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Zhang, X.; Tryk, D.A. Heterogeneous photocatalysis: From water photolysis to applications in environmental cleanup. Int. J. Hydrog. Energy 2007, 32, 2664–2672. [Google Scholar] [CrossRef]
- Bengtsson, N.; Castellote, M. Photocatalytic Activity for NO Degradation by Construction Materials: Parametric Study and Multivariable. Correlations. J. Adv. Oxid. Technol. 2010, 13, 341–349. [Google Scholar]
- Laplaza, A.; Jiménez-Relinque, E.; Campos, J.; Castellote, M. Photocatalytic behavior of colored mortars containing TiO2 and iron oxide based pigments. Constr. Build Mater. 2017, 144, 300–310. [Google Scholar] [CrossRef]
- Calvo, J.G.; Carballosa, P.; Castillo, A.; Revuelta, D.; Gutiérrez, J.; Castellote, M. Expansive concretes with photocatalytic activity for pavements: Enhanced performance and modifications of the expansive hydrates composition. Constr. Build. Mater. 2019, 218, 394–403. [Google Scholar] [CrossRef]
- Diamanti, M.; Del Curto, B.; Ormellese, M.; Pedeferri, M. Photocatalytic and self-cleaning activity of colored mortars containing TiO2. Constr. Build. Mater. 2013, 46, 167–174. [Google Scholar] [CrossRef]
- Farzadnia, N.; Ali, A.A.A.; Demirboga, R.; Anwar, M.P. Characterization of high strength mortars with nano Titania at elevated temperatures. Constr. Build. Mater. 2013, 43, 469–479. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Castellote, M. Rapid assessment of the photocatalytic activity in construction materials: Pros and cons of reductive inks and oxidative fluorescence probes versus standardized NOx testing. Catal. Today 2019, 358, 164–171. [Google Scholar] [CrossRef]
- Macphee, D.E.; Folli, A. Photocatalytic concretes—The interface between photocatalysis and cement chemistry. Cem. Concr. Res. 2016, 85, 48–54. [Google Scholar] [CrossRef]
- Folli, A.; Strøm, M.; Madsen, T.P.; Henriksen, T.; Lang, J.; Emenius, J.; Klevebrant, T.; Nilsson, Å. Field study of air purifying paving elements containing TiO2. Atmos. Environ. 2015, 107, 44–51. [Google Scholar] [CrossRef]
- Jiménez-Relinque, E.; Hingorani, R.; Rubiano, F.; Grande, M.; Castillo, Á.; Castellote, M. In situ evaluation of the NOx removal efficiency of photocatalytic pavements: Statistical analysis of the relevance of exposure time and environmental variables. Environ. Sci. Pollut. Res. 2019, 26, 36088–36095. [Google Scholar] [CrossRef]
- Ballari, M.M.; Brouwers, H.J.H. Full scale demonstration of air-purifying pavement. J. Hazard. Mater. 2013, 254–255, 406–414. [Google Scholar] [CrossRef] [Green Version]
- Guerrini, G.L.; Crespo, R.; Jurado, R. Use of photocatalytic cements on urban roads with high traffic volumes. Carreteras 2017, 4, 49–55. [Google Scholar]
- Cordero, J.; Hingorani, R.; Jiménez-Relinque, E.; Grande, M.; Borge, R.; Narros, A.; Castellote, M. NOx removal efficiency of urban photocatalytic pavements at pilot scale. Sci. Total Environ. 2020, 719, 137459. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Relinque, E.; Castellote, M. Influence of the inlet air in efficiency of photocatalytic devices for mineralization of VOCs in air-conditioning installations. Environ. Sci. Pollut. Res. 2014, 21, 11198–11207. [Google Scholar] [CrossRef]
- Auvinen, J.; Wirtanen, L. The influence of photocatalytic interior paints on indoor air quality. Atmos. Environ. 2008, 42, 4101–4112. [Google Scholar] [CrossRef]
- Ai, Z.; Ho, W.; Lee, S.; Zhang, L.J.E.s. Efficient photocatalytic removal of NO in indoor air with hierarchical bismuth oxybromide nanoplate microspheres under visible light. Environ. Sci. Technol. 2009, 43, 4143–4150. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Zhang, Y.; Xu, Q.; Lamson, J.J.; Zhao, R. Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmos. Environ. 2009, 43, 2229–2246. [Google Scholar] [CrossRef]
- European Environmental Agency (EEA). Report, no. 15/2021. Air quality in Europe 2021. Available online: https://www.eea.europa.eu/publications/air-quality-in-europe-2021 (accessed on 1 June 2022).
- Chen, J.; Kou, S.-c.; Poon, C.-s. Photocatalytic cement-based materials: Comparison of nitrogen oxides and toluene removal potentials and evaluation of self-cleaning performance. Build. Environ. 2011, 46, 1827–1833. [Google Scholar] [CrossRef]
- Cassar, L. Photocatalysis of cementitious materials: Clean buildings and clean air. MRS Bull. 2004, 29, 328–331. [Google Scholar] [CrossRef]
- Folli, A.; Pade, C.; Hansen, T.B.; De Marco, T.; Macphee, D.E. TiO2 photocatalysis in cementitious systems: Insights into self-cleaning and depollution chemistry. Cem. Concr. Res. 2012, 42, 539–548. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Rodriguez-Garcia, J.; Castillo, A.; Castellote, M. Characteristics and efficiency of photocatalytic cementitious materials: Type of binder, roughness and microstructure. Cem. Concr. Res. 2015, 71, 124–131. [Google Scholar] [CrossRef]
- Enea, D.; Guerrini, G.L. Photocatalytic properties of cement-based plasters and paints containing mineral pigments. Transp. Res. Rec. 2010, 52–60. [Google Scholar] [CrossRef]
- Maggos, T.; Bartzis, J.; Liakou, M.; Gobin, C. Photocatalytic degradation of NOx gases using TiO2-containing paint: A real scale study. J. Hazard. Mater. 2007, 146, 668–673. [Google Scholar] [CrossRef]
- Mills, A.; O’Rourke, C.; Lawrie, K.; Elouali, S. Assessment of the Activity of Photocatalytic Paint Using a Simple Smart Ink Designed for High Activity Surfaces. ACS Appl. Mater. Interfaces 2014, 6, 545–552. [Google Scholar] [CrossRef]
- Luévano-Hipólito, E.; Martínez-De La Cruz, A. Photocatalytic stucco for NOx removal under artificial and by real weatherism. Constr. Build. Mater. 2018, 174, 302–309. [Google Scholar] [CrossRef]
- da Silva, A.L.; Dondi, M.; Raimondo, M.; Hotza, D. Photocatalytic ceramic tiles: Challenges and technological solutions. J. Eur. Ceram. Soc. 2018, 38, 1002–1017. [Google Scholar] [CrossRef]
- Carrillo, H.G.; Relinque, E.J.; Ramírez, A.M.; Castellote, M.; Pérez, M.R. Optimising processing conditions for the functionalisation of photocatalytic glazes by ZnO nanoparticle deposition. Mater. Construcción 2021, 71, 344. [Google Scholar]
- Chengyu, W.; Huamei, S.; Ying, T.; Tongsuo, Y.; Guowu, Z. Properties and morphology of CdS compounded TiO2 visible-light photocatalytic nanofilms coated on glass surface. Sep. Purif. Technol. 2003, 32, 357–362. [Google Scholar] [CrossRef]
- Hurum, D.C.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Recombination pathways in the Degussa P25 formulation of TiO2: Surface versus lattice mechanisms. J. Phys. Chem. B 2005, 109, 977–980. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Castellote, M. Hydroxyl radical and free and shallowly trapped electron generation and electron/hole recombination rates in TiO2 photocatalysis using different combinations of anatase and rutile. Appl. Catal. A Gen. 2018, 565, 20–25. [Google Scholar] [CrossRef]
- Landsberg, P.T. Recombination in Semiconductors 1991, 600. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.19930050123 (accessed on 1 June 2022).
- David Dankovic, E.K.; Geraci, C.; Gilbert, S.; Rice, F.; Schulte, P.; Smith, R.; Sofge, C.; Wheeler, M.; Zumwalde, R. Occupational Exposure to Titanium Dioxide; Department of health and human services, Centers for disease control and prevention, National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 2011; p. 120. [Google Scholar]
- Chen, R.; Hu, B.; Liu, Y.; Xu, J.; Yang, G.; Xu, D.; Chen, C. Beyond PM2.5: The role of ultrafine particles on adverse health effects of air pollution. Biochim. Biophys. Acta BBA Gen. Subj. 2016, 1860, 2844–2855. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Delegated Regulation (EU) 2020/217 of 4 October 2019 amending, for the purposes of its adaptation to technical and scientific progress, Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures and correcting that Regulation (Text with EEA relevance). 2020. Available online: https://www.legislation.gov.uk/eur/2020/217 (accessed on 1 June 2022).
- Nava Núñez, M.; Martínez-de la Cruz, A.; López-Cuéllar, E. Preparation of BiOI microspheres in 2-propanol/ethylene glycol by microwave method with high visible-light photocatalytic activity. Res. Chem. Intermed. 2019, 45, 1475–1492. [Google Scholar] [CrossRef]
- Montoya-Zamora, J.; Martinez-De La Cruz, A.; Lopez-Cuellar, E.; González, F.P. BiOBr photocatalyst with high activity for NOx elimination. Adv. Powder Technol. 2020, 31, 3618–3627. [Google Scholar] [CrossRef]
- Reyna-Cavazos, K.; la Cruz, A.; Longoria Rodríguez, F.; López-Cuellar, E. Synthesis of bismuth oxyiodide (BiOI) by means of microwaves in glycerol with high photocatalytic activity for the elimination of NOx and SO2. Res. Chem. Intermed. 2020, 46, 923–941. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Li, L.; Gao, H.; Zhang, X. BiOCl nanosheet/Bi4Ti3O12 nanofiber heterostructures with enhanced photocatalytic activity. Catal. Commun. 2015, 58, 122–126. [Google Scholar] [CrossRef]
- Geng, Z.; Xin, M.; Zhu, X.; Xu, H.; Cheng, X.; Wang, D. A new method for preparing photocatalytic cement-based materials and the investigation on properties and mechanism. J. Build. Eng. 2021, 35, 102080. [Google Scholar] [CrossRef]
- Wang, D.; Hou, P.; Yang, P.; Cheng, X. BiOBr@ SiO2 flower-like nanospheres chemically-bonded on cement-based materials for photocatalysis. Appl Surf. Sci 2018, 430, 539–548. [Google Scholar] [CrossRef]
- Nava-Núñez, M.Y.; Jimenez-Relinque, E.; Grande, M.; Martínez-de la Cruz, A.; Castellote, M. Photocatalytic BiOX Mortars under Visible Light Irradiation: Compatibility, NOx Efficiency and Nitrate Selectivity. Catalysts 2020, 10, 226. [Google Scholar] [CrossRef]
- Kaja, A.M.; Brouwers, H.J.H.; Yu, Q.L. NOx degradation by photocatalytic mortars: The underlying role of the CH and C-S-H carbonation. Cem. Concr. Res. 2019, 125, 105805. [Google Scholar] [CrossRef]
- Yang, L.; Hakki, A.; Wang, F.; Macphee, D.E. Photocatalyst efficiencies in concrete technology: The effect of photocatalyst placement. Appl. Catal. B Environ. 2018, 222, 200–208. [Google Scholar] [CrossRef]
- Chen, X.-F.; Kou, S.-C.; Poon, C.S. Rheological behaviour, mechanical performance, and NOx removal of photocatalytic mortar with combined clay brick sands-based and recycled glass-based nano-TiO2 composite photocatalysts. Constr. Build. Mater. 2020, 240, 117698. [Google Scholar] [CrossRef]
- Yang, L.; Hakki, A.; Zheng, L.; Jones, M.R.; Wang, F.; Macphee, D.E. Photocatalytic concrete for NOx abatement: Supported TiO2 efficiencies and impacts. Cem. Concr. Res. 2019, 116, 57–64. [Google Scholar] [CrossRef]
- He, R.; Huang, X.; Zhang, J.; Geng, Y.; Guo, H. Preparation and evaluation of exhaust-purifying cement concrete employing titanium dioxide. Materials 2019, 12, 2182. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Clack, H.; Xia, T.; Bao, Y.; Wu, K.; Shi, H.; Li, V. Effect of TiO2 and fly ash on photocatalytic NOx abatement of engineered cementitious composites. Constr. Build. Mater. 2020, 236, 117559. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Maury-Ramirez, A.; Poon, C.S. Photocatalytic activities of titanium dioxide incorporated architectural mortars: Effects of weathering and activation light. Build. Environ. 2015, 94, 395–402. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Grande, M.; Duran, T.; Castillo, Á.; Castellote, M. Environmental impact of nano-functionalized construction materials: Leaching of titanium and nitrates from photocatalytic pavements under outdoor conditions. Sci. Total Environ. 2020, 140817. [Google Scholar] [CrossRef] [PubMed]
- Bloh, J.Z.; Folli, A.; Macphee, D.E.J.R.A. Photocatalytic NOx abatement: Why the selectivity matters. RSC Adv. 2014, 4, 45726–45734. [Google Scholar] [CrossRef]
- Dantas, S.R.A.; Vittorino, F.; Loh, K. Photocatalytic performance of white cement mortars exposed in urban atmosphere. Glob. J. Res. Eng. 2019, 19, 2. [Google Scholar]
- Castellote, M.; Andrade, C.; Turrillas, X.; Campo, J.; Cuello, G. Accelerated carbonation of cement pastes in situ monitored by neutron diffraction. Cem. Concr. Res. 2008, 38, 1365–1373. [Google Scholar] [CrossRef]
- Hingorani, R.; Jimenez-Relinque, E.; Grande, M.; Castillo, A.; Nevshupa, R.; Castellote, M. From analysis to decision: Revision of a multifactorial model for the in situ assessment of NOx abatement effectiveness of photocatalytic pavements. Chem. Eng. J. 2020, 402, 126250. [Google Scholar] [CrossRef]
- Anderson, C.; Bard, A.J. Improved photocatalytic activity and characterization of mixed TiO2/SiO2 and TiO2/Al2O3 materials. J. Phys. Chem. B 1997, 101, 2611–2616. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Rubiano, F.; Hingorani, R.; Grande, M.; Castillo, A.; Nevshupa, R.; Castellote, M. New Holistic Conceptual Framework for the Assessment of the Performance of Photocatalytic Pavement. Front. Chem. 2020, 8, 743. [Google Scholar] [CrossRef]
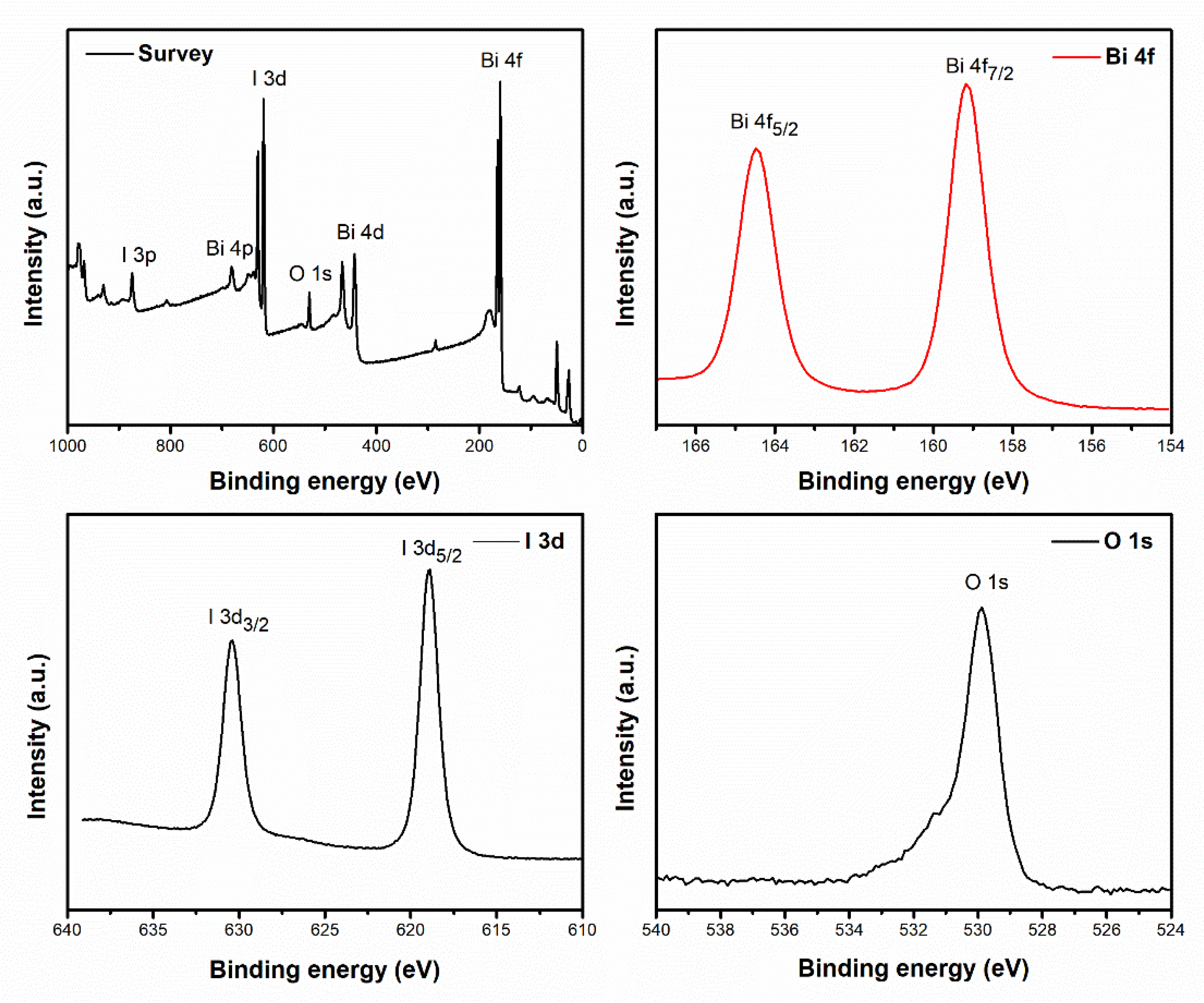


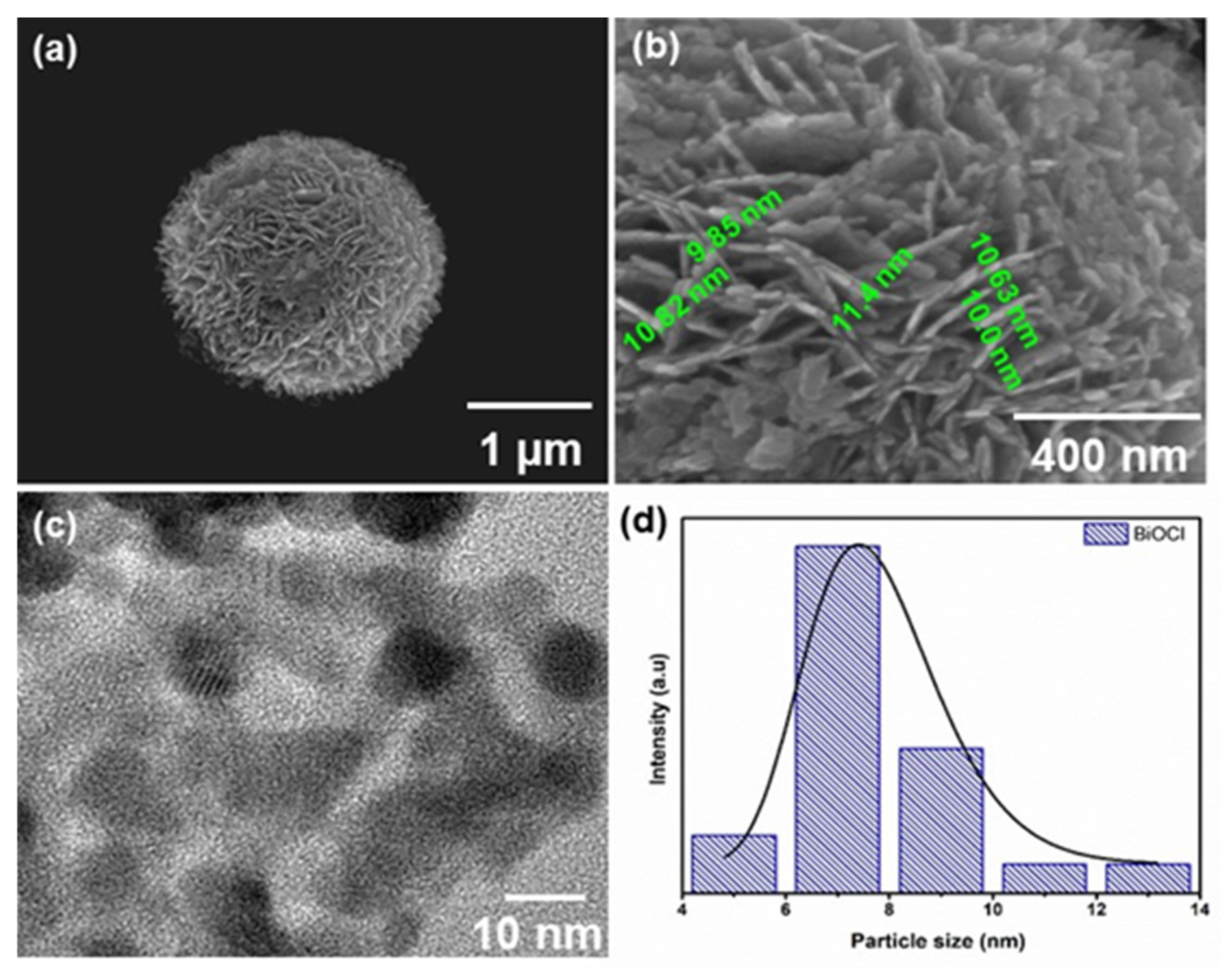

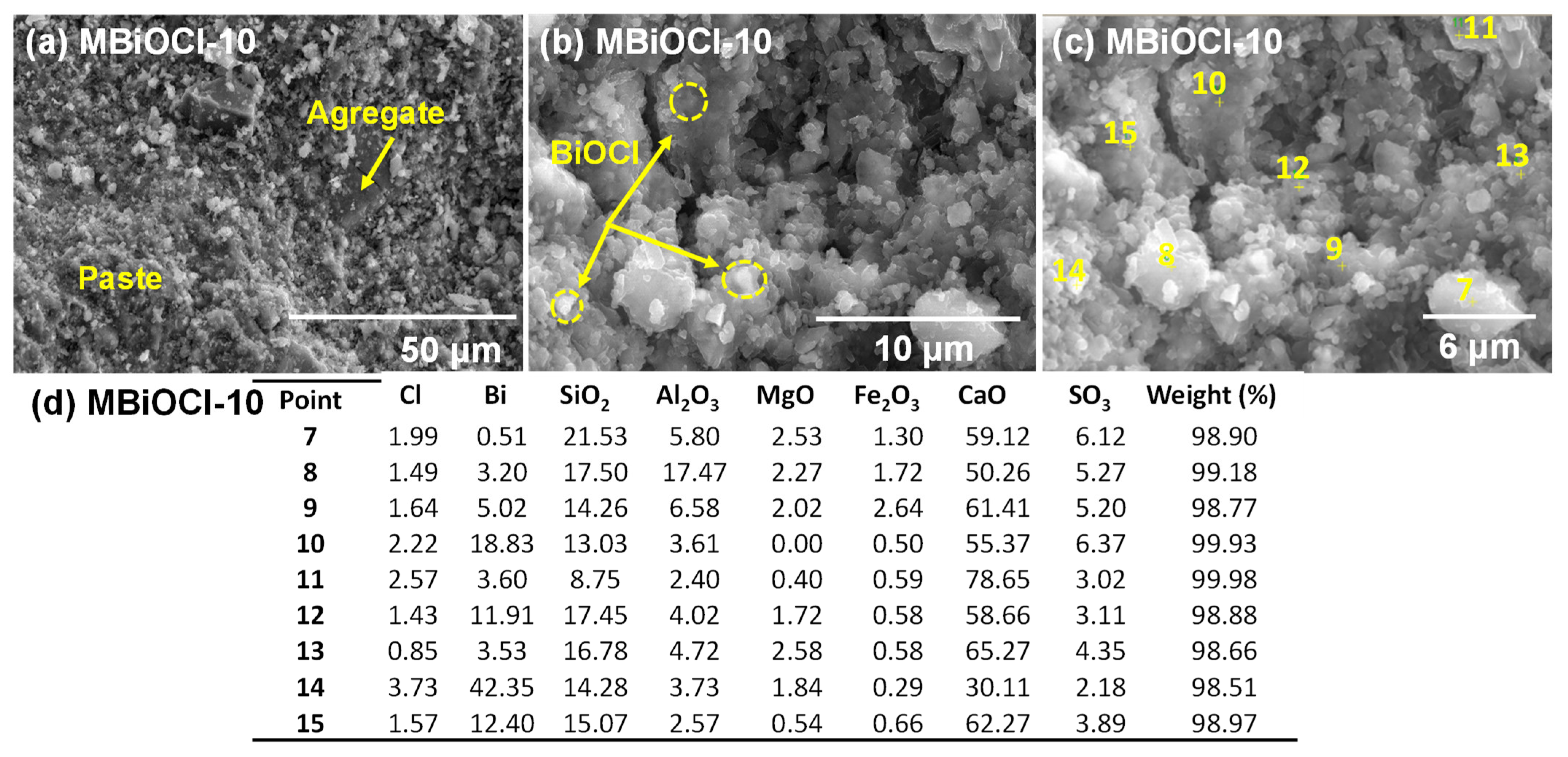

| Sample | Crystallite Size (nm) a | Band Gap (eV) b | Surface Area (m2.g−1) c |
|---|---|---|---|
| BiOI | 8 ± 3 | 1.9 | 57 |
| BiOCl | 12 ± 4 | 3.4 | 40 |
| TiO2 | 35 * | 3.2 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nava-Núñez, M.Y.; Jimenez-Relinque, E.; Martínez-de la Cruz, A.; Castellote, M. Photocatalytic NOx Removal in Bismuth-Oxyhalide (BiOX, X = I, Cl) Cement-Based Materials Exposed to Outdoor Conditions. Catalysts 2022, 12, 982. https://doi.org/10.3390/catal12090982
Nava-Núñez MY, Jimenez-Relinque E, Martínez-de la Cruz A, Castellote M. Photocatalytic NOx Removal in Bismuth-Oxyhalide (BiOX, X = I, Cl) Cement-Based Materials Exposed to Outdoor Conditions. Catalysts. 2022; 12(9):982. https://doi.org/10.3390/catal12090982
Chicago/Turabian StyleNava-Núñez, Magaly Y., Eva Jimenez-Relinque, Azael Martínez-de la Cruz, and Marta Castellote. 2022. "Photocatalytic NOx Removal in Bismuth-Oxyhalide (BiOX, X = I, Cl) Cement-Based Materials Exposed to Outdoor Conditions" Catalysts 12, no. 9: 982. https://doi.org/10.3390/catal12090982





