Green Synthesis of CuO-TiO2 Nanoparticles for the Degradation of Organic Pollutants: Physical, Optical and Electrochemical Properties
Abstract
1. Introduction
2. Results and Discussions
2.1. FTIR Characterization
2.2. Optical Properties of CuO-TiO2 Nanocomposites
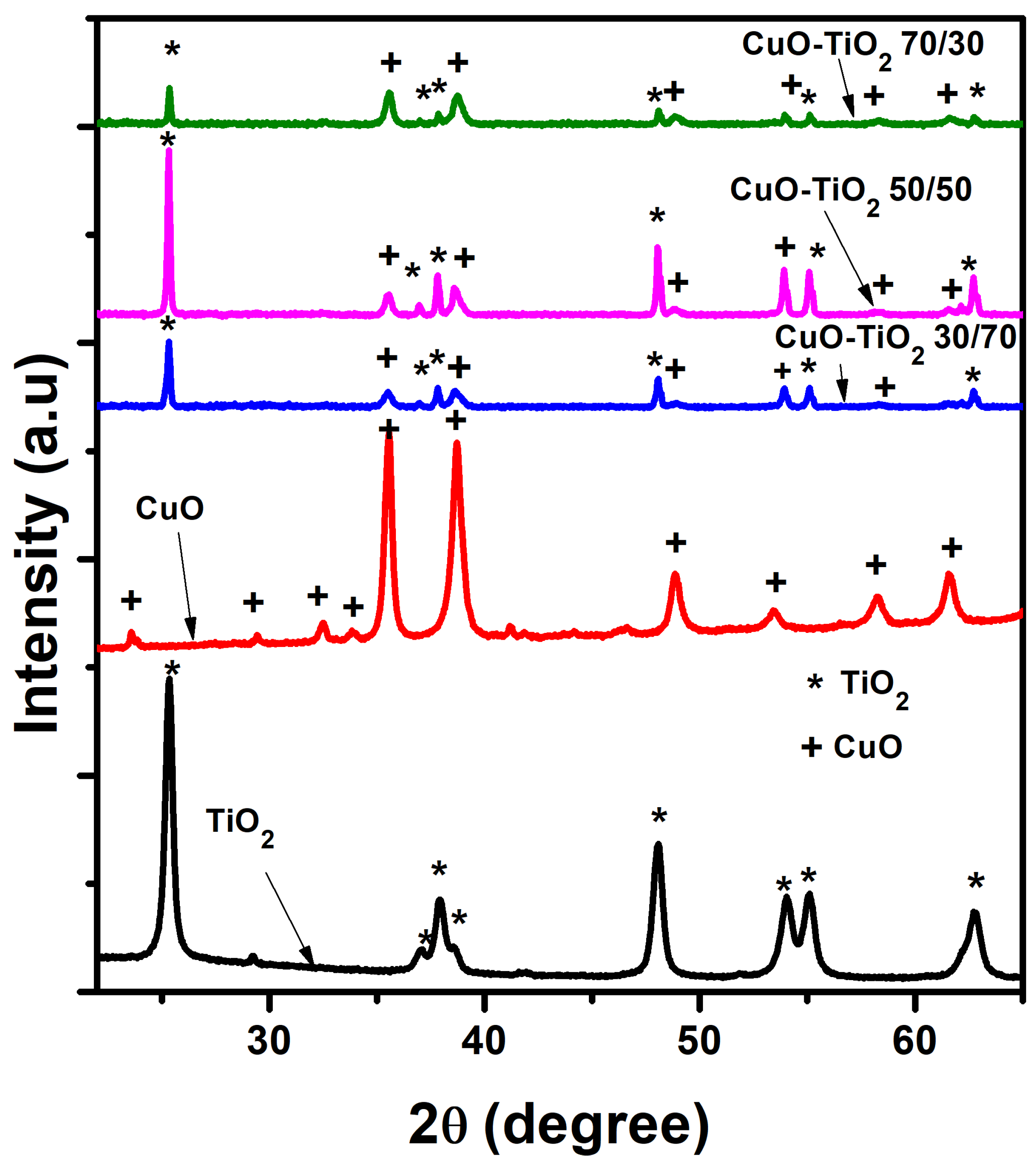
2.3. Structural and Phase Composition Analysis
- λ = X-ray wavelength (0.154060 nm)
- β = Full width at half-maximum of the (101) XRD peak
- θ = The Bragg diffraction angle (degree).
2.4. Morphological Analysis of the Various Materials
2.5. TGA and DTA Analysis
2.6. N2 Adsorption-Desorption Studies, BJH Pore Volume, and BET Surface Area
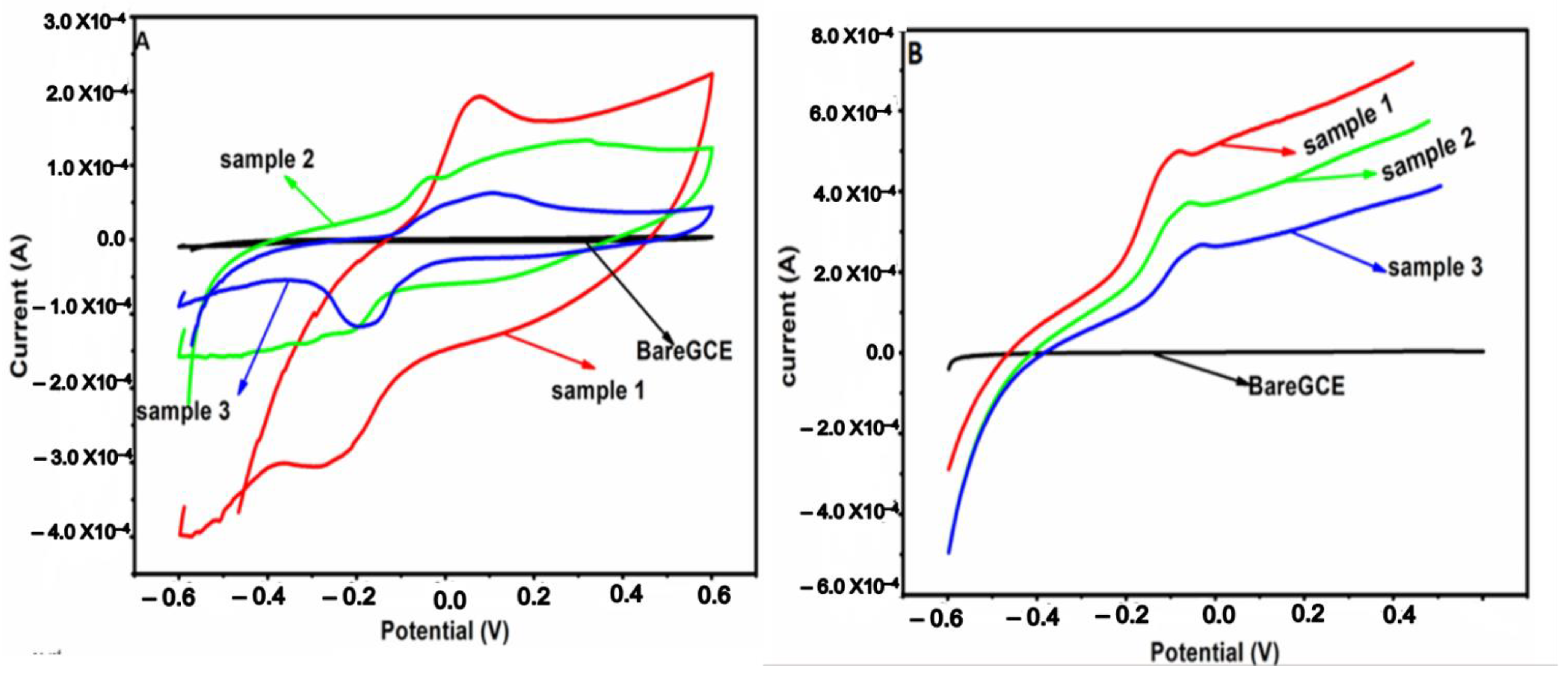
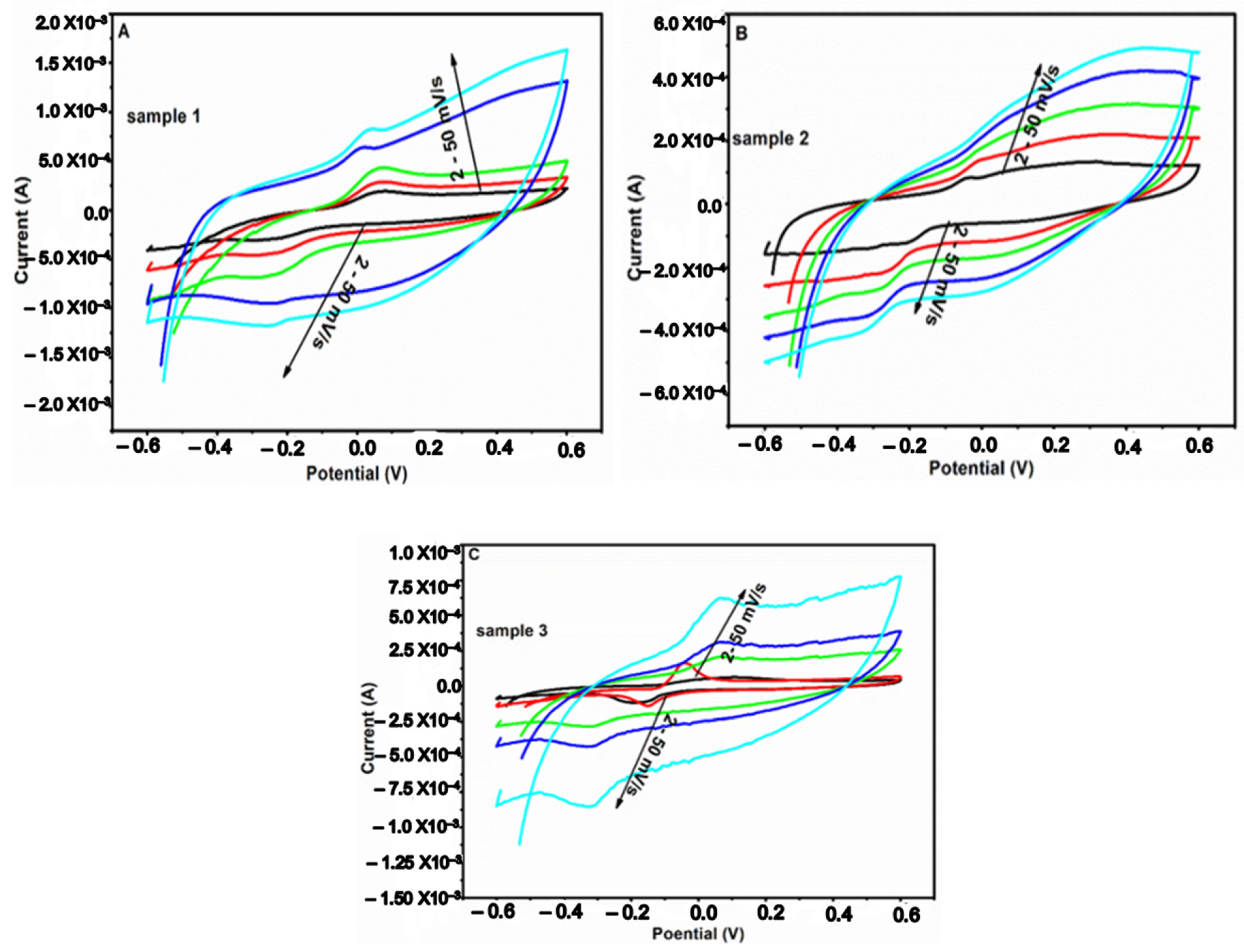
2.7. Electrochemical Characterization of the Composites
2.8. Photodegradation of MB Dye Using the CuO-TiO2 Nanocomposite
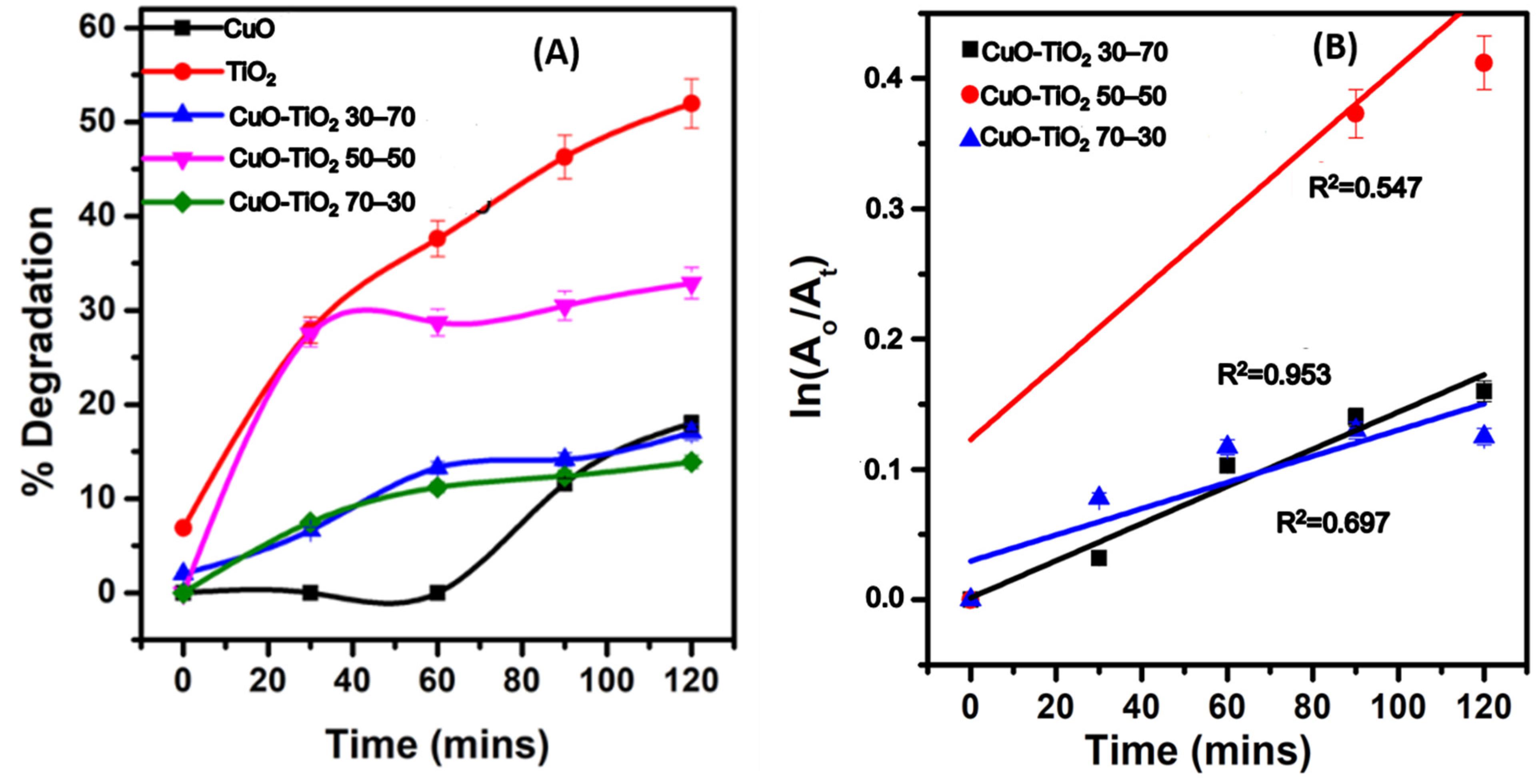
2.8.1. Photodegradation of MB UV Light
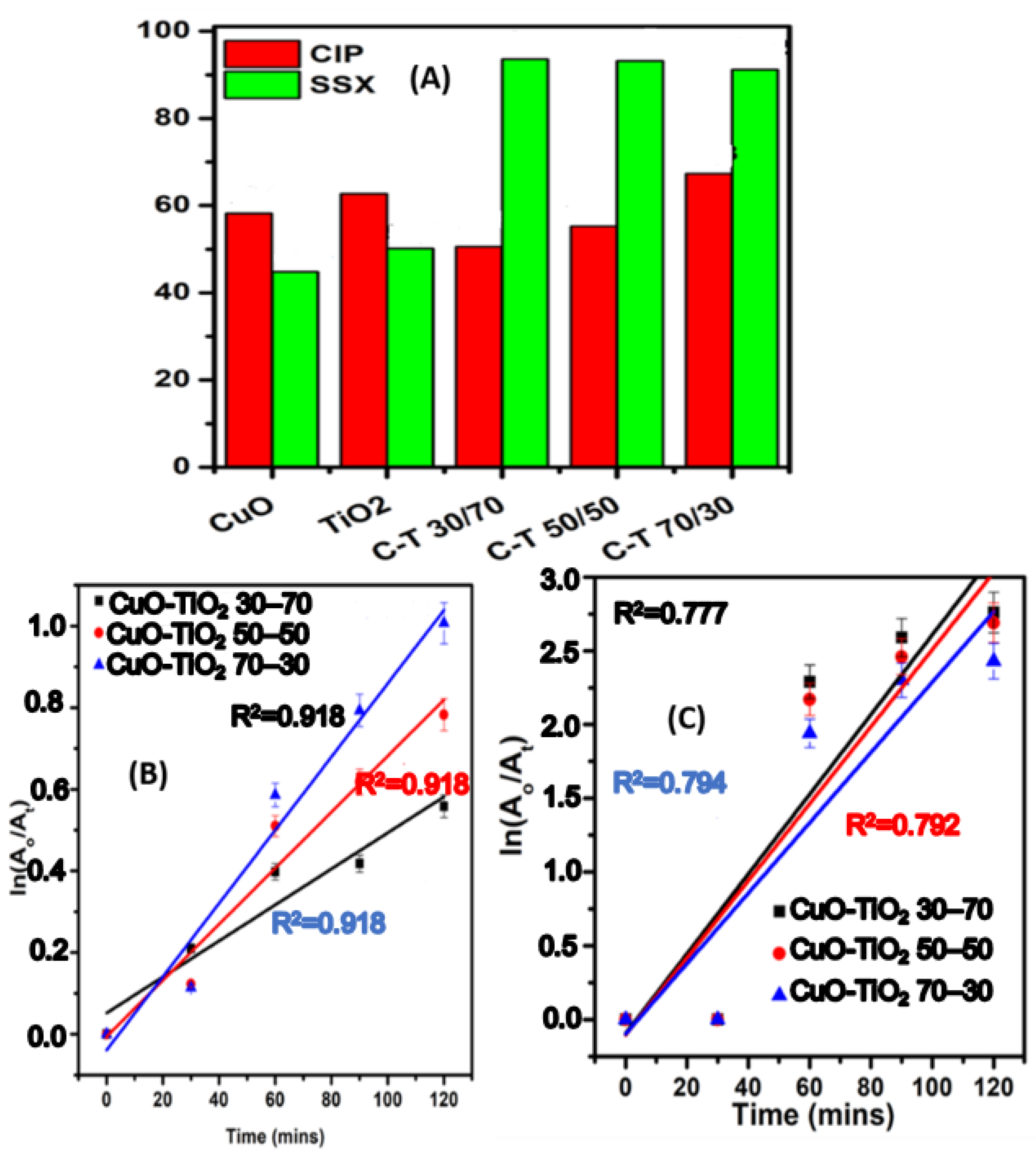
2.8.2. Photodegradation of CIP and SSX Using UV light
2.8.3. Effect of Trapping and Mechanism of Degradation
3. Experimental Procedure
3.1. Chemicals and Materials
3.2. Preparation of the Plant Extracts
3.3. Preparation of C. benghalensis-Mediated TiO2 Nanoparticle Synthesis
3.4. Preparation of C. benghalensis-Mediated CuO-NPs
3.5. Synthesis of CuO-TiO2 Nanocomposite
3.6. Characterization of CuO-TiO2 Materials
3.7. Electrochemical Experiment
3.8. Preparation of the Modified Electrode
3.9. Photocatalytic Degradation of Dyes and Antibiotics
- Ao = is the initial absorbance at 0 min (adsorption-desorption).
- Af = is the final absorbance of 30, 60, 90, and 120 min of photodegradation
3.10. Recyclability Studies of SSX
3.11. Effect of Scavengers on the Degradation of SSX
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khoshbin, S.; Seyyedi, K. Removal of acid red 1 dye pollutant from contaminated waters by electrocoagulation method using a recirculating tubular reactor. Lat. Am. Appl. Res. 2017, 47, 101–105. [Google Scholar] [CrossRef]
- Santhy, K.; Selvapathy, P. Removal of reactive dyes from wastewater by adsorption on coir pith activated carbon. Bioresour. Technol. 2006, 97, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.J.; Gómez-Ramos, M.M.; Agüera, A.; Mezcua, M.; Herrera, S.; Fernández-Alba, A.R. A new gas chromatography/mass spectrometry method for the simultaneous analysis of target and non-target organic contaminants in waters. J. Chromatogr. A 2009, 1216, 4071–4082. [Google Scholar] [CrossRef] [PubMed]
- Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; Okundaye, B.; Pal, K.; Osibote, O.A.; Esiekpe, E.L.; Kusuma, H.S.; Darmokoesoemo, H. A facile review on the sorption of heavy metals and dyes using bionanocomposites. Adsorpt. Sci. Technol. 2022, 2022, 8030175. [Google Scholar] [CrossRef]
- Arismendi, D.; Becerra-Herrera, M.; Cerrato, I.; Richter, P. Simultaneous determination of multiresidue and multiclass emerging contaminants in waters by rotating-disk sorptive extraction–derivatization-gas chromatography/mass spectrometry. Talanta 2019, 201, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, D.; Dhandapani, B.; Authilingam, S.; Sivakumar, S.V. A comprehensive review of effective adsorbents used for the removal of dyes from wastewater. Curr. Anal. Chem. 2022, 18, 255–268. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kapoor, A.; Prabhakar, S. Valorization of food waste as adsorbents for toxic dye removal from contaminated waters: A review. J. Hazard. Mater. 2022, 424, 127432. [Google Scholar] [CrossRef]
- Aftab, S.; Shabir, T.; Shah, A.; Nisar, J.; Shah, I.; Muhammad, H.; Shah, N.S. Highly efficient visible light active doped ZnO photocatalysts for the treatment of wastewater contaminated with dyes and pathogens of emerging concern. Nanomaterials 2022, 12, 486. [Google Scholar] [CrossRef]
- Zhao, X.; Baharinikoo, L.; Farahani, M.D.; Mahdizadeh, B.; Farizhandi, A.A.K. Experimental modelling studies on the removal of dyes and heavy metal ions using ZnFe2O4 nanoparticles. Sci. Rep. 2022, 12, 5987. [Google Scholar] [CrossRef]
- Ariaeenejad, S.; Motamedi, E.; Salekdeh, G.H. Highly efficient removal of dyes from wastewater using nanocellulose from quinoa husk as a carrier for immobilization of laccase. Bioresour. Technol. 2022, 349, 126833. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, Y.; Wang, W.; Zhang, T.; Chen, T.; Yi, H.; Rao, F.; Song, S. Removal of methylene blue from water with montmorillonite nanosheets/chitosan hydrogels as adsorbent. Appl. Surf. Sci. 2018, 448, 203–211. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Manzoor, O.; Mohsin, M.; Chaudhry, S.A. Nigella sativa seed based nanocomposite-MnO2/BC: An antibacterial material for photocatalytic degradation, and adsorptive removal of Methylene blue from water. Environ. Res. 2019, 171, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Jian, H.; Dou, Q.; Shi, X.; Su, R. Indirect photodegradation of sulfisoxazole: Effects of environmental factors (CDOM, pH, salinity, HCO3−, metal ions, halogen ions and NO3−). Mar. Pollut. Bull. 2022, 174, 113320. [Google Scholar] [CrossRef] [PubMed]
- Orachorn, N.; Bunkoed, O. Nanohybrid magnetic composite optosensing probes for the enrichment and ultra-trace detection of mafenide and sulfisoxazole. Talanta 2021, 228, 122237. [Google Scholar] [CrossRef] [PubMed]
- Uhlemann, T.; Seidel, S.; Müller, C.W. Site-specific binding of a water molecule to the sulfa drugs sulfamethoxazole and sulfisoxazole: A laser-desorption isomer-specific UV and IR study. Phys. Chem. Chem. Phys. 2018, 20, 6891–6904. [Google Scholar] [CrossRef]
- Girardi, C.; Greve, J.; Lamshöft, M.; Fetzer, I.; Miltner, A.; Schäffer, A.; Kästner, M. Biodegradation of ciprofloxacin in water and soil and its effects on the microbial communities. J. Hazard. Mater. 2011, 198, 22–30. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Oba, S.N.; Aniagor, C.O.; Adeniyi, A.G.; Ighalo, J.O. Adsorption of ciprofloxacin from water: A comprehensive review. J. Ind. Eng. Chem. 2021, 93, 57–77. [Google Scholar] [CrossRef]
- Ojo, B.O.; Arotiba, O.A.; Mabuba, N. Sonoelectrochemical degradation of ciprofloxacin in water on a Ti/BaTiO3 electrode. J. Environ. Chem. Eng. 2022, 10, 107224. [Google Scholar] [CrossRef]
- Xing, X.; Du, Z.; Zhuang, J.; Wang, D. Removal of ciprofloxacin from water by nitrogen doped TiO2 immobilized on glass spheres: Rapid screening of degradation products. J. Photochem. Photobiol. A Chem. 2018, 359, 23–32. [Google Scholar] [CrossRef]
- Soto-Robles, C.A.; Luque, P.A.; Gómez-Gutiérrez, C.M.; Nava, O.; Vilchis-Nestor, A.R.; Lugo-Medina, E.; Ranjithkumar, R.; Castro-Beltrán, A. Study on the effect of the concentration of Hibiscus sabdariffa extract on the green synthesis of ZnO nanoparticles. Results Phys. 2019, 15, 102807. [Google Scholar] [CrossRef]
- Ngoepe, N.M.; Mathipa, M.M.; Hintsho-Mbita, N.C. Biosynthesis of titanium dioxide for the degradation of methylene blue and removal of bacteria. Optik 2020, 224, 165728. [Google Scholar] [CrossRef]
- Jang, Y.J.; Simer, C.; Ohm, T. Comparison of zinc oxide nanoparticles and its nano-crystalline particles on the photocatalytic degradation of methylene blue. Mater. Res. Bull. 2006, 41, 67–77. [Google Scholar] [CrossRef]
- Munyai, S.; Hintsho-Mbita, N.C. Green derived metal suphides as photocatalysts for wastewater treatment. A review. Curr. Res. Green Sustain. Chem. 2021, 4, 100163. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, J.; Zhang, Z.; Zhang, W.; Wang, A.; Tian, F.; Zhao, W.; Yan, J. Green synthesis of CuO nanoparticles-loaded ZnO nanowires arrays with enhanced photocatalytic activity. Mater. Chem. Phys. 2021, 267, 124703. [Google Scholar] [CrossRef]
- Bopape, D.A.; Motaung, D.E.; Hintsho-Mbita, N.C. Green synthesis of ZnO: Effect of plant concentration on the morphology, optical properties and photodegradation of dyes and antibiotics in wastewater. Optik 2022, 251, 168459. [Google Scholar] [CrossRef]
- Prabu, H.J.; Varghese, R.; Johnson, I.; Sundaram, S.J.; Raj, A.D.; Rajakrishnan, R.; Kuppusamy, P.; Sathya, R.; Kaviyarasu, K. Laser induced plant leaf extract mediated synthesis of CuO nanoparticles and its photocatalytic activity. Environ. Res. 2022, 212, 113295. [Google Scholar] [CrossRef]
- Chowdhury, I.H.; Ghosh, S.; Basak, S.; Naskar, M.K. Mesoporous CuO-TiO2 microspheres for efficient catalytic oxidation of CO and photodegradation of methylene blue. J. Phys. Chem. Solids 2017, 104, 103–110. [Google Scholar] [CrossRef]
- Patil, B.N.; Taranath, T.C. Limonia acidissima L. leaf mediated synthesis of silver and zinc oxide nanoparticles and their antibacterial activities. Microb. Pathog. 2018, 115, 227–232. [Google Scholar] [CrossRef]
- Lu, D.; Zelekew, O.A.; Abay, A.K.; Huang, Q.; Chen, X.; Zheng, Y. Synthesis and photocatalytic activities of a CuO/TiO2 composite catalyst using aquatic plants with accumulated copper as a template. RSC Adv. 2019, 9, 2018–2025. [Google Scholar] [CrossRef]
- Khodadadi, B.; Yeganeh Faal, A.; Shahvarughi, A. Tilia platyphyllos extract assisted green synthesis of CuO/TiO2 nanocomposite: Application as a reusable catalyst for the reduction of organic dyes in water. J. Appl. Chem. Res. 2019, 13, 51–65. [Google Scholar]
- Ibrahim, J.; Ajaegbu, V.C.; Egharevba, H.O. Pharmacognostic and phytochemical analysis of Commelina benghalensis L. Ethnobot. Leafl. 2010, 2010, 7. [Google Scholar]
- Johnson, I.; Prabu, H.J. Green synthesis and characterization of silver nanoparticles by leaf extracts of Cycas circinalis, Ficus amplissima, Commelina benghalensis and Lippia nodiflora. Int. Nano Lett. 2015, 5, 43–51. [Google Scholar] [CrossRef]
- Feng, Y.; Yan, X.; Liu, C.; Hong, Y.; Zhu, L.; Zhou, M.; Shi, W. Hydrothermal synthesis of CdS/Bi2MoO6 heterojunction photocatalysts with excellent visible-light-driven photocatalytic performance. Appl. Surf. Sci. 2015, 353, 87–94. [Google Scholar] [CrossRef]
- Karim, K.M.R.; Jebi, M.A.P.; Ong, H.R.; Abdullah, H.; Tarek, M.; Cheng, C.K.; Khan, M.M.R. CuO-TiO2 as a visible light responsive photocatalyst for the photoelectroreduction of CO2 to methanol. Natl. Conf. Postgrad. Res. 2018, 103–111. [Google Scholar]
- Gnanasekaran, L.; Pachaiappan, R.; Kumar, P.S.; Hoang, T.K.; Rajendran, S.; Durgalakshmi, D.; Soto-Moscoso, M.; Cornejo-Ponce, L.; Gracia, F. Visible light driven exotic p (CuO)-n (TiO2) heterojunction for the photodegradation of 4-chlorophenol and antibacterial activity. Environ. Pollut. 2021, 287, 117304. [Google Scholar] [CrossRef] [PubMed]
- Muzakki, A.; Shabrany, H.; Saleh, R. Synthesis of ZnO/CuO and TiO2/CuO nanocomposites for light and ultrasound assisted degradation of a textile dye in aqueous solution. AIP Conf. Proc. 2016, 1725, 020051. [Google Scholar]
- Kubiak, A.; Bielan, Z.; Kubacka, M.; Gabała, E.; Zgoła-Grześkowiak, A.; Janczarek, M.; Zalas, M.; Zielińska-Jurek, A.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Microwave-assisted synthesis of a TiO2-CuO heterojunction with enhanced photocatalytic activity against tetracycline. Appl. Surf. Sci. 2020, 520, 146344. [Google Scholar] [CrossRef]
- Zangeneh, H.; Farhadian, M.; Zinatizadeh, A.A. N (Urea) and CN (L-Asparagine) doped TiO2-CuO nanocomposites: Fabrication, characterization and photodegradation of direct red 16. J. Environ. Chem. Eng. 2020, 8, 103639. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, X.; Shi, Q.; Ping, G.; Xu, H.; Waleed, A.; Li, G. CuO nanoparticle-decorated TiO2-nanotube heterojunctions for direct synthesis of methyl formate via photo-oxidation of methanol. ACS Omega 2020, 5, 15942–15948. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, Z.; Yu, C.; Waheed, A.; Xu, H.; Gao, Y.; Abroshan, H.; Li, G. Experimental and mechanistic understanding of photo-oxidation of methanol catalyzed by CuO/TiO2-spindle nanocomposite: Oxygen vacancy engineering. Nano Res. 2020, 13, 939–946. [Google Scholar] [CrossRef]
- Yu, Y.H.; Chen, Y.P.; Cheng, Z. Microwave-assisted synthesis of rod-like CuO/TiO2 for high-efficiency photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2015, 40, 15994–16000. [Google Scholar] [CrossRef]
- Ashok, C.H.; Venkateswara Rao, K. Microwave-assisted synthesis of CuO/TiO2 nanocomposite for humidity sensor application. J. Mater. Sci. Mater. Electron. 2016, 27, 8816–8825. [Google Scholar] [CrossRef]
- Fouda, A.; Salem, S.S.; Wassel, A.R.; Hamza, M.F.; Shaheen, T.I. Optimization of green biosynthesized visible light active CuO/ZnO nano-photocatalysts for the degradation of organic methylene blue dye. Heliyon 2020, 6, e04896. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Guo, Z. Hybrid Hydrophilic–Hydrophobic CuO@ TiO2-Coated Copper Mesh for Efficient Water Harvesting. Langmuir 2019, 36, 64–73. [Google Scholar] [CrossRef]
- Tayeb, A.M.; Hussein, D.S. Synthesis of TiO2 nanoparticles and their photocatalytic activity for methylene blue. Am. J. Nanomater. 2015, 3, 57–63. [Google Scholar]
- Sankar, R.; Manikandan, P.; Malarvizhi, V.; Fathima, T.; Shivashangari, K.S.; Ravikumar, V. Green synthesis of colloidal copper oxide nanoparticles using Carica papaya and its application in photocatalytic dye degradation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 121, 746–750. [Google Scholar] [CrossRef]
- Lingaraju, K.; Naika, H.R.; Manjunath, K.; Nagaraju, G.; Suresh, D.; Nagabhushana, H. Rauvolfia serpentina-mediated green synthesis of CuO nanoparticles and its multidisciplinary studies. Acta Metall. Sin. (Engl. Lett.) 2015, 28, 1134–1140. [Google Scholar] [CrossRef]
- Mofokeng, L.E.; Hlekelele, L.; Moma, J.; Tetana, Z.N.; Chauke, V.P. Energy-efficient CuO/TiO2@ GCN cellulose acetate-based membrane for concurrent filtration and photodegradation of ketoprofen in drinking and groundwater. Appl. Sci. 2022, 12, 1649. [Google Scholar] [CrossRef]
- Castañeda, C.; Martínez, J.J.; Santos, L.; Rojas, H.; Osman, S.M.; Gómez, R.; Luque, R. Caffeine photocatalytic degradation using composites of NiO/TiO2–F and CuO/TiO2–F under UV irradiation. Chemosphere 2022, 288, 132506. [Google Scholar] [CrossRef]
- Hajipour, P.; Eslami, A.; Bahrami, A.; Hosseini-Abari, A.; Saber, F.Y.; Mohammadi, R.; Mehr, M.Y. Surface modification of TiO2 nanoparticles with CuO for visible-light antibacterial applications and photocatalytic degradation of antibiotics. Ceram. Int. 2021, 47, 33875–33885. [Google Scholar] [CrossRef]
- Cosma, D.; Urda, A.; Radu, T.; Rosu, M.C.; Mihet, M.; Socaci, C. Evaluation of the Photocatalytic Properties of Copper Oxides/Graphene/TiO2 Nanoparticles Composites. Molecules 2022, 27, 5803. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Y.; Zhu, M. Green synthesis of 3D tripyramid TiO2 architectures with assistance of aloe extracts for highly efficient photocatalytic degradation of antibiotic ciprofloxacin. Appl. Catal. B Environ. 2020, 260, 118149. [Google Scholar] [CrossRef]
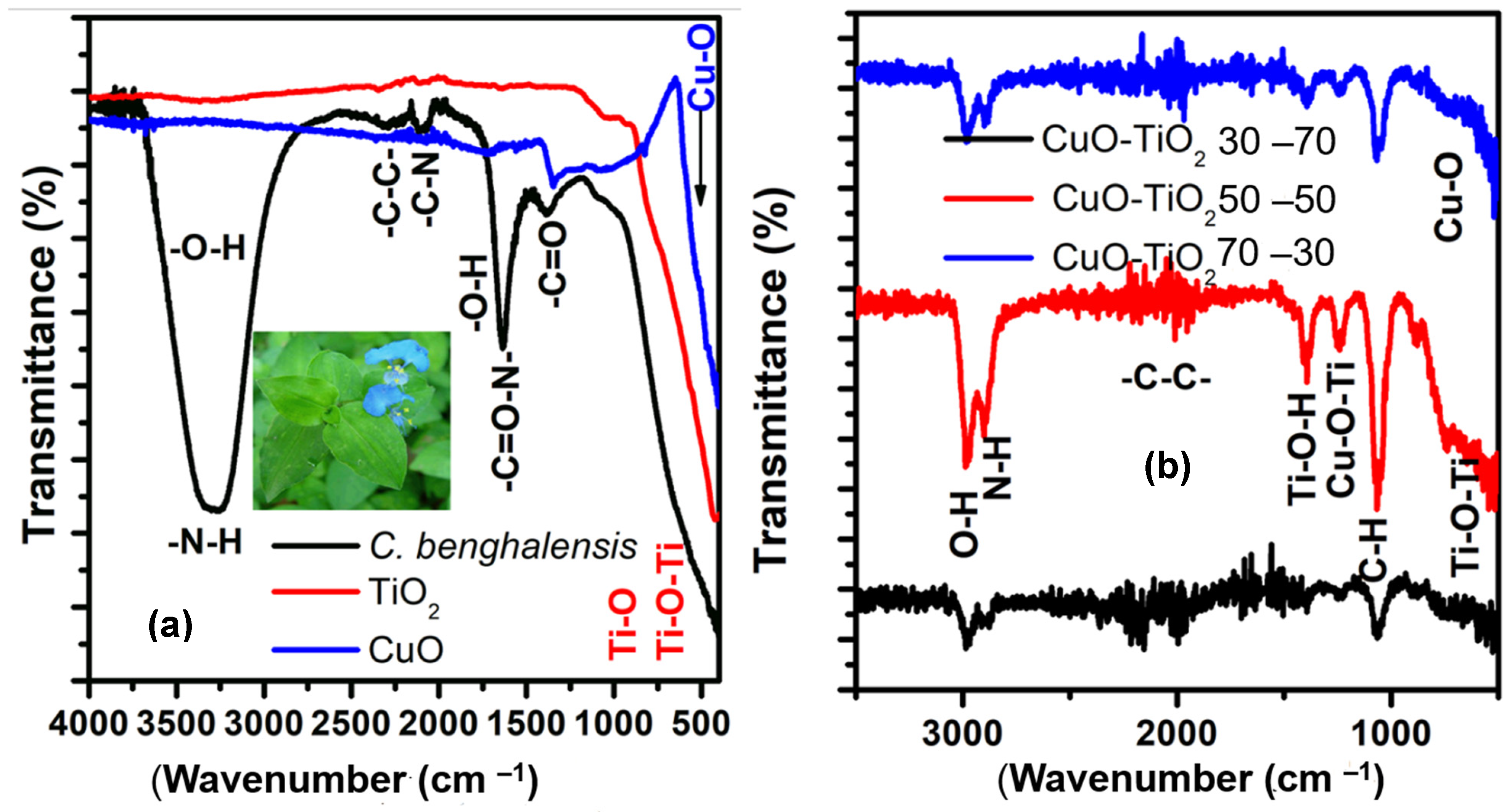









| Wavenumber | Compound | Correspondence | Functional Group | Refs. |
| 3345 cm−1 3356 cm−1 3335 cm−1 | 30/70 CuO-TiO2 50/50 CuO-TiO2 70/30 CuO-TiO2 | O-H (stretch) H- (bonded) | Phenols | [31,32,33,34] |
| 3335 cm−1 | C. benghalensis | N-H (vibrations) | Amide proteins | [31,34] |
| 2210 2335 | TiO2 | C-H (stretch) | Alkanes | [35] |
| 1925 2094 | TiO2 CuO | -C-C- | Stretch | [35] |
| 1641 cm−1 | CuO TiO2 C. benghalensis | (NH)C=O | II amines | [31,32,33,34] |
| 1557 cm−1 | CuO | N-H (bending) | I amines | [35] |
| 1361 cm−1 | CuO-TiO2 | Cu-O-Ti (stretch) | Metal oxides | [37,38] |
| 1010 cm−1 | TiO2 | O-H (bending) | Alkyl halides | [35] |
| 411 cm−1 916 cm−1 1010 cm−1 | TiO2 | Ti-O (bending) Ti-O-Ti (bending) | Alkyl halides | [36,37,38] |
| 516 cm−1 832 cm−1 1052 cm−1 | CuO | Cu-O (stretch) | Metal oxide | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bopape, D.A.; Mathobela, S.; Matinise, N.; Motaung, D.E.; Hintsho-Mbita, N.C. Green Synthesis of CuO-TiO2 Nanoparticles for the Degradation of Organic Pollutants: Physical, Optical and Electrochemical Properties. Catalysts 2023, 13, 163. https://doi.org/10.3390/catal13010163
Bopape DA, Mathobela S, Matinise N, Motaung DE, Hintsho-Mbita NC. Green Synthesis of CuO-TiO2 Nanoparticles for the Degradation of Organic Pollutants: Physical, Optical and Electrochemical Properties. Catalysts. 2023; 13(1):163. https://doi.org/10.3390/catal13010163
Chicago/Turabian StyleBopape, Dineo A., Sarah Mathobela, Nolubabalo Matinise, David E. Motaung, and Nomso C. Hintsho-Mbita. 2023. "Green Synthesis of CuO-TiO2 Nanoparticles for the Degradation of Organic Pollutants: Physical, Optical and Electrochemical Properties" Catalysts 13, no. 1: 163. https://doi.org/10.3390/catal13010163
APA StyleBopape, D. A., Mathobela, S., Matinise, N., Motaung, D. E., & Hintsho-Mbita, N. C. (2023). Green Synthesis of CuO-TiO2 Nanoparticles for the Degradation of Organic Pollutants: Physical, Optical and Electrochemical Properties. Catalysts, 13(1), 163. https://doi.org/10.3390/catal13010163









