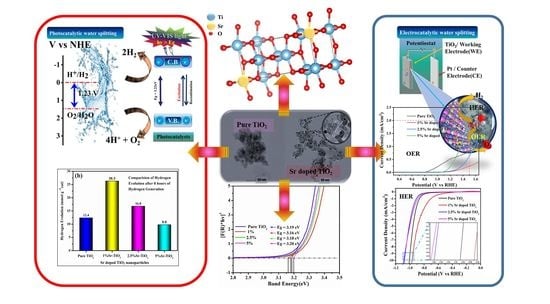
Pristine TiO2 and Sr-Doped TiO2 Nanostructures for Enhanced Photocatalytic and Electrocatalytic Water Splitting Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Pristine and Sr-Doped TiO2 Nanoparticles (NPs)
2.3. Characterizations
2.4. Photocatalytic Hydrogen Evolution Measurements
2.5. Electrode Preparation and Electrocatalytic Measurements
3. Results and Discussion
3.1. XRD Analysis
3.2. TEM Analysis
3.3. SEM Analysis
3.4. UV-Visible DRS Analysis
3.5. Raman Analysis
3.6. BET Surface Area Studies
3.7. Photocatalytic Water Splitting for Hydrogen Generation
3.8. Possible Photocatalytic Reaction Mechanism of Sr-doped TiO2 Nanoparticles
3.9. Electrocatalytic Water Splitting Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Mehtab, A.; Banerjee, S.; Mao, Y.; Ahmad, T. Type-II CuFe2O4/Graphitic Carbon Nitride Heterojunctions for High-Efficiency Photocatalytic and Electrocatalytic Hydrogen Generation. ACS Appl. Mater. Interfaces. 2022, 14, 44317–44329. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Sk, S.; Pal, U. Recent Advances in Solution Assisted Synthesis of Transition Metal Chalcogenides for Photo-electro Catalytic Hydrogen Evolution. Phys. Chem. 2022, 24, 20638–20673. [Google Scholar]
- Mehtab, A.; Alshehri, S.M.; Ahmad, T. Photocatalytic and Photoelectrocatalytic Water Splitting by Porous g-C3N4 Nanosheets for Hydrogen Generation. ACS Appl. Nano Mater. 2022, 5, 12656–12665. [Google Scholar] [CrossRef]
- Ali, S.A.; Ahmad, T. Chemical strategies in molybdenum based chalcogenides nanostructures for photocatalysis. Int. J. Hydrog. Energy 2022, 29, 29255–29283. [Google Scholar] [CrossRef]
- Xu, X.; Song, F.; Hu, X. A nickel iron diselenide-derived efficient oxygen-evolution catalyst. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pandit, N.A.; Ahmad, T. Tin Oxide Based Hybrid Nanostructures for Efficient Gas Sensing. Molecules 2022, 27, 7038–7082. [Google Scholar] [CrossRef]
- Mehtab, A.; Ahmed, J.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Rare earth doped metal oxide nanoparticles for photocatalysis: A perspective. Nanotechnology 2022, 33, 142001. [Google Scholar] [CrossRef]
- Joy, J.; Mathew, J.; George, S.C. Nanomaterials for photoelectrochemical water splitting-review. Int. J. Hydrog. Energy 2018, 43, 4804–4817. [Google Scholar] [CrossRef]
- Naaz, F.; Sharma, A.; Shahazad, M.; Ahmad, T. Hydrothermally Derived Hierarchical CuO Nanoflowers as an Efficient Photocatalyst and Electrocatalyst for Hydrogen Evolution. ChemistrySelect 2022, 7, e202201800. [Google Scholar] [CrossRef]
- Farooq, U.; Ahmed, J.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Self-Assembled Interwoven Nanohierarchitectures of NaNbO3 and NaNb1-xTaxO3 (0.05≤ x≤ 0.20): Synthesis, Structural Characterization, Photocatalytic Applications, and Dielectric Properties. ACS Omega 2022, 7, 16952–16967. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrog. Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Samuel, E.; Joshi, B.; Kim, M.; Swihart, M.T.; Yoon, S.S. Morphology engineering of photoelectrodes for efficient photoelectrochemical water splitting. Nano Energy 2020, 72, 104648. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Xu, Z.; Zhang, B.; Liu, H.; Lin, Y.; Gao, F.; Li, S.; Li, G. InGaN Nanorods Decorated with Au Nanoparticles for Enhanced Water Splitting Based on Surface Plasmon Resonance Effects. Nanomaterials 2020, 10, 912. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, S.; Liang, J.; Lin, J.; Yu, Y.; Li, R.; Gao, F.; Li, G. Surface passivation of InGaN nanorods using H3PO4 treatment for enhanced photoelectrochemical performance. J. Power Sources 2019, 419, 65–71. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, S.; Fu, X.; Xu, Y. Synthesis of M@TiO2 (M= Au, Pd, Pt) core–shell nanocomposites with tunable photoreactivity. J. Phys. Chem. C 2011, 115, 9136–9145. [Google Scholar] [CrossRef]
- Idriss, H. The elusive photocatalytic water splitting reaction using sunlight on suspended nanoparticles: Is there a way forward? Catal. Sci. Technol. 2020, 10, 304–310. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Liang, C.; Liu, J.; Tian, Z.; Wang, G.; Cai, W. Defect-mediated formation of Ag cluster-doped TiO2 nanoparticles for efficient photodegradation of pentachlorophenol. Langmuir 2012, 28, 3938–3944. [Google Scholar] [CrossRef]
- Wang, W.; Jin, C.; Qi, L. Hierarchical CdS nanorod@SnO2 nanobowl arrays for efficient and stable photoelectrochemical hydrogen generation. Small 2018, 14, 1801352. [Google Scholar] [CrossRef]
- Guo, C.X.; Xie, J.; Yang, H.; Li, C.M. Au@CdS Core–Shell Nanoparticles-Modified ZnO Nanowires Photoanode for Efficient Photoelectrochemical Water Splitting. Adv. Sci. Lett. 2015, 2, 1500135. [Google Scholar] [CrossRef]
- Cao, S.; Yin, Z.; Barber, J.; Boey, F.Y.; Loo, S.C.J.; Xue, C. Preparation of Au-BiVO4 heterogeneous nanostructures as highly efficient visible-light photocatalysts. ACS Appl. Mater. Interfaces 2012, 4, 418–423. [Google Scholar] [CrossRef]
- Valero, J.M.; Obregόn, S.; Colόn, G. Active site considerations on the photocatalytic H2 evolution performance of Cu-doped TiO2 obtained by different doping methods. ACS Catal. 2014, 4, 3320–3329. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Dai, J.; Li, J. Efficient visible-light-driven photocatalytic degradation with Bi2O3 coupling silica doped TiO2. Ind. Eng. Chem. Res. 2014, 53, 12575–12586. [Google Scholar] [CrossRef]
- Khan, H.; Lone, I.H.; Lofland, S.E.; Ramanujachary, K.V.; Ahmad, T. Exploiting Multiferroicity of TbFeO3 Nanoparticles for Hydrogen Generation through Photo/Electro/Photoelectro-catalytic Water Splitting. Int. J. Hydrog. Energy 2022, in press. [Google Scholar] [CrossRef]
- Jain, S.K.; Fazil, M.; Naaz, F.; Pandit, N.A.; Ahmed, J.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Silver-doped SnO2 nanostructures for photocatalytic water splitting and catalytic nitrophenol reduction. New J. Chem. 2022, 46, 2846–2857. [Google Scholar] [CrossRef]
- Wu, M.; Wu, P.; Lin, T.; Lin, T. Photocatalytic performance of Cu-doped TiO2 nanofibers treated by the hydrothermal synthesis and air-thermal treatment. Appl. Surf. Sci. 2018, 430, 390–398. [Google Scholar] [CrossRef]
- Sulaiman, S.N.A.; Noh, M.Z.; Adnan, N.N.; Bidin, N.; Razak, S.N.A. Effects of photocatalytic activity of metal and non-metal doped TiO2 for hydrogen production enhancement-a review. J. Phys. Conf. Ser. 2018, 1027, 012006. [Google Scholar] [CrossRef]
- Ko, S.; Banerjee, C.K.; Sankar, J. Photochemical synthesis and photocatalytic activity in simulated solar light of nanosized Ag doped TiO2 nanoparticle composite. Compos. B. Eng. 2011, 42, 579–583. [Google Scholar] [CrossRef]
- Emran, K.M. Catalytic Activity of Strontium Modified TiO2 Nanotubes for Hydrogen Evolution Reaction. Int. J. Electrochem. Sci. 2020, 15, 4218–4231. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Karunakaran, C.; Abiramasundari, G.; Gomathisankar, P.; Manikandan, G.; Anandi, V. Cu-doped TiO2 nanoparticles for photocatalytic disinfection of bacteria under visible light. J. Colloid Interface Sci. 2010, 352, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Rohilla, S. Rietveld refinement and structural characterization of TiO2/CoFe2O4 nanocomposites. In IOP Conference Series: Mater. Sci. Eng. 2020, 872, 012171. [Google Scholar]
- Yadav, H.M.; Otari, S.V.; Koli, V.B.; Mali, S.S.; Hong, C.K.; Pawar, S.H.; Delekar, S.D. Preparation and characterization of copper-doped anatase TiO2 nanoparticles with visible light photocatalytic antibacterial activity. J. Photochem. Photobiol. A 2014, 280, 32–38. [Google Scholar] [CrossRef]
- Gogoi, D.; Namdeo, A.; Golder, A.K.; Peela, N.R. Ag-doped TiO2 photocatalysts with effective charge transfer for highly efficient hydrogen production through water splitting. Int. J. Hydrog. Energy 2020, 45, 2729–2744. [Google Scholar] [CrossRef]
- Tsebriienko, T.; Popov, A.I. Effect of poly (titanium oxide) on the viscoelastic and thermophysical properties of interpenetrating polymer networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Jang, D.M.; Kwak, I.H.; Kwon, E.L.; Jung, C.S.; Im, H.S.; Park, K.; Park, J. Transition-metal doping of oxide nanocrystals for enhanced catalytic oxygen evolution. J. Phys. Chem. C 2015, 119, 1921–1927. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Ahmed, J.; Ahamad, T.; Alhokbany, N.; Arunachalam, P.; Al-Mayouf, A.M.; Ahmad, T. Synthesis, characterization, multifunctional electrochemical (OGR/ORR/SCs) and photodegradable activities of ZnWO4 nanobricks. J. Solgel Sci. Technol. 2018, 87, 137–146. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Alizade, B.; Modirshahla, N. Synthesis of Mg-doped TiO2 nanoparticles under different conditions and its photocatalytic activity. Photochem. Photobiol. 2011, 87, 1308–1314. [Google Scholar] [CrossRef]
- Farooq, U.; Ahmed, J.; Alshehri, S.M.; Ahmad, T. High-surface-area sodium tantalate nanoparticles with enhanced photocatalytic and electrical properties prepared through polymeric citrate precursor route. ACS Omega 2019, 4, 19408–19419. [Google Scholar] [CrossRef]
- Kumar, A.; Patel, A.S.; Mohanty, T. Correlation of photodegradation efficiency with surface potential of silver-TiO2 nanocomposite thin films. J. Phys. Chem. C 2012, 116, 20404–20408. [Google Scholar] [CrossRef]
- Jung, H.; Yeo, I.; Kim, T.; Ki, H.; Gu, H. Surface plasmon resonance effect of silver nanoparticles on a TiO2 electrode for dye-sensitized solar cells. Appl. Surf. Sci. 2018, 432, 266–271. [Google Scholar] [CrossRef]
- Barad, H.; Ginsburg, A.; Cohen, H.; Rietwyk, K.J.; Keller, D.A.; Tirosh, S.; Bouhadana, Y.; Anderson, A.Y.; Zaban, A. Hot Electron-Based Solid State TiO2|Ag Solar Cells. Adv. Mater. Interfaces 2016, 3, 1500789. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Zhou, Q.; Liang, X.; Liu, D. Facile synthesis of novel gully-like double-sized mesoporous structural Sr-doped ZrO2-TiO2 composites with improved photocatalytic efficiency. J. Solid State Chem. 2019, 269, 375–385. [Google Scholar] [CrossRef]
- Hamad, H.; Elsenety, M.M.; Sadik, W.; El-Demerdash, A.; Nashed, A.; Mostafa, A.; Elyamny, S. The superior photocatalytic performance and DFT insights of S-scheme CuO@TiO2 heterojunction composites for simultaneous degradation of organics. Sci. Rep. 2022, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Sahoo, B.; Khastgir, D. Flexible Nanocomposites Comprised of Poly (dimethylsiloxane) and High-Permittivity TiO2 Nanoparticles Doped with La3+/Cu+ for Dielectric Applications. ACS Appl. Nano Mater. 2019, 2, 4211–4221. [Google Scholar] [CrossRef]
- Farooq, U.; Chaudhary, P.; Ingole, P.P.; Kalam, A.; Ahmad, T. Development of cuboidal KNbO3@α-Fe2O3 hybrid nanostructures for improved photocatalytic and photoelectrocatalytic applications. ACS Omega 2020, 5, 20491–20505. [Google Scholar] [CrossRef]
- Baruah, M.; Ezung, S.L.; Sharma, S.; Sinha, U.B.; Sinha, D. Synthesis and characterization of Ni-doped TiO2 activated carbon nanocomposite for the photocatalytic degradation of Anthracene. Inorg. Chem. Commun. 2022, 144, 109905. [Google Scholar] [CrossRef]
- Peng, B.; Meng, X.; Tang, F.; Ren, X.; Chen, D.; Ren, J. General synthesis and optical properties of monodisperse multifunctional metal-ion-doped TiO2 hollow particles. J. Phys. Chem. C 2009, 113, 20240–20245. [Google Scholar] [CrossRef]
- Hamedani, H.A.; Allam, N.K.; Garmestani, H.; El-Sayed, M.A. Electrochemical fabrication of strontium-doped TiO2 nanotube array electrodes and investigation of their photoelectrochemical properties. J. Phys. Chem. C 2011, 115, 13480–13486. [Google Scholar] [CrossRef]
- Gobal, F.; Faraji, M. Fabrication of nanoporous nickel oxide by de-zincification of Zn–Ni/(TiO2-nanotubes) for use in electrochemical supercapacitors. Electrochim. Acta 2013, 100, 133–139. [Google Scholar] [CrossRef]
- Wei, X.; Cao, J.; Fang, F. A novel multifunctional Ag and Sr2+ co-doped TiO2@ rGO ternary nanocomposite with enhanced p-nitrophenol degradation, and bactericidal and hydrogen evolution activity. RSC Adv. 2018, 8, 31822–31829. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.C.; Doan, T.L.L.; Prabhakaran, S.; Tran, D.T.; Kim, D.H.; Lee, J.H.; Kim, N.H. Hierarchical Co and Nb dual-doped MoS2 nanosheets shelled micro-TiO2 hollow spheres as effective multifunctional electrocatalysts for HER, OER, and ORR. Nano Energy 2021, 82, 105750. [Google Scholar] [CrossRef]
- Farooq, U.; Phul, R.; Alshehri, S.M.; Ahmed, J.; Ahmad, T. Electrocatalytic and enhanced photocatalytic applications of sodium niobate nanoparticles developed by citrate precursor route. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahiro, N.; Shinagawa, T.; Nishimoto, T.; Takanabe, K. Recent advances in understanding oxygen evolution reaction mechanisms over iridium oxide. Inorg. Chem. Front. 2021, 8, 2900–2917. [Google Scholar]
- Ahmed, J.; Ahamad, T.; Alhokbany, N.; Almaswari, B.M.; Ahmad, T.; Hussain, A.; Al-Farraj, E.S.S.; Alshehri, S.M. Molten salts derived copper tungstate nanoparticles as bifunctional electro-catalysts for electrolysis of water and supercapacitor applications. ChemElectroChem 2018, 5, 3938–3945. [Google Scholar] [CrossRef]
- Haase, F.T.; Rabe, A.; Schmidt, F.P.; Herzog, A.; Jeon, H.S.; Frandsen, W.; Narangoda, P.V.; Spanos, I.; Friedel Ortega, K.; Timoshenko, J.; et al. Role of Nanoscale Inhomogeneities in Co2FeO4 Catalysts during the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2022, 144, 12007–12019. [Google Scholar] [CrossRef]
- Grimes, C.A.; Varghese, O.K.; Ranjan, S. Photoelectrolysis. In Light, Water, Hydrogen; Springer: Berlin/Heidelberg, Germany, 2008; pp. 115–190. [Google Scholar]
- Zhu, C.; Li, C.; Zheng, M.; Delaunay, J. Plasma-induced oxygen vacancies in ultrathin hematite nanoflakes promoting photoelectrochemical water oxidation. ACS Appl. Mater. Interfaces 2015, 7, 22355–22363. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, Y.; Liu, S.; Xu, W.; Wu, L.; Hsieh, Y.; Liu, P.; Zhu, Y.; Sasaki, K.; Renner, J.N.; et al. Reaction mechanism for oxygen evolution on RuO2, IrO2, and RuO2@ IrO2 core-shell nanocatalysts. J. Electroanal. Chem. 2018, 819, 296–305. [Google Scholar] [CrossRef]
- Ahmed, J.; Ubaidullah, M.; Ahmad, T.; Alhokbany, N.; Alshehri, S.M. Synthesis of graphite oxide/cobalt molybdenum oxide hybrid nanosheets for enhanced electrochemical performance in supercapacitors and the oxygen evolution reaction. ChemElectroChem 2019, 6, 2524–2530. [Google Scholar] [CrossRef]
- Ahmad, T.; Ganguli, A.K. Synthesis of nanometer-sized particles of barium orthotitanate prepared through a modified reverse micellar route: Structural characterization, phase stability and dielectric properties. J. Mater. Res. 2004, 19, 2905–2912. [Google Scholar] [CrossRef]
- Ahmad, T.; Ganguli, A.K. Reverse micellar route to nanocrystalline titanates (SrTiO3, Sr2TiO4, and PbTiO3): Structural aspects and dielectric properties. J. Am. Ceram. Soc. 2006, 89, 1326–1332. [Google Scholar] [CrossRef]
- Jain, S.; Pandit, N.A.; Fazil, M.; Ali, S.A.; Ahmed, J.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Chemical Fabrication, Structural Characterization and Photocatalytic Water Splitting Application of Sr-Doped SnO2 Nanoparticles. Nanotechnology 2022, 33, 355706. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Fazil, M.; Pandit, N.A.; Ali, S.A.; Naaz, F.; Khan, H.; Mehtab, A.; Ahmed, J.; Ahmad, T. Modified, Solvothermally Derived Cr-doped SnO2 Nanostructures for Enhanced Photocatalytic and Electrochemical Water-Splitting Applications. ACS Omega 2022, 7, 14138–14147. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Hua, N.; Jia, H.; Zhu, Y.; Wang, Z.; Xu, J.; Wang, C. Synthesis and evaluation of visible-light photocatalyst: Nitrogen-doped TiO2/Bi2O3 heterojunction structures. Sci. Adv. Mater. 2014, 6, 1892–1899. [Google Scholar] [CrossRef]
- Mohamed, I.M.; Dao, V.; Yasin, A.S.; Barakat, N.A.; Choi, H. Design of an efficient photoanode for dye-sensitized solar cells using electrospun one-dimensional GO/N-doped nanocomposite SnO2/TiO2. Appl. Surf. Sci. 2017, 400, 355–364. [Google Scholar] [CrossRef]
- Brik, M.G.; Srivastava, A.M.; Popov, A.I. A few common misconceptions in the interpretation of experimental spectroscopic data. Opt. Mater. 2022, 127, 112276. [Google Scholar] [CrossRef]
- Saqib, M.; Althubeiti, K.; Abualnaja, K.M.; Rahman, N.; Khan, R. Effect of Sr and Co co-doping on the TiO2-diluted magnetic semiconductor for spintronic applications. J. Mater. Sci.: Mater. Electron. 2021, 32, 28718–28729. [Google Scholar]
- Ekoi, E.J.; Gowen, A.; Dorrepaal, R.; Dowling, D.P. Characterisation of titanium oxide layers using Raman spectroscopy and optical profilometry: Influence of oxide properties. Results Phys. 2019, 12, 1574–1585. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, Z.; Shi, H.; Xiao, T.; Yan, Z. Preparation of highly visible-light active N-doped TiO2 photocatalyst. J. Mater. Chem. 2010, 20, 5301–5309. [Google Scholar] [CrossRef]
- Kumar, M.K.; Bhavani, K.; Srinivas, B.; Kumar, S.N.; Sudhakar, M.; Naresh, G.; Venugopal, A. Nano structured bismuth and nitrogen co-doped TiO2 as an efficient light harvesting photocatalyst under natural sunlight for the production of H2 by H2O splitting. Appl. Catal. A 2016, 515, 91–100. [Google Scholar] [CrossRef]
- Zhang, X.; Song, P.; Cui, X. Nitrogen-doped TiO2 photocatalysts synthesized from titanium nitride: Characterizations and photocatalytic hydrogen evolution performance. J. Adv. Oxid. Technol. 2013, 16, 131–136. [Google Scholar] [CrossRef]
- Tristantini, D.; Ibadurrohman, M. Photocatalytic hydrogen production from glycerol–water mixture over Pt-N-TiO2 nanotube photocatalyst. Int. J. Energy Res. 2013, 11, 1372–1381. [Google Scholar]
- Wei, X.; Li, J.; Liu, Z.; Yang, X.; Naraginti, S.; Xu, X.; Wang, X. Visible light photocatalytic mineralization of 17α-ethinyl estradiol (EE2) and hydrogen evolution over silver and strontium modified TiO2 nanoparticles: Mechanisms and phytotoxicity assessment. RSC Adv. 2018, 8, 4329–4339. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Gao, H.; Yao, Y.; Zhu, L.; Zhou, X.; Peng, X.; Zhang, X. Pd loading, Mn+ (n= 1, 2, 3) metal ions doped TiO2 nanosheets for enhanced photocatalytic H2 production and reaction mechanism. Int. J. Hydrog. Energy 2022, 47, 10250–10260. [Google Scholar] [CrossRef]
- Nguyen, C.; Dinh, C.; Do, T. Hollow Sr/Rh-codoped TiO2 photocatalyst for efficient sunlight-driven organic compound degradation. RSC Adv. 2017, 7, 3480–3487. [Google Scholar] [CrossRef]
- Li, C.; Hu, P.; Meng, H.; Jiang, Z. Role of sulfites in the water splitting reaction. J. Sol. Chem. 2016, 45, 67–80. [Google Scholar] [CrossRef]
- Chandra, M.; Bhunia, K.; Pradhan, D. Controlled synthesis of CuS/TiO2 heterostructured nanocomposites for enhanced photocatalytic hydrogen generation through water splitting. Inorg. Chem. 2018, 57, 4524–4533. [Google Scholar] [CrossRef]
- da Silva, G.C.; Venturini, S.I.; Zhang, S.; Löffler, M.; Scheu, C.; Mayrhofer, K.J.; Ticianelli, E.A.; Cherevko, S. Oxygen evolution reaction on tin oxides supported iridium catalysts: Do we need dopants? ChemElectroChem 2020, 7, 2330–2339. [Google Scholar] [CrossRef]
- Phul, R.; Perwez, M.; Ahmed, J.; Sardar, M.; Alshehri, S.M.; Alhokbany, N.; Khan, M.A.A.; Ahmad, T. Efficient multifunctional catalytic and sensing properties of synthesized ruthenium oxide nanoparticles. Catalysts 2020, 10, 780. [Google Scholar] [CrossRef]













| Sample | SBET/(m2g−1) | Average Pore Size/Å | Vtotal/(cm3g−1) | Eg/eV |
|---|---|---|---|---|
| Pristine TiO2 | 169.9 | 17.02 ± 0.85 | 0.359 | 3.19 |
| 1% Sr-doped TiO2 | 182.2 | 16.95± 0.85 | 0.351 | 3.16 |
| 2.5% Sr-doped TiO2 | 178.3 | 16.97 ± 0.85 | 0.426 | 3.18 |
| 5% Sr-doped TiO2 | 141.2 | 16.94 ± 0.85 | 0.294 | 3.20 |
| S.No. | Dopant | Synthesis Method | Parameters | Hydrogen Production | Ref. |
|---|---|---|---|---|---|
| 1. | Bismuth, nitrogen | Sol-gel method | Solar light, methanol | 1800 μmol/g | [71] |
| 2. | Nitrogen | Solid state/calcination method | UV–Vis light irradiation, Na2S/Na2SO3 | 18 μmol | [72] |
| 3. | Platinum, nitrogen | Photodeposition method | 1150 mL Pyrex vessel, UV light | 3200 μmol | [73] |
| 4. | Strontium, silver | Sol–gel method, | 500 W Xe arc lamp | 49.4 μmol/h | [74] |
| 5. | Pd/0.2%K+ | Hydrothermal method | _ | 76.6 μmol h−1 | [75] |
| 6. | Strontium | Hydrothermal method | 200 W, Hg−Xe arc lamp | 3.3 mmolh−1 | In this work |
| S. No | Materials | HER | OER | |||
|---|---|---|---|---|---|---|
| Overpotential (V) to Attain 10 mA/cm2 | Tafel Slope (mV/dec) | Onset Potential (V) | Anodic Current Density (mA/cm2) at 1.55 V | Tafel Slope (mV/dec) | ||
| 1. | Pristine TiO2 | 1.00 | 133.33 | 0.8 | 0.86 | 272.94 |
| 2. | 1% Sr-doped TiO2 | 0.96 | 84.09 | 1.23 | 0.30 | 135.09 |
| 3. | 2.5% Sr-doped TiO2 | 1.07 | 139.07 | 1.34 | 0.87 | 170.66 |
| 4. | 5% Sr-doped TiO2 | 1.08 | 146.16 | 1.1 | 2.49 | 91.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazil, M.; Ahmad, T. Pristine TiO2 and Sr-Doped TiO2 Nanostructures for Enhanced Photocatalytic and Electrocatalytic Water Splitting Applications. Catalysts 2023, 13, 93. https://doi.org/10.3390/catal13010093
Fazil M, Ahmad T. Pristine TiO2 and Sr-Doped TiO2 Nanostructures for Enhanced Photocatalytic and Electrocatalytic Water Splitting Applications. Catalysts. 2023; 13(1):93. https://doi.org/10.3390/catal13010093
Chicago/Turabian StyleFazil, Mohd, and Tokeer Ahmad. 2023. "Pristine TiO2 and Sr-Doped TiO2 Nanostructures for Enhanced Photocatalytic and Electrocatalytic Water Splitting Applications" Catalysts 13, no. 1: 93. https://doi.org/10.3390/catal13010093
APA StyleFazil, M., & Ahmad, T. (2023). Pristine TiO2 and Sr-Doped TiO2 Nanostructures for Enhanced Photocatalytic and Electrocatalytic Water Splitting Applications. Catalysts, 13(1), 93. https://doi.org/10.3390/catal13010093