Co-Immobilization of D-Amino Acid Oxidase, Catalase, and Transketolase for One-Pot, Two-Step Synthesis of L-Erythrulose
Abstract
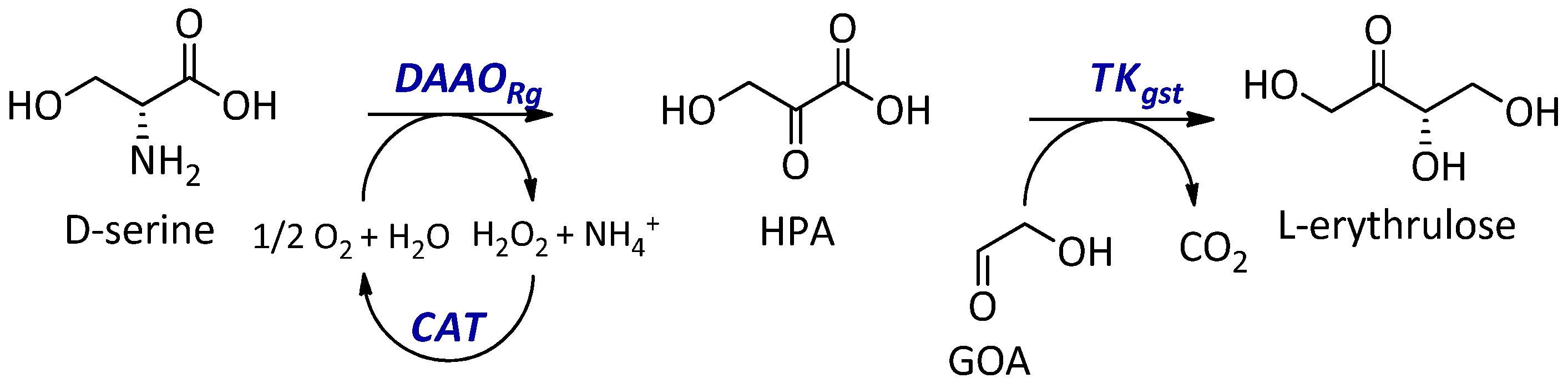
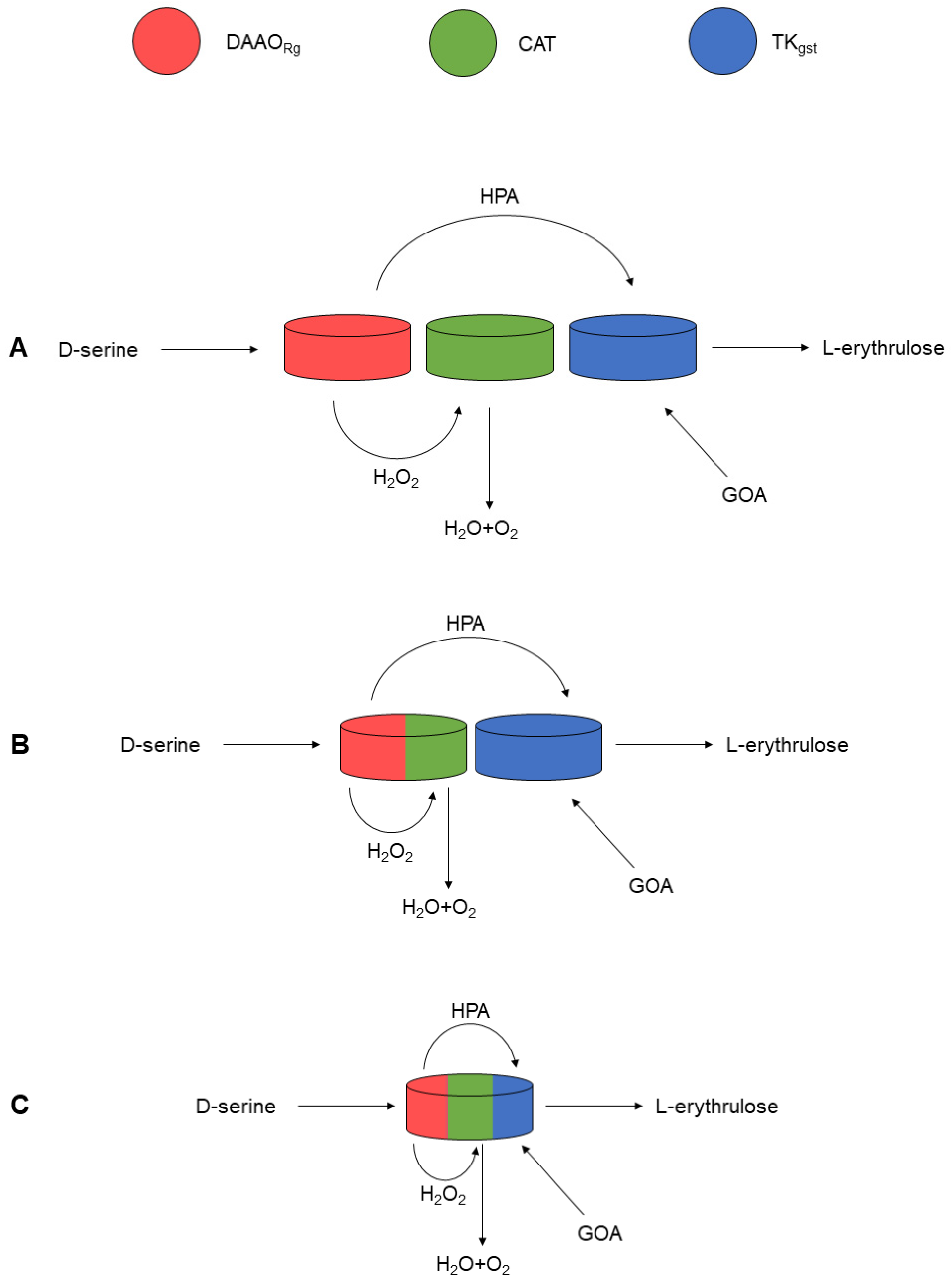
:1. Introduction
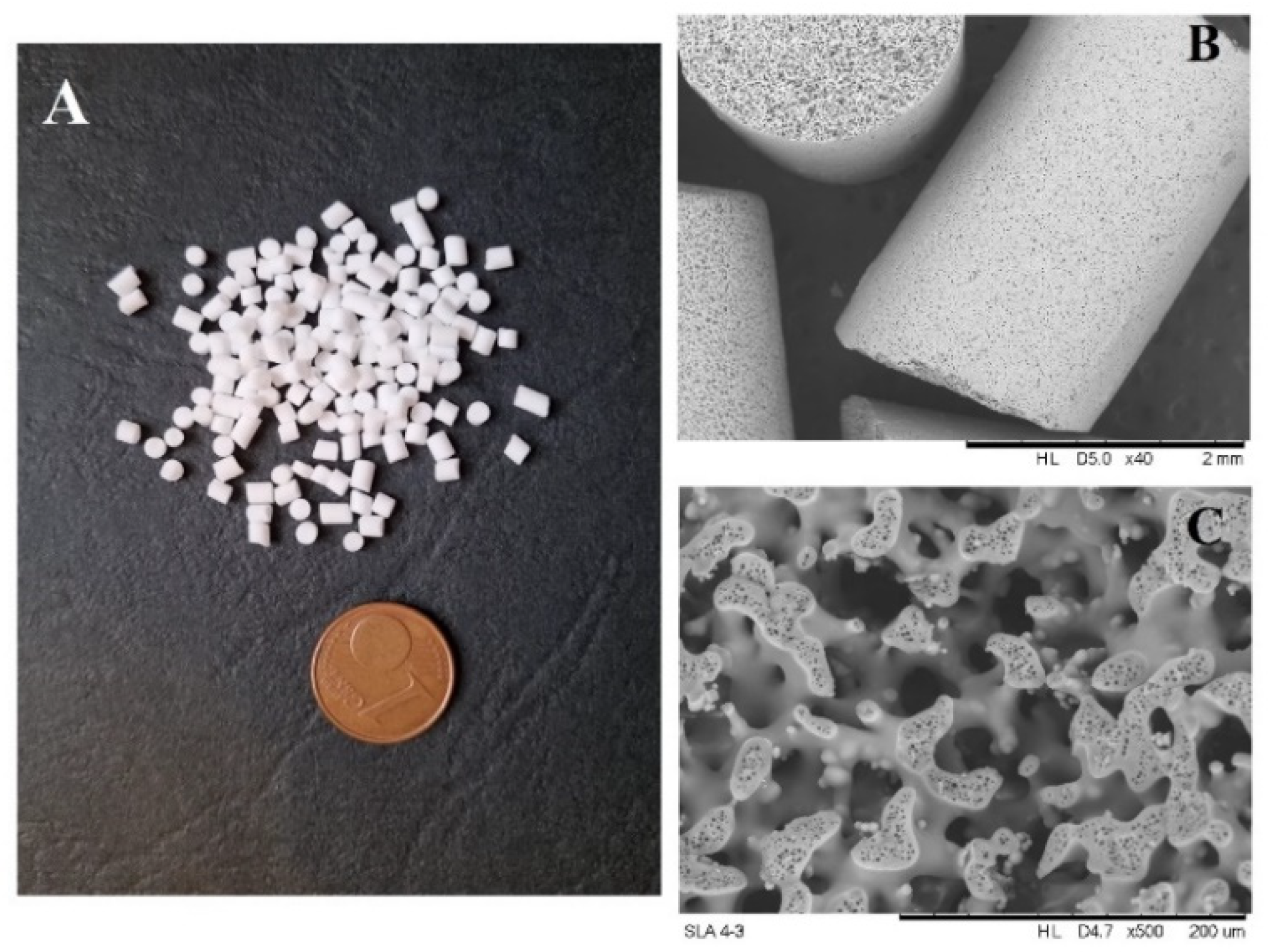
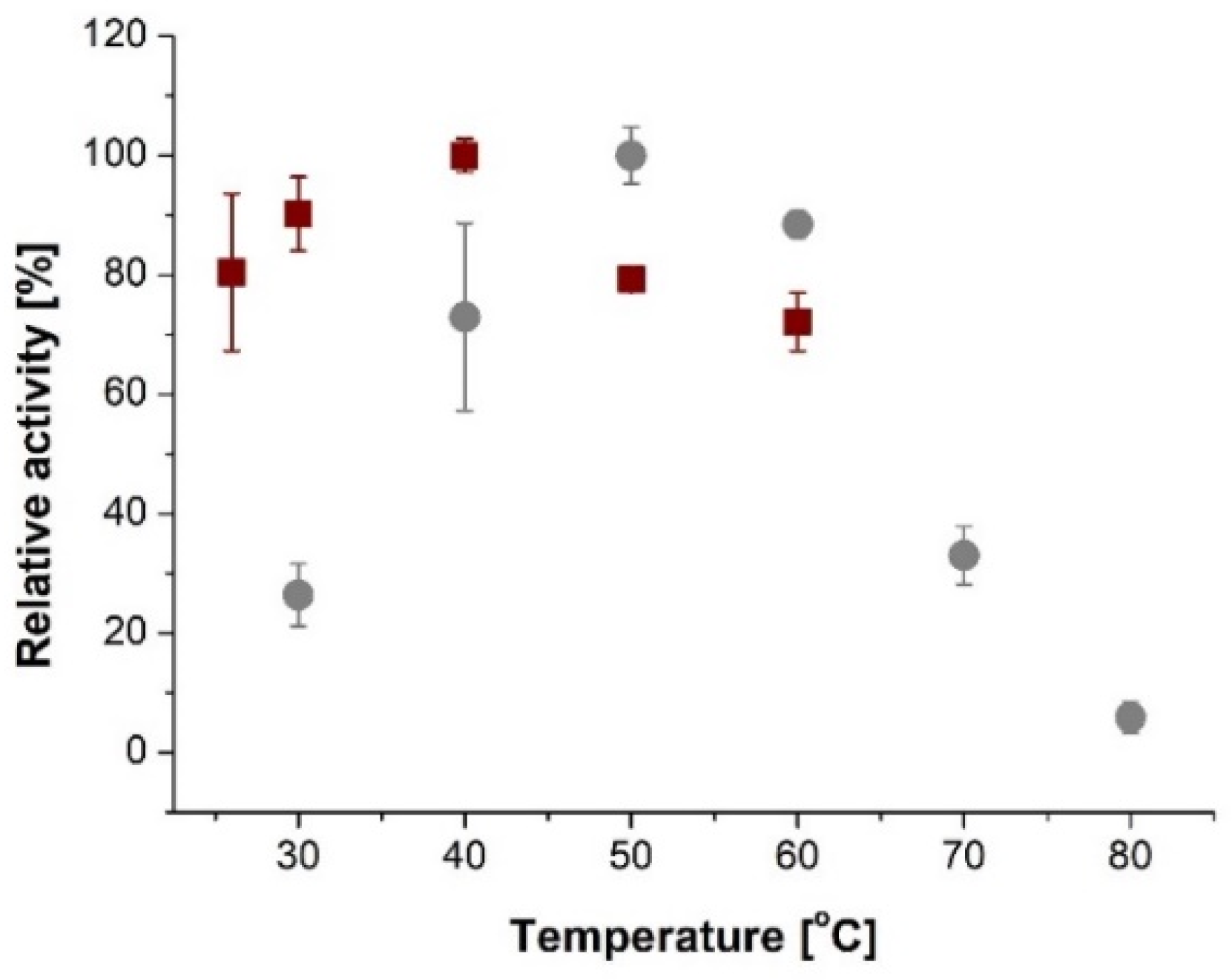
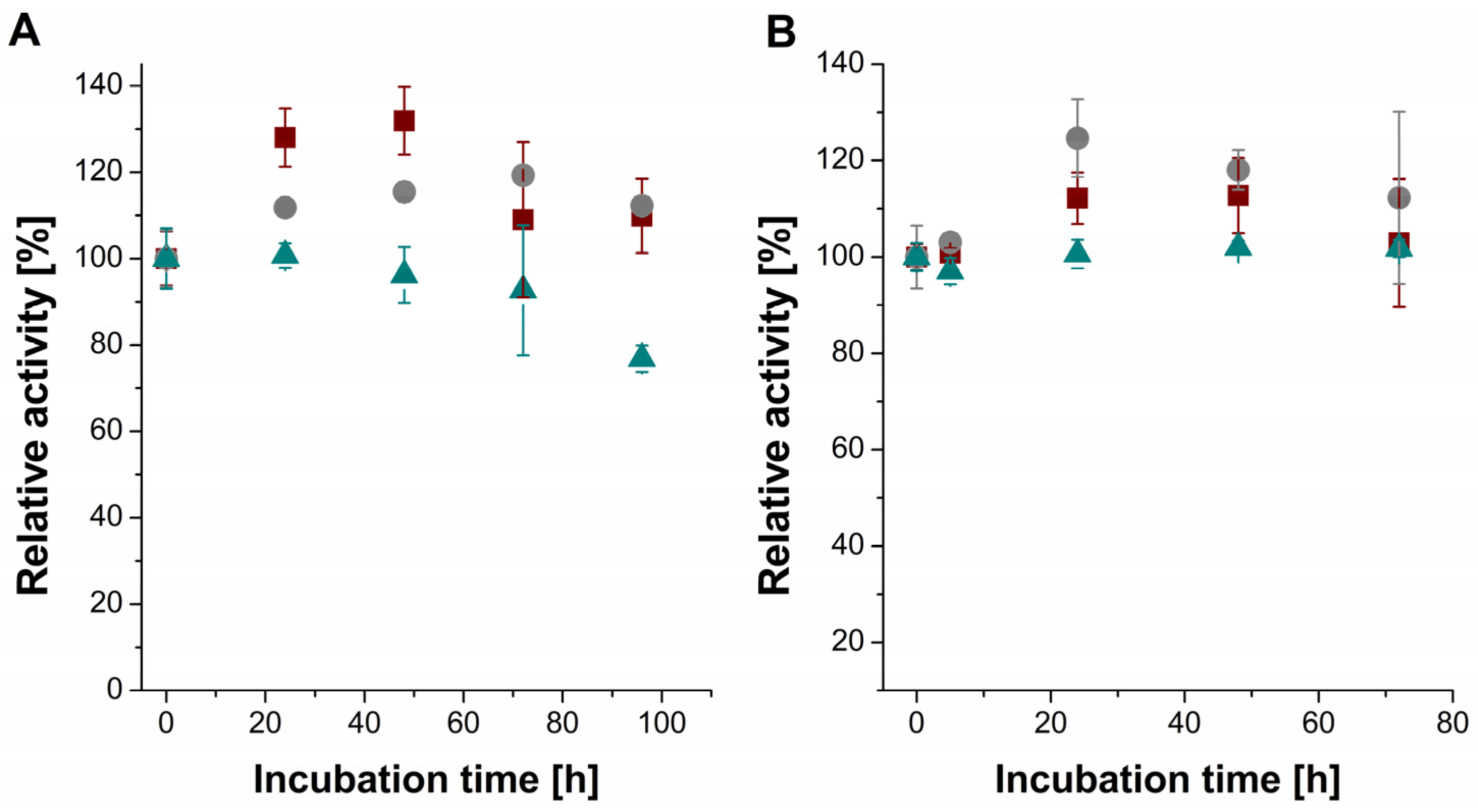
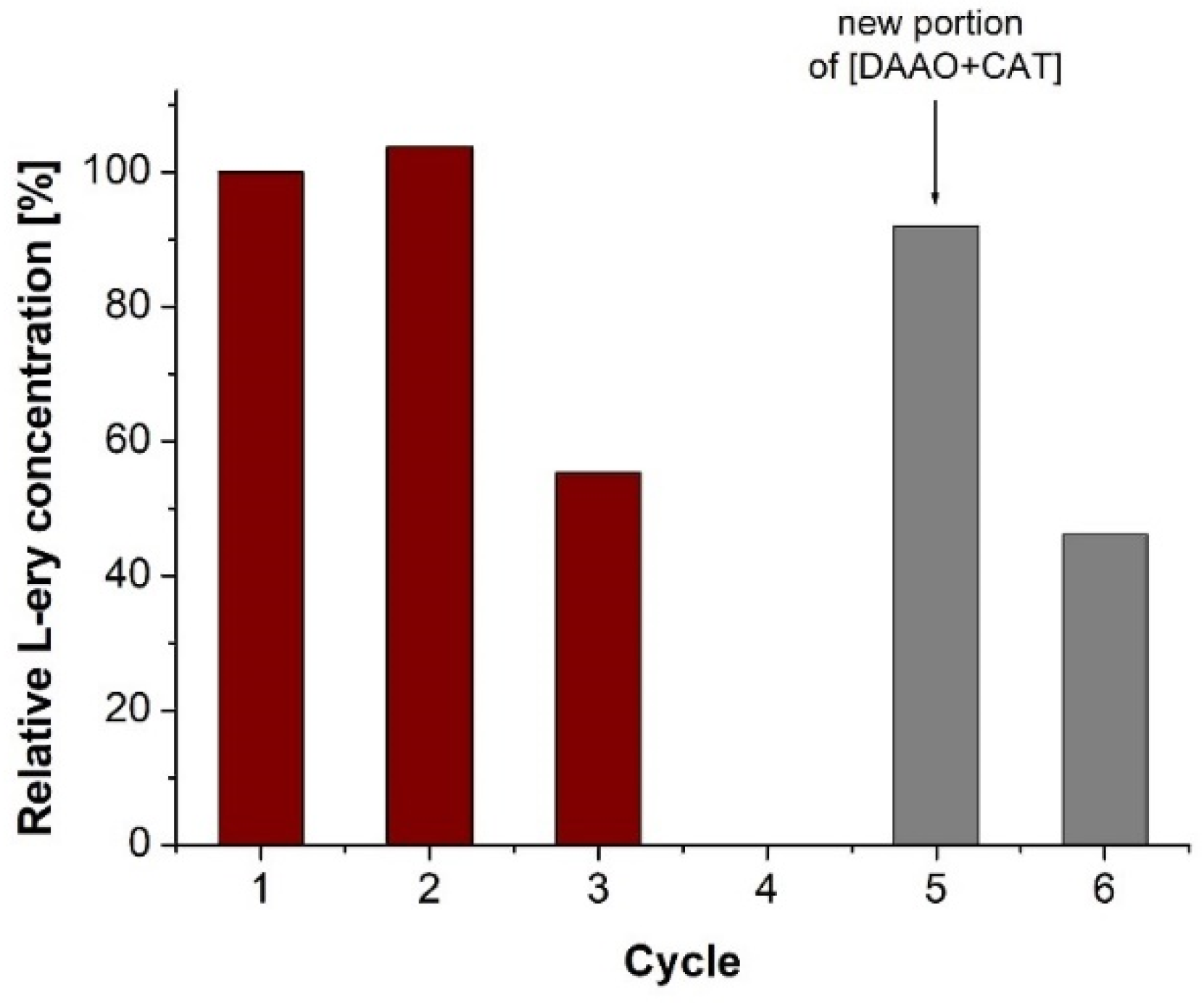
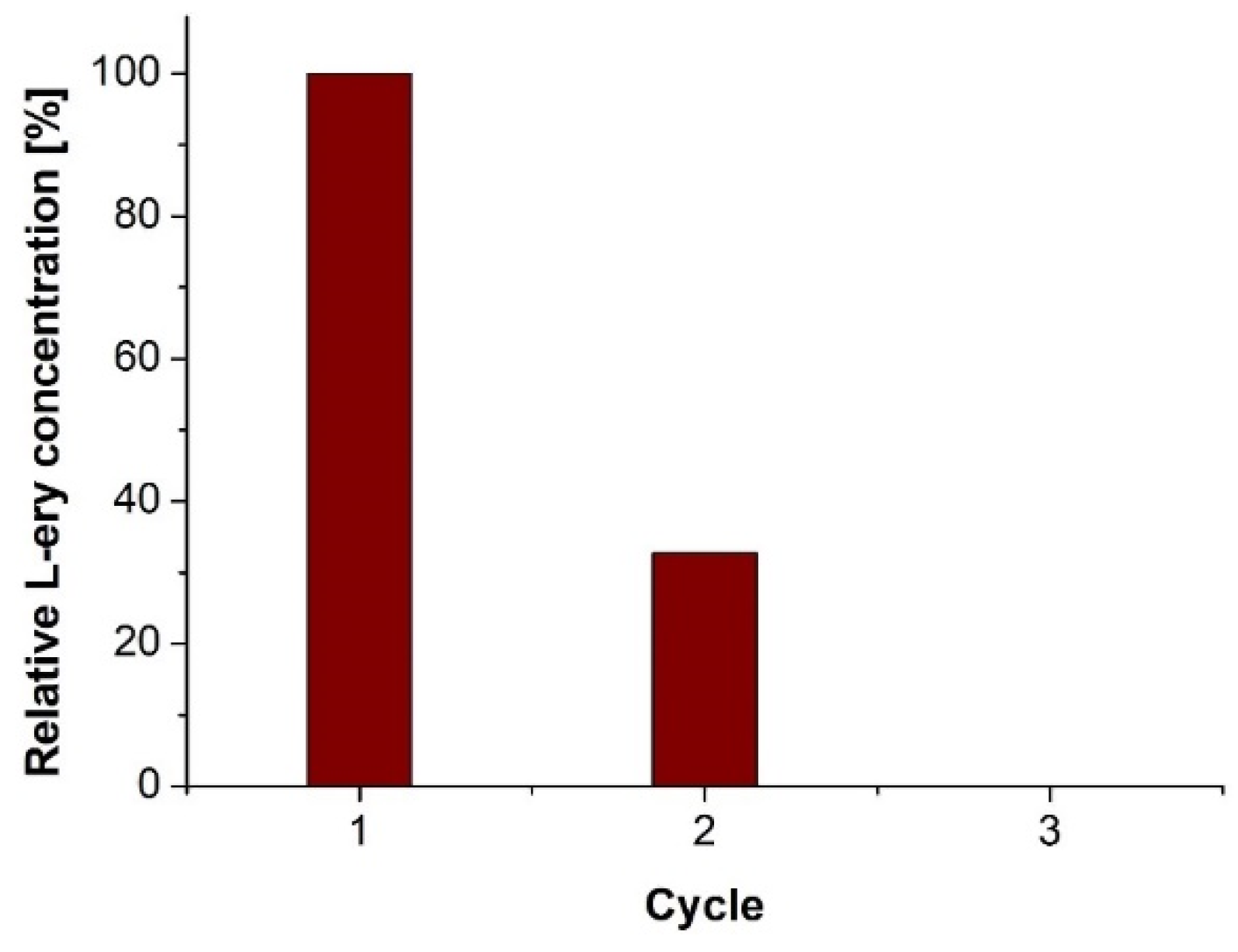
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Expression of TKgst from Geobacillus stearothermophillus and DAAORg from Rhodotorula gracilis
3.3. Purification of TKgst and DAAORg
3.4. Synthesis and Functionalization of Silica Monoliths (MH)
3.5. Immobilization of Enzymes on Amino-Modified MH
3.6. Activity of Single Immobilized DAAORg
3.7. Activity of Single Immobilized TKgst
3.8. L-Erythrulose Synthesis by Immobilized DAAORg, TKgst, and CAT
3.9. Determination of HPA, Glycolaldehyde, and L-Erythrulose Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Li, Z.; Li, F.; Wang, M.; Wang, N.; Gao, X.-D. Cascade synthesis of rare ketoses by whole cells based on l-rhamnulose-1-phosphate aldolase. Enzyme Microb. Technol. 2020, 133, 109456. [Google Scholar] [CrossRef]
- Li, Z.; Li, F.; Cai, L.; Chen, Z.; Qin, L.; Gao, X.-D. One-Pot Multienzyme Synthesis of Rare Ketoses from Glycerol. J. Agric. Food Chem. 2020, 68, 1347–1353. [Google Scholar] [CrossRef]
- Ocal, N.; L’enfant, M.; Charmantray, F.; Pollegioni, L.; Martin, J.; Auffray, P.; Collin, J.; Hecquet, L. D-serine as a Key Building block: Enzymatic process development and smart applications within the cascade enzymatic concept. Org. Proces. Res. Dev. 2020, 24, 769–775. [Google Scholar] [CrossRef]
- L’enfant, M.; Bruna, F.; Lorilliere, M.; Ocal, N.; Fessner, W.-D.; Pollegioni, L.; Charmantray, F.; Hecquet, L. One-pot cascade synthesis pf (3S)-hydroxyketones catalyzed by transketolase via hydroxypyruvate generated in situ from D-serine by D-aminoacid oxidase. Adv. Synth. Catal. 2019, 361, 2550–2558. [Google Scholar] [CrossRef] [Green Version]
- Lorillere, M.; De Sousa, M.; Bruna, F.; Heuson, E.; Gefflaut, T.; de Bererdinis, V.; Saravanan, T.; Yi, D.; Fessner, W.-D.; Charmantray, F.; et al. One-pot, two-step cascade synthesis of naturally rare L-erythro (3S,4S) ketoses by coupling a thermostable transaminase and transketolase. Green Chem. 2017, 19, 425–435. [Google Scholar] [CrossRef]
- Zhong, C.; Duić, B.; Bolivar, J.M.; Nidetzky, B. Three-Enzyme Phosphorylase Cascade Immobilized on Solid Support for Biocatalytic Synthesis of Cellooligosaccharides. ChemCatChem 2020, 12, 1350–1358. [Google Scholar] [CrossRef]
- Kumpf, A.; Kowalczykiewicz, D.; Szymańska, K.; Mehnert, M.; Bento, I.; Łochowicz, A.; Pollender, A.; Jarzȩbski, A.; Tischler, D. Immobilization of the Highly Active UDP-Glucose Pyrophosphorylase from Thermocrispum agreste Provides a Highly Efficient Biocatalyst for the Production of UDP-Glucose. Front. Bioeng. Biotechnol. 2020, 8, 740. [Google Scholar] [CrossRef] [PubMed]
- Stradomska, D.; Heba, M.; Czernek, A.; Kuźnik, N.; Gillner, D.; Maresz, K.; Pudło, W.; Jarzębski, A.; Szymańska, K. Lipase immobilized on MCFs as biocatalysts for kinetic and dynamic kinetic resolution of sec-alcohols. Catalysts 2015, 11, 518. [Google Scholar] [CrossRef]
- Kuan, I.; Liao, R.; Hsieh, H.; Chen, K.; Yu, C. Properties of Rhodotorula gracilis d-Amino Acid Oxidase Immobilized on Magnetic Beads through His-Tag. J. Biosci. Bioeng. 2008, 105, 110–115. [Google Scholar] [CrossRef]
- Wang, M.; Qi, W.; Xu, H.; Yu, H.; Zhang, S.; Shen, Z. Affinity-binding immobilization of d-amino acid oxidase on mesoporous silica by a silica-specific peptide. J. Ind. Microbiol. Biotech. 2019, 46, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Sun, J.; Fu, Y.; Du, K.; Cai, M.; Ji, P.; Feng, W. Immobilization of genetically-modified D-amino acid oxidase and catalase on carbon nanotubes to improve the catalytic efficiency. Catalysts 2016, 6, 66. [Google Scholar] [CrossRef] [Green Version]
- Dang, P.T.; Le, H.G.; Hoang, V.T.; Tran, H.T.H.; Dao, C.D.; Nguyen, K.T.; Le, G.H.; Nguyen, Q.K.; Nguyen, T.V.; Vu, T.A. Immobilization of D-amino acid oxidase enzyme on hybrid mesoporous MCF, SBA-15 and MCM-41 nanomaterials. J. Nanosci. Nanotechnol. 2017, 7, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Matosevic, S.; Lye, G.J.; Baganz, F. Design and Characterization of a Prototype Enzyme Microreactor: Quantification of Immobilized Transketolase Kinetics. Biotechnol. Prog. 2010, 26, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Benaissi, K.; Helaine, V.; Prevot, V.; Forano, C.; Hecquet, L. Efficient Immobilization of Yeast Transketolase on Layered Double Hydroxides and Application for Ketose synthesis. Adv. Synth. Catal. 2011, 353, 1497–1509. [Google Scholar] [CrossRef]
- Ali, G.; Moreau, T.; Forano, C.; Mousty, C.; Prevot, V.; Charmantray, F.; Hecquet, L. Chiral polyol synthesis catalyzed by a thermostable Transketolase immobilized on Layered Double Hydroxides in Ionic liquids. ChemCatChem 2015, 7, 3163–3170. [Google Scholar] [CrossRef]
- Xu, K.; Chen, X.; Zheng, R.; Zheng, Y. Immobilization of Multi-Enzymes on Support Materials for Efficient Biocatalysis. Front. Bioeng. Biotechnol. 2020, 8, 660. [Google Scholar] [CrossRef]
- Arana-Pena, S.; Carballares, D.; Morellon-Sterling, R.; Berenguer-Murcia, A.; Alcantara, A.R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Enzyme co-immobilization: Always the biocatalyst designers’ choice…or not? Biotechnolog. Adv. 2021, 51, 107584. [Google Scholar] [CrossRef]
- Bilal, M.; Hussain, N.; Americo-Pinheiro, J.H.P.; Almulaiky, Y.Q.; Iqbal, H.M.N. Multi-enzyme co-immobilized nano-assemblies: Bringing enzymes together for expanding bio-catalysis scope to meet biotechnological challenges. Int. J. Biol. Macromol. 2021, 186, 735–749. [Google Scholar] [CrossRef]
- Hwang, E.T.; Lee, S. Multienzymatic Cascade Reactions via Enzyme Complex by Immobilization. ACS Catal. 2019, 9, 4402–4425. [Google Scholar] [CrossRef]
- Ren, S.; Li, C.; Jiao, X.; Jia, S.; Jiang, Y.; Bilal, M.; Cui, J. Recent progress in multienzymes co-immobilization and multienzyme system applications. Chem. Eng. J. 2019, 373, 1254–1278. [Google Scholar] [CrossRef]
- Sanchez- Otero, M.G.; Quintana-Castro, R.; Rojas-Vazquez, A.S.; Badillo-Zeferino, G.L.; Mondragón-Vazquez, K.; Espinosa-Luna, G.; Nadda, A.K.; Oliart-Ros, R.M. Polypropylene as a selective support for the immobilization of lipolytic enzymes: Hyper-activation, purification and biotechnological applications. J. Chem. Technol. Biotechnol. 2022, 97, 436–445. [Google Scholar] [CrossRef]
- Rather, H.; Khan, R.S.; Wani, T.U.; Beigh, M.A.; Sheikh, F.A. Overview on immobilization of enzymes on synthetic polymeric nanofibers fabricated by electrospinning. Biotechnol. Bioeng. 2022, 119, 9–23. [Google Scholar] [CrossRef]
- Singh, A.; Rai, S.K.; Manisha, M.; Yadav, S.K. Immobilized L-ribose isomerase for the sustained synthesis of a rare sugar D-talose. Mol. Catal. 2021, 511, 11723. [Google Scholar] [CrossRef]
- Gentil, S.; Pifferi, C.; Rousselot-Pailley, P.; Tron, T.; Renaudet, O.; Le Goff, A. Clicked Bifunctional Dendrimeric and Cyclopeptidic Addressable Redox Scaffolds for the Functionalization of Carbon Nanotubes with Redox Molecules and Enzymes. Langmuir 2021, 37, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Cristovao, R.O.; Almeida, M.R.; Barros, M.A.; Nunes, J.C.F.; Boaventura, R.A.R.; Loureiro, J.M.; Faria, J.L.; Neves, M.C.; Freire, M.G.; Ebinuma-Santos, V.C.; et al. Development and characterization of a novel L-asparaginase/MWCNT nanobioconjugate. RSC Adv. 2020, 10, 31205. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, K.; Odrozek, K.; Zniszczoł, A.; Pudło, W.; Jarzębski, A. A novel hierarchically structured siliceous packing to boost the performance of rotating bed enzymatic reactors. Chem. Eng. J. 2017, 315, 18–24. [Google Scholar] [CrossRef]
- Hou, C.; Ghéczy, N.; Messmer, D.; Szymańska, K.; Adamcik, J.; Mezzenga, R.; Jarzębski, A.B.; Walde, P. Stable immobilization of enzymes in a macro- and mesoporous silica monolith. ACS Omega 2019, 4, 7795–7806. [Google Scholar] [CrossRef]
- Kowalczykiewicz, D.; Szymańska, K.; Gillner, D.; Jarzębski, A.B. Rotating bed reactor packed with heterofunctional structured silica-supported lipase. Developing an effective system for the organic solvent and aqueous phase reactions. Microporous Mesoporous Mater. 2021, 312, 110789. [Google Scholar] [CrossRef]
- Butò, S.; Pollegioni, L.; D’Angiuro, L.; Pilone, M.S. Evaluation of D-amino acid oxidase from Rhodotorula gracilis for the production of α-keto acids: A reactor system. Biotechnol. Bioeng. 1994, 44, 1288–1294. [Google Scholar] [CrossRef]
- Sun, J.; Du, K.; Song, X.; Gao, Q.; Wu, H.; Ma, J.; Ji, P.; Feng, W. Specific immobilization of D-amino acid oxidase on hematin functionalized support mimicking multi enzyme catalysis. Green Chem. 2015, 17, 4465. [Google Scholar] [CrossRef]
- Ranoux, A.; Karmee, S.K.; Jin, J.; Bhaduri, A.; Caiazzo, A.; Arends, I.W.; Hanefeld, U. Enhancement of the Substrate Scope of Transketolase. ChemBioChem. 2012, 13, 1921–1931. [Google Scholar] [CrossRef]
- Subrizi, F.; Cárdenas-Fernández, M.; Lye, G.J.; Ward, J.M.; Dalby, P.A.; Sheppard, T.D.; Hailes, H.C. Transketolase catalysed upgrading of l-arabinose: The one-step stereoselective synthesis of l-gluco-heptulose. Green Chem 2016, 18, 3158–3165. [Google Scholar] [CrossRef] [Green Version]
- Bawn, M.; Subrizi, F.; Lye, G.J.; Sheppard, T.D.; Hailes, H.C.; Ward, J.M. One-pot, two-step transaminase and transketolase synthesis of l-gluco-heptulose from l-arabinose. Enzyme Microb. Technol. 2018, 116, 16–22. [Google Scholar] [CrossRef] [PubMed]
- James, P.; Isupov, M.N.; De Rose, S.A.; Sayer, C.; Cole, I.S.; Littlechild, J.A. A ‘Split-Gene’ Transketolase from the Hyper-Thermophilic Bacterium Carboxydothermus hydrogenoformans: Structure and Biochemical Characterization. Front. Microbiol. 2020, 11, 592353. [Google Scholar] [CrossRef]
- Cardenas-Fernanadez, M.; Subrizi, F.; Dobrijevic, D.; Hailes, H.C.; Ward, J.M. Characterisation of a hyperthermophilic transketolase from Thermotoga maritima DSM3109 as a biocatalyst for 7-keto-octuronic acid synthesis. Org. Biomol. Chem. 2021, 19, 6493–6500. [Google Scholar] [CrossRef]
- Abdoul Zabar, J.; Sorel, I.; Helaine, V.; Charmantray, F.; Devamani, T.; Yi, D.; de Berardinis, V.; Louis, D.; Marilere, P.; Fessner, W.D.; et al. Thermostable transketolase from Geobacillus starothermophilus: Characterization and catalytic properties. Adv. Synth. Catal. 2013, 355, 116–128. [Google Scholar] [CrossRef]
- Lorillière, M.; Dumoulin, R.; L’Enfant, M.; Rambourdin, A.; Thery, V.; Nauton, L.; Fessner, W.D.; Charmantray, F.; Hecquet, L. Evolved Thermostable Transketolase for Stereoselective Two-Carbon Elongation of Non-Phosphorylated Aldoses to Naturally Rare Ketoses. ACS Catal. 2019, 9, 4754–4763. [Google Scholar] [CrossRef]
- Szymańska, K.; Pietrowska, M.; Kocurek, J.; Maresz, K.; Koreniuk, A.; Mrowiec-Białoń, J.; Widłak, P.; Magner, E.; Jarzębski, A. Low back-pressure hierarchically structured multichannel microfluidic bioreactors for rapid protein digestion–Proof of concept. Chem. Eng. J. 2016, 287, 148–154. [Google Scholar] [CrossRef]
- Nezhad, M.K.; Aghaei, H. Tosylated cloisite as a new heterofunctional carrier for covalent immobilization of lipase and its utilization for production of biodiesel from waste frying oil. Renew. Energy 2019, 275, 95–104. [Google Scholar] [CrossRef]
- Bedade, D.K.; Sutar, Y.B.; Singhal, R.S. Chitosan coated calcium alginate beads for covalent immobilization of acrylamidase: Process parameters and removal of acrylamide from coffee. Food Chem. 2019, 275, 95–104. [Google Scholar] [CrossRef]
- Pollegioni, L.; Falbo, A.; Pilone, M.S. Specificity and kinetics of Rhodotorula gracillis d-amino acid oxidase. Biochem. Biophys. Acta 1992, 1120, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Groth, L. Heat and pH dependence of catalase. A comparative study. Acta Biol. Hung. 1987, 38, 279–285. [Google Scholar]
- Alptekin, O.; Seyhan Tukel, S.; Yildirim, D. Immobilization and characterization of bovine liver catalase on eggshell. J. Serb. Chem. Soc. 2008, 73, 609–618. [Google Scholar] [CrossRef]
- Mathesh, M.; Liu, J.; Barrow, C.J.; Yang, W. Graphene-Oxide-Based Enzyme Nanoarchitectonics for Substrate Channeling. Chem. Eur. J. 2016, 23, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Fantinato, S.; Pollegioni, L.; Pilone, M.S. Engineering, expression and purification of his-tagged chimeric D-amino acid oxidase from Rhodotorula gracilis. Enzyme Microb. Technol. 2001, 29, 407–412. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kowalczykiewicz, D.; Przypis, M.; Mestrom, L.; Kumpf, A.; Tischler, D.; Hagedoorn, P.L.; Hanefeld, U.; Jarzębski, A.; Szymańska, K. Engineering of continuous bienzymatic cascade process using monolithic microreactors–In flow synthesis of trehalose. Chem. Eng. J. 2022, 427, 131439. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świętochowska, D.; Łochowicz, A.; Ocal, N.; Pollegioni, L.; Charmantray, F.; Hecquet, L.; Szymańska, K. Co-Immobilization of D-Amino Acid Oxidase, Catalase, and Transketolase for One-Pot, Two-Step Synthesis of L-Erythrulose. Catalysts 2023, 13, 95. https://doi.org/10.3390/catal13010095
Świętochowska D, Łochowicz A, Ocal N, Pollegioni L, Charmantray F, Hecquet L, Szymańska K. Co-Immobilization of D-Amino Acid Oxidase, Catalase, and Transketolase for One-Pot, Two-Step Synthesis of L-Erythrulose. Catalysts. 2023; 13(1):95. https://doi.org/10.3390/catal13010095
Chicago/Turabian StyleŚwiętochowska, Daria, Aleksandra Łochowicz, Nazim Ocal, Loredano Pollegioni, Franck Charmantray, Laurence Hecquet, and Katarzyna Szymańska. 2023. "Co-Immobilization of D-Amino Acid Oxidase, Catalase, and Transketolase for One-Pot, Two-Step Synthesis of L-Erythrulose" Catalysts 13, no. 1: 95. https://doi.org/10.3390/catal13010095
APA StyleŚwiętochowska, D., Łochowicz, A., Ocal, N., Pollegioni, L., Charmantray, F., Hecquet, L., & Szymańska, K. (2023). Co-Immobilization of D-Amino Acid Oxidase, Catalase, and Transketolase for One-Pot, Two-Step Synthesis of L-Erythrulose. Catalysts, 13(1), 95. https://doi.org/10.3390/catal13010095






