Synthesis of Precursors to Ethylene Glycol via the Acid-Catalyzed Carbonylation of Formaldehyde
Abstract
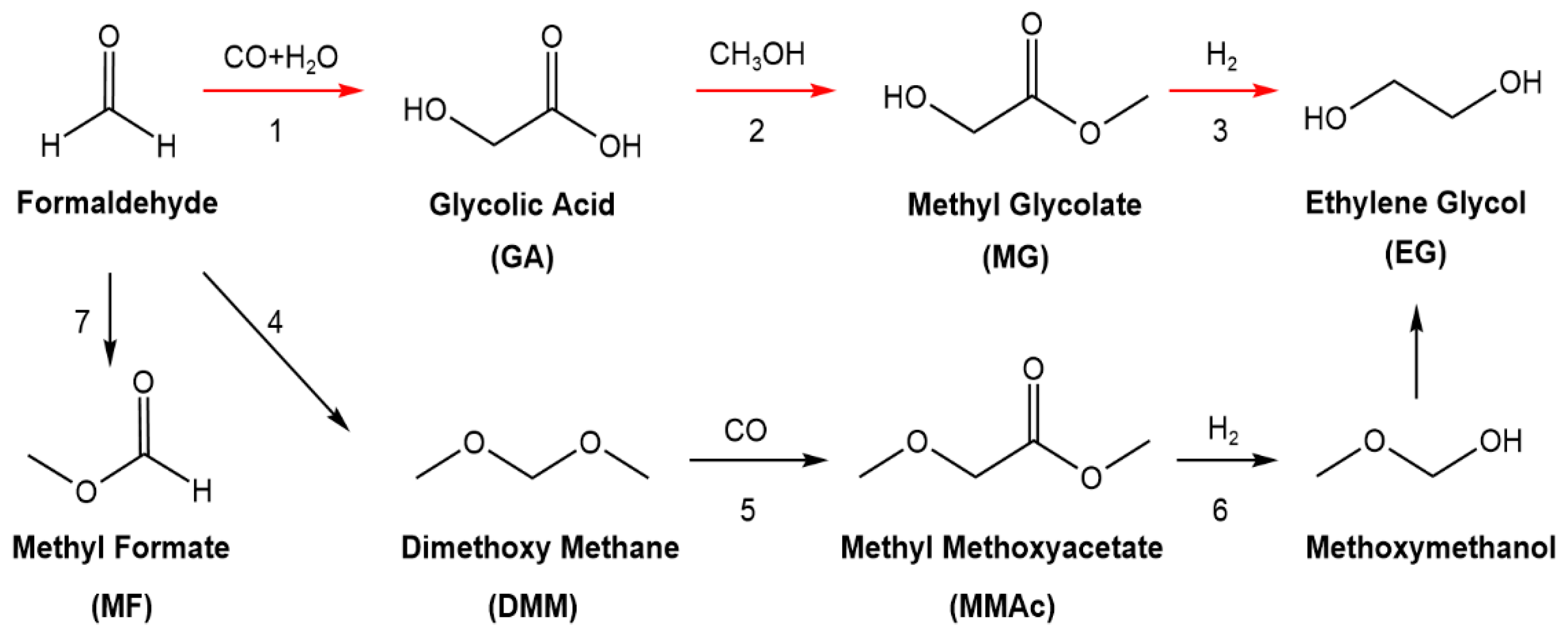
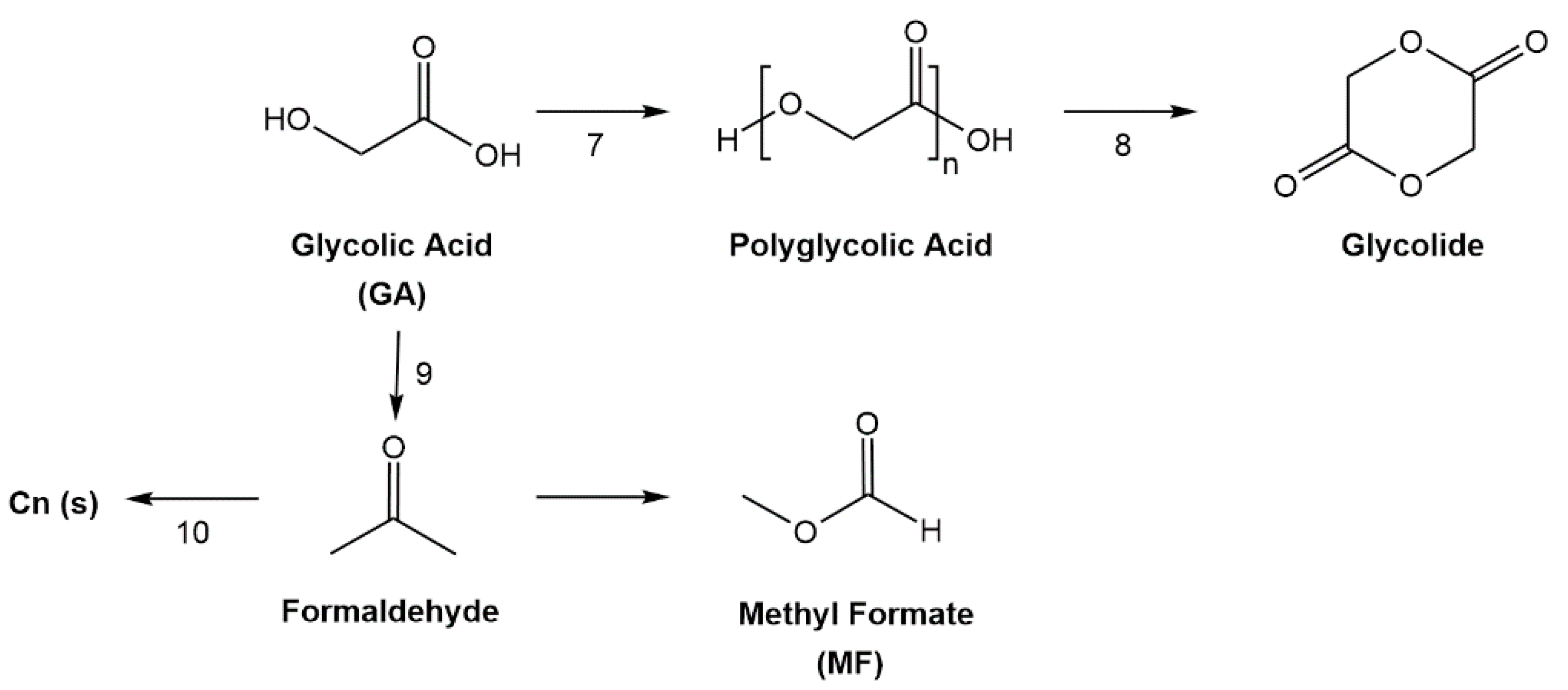
:1. Introduction
2. Results and Discussion
2.1. Optimization of Reaction Process
2.1.1. Modification of Solvents
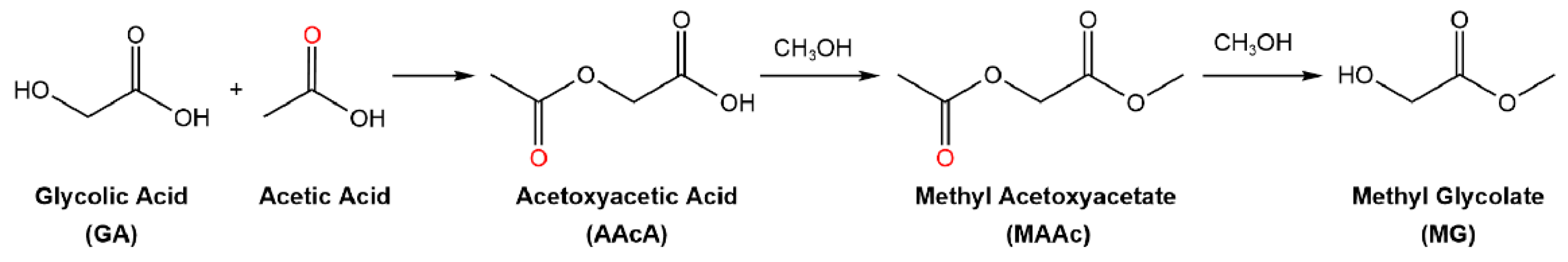
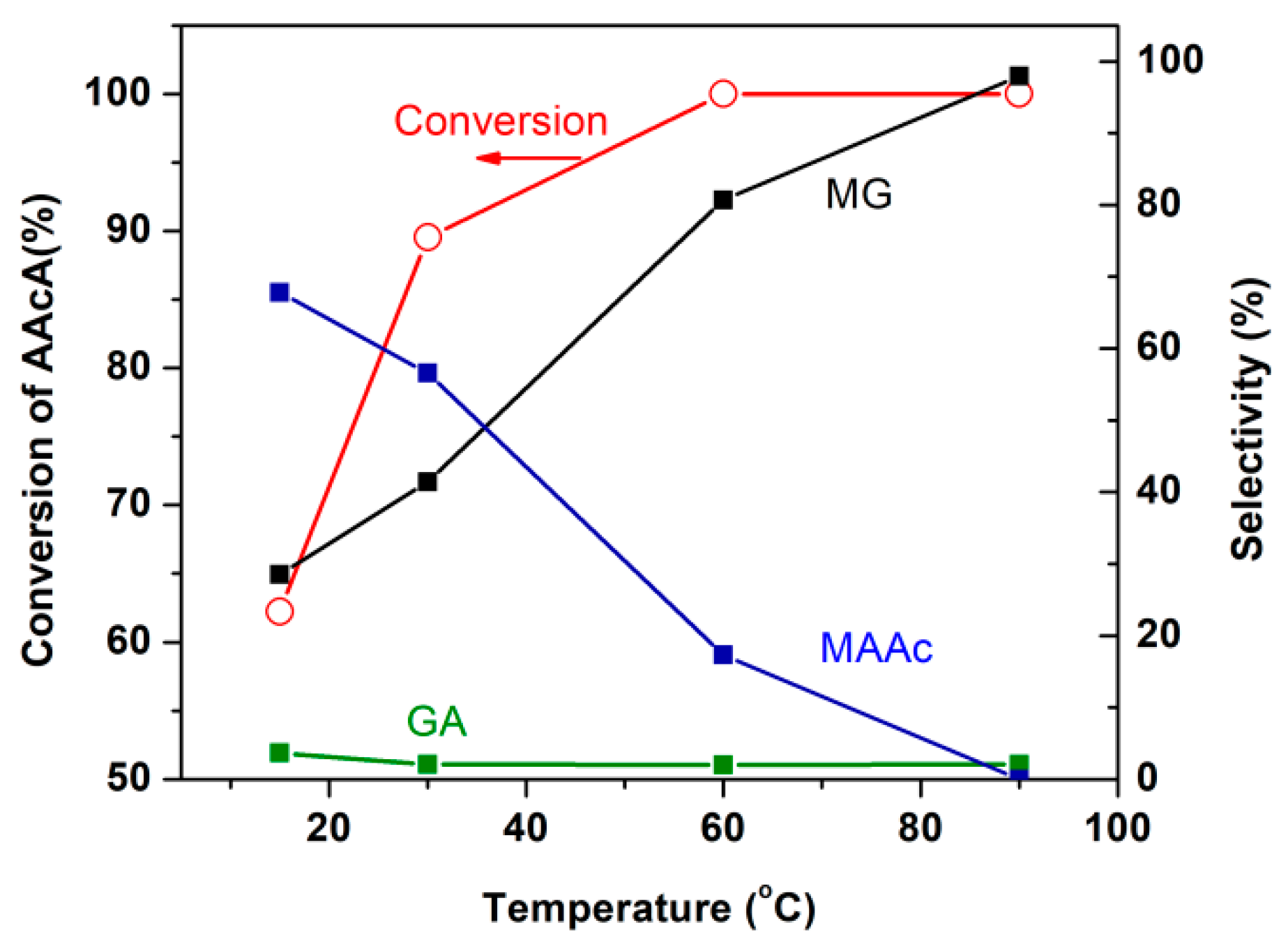
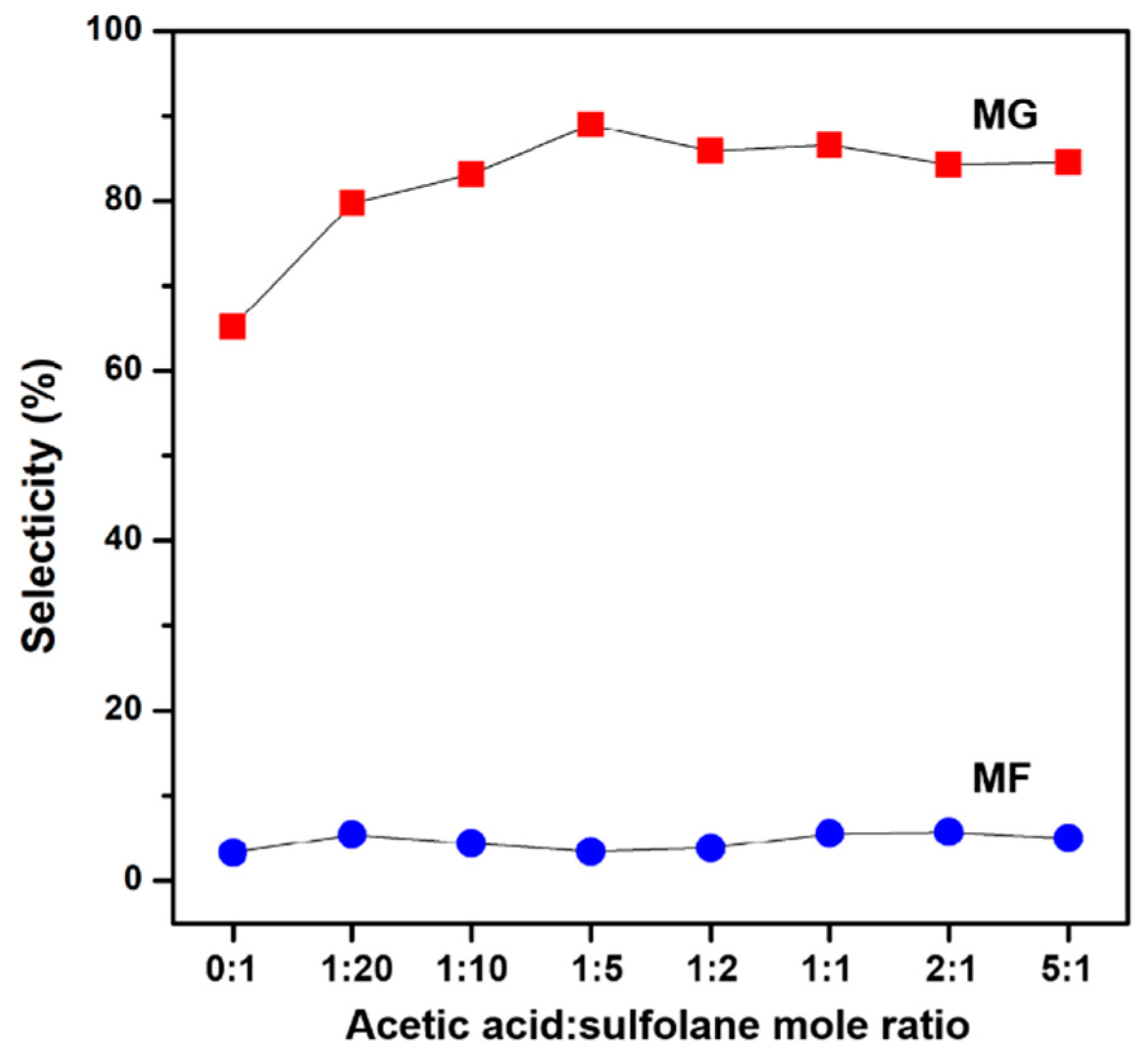
2.1.2. Study on Carboxylic Acid Protection Strategy
- (1)
- The esterification reaction of AAcA takes place first to form MAAc, followed by transesterification of MAAc to form MG;
- (2)
- The transesterification reaction of AAcA takes place first to form GA, followed by the esterification of GA to form MG;
- (3)
- The esterification and transesterification reactions take place simultaneously.
2.2. Catalyst Design for Carbonylation
2.2.1. Studies on Liquid Acid Catalysts
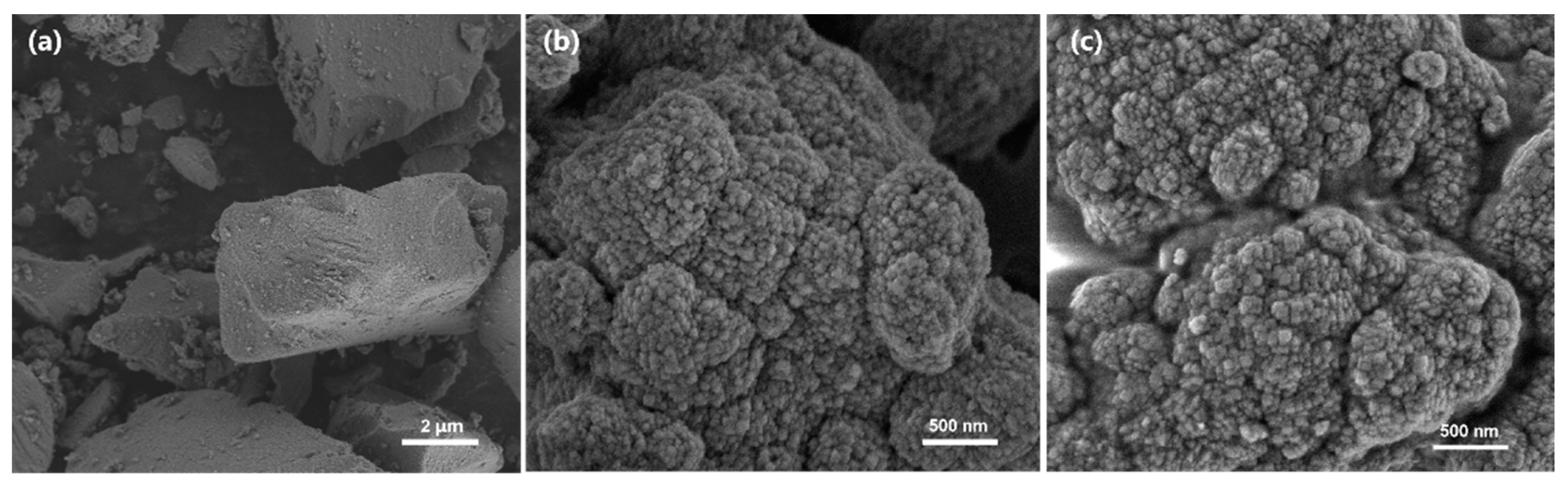
2.2.2. Studies on Solid Acid Catalysts
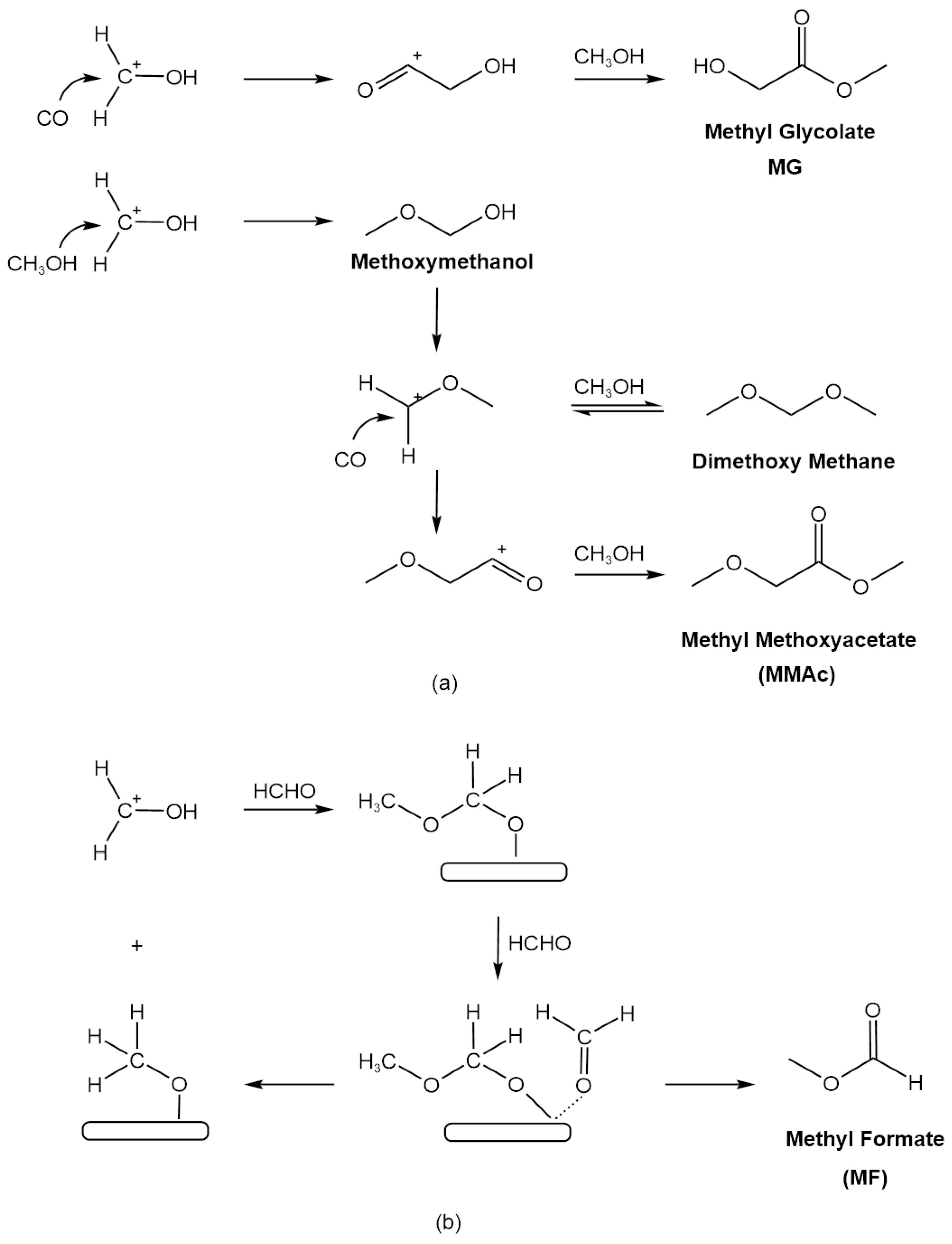
2.2.3. Possible Reaction Mechanism
3. Materials and Methods
3.1. Materials
3.2. Catalyst Synthesis
3.3. Characterizations
3.4. Catalytic Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salusjärvi, L.; Havukainen, S.; Koivistoinen, O.; Toivari, M. Biotechnological production of glycolic acid and ethylene glycol: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 2525–2535. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ye, Y.; Tang, Y.; Chen, L.; Tang, K. Efficient hydrogenation of dimethyl oxalate to ethylene glycol via nickel stabilized copper catalysts. RSC Adv. 2016, 112, 111415–111420. [Google Scholar] [CrossRef]
- Shen, Z.; Xie, S.; Fan, W.; Zhang, O.; Xie, Z.; Yang, W.; Wang, Y.; Lin, J.; Wu, X.; Wan, H.; et al. Direct conversion of formaldehyde to ethylene glycol via photocatalytic carbon–carbon coupling over bismuth vanadate. Catal. Sci. Technol. 2016, 6, 6485–6489. [Google Scholar] [CrossRef]
- Fan, Y.; Bao, J.; Shi, L.; Li, S.; Lu, Y.; Liu, H.; Wang, H.; Zhong, L.; Sun, Y. Photocatalytic Coupling of Methanol and Formaldehyde into Ethylene Glycol with High Atomic Efficiency. Catal. Lett. 2018, 148, 2274–2282. [Google Scholar] [CrossRef]
- Novoselov, A.I.; Silaev, M.M.; Bugaenko, L.T. γ-Induced single-step synthesis of ethylene glycol from methanol-formaldehyde solutions. Theor. Found. Chem. Eng. 2010, 44, 432–435. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, Q.; Zhang, J.; Li, T.; Guo, H. Direct synthesis of ethylene glycol from methanol by dielectric barrier discharge. Chem. Commun. 2013, 49, 10106–10108. [Google Scholar] [CrossRef]
- Song, H.; Jin, R.; Kang, M.; Chen, J. Progress in synthesis of ethylene glycol through C1 chemical industry routes. Chin. J. Catal. 2013, 34, 1035–1050. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, J.C.; Lee, J.S.; Kim, Y.G. Carbonylation of formaldehyde over ion exchange resin catalysts. 1. Batch reactor studies. Ind. Eng. Chem. Res. 1993, 32, 253–259. [Google Scholar] [CrossRef]
- E. I. du Pont de Nemours and Company. Process for Manufacture of Glycolic Acid. U.S. Patent 2152852, 4 April 1939. [Google Scholar]
- Chevron Research Company. Process for the Production of Glycolic Acid and Oxydiacetic Acid. U.S. Patent 3911003, 7 October 1975. [Google Scholar]
- Barri, S.A.I.; Chadwick, D. Carbonylation of Formaldehyde with Zeolite Catalysts. Catal. Lett. 2011, 141, 749–753. [Google Scholar] [CrossRef]
- Shapovalov, V.; Bell, T.A. Theoretical Study of Zeolite-Catalyzed Dimethoxymethane Carbonylation to Methyl Methoxyacetate. J. Phys. Chem. C 2010, 114, 17753–17760. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, W.; Shi, L.; Liu, H.; Liu, Y.; Ni, Y.; Li, L.; Zhou, H.; Xu, S.; Liu, Z. A highly efficient Nafion-H catalyst for vapour phase carbonylation of dimethoxymethane. RSC Adv. 2014, 4, 40999–41002. [Google Scholar] [CrossRef]
- Wang, Z.; Shimada, T.; Takagi, H.; Ahn, C.; Sano, T.; Soga, K.; Takahashi, I.; Masuda, T. Carbonylation of formaldehyde with carbon monoxide over cation-exchange resin catalysts. Bull. Chem. Soc. Jpn. 1999, 72, 1935–1940. [Google Scholar] [CrossRef]
- Celik, F.E.; Lawrence, H.; Bell, A.T. Synthesis of precursors to ethylene glycol from formaldehyde and methyl formate catalyzed by heteropoly acids. J. Mol. Catal. A Chem. 2008, 288, 87–96. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Shen, J.; Liu, H.; Liu, Z. Highly effective synthesis of methyl glycolate with heteropolyacids as catalysts. Catal. Commun. 2009, 10, 678–681. [Google Scholar] [CrossRef]
- Badmaev, S.D.; Potemkin, D.I.; Pechenkin, A.A.; Volkova, G.G.; Sobyanin, V.A.; Parmon, V.N. Gas-phase carbonylation of dimethoxymethane to methyl methoxyacetate over the Cs2.5H0.5PW12O40 catalyst. Dokl. Phys. Chem. 2016, 468, 85–88. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Song, H.; Chen, J. Heteropolyacids as Efficient Catalysts for the Synthesis of Precursors to Ethylene Glycol by the Liquid-phase Carbonylation of Dimethoxymethane. Catal. Lett. 2015, 44, 806–808. [Google Scholar] [CrossRef]
- Song, H.; Jing, F.; Jin, R.; Chen, J. Novel Functional Ionic Liquids as Metal-Free, Efficient and Recyclable Catalysts for the Carbonylation of Formaldehyde. Catal. Lett. 2014, 144, 711–716. [Google Scholar] [CrossRef]
- Lapidus, A.L.; Eliseev, O.L. Catalytic carbonylation in ionic liquids. Solid Fuel Chem. 2010, 44, 197–202. [Google Scholar] [CrossRef]
- Li, T.; Souma, Y.; Xu, Q. Carbonylation of formaldehyde catalyzed by p-toluenesulfonic acid. Catal. Today 2006, 111, 288–291. [Google Scholar] [CrossRef]
- Souma, Y.; Sano, H. Carbonylation of formaldehyde catalyzed by Copper(I), Silver carbonyls. Nippon. Kagaku Kaishi 1982, 2, 263–267. [Google Scholar] [CrossRef]
- Ayyoob, M.; Lee, D.H.; Kim, J.H.; Nam, S.W.; Kim, Y.J. Synthesis of poly(glycolic acids) via solution polycondensation and investigation of their thermal degradation behaviors. Fibers Polym. 2017, 18, 407–415. [Google Scholar] [CrossRef]
- Zhu, H.; Cao, H.; Li, T. A theoretical study on the decomposition mechanisms of deprotonated glycolic acid. Comput. Theor. Chem. 2013, 1008, 32–38. [Google Scholar] [CrossRef]
- Jo, H.J.; Kim, J.H.; Kim, Y.N. Glyoxylate carboligase-based whole-cell biotransformation of formaldehyde into ethylene glycol via glycolaldehyde. Green Chem. 2022, 24, 218–226. [Google Scholar] [CrossRef]
- Walker, J.F. Formaldehyde, American Chemical Society Monograph Series; Reinhold: London, UK, 1964. [Google Scholar]
- Ragazzini, M.; Modena, M.; Gallinella, E.; Cevidalli, G. Preparation and structure of some carbon monoxide-formaldehyde copolymers. J. Polym. Sci. 1964, 2, 5203. [Google Scholar] [CrossRef]
- Chevron Research Company. Process for Preparing Glycolic Acid from Formaldehyde Using Acetic Acid. U.S. Patent 4136112, 23 January 1979. [Google Scholar]
- Pirozhkov, S.; Stepanyan, A.; Myshenkova, T.N.; Ordyan, M.; Lapidus, A. Carbonylation of olefins and alcohols at atmospheric pressure in the presence of acid catalysts with added Ag2O or Cu2O. Russ. Chem. Bull. 1982, 31, 1852–1858. [Google Scholar] [CrossRef]
- Song, H.; Chen, J.; Xia, C. Brönsted acidic ionic liquids as efficient and recyclable catalysts for the carbonylation of formaldehyde. Catal. Lett. 2012, 142, 81–86. [Google Scholar] [CrossRef]
- Alexandratos, S.D. Ion-exchange resins: A retrospective from industrial and engineering chemistry research. Ind. Eng. Chem. Res. 2009, 48, 388–398. [Google Scholar] [CrossRef]
- Harmer, M.A.; Farneth, W.E.; Sun, Q. High surface area Nafion resin/silica nanocomposites: A new class of solid acid catalyst. J. Am. Chem. Soc. 1996, 118, 7708–7715. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Lafon, O.; Trébosc, J.; Kim, K.D.; Stampfl, C.; Baiker, A.; Amoureus, J.; Huang, J. Brönsted acid sites based on penta-coordinated aluminum species. Nat. Commun. 2016, 7, 13820. [Google Scholar] [CrossRef]
- Marmoru, A. The reaction of formaldehyde on various metal oxide catalysts. J. Catal. 1983, 83, 141–150. [Google Scholar]
- Tanabe, K.; Saito, K. The conversion of benzaldehyde into benzyl benzoate with alkaline earth metal oxide catalysts. J. Catal. 1974, 35, 247–255. [Google Scholar] [CrossRef]
- Tsunetake, S.; Tetsuo, N.; Makoto, O. The Tishchenko Reaction: A classic and practical tool for ester synthesis. Chem. Lett. 2006, 35, 824–829. [Google Scholar]
- Timofeeva, M.N. Acid catalysis by heteropoly acids. Appl. Catal. A 2003, 256, 19–35. [Google Scholar] [CrossRef]
- Morooka, S.; Matubayasi, N.; Nakahara, M. Kinetic Study on Disproportionations of C1 Aldehydes in Supercritical Water: Methanol from Formaldehyde and Formic Acid. J. Phys. Chem. 2007, 111, 2697–2705. [Google Scholar] [CrossRef]
- Jiang, Y.; Hunger, M.; Wang, W. On the Reactivity of Surface Methoxy Species in Acidic Zeolites. J. Am. Chem. Soc. 2006, 128, 11679–11692. [Google Scholar] [CrossRef]
- Boronat, M.; Martinez-Sanchez, C.; Law, D.; Corma, A. Enzyme-like specificity in zeolites: A unique site position in mordenite for selective carbonylation of methanol and dimethyl ether with CO. J. Am. Chem. Soc. 2008, 130, 16316–16323. [Google Scholar] [CrossRef]
| Solvents | HCHO Conversion (%) | Selectivity (%) | ||
|---|---|---|---|---|
| MG | MMAc | MF | ||
| Sulfolane | 99 | 65.9 | 8.8 | 6.3 |
| n-pentane | 99 | 59.2 | 9.7 | 9.4 |
| 1,3-dioxolane | 83 | 36.4 | 16.1 | - |
| 1,4-dioxane | 82 | 53.2 | 21.8 | 13.4 |
| Dimethylsulfoxide | 35 | - | 44.6 | 27.4 |
| Cyclohexane | 99 | 61.1 | 6.6 | 8.0 |
| n-octane | 98 | 60.1 | 8.5 | 11.0 |
| i-octane | 99 | 57.0 | 6.3 | 9.0 |
| Toluene | 88 | 15.9 | 9.8 | 12.7 |
| Solvents * | HCHO Conversion (%) | Selectivity (%) | ||
|---|---|---|---|---|
| MG | MMAc | MF | ||
| No carboxylic acid | 99 | 65.9 | 8.8 | 6.3 |
| Acetic acid | 99 | 81.2 | 3.5 | 2.4 |
| Propanoic acid | 99 | 79.8 | 3.6 | 3.7 |
| Isobutyric acid | 98 | 73.5 | 5.9 | 6.0 |
| Oxalic acid | 91 | 56.8 | 11.6 | 23.6 |
| Liquid Acid | Selectivity (%) | ||||
|---|---|---|---|---|---|
| Name | Formula | b.p. (lit.) | MG | MMAc | MF |
| Trifluoromethanesulfonic acid | CF3SO3H | 162 °C | 90.0 | 4.1 | 3.5 |
| Methanesulfonic acid | CH3SO3H | 167 °C (10 mm Hg) | 11.1 | 11.3 | 4.2 |
| P-toluenesulfonic acid | C7H7SO3H | 140 °C (20 mm Hg) | 12.4 | 10.9 | 4.1 |
| Nonafluoro-1-butanesulfonic acid | C4F9SO3H | 112–114 °C (14 mm Hg) | 85.8 | 9.1 | 3.6 |
| Heptadecafluorooctanesulfonic acid | C8F17SO3H | 260 °C | 3.2 | 8.4 | 3.3 |
| Dodecyl benzene sulphonic acid | C18H29SO3H | 315 °C | 10.2 | 1.5 | 5.4 |
| Catalyst | Functional Group | HCHO Conversion (%) | Selectivity (%) | |
|---|---|---|---|---|
| MG | MF | |||
| Purolite CT 251 | −SO3H | 90 | 85.4 | 4.0 |
| Amberlite IR 120 | −SO3H | 95 | 81.1 | 3.2 |
| Amberlyst-15 | −SO3H | 91 | 83.4 | 5.8 |
| Nafion | −CF2SO3H | 52 | 92.9 | 1.2 |
| Catalyst | HCHO Conversion (%) | Selectivity (%) | ||
|---|---|---|---|---|
| MG | MMAc | MF | ||
| 10%Nafion/SiO2 | 66 | 73.3 | 15.6 | 3.1 |
| 20%Nafion/SiO2 | 84 | 78.2 | 6.3 | 1.3 |
| 30%Nafion/SiO2 | 93 | 80.0 | 6.4 | 1.4 |
| 45%Nafion/SiO2 | 97 | 80.9 | 6.1 | 1.3 |
| Supported Catalyst | HCHO Conversion (%) | Selectivity (%) | ||
|---|---|---|---|---|
| MG | MMAc | MF | ||
| 45%Nafion/SiO2 | 90 | 79.3 | 5.7 | 1.5 |
| 45%Nafion/ZEO | 100 | 82.4 | 7.0 | 3.9 |
| 45%Nafion/m-ZEO | 100 | 87.8 | 4.4 | 0.2 |
| Catalyst | Nafion Loading | BET Surface Area (m2/g) | Acid Site Concentration * (mmol/g) |
|---|---|---|---|
| 45%Nafion/SiO2 | 45% | 107.9 | 0.30 |
| 45%Nafion/ZEO | 45% | 325.6 | 0.49 |
| 45%Nafion/m-ZEO | 45% | 314.3 | 0.55 |
| Sample | Temperature (°C) | Brönsted Acid (μmol/g) | Lewis Acid (μmol/g) | Brönsted Acid/ Lewis Acid |
|---|---|---|---|---|
| ZEO | 150 | 375 | 126 | 3.0 |
| 250 | 314 | 83 | 3.8 | |
| 350 | 265 | 75 | 3.5 | |
| m-ZEO | 150 | 470 | 54 | 8.7 |
| 250 | 458 | 62 | 7.4 | |
| 350 | 353 | 51 | 6.9 |
| Zeolite Catalyst | SiO2/Al2O3 1 | Brönsted Acid/ Lewis Acid 2 | HCHO Conversion (%) 3 | Selectivity (%) | |
|---|---|---|---|---|---|
| MG | MF | ||||
| MOR | 80 | 0.9 | 93 | 1.6 | 32.6 |
| Beta | 75 | 1.7 | 80 | 25.8 | 22.5 |
| ZSM-5 | 79 | 2.4 | 79 | 39.6 | 10.4 |
| Catalyst | CeO2 | ZrO2 | Amberlyst-15 | H-Y | SiO2 |
|---|---|---|---|---|---|
| Activity (10−3 mol MF·g−1·h−1) | 8.7 | 3.6 | 0.04 | 1.2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Lv, J. Synthesis of Precursors to Ethylene Glycol via the Acid-Catalyzed Carbonylation of Formaldehyde. Catalysts 2023, 13, 1327. https://doi.org/10.3390/catal13101327
Wang D, Lv J. Synthesis of Precursors to Ethylene Glycol via the Acid-Catalyzed Carbonylation of Formaldehyde. Catalysts. 2023; 13(10):1327. https://doi.org/10.3390/catal13101327
Chicago/Turabian StyleWang, Di, and Jiangang Lv. 2023. "Synthesis of Precursors to Ethylene Glycol via the Acid-Catalyzed Carbonylation of Formaldehyde" Catalysts 13, no. 10: 1327. https://doi.org/10.3390/catal13101327
APA StyleWang, D., & Lv, J. (2023). Synthesis of Precursors to Ethylene Glycol via the Acid-Catalyzed Carbonylation of Formaldehyde. Catalysts, 13(10), 1327. https://doi.org/10.3390/catal13101327






