Abstract
The use of photocatalysis to address environmental pollution and energy shortage is an attractive choice. Herein, we successfully synthesized a novel 3D interconnected porous carbon-rich g-C3N4 catalyst via facile thermal polymerization to enhance photocatalytic hydrogen production and photodegradation of dye contaminants. Enhanced hydrogen evolution (1956.23 μmol g−1 h−1) and photocatalytic RhB degradation (96.74%) efficiency were achieved with the as-obtained catalysts. Based on the photocatalytic experimental data and characterization analyses, an enhancement mechanism was proposed. The 3D interconnected porous structure endowed the g-C3N4 with numerous active sites and a large specific surface area, and the carbon modification facilitated the separation and transfer of the photoinduced charge carriers. Nanoshape engineering and the carbon-rich structure showed a synergetic effect in increasing photocatalytic performance. This study offers an applicable methodology for the exploitation of an economical catalyst to alleviate environmental pollution and energy shortages.
1. Introduction
The environmental pollution and energy crises faced by human beings have become urgent issues related to sustainable development [1,2,3,4]. As an efficient, low-energy, and environmentally friendly technology, photocatalysis has emerged as an effective strategy for addressing these two issues [5,6,7,8]. To date, photocatalysis has been applied to hydrogen evolution [9], CO2 reduction [10], pollutant degradation [11], microbial inactivation [12], and so on. Nevertheless, the need for efficient and robust photocatalysts is an important factor restricting the development of photocatalysis. For this reason, researchers have devoted many studies to developing high-efficiency and durable catalysts [13,14,15].
In this respect, graphitic carbon nitride (g-C3N4) performs very well due to its special electronic band structure, tendency toward stability, and VIS irradiation response [16,17]. However, bulk g-C3N4 still has a series of shortcomings restricting its application, such as a low specific surface area (SSA), limited light absorption (g-C3N4 has a moderate band gap of 2.7–2.8 eV), and rapid recombination rate for photogenerated charges [18,19]. Considerable effort has been devoted to addressing these inherent shortcomings, such as with element doping, structural control, heterojunction construction, and surface modifications [20,21,22,23]. Therefore, doping with nonmetallic elements has been deemed an efficient and facile strategy for improving photocatalytic performance [12]. This strategy maintains the metal-free nature of g-C3N4 and enhances the efficiency of photoinduced carrier separation, which enhances the photocatalytic activity. To date, many nonmetallic element atoms (such as P, O, B, S, and C) have been successfully doped onto the skeletons of graphitic carbon nitride to increase the photocatalytic efficiency [24,25,26]. For example, Yu et al. [27] synthesized dual P-doped g-C3N4, which displayed enhanced photocatalytic H2O2 production. Among these atoms, self-doping is the preferred choice since the introduction of heteroatoms can provide a new center for charge recombination. Moreover, self-doping can change the structure and conductivity of g-C3N4 without introducing other impurity elements, thereby improving its photocatalytic activity. Herein, we prepared C-doped g-C3N4 with significantly improved photocatalytic performance by the dopant of cytosine.
In addition, with an appealing electronic band structure and relatively low recombination rate for the charge carriers, the key factor limiting the photocatalytic activity is the paucity of active sites [17]. Pristine g-C3N4 presents limited photocatalytic activity because of the small SSA and resulting poor solvent dispersibility. In this regard, various g-C3N4 structures, such as 0D nanodots [28], 1D nanoribbons [29], 2D nanosheets [30], and 3D nanospheres [31], have been synthesized to provide additional active sites and promote photocatalytic efficiency. In particular, 2D nanosheets have been prepared due to their large SSA and abundant reactive sites. However, their thinness and smaller particle sizes make them difficult to separate from suspensions, thereby causing secondary pollution [32,33]. However, 3D structural g-C3N4 catalysts assembled on 2D nanosheets maintain a larger SSA and reactive sites while avoiding structural conglutination during the photocatalytic process [34]. Based on these considerations, a combination of nonmetallic element doping and nanoshape engineering strategies may be a conceivable way to modify the photocatalytic efficiency.
In this study, we coupled carbon self-doping with morphological adjustments and fabricated 3D interconnected porous carbon-rich graphitic carbon nitride catalysts with enhanced VIS radiation photocatalytic activity. The 3D porous structure endows g-C3N4 with more active sites and adsorption sites, and C doping increases the VIS irradiation response range of the catalyst. The two work together to result in the enhancement of photocatalytic activity. Hydrogen generation via water decomposition and RhB elimination tests were conducted to verify the capability of the as-prepared catalysts. Furthermore, based on characterization analyses and photocatalytic experimental data, a reasonable photocatalytic mechanism was developed.
2. Results and Discussion
2.1. Morphologies and Structures
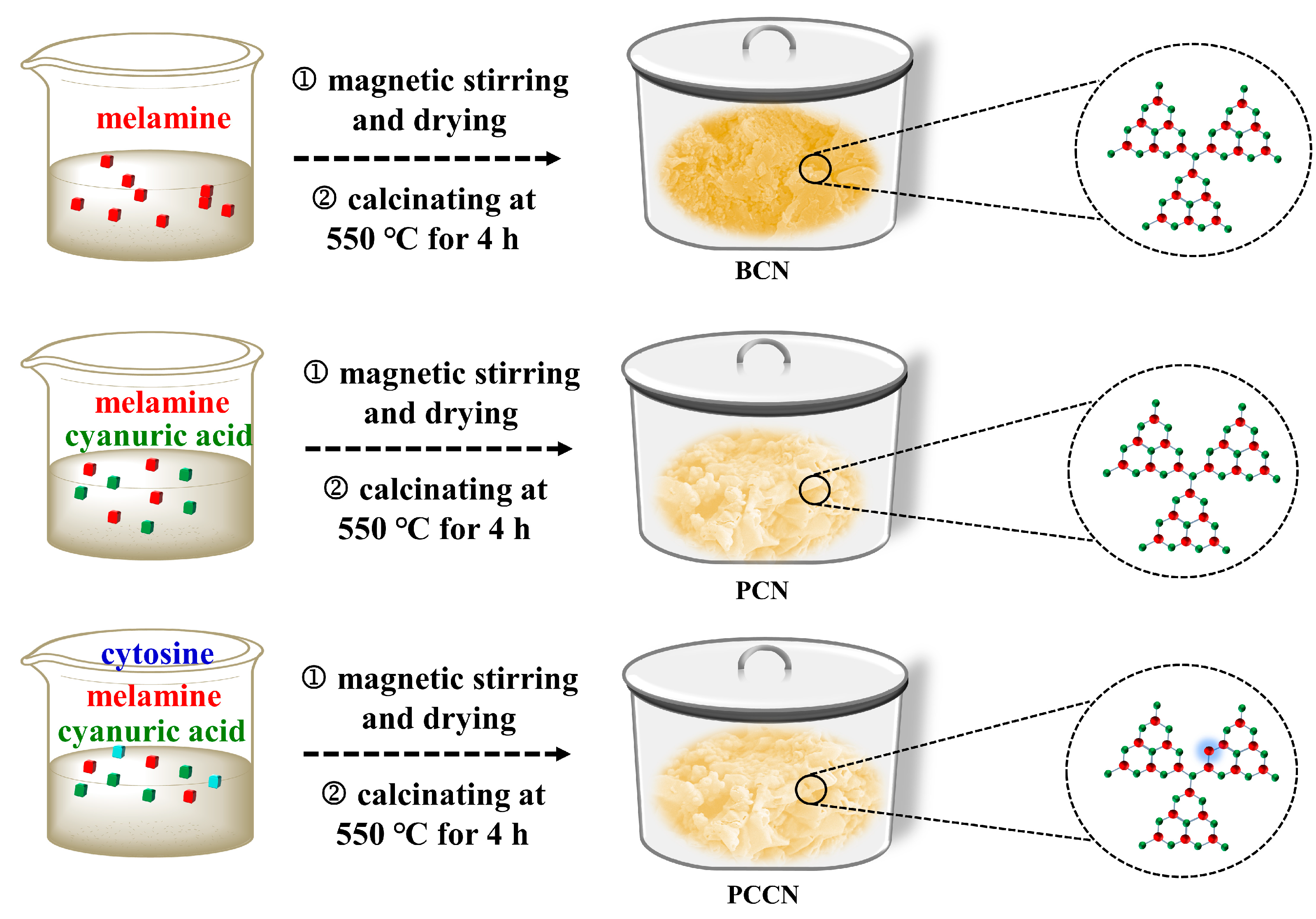
The formation mechanism of carbon-rich g-C3N4 is shown in Figure S1. In the synthesis process of PCN, the melamine and cyanuric acid would form melamine-cyanuric acid supramolecular (MCS) under magnetic stirring due to the self-assembly effect. The cyanuric acid in the MCS decomposes into gases during the calcination at high temperatures because of its instability, and thus, the 3D interconnected porous g-C3N4 catalysts were obtained. In the same way, the MCS came into being in the preparation of PCCN catalysts. At the same time, cytosine is attached to the surface of MCS due to hydrogen bonding. During the calcination at high temperatures, the C atoms from the cytosine ring substituted melamine in the MCS and doped into the g-C3N4 framework, forming carbon-rich g-C3N4.
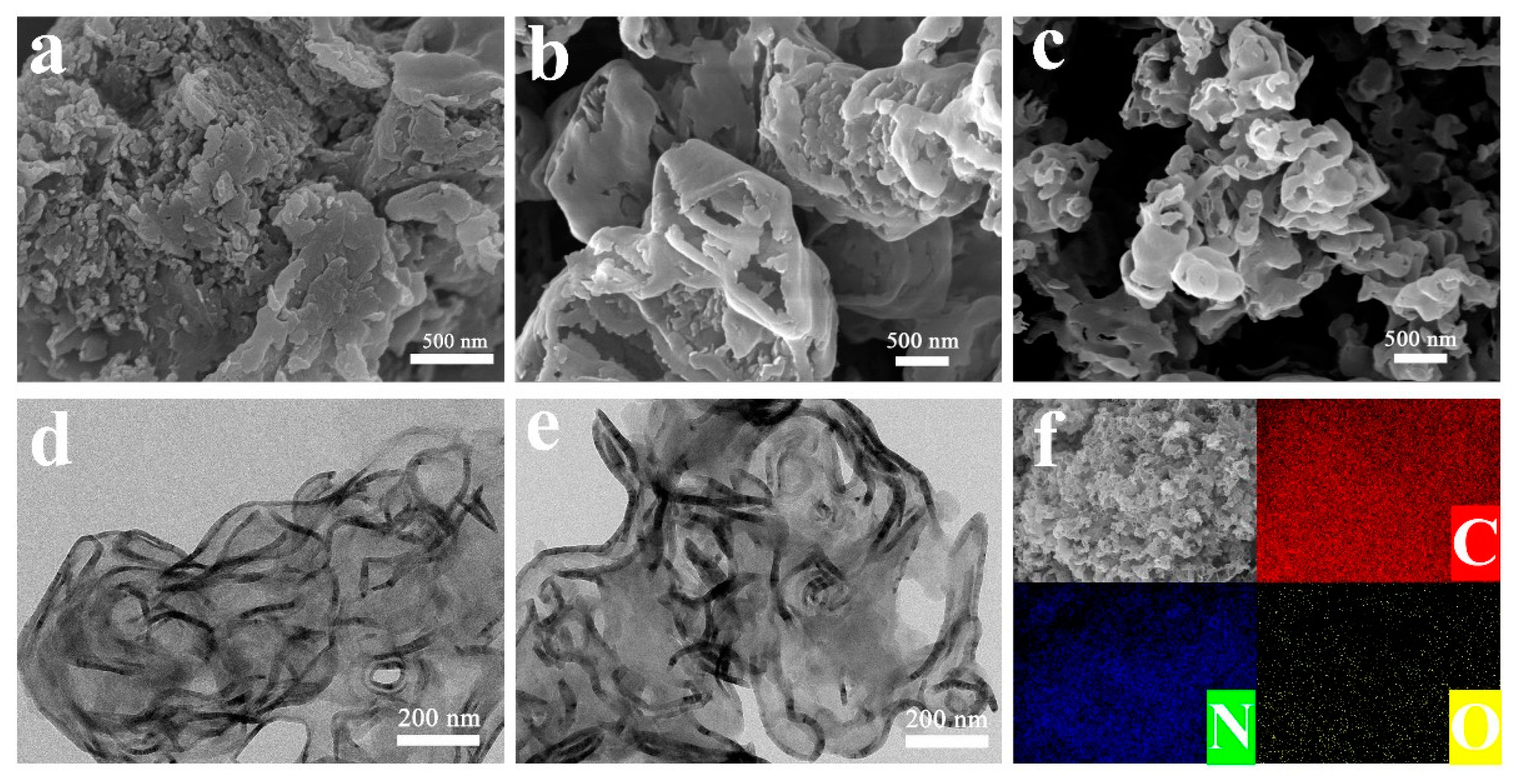
To determine the morphologies and structures of the as-synthesized samples, we used transmission electron microscopy (TEM, JEM-2010, JEOL Co., Ltd., Beijing, China) and field emission scanning electron microscopy (FESEM, S-4800, Hitachi, Tokyo, Japan), and the morphological results are illustrated in Figure 1 and Figure S2. As expected, the BCN samples prepared via directly calcinating melamine displayed a typical tight and large blocky shape and irregular agglomerations (Figure 1a), which was undesirable for photocatalytic reactions. However, the PCN samples obtained by the precursor system of melamine and cyanuric acid exhibited 3D interlinked porous structures, as shown in Figure 1b. The 3D interconnected structure endowed the g-C3N4 catalysts with stronger adsorption capacity during the photocatalytic reactions and provided abundant active sites to enhance the photocatalytic performance. In addition, the PCCN, fabricated through annealing the supermolecules of melamine, cyanuric acid, and cytosine, still had a 3D interconnected porous structure (Figure 1c and Figure S2). Of note, the 3D interconnected porous structures of PCCN samples became thinner as the cytosine content was increased, which was consistent with the increased SSA (Figure 1a and Table 1). Furthermore, TEM provided more details about the 3D structure characteristics of the 3D interconnected porous form of the unit cross-linking 2D g-C3N4 nanosheet, which was consistent with our previous study [31]. The elemental mapping plotted in Figure 1f,g verified the presence of C and N elements in the PCCN-20 samples.
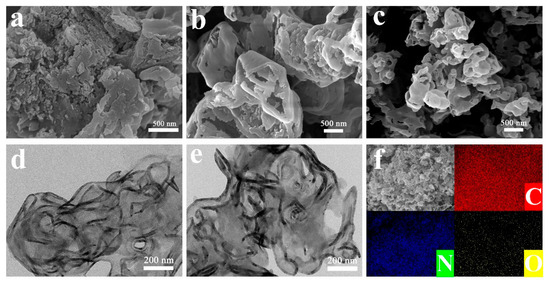
Figure 1.
FESEM images of (a) BCN, (b) PCN, and (c) PCCN-20; TEM images of (d) PCN and (e) PCCN-20; and (f) elemental mapping of PCCN-20.

Table 1.
Specific surface areas and total pore volumes of BCN, PCN, PCCN-10, PCCN-20, and PCCN-30.
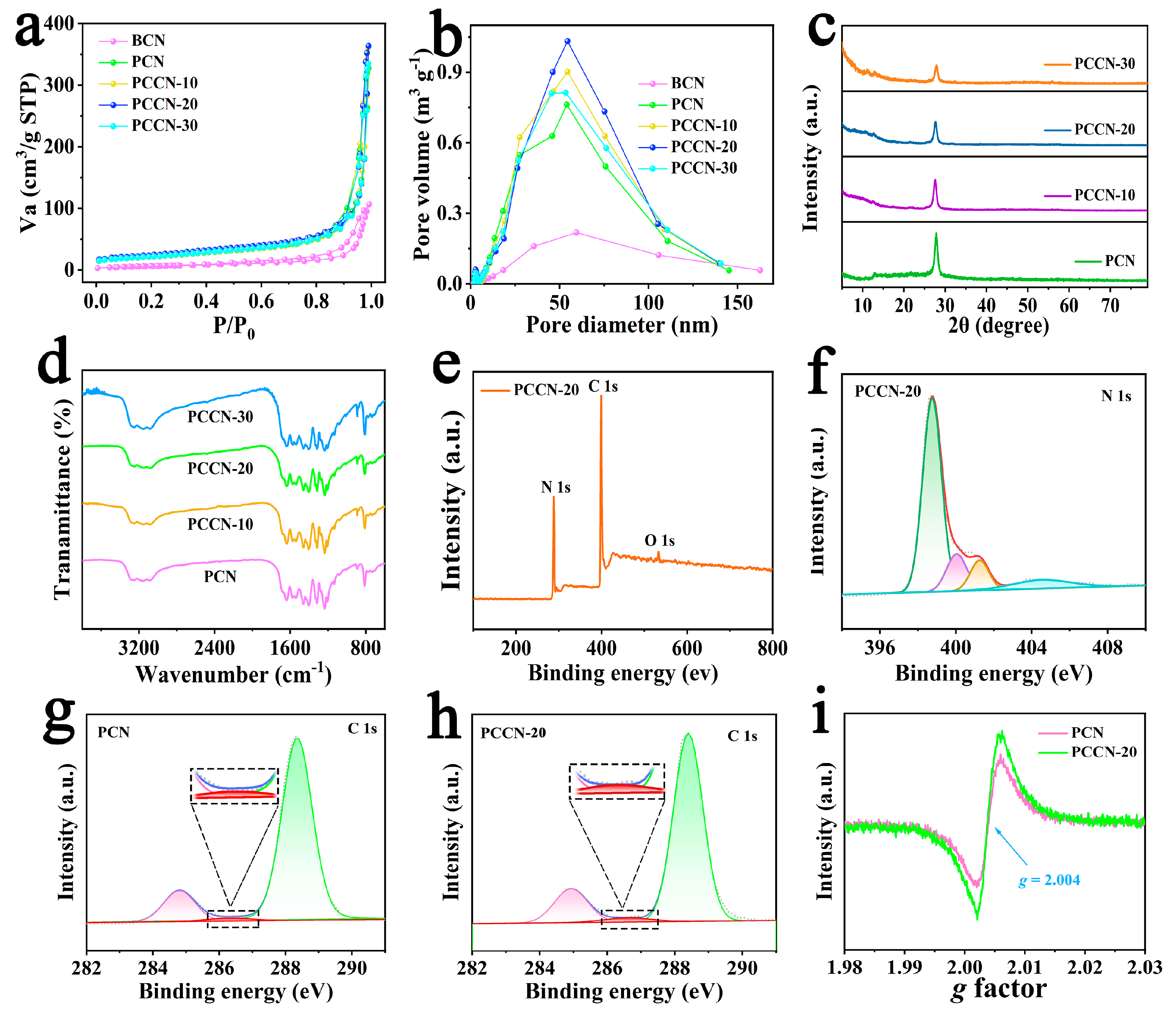
Nitrogen adsorption–desorption isotherms were used to characterize the texture and porosity of the as-fabricated catalysts. The results summarized in Figure 2a,b show that all samples presented classical H3-type hysteresis loops, indicating the formation of mesoporous materials [35]. Moreover, the BCN had the lowest SSA of 15.16 m2 g−1 (Table 1), which was consistent with the SEM results. Obviously, the PCN prepared by calcination of melamine and cyanuric acid exhibited a greatly increased SSA with a 3D interlinked porous structure, which contained ample active sites and adsorption sites for photocatalytic reactions. Fortunately, the SSAs of the PCCN samples were increased slightly by the addition of cytosine and reached 85.68 m2 g−1 under the optimal conditions (PCCN-30), which indicated that the carbon-rich graphitic carbon nitride structure would further increase the SSA of 3D g-C3N4. Generally, the pore size distribution curves presented in Figure 2b were consistent with the SSA of the catalysts, which verified the effect of the 3D structure and C-doping on the morphology of the catalyst.
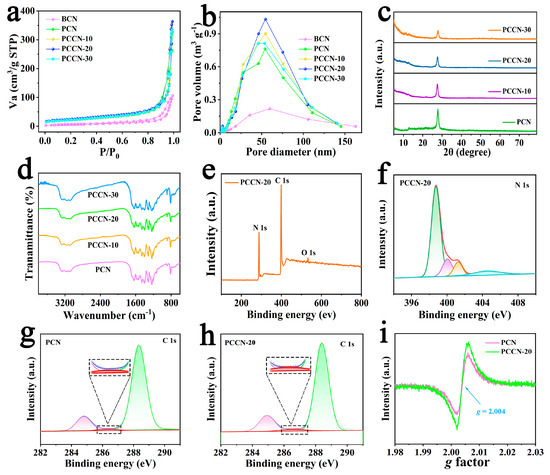
Figure 2.
(a) N2 isotherms and (b) pore size distribution images of BCN, PCN, and PCCN samples; (c) XRD and (d) FT-IR spectra of BCN, PCN, and PCCN samples; (e) XPS survey spectrum and (f) N 1 s spectrum for PCCN-20; XPS C 1 s spectra of (g) PCN and (h) PCCN-20; and (i) EPR spectra of PCN and PCCN-20.
To understand the crystal structure and surface groups of the as-prepared catalysts, X-ray diffraction (XRD, PANalytical, EA Almelo, The Netherlands) patterns along with Fourier transform infrared (FT-IR, NEXUS 470, Nicolet, Wisconsin, US) spectra were recorded. As shown in Figure 2c, two peaks were observed at approximately 27.8° and 12.7° for the (002) and (100) planes of g-C3N4, which was consistent with the interlayer-stacking of aromatic units and the repeated planar tri-s-triazine (heptazine) units [36], respectively. In addition, the intensity of the diffraction peak centered at 27.8° for the PCCN samples gradually weakened compared with that of the PCN. This indicated that carbon derived from the introduction of cytosine inhibited polymeric condensation of the g-C3N4. In addition, the FT-IR spectra of the as-prepared samples in Figure 2d exhibited similar absorption peaks at approximately 810 cm−1, which were ascribed to a tri-s-triazine bending vibration [35]. Many peaks from 1200 to 1700 cm−1 arose from stretching vibrations of the CN heterocyclic rings [37], and numerous peaks centered at approximately 3200 cm−1 were attributed to N–H stretching vibrations [38]. In summary, the XRD and FT-IR results demonstrated that added elements did not destroy the pristine structure of g-C3N4.
We also used X-ray photoelectron spectroscopy (XPS, Axis Ultra, Kratos Analytical, Manchester, UK) to further analyze the structures and surface groups of the as-synthesized samples, and the results are shown in Figure 2e–h. The XPS survey spectrum for PCCN-20 contained signals for the C, N, and O elements, and the trace amount of O may be attributed to H2O or CO2 adsorbed in the catalyst. As shown in Figure 2f, the N 1 s signal was deconvoluted into four peaks at 398.8, 400.0, 401.3, and 404.5 eV, which were ascribed to C–N=C groups, tertiary nitrogen (N–(C)3) groups, C–N–Hx, and π excitation [39], respectively. In addition, in the C 1 s spectrum of PCCN-20 (Figure 2g), the peaks at 288.3, 286.5, and 284.8 eV were attributed to sp2 hybridized carbons in N–C=N bonds [40], N–sp2 carbons in the triazine rings, and carbon impurities, respectively. Additionally, it was noteworthy that the C content in the PCCN-20 catalyst had increased due to the introduction of cytosine (inset image in Figure 2g,h), indicating the successful introduction of carbon. These results are shown more clearly in Table 2, which indicates that the C content of PCCN-20 increased from 47.39% to 48.19%.

Table 2.
The contents of C and N in PCN and PCCN-20 catalysts according to the XPS measurement.
Electron paramagnetic resonance (EPR, MEX-nano, Bruker, Karlsruhe, Germany) was performed to assess unpaired electrons formed by the as-fabricated samples. Obviously, both PCN and PCCN-20 exhibit the same Lorentzian lines located at g = 2.004 (Figure 2i). In addition, PCCN-20 showed a higher single intensity, identifying more delocalized electrons generated from rich carbon [41], which was helpful for the separation of photoinduced charge carriers.
2.2. Optical and Electrochemical Performance
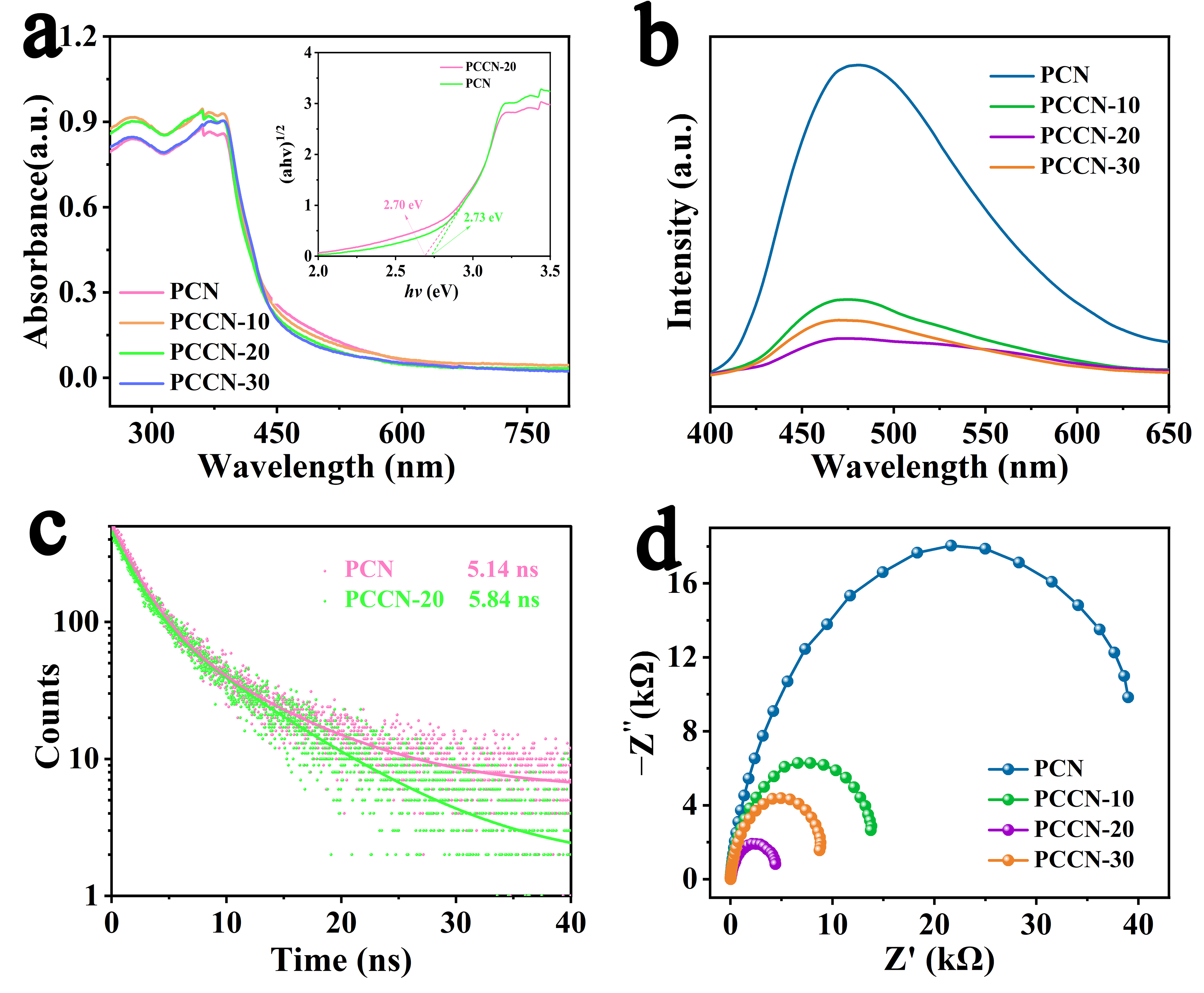
UV–VIS diffuse reflectance spectroscopy (UV–VIS DRS, U-3900, Hitachi, Tokyo, Japan) was used to assess the absorption capacity of the as-fabricated catalysts, and the results are illustrated in Figure 3a. All as-prepared samples showed similar spectra with absorption peaks in the VIS radiation region. In comparison with that for PCN, the visible absorption peaks of the as-prepared PCCN catalysts were slightly enhanced, suggesting slightly greater light absorption. In addition, the band gap energy (Eg) was calculated with a Tauc plot, as shown in the inset in Figure 3a [42]. The narrowed band gap energy was consistent with the UV–VIS DRS results, indicating that more charge carriers were generated for the photocatalytic reactions.
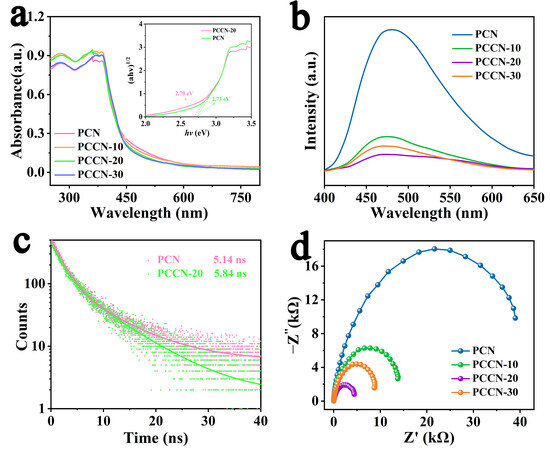
Figure 3.
(a) UV–VIS DRS and Tauc’s plot of PCN and PCCN-20 (inset), (b) PL, (c) RTPL spectra, and (d) EIS of BCN, PCN, and PCCN catalysts.
It is commonly recognized that charge separation and migration in catalysts are crucial aspects of their photocatalytic capacity. Therefore, steady-state photoluminescence (PL, Fluoromax-4, HORIBA Jobin Yvon, Kyoto, Japan) spectroscopy was used to assess charge separation in the as-prepared samples. As displayed in Figure 3b for PCN, an obvious emission peak at approximately 470 nm was ascribed to band-to-band recombination. Compared with PCN, the PCCN samples showed decreased emission peak intensity, suggesting a significantly suppressed combination of the photoinduced charge carriers. Time-resolved photoluminescence (RTPL, FLSP920, EI, Edinburgh, UK) decay spectra (Figure 3c) were also obtained, and they supplied more detail on the photogenerated charge carriers, from which the average recovery lifetimes (τ) were derived, as in Equation (1) [43]. Apparently, PCCN-20 (5.84 ns) showed a longer emission lifetime than PCN (5.14 ns), which was displayed in Table 3, indicating that the doped carbon enhanced the migration of the photogenerated charge carriers.

Table 3.
Lifetime profile and corresponding carrier dynamics information of the PCN and PCCN-20.
The charge-transfer efficiency of the catalysts was obtained with electrochemical impedance spectroscopy (EIS), as illustrated in Figure 3d. Based on the equivalent circuit model, PCN and PCCN-20 had the highest and smallest EIS Nyquist arc radii, respectively, indicating the worst and best charge separation and transfer capacities [44].
2.3. Photocatalytic Capacity for H2 Production and RhB Degradation
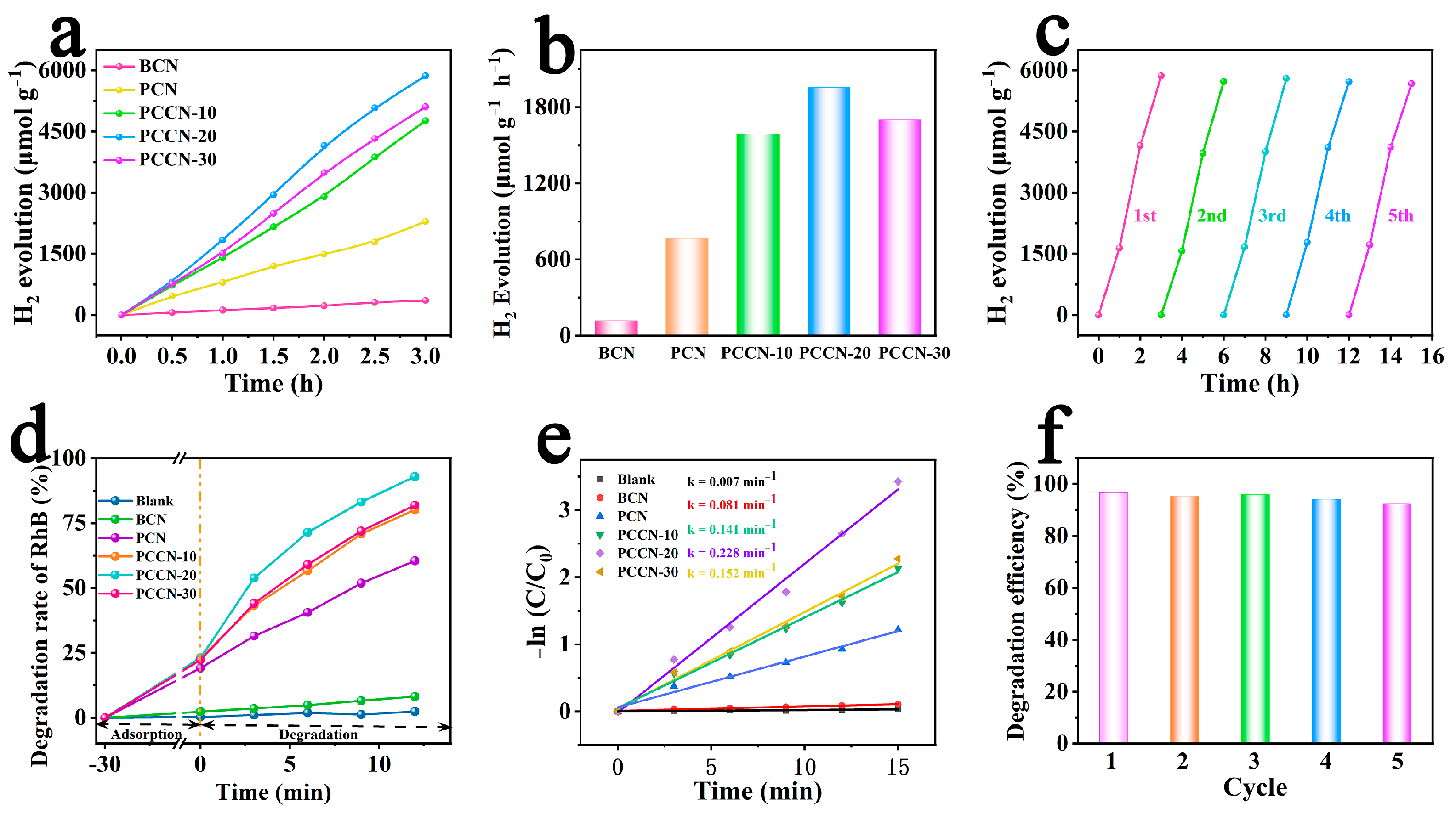
To assess the photocatalytic activity of the as-fabricated catalysts, catalytic water splitting experiments were performed with irradiation in the VIS radiation region (λ > 420 nm). Figure 4a shows that the BCN with a blocky shape displayed poor reactivity and released only 119.21 μmol g−1 h−1 H2 within 1 h. Due to the 3D interconnected structure, the H2 evolution yield for PCN reached 765.06 μmol g−1 h−1, which was 6.42 times that of BCN, suggesting that the 3D interconnected structure significantly enhanced the photocatalytic capacity. For the PCCN catalysts, the H2 evolution rates were substantially higher than that of PCN, strongly confirming that the carbon-modified 3D porous g-C3N4 enhanced the photocatalytic capacity. In particular, PCCN-20 exhibited a significantly enhanced H2 production rate of 1956.23 μmol g−1 h−1, which was approximately 16.41 times and 2.56 times those of the BCN and PCN samples, respectively. Nevertheless, the decreased H2 production efficiency with the excessive carbon doping (from 1956.23 μmol g−1 h−1 to 1701.07 μmol g−1 h−1) may be attributed to the generation of more recombination centers for the photogenerated charge carriers.
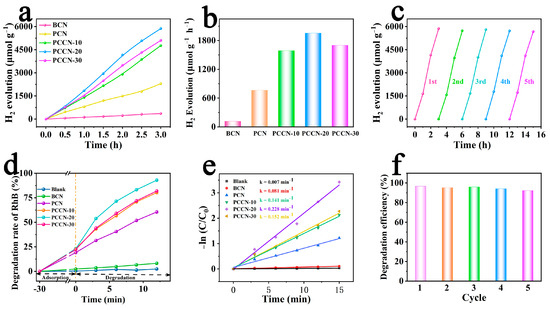
Figure 4.
(a) H2 production and (b) the corresponding production rate of the as-synthesized catalysts; (c) stability of the PCCN-20 catalysts during H2 production; (d) degradation rate and (e) corresponding k values for RhB degradation in different conditions; and (f) stability of RhB degradation by PCCN-20 catalyst.
In addition, long-term photocatalytic stability is necessary for practical applications [45]. Therefore, we used a cycling experiment to probe the persistence and reusability of the as-synthesized catalysts. The as-synthesized rich-carbon PCCN-20 catalysts still showed H2 evolution yields of 1890.34 μmol g−1 h−1 after 15 h of consecutive continuous photocatalytic H2 evolution (Figure 4c). However, the slight decrease in the H2 evolution rate may have resulted from a slight loss of the PCCN-20 catalyst during the washing process. The conclusion was reached from the cycling results showing that the as-synthesized catalysts showed outstanding stability and reusability.
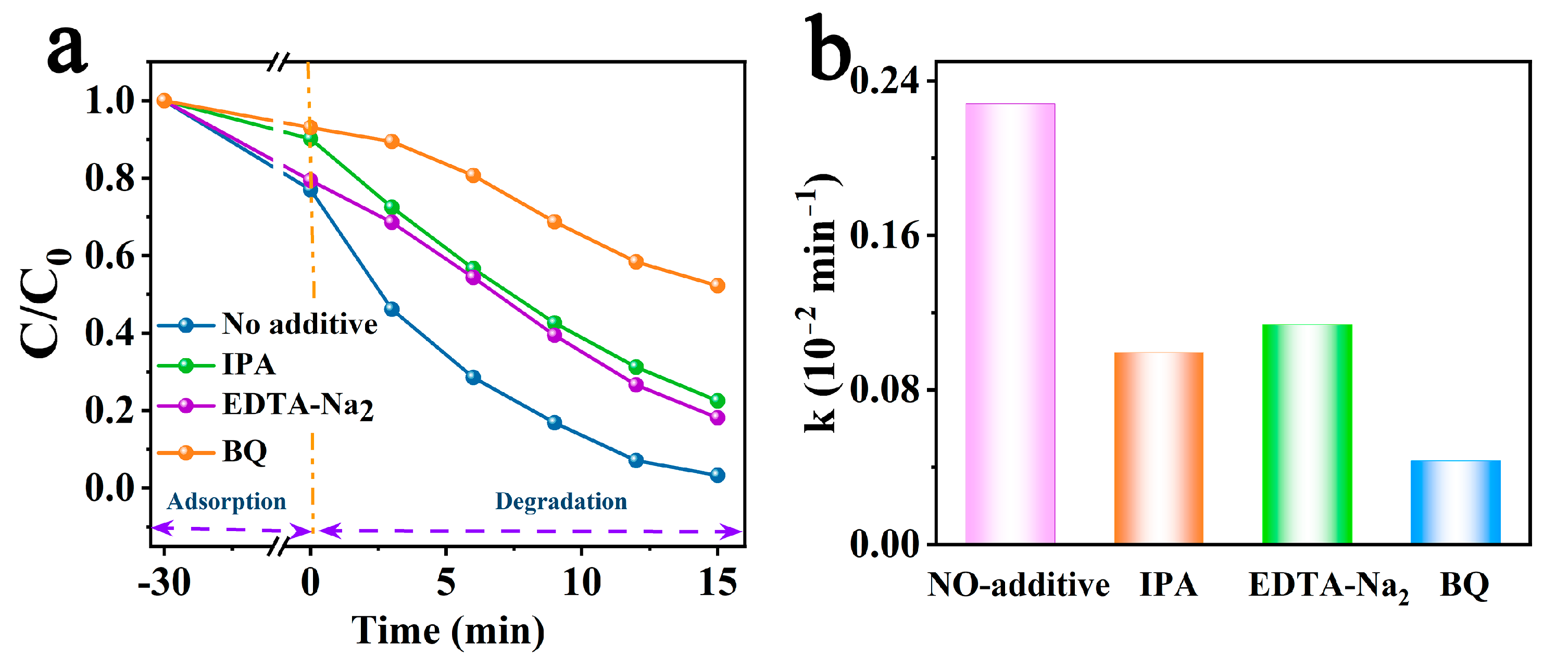
Rhodamine B (RhB), a synthetic dye, is widely used in the textile printing and food industries and has carcinogenicity. It is one of the common pollutants in industrial wastewater because of its solubility and good stability [46]. However, its toxicity, carcinogenicity, and mutagenicity make it an urgent issue to remove it from wastewater. Therefore, using RhB as a probe pollutant, we performed pollutant degradation experiments to appraise the photocatalytic activity of the as-synthesized catalysts. Figure 4d displays the concentration of RhB as a function of irradiation time in the presence of different catalysts. The first-order kinetic equation (Equation (2)) was used to simulate the degradation process [47], in which C0 is the initial RhB concentration, and C is that at time t (min).
First, we can notice that the degradation of RhB without a catalyst can be negligible (only 3.2% within 15 min). BCN showed the slowest degradation rate (10.32% within 15 min) with a k value equal to 0.007 min−1, and PCN showed a higher degradation efficiency of 70.43% within 15 min. As with the H2 evolution results, the PCCN catalysts showed significantly higher RhB degradation rates. The RhB degradation rates for the PCCN-20 catalysts reached 96.74% within 15 min of VIS radiation irradiation, and the k value (0.228 min−1) was approximately 32.57 times and 2.81 times those of BCN (0.007 min−1) and PCN (0.081 min−1), respectively. However, additionally, the cycling experiments verified the good stability and reusability of the PCCN-20 catalyst in RhB photodegradation. These results for H2 evolution and RhB degradation proved that the morphology and carbon-rich g-C3N4 structure showed synergism in enhancing photocatalytic performance.
It is widely accepted that photogenerated active species such as hydroxyl radicals (•OH) and superoxide radicals (O2•−) are involved in contaminant degradation. They can be generated by the following reactions (Equations (3)–(8)):
PCCN catalyst + hv → h+ + e−
2e− + O2 + 2H+ → H2O2
H2O2 + e− →•OH + OH−
e− + O2 →O2•−
H2O2 + O2•− → •OH + OH− + O2
h+ + OH− →•OH
Therefore, radical quenching experiments were used to further explore the contributions of generated active substances, where h+, O2•−, and •OH were captured by the trapping agents EDTA-Na2, BQ, and IPA [48], respectively. As shown in Figure 5a,b, the various scavengers showed different degrees of inhibition for the photocatalytic degradation of RhB by PCCN-20. The degradation rates were slightly suppressed by the presence of EDTA-Na2 and IPA, from 96.74 to 81.88 and 77.51% (the k values were reduced from 0.228 to 0.114 and 0.100 min−1), respectively, indicating that h+ and •OH participated in RhB degradation. The efficiency for the removal of RhB showed a dramatic decline after adding BQ, reaching 52.20%, which suggested that O2•− played an irreplaceable role in the process of RhB degradation by PCCN-20.
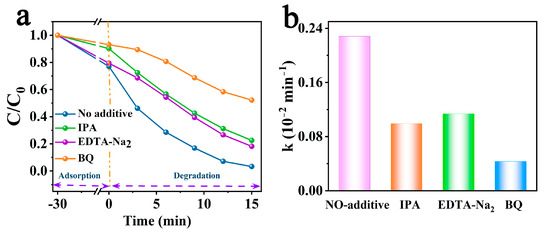
Figure 5.
(a) RhB degradation rates in the radical quenching experiments and (b) the corresponding k values.
2.4. Mechanism of Enhanced Photocatalytic Activity
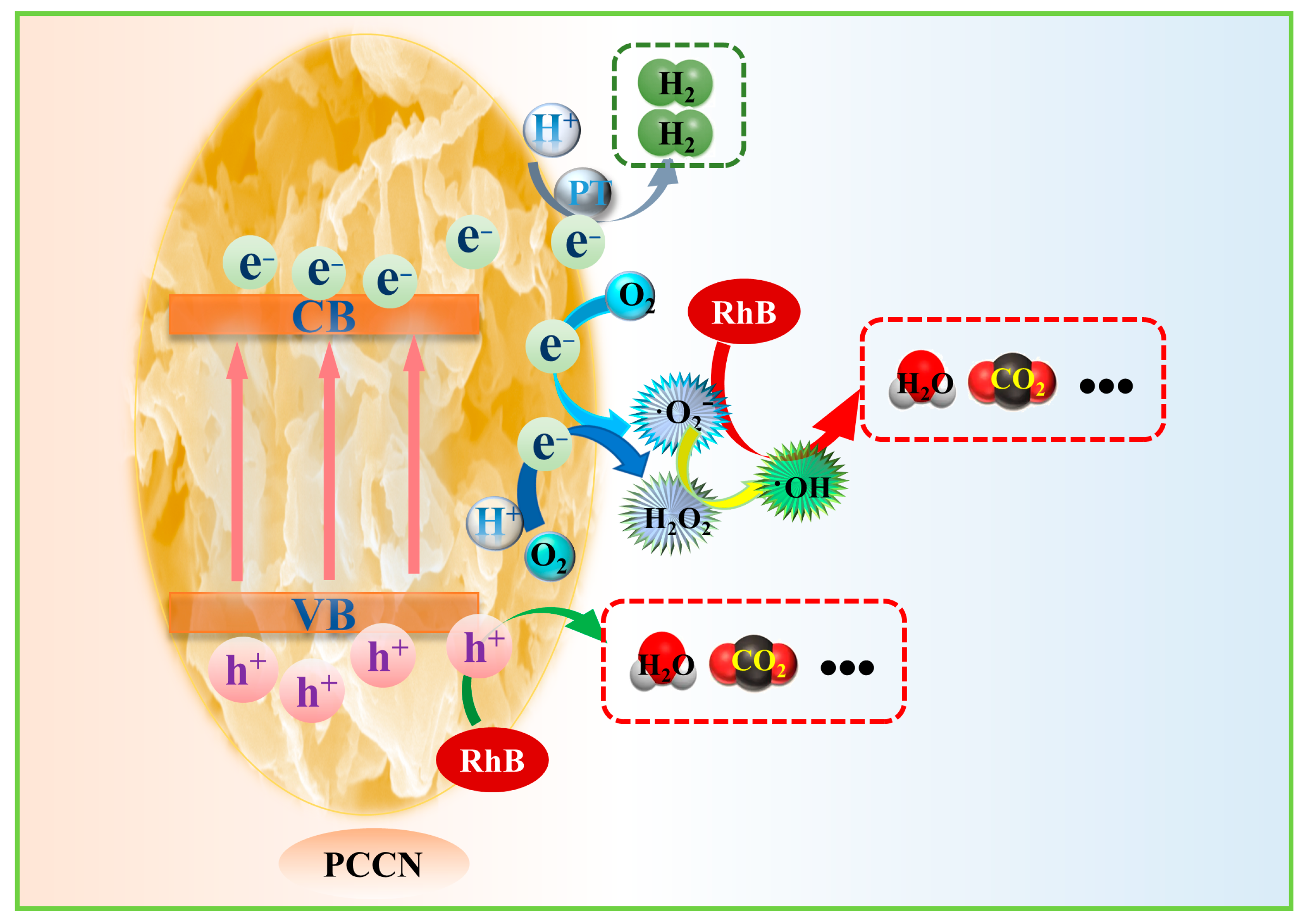
Based on the photocatalytic experimental data and characterization analyses, a photocatalytic mechanism was developed in this study (Figure 6). Under VIS radiation, electrons in the VB of the PCCN catalysts were promoted to the CB, leaving holes in the VB and resulting in the separation of the photogenerated charge carriers. The photogenerated holes were transferred to the surface of the catalysts and participated in RhB degradation, while the photoinduced electrons were transported to the surface and participated in hydrogen evolution. During the whole process, the 3D interconnected porous structure of g-C3N4 provided copious active sites and a large SSA, and the carbon-rich g-C3N4 structure facilitated the separation and migration of the photogenerated charge carriers, thus resulting in enhanced RhB degradation efficiency and hydrogen evolution rate. In summary, nanoshape engineering and carbon doping had a synergetic effect in promoting photocatalytic performance.
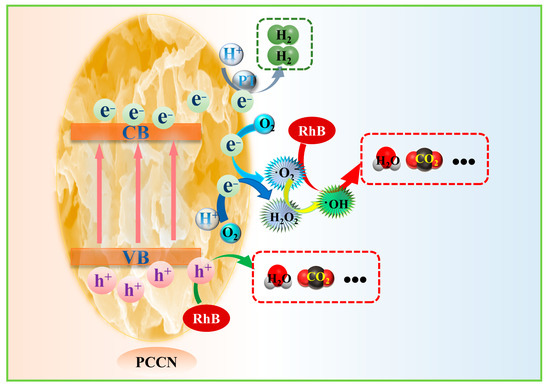
Figure 6.
Potential mechanism of hydrogen generation with water decomposition and RhB elimination by PCCN-20.
3. Materials and Methods
3.1. Materials and Reagents
The analytical reagent (AR) melamine, cyanuric acid, cytosine, and rhodamine B (RhB) were purchased from Shanghai Maclin Biochemical Co., Ltd. (Shanghai, China). In addition, other chemical reagents, including ethylene diamine tetraacetic acid disodium salt (EDTA-Na2), isopropanol (IPA), and benzoquinone (BQ), were purchased from Sigma-Aldrich (St. Louis, MO, USA). All reagents were used directly without further purification.
3.2. Fabrication of the Catalysts
Figure 7 displays the synthetic procedure used to prepare the catalysts. All of the g-C3N4 catalysts were synthesized through simple calcination.
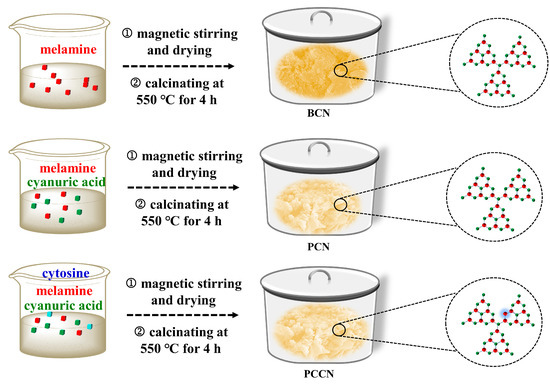
Figure 7.
Synthetic procedure for the PCCN, PCN, and BCN samples.
3.2.1. Synthesis of BCN
Bulk g-C3N4 (denoted as BCN) catalysts were synthesized by annealing melamine at 550 °C for 4 h (with a ramping rate of 2 °C min−1) directly.
3.2.2. Preparation of PCN
The 3D interlinked porous g-C3N4 catalysts were prepared through simple calcination. Typically, 1.26 g of melamine and 1.29 g of cyanuric acid were dissolved in distilled water (100 mL) and stirred magnetically for 10 h. The product was dried at 60 °C, and it was calcined at 550 °C for 4 h (with a ramping rate of 2 °C min−1). The resulting faint yellow powder was the 3D interconnected porous g-C3N4 catalyst.
3.2.3. Preparation of PCCN
The 3D interlinked porous g-C3N4 catalysts were prepared by the same synthesis method as PCN; the only difference was that the precursors of PCCN were 1.26 g of melamine, 1.29 g of cyanuric acid, and a certain amount of cytosine. The as-synthesized 3D interconnected porous carbon-rich g-C3N4 samples with 10, 20, and 30 mg of cytosine were labeled PCCN-10, PCCN-20, and PCCN-30, respectively.
3.3. Characterization Measurements
X-ray diffraction (XRD) patterns of the catalysts were collected on a Holland Panalytical PRO PW3040/60. The surface chemical status of the samples was characterized by X-ray photoelectron spectroscopy (XPS, Kratos-AXIS ULTRA DLD, and Al Kα X-ray source). The morphologies and structures of the prepared samples were characterized by field-emission scanning electron microscopy (FESEM, Hitachi S-4800, Tokyo, Japan) and transmission electron microscopy (TEM, JEM-2010, JEOL Co., Ltd, Beijing, China). UV-Vis diffuse reflectance spectra (DRS) of the as-prepared samples were obtained by a Hitachi U-3900 spectrophotometer (Tokyo, Japan). The room temperature photoluminescence (PL) spectra were examined by fluorescence spectrophotometer (HORIBA Jobin Yvon fluoromax-4, Kyoto, Japan) with an excitation wavelength of 385 nm. The time-resolved PL (RTPL) spectra were recorded by a Multifunction Steady State and Transient State Fluorescence Spectrometer (FES920, Edinburgh Instruments, Edinburgh, UK) at 300 nm. The Brunauer–Emmett–Teller (BET) specific surface areas of the samples were evaluated on the basis of nitrogen adsorption isotherms measured at 77 K using a BELSORP-max nitrogen adsorption apparatus (Micrometitics, Norcross, GA, USA). The photocurrent was measured on an electrochemical workstation (CHI760E) with a standard three-electrode configuration. Catalyst (10 mg)-loaded indium tin oxide (ITO) electrodes (6 × 6 mm) served as the working electrode, a Pt foil as the counter electrode, and an Ag/AgCl electrode as the reference electrode under Xe light irradiation in 0.1 M Na2SO4 solution. EIS was performed over a frequency range from 0.1 Hz to 1 MHz at open-circuit potential in 0.1 M Na2SO4 solution under Xe light irradiation.
3.4. Photocatalytic Measurements
Hydrogen generation with water decomposition and RhB elimination tests were executed under VIS radiation irradiation to estimate the capability of the as-prepared samples.
Photocatalytic H2 evolution was conducted using a photocatalytic analysis system (Labsolar 6A, Perfectlight, Technology Co., Ltd, Beijing, China). VIS radiation was obtained from a 300 W Xe lamp with a 420 nm cutoff filter. Amounts of 50 mg of photocatalysts were added into 100 mL solution (90 mL deionized water and 10 mL triethanolamine as sacrificial agent). Amounts of 1wt.% Pt as co-catalysts were loaded on the photocatalysts by in situ photo-deposition method using H2PtCl6. Before light irradiation, the suspensions were ultrasonically dispersed in the dark for 30 min to achieve absorption–desorption equilibrium. At given time intervals (30 min), a certain amount of produced gas was measured by an online gas chromatograph (GC-2002 N/TFF) equipped with a thermal conductive detector (TCD) and a 5 Å molecular sieve column, using argon as the carrier gas. Product gases were calibrated with standard H2 gas, and their identities were determined according to the retention time.
The catalytic performance of catalysts was evaluated by degradation of RhB. All reactions were carried out in a photochemical reactor (Yanzheng Co., Ltd., Shanghai, China) with condensed water under stirring. A 500 W metal halide lamp with a 420 nm cutoff filter (average light intensity: 30 mW cm−2) was used as the light source. Briefly, 40 mg of catalyst was ultrasonically dispersed into organic contaminant solution (80 mL, 20 mg L−1), then stirred in darkness for 30 min to achieve an adsorption–desorption balance between catalyst and RhB. During catalytic reaction, 3 mL of suspension was taken out at given time intervals and immediately centrifuged to remove the catalyst. The filtrate of RhB was determined with a Hitachi U-3900 UV-vis spectrophotometer, and the absorbance value of RhB was used to calculate the concentration at the maximum absorption wavelength.
4. Conclusions
In this study, combining morphological control and carbon doping, we successfully synthesized a novel 3D interconnected porous carbon-rich g-C3N4 catalyst via facile thermal polymerization. The obtained catalysts of PCCN-20 showed the best photocatalytic performance with a 96.74% RhB degradation efficiency and a 1956.23 μmol g−1 h−1 hydrogen evolution rate, which were approximately 2.81 and 2.56 times that of PCN. However, the photocatalytic capacity of PPCN catalysts declined with the decrease in cytosine addition, indicating that the addition of excessive C element would lead to a photogenerated charge recombination center, which would affect the photocatalytic performance. Moreover, the mechanistic analysis showed that morphological control and carbon doping had a synergetic impact in enhancing photocatalytic performance. In a word, this research offered a possible and imputable reference for the development of economical catalysts for alleviating environmental pollution and energy shortages.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal13101345/s1. Figure S1: The schematic copolymerization processes of PCCN from melamine, cyanuric acid and cytosine; Figure S2: The SEM images of (a) PCCN-10 and (b) PCCN-30.
Author Contributions
Conceptualization, D.L.; methodology, C.Z., D.L. and X.H.; software, C.L. and C.Z.; investigation, C.T., C.L. and X.H.; writing—original draft preparation, C.T., C.L., C.Z., D.L. and X.H.; writing—review and editing, C.T.; supervision, D.L. and X.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Starting Research Fund of Xinxiang Medical University [grant numbers XYBSKYZZ201911] and the Excellent Young Teachers Training Program of Sanquan College of Xinxiang Medical University [grant numbers SQ2021YQJH14 and SQ2022YQJH12].
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shao, Y.; Hao, X.; Lu, S.; Jin, Z. Molten salt-assisted synthesis of nitrogen-vacancy crystalline graphitic carbon nitride with tunable band structures for efficient photocatalytic overall water splitting. Chem. Eng. J. 2023, 454, 140123. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, Z.; Xiao, K.; Sun, H.; Chan, H.S.; Tsang, T.H.; Yang, S.; Wong, P.K. Photo-assisted separation of noble-metal-free oxidation and reduction cocatalysts for graphitic carbon nitride nanosheets with efficient photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2021, 280, 119456. [Google Scholar] [CrossRef]
- Thangamuthu, M.; Vankayala, K.; Xiong, L.; Conroy, S.; Zhang, X.; Tang, J. Tungsten oxide-based Z-scheme for visible light-driven hydrogen production from water splitting. ACS Catal. 2023, 13, 9113–9124. [Google Scholar] [CrossRef]
- Tang, R.; Gong, D.; Zhou, Y.; Deng, Y.; Feng, C.; Xiong, S.; Huang, Y.; Peng, G.; Li, L. Unique g-C3N4/PDI-g-C3N4 homojunction with synergistic piezo-photocatalytic effect for aquatic contaminant control and H2O2 generation under visible light. Appl. Catal. B Environ. 2022, 303, 120929. [Google Scholar] [CrossRef]
- Carey, J.H.; Lawrence, J.; Tosine, H.M. Photodechlorination of PCB’s in the presence of titanium dioxide in aqueous sus-pensions. Bull. Environ. Contam. Toxicol. 1976, 16, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.J.; Foo, J.J.; Siang, T.J.; Ong, W. Shining light on carbon aerogel photocatalysts: Unlocking the potentials in the quest for revolutionizing solar-to-chemical conversion and environmental remediation. Adv. Funct. Mater. 2023, e2306014. [Google Scholar] [CrossRef]
- Ai, M.; Pan, L.; Shi, C.; Huang, Z.-F.; Zhang, X.; Mi, W.; Zou, J.-J. Spin selection in atomic-level chiral metal oxide for photocatalysis. Nat. Commun. 2023, 14, 4562. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Q.; Li, J.; Gao, G.; Zhi, J. Onion-liked carbon-embedded graphitic carbon nitride for enhanced photocatalytic hydrogen evolution and dye degradation. Appl. Catal. B Environ. 2022, 308, 121216. [Google Scholar] [CrossRef]
- Wang, L.; Lian, R.; Zhang, Y.; Ma, X.; Huang, J.; She, H.; Liu, C.; Wang, Q. Rational preparation of cocoon-like g-C3N4/COF hybrids: Accelerated intramolecular charge delivery for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2022, 315, 121568. [Google Scholar] [CrossRef]
- Yang, M.; Wang, P.; Li, Y.; Tang, S.; Lin, X.; Zhang, H.; Zhu, Z.; Chen, F. Graphene aerogel-based NiAl-LDH/g-C3N4 with ultratight sheet-sheet heterojunction for excellent visible-light photocatalytic activity of CO2 reduction. Appl. Catal. B Environ. 2022, 306, 121065. [Google Scholar] [CrossRef]
- Liu, M.; Ning, Y.; Ren, M.; Fu, X.; Cui, X.; Hou, D.; Wang, Z.; Cui, J.; Lin, A. Internal electric field-modulated charge migration behavior in MoS(2)/MIL-53(Fe) S-scheme heterojunction for boosting visible-light-driven photocatalytic chlorinated antibiotics degradation. Small 2023, e2303876. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Huang, H. Coupled adsorption and photocatalysis of g-C3N4 based composites: Material synthesis, mechanism, and environmental applications. Chem. Eng. J. 2023, 453, 139755. [Google Scholar] [CrossRef]
- Li, H.; Li, R.; Liu, G.; Zhai, M.; Yu, J. Noble-metal-free single- and dual-atom catalysts for artificial photosynthesis. Adv. Mater. 2023, e2301307. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Kong, F.; Xing, M. Spatial separation of photo-generated carriers in g-C3N4/MnO2/Pt with enhanced H2 evolution and organic pollutant control. Res. Chem. Intermed. 2022, 48, 2837–2855. [Google Scholar] [CrossRef]
- Sun, J.; Yang, S.; Liang, Z.; Liu, X.; Qiu, P.; Cui, H.; Tian, J. Two-dimensional/one-dimensional molybdenum sulfide (MoS2) nanoflake/graphitic carbon nitride (g-C3N4) hollow nanotube photocatalyst for enhanced photocatalytic hydrogen production activity. J. Colloid Interf. Sci. 2020, 567, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Marchi, M.; Raciti, E.; Gali, S.M.; Piccirilli, F.; Vondracek, H.; Actis, A.; Salvadori, E.; Rosso, C.; Criado, A.; D’Agostino, C.; et al. Carbon vacancies steer the activity in dual Ni carbon nitride photocatalysis. Adv. Sci. 2023, 10, e2303781. [Google Scholar] [CrossRef]
- Xie, K.; Fang, J.; Li, L.; Deng, J.; Liang, Z. Enhancement of the Photodegradation Activity of methylene blue by the low-temperature regulation of oxide-rich graphitic carbon nitride. ChemistrySelect 2021, 6, 11407–11416. [Google Scholar] [CrossRef]
- Lei, Z.; Cao, X.; Fan, J.; Hu, X.; Hu, J.; Li, N.; Sun, T.; Liu, E. Efficient photocatalytic H2 generation over In2.77S4/NiS2/g-C3N4 S-scheme heterojunction using NiS2 as electron-bridge. Chem. Eng. J. 2023, 457, 141249. [Google Scholar] [CrossRef]
- Yuan, J.; Tian, N.; Zhu, Z.; Yu, W.; Li, M.; Zhang, Y.; Huang, H. P, K doped crystalline g-C3N4 grafted with cyano groups for efficient visible-light-driven H2O2 evolution. Chem. Eng. J. 2023, 467, 143379. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Ni, T.; Gao, R.; Ge, J.; Zhang, F.; Wu, W.; Li, J.; Zhao, Q. 3D interconnected porous g-C3N4 hybridized with Fe2O3 quantum dots for enhanced photo-Fenton performance. Appl. Surf. Sci. 2021, 555, 149677. [Google Scholar] [CrossRef]
- Sun, B.; Lu, N.; Su, Y.; Yu, H.; Meng, X.; Gao, Z. Decoration of TiO2 nanotube arrays by graphitic-C3N4 quantum dots with improved photoelectrocatalytic performance. Appl. Surf. Sci. 2017, 394, 479–487. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Sun, X.; Ji, H.; Liu, W.; Cai, Z. Construction of Z-scheme Ag/AgVO3/carbon-rich g-C3N4 heterojunction for enhanced photocatalytic degradation of sulfamethiadiazole: DFT calculation and mechanism study. Chem. Eng. J. 2022, 433, 133604. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Z.; Wang, H.; Liao, G.; Bai, S.; Zou, J.; Wu, P.; Zhang, P.; Li, X. Sulfur-doped g-C3N4/g-C3N4 isotype step-scheme heterojunction for photocatalytic H2 evolution. J. Mater. Sci. Technol. 2022, 118, 15–24. [Google Scholar] [CrossRef]
- Huang, J.; Li, D.; Li, R.; Zhang, Q.; Chen, T.; Liu, H.; Liu, Y.; Lv, W.; Liu, G. An efficient metal-free phosphorus and oxygen co-doped g-C3N4 photocatalyst with enhanced visible light photocatalytic activity for the degradation of fluoroquinolone antibiotics. Chem. Eng. J. 2019, 374, 242–253. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Y.; Lyu, L.; Zeng, Q.; Xing, X.; Hu, C. Electronic structure modulation of graphitic carbon nitride by oxygen doping for enhanced catalytic degradation of organic pollutants through peroxymonosulfate activation. Environ. Sci. Technol. 2018, 52, 14371–14380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P.; Ren, T.Z.; Yuan, Z.Y. Mesoporous Phosphorus-doped g-C3N4 nanostructured flowers with superior photocatalytic hydrogen evolution performance. ACS Appl. Mater. Inter. 2015, 7, 16850–16856. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Gong, K.; Xing, C.; Hu, L.; Huang, H.; Gao, L.; Wang, D.; Li, X. Dual P-doped-site modified porous g-C3N4 achieves high dissociation and mobility efficiency for photocatalytic H2O2 production. Chem. Eng. J. 2023, 461, 142140. [Google Scholar] [CrossRef]
- Cui, Q.; Xu, J.; Wang, X.; Li, L.; Antonietti, M.; Shalom, M. Phenyl-modified carbon nitride quantum dots with distinct photoluminescence behavior. Angew. Chem. Int. Ed. Engl. 2016, 55, 3672–3676. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, F.; Wang, X.; Xu, C.; Zhang, Z.; Shi, G.; Qu, L. Graphitic carbon nitride nanoribbons: Graphene-assisted formation and synergic function for highly efficient hydrogen evolution. Angew. Chem. Int. Ed. Engl. 2014, 53, 13934–13939. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Wei, Y.; Gao, C.; Bao, J.; Zhang, N. In situ grown Co3O4 nanosheets in the interlayer space of g-C3N4 for efficient removal of Hg0 from flue gas. Fuel 2022, 324, 124660. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Zhao, C.; Zhao, Q.; Niu, T.; Pan, L.; Xu, P.; Zhang, F.; Wu, W.; Ni, T. Facile synthesis of three-dimensional hollow porous carbon doped polymeric carbon nitride with highly efficient photocatalytic performance. Chem. Eng. J. 2022, 438, 135623. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, Y.; Zhang, H.S.; Liu, Y.; Zhao, Y.J.; Qiu, J.; Dong, G. 3D foam strutted graphene carbon nitride with highly stable optoelectronic properties. Adv. Funct. Mater. 2017, 27, 1703711. [Google Scholar] [CrossRef]
- Tian, N.; Zhang, Y.; Li, X.; Xiao, K.; Du, X.; Dong, F.; Waterhouse, G.I.N.; Zhang, T.; Huang, H. Precursor-reforming protocol to 3D mesoporous g-C3N4 established by ultrathin self-doped nanosheets for superior hydrogen evolution. Nano Energy 2017, 38, 72–81. [Google Scholar] [CrossRef]
- Chen, X.; Shi, R.; Chen, Q.; Zhang, Z.; Jiang, W.; Zhu, Y.; Zhang, T. Three-dimensional porous g-C3N4 for highly efficient photocatalytic overall water splitting. Nano Energy 2019, 59, 644–650. [Google Scholar] [CrossRef]
- Hu, S.; Ma, L.; You, J.; Li, F.; Fan, Z.; Lu, G.; Liu, D.; Gui, J. Enhanced visible light photocatalytic performance of g-C3N4 photocatalysts co-doped with iron and phosphorus. Appl. Surf. Sci. 2014, 311, 164–171. [Google Scholar] [CrossRef]
- Bhuvaneswari, K.; Palanisamy, G.; Pazhanivel, T.; Maiyalagan, T.; Bharathi, G. Photodegradation activity of nitrogen-rich graphitic carbon nitride intercalated ZnO\Mg-Al layered double hydroxide ternary nanocomposites on methylene blue dye. ChemistrySelect 2019, 4, 2982–2990. [Google Scholar] [CrossRef]
- Sun, L.; Wang, W.; Zhang, C.; Cheng, M.; Zhou, Y.; Yang, Y.; Luo, H.; Qin, D.; Huang, C.; Ouyang, Z. Multiple optimization strategies for improving photocatalytic performance of the h-BN/flower-ring g-C3N4 heterostructures: Morphology engineering and internal electric field effect. Chem. Eng. J. 2022, 446, 137027. [Google Scholar] [CrossRef]
- Qin, J.; Jiao, Y.; Liu, M.; Li, Y.; Wang, J. Heat treatment to prepare boron doped g-C3N4 nanodots/carbon-rich g-C3N4 nanosheets heterojunction with enhanced photocatalytic performance for water splitting hydrogen evolution. J. Alloy. Compd. 2022, 898, 162846. [Google Scholar] [CrossRef]
- Sheng, Y.; Wei, Z.; Miao, H.; Yao, W.; Li, H.; Zhu, Y. Enhanced organic pollutant photodegradation via adsorption/photocatalysis synergy using a 3D g-C3N4/TiO2 free-separation photocatalyst. Chem. Eng. J. 2019, 370, 287–294. [Google Scholar] [CrossRef]
- Luo, W.; Chen, X.; Wei, Z.; Liu, D.; Yao, W.; Zhu, Y. Three-dimensional network structure assembled by g-C3N4 nanorods for improving visible-light photocatalytic performance. Appl. Catal. B Environ. 2019, 255, 117761. [Google Scholar] [CrossRef]
- Cui, M.; Cui, K.; Liu, X.; Chen, X.; Guo, Z.; Chen, Y.; Li, C.X. Insights into the photocatalytic peroxymonosulfate activation over defective boron-doped carbon nitride for efficient pollutants degradation. J. Hazard. Mater. 2021, 418, 126338. [Google Scholar] [CrossRef]
- Zhan, H.; Zhou, Q.; Li, M.; Zhou, R.; Mao, Y.; Wang, P. Photocatalytic O2 activation and reactive oxygen species evolution by surface B-N bond for organic pollutants degradation. Appl. Catal. B Environ. 2022, 310, 121329. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Bi, Y.; Jin, J.; Ehsan, M.F.; Fu, M.; He, T. Preparation of 2D hydroxyl-rich carbon nitride nanosheets for photocatalytic reduction of CO2. RSC Adv. 2015, 5, 33254–33261. [Google Scholar] [CrossRef]
- Wang, K.; Shao, X.; Zhang, K.; Wang, J.; Wu, X.; Wang, H. 0D/3D Bi3TaO7/ZnIn2S4 heterojunction photocatalyst towards degradation of antibiotics coupled with simultaneous H2 evolution: In situ irradiated XPS investigation and S-scheme mechanism insight. Appl. Surf. Sci. 2022, 596, 153444. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Liu, F.; Jiang, W.; Zhang, D.; Liang, J. Visible-light-driven photocatalytic degradation of diclofenac by carbon quantum dots modified porous g-C3N4: Mechanisms, degradation pathway and DFT calculation. Water Res. 2019, 151, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Sun, H.; Li, C.; Wang, M.; Wu, J.; Chen, M.; Jiang, S.; Niu, T.; Liu, D. Facile Synthesis of 3D Interconnected Porous g-C3N4/rGO Composite for Hydrogen Production and Dye Elimination. Catalysts 2023, 13, 1079. [Google Scholar] [CrossRef]
- Huang, D.; Sun, X.; Liu, Y.; Ji, H.; Liu, W.; Wang, C.-C.; Ma, W.; Cai, Z. A carbon-rich g-C3N4 with promoted charge separation for highly efficient photocatalytic degradation of amoxicillin. Chin. Chem. Lett. 2021, 32, 2787–2791. [Google Scholar] [CrossRef]
- Zhao, C.; Li, C.; Chen, M.; Niu, T.; Zhao, Q.; Ni, T.; Yan, D.; Wu, W.; Liu, D. Effective removal of antineoplastic doxorubicin by 0D Nb2O5 quantum dots embed 3D porous C-doped g-C3N4: Degradation mechanism, pathway and toxicity assessment. Appl. Surf. Sci. 2023, 612, 155861. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).