Production of Prebiotic Galacto-Oligosaccharides from Acid Whey Catalyzed by a Novel β-Galactosidase from Thermothielavioides terrestris and Commercial Lactases: A Comparative Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Acid Whey Characterization
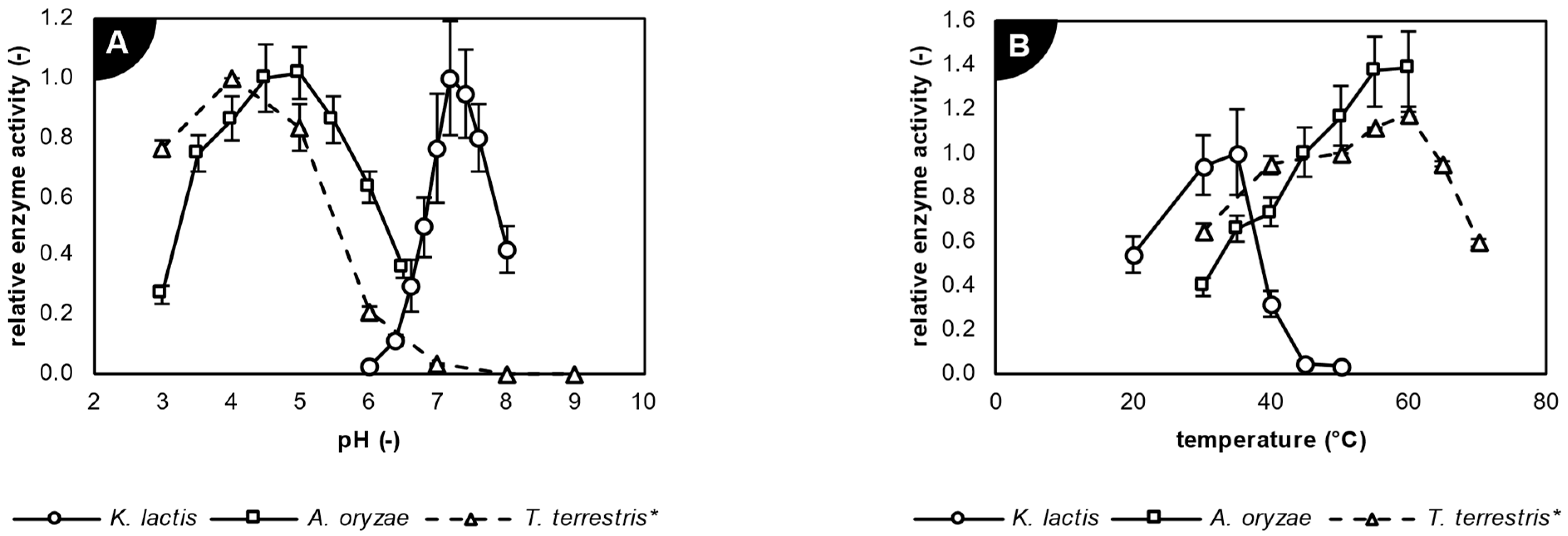
2.2. Characterization of Studied Biocatalysts
2.3. Enzymatic Transgalactosylation of Acid Whey Lactose
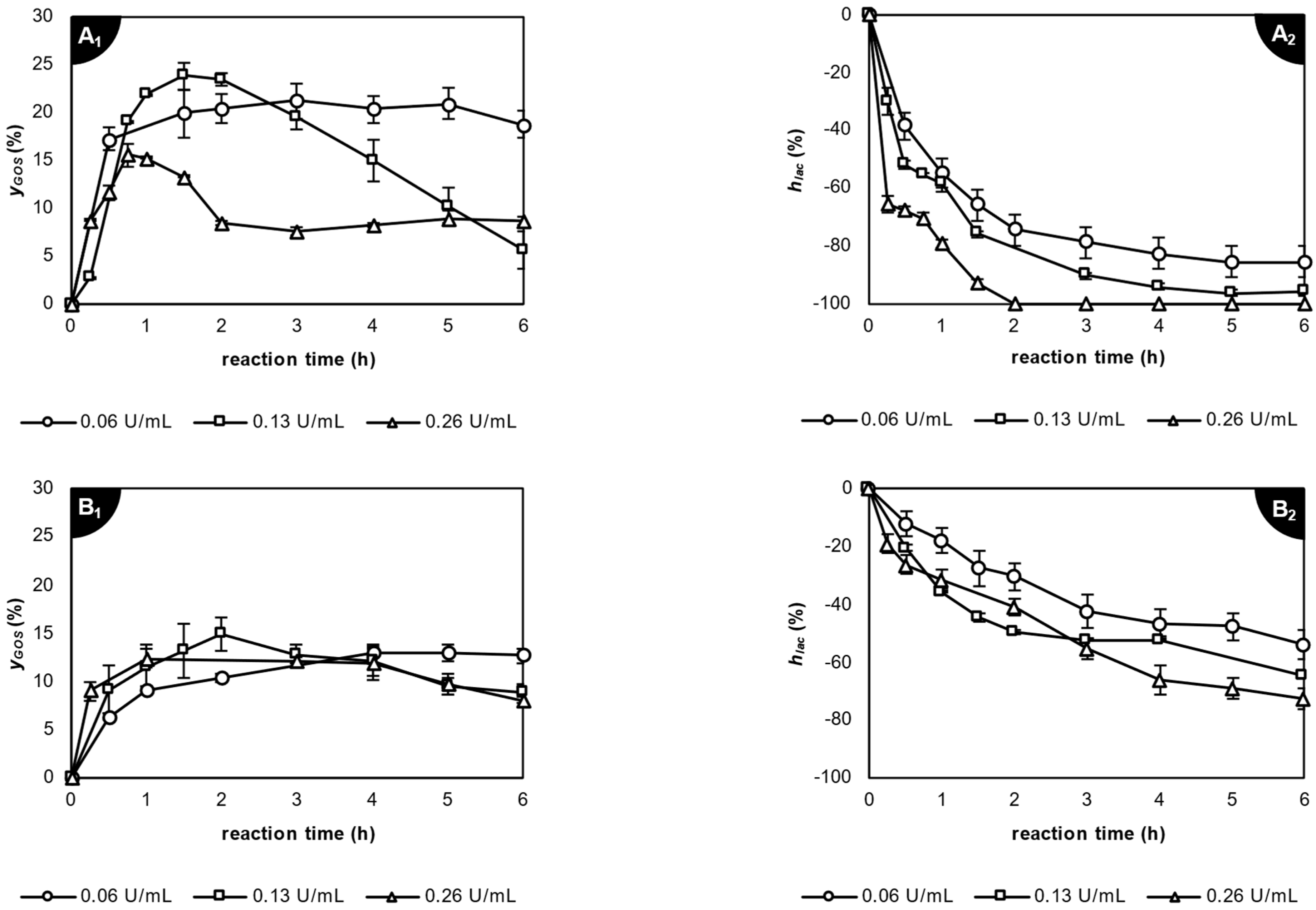
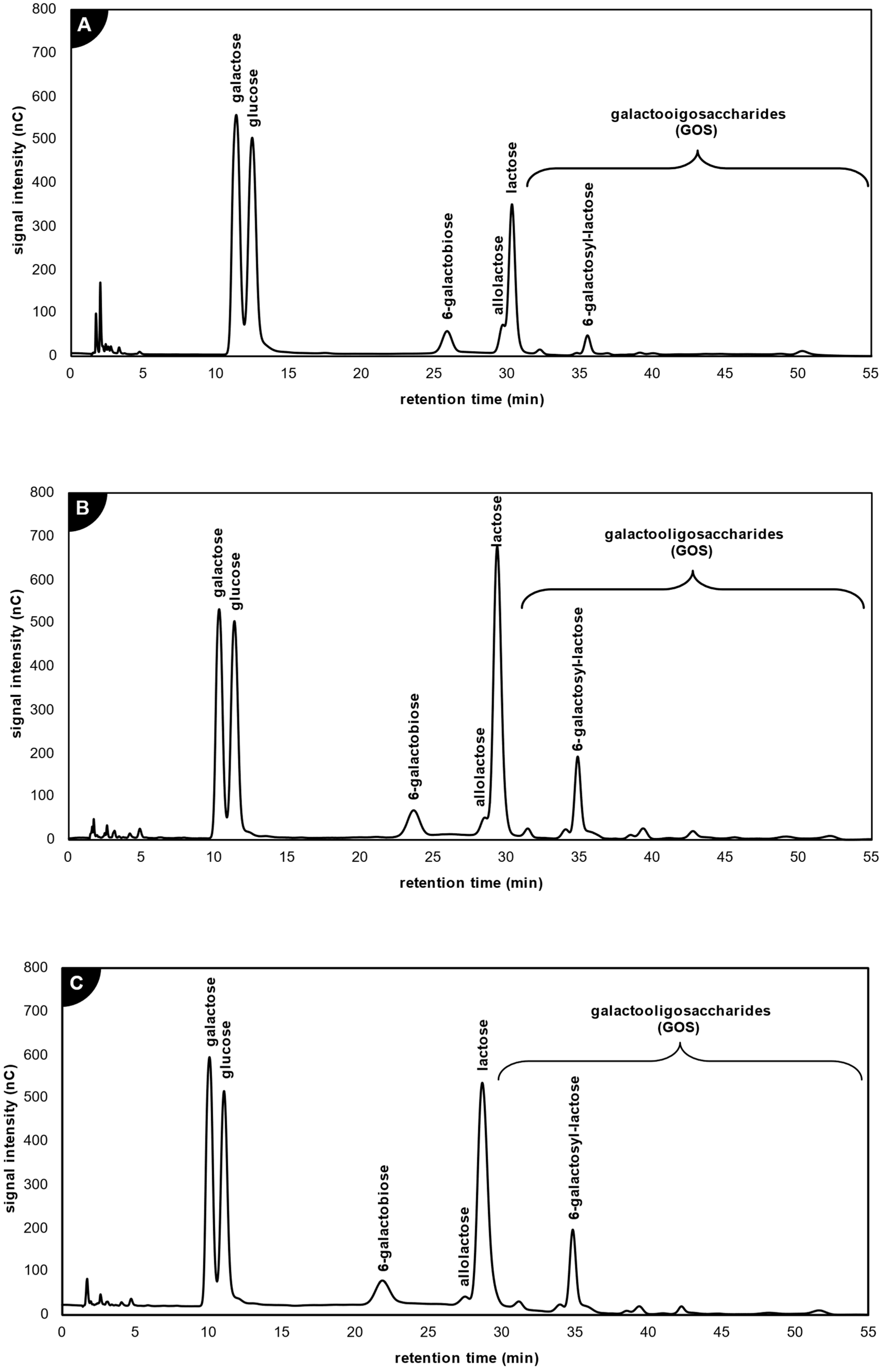
2.3.1. Application of β-Galactosidase from Kluyveromyces lactis
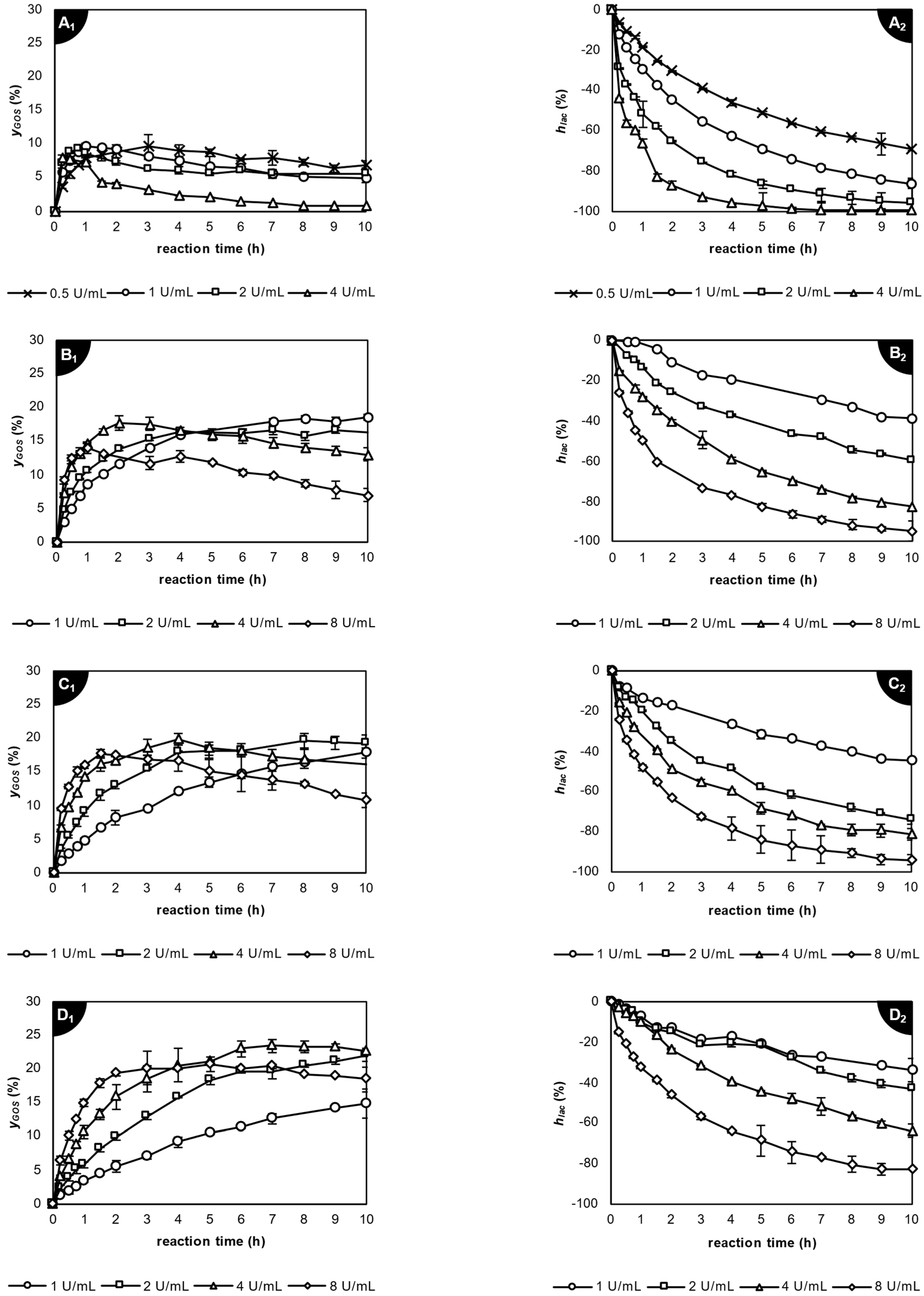
2.3.2. Application of β-Galactosidase from Aspergillus oryzae
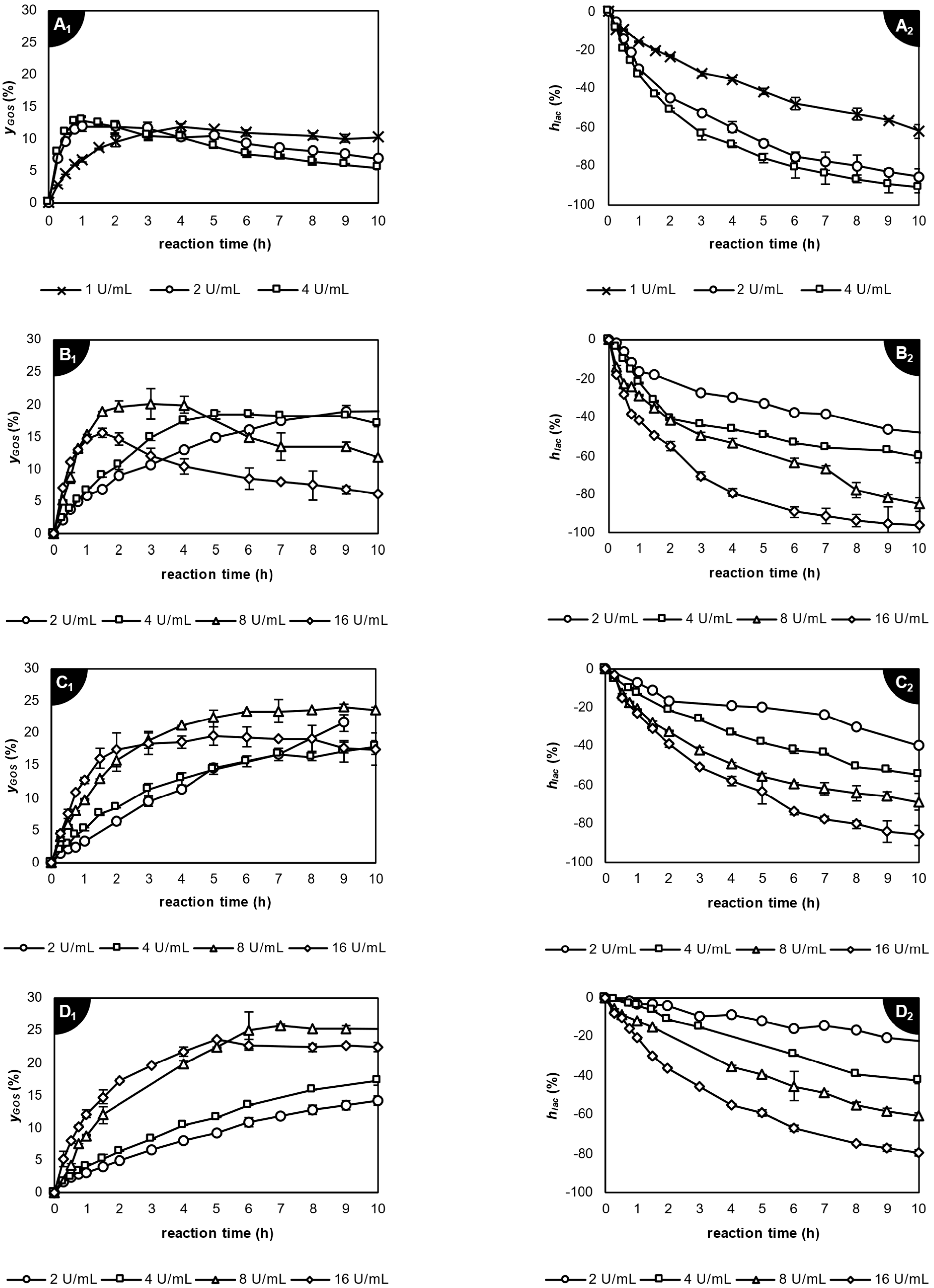
2.3.3. Application of β-Galactosidase from Thermothielavioides terrestris
2.4. Comparison of Galacto-Oligosaccharide Profile Produced by the Studied Lactases
3. Materials and Methods
3.1. Materials
3.2. Characterization and Pretreatment of Acid Whey
3.3. Characterization of Biocatalysts
3.4. Enzymatic Reaction for GOS Production
3.5. Analysis of Enzymatic Reaction Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rocha-Mendoza, D.; Kosmerl, E.; Krentz, A.; Zhang, L.; Badiger, S.; Miyagusuku-Cruzado, G.; Mayta-Apaza, A.; Giusti, M.; Jiménez-Flores, R.; García-Cano, I. Invited Review: Acid Whey Trends and Health Benefits. J. Dairy Sci. 2021, 104, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Menchik, P.; Zuber, T.; Zuber, A.; Moraru, C.I. Short Communication: Composition of Coproduct Streams from Dairy Processing: Acid Whey and Milk Permeate. J. Dairy Sci. 2019, 102, 3978–3984. [Google Scholar] [CrossRef]
- Erickson, B.E. Acid Whey: Is the Waste Product an Untapped Goldmine? Companies, Food Scientists Develop Innovative Solutions to Handle Tons of Greek Yogurt Byproduct. Chem. Eng. News 2017, 95, 26–30. [Google Scholar]
- Greek Yogurt Market. 2022. Available online: https://www.factmr.com/report/269/greek-yogurt-market (accessed on 25 July 2023).
- Ketterings, Q.; Czymmek, K.; Gami, S.; Godwin, G.; Ganoe, K. Guidelines for Land Application of Acid Whey. Cornell University Department of Animal Science Publication Series No. 247. 2017. Available online: http://nmsp.cals.cornell.edu/publications/extension/AcidWheyGuidelines2017.pdf (accessed on 25 July 2023).
- Smithers, G.W. Whey and Whey Proteins-From “Gutter-to-Gold”. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Fischer, C.; Kleinschmidt, T. Synthesis of Galactooligosaccharides in Milk and Whey: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 678–697. [Google Scholar] [CrossRef]
- Torres, D.P.M.; Gonçalves, M.d.P.F.; Teixeira, J.A.; Rodrigues, L.R. Galacto-Oligosaccharides: Production, Properties, Applications, and Significance as Prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef]
- Erich, S.; Kuschel, B.; Schwarz, T.; Ewert, J.; Böhmer, N.; Niehaus, F.; Eck, J.; Lutz-Wahl, S.; Stressler, T.; Fischer, L. Novel High-Performance Metagenome β-Galactosidases for Lactose Hydrolysis in the Dairy Industry. J. Biotechnol. 2015, 210, 27–37. [Google Scholar] [CrossRef]
- Gosling, A.; Stevens, G.W.; Barber, A.R.; Kentish, S.E.; Gras, S.L. Recent Advances Refining Galactooligosaccharide Production from Lactose. Food Chem. 2010, 121, 307–318. [Google Scholar] [CrossRef]
- Yin, H.; Bultema, J.B.; Dijkhuizen, L.; van Leeuwen, S.S. Reaction Kinetics and Galactooligosaccharide Product Profiles of the β-Galactosidases from Bacillus circulans, Kluyveromyces lactis and Aspergillus oryzae. Food Chem. 2017, 225, 230–238. [Google Scholar] [CrossRef]
- Vera, C.; Córdova, A.; Aburto, C.; Guerrero, C.; Suárez, S.; Illanes, A. Synthesis and Purification of Galacto-Oligosaccharides: State of the Art. World J. Microbiol. Biotechnol. 2016, 32, 197. [Google Scholar] [CrossRef] [PubMed]
- Barile, D.; Rastall, R.A. Human Milk and Related Oligosaccharides as Prebiotics. Curr. Opin. Biotechnol. 2013, 24, 214–219. [Google Scholar] [CrossRef]
- Rodriguez-Colinas, B.; Fernandez-Arrojo, L.; Ballesteros, A.O.; Plou, F.J. Galactooligosaccharides Formation during Enzymatic Hydrolysis of Lactose: Towards a Prebiotic-Enriched Milk. Food Chem. 2014, 145, 388–394. [Google Scholar] [CrossRef]
- Urrutia, P.; Rodriguez-Colinas, B.; Fernandez-Arrojo, L.; Ballesteros, A.O.; Wilson, L.; Illanes, A.; Plou, F.J. Detailed Analysis of Galactooligosaccharides Synthesis with β-Galactosidase from Aspergillus oryzae. J. Agric. Food Chem. 2013, 61, 1081–1087. [Google Scholar] [CrossRef]
- Srivastava, A.; Mishra, S.; Chand, S. Transgalactosylation of Lactose for Synthesis of Galacto-oligosaccharides Using Kluyveromyces marxianus NCIM 3551. New Biotechnol. 2015, 32, 412–418. [Google Scholar] [CrossRef]
- Yañez-Ñeco, C.V.; Cervantes, F.V.; Amaya-Delgado, L.; Ballesteros, A.O.; Plou, F.J.; Arrizon, J. Synthesis of β(1→3) and β(1→6) Galactooligosaccharides from Lactose and Whey Using a Recombinant β-Galactosidase from Pantoea anthophila. Electron. J. Biotechnol. 2021, 49, 14–21. [Google Scholar] [CrossRef]
- Zeuner, B.; Nyffenegger, C.; Mikkelsen, J.D.; Meyer, A.S. Thermostable β-Galactosidases for the Synthesis of Human Milk Oligosaccharides. New Biotechnol. 2016, 33, 355–360. [Google Scholar] [CrossRef]
- Fischer, C.; Kleinschmidt, T. Synthesis of Galactooligosaccharides Using Sweet and Acid Whey as a Substrate. Int. Dairy J. 2015, 48, 15–22. [Google Scholar] [CrossRef]
- Fischer, C.; Kleinschmidt, T. Synthesis of Galactooligosaccharides by Cryptococcus laurentii and Aspergillus oryzae Using Different Kinds of Acid Whey. Int. Dairy J. 2021, 112, 104867. [Google Scholar] [CrossRef]
- Zerva, A.; Limnaios, A.; Kritikou, A.S.; Thomaidis, N.S.; Taoukis, P.; Topakas, E. A Novel Thermophile β-Galactosidase from Thermothielavioides terrestris Producing Galactooligosaccharides from Acid Whey. New Biotechnol. 2021, 63, 45–53. [Google Scholar] [CrossRef]
- Jelen, P. WHEY PROCESSING|Utilization and Products. Encycl. Dairy Sci. 2011, 731–737. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kagamiishi, A.; Kiuchi, A.; Horiuchi, T. Purification and Properties of β-Galactosidase from Aspergillus oryzae. J. Biochem. 1975, 77, 241–247. [Google Scholar]
- Tanriseven, A.; Dogan, S. A Novel Method for the Immobilization of β-Galactosidase. Process Biochem. 2002, 38, 27–30. [Google Scholar] [CrossRef]
- Vera, C.; Guerrero, C.; Illanes, A. Determination of the Transgalactosylation Activity of Aspergillus oryzae β-Galactosidase: Effect of pH, Temperature, and Galactose and Glucose Concentrations. Carbohydr. Res. 2011, 346, 745–752. [Google Scholar] [CrossRef]
- Fujimura, Y.; Rokushika, S.; Ohnishi, M. Purification and Molecular Characterization of β-Galactosidase from Yeast Kluyveromyces lactis. Int. J. Biol. Macromol. 2003, 3, 97–103. [Google Scholar]
- Dickson, R.C.; Dickson, L.R.; Markin, J.S. Purification and Properties of an Inducible β-Galactosidase Isolated from the Yeast Kluyveromyces lactis. J. Bacteriol. 1979, 137, 51–61. [Google Scholar] [CrossRef]
- González-Delgado, I.; López-Muñoz, M.J.; Morales, G.; Segura, Y. Optimisation of the Synthesis of High Galacto-Oligosaccharides (GOS) from Lactose with β-Galactosidase from Kluyveromyces lactis. Int. Dairy J. 2016, 61, 211–219. [Google Scholar] [CrossRef]
- Mano, M.C.R.; Paulino, B.N.; Pastore, G.M. Whey Permeate as the Raw Material in Galacto-oligosaccharide Synthesis Using Commercial Enzymes. Food Res. Int. 2019, 124, 78–85. [Google Scholar] [CrossRef]
- Jenab, E.; Omidghane, M.; Mussone, P.; Armada, D.H.; Cartmell, J.; Montemagno, C. Enzymatic Conversion of Lactose into Galacto-oligosaccharides: The Effect of Process Parameters, Kinetics, Foam Architecture, and Product Characterization. J. Food Eng. 2018, 222, 63–72. [Google Scholar] [CrossRef]
- Yadav, A.; Kayastha, A.M. Lens culinaris β-Galactosidase (Lsbgal): Insights into Its Purification, Biochemical Characterization and Trisaccharides Synthesis. Bioorg. Chem. 2020, 95, 103543. [Google Scholar] [CrossRef]
- Rico-Rodríguez, F.; Noriega, M.A.; Lancheros, R.; Serrato-Bermúdez, J.C. Kinetics of Galactooligosaccharide (GOS) Production with Two β-Galactosidases Combined: Mathematical Model and Raw Material Effects. Int. Dairy J. 2021, 118, 105015. [Google Scholar] [CrossRef]
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoffs in Organischen Körpern. Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- van Boekel, M.A.J.S.; Ribadeau Dumas, B. Addendum to the Evaluation of the Kjeldahl Factor for Conversion of the Nitrogen Content of Milk and Milk Products to Protein Content. Neth. Milk Dairy J. 1987, 41, 281–284. [Google Scholar]
- O’Connor, T.P.; O’Brien, N.M. LIPIDS|Analytical Methods. Encycl. Dairy Sci. 2002, 1583–1587. [Google Scholar] [CrossRef]
- Borshchevskaya, L.N.; Gordeeva, T.L.; Kalinina, A.N.; Sineokii, S.P. Spectrophotometric Determination of Lactic Acid. J. Anal. Chem. 2016, 71, 755–758. [Google Scholar] [CrossRef]
- Sulphate Turbidimetric Method 9038; United States Environmental Protection Agency (EPA): Washington, DC, USA, 1986.
- Yalkowsky, S.H.; He, Y.; Jain, P. Handbook of Aqueous Solubility Data, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 13: 978-1-4398-0246-5. [Google Scholar]
- DIONEX Application Note 155 Determination of Trans-Galactooligosaccharides in Foods by AOAC Method 2001.02. 2016. Available online: https://assets.thermofisher.com/TFS-Assets/CMD/Application-Notes/AN-155-IC-Trans-Galactooligosaccharides-Foods-LPN1547-EN.pdf (accessed on 25 July 2023).


| Component | Concentration (g/L) | Component | Concentration (mg/L) |
|---|---|---|---|
| Total sugars | 38.72 ± 2.81 | K+ | 1131 ± 38 |
| Lactose | 31.18 ± 2.34 | Na+ | 343.2 ± 50.4 |
| Galactose | 7.42 ± 1.56 | Ca2+ | 1175 ± 28 |
| Glucose | 0.12 ± 0.09 | Mg2+ | 29.26 ± 6.20 |
| Total proteins | 2.752 ± 0.421 | Mn2+ | 0.019 ± 0.003 |
| Fat | 0.719 ± 0.120 | Zn2+ | 1.746 ± 0.121 |
| Lactic acid | 1.37 ± 0.05 | Cu2+ | <0.01 |
| Solid residue | 53.75 ± 2.50 | SO42− | 65.4 ± 0.6 |
| Ash | 8.964 ± 0.198 |
| Enzyme Source | Concentration (mM) | K+ | Na+ | Ca2+ | Mg2+ | Mn2+ | Zn2+ | Cu2+ |
|---|---|---|---|---|---|---|---|---|
| Kluyveromyces lactis | 0 | 100 ± 9 | ||||||
| 1 | 231 ± 4 | 118 ± 3 | 183 ± 16 | 29 ± 3 | 25 ± 2 | 1.4 ± 0.3 | n.d. | |
| 10 | 393 ± 6 | 195 ± 9 | 256 ± 12 | 38 ± 5 | n.d. | n.d. | n.d. | |
| 100 | 651 ± 9 | 389 ± 7 | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Aspergillus oryzae | 0 | 100 ± 2 | ||||||
| 1 | 92 ± 2 | 99 ± 2 | 97 ± 3 | 97 ± 4 | 97 ± 3 | 104 ± 3 | 108 ± 2 | |
| 10 | 98 ± 3 | 98 ± 3 | 104 ± 7 | 100 ± 3 | 90 ± 6 | 103 ± 2 | 104 ± 3 | |
| 100 | 99 ± 2 | 101 ± 2 | n.d. | 101 ± 3 | 98 ± 10 | n.d. | n.d. | |
| Enzyme Source | T (°C) | pH | clac(0) (% w/v) | AE (U/mL) | yGOS(max) (%) | tGOS(max) (h) | hlac (%) | ygal-gal (%) |
|---|---|---|---|---|---|---|---|---|
| Kluyveromyces lactis | 35 | 7.2 | 3.1 | 0.06 | 21.2 ± 1.8 e | 3 | −78.9 | 3.70 ± 0.01 |
| 0.13 | 23.7 ± 1.4 g | 1.5 | −76.0 | 3.72 ± 0.29 | ||||
| 0.26 | 15.6 ± 1.2 b | 0.75 | −70.6 | 3.62 ± 0.17 | ||||
| 10 | 0.06 | 13.0 ± 0.6 a,b,c | 4 | −46.3 | 6.56 ± 0.11 | |||
| 0.13 | 14.9 ± 1.8 a,b | 2 | −49.3 | 5.72 ± 0.09 | ||||
| 0.26 | 12.4 ± 1.0 a,b,c | 1 | −31.2 | 6.25 ± 0.17 | ||||
| 14 | 0.06 | 21.3 ± 1.1 e | 6 | −68.3 | 6.40 ± 0.29 | |||
| 0.13 | 14.8 ± 1.1 a,b | 3 | −54.0 | 4.61 ± 0.11 | ||||
| 0.26 | 18.8 ± 2.3 f | 1.5 | −57.0 | 4.34 ± 0.01 | ||||
| 18 | 0.06 | 9.11 ± 1.39 d | 3 | −37.4 | 7.19 ± 0.01 | |||
| 0.13 | 8.33 ± 1.24 d | 4 | −53.9 | 7.44 ± 0.34 | ||||
| 0.26 | 11.9 ± 1.2 c | 2 | −49.8 | 6.63 ± 0.21 | ||||
| Aspergillus oryzae | 45 | 4.5 | 3.1 | 0.5 | 9.57 ± 1.81 a | 3 | −38.8 | 1.84 ± 0.01 |
| 1 | 9.59 ± 0.56 a | 1 | −29.6 | 1.66 ± 0.02 | ||||
| 2 | 9.14 ± 0.53 a | 0.75 | −44.1 | 1.95 ± 0.02 | ||||
| 4 | 7.99 ± 0.45 a | 0.25 | −43.8 | 1.21 ± 0.01 | ||||
| 10 | 1 | 18.5 ± 0.4 c,d | 10 | −38.5 | 5.32 ± 0.01 | |||
| 2 | 16.7 ± 0.7 c | 7 | −48.2 | 8.97 ± 0.09 | ||||
| 4 | 17.7 ± 1.0 c | 2 | −40.0 | 6.38 ± 0.10 | ||||
| 8 | 13.9 ± 0.7 b | 1 | −49.6 | 7.86 ± 0.02 | ||||
| 15 | 1 | 17.8 ± 0.7 c | 10 | −44.7 | 8.25 ± 0.18 | |||
| 2 | 19.7 ± 1.1 d,e | 8 | −68.6 | 4.71 ± 0.10 | ||||
| 4 | 19.8 ± 0.9 d,e | 4 | −59.9 | 5.97 ± 0.08 | ||||
| 8 | 17.7 ± 0.6 c | 1.5 | −55.6 | 6.89 ± 0.09 | ||||
| 20 | 1 | 14.8 ± 2.1 b | 10 | −33.7 | 4.19 ± 0.06 | |||
| 2 | 22.0 ± 0.9 f,g | 10 | −43.4 | 5.60 ± 0.38 | ||||
| 4 | 23.4 ± 0.9 g | 7 | −51.9 | 4.78 ± 0.40 | ||||
| 8 | 20.7 ± 1.0 e,f | 5 | −68.7 | 4.93 ± 0.54 | ||||
| Thermothielavioides terrestris | 50 | 4.0 | 3.1 | 1 | 11.8 ± 0.6 a | 4 | −35.2 | 2.99 ± 0.06 |
| 2 | 12.0 ± 0.6 a | 2 | −44.5 | 3.39 ± 0.05 | ||||
| 4 | 12.9 ± 0.6 a,b | 1 | −32.4 | 3.03 ± 0.05 | ||||
| 10 | 2 | 19.0 ± 1.0 f,g,h | 9 | −45.9 | 5.71 ± 0.29 | |||
| 4 | 18.4 ± 0.6 e,f,g | 5 | −49.3 | 4.65 ± 0.04 | ||||
| 8 | 20.2 ± 2.4 h | 3 | −49.2 | 5.34 ± 0.15 | ||||
| 16 | 15.6 ± 0.8 c,d | 1.5 | −49.0 | 3.95 ± 0.06 | ||||
| 15 | 2 | 16.7 ± 0.8 d,e | 9 | −39.9 | 3.86 ± 0.01 | |||
| 4 | 17.8 ± 1.0 e,f | 10 | −55.1 | 4.16 ± 0.23 | ||||
| 8 | 23.7 ± 0.4 i | 9 | −65.6 | 6.88 ± 0.26 | ||||
| 16 | 19.6 ± 1.5 g,h | 5 | −63.8 | 5.83 ± 0.55 | ||||
| 20 | 2 | 14.3 ± 0.7 b,c | 10 | −20.4 | 2.94 ± 0.01 | |||
| 4 | 17.2 ± 0.6 d,e | 10 | −42.3 | 4.09 ± 0.16 | ||||
| 8 | 25.7 ± 0.3 j | 7 | −48.8 | 6.07 ± 0.07 | ||||
| 16 | 23.6 ± 0.2 i | 5 | −59.1 | 5.91 ± 0.18 |
| β-Galactosidase Source | Buffer Solution | pH Range | Temperature Range (°C) |
|---|---|---|---|
| Kluyveromyces lactis | Na2HPO4—NaH2PO4 | 6.0–8.0 | 20–50 |
| Aspergillus oryzae | citric acid—NaOH | 3.0–6.5 | 30–60 |
| Thermothielavioides terrestris * | citrate, phosphate, Tris—HCl | 3.0–9.0 | 30–70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limnaios, A.; Tsevdou, M.; Tsika, E.; Korialou, N.; Zerva, A.; Topakas, E.; Taoukis, P. Production of Prebiotic Galacto-Oligosaccharides from Acid Whey Catalyzed by a Novel β-Galactosidase from Thermothielavioides terrestris and Commercial Lactases: A Comparative Study. Catalysts 2023, 13, 1360. https://doi.org/10.3390/catal13101360
Limnaios A, Tsevdou M, Tsika E, Korialou N, Zerva A, Topakas E, Taoukis P. Production of Prebiotic Galacto-Oligosaccharides from Acid Whey Catalyzed by a Novel β-Galactosidase from Thermothielavioides terrestris and Commercial Lactases: A Comparative Study. Catalysts. 2023; 13(10):1360. https://doi.org/10.3390/catal13101360
Chicago/Turabian StyleLimnaios, Athanasios, Maria Tsevdou, Elena Tsika, Nausika Korialou, Anastasia Zerva, Evangelos Topakas, and Petros Taoukis. 2023. "Production of Prebiotic Galacto-Oligosaccharides from Acid Whey Catalyzed by a Novel β-Galactosidase from Thermothielavioides terrestris and Commercial Lactases: A Comparative Study" Catalysts 13, no. 10: 1360. https://doi.org/10.3390/catal13101360
APA StyleLimnaios, A., Tsevdou, M., Tsika, E., Korialou, N., Zerva, A., Topakas, E., & Taoukis, P. (2023). Production of Prebiotic Galacto-Oligosaccharides from Acid Whey Catalyzed by a Novel β-Galactosidase from Thermothielavioides terrestris and Commercial Lactases: A Comparative Study. Catalysts, 13(10), 1360. https://doi.org/10.3390/catal13101360









