Enzymatic Glycosylation Strategies in the Production of Bioactive Compounds
Abstract
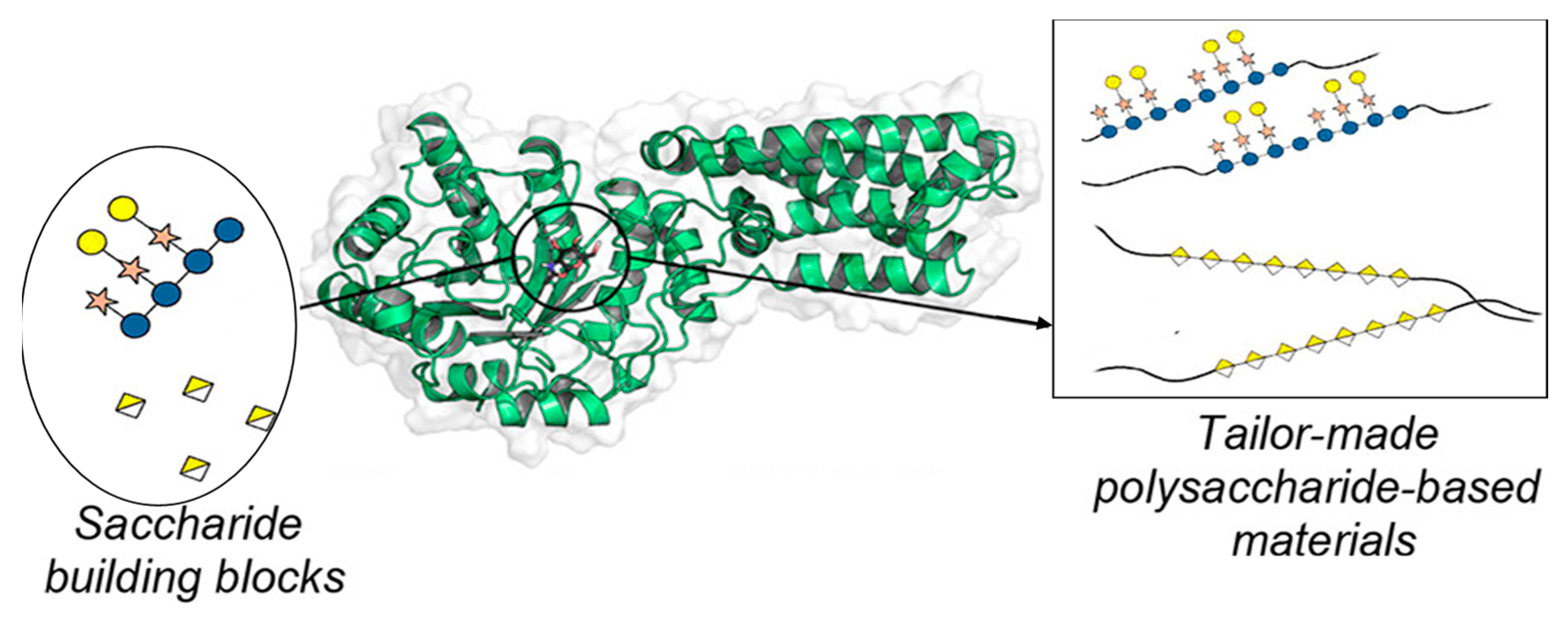
1. Introduction
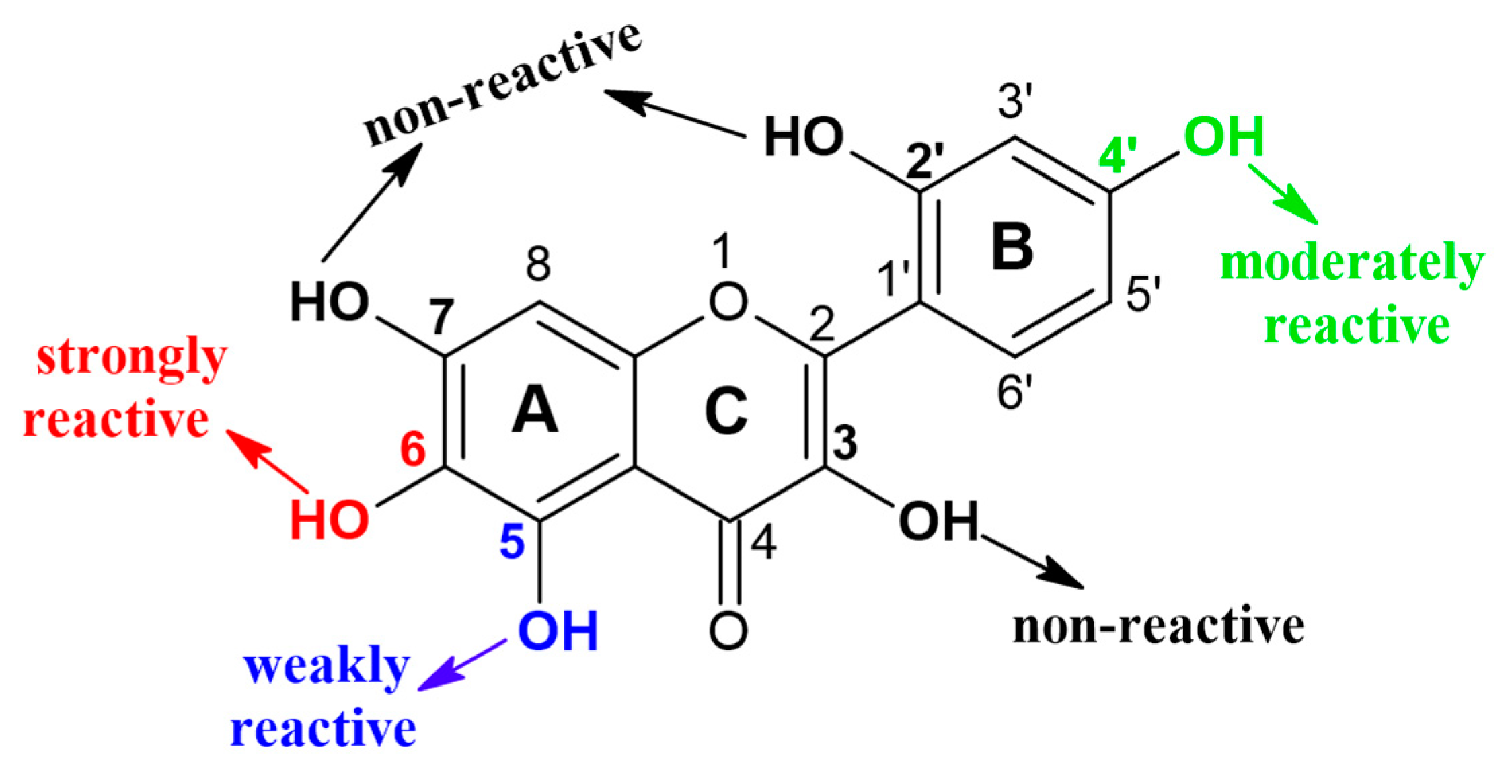
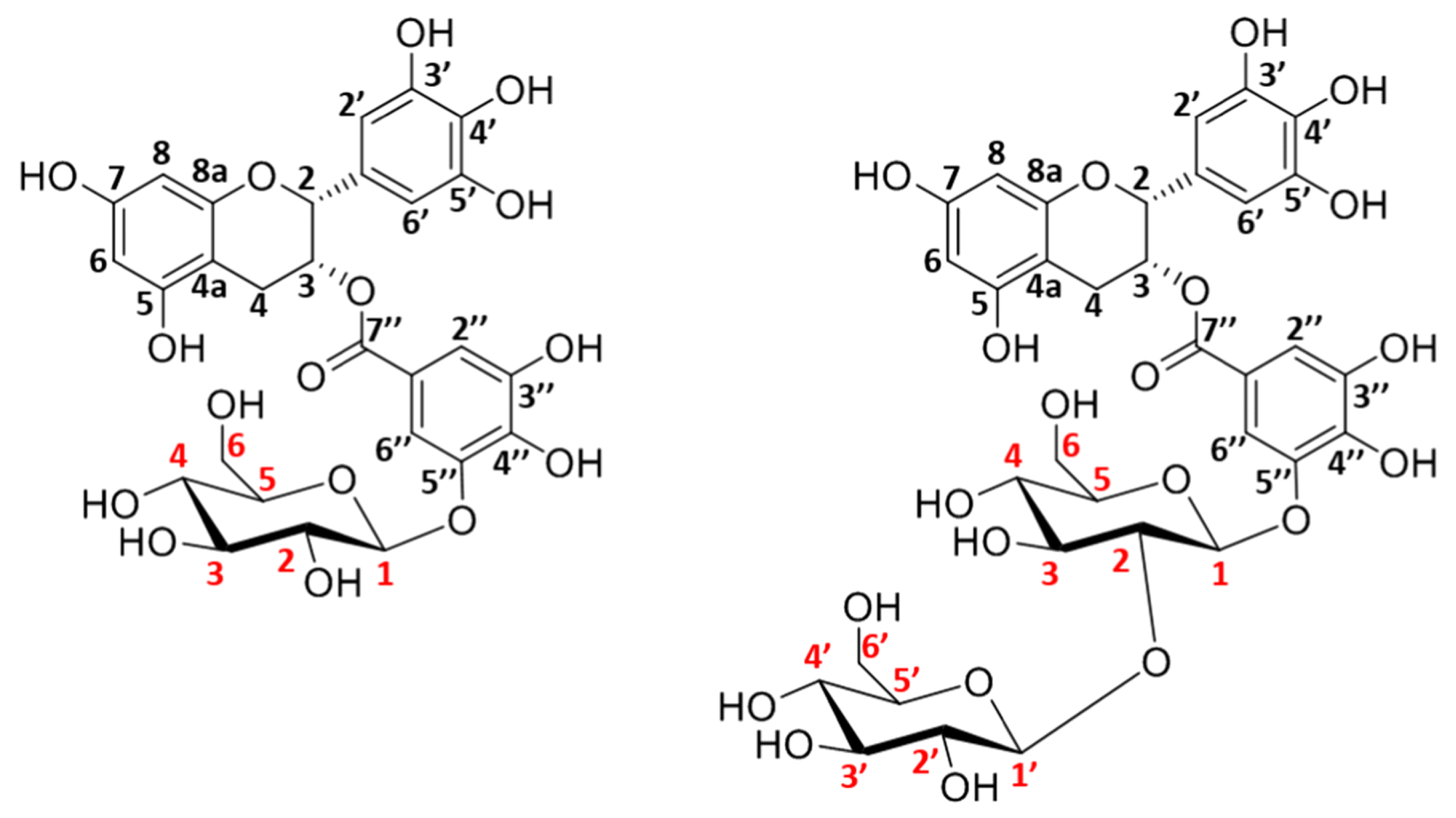
2. Enzymatic Glycosylation of Phenolic Compounds
2.1. Glycosyltransferase
2.1.1. Leloir Glycosyltransferase
| Enzyme | Glycosyl Acceptor | Glycosyl Donor | Conditions/Products | Yield | Refs. |
|---|---|---|---|---|---|
| glycosyltransferase MhGT1 from the filamentous fungus Mucor hiemalis | glycycoumarin and 93 phenolic substrates | UDP Glu | 30–40 °C, pH 8.0–9.0 enzyme activity enhanced by Ca2+, Mg2+ and Ba2+ ions | average conversion 89% for substrates containing prenyl groups | [18] |
| UDP glycosyltransferases UGT58A1 from Absidia coerulea UGT59A1 from Rhizopus japonicas | 13 different phenolic acceptors including lignans, flavonoids, anthraquinon, stilbene, benzophenone, curcuminoid, xanthones, and coumarin | UDP Glu UDP GluA | 50 mM Tris-HCl pH 8–9, 5 mM MgCl2, 400 μM UDP-Glc, 200 μM magnolol, and 200 μg of protein, 45 °C, 2 h. Mg2+, Mn2+, Ca2+, Ba2+ increase activity Ni2+, Co2+ and Cu2+, decrease activity | 94% | [20] |
| UDP glycosyltransferase, Bs-PUGT from B. subtilis PI18, (cloned and expressed in E. coli BL21 (DE3) and purified) | tyrosol, cinnamic alcohol, vanillin, ferulic acid, and caffeic acid | trehalose, sucrose, lactose. maltose, starch, glucose | pH 8.0, 30 °C, 25 g/L sucrose, 10 mM Ca2+, and 0.5 g/L caffeic acid | 78.3% with caffeic acid as acceptor | [21] |
| UDP-glucosyltransferase from Beauveria bassiana ATCC 7159 expressed in E. coli | quercetin resveratrol, curcumin and zearalenone | UDP Glc | 2.5 mM quercetin, 2 mM sugar donor, 1 mM MgCl2, 20 mM Tris–HCl pH 8 and 0.125 mg/mL of purified recombinant BbGT protein, 35 °C Ca2+, Mg2+, and Mn2+ stimulate the activity of BbGT, while Zn2+ inhibit it. five monoglucosylated and one diglucosylated quercetin derivatives by BbGT-harboring E. coli | quercetin-7-O-β-d-glucosides 0.34 mM from 0.83 mM quercetin in 24 h by BbGT-harboring E. coli quercetin-3-O-β-d-glucoside 0.22 mM from 0.41 mM quercetin in 12 h by BbGT-harboring S. cerevisiae | [22] |
2.1.2. Non-Leloir Glycosyltransferase
2.1.3. C-Glycosyltransferases
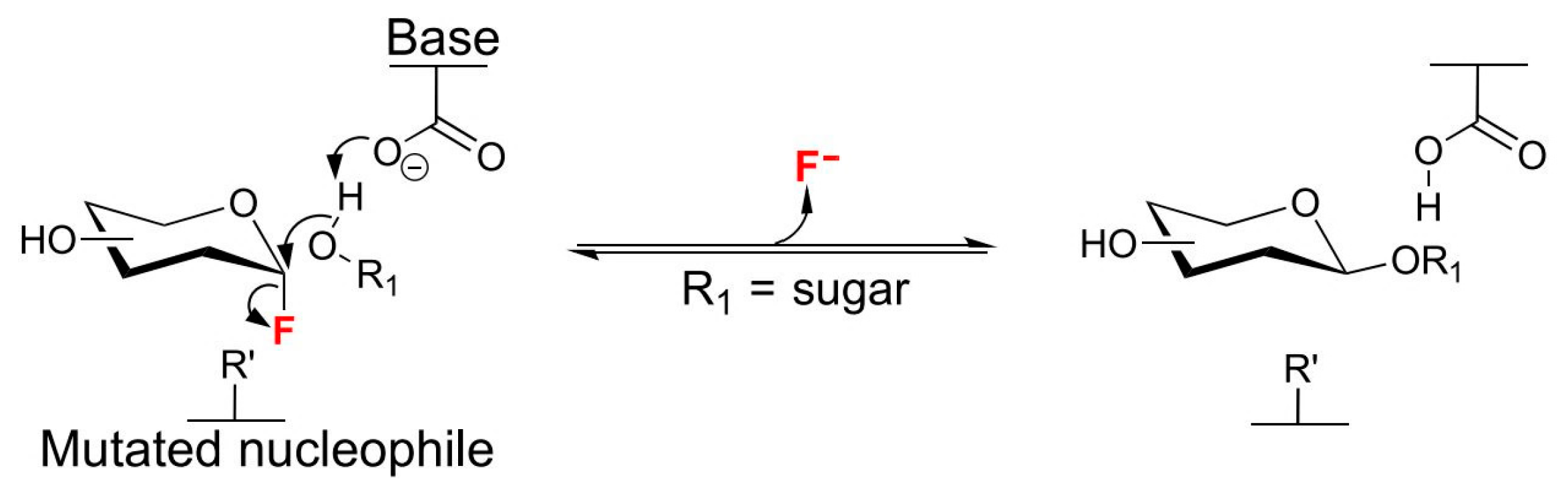
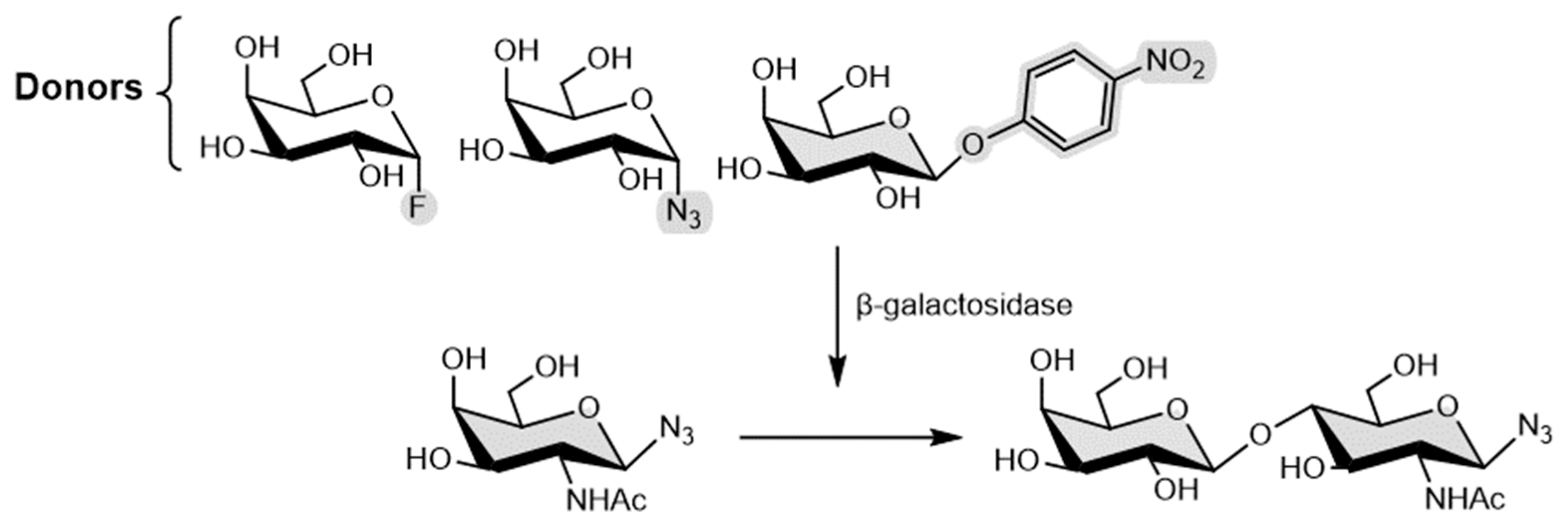
2.2. Glycosidases
Microbial Glycosylation
3. Enzymatic Glycosylation of Other Alcohols
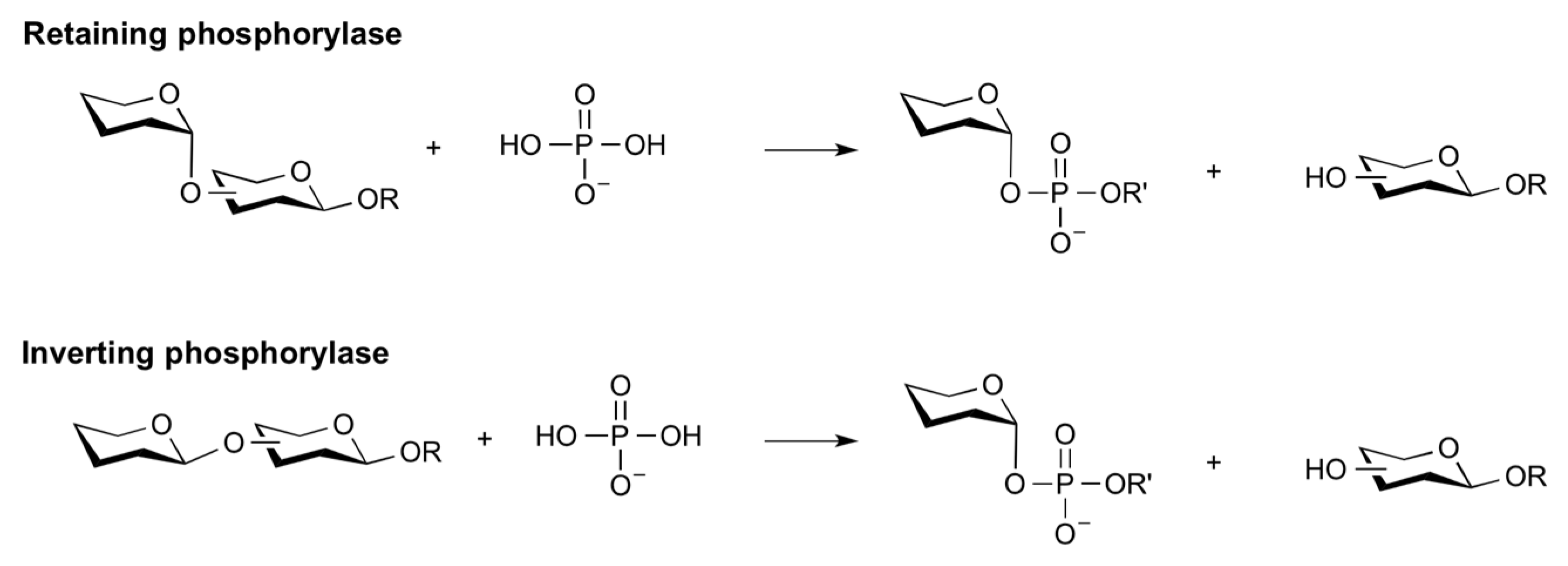
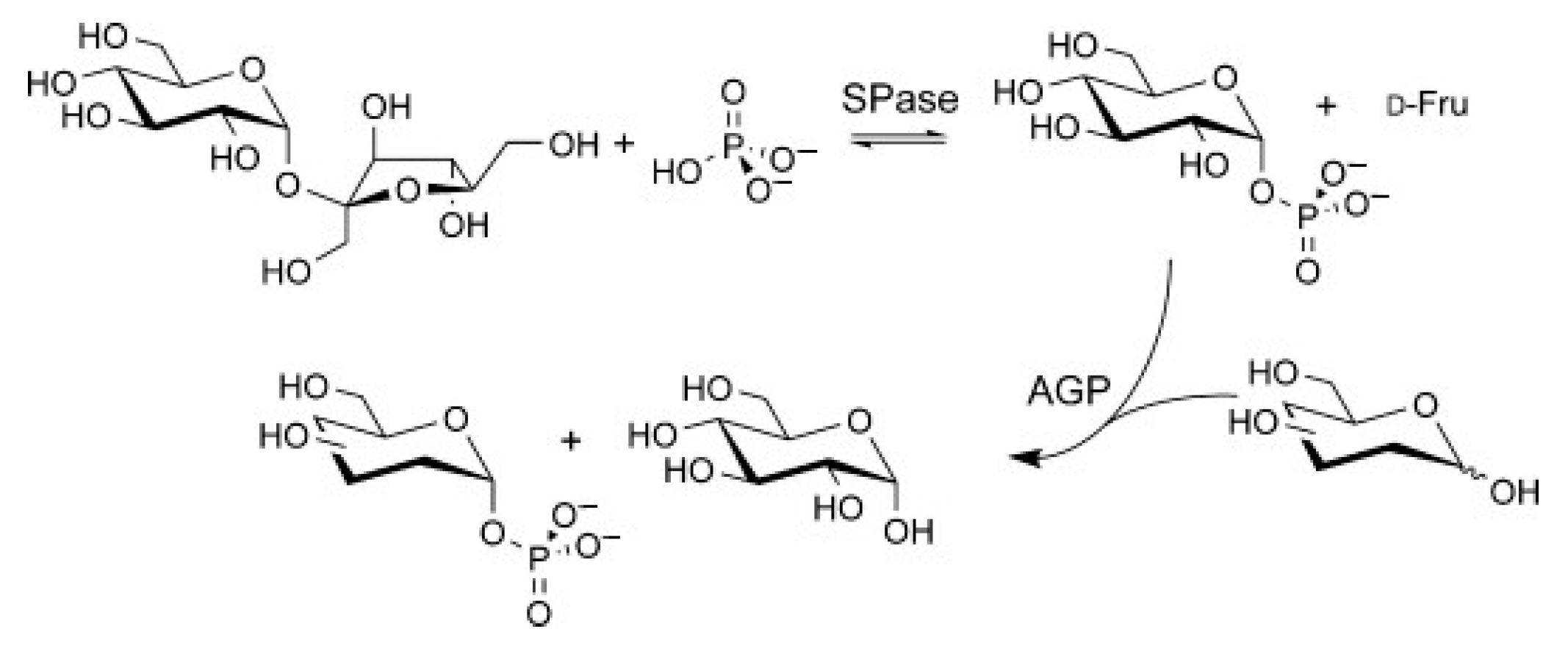
Glycan Phosphorylases (GP)
4. Enzymatic Synthetic Glycosylation of Oligosaccharides
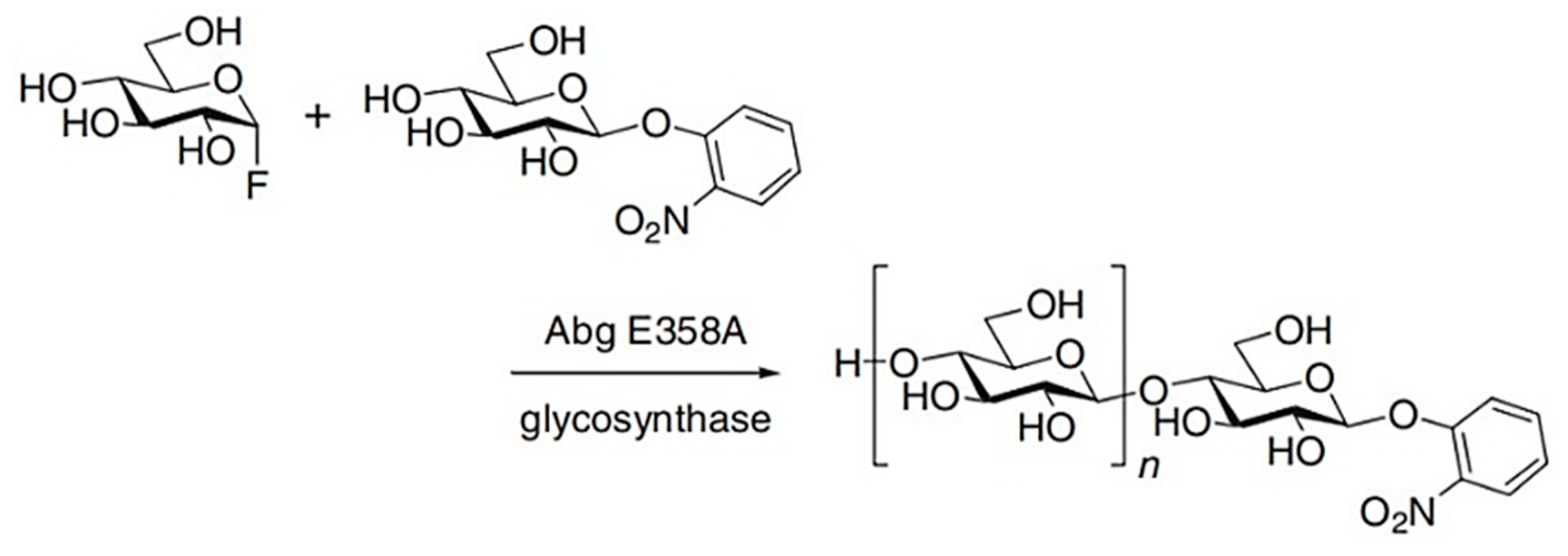
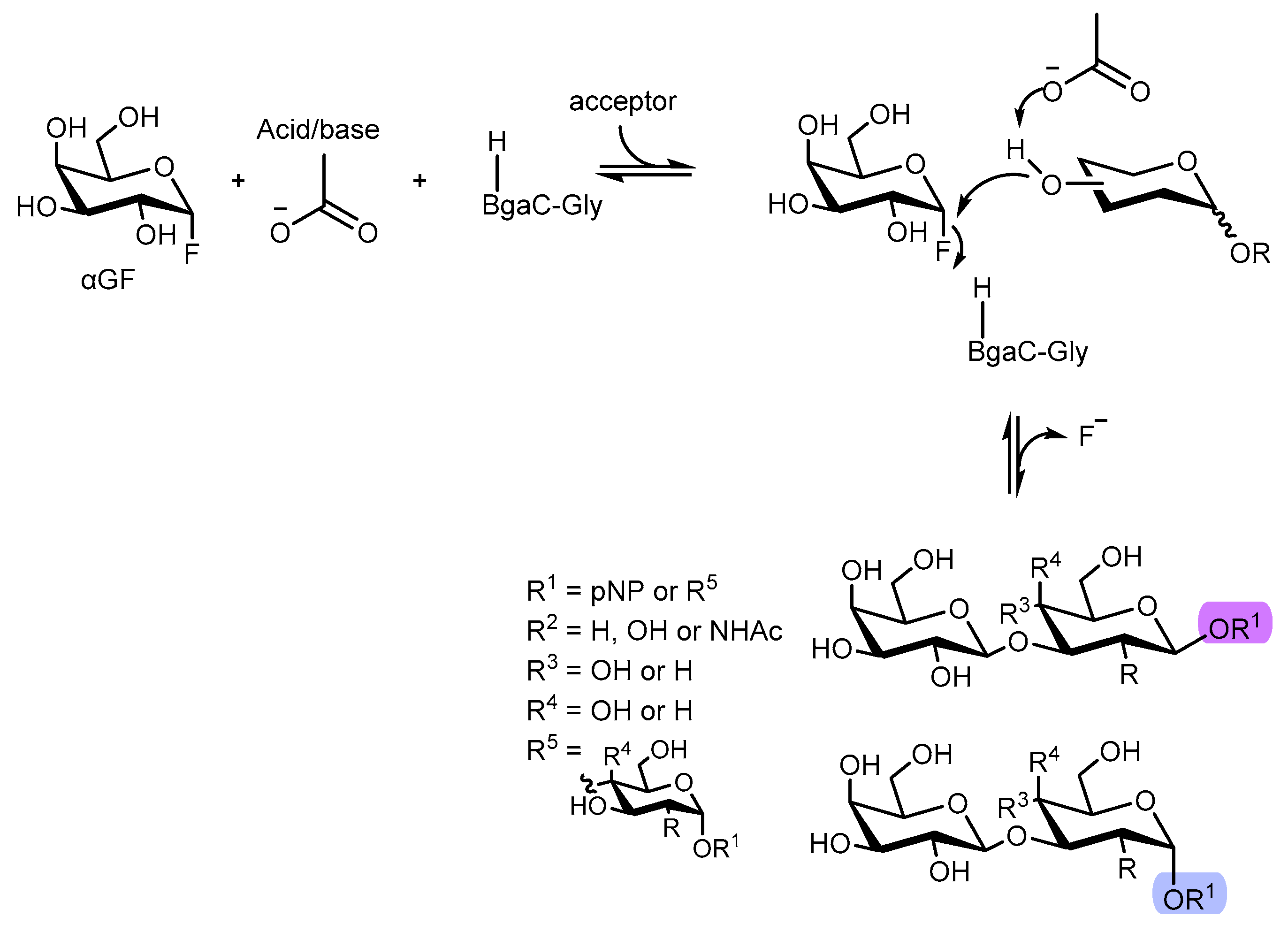
4.1. Glucosynthases
4.2. Galactosynthases
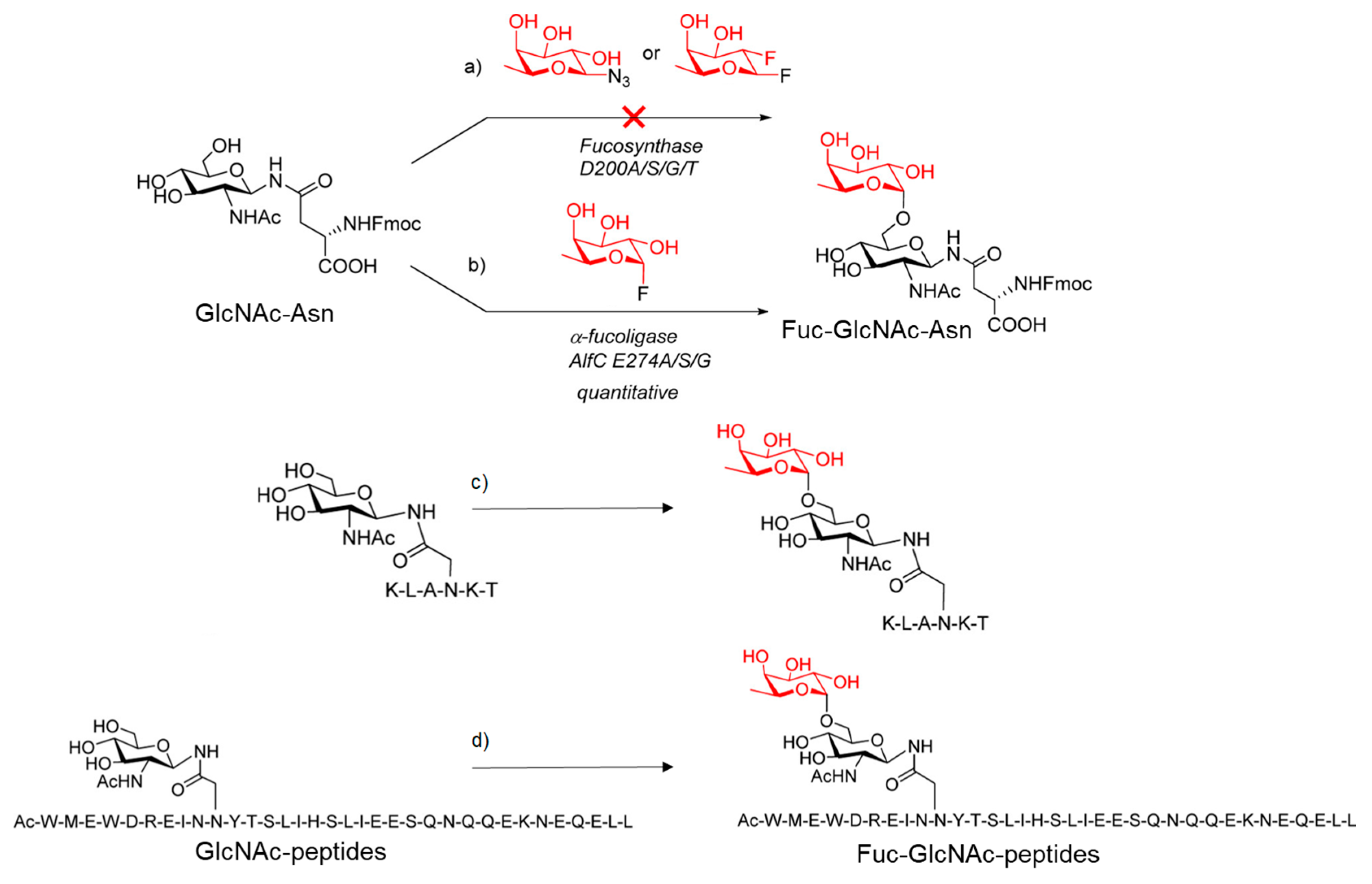
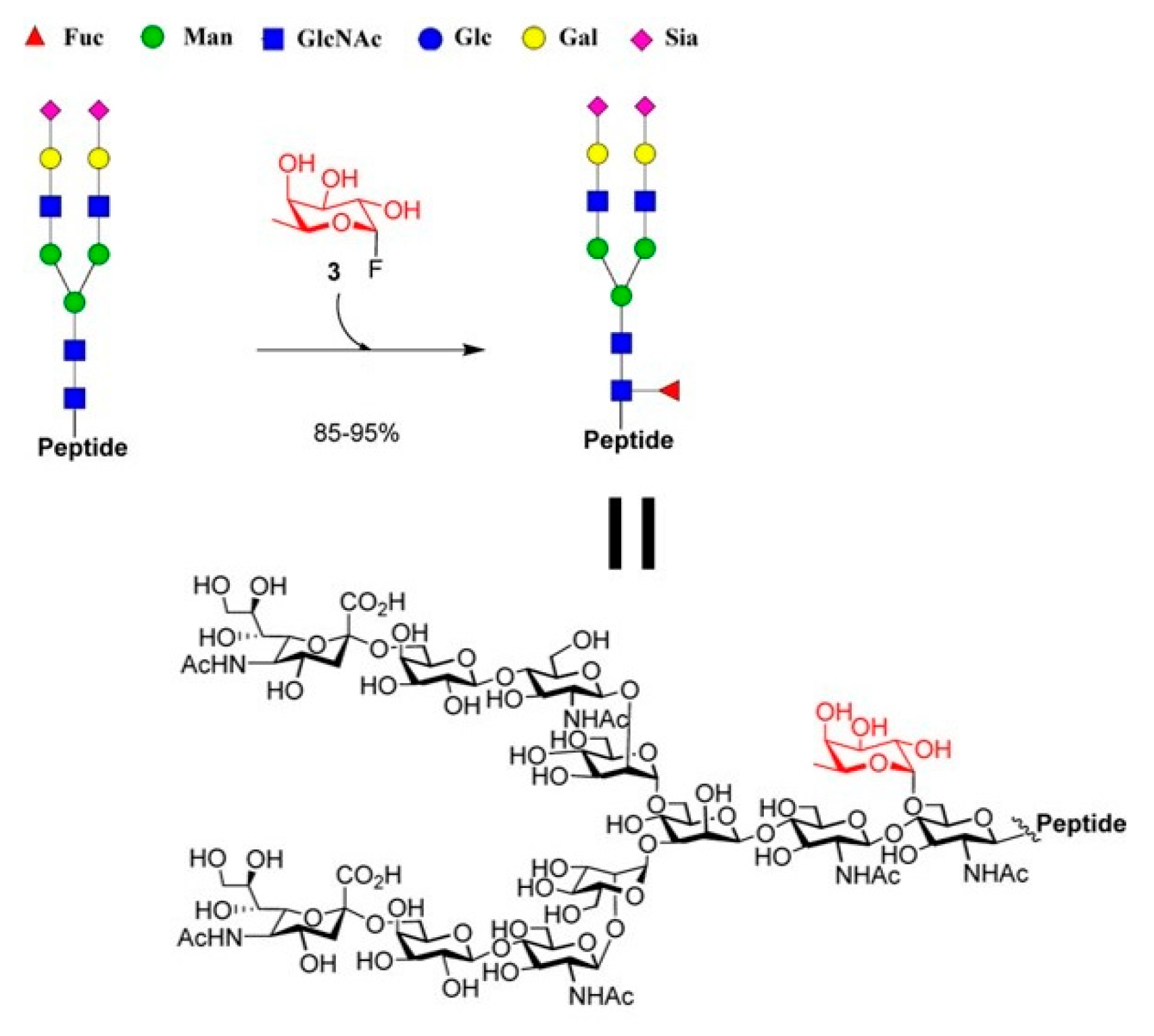
4.3. Fucosynthases
4.4. Chitinases
5. Conclusions and Future Prospect
- (i)
- Drug development: Enzymatic glycosylation can be used to enhance the pharmacokinetics and therapeutic efficacy of drugs. Future developments may focus on designing glycosylation strategies that improve drug targeting, bioavailability, and reduce side effects. This approach could lead to the creation of more effective and safer pharmaceuticals.
- (ii)
- Precision medicine: Tailoring glycosylation patterns in bioactive compounds to match individual patient profiles could become a reality. This personalized medicine approach may involve using enzymatic glycosylation to produce glycoconjugates that are optimized for specific patient populations, potentially improving treatment outcomes.
- (iii)
- Immunotherapy: Glycosylation plays a crucial role in modulating the immune response. Enzymatic glycosylation strategies could be used to develop glycoconjugates that enhance the efficacy of immunotherapies, such as cancer vaccines and monoclonal antibodies, by improving their interaction with immune cells and reducing immune evasion.
- (iv)
- Functional foods and nutraceuticals: Enzymatic glycosylation can be applied to enhance the functional properties of food ingredients and nutraceuticals. Future research may focus on producing glycoconjugates that offer improved bioavailability of nutrients, better taste, and enhanced health benefits.
- (v)
- Biotechnology: Enzymatic glycosylation is essential in the biotechnology industry for the production of therapeutic glycoproteins, vaccines, and glycolipids. Ongoing advancements may lead to more efficient and scalable processes, reducing production costs and improving the quality of biopharmaceuticals.
- (vi)
- Sustainability: Enzymatic glycosylation is considered an environmentally friendly approach compared to chemical methods. The future may see increased emphasis on developing more sustainable glycosylation processes, using renewable feedstocks, and reducing waste generation.
- (vii)
- Glycoengineering: Advances in glycoengineering may enable the precise control of glycosylation patterns, allowing for the creation of bioactive compounds with defined glycan structures. This could lead to the development of novel therapeutics and diagnostics.
- (viii)
- Enzyme engineering: Continued research in enzyme engineering may result in the discovery or design of more efficient and robust glycosyltransferases and other glycosylation enzymes, expanding the range of substrates and glycan structures that can be synthesized.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gantt, R.W.; Peltier-Pain, P.P.; Thorson, J.S. Enzymatic methods for glyco(diversification/randomization) of drugs and small molecules. Nat. Prod. Rep. 2011, 28, 1811–1853. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Phillips, G.N., Jr.; Thorson, J.S. The structural biology of enzymes involved in natural product glycosylation. Nat. Prod. Rep. 2012, 29, 1201–1237. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, M.C.; Kim, E.; Ban, Y.H.; Yoo, Y.J.; Kim, E.J.; Park, S.R.; Pandey, R.P.; Sohng, J.K.; Yoon, Y.J. Achievements and impacts of glycosylation reactions involved in natural product biosynthesis in prokaryotes. Appl. Microbiol. Biotechnol. 2013, 97, 5691–5704. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Butler, M.S. Natural products to drugs: Natural product-derived compounds in clinical trials. Nat. Prod. Rep. 2008, 25, 475–516. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nguyen, T.T.H.; Kim, N.M.; Moon, Y.-H.; Ha, J.-M.; Park, N.; Lee, D.-G.; Hwang, K.-H.; Park, J.-S.; Kim, D. Functional properties of novel epigallocatechin gallate glucosides synthesized by using dextransucrase from Leuconostoc mesenteroides B-1299CB4. J. Agric. Food Chem. 2016, 64, 9203–9213. [Google Scholar] [CrossRef]
- Faber, K. Biotransformations in Organic Chemistry: A Textbook, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Lyu, J.; Zhang, J.; Zhu, J.; Chen, S.; Han, T.; Zhang, Y.; Gao, R.; Xie, G.; Guo, Z. Molecular dynamics simulation guided distal mutation of Thermotoga naphthophila β-glucosidase for significantly enhanced synthesis of galactooligosaccharides and expanded product scope. Int. J. Biol. Macromol. 2022, 210, 21–32. [Google Scholar] [CrossRef]
- Paliya, B.S.; Sharma, V.K.; Tuohy, M.G.; Singh, H.B.; Koffas, N.; Benhida, R.; Tiwari, B.K.; Kalaskar, D.M.; Singh, B.N.; Gupta, V.K. Bacterial glycobiotechnology: A biosynthetic route for the production of biopharmaceutical glycans. Biotechnol. Adv. 2023, 67, 108180. [Google Scholar] [CrossRef]
- Xu, L.; Qi, T.; Xu, L.; Lu, L.; Xiao, M. Recent progress in the enzymatic glycosylation of phenolic compounds. J. Carbohyd. Chem. 2016, 35, 1–23. [Google Scholar] [CrossRef]
- Saranraj, P.; Behera, S.S.; Ray, R.C. Traditional Foods from Tropical Root and Tuber Crops: Innovations and Challenges, Innovations in Traditional Foods; Elsevier: Amsterdam, The Netherlands, 2019; pp. 159–191. [Google Scholar]
- Sun, Q.; Heilmann, J.; König, B. Natural phenolic metabolites with anti-angiogenic properties–a review from the chemical point of view. Beilstein J. Org. Chem. 2015, 11, 249–264. [Google Scholar] [CrossRef]
- Orhan, D.D.; Özçelik, B.; Özgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Zarrouk, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. The Occurrence and Bioactivity of Polyphenols in Tunisian Olive Products and by-Products: A Review. J. Food Sci. 2012, 77, R83–R92. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Líter, J.A.; Tundidor, I.; Nieto-Domínguez, M.; de Toro, B.F.; Santana, A.G.; de Eugenio, L.I.; Prieto, A.; Asensio, J.L.; Sánchez, C.; Martínez, M.J. Transglycosylation products generated by Talaromyces amestolkiae GH3 β-glucosidases: Effect of hydroxytyrosol, vanillin and its glucosides on breast cancer cells. Microb. Cell Factories 2019, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, B.; Qiao, M.; Li, J.; Xu, H.; Zhang, L.; Zhang, X. Advances on the in vivo and in vitro glycosylations of flavonoids. Appl. Microbiol. Biotechnol. 2020, 104, 6587–6600. [Google Scholar] [CrossRef]
- Schmid, J.; Heider, D.; Wendel, N.J.; Sperl, N.; Sieber, V. Bacterial glycosyltransferases: Challenges and opportunities of a highly diverse enzyme class toward tailoring natural products. Front. Microbiol. 2016, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, P.; Cui, Y.; Li, K.; Qiao, X.; Zhang, Y.T.; Li, S.M.; Cox, R.J.; Wu, B.; Ye, M. Regio- and Stereospecific O-Glycosylation of Phenolic Compounds Catalyzed by a Fungal Glycosyltransferase from Mucor hiemalis. Adv. Synth. Catal. 2017, 359, 995–1006. [Google Scholar] [CrossRef]
- Chang, S.K.; Jiang, Y.; Yang, B. An update of prenylated phenolics: Food sources, chemistry and health benefits. Trends Food Sci. Technol. 2021, 108, 197–213. [Google Scholar] [CrossRef]
- Xie, K.; Dou, X.; Chen, R.; Chen, D.; Fang, C.; Xiao, Z.; Dai, J. Two novel fungal phenolic UDP glycosyltransferases from Absidia coerulea and Rhizopus japonicus. Appl. Environ. Microbiol. 2017, 83, e03103-16. [Google Scholar] [CrossRef]
- Li, B.; Chang, S.; Jin, D.; Zhang, S.; Chen, T.; Pan, X.; Fan, B.; Lv, K.; He, X. Ca2+ assisted glycosylation of phenolic compounds by phenolic-UDP-glycosyltransferase from Bacillus subtilis PI18. Int. J. Biol. Macromol. 2019, 135, 373–378. [Google Scholar] [CrossRef]
- Ren, J.; Tang, W.; Barton, C.D.; Price, O.M.; Mortensen, M.W.; Phillips, A.; Wald, B.; Hulme, S.E.; Stanley, L.P.; Hevel, J. A highly versatile fungal glucosyltransferase for specific production of quercetin-7-O-β-d-glucoside and quercetin-3-O-β-d-glucoside in different hosts. Appl. Microbiol. Biotechnol. 2021, 106, 227–245. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Lei, H.; Chen, R.; Wang, X.; Peng, Y.; Dai, J. Neuroprotective glucosides of magnolol and honokiol from microbial-specific glycosylation. Tetrahedron 2014, 70, 8244–8251. [Google Scholar] [CrossRef]
- Rha, C.-S.; Choi, J.-M.; Jung, Y.S.; Kim, E.-R.; Ko, M.J.; Seo, D.-H.; Kim, D.-O.; Park, C.-S. High-efficiency enzymatic production of α-isoquercitrin glucosides by amylosucrase from Deinococcus geothermalis. Enzym. Microb. Technol. 2019, 120, 84–90. [Google Scholar] [CrossRef]
- Rha, C.-S.; Jung, Y.S.; Seo, D.-H.; Kim, D.-O.; Park, C.-S. Site-specific α-glycosylation of hydroxyflavones and hydroxyflavanones by amylosucrase from Deinococcus geothermalis. Enzym. Microb. Technol. 2019, 129, 109361. [Google Scholar] [CrossRef]
- Gonzalez-Alfonso, J.L.; Leemans, L.; Poveda, A.; Jimenez-Barbero, J.S.; Ballesteros, A.O.; Plou, F.J. Efficient α-glucosylation of epigallocatechin gallate catalyzed by cyclodextrin glucanotransferase from Thermoanaerobacter species. J. Agric. Food Chem. 2018, 66, 7402–7408. [Google Scholar] [CrossRef] [PubMed]
- González-Alfonso, J.L.; Míguez, N.; Padilla, J.D.; Leemans, L.; Poveda, A.; Jiménez-Barbero, J.; Ballesteros, A.O.; Sandoval, G.; Plou, F.J. Optimization of regioselective α-glucosylation of hesperetin catalyzed by cyclodextrin glucanotransferase. Molecules 2018, 23, 2885. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Líter, J.A.; Nieto-Domínguez, M.; de Toro, B.F.; Santana, A.G.; Prieto, A.; Asensio, J.L.; de Eugenio, L.I.; Martínez, M.J. A glucotolerant β-glucosidase from the fungus Talaromyces amestolkiae and its conversion into a glycosynthase for glycosylation of phenolic compounds. Microb. Cell Factories 2020, 19, 127. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- He, J.B.; Zhao, P.; Hu, Z.M.; Liu, S.; Kuang, Y.; Zhang, M.; Li, B.; Yun, C.H.; Qiao, X.; Ye, M. Molecular and Structural Characterization of a Promiscuous C-Glycosyltransferase from Trollius chinensis. Angew. Chem. Int. Ed. 2019, 131, 11637–11644. [Google Scholar] [CrossRef]
- Pei, J.; Sun, Q.; Gu, N.; Zhao, L.; Fang, X.; Tang, F.; Cao, F. Production of isoorientin and isovitexin from luteolin and apigenin using coupled catalysis of glycosyltransferase and sucrose synthase. Appl. Biochem. Biotechnol. 2020, 190, 601–615. [Google Scholar] [CrossRef]
- Pei, J.; Sun, Q.; Zhao, L.; Shi, H.; Tang, F.; Cao, F. Efficient biotransformation of luteolin to isoorientin through adjusting induction strategy, controlling acetic acid, and increasing UDP-glucose supply in Escherichia coli. J. Agric. Food Chem. 2018, 67, 331–340. [Google Scholar] [CrossRef]
- Carević, M.; Veličković, D.; Stojanović, M.; Milosavić, N.; Rogniaux, H.; Ropartz, D.; Bezbradica, D. Insight in the regioselective enzymatic transgalactosylation of salicin catalyzed by β-galactosidase from Aspergillus oryzae. Process. Biochem. 2015, 50, 782–788. [Google Scholar] [CrossRef]
- Gonzalez-Alfonso, J.L.; Ubiparip, Z.; Jimenez-Ortega, E.; Poveda, A.; Alonso, C.; Coderch, L.; Jimenez-Barbero, J.; Sanz-Aparicio, J.; Ballesteros, A.O.; Desmet, T. Enzymatic Synthesis of Phloretin α-Glucosides Using a Sucrose Phosphorylase Mutant and its Effect on Solubility, Antioxidant Properties and Skin Absorption. Adv. Synth. Catal. 2021, 363, 3079–3089. [Google Scholar] [CrossRef]
- Murguiondo, C.; Mestre, A.; Méndez-Líter, J.A.; Nieto-Domínguez, M.; de Eugenio, L.I.; Molina-Gutiérrez, M.; Martínez, M.J.; Prieto, A. Enzymatic glycosylation of bioactive acceptors catalyzed by an immobilized fungal β-xylosidase and its multi-glycoligase variant. Int. J. Biol. Macromol. 2021, 167, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Sordon, S.; Popłoński, J.; Huszcza, E. Microbial glycosylation of flavonoids. Pol. J. Microbiol. 2016, 65, 7. [Google Scholar] [CrossRef] [PubMed]
- Sordon, S.; Popłoński, J.; Tronina, T.; Huszcza, E. Microbial glycosylation of daidzein, genistein and biochanin A: Two new glucosides of biochanin A. Molecules 2017, 22, 81. [Google Scholar] [CrossRef] [PubMed]
- Sordon, S.; Popłoński, J.; Tronina, T.; Huszcza, E. Regioselective O-glycosylation of flavonoids by fungi Beauveria bassiana, Absidia coerulea and Absidia glauca. Bioinorg. Chem. 2019, 93, 102750. [Google Scholar] [CrossRef]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Biotransformations of flavones and an isoflavone (daidzein) in cultures of entomopathogenic filamentous fungi. Molecules 2018, 23, 1356. [Google Scholar] [CrossRef]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Glycosylation of 3-hydroxyflavone, 3-methoxyflavone, quercetin and baicalein in fungal cultures of the genus Isaria. Molecules 2018, 23, 2477. [Google Scholar] [CrossRef]
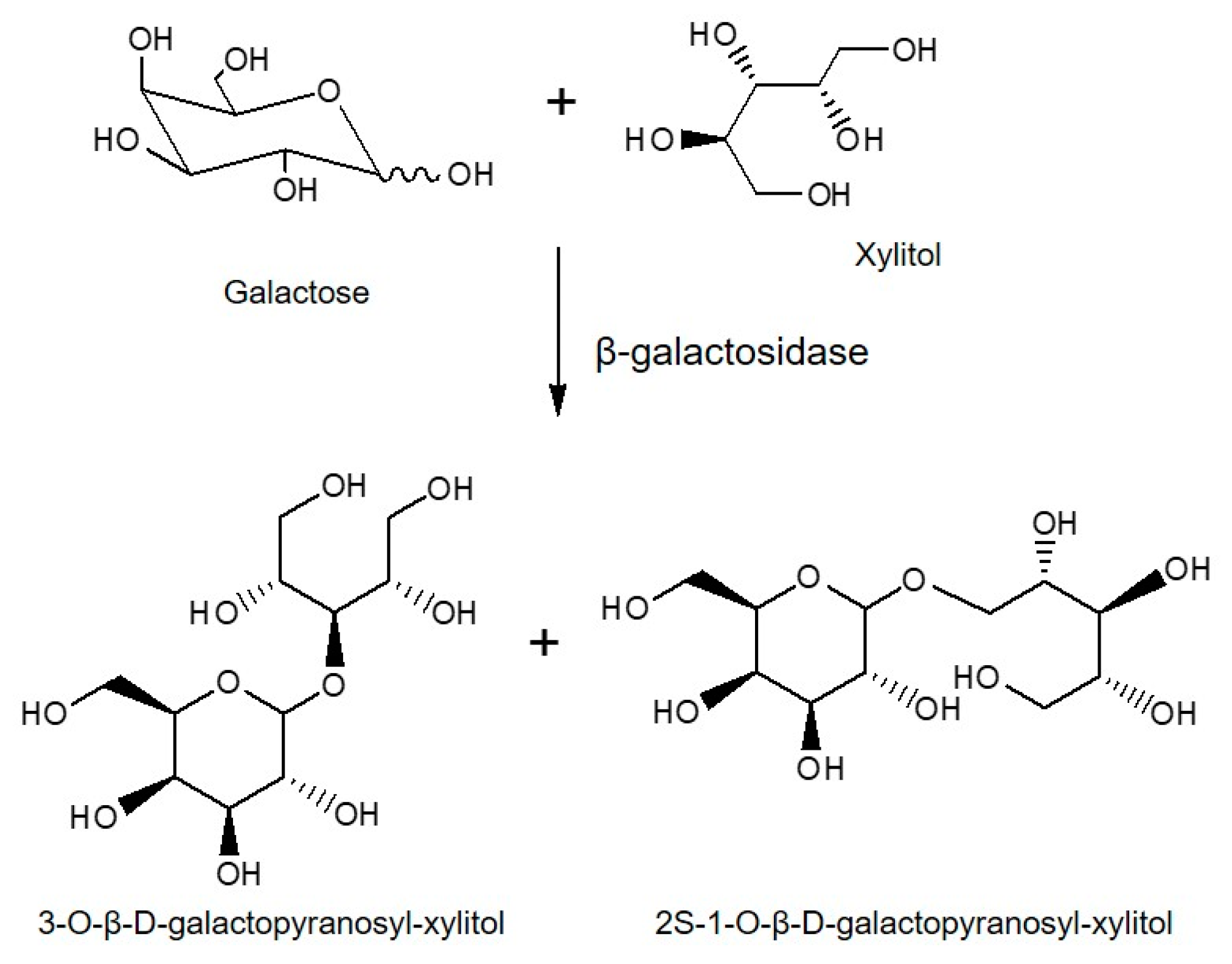
- Rosado, E.; Delgado-Fernandez, P.; de las Rivas, B.; Muñoz, R.; Moreno, J.; Corzo, N.; Mateo, C. Production of β-Galactosyl Xylitol Derivatives Using Heterogeneous Catalysts of LacA β-Galactosidase from Lactobacillus plantarum WCFS1. Molecules 2022, 27, 1235. [Google Scholar] [CrossRef]
- Cheng, K.L.; Bradely, T.; Budman, D.R. Novel microtubule-targeting agents- the epothilones. Biologics 2008, 2, 789–811. [Google Scholar]
- Hofle, G.; Reichenbach, H. Epothilone, a myxobacterial metabolite with promising antitumor activity. In Anticancer Agents from Natural Products; Cragg, G.M., Kingston, D.G.I., Newman, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 413–450. [Google Scholar]
- Hardt, I.H.; Steinmetz, H.; Gerth, K.; Sasse, F.; Reichenbach, H.; Hofle, G. New natural epothilones from Sorangium cellulosum, strains So ce90/B2 and So ce90/D13: Isolation, structure elucidation, and SAR studies. J. Nat. Prod. 2001, 64, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.Y.; Borzilleri, R.; Fairchild, C.R.; Kim, S.H.; Long, B.H.; Reventos-Suarez, C.; Vite, G.D.; Rose, W.C.; Kramer, R.A. BMS-247550: A novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin. Cancer Res. 2001, 7, 1429–1437. [Google Scholar]
- Thomas, E.; Tabemero, J.; Fornier, M.; Conte, P.; Fumoleau, P.; Liuch, A.; Vahdat, L.T.; Bunnell, C.A.; Burris, H.A.; Viens, P.; et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxane-resistant metastatic breast cancer. J. Clin. Oncol. 2007, 25, 3399–3406. [Google Scholar] [CrossRef] [PubMed]
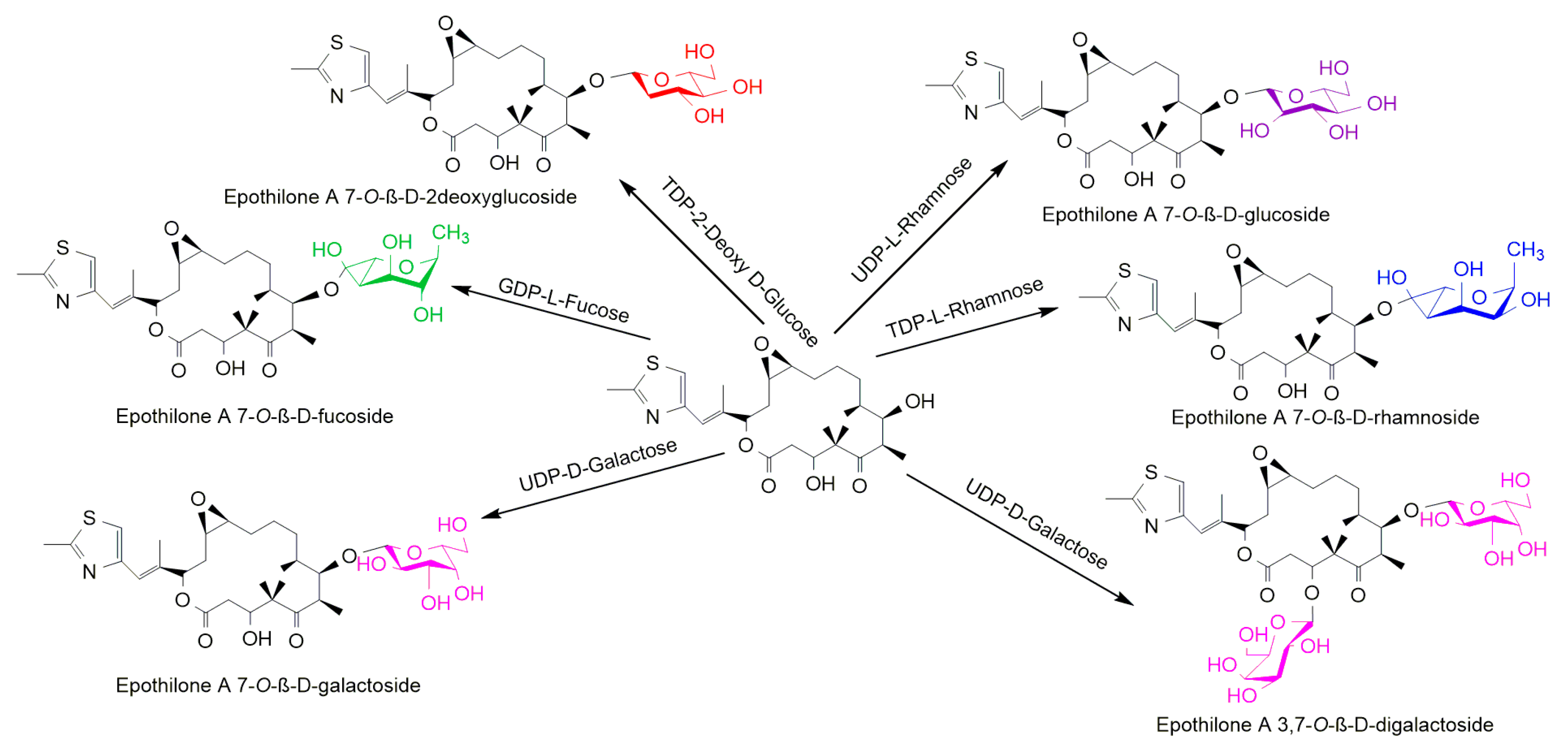
- Parajuli, P.; Pandey, R.P.; Koirala, N.; Yoon, Y.J.; Kim, B.-G.; Sohng, J.K. Enzymatic synthesis of epothilone A glycosides. AMB Express 2014, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.C.; Field, R.A. Enzymatic synthesis using glycoside phosphorylases. Carbohydr. Res. 2015, 403, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Puchart, V. Glycoside phosphorylases: Structure, catalytic properties and biotechnological potential. Biotechnol. Adv. 2015, 33, 261–276. [Google Scholar] [CrossRef]
- Nakai, H.; Kitaoka, M.; Svensson, B.; Ohtsubo, K.I. Recent development of phosphorylases possessing large potential for oligosaccharide synthesis. Curr. Opin. Chem. Biol. 2013, 17, 301–309. [Google Scholar] [CrossRef]
- Pergolizzia, G.; Kuhaudomlarpa, S.; Kalitaa, E.; Fielda, R.A. Glycan Phosphorylases in Multi-Enzyme Synthetic Processes. Protein Pept. Lett. 2017, 24, 696–709. [Google Scholar] [CrossRef]
- Wildberger, P.; Pfeiffer, M.; Brecker, L.; Nidetzky, B. Diastereoselective synthesis of glycosyl phosphates by using a phosphorylase-phosphatase combination catalyst. Angew. Chem. Int. Ed. 2015, 54, 15867–15871. [Google Scholar] [CrossRef]
- Hancock, S.M.; Vaughan, M.D.; Withers, S.G. Engineering of glycosidases and glycosyltransferases. Curr. Opin. Chem. Biol. 2006, 10, 509–519. [Google Scholar] [CrossRef]
- Schmölzer, K.; Weingarten, M.; Baldenius, K.; Nidetzky, B. Glycosynthase Principle Transformed into Biocatalytic Process Technology: Lacto N triose II Production with Engineered exo-Hexosaminidase. ACS Catal. 2019, 9, 5503–5514. [Google Scholar] [CrossRef]
- Bulmer, G.S.; de Andrade, P.; Field, R.A.; van Munster, J.M. Recent advances in enzymatic synthesis of β-glucan and cellulose. Carbohydr. Res. 2021, 508, 108411. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, Z.; Nekvasilová, P.; Bojarová, P.; Kren, V.; Fernández Slámová, K. Advanced glycosidases as ingenious biosynthetic instruments. Biotechnol. Adv. 2021, 49, 107733. [Google Scholar] [CrossRef]
- Williams, S.J.; Withers, S.G. Glycosynthases: Mutant Glycosidases for Glycoside Synthesis. Aus. J. Chem. 2002, 55, 3–12. [Google Scholar] [CrossRef]
- Hrmova, M.; Imai, T.; Rutten, S.J.; Fairweather, J.K.; Pelosi, L.; Bulone, V.; Driguez, H.; Fincher, G.B. Mutated Barley (1,3)-β-d-Glucan Endohydrolases Synthesize Crystalline (1,3)-β-d-Glucans. J. Biol. Chem. 2002, 277, 30102–30111. [Google Scholar] [CrossRef]
- Pérez, X.; Faijes, M.; Planas, A. Artificial Mixed-Linked β-Glucans Produced by Glycosynthase-Catalyzed Polymerization: Tuning Morphology and Degree of Polymerization. Biomacromolecules 2011, 12, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.J.; Ortiz-Soto, M.E.; Roth, C.; Barnes, W.J.; Seibel, J.; Urbanowicz, B.R.; Pfrengle, F. Enzymatic Synthesis of Artificial Polysaccharides. ACS Sustain. Chem. Eng. 2020, 8, 11853–11871. [Google Scholar] [CrossRef]
- Van Den Akker, F.; Steensma, E.; Hol, W.G.J. Tumor marker disaccharide D-Gal-β1,3-GalNAc complexed to heat-labile enterotoxin from Escherichia coli. Protein Sci. 1996, 5, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, R.; Morris, L.M.; Woods, R.J.; Weimar, T. Synthesis and conformational analysis of the T-antigen disaccharide (β-D-Gal-(1→3)-α-D-GalNAc-OMe). Eur. J. Org. 2001, 14, 2697–2705. [Google Scholar] [CrossRef]
- Mocchetti, I. Exogenous gangliosides, neuronal plasticity and repair, and the neurotrophins. Cell Mol. Life Sci. 2005, 62, 2283–2294. [Google Scholar] [CrossRef]
- Brockhausen, I. Mucin-type O-glycans in human colon and breast cancer: Glycodynamics and functions. EMBO Rep. 2006, 7, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Urashima, T.; Asakuma, S.; Leo, F.; Fukuda, K.; Messer, M.; Oftedal, O.T. The predominance of type I oligosaccharides is a feature specific to human breast milk. Adv. Nutr. 2012, 3, 473s–482s. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Kim, Y.W. Characterization of a Galactosynthase Derived from Bacillus Circulans Beta-Galactosidase: Facile Synthesis of D-Lacto- and D-Galacto-N-Bioside. ChemBioChem 2014, 15, 522–526. [Google Scholar] [CrossRef]
- Yamamoto, K. Recent advances in glycotechnology for glycoconjugate synthesis using microbial endoglycosidases. Biotechnol. Lett. 2013, 35, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Damborsky, J.; Brezovsky, J. Computational tools for designing and engineering biocatalysts. Curr. Opin. Chem. Biol. 2009, 13, 26–34. [Google Scholar] [CrossRef]
- Armstrong, Z.; Withers, S.G. Synthesis of glycans and glycopolymers through engineered enzymes. Biopolymers 2013, 99, 666–674. [Google Scholar] [CrossRef]
- Cobucci-Ponzano, B.; Strazzulli, A.; Rossi, M.; Moracci, M. Glycosynthases in biocatalysis. Adv. Synth. Catal. 2011, 353, 2284–2300. [Google Scholar] [CrossRef]
- Mackenzie, L.F.; Wang, Q.; Warren, R.A.J.; Withers, S.G. Glycosynthases: Mutant Glycosidases for Oligosaccharide synthesis. J. Am. Chem. Soc. 1998, 120, 5583–5584. [Google Scholar] [CrossRef]
- Ruzic, L.; Bolivar, J.M.; Nidetzky, B. Glycosynthase reaction meets the flow: Continuous synthesis of lacto-N-triose II by engineered β-hexosaminidase immobilized on solid support. Biotechnol. Bioeng. 2020, 117, 1597–1602. [Google Scholar] [CrossRef]
- Hovorková, M.; Kulik, N.; Konvalinková, D.; Petrásková, L.; Křen, V.; Bojarová, P. Mutagenesis of Catalytic Nucleophile of β-Galactosidase Retains Residual Hydrolytic Activity and Affords a Transgalactosidase. ChemCatChem 2021, 13, 4532–4542. [Google Scholar] [CrossRef]
- Mészáros, Z.; Nekvasilová, P.; Bojarová, P.; Křen, V.; Slámová, K. Reprint of: Advanced glycosidases as ingenious biosynthetic instruments. Biotechnol. Adv. 2021, 51, 107820. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, M.; Matsunaga, K.; Watanabe, K.I.; Yamashita, K.; Tagami, T.; Kikuchi, A.; Ma, M.; Klahan, P.; Mori, H.; Yao, M.; et al. Efficient synthesis of α-galactosyl oligosaccharides using a mutant Bacteroides thetaiotaomicron retaining α-galactosidase (BtGH97b). FEBS J. 2017, 284, 766–783. [Google Scholar] [CrossRef]
- Dwek, R.A. Glycobiology: Toward Understanding the Function of Sugars. Chem. Rev. 1996, 96, 683. [Google Scholar] [CrossRef]
- Helenius, A.; Aebi, M. Intracellular Functions of N-linked Glycans. Science 2001, 291, 2364. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Kizuka, Y. Glycans and Cancer: Role of N-Glycans in cancer Biomarker, progression and metastasis, and therapeutics. Adv. Cancer Res. 2015, 126, 11. [Google Scholar] [PubMed]
- Pinho, S.S.; Reis, C.A. Glycosilation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Jan, Y.H.; Juan, Y.H.; Yang, C.J.; Huang, M.S.; Yu, C.J.; Yang, P.C.; Hsiao, M.; Hsu, T.L.; Wong, C.H. Fructosyltransferase 8 as a function regulator of nonsmall cell lung cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 630. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Ma, C.; Qu, J.; Calderon, A.D.; Wu, B.; Wei, N.; Wang, X.; Guo, Y.; Xiao, Z.; et al. Efficient Chemoenzymatic synthesis of an N-glycan isomer library. Chem. Sci. 2015, 6, 5652. [Google Scholar] [CrossRef]
- Brzezicka, K.; Echeverria, B.; Serna, S.; van Diepen, A.; Hokke, C.H.; Reichardt, N.C. Synthesis and microarray-assisted binding studies of core xylose and fucose containing N-glycans. ACS Chem. Biol. 2015, 10, 1290. [Google Scholar] [CrossRef]
- Calderon, A.D.; Liu, Y.; Li, X.; Wang, X.; Chen, X.; Li, L.; Wang, P.G. Substrate specificity of FUT8 and chemoenzymatic synthesis of core-fucosylated asymmetric N-glycans. Org. Biomol. Chem. 2016, 14, 4027. [Google Scholar]
- Tseng, T.H.; Lin, T.W.; Chen, C.Y.; Chen, C.H.; Lin, J.L.; Hsu, T.L.; Wong, C.H. Substrate preference and Interplay of Fructosyltransferase 8 and N-Acetylglucosaminyltransferases. J. Am. Chem. Soc. 2017, 139, 9431. [Google Scholar] [CrossRef] [PubMed]
- Chao Li, C.; Zhu, S.S.; Ma, C.C.; Wang, L.-X. Designer α1,6-Fucosidase Mutants Enable Direct Core Fucosylation of Intact N-Glycopeptides and N-Glycoproteins. J. Am. Chem. Soc. 2017, 139, 15074–15087. [Google Scholar]
- MacKenzie, L.F.; Wang, Q.; Warren, R.A.J.; Withers, S.G. Glycosynthase mediated synthesis of glycosphingolipids J. Am. Chem. Soc. 1998, 120, 5583. [Google Scholar] [CrossRef]
- Armand, S.; Tomita, H.; Heyraud, A.; Gey, C.; Watanabe, T.; Henrissat, B. Stereochemical course of the hydrolysis reaction catalyzed by chitinases Al and D from Bacillus circulans WL-12. FEBS Lett. 1994, 343, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Henrissat, B.; Davies, G. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 1997, 7, 637–644. [Google Scholar] [CrossRef]
- Alsina, C.; Faijes, M.; Planas, A. Glycosynthase-type GH18 mutant chitinases at the assisting catalytic residue for polymerization of chitooligosaccharides. Carbohydr. Res. 2019, 478, 1–9. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Sharma, L.; Dela Cruz, C.S. Chitin and its effects on inflammatory and immune responses Clin. Rev. Allergy Immunol. 2018, 54, 213–223. [Google Scholar] [CrossRef]
- Yoon, H.J.; Moon, M.E.; Park, H.S.; Im, S.Y.; Kim, Y.H. Chitosan oligosaccharide (COS) inhibits LPS-induced inflammatory effects in RAW 264.7 macrophage cells. Biochem. Biophys. Res. Commun. 2007, 358, 954–959. [Google Scholar] [CrossRef]
- Yousef, M.; Pichyangkura, R.; Soodvilai, S.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: Therapeutic efficacy and possible mechanisms of action. Pharmacol. Res. 2012, 66, 66–79. [Google Scholar] [CrossRef]
- Chung, M.J.; Park, J.K.; Park, Y. II. Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells and asthma model mice. Int. Immunopharmacol. 2012, 12, 453–459. [Google Scholar] [CrossRef]
- Shen, K.-T.; Chen, M.-H.; Chan, H.-Y.; Jeng, J.-H.; Wang, Y.-J. Inhibitory effects of chitooligosaccharides on tumor growth and metastasis. Food Chem. Toxicol. 2009, 47, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Chung, M.J.; Choi, H.N.; Park, Y. II. Effects of the molecular weight and the degree of deacetylation of chitosan oligosaccharides on antitumor activity. Int. J. Mol. Sci. 2011, 12, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Hadwiger, L.A. Plant science review: Multiple effects of chitosan on plant systems: Solid science or hype. Plant. Sci. 2013, 208, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, D.; Hemantaranjan, A.; Singh, B.; Bhanu, A.N. A future perspective in crop protection: Chitosan and its oligosaccharides. Adv. Plants Agric. Res. 2014, 1, 4–11. [Google Scholar]
- Azuma, K.; Ifuku, S.; Osaki, T.; Okamoto, Y.; Minami, S. Preparation and biomedical applications of chitin and chitosan nanofibers. J. Biomed. Nanotechnol. 2014, 10, 2891–2920. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Philibert, T.; Lee, B.H.; Fabien, N. Current status and new perspectives on chitin and chitosan as functional biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Martinez, E.A.; Boer, H.; Koivula, A.; Samain, E.; Driguez, H.; Armand, S.; Cottaz, S. Engineering chitinases for the synthesis of chitin oligosaccharides: Catalytic aminoacid mutations convert the GH-18 family glycoside hydrolases into transglycosylases. J. Mol. Catal. B Enzym. 2012, 74, 89–96. [Google Scholar] [CrossRef]
- Alsina, C.; Sancho-Vaello, E.; Aranda-Martínez, A.; Faijes, M.; Planas, A. Auxiliary active site mutations enhance the glycosynthase activity of a GH18 chitinase for polymerization of chitooligosaccharides. Carbohydr. Polym. 2021, 252, 117121. [Google Scholar] [CrossRef] [PubMed]
- Ohnuma, T.; Tanaka, T.; Urasaki, A.; Dozen, S.; Fukamizo, T. A novel method for chemo-enzymatic synthesis of chitin oligosaccharide catalyzed by the mutant of inverting family GH19 chitinase using 4,6-dimethoxy-1,3,5-triazin-2-yl α-chitobioside as a glycosyl donor. J. Biochem. 2019, 165, 497–503. [Google Scholar] [CrossRef] [PubMed]



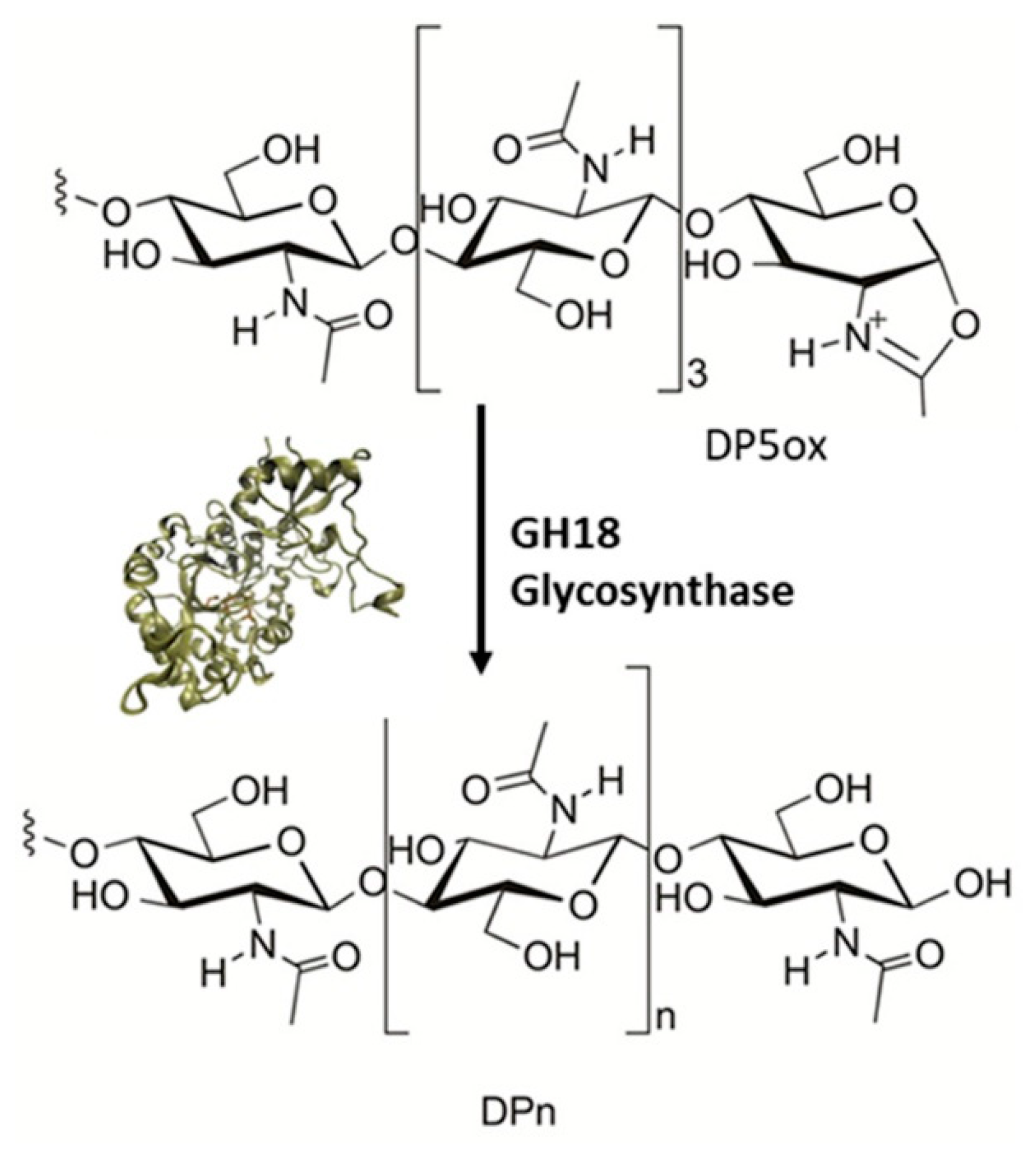
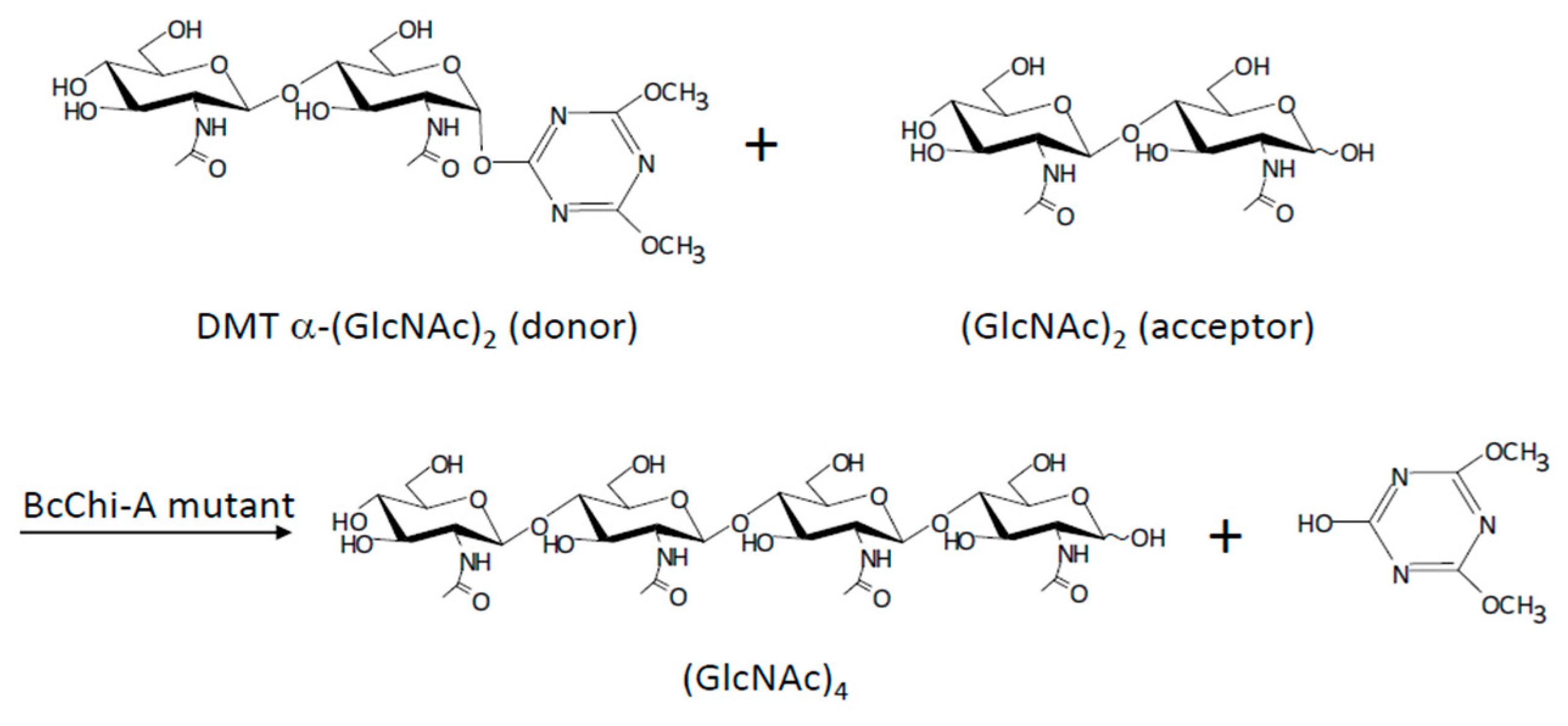
| Enzyme | Glycosyl Acceptor | Glycosyl Donor | Conditions/Products | Yield | Refs. |
|---|---|---|---|---|---|
| cyclodextrin glucanotransferase (CGTase) amylosucrase from Deinococcus geothermalis (DGAS) | isoquercitrin (IQ) | sucrose | For DGAS: 50 mg/mL sucrose, 1 mg/mL of isoquercitrin, in 50 mM Tris-HCl buffer pH-7 at 45 °C, 1 units/mL, 24 h For CGTase: 50 mg/mL of soluble starch, 1 mg/mL of isoquercitrin, in 50 mM citrate phosphate buffer pH7 at 70 °C, 1 units/mL, 24 h Products: IQ-glucoside, IQ-diglucoside and IQ-triglucoside | 97% conversion with DGAS 75% conversion with CGTase | [24] |
| amylosucrase from Deinococcus geothermalis (DGAS) | 15 different flavonoids, various mono-, di- and tri-hydroxyflavones (HFVO) and -hydroxyflavanones (HFVA) | sucrose | 200 mM of sucrose, 50 μM of HFVO or HFVA, 1 unit/mL of DGAS, 50 mM buffer pH 5–8.8 at 45 °C | 100% for HFVAs and HFVOs with 6-hydroxyl group 50–60% for HFVAs and HFVOs with 4′-hydroxyl group | [25] |
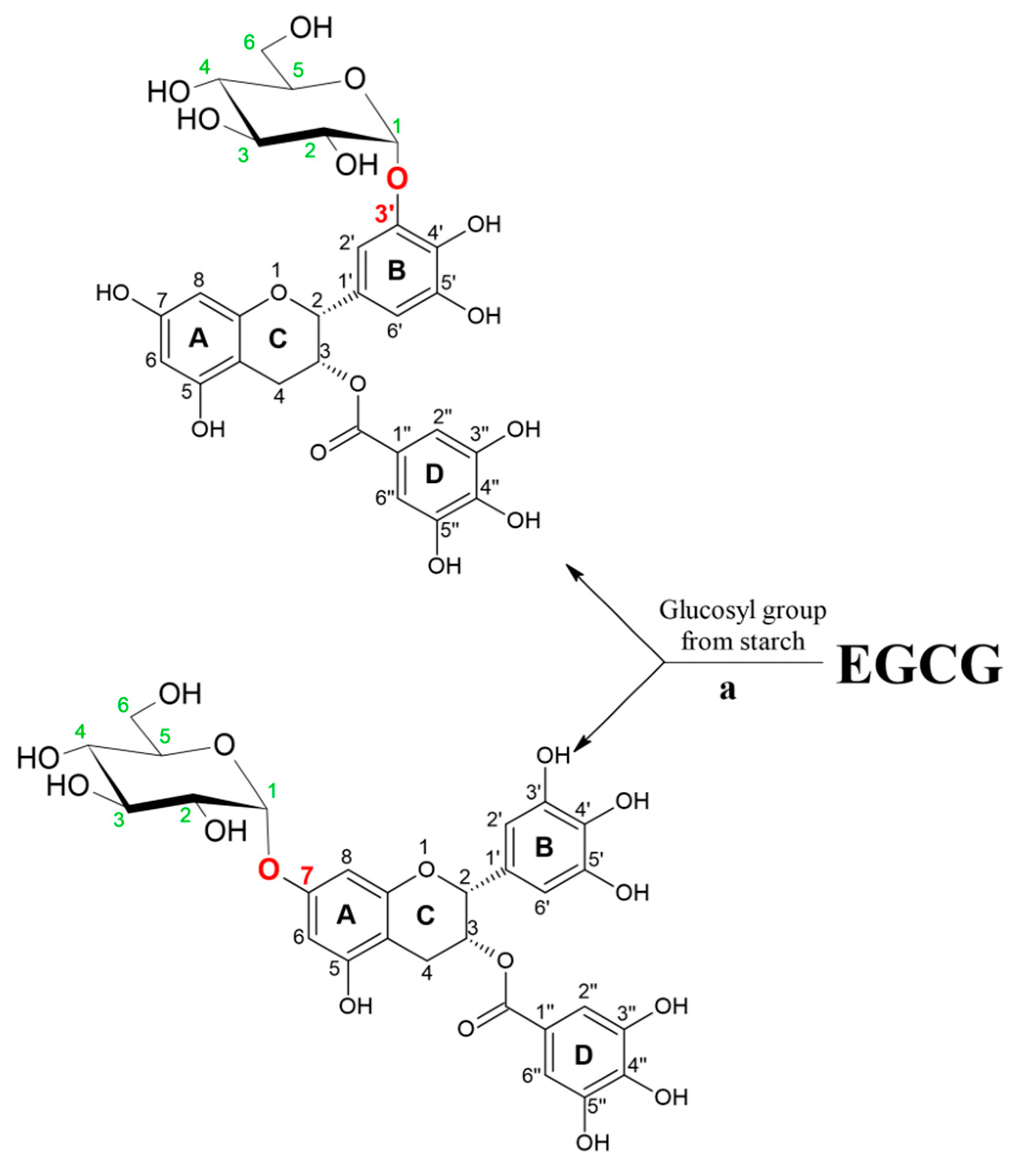
| dextransucrase from Leuconostoc mesenteroides B-1299CB4 expressed in E. coli | epigallocatechin gallate (EGCG) | sucrose | Nine different reaction products were purified | 91.43% conversion of EGCG | [6] |
| cyclodextrin glycosyltransferase from Thermoanaerobacter sp. | epigallocatechin gallate (EGCG) | hydrolyzed potato starch | EGCG (20 mM), hydrolyzed potato starch (20 mg/mL), partially purified Toruzyme 3.0 L (5% v/v), 50 °C, 150 rpm Products: EGCG-3′-O-α-D-glucopyranoside and EGCG-7-O-α-D-glucopyranoside | 58% EGCG 3′-O-α-D-glucopyranoside 13% EGCG 7-O-α-D-glucopyranoside | [26] |
| cyclodextrin glycosyltransferase (CGTase) from Thermoanaerobacter sp. | hesperetin | soluble starch | hesperetin 15 mg/mL, soluble starch 180 mg/mL, CGTase 10% v/v, bis(2-methoxyethyl) ether 30% v/v, 10 mM sodium citrate buffer pH 5.0 60% v/v, 60 °C, 1000 rpm main product: hesperetin-7-O-α-glucopyranosid | 4.1% | [27] |
| Enzyme | Glycosyl Acceptor | Glycosyl Donor | Conditions/ Products | Yield | Refs. |
|---|---|---|---|---|---|
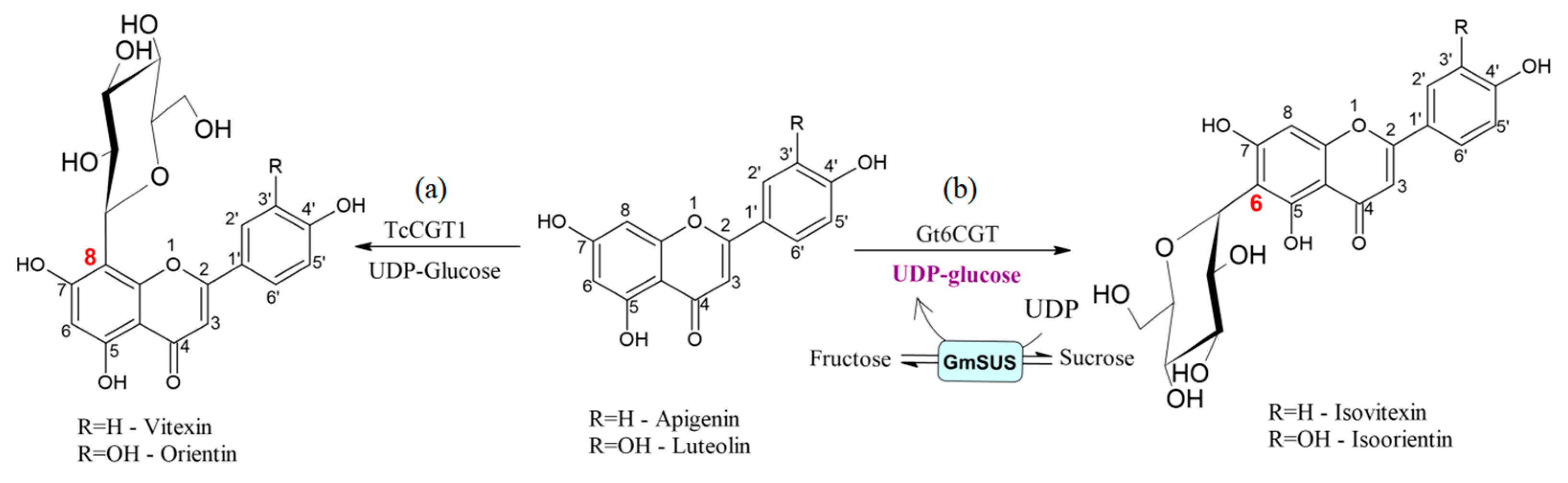
| C-glycosyltransferase TcCGT1 from medicinal plant Trollius chinensis | apigenin, luteolina and 36 more structurally different flavonoids | UDP Glc | 50 mm phosphate buffer pH 8 0.5 mM UDP–Glc, 0.2 mM aglycone, 50 mg purified recombinant TcCGT1, 30 °C for 12 h | 100% conversion of apigenin and luteolin >77% for 12 substrates | [30] |
| C-glucosyltransferase (Gt6CGT) from Gentiana triflora expressed in E. coli BL21 combined with Glycine max sucrose synthase (GmSUS) | luteoilin and apigenin | UDP Glc | 500 mM sucrose, 1.0 mM luteolin, 0.3 mM UDP and 50 mM phosphate buffer pH 7.5, 3% DMSO (v/v), 5 mU/mL Gt6CGT, and 20 mU/mL GmSUS, 48 h at 45 °C products: isoorientin and isovitexin | 94.7% conversion of luteolin 97.1% conversion of apigenin | [31] |
| Enzyme | Glycosyl Acceptor | Glycosyl Donor | Conditions/Products | Yield | Refs. |
|---|---|---|---|---|---|
| GH3 β-glucosidases BGL-2 from Talaromyces amestolkiae | vanillyl alcohol and hydroxyl tyrosol | cellobiose | 4 U of β-glucosidase activity of BGL-2, 350 mM of cellobiose 195 mM of hydroxytyrosol or vanillyl alcohol, 50 mM acetate pH 4 and 0.1% BSA At 50 °C for 5 h for maximum yield 32 mM hydroxytyrosol and vanillyl alcohol for maximum relative conversion of the acceptors | 2.55 g/L for hydroxytyrosol glucoside 3.8 g/L for vanillyl glucoside Maximum conversion 13.8% for hydroxytyrosol glucoside 19% for vanillyl glucoside | [15] |
| β-Galactosidase from Aspergillus oryzae | salicin | lactose | 40 mM of lactose, 110 mM of salicin and enzyme amount of 360 IU | 77% conversion | [33] |
| mutant of sucrose phosphorylase from Thermoanaerobacterium thermosaccharolyticum | phloretin | sucrose | phloretin (5 mg/mL), sucrose (342 mg/mL), MOPS buffer (50 mM, pH 5.6), acetone (10% v/v) and enzyme 5 U/mL, at 48 °C. Products: phloretin 4′-O-α-D-glucopyranoside (Glc-Phoretin-1) and phloretin 4′-O-[α-D-glucopyranosyl-(1→3)-O-α-D-glucopyranoside (Glc-Phoretin-2) | 53% conversion yield of Glc-Phoretin-1 after 12 h 73% conversion yield of Glc-Phoretin-2 after 150 h | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreu, A.; Ćorović, M.; Garcia-Sanz, C.; Santos, A.S.; Milivojević, A.; Ortega-Nieto, C.; Mateo, C.; Bezbradica, D.; Palomo, J.M. Enzymatic Glycosylation Strategies in the Production of Bioactive Compounds. Catalysts 2023, 13, 1359. https://doi.org/10.3390/catal13101359
Andreu A, Ćorović M, Garcia-Sanz C, Santos AS, Milivojević A, Ortega-Nieto C, Mateo C, Bezbradica D, Palomo JM. Enzymatic Glycosylation Strategies in the Production of Bioactive Compounds. Catalysts. 2023; 13(10):1359. https://doi.org/10.3390/catal13101359
Chicago/Turabian StyleAndreu, Alicia, Marija Ćorović, Carla Garcia-Sanz, A. Sofia Santos, Ana Milivojević, Clara Ortega-Nieto, Cesar Mateo, Dejan Bezbradica, and Jose M. Palomo. 2023. "Enzymatic Glycosylation Strategies in the Production of Bioactive Compounds" Catalysts 13, no. 10: 1359. https://doi.org/10.3390/catal13101359
APA StyleAndreu, A., Ćorović, M., Garcia-Sanz, C., Santos, A. S., Milivojević, A., Ortega-Nieto, C., Mateo, C., Bezbradica, D., & Palomo, J. M. (2023). Enzymatic Glycosylation Strategies in the Production of Bioactive Compounds. Catalysts, 13(10), 1359. https://doi.org/10.3390/catal13101359








