1. Introduction
The continuous societal development and industrial modernization has resulted in an increased discharge of industrial wastewater, thereby polluting water bodies and causing water shortages. Water pollution severely restricts economic and societal development [
1,
2,
3,
4,
5]. Sewage is complex because it contains various toxic substances, and is difficult to degrade. If the sewage is discharged without treatment, it can damage the environment and endanger human health. Therefore, developing an efficient, biodegradable, and environment-friendly sewage treatment methods is crucial. To treat the organic compounds industial wastewater, which are difficult to degrade, various chemical, physical, and biological methodsare commonly used [
6,
7]. In particular, attention has been paid to the development of solar-driven catalytic technologies and other emerging processes, such as microwave assisted catalysis, plasma-catalytic processes, and biocatalytic remediation [
8]. Bahnemann et al. used TiO
2 photocatalysis to transform aromatic water pollutants into fuels [
9]. They used a dual-function system of B/N-graphene-coated Cu/TiO
2 as a photocatalyst to convert the organic pollutants into hydrogen for waste recycling [
10]. A comprehensive review of the literature on solving organic pollution problems through Fenton reaction and related process experiments [
11]. These methods generate strong oxidizing free radicals to degrade pollutants. Therefore, the Fenton method, used in advanced wastewater oxidation processes, is effective and environment friendly [
12,
13,
14]. In the Fenton catalytic oxidation water treatment method, the released metal ions catalyze the generation of hydroxyl radicals from H
2O
2 with high activity, low cost, and mild conditions. However, the traditional Fenton reaction has many limitations, including the necessity for acidic environments, formation of iron sludge, and introduction of excess chemicals [
15]. Heterogeneous Fenton catalysis solves the above problems by imparting a wide range of degradable pH values, reduced production of metal sludge, multiple sustainable degradation pathways, and maintenance of high degradation efficiency.
Fenton’s reagents, H
2O
2 and Fe
2+/Fe
3+, are powerful sources of hydroxyl radicals [
16]. The reaction between Fe
2+ and H
2O
2 generates hydroxyl radicals (Equation (1)), and Fe
3+ reacts with H
2O
2 to regenerate Fe
2+, thereby prolonging the Fenton reaction [
17] (Equations (2) and (3)).
Fenton catalysts can be classified as either homogeneous or heterogeneous. In the homogeneous Fenton process, Fe
2+ and H
2O
2 undergo a homogeneous reaction in the solution to produce reactive oxygen species. In the heterogeneous Fenton process, iron or other metal ions are loaded onto carrier substrates as catalysts, and heterogeneous reactions occur on the surface to generate free radicals [
18,
19]. Owing to the drawbacks of homogeneous Fenton catalysis, including the production of a large amount of metal sludge, narrow pH range, and difficult recycling, heterogeneous Fenton catalysis is commonly used to improve the stability, dispersion, and ability of catalysts to adsorb pollutants. Fenton catalyst carriers are usually porous materials with large specific surface areas and high stabilities, such as alumina, activated carbon [
20], diatomaceous earth [
21], and kaolin [
22].
Catalysts supported by multiple metals can achieve synergistic catalysis and improved catalytic efficiency. The active metal is the basis for improving catalyst performance and determining the reaction rate and direction [
23]. Equation (4) describes the concerted copper and ferric reactions that generate the effective catalytic components.
To improve the synergistic catalysis efficiency, researchers have introduced transition metals (including Cu and Mn) and rare earth elements (such as Ce) into iron-based materials, resulting in improved catalytic activities [
24,
25]. The experimental equations for the synergistic catalytic degradation of Fe and Ce are as follows:
The standard oxidation reduction potential of Ce
4+/Ce
3+ and Fe
3+/Fe
2+ is 1.44 and 0.77 V, respectively. Therefore, the electron transfer from Fe
2+ to Ce
4+ is thermodynamically advantageous. The generated Ce
3+ can further react with H
2O
2 to generate ·OH. Therefore, this reaction system can stably provide Fe
2+ and Ce
3+ for H
2O
2 decomposition, generating additional ·OH for methylene blue (MB) oxidation [
26]. Synergistic catalysis involves two metal ions and produces excess ·OH, which efficiently degrades pollutants.
In this study, we used activated carbon (AC) as a carrier and loaded it with Fe, Cu, Mn, and Ce to prepare Fenton catalysts. Further, using MB as a model pollutant, we studied the degradation performance of the Fenton catalysts, and expored their catalytic degradation efficiency at different pH values, H2O2 concentrations, calcination temperatures, and metal impregnation ratios to determine the optimal solution for the degradation of organic pollutants (especially dyes) in water.
4. Conclusions
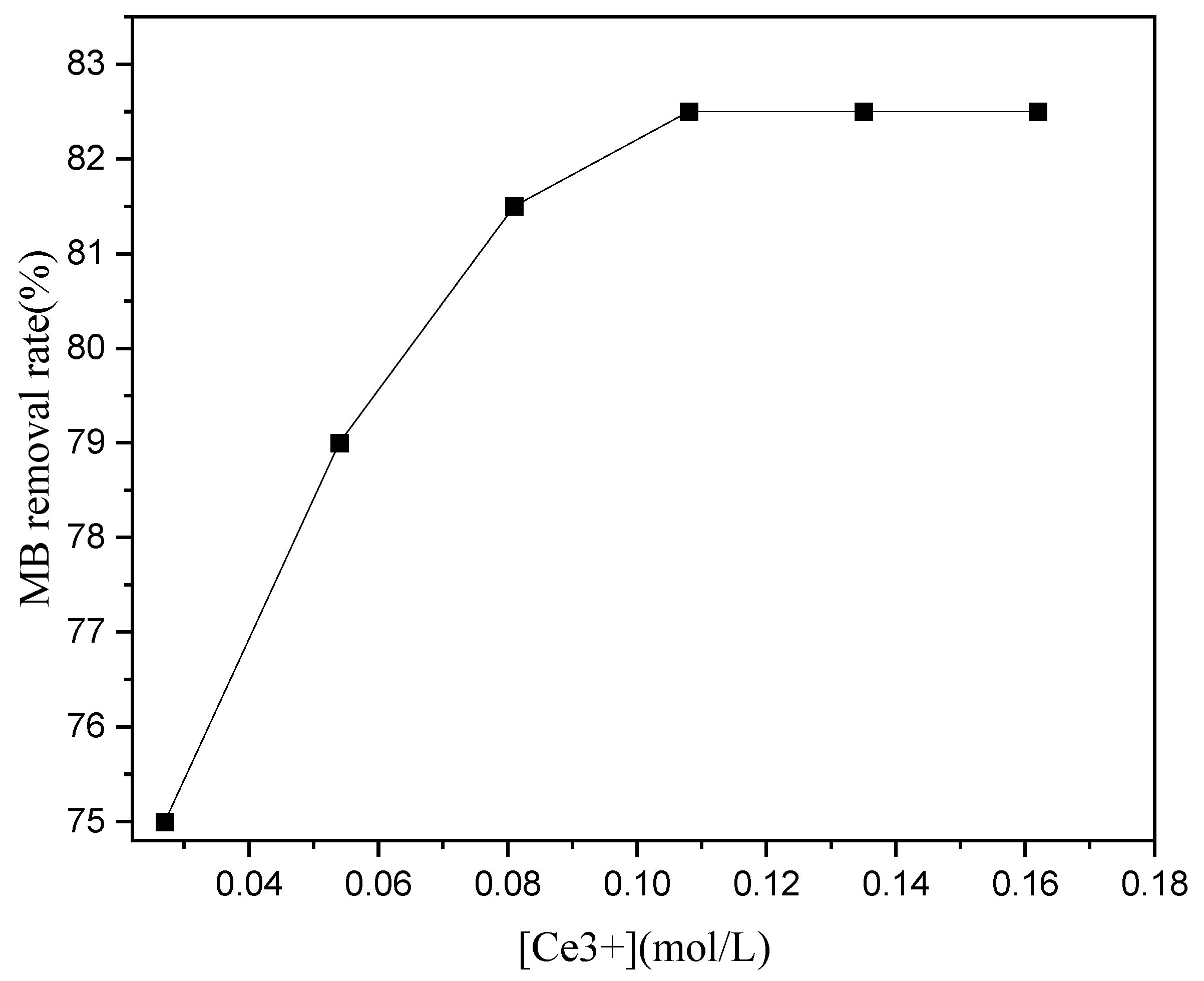
With societal development, water pollution has become a critical problem and has peaked the research interest on sewage treatment. While some of the proposed solutions have high sewage treatment cost or difficult and complex catalyst preparation methods, others have low reuse rates. However, the catalyst preparation method is simple and reusable, has high catalytic efficiency, has a long service life, and is easy to implement for industrial use.
The thermal weight loss of the AC impregnation at different sintering temperatures was studied through TGA/DSC. At low sintering temperatures, the metal salts loaded onto the AC did not fully decompose and could not form metals or alloy particles. However, when the sintering temperature is excessive, the metal or alloy particles of the catalyst fuse into large particles and the catalytic efficiency decreases. XRD, SEM and SEM-EDS analysis showed that the catalyst particles on the AC were mostly alloys, laying the foundation for multi-metal synergistic catalysis. We used XPS to study the activity of the catalyst based on the electron binding energies of the atoms. The EPR analysis has shown that sufficient light is the optimal solution for accelerating the Fenton catalytic degradation. The porous structure of the AC was not affected by catalyst loading and the catalyst particles fully utilized the large specific surface area of the AC carrier. The optimal impregnation solution for preparation of the Fenton heterogeneous catalyst was as follows: 0.45 mol/L Fe3+, 0.72 mol/L Cu2+, 0.16 mol/L Mn2+, and 0.11 mol/L Ce3+. Optimal calcination conditions were 800 or 900 °C for 1 h. The optimal degradation conditions for the catalyst were pH 5, 5 mM H2O2, and 10 g/L catalyst loading.