Enhancing the Catalytic Performance of Platinum-Doped Lanthanum Hexaaluminate through Reduction Method Variation
Abstract
:1. Introduction
2. Experimental Section
2.1. Material and Methods
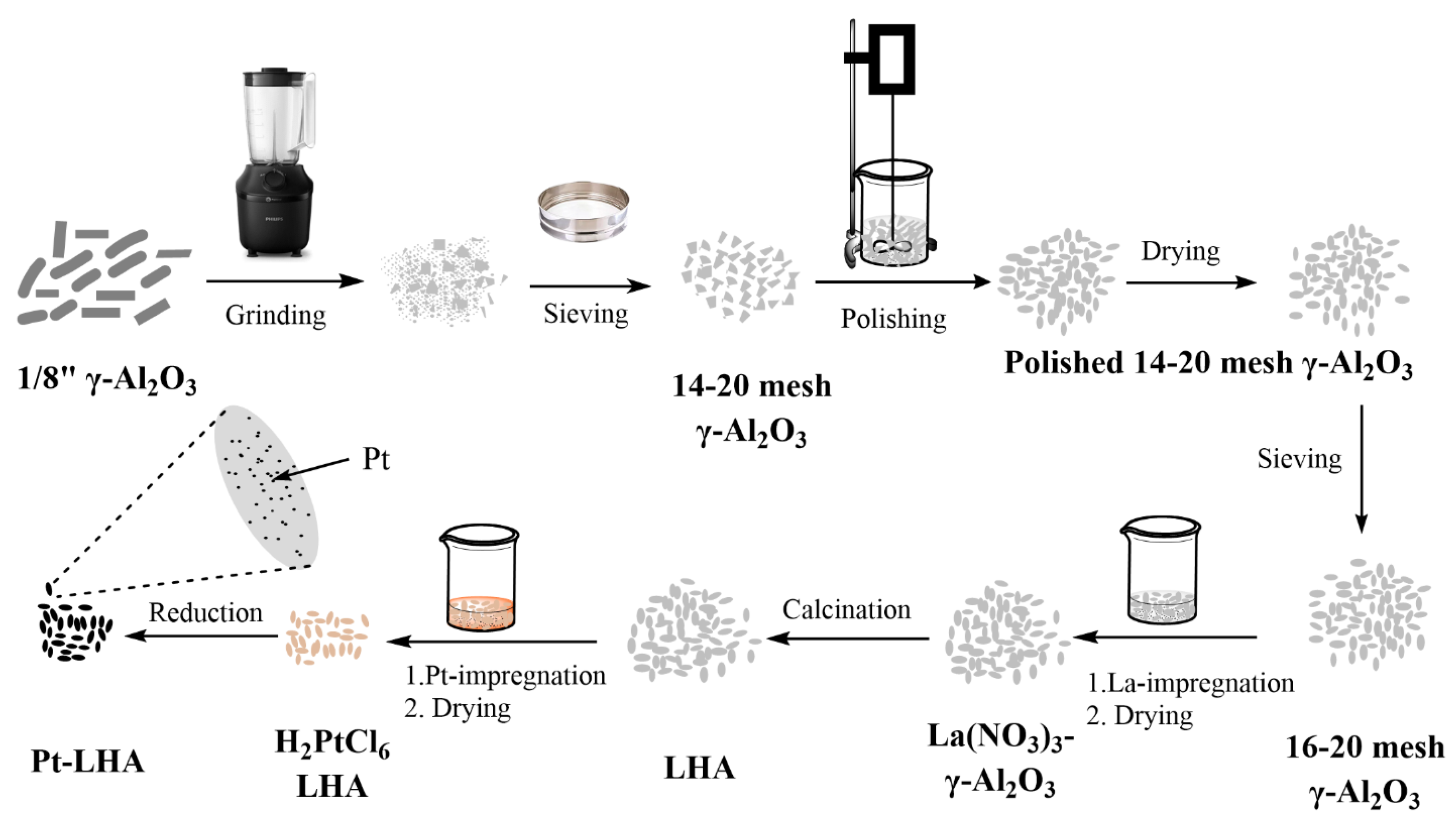
2.2. Catalyst Synthesis
2.2.1. Synthesis of Lanthanum Hexaaluminate (LHA)
2.2.2. Pt Impregnation on LHA
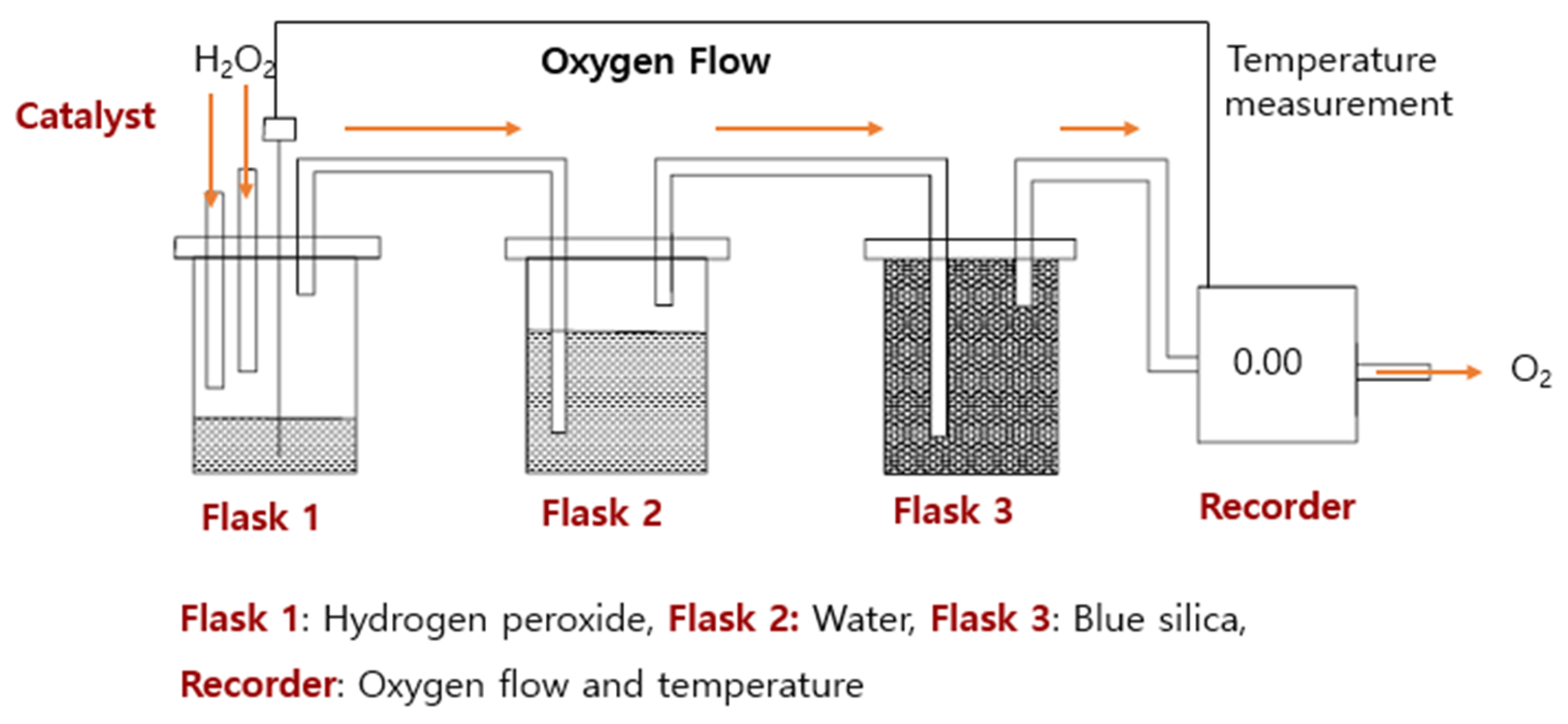
2.3. Catalytic Activity Test Using an H2O2 Reactor
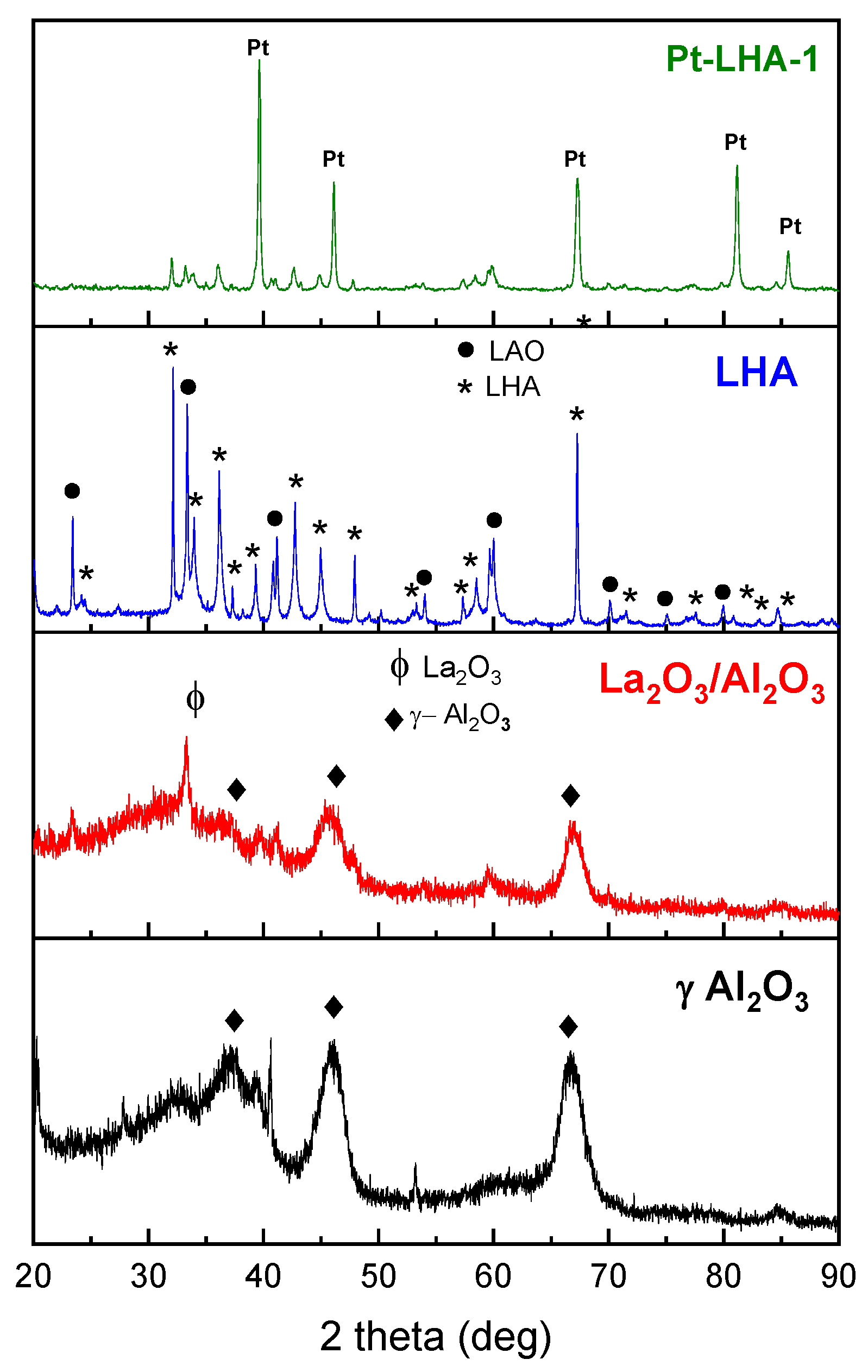
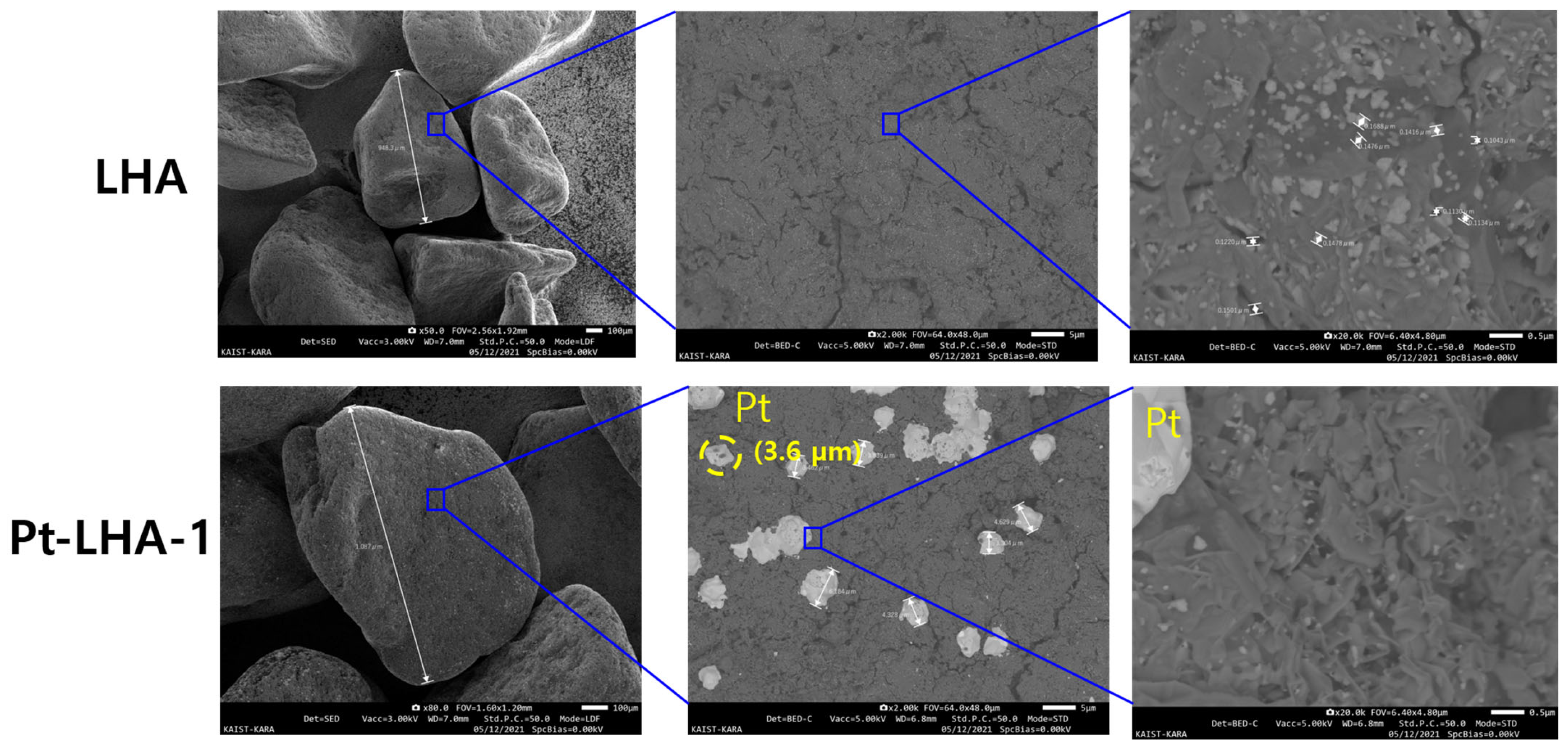
3. Results and Discussion
3.1. Effect of Calcination Temperature Heating Rate
3.1.1. Calcination of Catalyst without Hydrogen Flow
3.1.2. Reduction of Catalyst with Hydrogen Flow
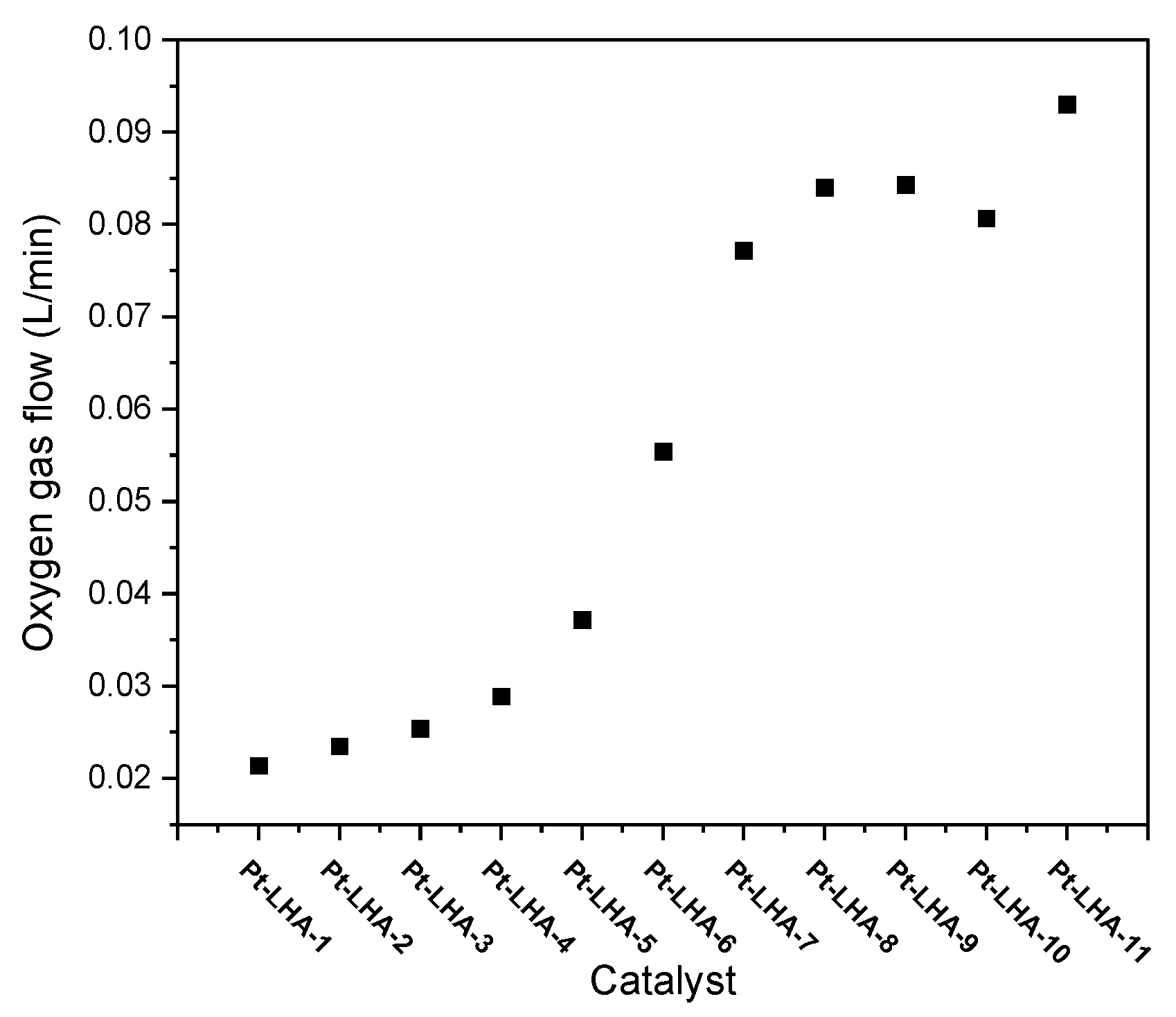
3.2. Catalyst Activity Using an H2O2 Reactor
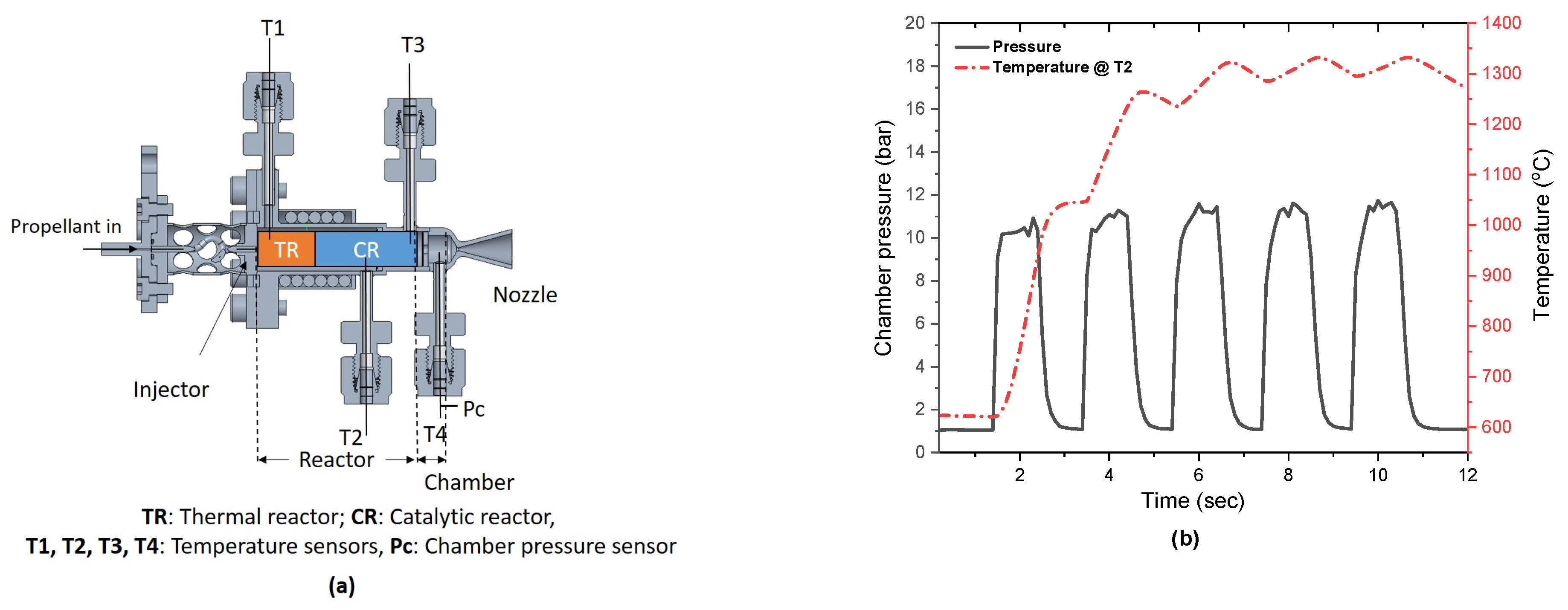
3.3. Hot Fire Test
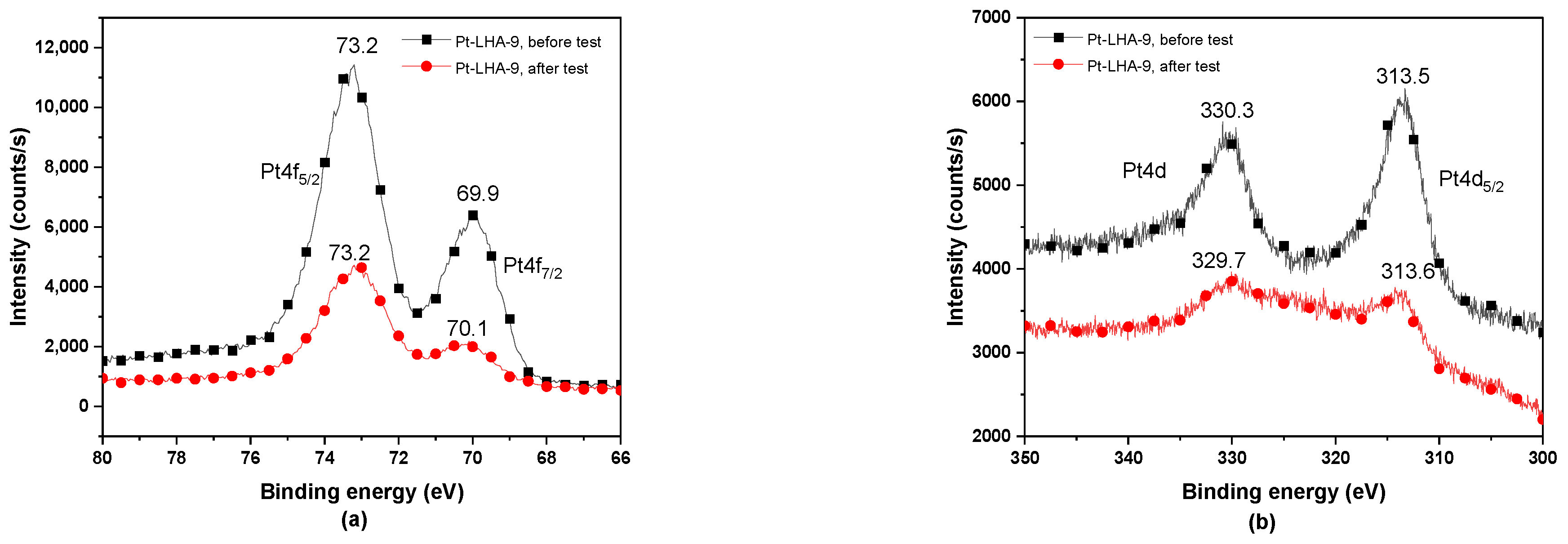
3.4. XPS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torrez-Herrera, J.J.; Korili, S.A.; Gil, A. Progress in the Synthesis and Applications of Hexaaluminate-Based Catalysts. Catal. Rev.—Sci. Eng. 2022, 64, 592–630. [Google Scholar] [CrossRef]
- Svintsitskiy, D.A.; Kibis, L.S.; Stadnichenko, A.I.; Slavinskaya, E.M.; Romanenko, A.V.; Fedorova, E.A.; Stonkus, O.A.; Doronkin, D.E.; Marchuk, V.; Zimina, A.; et al. Insight into the Nature of Active Species of Pt/Al2O3 Catalysts for Low Temperature NH3 Oxidation. ChemCatChem 2020, 12, 867–880. [Google Scholar] [CrossRef]
- Woo, J.; Yoo, D.; Oh, S.; Jeon, J.-K. Decomposition of Energetic Ionic Liquid Over IrCu/Honeycomb Catalysts. J. Nanosci. Nanotechnol. 2020, 20, 7065–7069. [Google Scholar] [CrossRef]
- Yoo, D.; Jeon, J.-K. Decomposition of Eco-Friendly Liquid Propellants over Ruthenium/Al2O3/Metal Foam Catalyst. Clean Technol. 2019, 25, 256–262. [Google Scholar] [CrossRef]
- Cimino, S.; Nigro, R.; Weidmann, U.; Holzner, R. Catalytic Combustion of Methanol over La, Mn-Hexaaluminate Catalysts. Fuel Process. Technol. 2015, 133, 1–7. [Google Scholar] [CrossRef]
- Reddy, V.; Koopmans, R.; Seyidoglu, T.; Vitztum, E.; Hatzenbichler, M.; Plesescu, F.; Matkovits, C.; Happl, M.; Seifert, B.; Bel, F.V.; et al. Vacuum Testing of 3D Printed 1 N Hydrogen Peroxide Thruster and a Novel Catalyst. In Proceedings of the 73rd International Astronautical Congress (IAC), Paris, France, 18–22 September 2022; pp. 1–13. [Google Scholar]
- Kang, S.; Kwon, S. Preparation and Performance Evaluation of Platinum Barium Hexaaluminate Catalyst for Green Propellant Hydroxylamine Nitrate Thrusters. Materials 2021, 14, 2828. [Google Scholar] [CrossRef] [PubMed]
- Maleix, C.; Chabernaud, P.; Brahmi, R.; Beauchet, R.; Batonneau, Y.; Kappenstein, C.; Schwentenwein, M.; Koopmans, R.J.; Schuh, S.; Scharlemann, C. Development of Catalytic Materials for Decomposition of ADN-Based Monopropellants. Acta Astronaut. 2019, 158, 407–415. [Google Scholar] [CrossRef]
- Abdelouahab-Reddam, Z.; Mail, R.E.; Coloma, F.; Sepúlveda-Escribano, A. Platinum Supported on Highly-Dispersed Ceria on Activated Carbon for the Total Oxidation of VOCs. Appl. Catal. A Gen. 2015, 494, 87–94. [Google Scholar] [CrossRef]
- Courtheoux, L.; Gautron, E.; Rossignol, S.; Kappenstein, C. Transformation of Platinum Supported on Silicon-Doped Alumina during the Catalytic Decomposition of Energetic Ionic Liquid. J. Catal. 2005, 232, 10–18. [Google Scholar] [CrossRef]
- Heveling, J. La-Doped Alumina, Lanthanum Aluminate, Lanthanum Hexaaluminate, and Related Compounds: A Review Covering Synthesis, Structure, and Practical Importance. Ind. Eng. Chem. Res. 2023, 62, 2353–2386. [Google Scholar] [CrossRef]
- Ropp, R.C.; Carroll, B. Solid-State Kinetics of LaAl11O18. J. Am. Ceram. Soc. 1980, 63, 416–419. [Google Scholar] [CrossRef]
- Gasperin, M.; Saine, M.C.; Kahn, A.; Laville, F.; Lejus, A.M. Influence of M2+ Ions Substitution on the Structure of Lanthanum Hexaaluminates with Magnetoplumbite Structure. J. Solid State Chem. 1984, 54, 61–69. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, Z.; Wei, Z. Highly Active Ruthenium Catalyst Supported on Barium Hexaaluminate for Ammonia Decomposition to COx-Free Hydrogen. ACS Sustain. Chem. Eng. 2019, 7, 8226–8235. [Google Scholar] [CrossRef]
- Negri, M.; Wilhelm, M.; Hendrich, C.; Wingborg, N.; Gediminas, L.; Adelöw, L.; Maleix, C.; Chabernaud, P.; Brahmi, R.; Beauchet, R.; et al. New Technologies for Ammonium Dinitramide Based Monopropellant Thrusters—The Project RHEFORM. Acta Astronaut. 2018, 143, 105–117. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, X.; Zhao, X.; Yang, J.; Hou, Z.; Bai, L.; Chang, H.; Liu, Y.; Deng, J.; Guo, G.; et al. Preparation, Characterization, and Catalytic Performance of PdPt/3DOM LaMnAl11O19 for the Combustion of Methane. Appl. Catal. A Gen. 2018, 562, 284–293. [Google Scholar] [CrossRef]
- Tian, M.; Wang, X.D.; Zhang, T. Hexaaluminates: A Review of the Structure, Synthesis and Catalytic Performance. Catal. Sci. Technol. 2016, 6, 1984–2004. [Google Scholar] [CrossRef]
- Gugulothu, R.; Macharla, A.K.; Chatragadda, K.; Vargeese, A.A. Catalytic Decomposition Mechanism of Aqueous Ammonium Dinitramide Solution Elucidated by Thermal and Spectroscopic Methods. Thermochim. Acta 2020, 686, 178544. [Google Scholar] [CrossRef]
- Maleix, C.; Chabernaud, P.; Artault, M.; Rivet, Q.; Brahmi, R.; Beauchet, R.; Batonneau, Y.; Kappenstein, C. Development and Activity Assessment of New Catalytic Materials for Decomposition of ADN-Based Monopropellants. In Proceedings of the 53rd AIAA/SAE/ASEE Joint Propulsion Conference, Atlanta, GA, USA, 10–12 July 2017; pp. 1–10. [Google Scholar] [CrossRef]
- Jeon, J.-K.; Heo, S.; Jo, Y.M.; Kim, T. A Study of Catalysts for Decomposition of ADN-Based Liquid Monopropellant. In Proceedings of the 2017 KSPE Spring Conference, Jeju, Republic of Korea, 31 May–2 June 2017; pp. 412–415.
- Nishio, Y.; Ozawa, M. Microstructure of La-Modified Al2O3 Support with LaAlO3. J. Ceram. Soc. Japan 2008, 116, 1295–1298. [Google Scholar] [CrossRef]
- Schweizer, A.E.; Kerr, G.T. Thermal Decomposition of Hexachloroplatinic Acid. Inorg. Chem. 1978, 17, 2326–2627. [Google Scholar] [CrossRef]
- Barbier, J.; Bahloul, D.; Marecot, P. Reduction of Pt/Al2O3 Catalysts: Effect of Hydrogen and of Water and Hydrochloric Acid Vapor on the Accessibility of Platinum. J. Catal. 1992, 137, 377–384. [Google Scholar] [CrossRef]
- Slavinskaya, E.M.; Kibis, L.S.; Stonkus, O.A.; Svintsitskiy, D.A.; Stadnichenko, A.I.; Fedorova, E.A.; Romanenko, A.V.; Marchuk, V.; Doronkin, D.E.; Boronin, A.I. The Effects of Platinum Dispersion and Pt State on Catalytic Properties of Pt/Al2O3 in NH3 Oxidation. ChemCatChem 2021, 13, 313–327. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corporation Physical Electronics Division 6509 Flying Cloud Drive: Eden Prairie, MN, USA, 2005; ISBN 9780470014226. [Google Scholar]
- Kim, K.S.; Winograd, N.; Davis, R.E. Electron Spectroscopy of Platinum-Oxygen Surfaces and Application to Electrochemical Studies. J. Am. Chem. Soc. 1971, 93, 6296–6297. [Google Scholar] [CrossRef]

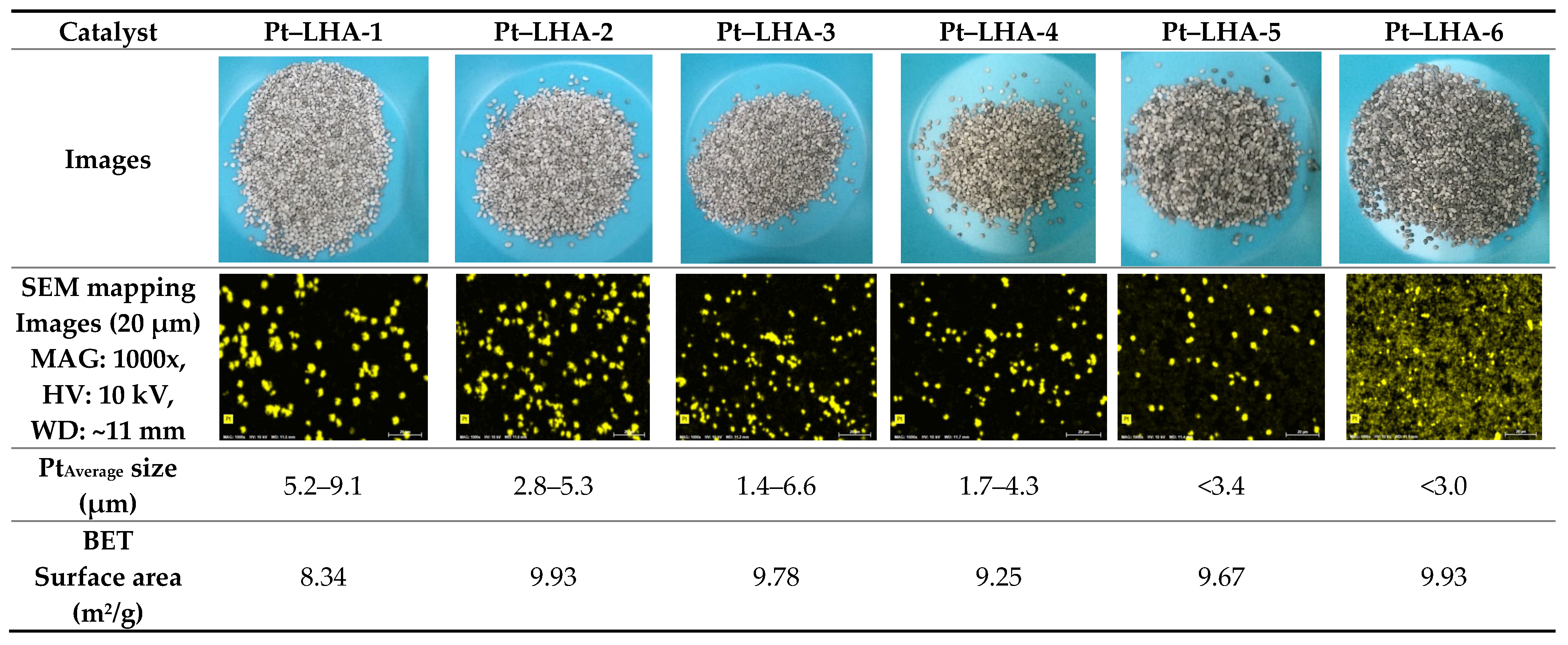


| Catalyst | Weight before Calcination, g | Calcination Temperature, °C | Heating Rate, °C/min | Gas Flow, (0.2 L/min) | Weight after Calcination, g | Weight Loss (%) |
|---|---|---|---|---|---|---|
| Pt–LHA-1 | 3.02 | 600 | 5 | - | 2.53 | 16.05 |
| Pt–LHA-2 | 3.10 | 600 | 4 | - | 2.61 | 15.84 |
| Pt–LHA-3 | 3.21 | 600 | 3 | - | 2.71 | 45.39 |
| Pt–LHA-4 | 3.28 | 600 | 2 | - | 2.79 | 15.06 |
| Pt–LHA-5 | 3.64 | 600 | 1 | - | 3.11 | 14.55 |
| Pt–LHA-6 | 3.15 | 600 | 0.5 | - | 2.76 | 13.84 |
| Pt–LHA-7 | 2.29 | 600 | 5 | 4% H2 + 96% N2 | 1.93 | 15.74 |
| Pt–LHA-8 | 2.29 | 600 | 4 | 4% H2 + 96% N2 | 1.94 | 15.31 |
| Pt–LHA-9 | 2.94 | 600 | 3 | 4% H2 + 96% N2 | 2.49 | 15.10 |
| Pt–LHA-10 | 2.69 | 600 | 2 | 4% H2 + 96% N2 | 2.27 | 15.51 |
| Pt–LHA-11 | 2.40 | 600 | 1 | 4% H2 + 96% N2 | 2.034 | 15.34 |
| Catalyst | Catalyst Weight Taken (g) | Weight of 69.42 wt% H2O2 (g) | Concentration of H2O2 after Reaction (wt%) |
|---|---|---|---|
| Pt–LHA-1 | 0.0201 | 50.6 | 68.46 |
| Pt–LHA-2 | 0.0200 | 50.7 | 68.21 |
| Pt–LHA-3 | 0.0200 | 50.3 | 67.98 |
| Pt–LHA-4 | 0.0201 | 50.6 | 67.96 |
| Pt–LHA-5 | 0.0202 | 50.6 | 67.72 |
| Pt–LHA-6 | 0.0199 | 50.3 | 66.33 |
| Pt–LHA-7 | 0.0200 | 50.13 | 65.15 |
| Pt–LHA-8 | 0.0201 | 50.16 | 65.53 |
| Pt–LHA-9 | 0.0199 | 50.42 | 65.40 |
| Pt–LHA-10 | 0.0200 | 50.09 | 64.20 |
| Pt–LHA-11 | 0.0199 | 50.65 | 63.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhosale, V.K.; Yoon, W.; Yoon, H. Enhancing the Catalytic Performance of Platinum-Doped Lanthanum Hexaaluminate through Reduction Method Variation. Catalysts 2023, 13, 1413. https://doi.org/10.3390/catal13111413
Bhosale VK, Yoon W, Yoon H. Enhancing the Catalytic Performance of Platinum-Doped Lanthanum Hexaaluminate through Reduction Method Variation. Catalysts. 2023; 13(11):1413. https://doi.org/10.3390/catal13111413
Chicago/Turabian StyleBhosale, Vikas Khandu, Wonjae Yoon, and Hosung Yoon. 2023. "Enhancing the Catalytic Performance of Platinum-Doped Lanthanum Hexaaluminate through Reduction Method Variation" Catalysts 13, no. 11: 1413. https://doi.org/10.3390/catal13111413





