Promoter Effect on Carbon Nanosphere-Encapsulated Fe-Co Catalysts for Converting CO2 to Light Olefins
Abstract
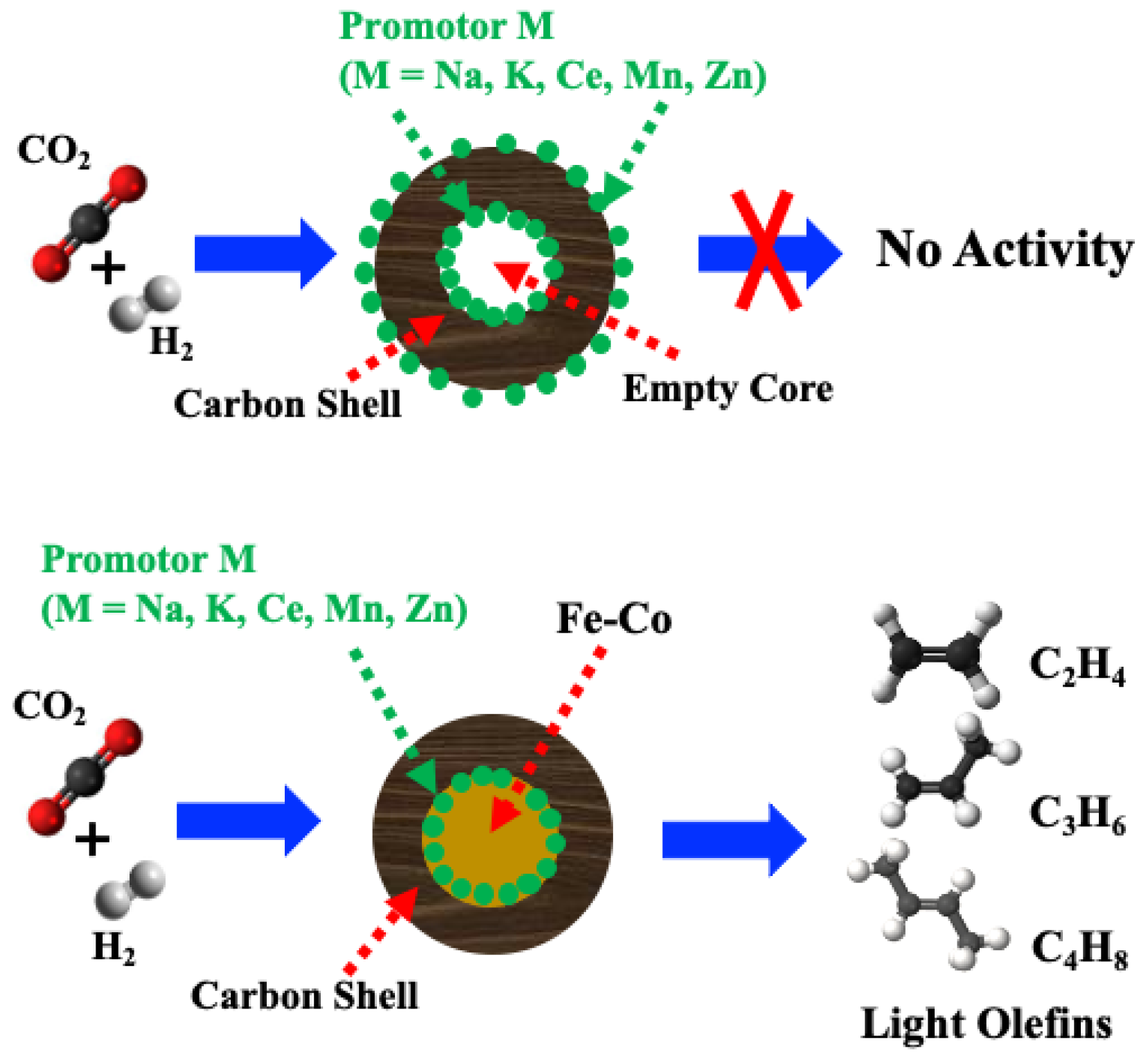
:1. Introduction
2. Results and Discussion
2.1. Diffusion Process Used to Synthesize Promoted M-CNS-FeCo (M = Ce, Na, K, Mn, Zn)
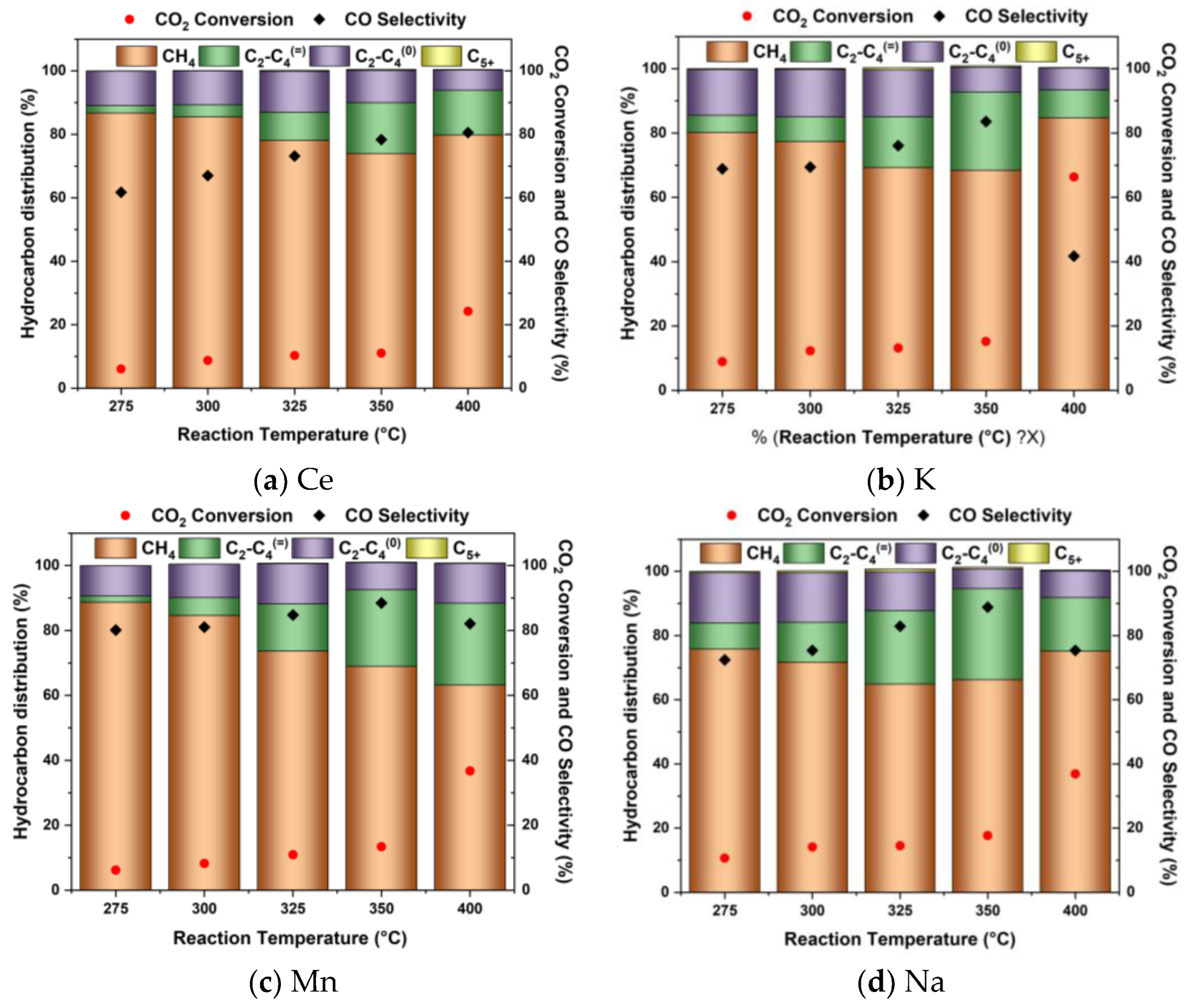
2.2. Catalytic Performance of Promoted M-CNS-FeCo (M = Ce, Na, K, Mn, Zn)
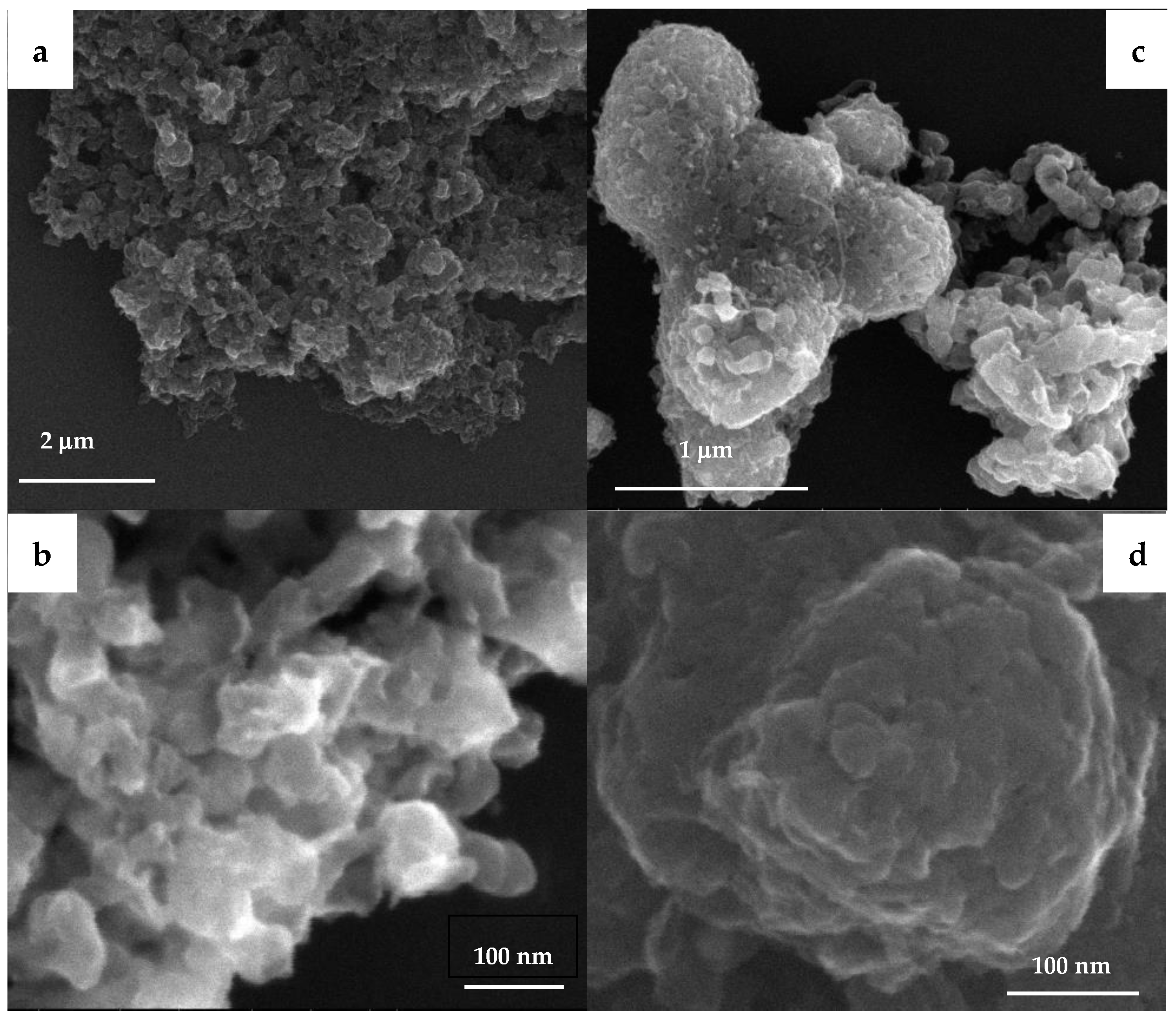
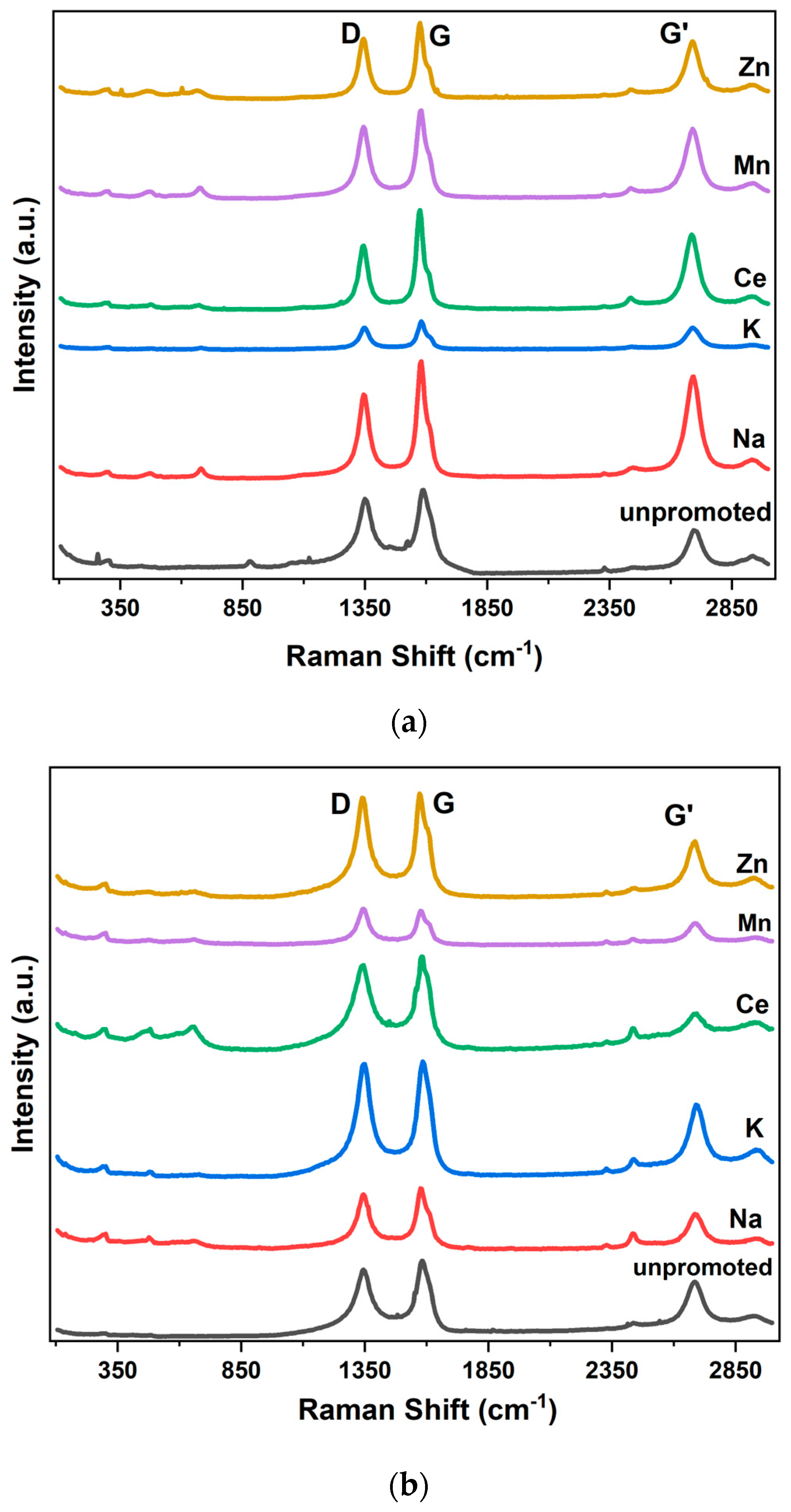
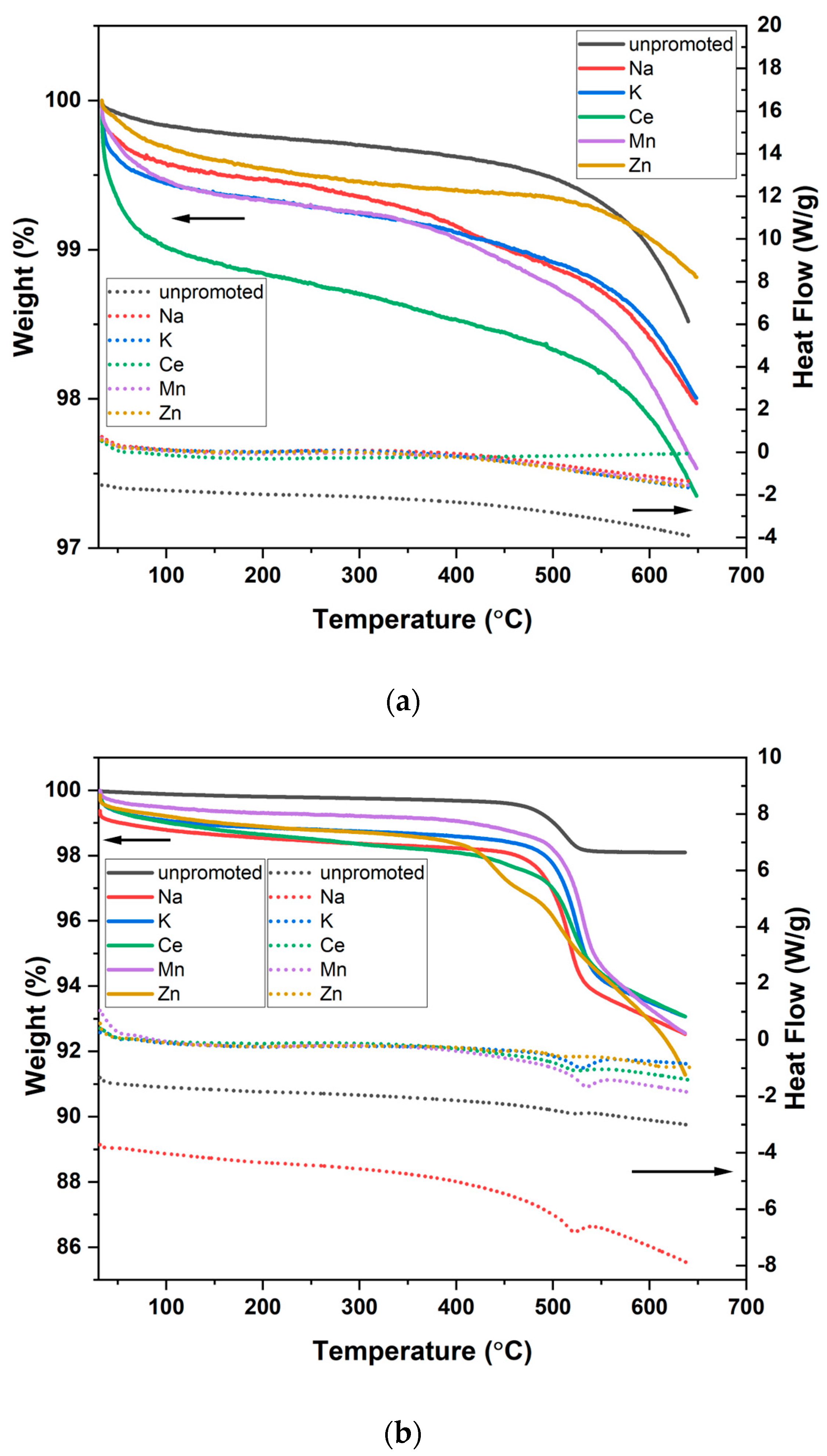
2.3. Physicochemical Properties of Promoted M-CNS-FeCo (M = Ce, Na, K, Mn, Zn)
2.4. Mechanistic Insights of Promoted M-CNS-FeCo for CO2 Hydrogenation
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Promoted M-CNS-FeCo (M = Ce, Na, K, Mn, Zn)
- (1)
- Synthesis of CNS-FeCo
- (2)
- Synthesis of M-CNS-FeCo (M = Ce, Na, K, Mn, Zn)
3.3. Catalyst Evaluation
3.4. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Zhang, A.; Jiang, X.; Zhu, J.; Liu, J.; Li, J.; Zhang, G.; Song, C.; Guo, X. Utilization of CO2 for aromatics production over ZnO/ZrO2-ZSM-5 tandem catalyst. J. CO2 Util. 2019, 29, 140–145. [Google Scholar] [CrossRef]
- Science Daily. Available online: https://www.sciencedaily.com/releases/2018/11/181108130533.htm (accessed on 15 June 2023).
- Ma, Z.; Porosoff, M. Development of tandem catalysts for CO2 hydrogenation to olefins. ACS Catal. 2019, 9, 2639–2656. [Google Scholar] [CrossRef]
- Li, W.; Zhang, G.; Jiang, X.; Liu, Y.; Zhu, J.; Ding, F.; Liu, Z.; Guo, X.; Song, C. CO2 hydrogenation on un-promoted and M-promoted Co/TiO2 catalysts (M = Zr, K, Cs): Effects of crystal phase of supports and metal–support interaction on tuning product distribution. ACS Catal. 2019, 9, 2739–2751. [Google Scholar] [CrossRef]
- Wang, D.; Xie, Z.; Porosoff, M.D.; Chen, J. Recent advances in carbon dioxide hydrogenation to produce olefins and aromatics. Chem 2021, 7, 2277–2311. [Google Scholar] [CrossRef]
- Wei, J.; Yao, R.W.; Ge, Q.J.; Wen, Z.Y.; Ji, X.W.; Fang, C.Y.; Zhang, J.X.; Xu, H.Y.; Sun, J. Catalytic hydrogenation of CO2 to isoparaffins over Fe-based multifunctional catalysts. ACS Catal. 2018, 8, 9958–9967. [Google Scholar] [CrossRef]
- Wei, J.; Ge, Q.; Yao, R.; Wen, Z.; Fang, C.; Guo, L.; Xu, H.; Sun, J. Directly converting CO2 into a gasoline fuel. Nat. Commun. 2018, 8, 15174. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Qu, Y.; Liu, H.; Tang, C.; Miao, S.; Feng, Z.; An, H.; Li, C. Highly selective conversion of carbon dioxide to lower olefins. ACS Catal. 2017, 7, 8544–8548. [Google Scholar] [CrossRef]
- Li, Z.L.; Qu, Y.Z.; Wang, J.J.; Liu, H.L.; Li, M.R.; Miao, S.; Li, C. Highly selective conversion of carbon dioxide to aromatics over tandem catalysts. Joule 2019, 3, 570–583. [Google Scholar] [CrossRef]
- Liu, J.H.; Zhang, A.F.; Jiang, X.; Liu, M.; Zhu, J.; Song, C.S.; Guo, X.W. Direct transformation of carbon dioxide to value-added hydrocarbons by physical mixtures of Fe5C2 and K-modified Al2O3. Ind. Eng. Chem. Res. 2018, 57, 9120–9126. [Google Scholar] [CrossRef]
- Xie, C.L.; Chen, C.; Yu, Y.; Su, J.; Li, Y.F.; Somorjai, G.A.; Yang, P.D. Tandem catalysis for CO2 hydrogenation to C2–C4 hydrocarbons. Nano Lett. 2017, 17, 3798–3802. [Google Scholar] [CrossRef]
- Liu, M.; Yi, Y.H.; Wang, L.; Guo, H.C.; Bogaerts, A. Hydrogenation of carbon dioxide to value-added chemicals by heterogeneous catalysis and plasma catalysis. Catalysts 2019, 9, 275. [Google Scholar] [CrossRef]
- Dang, S.S.; Gao, P.; Liu, Z.Y.; Chen, X.Q.; Yang, C.G.; Wang, H.; Zhong, L.S.; Li, S.G.; Sun, Y.H. Role of zirconium in direct CO2 hydrogenation to lower olefins on oxide/zeolite bifunctional catalysts. J. Catal. 2018, 364, 382–393. [Google Scholar] [CrossRef]
- Cordero-Lanzac, T.; Ramirez, A.; Navajas, A.; Gevers, L.; Brunialti, S.; Gandía, L.M.; Aguayo, A.T.; Sarathy, S.M.; Gascon, J. A techno-economic and life cycle assessment for the production of green methanol from CO2: Catalyst and process bottlenecks. J. Energy Chem. 2022, 68, 255–266. [Google Scholar] [CrossRef]
- Ateka, A.; Rodriguez-Vega, P.; Ereña, J.; Aguayo, A.T.; Bilbao, J. Kinetic modeling and reactor design of the direct synthesis of dimethyl ether for CO2 valorization. A review. Fuel 2022, 327, 125148. [Google Scholar] [CrossRef]
- Ateka, A.; Rodriguez-Vega, P.; Ereña, J.; Aguayo, A.T.; Bilbao, J. A review on the valorization of CO2. Focusing on the thermodynamics and catalyst design studies of the direct synthesis of dimethyl ether. Fuel Process Technol. 2022, 233, 107310. [Google Scholar] [CrossRef]
- Gao, P.; Dang, S.S.; Li, S.G.; Bu, X.N.; Liu, Z.Y.; Qiu, M.H.; Yang, C.G.; Wang, H.; Zhong, L.S.; Han, Y.; et al. Direct production of lower olefins from CO2 conversion via bifunctional catalysis. ACS Catal. 2018, 8, 571–578. [Google Scholar] [CrossRef]
- Saeidi, S.; Najari, S.; Hessel, V.; Wilson, K.; Keil, F.J.; Concepción, P.; Suib, S.L.; Rodrigues, A.E. Recent advances in CO2 hydrogenation to value-added products-Current challenges and future directions. Prog. Energy Combust. Sci. 2021, 85, 100905. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Jacobs, G.; Hamdeh, H.H.; Shafer, W.D.; Liu, F.; Hopps, S.D.; Thomas, G.A.; Davis, B.H. Hydrogenation of carbon dioxide over Co–Fe bimetallic catalysts. ACS Catal. 2016, 6, 913–927. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, Y.; Zheng, X.C.; Qi, L.; Li, Y.L. Loaded Cu-Er metal iso-atoms on graphdiyne for artificial photosynthesis. Mater. Today 2023, 66, 72–83. [Google Scholar] [CrossRef]
- Zheng, Z.Q.; Qi, L.; Gao, Y.Q.; Luan, X.Y.; Xue, Y.R.; He, F.; Li, Y.L. Ir0/graphdiyne atomic interface for selective epoxidation. Natl. Sci. Rev. 2023, 10, nwad156. [Google Scholar] [CrossRef]
- Cui, T.; Dong, J.H.; Pan, X.L.; Yu, T.; Fu, Q.; Bao, X.H. Enhanced hydrogen evolution reaction over molybdenum carbide nanoparticles confined inside single-walled carbon nanotubes. J. Energy Chem. 2019, 28, 123–127. [Google Scholar] [CrossRef]
- Xiao, J.P.; Pan, X.L.; Guo, S.J.; Ren, P.J.; Bao, X.H. Toward Fundamentals of Confined Catalysis in Carbon Nanotubes. J. Am. Chem. Soc. 2015, 137, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhang, Z.Y.; Hu, P.; Xue, N.H.; Peng, L.M.; Guo, X.F.; Lin, M.; Ding, W.P. Platinum Nanoparticles Encapsulated in MFI Zeolite Crystals by a Two-Step Dry Gel Conversion Method as a Highly Selective Hydrogenation Catalyst. ACS Catal. 2015, 5, 6893–6901. [Google Scholar] [CrossRef]
- Yang, G.H.; Kawata, H.; Lin, Q.H.; Wang, J.Y.; Jin, Y.Z.; Zeng, C.Y.; Yoneyama, Y.; Tsubaki, N. Oriented synthesis of target products in liquid-phase tandem reaction over a tripartite zeolite capsule catalyst. Chem. Sci. 2013, 4, 3958. [Google Scholar] [CrossRef]
- Jiang, K.; Siahrostami, S.; Zheng, T.T.; Hu, Y.F.; Hwang, S.; Stavitski, E.; Peng, Y.; Dynes, J.; Gangisetty, M.; Su, D.; et al. Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction. Energy Environ. Sci. 2018, 11, 893. [Google Scholar] [CrossRef]
- Zhan, G.; Zeng, H.C. ZIF-67-derived nanoreactors for controlling product selectivity in CO2 hydrogenation. ACS Catal. 2017, 7, 7509–7519. [Google Scholar] [CrossRef]
- Li, S.W.; Tuel, A.; Meunier, F.; Aouine, M.; Farrusseng, D. Platinum nanoparticles entrapped in zeolite nanoshells as active and sintering-resistant arene hydrogenation catalysts. J. Catal. 2015, 332, 25–30. [Google Scholar] [CrossRef]
- Wang, T.S.; Gao, L.J.; Hou, J.W.; Herou, S.J.A.; Griffiths, J.T.; Li, W.W.; Dong, J.H.; Gao, S.; Titirici, M.; Kumar, R.V.; et al. Rational approach to guest confinement inside MOF cavities for low-temperature catalysis. Nat. Commun. 2019, 10, 1340. [Google Scholar] [CrossRef]
- Goel, S.; Wu, Z.J.; Zones, I.; Iglesia, S.I.; Iglesia, E. Synthesis and Catalytic Properties of Metal Clusters Encapsulated within Small-Pore ((SOD, GIS, ANA) Zeolites. J. Am. Chem. Soc. 2012, 134, 17688–17695. [Google Scholar] [CrossRef]
- Wang, X.F.; Cui, Y.Z.; Wang, Y.; Song, X.W.; Yu, J.H. Fabrication and Catalytic Performance of Highly Stable Multifunc-tional Core–Shell Zeolite Composites. Inorg. Chem. 2013, 52, 10708–10710. [Google Scholar] [CrossRef]
- He, J.J.; Liu, Z.L.; Yoneyama, Y.; Nishiyama, N.; Tsubaki, N. Multiple-Functional Capsule Catalysts: A Tailor-Made Confined Reaction Environment for the Direct Synthesis of Middle Isoparaffins from Syngas. Chem. Eur. J. 2006, 12, 8296–8304. [Google Scholar] [CrossRef]
- Farrusseng, D.; Tuel, A. Perspectives on zeolite-encapsulated metal nanoparticles and their applications in catalysis. New J. Chem. 2016, 40, 3933–3949. [Google Scholar] [CrossRef]
- Ren, N.; Yang, Y.H.; Shen, J.; Zhang, Y.H.; Xu, H.L.; Gao, Z.; Tang, Y. Novel, efficient hollow zeolitically microcapsulized noble metal catalysts. J. Catal. 2007, 251, 182–188. [Google Scholar] [CrossRef]
- Weber, D.; He, T.; Wong, M.; Moon, C.; Zhang, A.; Foley, N.; Ramer, N.J.; Zhang, C. Recent Advances in Mitigating Catalyst Deactivation of CO2 Hydrogenation to Light Olefins. Catalysts 2021, 11, 1447. [Google Scholar] [CrossRef]
- Zhang, C.; Bhargava, G.; Elwell, M.D.; Parasher, S.; Zhou, B.; Yates, D.; Knoke, I.; Neitzel, I.; Gogotsi, Y. Hollow graphitic carbon nanospheres: Synthesis and properties. J. Mater. Sci. 2014, 49, 1947–1956. [Google Scholar] [CrossRef]
- Xiang, W.L.; Zhang, Y.P.; Lin, H.F.; Liu, C.J. Nanoparticle/metal–organic framework composites for catalytic applications: Current status and perspective. Molecules 2017, 22, 2103. [Google Scholar] [CrossRef]
- Li, W.H.; Wang, H.Z.; Jiang, X.; Zhu, J.; Liu, Z.M.; Guo, X.W.; Song, C.S. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. RSC Adv. 2018, 8, 7651. [Google Scholar] [CrossRef]
- Yan, Q.G.; Li, J.H.; Zhang, X.F.; Zhang, J.L.; Cai, Z.Y. Mass production of graphene materials from solid carbon sources using a molecular cracking and welding method. J. Mater. Chem. A 2019, 7, 13978–13985. [Google Scholar] [CrossRef]
- Gupta, S.; Jain, V.K.; Jagadeesan, D. Fine tuning the composition and nanostructure of Fe-based core–shell nanocatalyst for efficient CO2 hydrogenation. ChemNanoMat 2016, 2, 989–996. [Google Scholar] [CrossRef]
- Han, Y.Q.; Xu, H.T.; Su, Y.Q.; Xu, Z.L.; Wang, K.F.; Wang, W.Z. Noble metal (Pt, Au@Pd) nanoparticles supported on metal organic framework (MOF-74) nanoshuttles as high-selectivity CO2 conversion catalysts. J. Catal. 2019, 370, 70–78. [Google Scholar] [CrossRef]
- Jiang, Q.; Lan, D.P.; Zhao, G.F.; Xu, H.T.; Gong, X.D.; Liu, J.C.; Shi, Y.; Zhang, L.D.; Fang, H.M.; Cheng, D.H.; et al. Converting CO2 Hydrogenation Products from Paraffins to Olefins: Modification of Zeolite surface properties by a UIO-n membrane. ACS Catal. 2022, 12, 5894–5902. [Google Scholar] [CrossRef]
- Tu, J.L.; Ding, M.Y.; Zhang, Q.; Zhang, Y.L.; Wang, C.G.; Wang, T.J.; Ma, L.L.; Li, X.J. Design of carbon-encapsulated Fe3O4 nanocatalyst with enhanced performance for Fischer–Tropsch synthesis. ChemCatChem 2015, 7, 2323–2327. [Google Scholar] [CrossRef]
- Chen, W.; Fan, Z.; Pan, X.; Bao, X.; Chen, W.; Fan, Z.; Pan, X.; Bao, X. Effect of Confinement in carbon nanotubes on the activity of Fischer–Tropsch iron catalyst. J. Am. Chem. Soc. 2008, 130, 9414–9419. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, J.; Liu, P.; Wang, Y.; Xiao, H.; Yang, Q.; Feng, X.; Zhou, S. Carbon-confined magnesium hydride nano-lamellae for catalytic hydrogenation of carbon dioxide to lower olefins. J. Catal. 2019, 379, 121–128. [Google Scholar] [CrossRef]
- Sánchez-Contador, M.; Ateka, A.; Aguayo, A.T.; Bilbao, J. Direct synthesis of dimethyl ether from CO and CO2 over a core-shell structured CuO-ZnO-ZrO2@SAPO-11 catalyst. Fuel Process. Technol. 2018, 179, 258–268. [Google Scholar] [CrossRef]
- Ateka, A.; Sánchez-Contador, M.; Portillo, A.; Bilbao, J.; Aguayo, A.T. Kinetic modeling of CO2+CO hydrogenation to DME over a CuO-ZnO-ZrO2@SAPO-11 core-shell catalyst. Fuel Process Technol. 2020, 206, 106434. [Google Scholar] [CrossRef]
- Wu, T.; Lin, J.; Cheng, Y.; Tian, J.; Wang, S.; Xie, S.; Pei, Y.; Yan, S.; Qiao, M.; Xu, H.; et al. Porous graphene-conned Fe–K as highly ecient catalyst for CO2 direct hydrogenation to light olens. ACS Appl. Mater. Interfaces 2018, 10, 23439–23443. [Google Scholar] [CrossRef]
- Wei, J.; Sun, J.; Wen, Z.; Fang, C.; Ge, Q.; Xu, H. New insights into the effect of sodium on Fe3O4-based nanocatalysts for CO2 hydrogenation to light olefins. Catal. Sci. Technol. 2016, 6, 4786–4793. [Google Scholar] [CrossRef]
- Wezendonk, T.A.; Sun, X.; Dugulan, A.I.; van Hoof, A.J.F.; Hensen, E.J.M.; Kapteijn, F.; Gascon, J. Controlled formation of iron carbides and their performance in Fischer-Tropsch synthesis. J. Catal. 2018, 362, 106–117. [Google Scholar] [CrossRef]
- Weber, D.; Rui, N.; Zhang, F.; Zhang, H.; Vovchok, D.; Wildy, M.; Arizapana, K.; Saporita, A.; Zhang, J.Z.; Senanayake, S.D.; et al. Carbon Nanosphere-Encapsulated Fe Core–Shell Structures for Catalytic CO2 Hydrogenation. ACS Appl. Nano Mater. 2022, 5, 11605–11616. [Google Scholar] [CrossRef]
- Weber, D.; Zhang, H.; Gandotra, A.; Schossig, J.; Rui, N.; Wildy, M.; Wei, W.Y.; Arizapana, K.; Forte, N.; Zhang, J.Z.; et al. CO2 conversion to value-added chemicals over carbon nano-sphere encapsulated Fe-Co catalysts. ACS Appl. Mater. Interfaces 2023. submitted. [Google Scholar]
- Xu, Q.; Xu, X.; Fan, G.; Yang, L.; Li, F. Unveiling the roles of Fe-Co interactions over ternary spinel-type ZnCoxFe2−xO4 catalysts for highly efficient CO2 hydrogenation to produce light olefins. J. Catal. 2021, 400, 355–366. [Google Scholar] [CrossRef]
- Yuan, F.; Zhang, G.; Zhu, J.; Ding, F.; Zhang, A.; Song, C.; Guo, X. Boosting light olefin selectivity in CO2 hydrogenation by adding Co to Fe catalysts within close proximity. Catal. Today 2021, 371, 142–149. [Google Scholar] [CrossRef]
- Wei, C.; Tu, W.; Jia, L.; Liu, Y.; Lian, H.; Wang, P.; Zhang, Z. The evolutions of carbon and iron species modified by Na and their tuning effect on the hydrogenation of CO2 to olefins. Appl. Surf. Sci. 2020, 525, 146622. [Google Scholar] [CrossRef]
- Yang, S.; Chun, H.; Lee, S.; Han, S.J.; Lee, K.; Kim, Y.T. Comparative study of olefin production from CO and CO2 Using Na-and K-promoted zinc ferrite. ACS Catal. 2020, 10, 10742–10759. [Google Scholar] [CrossRef]
- Liang, B.; Duan, H.; Sun, T.; Ma, T.; Liu, X.; Xu, J.; Su, X.; Huang, Y.; Zhang, T. Effect of Na promoter on Fe-based catalyst for CO2 hydrogenation to alkenes. ACS Sustain. Chem. Eng. 2019, 7, 925–932. [Google Scholar] [CrossRef]
- Ramirez, A.; Gevers, L.; Bavykina, A.; Ould-Chikh, S.; Gascon, J. Metal organic framework-derived iron catalysts for the direct hydrogenation of CO2 to short chain olefins. ACS Catal. 2018, 8, 9174–9182. [Google Scholar] [CrossRef]
- Numpilai, T.; Chanlek, N.; Poo-Arporn, Y.; Cheng, C.K.; Siri-Nguan, N.; Sornchamni, T.; Chareonpanich, M.; Kongkachuichay, P.; Yigit, N.; Rupprechter, G.; et al. Tuning interactions of surface-adsorbed species over Fe-Co/K-Al2O3 catalyst by different K content: Selective CO2 hydrogenation to light olefins. ChemCatChem 2020, 12, 3306–3320. [Google Scholar] [CrossRef]
- Boreriboon, N.; Jiang, X.; Song, C.; Prasassarakich, P. Fe-based bimetallic catalysts supported on TiO2 for selective CO2 hydrogenation to hydrocarbons. J. CO2 Util. 2018, 25, 330–337. [Google Scholar] [CrossRef]
- Jiang, J.; Wen, C.; Tian, Z.; Wang, Y.; Zhai, Y.; Chen, L.; Li, Y.; Liu, Q.; Wang, C.; Ma, L. Manganese-promoted Fe3O4 microsphere for efficient conversion of CO2 to light olefins. Ind. Eng. Chem. Res. 2020, 59, 2155–2162. [Google Scholar] [CrossRef]
- Liang, B.; Sun, T.; Ma, J.; Duan, H.; Li, L.; Yang, X.; Zhang, Y.; Su, X.; Huang, Y.; Zhang, T. Mn decorated Na/Fe catalysts for CO2 hydrogenation to light olefins. Catal. Sci. Technol. 2019, 9, 456–464. [Google Scholar] [CrossRef]
- Zhang, J.; Su, X.; Wang, X.; Ma, Q.; Fan, S.; Zhao, T. Promotion effects of Ce added Fe–Zr–K on CO2 hydrogenation to light olefins. React. Kinet. Mech. Catal. 2018, 124, 575–585. [Google Scholar] [CrossRef]
- Chaipraditgul, N.; Numpilai, T.; Cheng, C.K.; Siri-Nguan, N.; Sornchamni, T.; Wattanakit, C.; Limtrakul, J.; Witoon, T. Tuning interaction of surface-adsorbed species over Fe/K-Al2O3 modified with transition metals (Cu, Mn, V, Zn or Co) on light olefins production from CO2 hydrogenation. Fuel 2021, 283, 119248. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Jacobs, G.; Hamdeh, H.H.; Shafer, W.D.; Liu, F.; Hopps, S.D.; Thomas, G.A.; Davis, B.H. Hydrogenation of carbon dioxide over iron carbide prepared from alkali metal promoted iron oxalate. Appl. Catal. A Gen. 2018, 564, 243–249. [Google Scholar] [CrossRef]
- Guo, L.; Sun, J.; Ji, X.; Wei, J.; Wen, Z.; Yao, R.; Xu, H.; Ge, Q. Directly converting carbon dioxide to linear α-olefins on bio-promoted catalysts. Commun. Chem. 2018, 1, 11. [Google Scholar] [CrossRef]
- Witoon, T.; Chaipraditgul, N.; Numpilai, T.; Lapkeatseree, V.; Ayodele, B.; Cheng, C.; Siri-Nguan, N.; Sorn-chamni, T.; Limtrakul, J. Highly active Fe-Co-Zn/K-Al2O3 catalysts for CO2 hydrogenation to light olefins. Chem. Eng. Sci. 2021, 233, 116428. [Google Scholar] [CrossRef]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, W.; Ho, D.L.; Winey, K.I.; Fischer, J.E.; Glinka, C.J.; Hobbie, E.K. Dispersing single-walled carbon nanotubes with surfactants: A small angle neutron scattering study. Nano. Lett. 2004, 4, 1789–1793. [Google Scholar] [CrossRef]
- Vix-Guterl, C.; Couzi, M.; Dentzer, J.; Trinquecoste, M.; Delhaes, P. Surface Characterizations of Carbon Multiwall Nanotubes: Comparison between Surface Active Sites and Raman Spectroscopy. J. Phys. Chem. B 2004, 108, 19361–19367. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, C.; Zhang, Y.; Liu, X.; Xu, J.; Zhu, M.; Tu, W.; Han, Y. Unraveling the role of zinc on bimetallic Fe5C2–ZnO catalysts for highly selective carbon dioxide hydrogenation to high carbon α-olefins. ACS Catal. 2021, 11, 2121–2133. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, C.; Balee, R. Polymeric Materials Incorporating Carbon Nanosphere Nanostructures. U.S. Patent 7935276B2, 9 December 2010. [Google Scholar]
- Zhang, C.; Fransson, M.; Liu, C.K.; Zhou, B. Carbon Nanostructures Manufactured from Catalytic Templating Nanoparticles. U.S. Patent 7718155B2, 18 May 2010. [Google Scholar]
- Zhang, C.; Gao, Q.H.; Parasher, S.; Yates, D. d-Glucose mitigates the agglomeration of the hollow graphitic carbon nano-spheres. J. Mater. Sci. 2017, 52, 5968–5980. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, Q.S.; Zhou, B.; Bhargava, G. Preparation, characterization, and surface conductivity of nanocomposites with hollow graphitic carbon nanosphere as fillers in polymethylmethacry-late matrix. J. Nanopart. Res. 2017, 19, 269. [Google Scholar] [CrossRef]



| Catalysts | Fe (wt%) | Co (wt%) | M (wt%) |
|---|---|---|---|
| CNS-FeCo | 6.38 ± 0.11 | 15.23 ± 0.17 | none |
| Ce-CNS-FeCo | 6.38 ± 0.11 | 15.23 ± 0.17 | Ce: 0.96 ± 0.05 |
| Na-CNS-FeCo | 6.38 ± 0.11 | 15.23 ± 0.17 | Na: 0.94 ± 0.05 |
| K-CNS-FeCo | 6.38 ± 0.11 | 15.23 ± 0.17 | K: 1.02 ± 0.07 |
| Mn-CNS-FeCo | 6.38 ± 0.11 | 15.23 ± 0.17 | Mn: 1.05 ± 0.07 |
| Zn-CNS-FeCo | 6.38 ± 0.11 | 15.23 ± 0.17 | Zn: 0.98 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, D.; Gandotra, A.; Schossig, J.; Zhang, H.; Wildy, M.; Wei, W.; Arizapana, K.; Zhang, J.Z.; Lu, P.; Zhang, C. Promoter Effect on Carbon Nanosphere-Encapsulated Fe-Co Catalysts for Converting CO2 to Light Olefins. Catalysts 2023, 13, 1416. https://doi.org/10.3390/catal13111416
Weber D, Gandotra A, Schossig J, Zhang H, Wildy M, Wei W, Arizapana K, Zhang JZ, Lu P, Zhang C. Promoter Effect on Carbon Nanosphere-Encapsulated Fe-Co Catalysts for Converting CO2 to Light Olefins. Catalysts. 2023; 13(11):1416. https://doi.org/10.3390/catal13111416
Chicago/Turabian StyleWeber, Daniel, Akash Gandotra, John Schossig, Heng Zhang, Michael Wildy, Wanying Wei, Kevin Arizapana, Jin Zhong Zhang, Ping Lu, and Cheng Zhang. 2023. "Promoter Effect on Carbon Nanosphere-Encapsulated Fe-Co Catalysts for Converting CO2 to Light Olefins" Catalysts 13, no. 11: 1416. https://doi.org/10.3390/catal13111416
APA StyleWeber, D., Gandotra, A., Schossig, J., Zhang, H., Wildy, M., Wei, W., Arizapana, K., Zhang, J. Z., Lu, P., & Zhang, C. (2023). Promoter Effect on Carbon Nanosphere-Encapsulated Fe-Co Catalysts for Converting CO2 to Light Olefins. Catalysts, 13(11), 1416. https://doi.org/10.3390/catal13111416








