Efficient Synthesis of 2-Aminoquinazoline Derivatives via Acid-Mediated [4+2] Annulation of N-Benzyl Cyanamides
Abstract
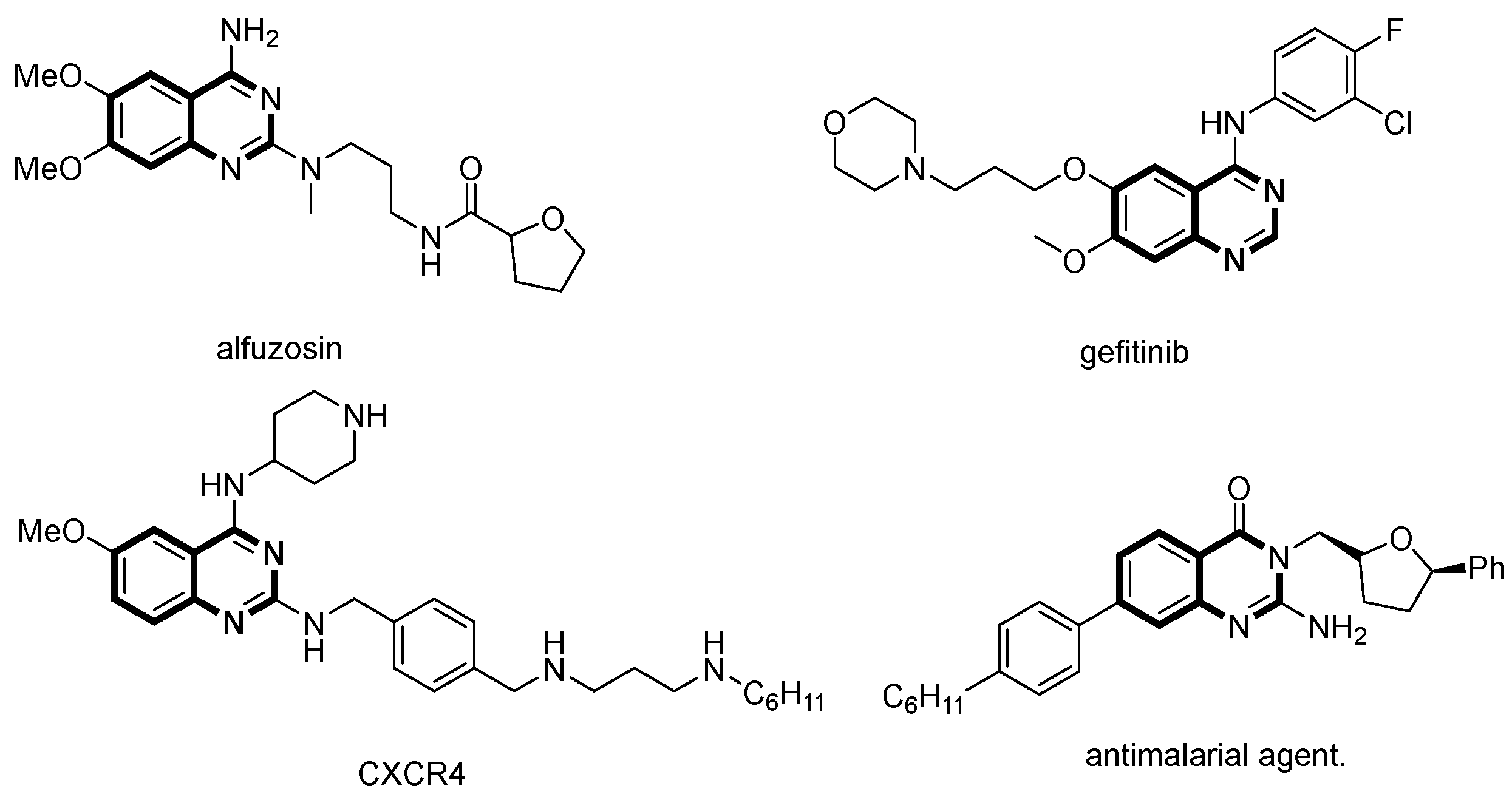
:1. Introduction
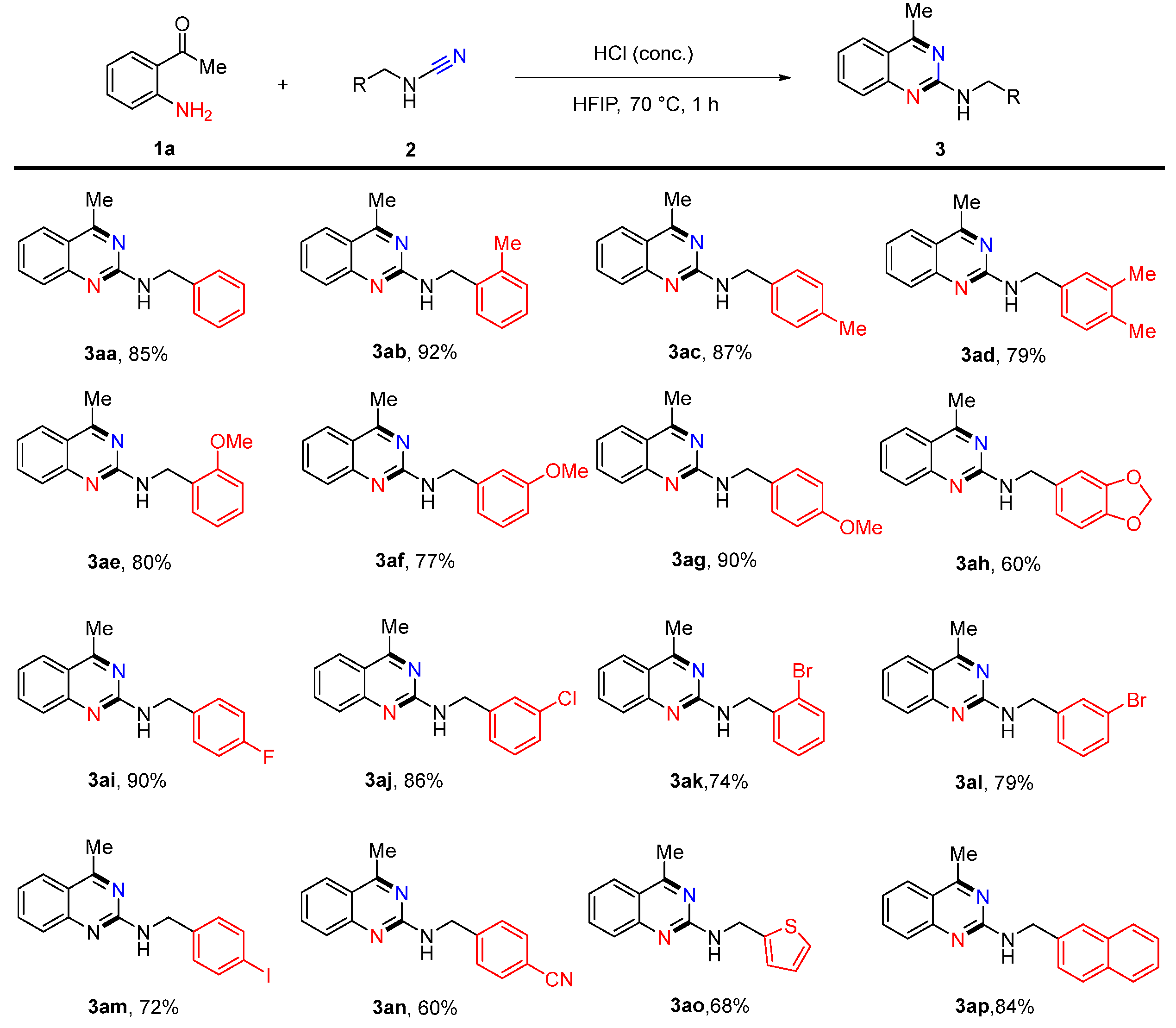
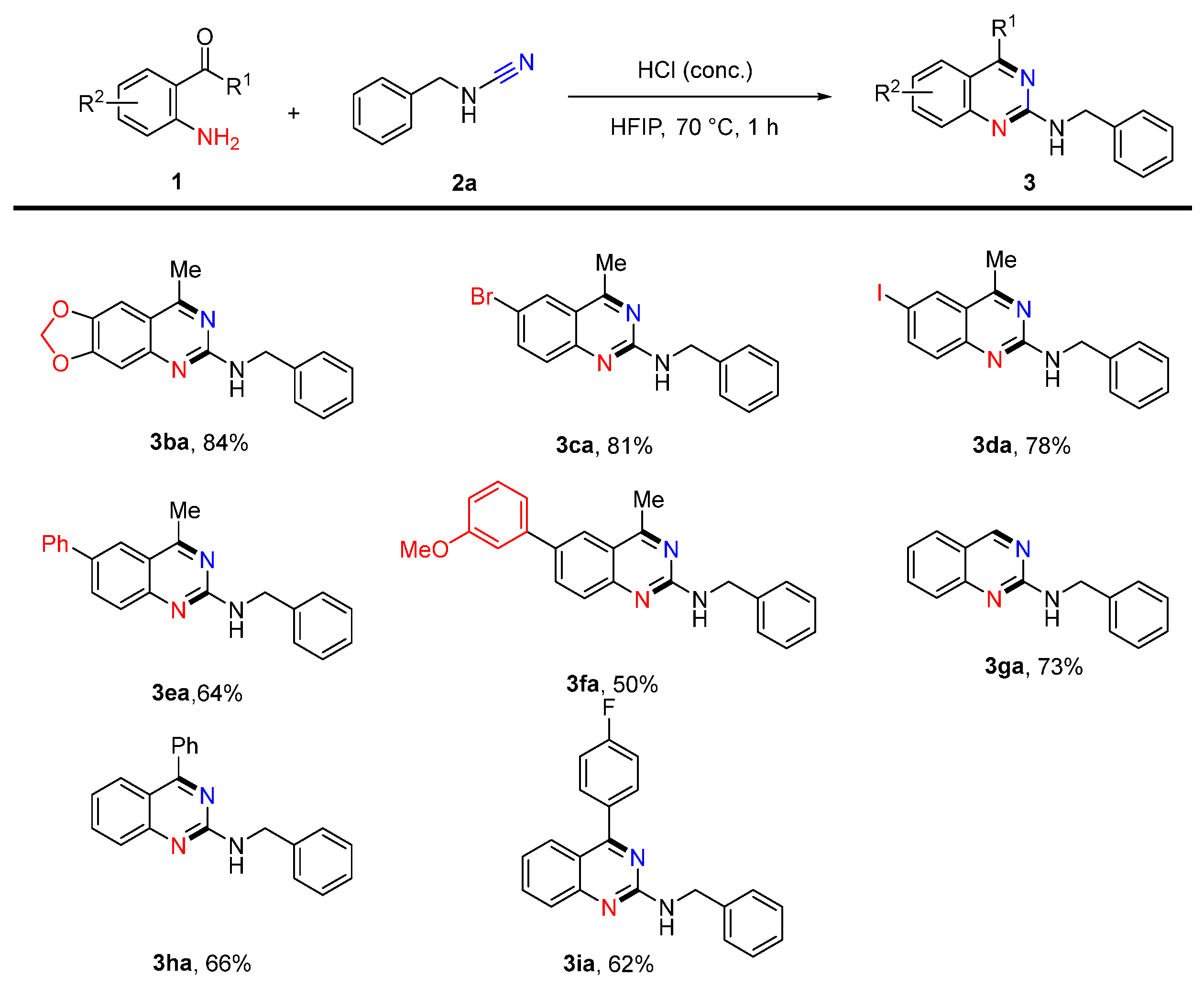
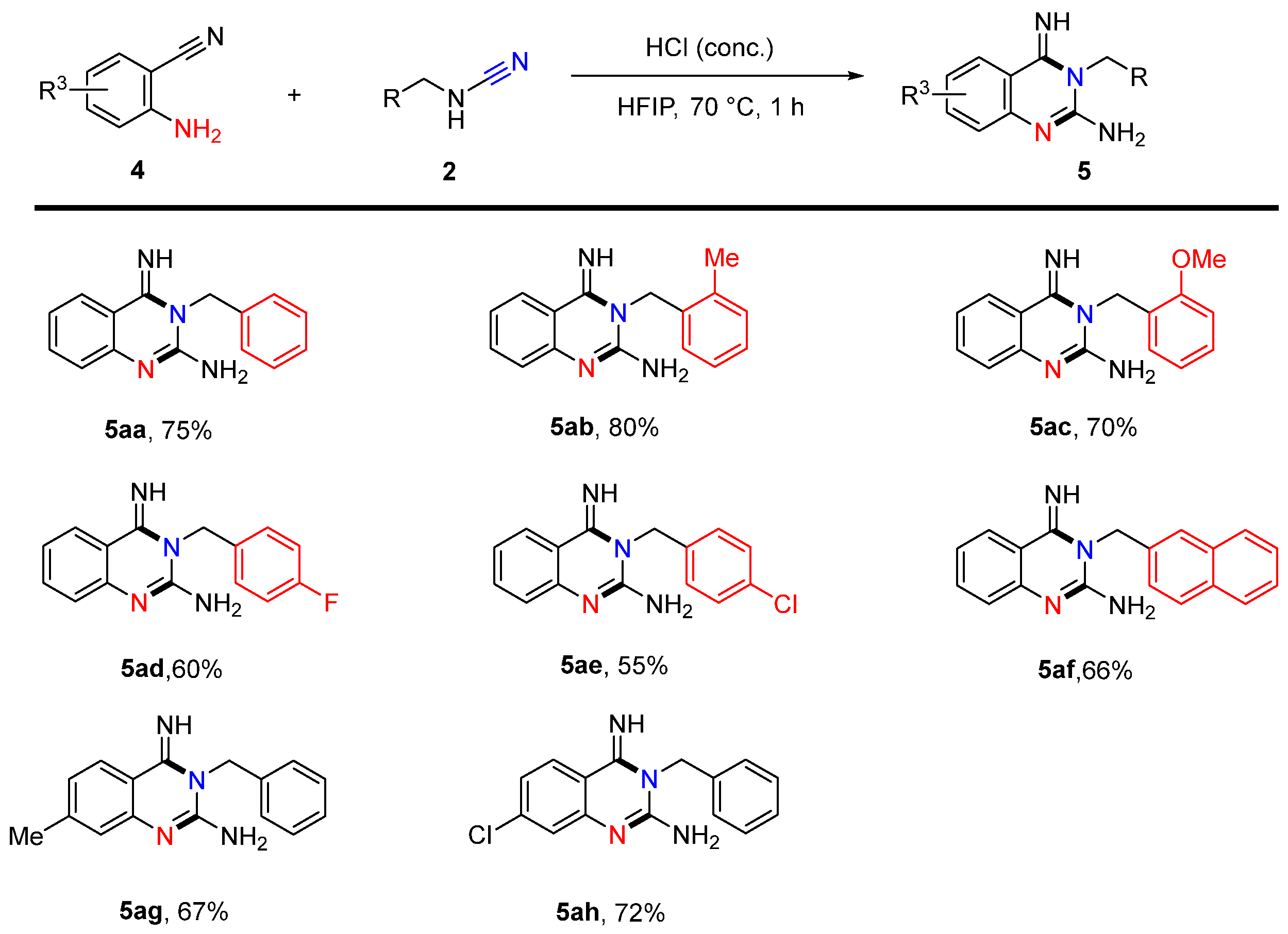
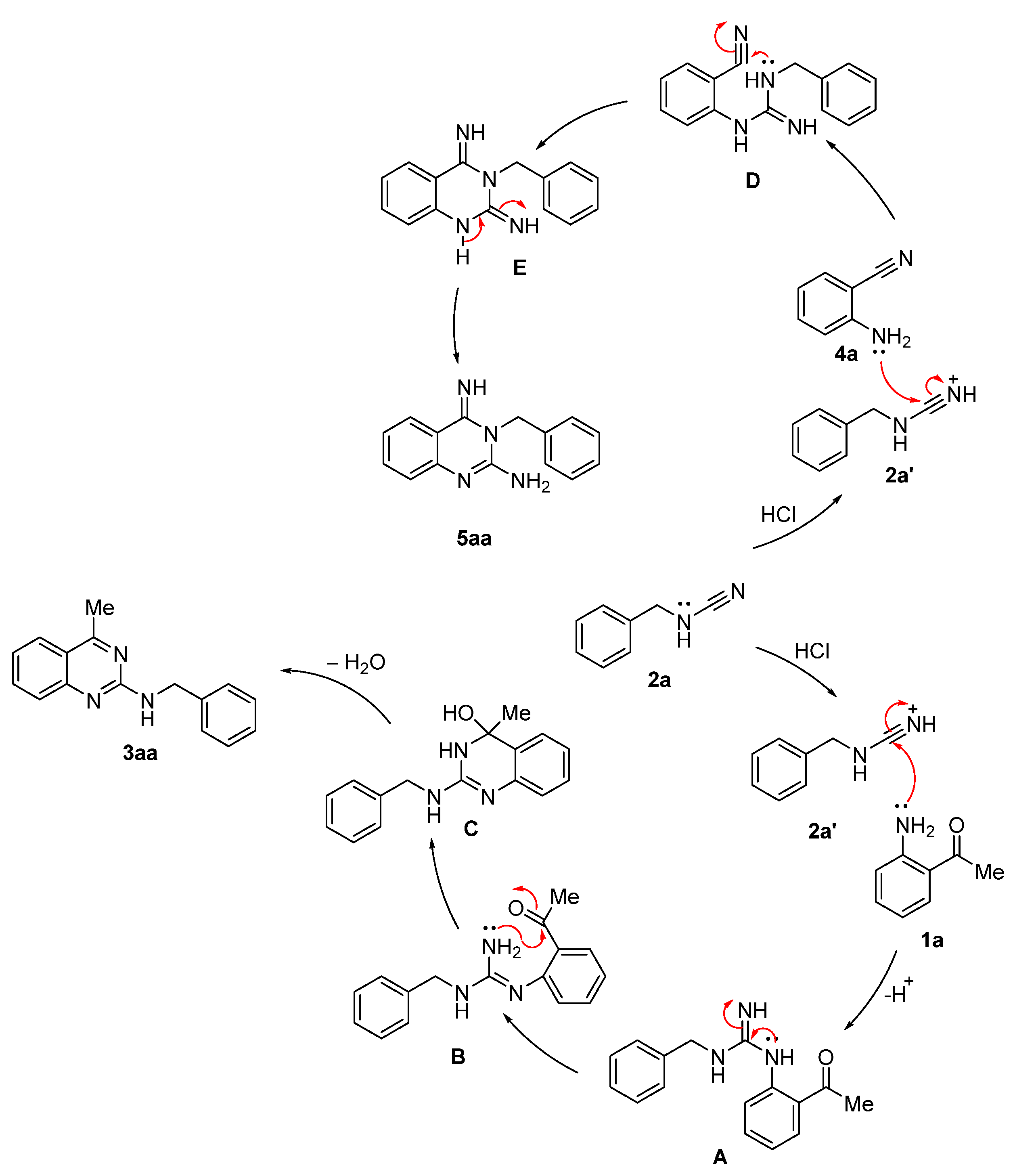
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. N-Benzyl-4-methylquinazolin-2-amine (3aa)
4.2. 4-Methyl-N-(2-methylbenzyl)quinazolin-2-amine (3ab)
4.3. 4-Methyl-N-(4-methylbenzyl)quinazolin-2-amine (3ac)
4.4. N-(3,4-Dimethylbenzyl)-4-methylquinazolin-2-amine (3ad)
4.5. N-(2-Methoxybenzyl)-4-methylquinazolin-2-amine (3ae)
4.6. N-(3-Methoxybenzyl)-4-methylquinazolin-2-amine (3af)
4.7. N-(4-Methoxybenzyl)-4-methylquinazolin-2-amine (3ag)
4.8. N-(Benzo[d][1,3]dioxol-5-ylmethyl)-4-methylquinazolin-2-amine (3ah)
4.9. N-(4-Fluorobenzyl)-4-methylquinazolin-2-amine (3ai)
4.10. N-(3-Chlorobenzyl)-4-methylquinazolin-2-amine (3aj)
4.11. N-(2-Bromobenzyl)-4-methylquinazolin-2-amine (3ak)
4.12. N-(3-Bromobenzyl)-4-methylquinazolin-2-amine (3al)
4.13. N-(4-Iodobenzyl)-4-methylquinazolin-2-amine (3am)
4.14. 4-(((4-Methylquinazolin-2-yl)amino)methyl)benzonitrile (3an)
4.15. 4-Methyl-N-(thiophen-2-ylmethyl)quinazolin-2-amine (3ao)
4.16. 4-Methyl-N-(naphthalen-2-ylmethyl)quinazolin-2-amine (3ap)
4.17. N-Benzyl-8-methyl-[1,3]dioxolo [4,5-g]quinazolin-6-amine (3ba)
4.18. N-Benzyl-6-bromo-4-methylquinazolin-2-amine (3ca)
4.19. N-Benzyl-6-iodo-4-methylquinazolin-2-amine (3da)
4.20. N-Benzyl-4-methyl-6-phenylquinazolin-2-amine (3ea)
4.21. N-Benzyl-6-(3-methoxyphenyl)-4-methylquinazolin-2-amine (3fa)
4.22. N-Benzylquinazolin-2-amine (3ga)
4.23. N-Benzyl-4-phenylquinazolin-2-amine (3ha)
4.24. N-Benzyl-4-(4-fluorophenyl)quinazolin-2-amine (3ia)
4.25. 3-Benzyl-4-imino-3,4-dihydroquinazolin-2-amine (5aa)
4.26. 4-Imino-3-(2-methylbenzyl)-3,4-dihydroquinazolin-2-amine (5ab)
4.27. 4-Imino-3-(2-methoxybenzyl)-3,4-dihydroquinazolin-2-amine (5ac)
4.28. 3-(4-Fluorobenzyl)-4-imino-3,4-dihydroquinazolin-2-amine (5ad)
4.29. 3-(4-Chlorobenzyl)-4-imino-3,4-dihydroquinazolin-2-amine (5ae)
4.30. 4-Imino-3-(naphthalen-2-ylmethyl)-3,4-dihydroquinazolin-2-amine (5af)
4.31. 3-Benzyl-4-imino-7-methyl-3,4-dihydroquinazolin-2-amine (5ag)
4.32. 3-Benzyl-7-chloro-4-imino-3,4-dihydroquinazolin-2-amine (5ah)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Da Silva, J.F.M.; Walters, M.; Al-Damluji, S.; Ganellin, C.R. Molecular features of the prazosin molecule required for activation of Transport-P. Bioorg. Med. Chem. 2008, 16, 7254–7263. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ibrar, A.; Ahmed, W.; Saeed, A. Synthetic approaches, functionalization and therapeutic potential of quinazoline and quinazolinone skeletons: The advances continue. Eur. J. Med. Chem. 2015, 90, 124–169. [Google Scholar] [CrossRef]
- Alagarsamy, V.; Chitra, K.; Saravanan, G.; Solomon, V.R.; Sulthana, M.T.; Narendhar, B. An overview of quinazolines: Pharmacological significance and recent developments. Eur. J. Med. Chem. 2018, 151, 628–685. [Google Scholar] [CrossRef]
- Gupta, T.; Rohilla, A.; Pathak, A.; Akhtar, M.J.; Haider, M.R.; Yar, M.S. Current perspectives on quinazolines with potent biological activities: A review. Synthetic. Commun. 2018, 48, 1099–1127. [Google Scholar] [CrossRef]
- Kung, P.-P.; Casper, M.D.; Cook, K.L.; Wilson-Lingardo, L.; Risen, L.M.; Vickers, T.A.; Ranken, R.; Blyn, L.B.; Wyatt, J.R.; Cook, P.D.; et al. Structure−Activity Relationships of Novel 2-Substituted Quinazoline Antibacterial Agents. J. Med. Chem. 1999, 42, 4705–4713. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.A.; Askari, S.; Rohi, H.; Soorki, A.A. Design, Synthesis, and Antibacterial Evaluation of Some Novel 3′-(Phenylamino)-1′H-spiro[indoline-3,2′-quinazoline]-2,4′(3′H)-dione Derivatives. Synthetic. Commun. 2014, 44, 457–467. [Google Scholar] [CrossRef]
- Seo, W.D.; Ryu, Y.B.; Curtis-Long, M.J.; Lee, C.W.; Ryu, H.W.; Jang, K.C.; Park, K.H. Evaluation of anti-pigmentary effect of synthetic sulfonylamino chalcone. Eur. J. Med. Chem. 2010, 45, 2010–2017. [Google Scholar] [CrossRef]
- Hess, H.J.; Cronin, T.H.; Scriabine, A. Antihypertensive 2-amino-4(3H)-quinazolinones. J. Med. Chem. 1968, 11, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Rasina, D.; Otikovs, M.; Leitans, J.; Recacha, R.; Borysov, O.V.; Kanepe-Lapsa, I.; Domraceva, I.; Pantelejevs, T.; Tars, K.; Blackman, M.J.; et al. Fragment-Based Discovery of 2-Aminoquinazolin-4(3H)-ones As Novel Class Nonpeptidomimetic Inhibitors of the Plasmepsins I, II, and IV. J. Med. Chem. 2016, 59, 374–387. [Google Scholar] [CrossRef]
- Shang, X.-F.; Morris-Natschke, S.L.; Liu, Y.-Q.; Guo, X.; Xu, X.-S.; Goto, M.; Li, J.-C.; Yang, G.-Z.; Lee, K.-H. Biologically active quinoline and quinazoline alkaloids part I. Med. Res. Rev. 2018, 38, 775–828. [Google Scholar] [CrossRef]
- Chandregowda, V.; Kush, A.K.; Chandrasekara Reddy, G. Synthesis and in vitro antitumor activities of novel 4-anilinoquinazoline derivatives. Eur. J. Med. Chem. 2009, 44, 3046–3055. [Google Scholar] [CrossRef] [PubMed]
- Pathania, D.; Sechi, M.; Palomba, M.; Sanna, V.; Berrettini, F.; Sias, A.; Taheri, L.; Neamati, N. Design and discovery of novel quinazolinedione-based redox modulators as therapies for pancreatic cancer. Bba-Gen. Subj. 2014, 1840, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Auti, P.S.; George, G.; Paul, A.T. Recent advances in the pharmacological diversification of quinazoline/quinazolinone hybrids. Rsc. Adv. 2020, 10, 41353–41392. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Hong, J. Recent advancements of 4-aminoquinazoline derivatives as kinase inhibitors and their applications in medicinal chemistry. Eur. J. Med. Chem. 2019, 170, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Wang, C.-J.; Chang, C.-P.; Cheng, Y.-C.; Song, J.-S.; Jan, J.-J.; Chou, M.-C.; Ke, Y.-Y.; Ma, J.; Wong, Y.-C.; et al. Function-Oriented Development of CXCR4 Antagonists as Selective Human Immunodeficiency Virus (HIV)-1 Entry Inhibitors. J. Med. Chem. 2015, 58, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, M.; Xiong, B.; Tan, Z.; Lv, W.; Jiang, H. A Novel Ruthenium-Catalyzed Dehydrogenative Synthesis of 2-Arylquinazolines from 2-Aminoaryl Methanols and Benzonitriles. Org. Lett. 2014, 16, 6028–6031. [Google Scholar] [CrossRef]
- Karnakar, K.; Kumar, A.V.; Murthy, S.N.; Ramesh, K.; Nageswar, Y.V.D. Recyclable graphite oxide promoted efficient synthesis of 2-phenyl quinazoline derivatives in the presence of TBHP as an oxidant. Tetrahedron Lett. 2012, 53, 4613–4617. [Google Scholar] [CrossRef]
- Lin, J.-P.; Zhang, F.-H.; Long, Y.-Q. Solvent/Oxidant-Switchable Synthesis of Multisubstituted Quinazolines and Benzimidazoles via Metal-Free Selective Oxidative Annulation of Arylamidines. Org. Lett. 2014, 16, 2822–2825. [Google Scholar] [CrossRef]
- Jatangi, N.; Palakodety, R.K. I2-Catalyzed oxidative synthesis of N,4-disubstituted quinazolines and quinazoline oxides. Org. Biomol. Chem. 2019, 17, 3714–3717. [Google Scholar] [CrossRef]
- McGowan, M.A.; McAvoy, C.Z.; Buchwald, S.L. Palladium-Catalyzed N-Monoarylation of Amidines and a One-Pot Synthesis of Quinazoline Derivatives. Org. Lett. 2012, 14, 3800–3803. [Google Scholar] [CrossRef]
- Theiling, L.F.; McKee, R.L. 2-Guanidinoquinazolines. J. Am. Chem. Soc. 1952, 74, 1834–1836. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, J.; Wang, Z. A facile synthesis of 2-methylquinolines via Pd-catalyzed aza-Wacker oxidative cyclization. Org. Lett. 2008, 10, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, F.; Wei, T.-Q.; Yin, L.; Wang, S.-Y.; Ji, S.-J. Palladium-Catalyzed Incorporation of Two C1 Building Blocks: The Reaction of Atmospheric CO2 and Isocyanides with 2-Iodoanilines Leading to the Synthesis of Quinazoline-2,4(1H,3H)-diones. Org. Lett. 2017, 19, 4484–4487. [Google Scholar] [CrossRef] [PubMed]
- Pathare, R.S.; Maurya, A.K.; Kumari, A.; Agnihotri, V.K.; Verma, V.P.; Sawant, D.M. Synthesis of quinazoline-3-oxides via a Pd(II) catalyzed azide-isocyanide coupling/cyclocondensation reaction. Org. Biomol. Chem. 2019, 17, 363–368. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, N.; Qi, M.; Qiao, H.; Lu, X.; Ma, L.; Zhou, Y.; Zhang, F.-L. Monodentate Transient Directing Group Assisted Ruthenium(II)-Catalyzed Direct ortho-C–H Imidation of Benzaldehydes for Diverse Synthesis of Quinazoline and Fused Isoindolinone. Org. Lett. 2021, 23, 3923–3927. [Google Scholar] [CrossRef]
- Monrad, R.N.; Madsen, R. Ruthenium-catalysed synthesis of 2-and 3-substituted quinolines from anilines and 1,3-diols. Org. Biomol. Chem. 2011, 9, 610–615. [Google Scholar] [CrossRef]
- Lou, M.; Deng, Z.; Mao, X.; Fu, Y.; Yang, Q.; Peng, Y. Rhodium-catalyzed C–H bond activation alkylation and cyclization of 2-arylquinazolin-4-ones. Org. Biomol. Chem. 2018, 16, 1851–1859. [Google Scholar] [CrossRef]
- Hu, F.-P.; Cui, X.-F.; Lu, G.-Q.; Huang, G.-S. Base-promoted Lewis acid catalyzed synthesis of quinazoline derivatives. Org. Biomol. Chem. 2020, 18, 4376–4380. [Google Scholar] [CrossRef]
- Xu, W.; Fu, H. Amino Acids as the Nitrogen-Containing Motifs in Copper-Catalyzed Domino Synthesis of N-Heterocycles. J. Org. Chem. 2011, 76, 3846–3852. [Google Scholar] [CrossRef]
- Xu, C.; Jia, F.-C.; Zhou, Z.-W.; Zheng, S.-J.; Li, H.; Wu, A.-X. Copper-Catalyzed Multicomponent Domino Reaction of 2-Bromoaldehydes, Benzylamines, and Sodium Azide for the Assembly of Quinazoline Derivatives. J. Org. Chem. 2016, 81, 3000–3006. [Google Scholar] [CrossRef]
- Wang, C.; Li, S.; Liu, H.; Jiang, Y.; Fu, H. Copper-Catalyzed Synthesis of Quinazoline Derivatives via Ullmann-Type Coupling and Aerobic Oxidation. J. Org. Chem. 2010, 75, 7936–7938. [Google Scholar] [CrossRef] [PubMed]
- Toh, K.K.; Sanjaya, S.; Sahnoun, S.; Chong, S.Y.; Chiba, S. Copper-Catalyzed Aerobic Intramolecular Carbo- and Amino-Oxygenation of Alkynes for Synthesis of Azaheterocycles. Org. Lett. 2012, 14, 2290–2292. [Google Scholar] [CrossRef]
- Arienzo, R.; Cramp, S.; Dyke, H.J.; Lockey, P.M.; Norman, D.; Roach, A.G.; Smith, P.; Wong, M.; Wren, S.P. Quinazoline and benzimidazole MCH-1R antagonists. Bioorg. Med. Chem. Lett. 2007, 17, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Jatangi, N.; Palakodety, R.K. Base-catalyzed synthesis of quinazolines in aqueous medium. Tetrahedron Lett. 2019, 60, 151186–151189. [Google Scholar] [CrossRef]
- Tran, L.Q.; Li, J.; Neuville, L. Copper-Catalyzed Domino Three-Component Approach for the Assembly of 2-Aminated Benzimidazoles and Quinazolines. J. Org. Chem. 2015, 80, 6102–6108. [Google Scholar] [CrossRef]
- Liu, S.; Wang, A.-J.; Li, M.; Zhang, J.; Yin, G.-D.; Shu, W.-M.; Yu, W.-C. Rh(III)-Catalyzed Tandem Reaction Access to (Quinazolin-2-yl)methanone Derivatives from 2,1-Benzisoxazoles and α-Azido Ketones. J. Org. Chem. 2022, 87, 11253–11260. [Google Scholar] [CrossRef]


| Entry | Solvent | Additive b | Temp (°C) | Yield (%) c |
|---|---|---|---|---|
| 1 | HFIP | MsOH | 90 | 57 |
| 2 | HFIP | TfOH | 90 | 45 |
| 3 | HFIP | TFA | 90 | 30 |
| 4 | HFIP | HCOOH | 90 | 30 |
| 5 | HFIP | AcOH | 90 | 45 |
| 6 | HFIP | TsOH | 90 | 60 |
| 7 | HFIP | H2SO4 | 90 | 47 |
| 8 | HFIP | HI | 90 | 55 |
| 9 | HFIP | HBr | 90 | 50 |
| 10 | HFIP | CuBr2 | 90 | 58 |
| 11 | HFIP | CuI | 90 | 63 |
| 12 | HFIP | FeCl3 | 90 | 57 |
| 13 | HFIP | HCl | 90 | 73 |
| 14 | HFIP | - | 90 | 0 |
| 15 | EtOAc | HCl | 90 | 20 |
| 16 | iPrOH | HCl | 90 | 32 |
| 17 | MeOH | HCl | 90 | 40 |
| 18 | CH3CN | HCl | 90 | 60 |
| 19 | EtOH | HCl | 90 | 42 |
| 20 | Dioxane | HCl | 90 | 30 |
| 21 | H2O | HCl | 90 | 35 |
| 22 | Et2O | HCl | 90 | 30 |
| 23 | HFIP | HCl | 70 | 80 |
| 24 | HFIP | HCl | 80 | 70 |
| 25 | HFIP | HCl | 60 | 62 |
| 26 | HFIP | HCl | 50 | 50 |
| 27 d | HFIP | HCl (2) | 70 | 85 |
| 28 e | HFIP | HCl (1) | 70 | 78 |
| 29 f | HFIP | HCl (0.5) | 70 | 72 |
| 30 g | HFIP | HCl (2) | 70 | 76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Z.-Y.; Sun, C.-X.; Zhang, W.-W.; Li, J.-R.; Gong, Y.-X.; Shu, W.-M.; Wu, A.-X. Efficient Synthesis of 2-Aminoquinazoline Derivatives via Acid-Mediated [4+2] Annulation of N-Benzyl Cyanamides. Catalysts 2023, 13, 1447. https://doi.org/10.3390/catal13111447
Qin Z-Y, Sun C-X, Zhang W-W, Li J-R, Gong Y-X, Shu W-M, Wu A-X. Efficient Synthesis of 2-Aminoquinazoline Derivatives via Acid-Mediated [4+2] Annulation of N-Benzyl Cyanamides. Catalysts. 2023; 13(11):1447. https://doi.org/10.3390/catal13111447
Chicago/Turabian StyleQin, Zhao-Yi, Chen-Xi Sun, Wen-Wen Zhang, Jun-Ru Li, Yin-Xiang Gong, Wen-Ming Shu, and An-Xin Wu. 2023. "Efficient Synthesis of 2-Aminoquinazoline Derivatives via Acid-Mediated [4+2] Annulation of N-Benzyl Cyanamides" Catalysts 13, no. 11: 1447. https://doi.org/10.3390/catal13111447
APA StyleQin, Z.-Y., Sun, C.-X., Zhang, W.-W., Li, J.-R., Gong, Y.-X., Shu, W.-M., & Wu, A.-X. (2023). Efficient Synthesis of 2-Aminoquinazoline Derivatives via Acid-Mediated [4+2] Annulation of N-Benzyl Cyanamides. Catalysts, 13(11), 1447. https://doi.org/10.3390/catal13111447






