A CoFe Bimetallic Catalyst for the Direct Conversion of Syngas to Olefins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization
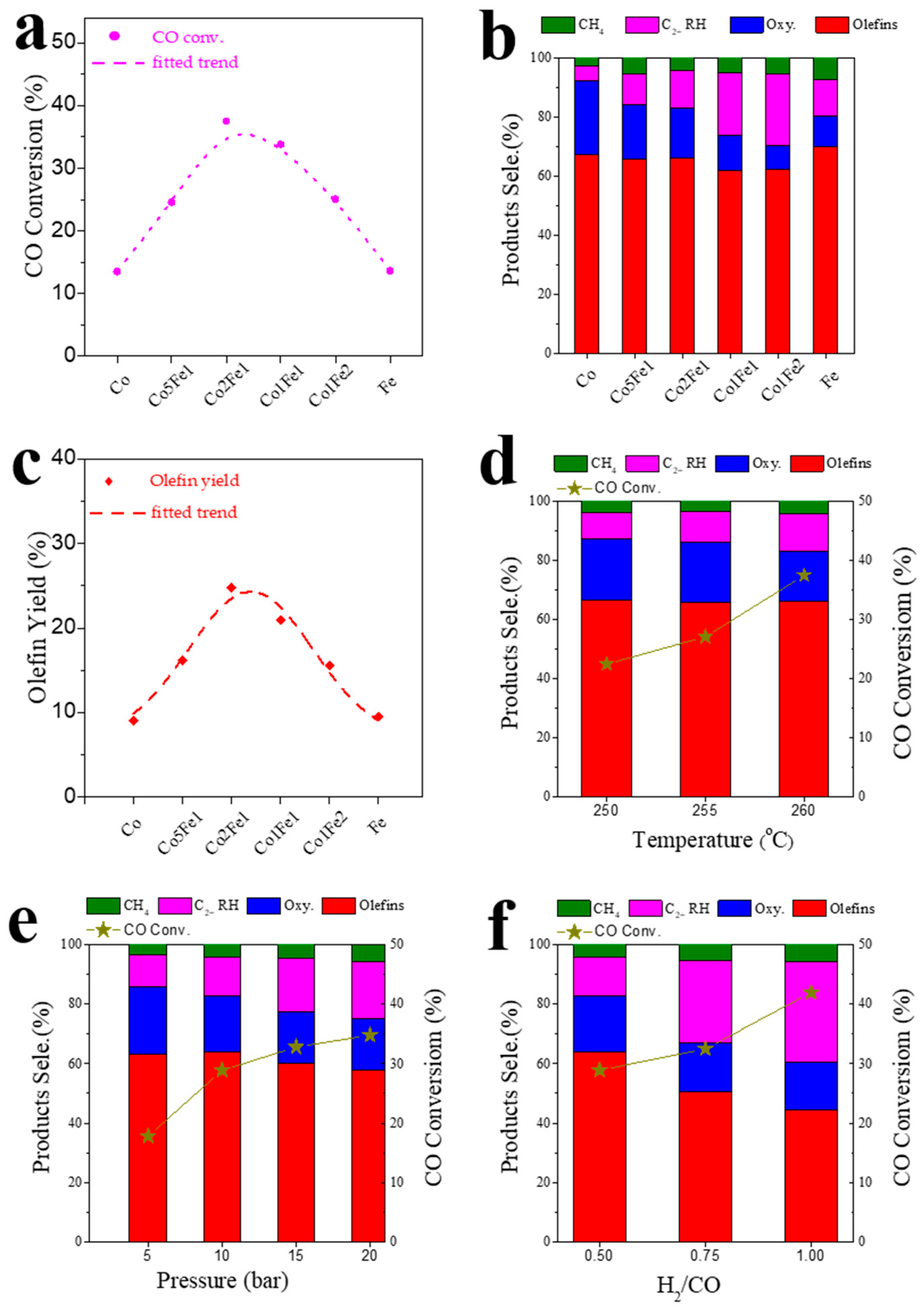
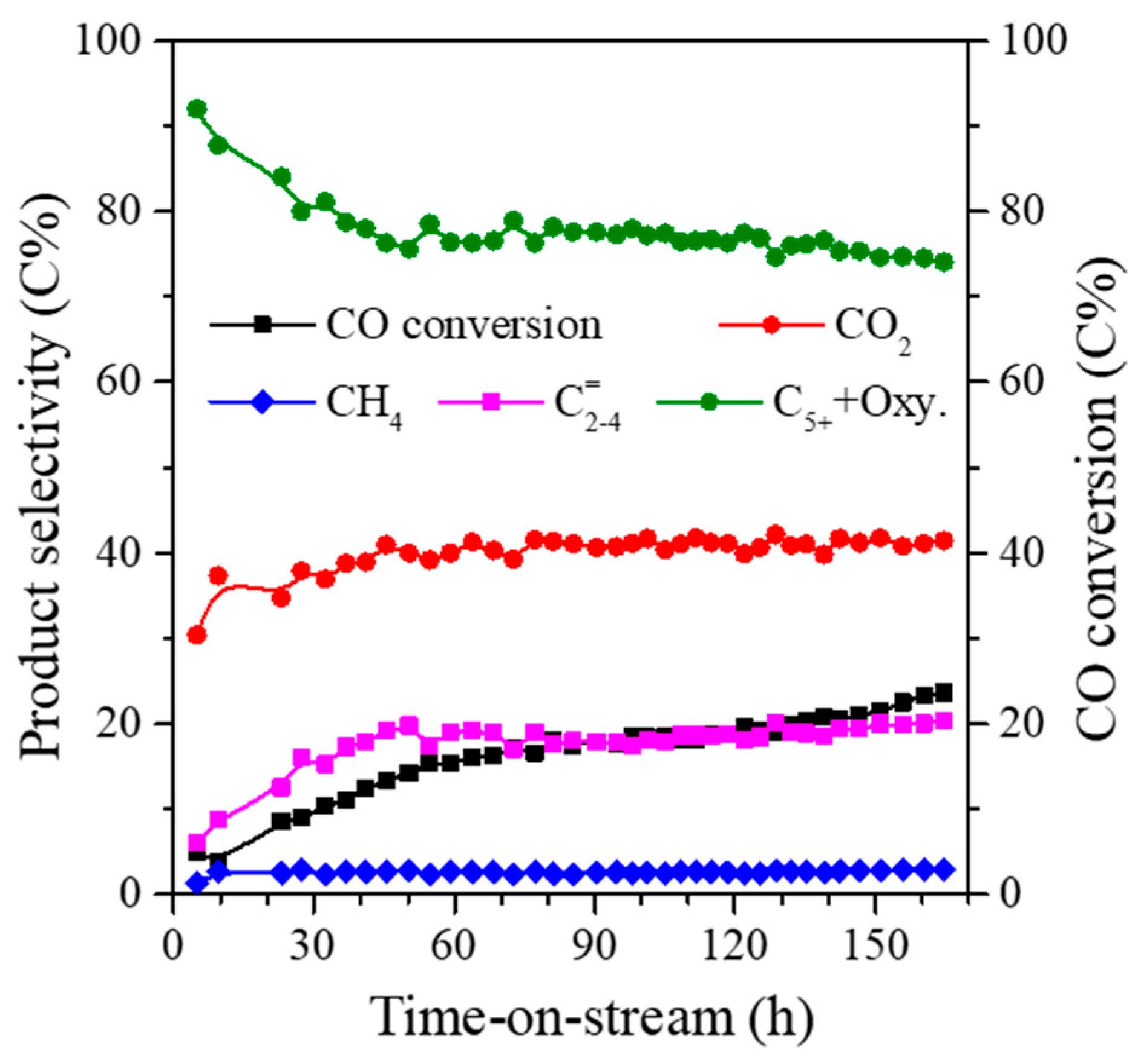
2.2. Catalytic Performance
2.3. Structural-Performance Discussion
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalyst Characterization
3.3. Catalytic Performance Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Iglesia, E. Design, synthesis, and use of cobalt-based Fischer-Tropsch synthesis catalysts. Appl. Catal. A Gen. 1997, 161, 59–78. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, W.; Wang, Y. Recent advances in understanding the key catalyst factors for Fischer-Tropsch synthesis. J. Energy Chem. 2013, 22, 27–38. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Chu, W.; Fongarland, P. Advances in the Development of Novel Cobalt Fischer−Tropsch Catalysts for Synthesis of Long-Chain Hydrocarbons and Clean Fuels. Chem. Rev. 2007, 107, 1692–1744. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Gu, B.; Liu, X.; Kang, J.; Zhang, Q.; Wang, Y. Direct and Highly Selective Conversion of Synthesis Gas into Lower Olefins: Design of a Bifunctional Catalyst Combining Methanol Synthesis and Carbon–Carbon Coupling. Angew. Chem. Int. Ed. 2016, 55, 4725–4728. [Google Scholar] [CrossRef]
- Jiao, F.; Li, J.; Pan, X.; Xiao, J.; Li, H.; Ma, H.; Wei, M.; Pan, Y.; Zhou, Z.; Li, M. Selective conversion of syngas to light olefins. Science 2016, 351, 1065–1068. [Google Scholar] [CrossRef]
- Pan, X.; Jiao, F.; Miao, D.; Bao, X. Oxide–Zeolite-Based Composite Catalyst Concept That Enables Syngas Chemistry beyond Fischer–Tropsch Synthesis. Chem. Rev. 2021, 121, 6588–6609. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Gao, J.; Wang, J.; Ma, G.; Wen, X.; Yang, Y.; Li, Y.; Ding, M. A hydrophobic FeMn@Si catalyst increases olefins from syngas by suppressing C1 by-products. Science 2021, 371, 610–613. [Google Scholar] [CrossRef]
- Cheng, K.; Kang, J.; King, D.L.; Subramanian, V.; Zhou, C.; Zhang, Q.; Wang, Y. Advances in Catalysis for Syngas Conversion to Hydrocarbons. In Advances in Catalysis; Song, C., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 60, pp. 125–208. [Google Scholar]
- Lyu, S.; Wang, L.; Li, Z.; Yin, S.; Chen, J.; Zhang, Y.; Li, J.; Wang, Y. Stabilization of ε-iron carbide as high-temperature catalyst under realistic Fischer–Tropsch synthesis conditions. Nat. Commun. 2020, 11, 6219. [Google Scholar] [CrossRef]
- Torres Galvis, H.M.; Bitter, J.H.; Khare, C.B.; Ruitenbeek, M.; Dugulan, A.I.; de Jong, K.P. Supported iron nanoparticles as catalysts for sustainable production of lower olefins. Science 2012, 335, 835–838. [Google Scholar] [CrossRef]
- Torres Galvis, H.M.; Koeken, A.C.J.; Bitter, J.H.; Davidian, T.; Ruitenbeek, M.; Dugulan, A.I.; de Jong, K.P. Effects of sodium and sulfur on catalytic performance of supported iron catalysts for the Fischer–Tropsch synthesis of lower olefins. J. Catal. 2013, 303, 22–30. [Google Scholar] [CrossRef]
- Koeken, A.C.; Torres Galvis, H.M.; Davidian, T.; Ruitenbeek, M.; de Jong, K.P. Suppression of carbon deposition in the iron-catalyzed production of lower olefins from synthesis gas. Angew. Chem. Int. Ed. 2012, 124, 7302–7305. [Google Scholar] [CrossRef]
- Zhai, P.; Xu, C.; Gao, R.; Liu, X.; Li, M.; Li, W.; Fu, X.; Jia, C.; Xie, J.; Zhao, M.; et al. Highly Tunable Selectivity for Syngas-Derived Alkenes over Zinc and Sodium-Modulated Fe5C2 Catalyst. Angew. Chem. Int. Ed. 2016, 55, 9902–9907. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Yu, F.; An, Y.; Zhao, Y.; Sun, Y.; Li, Z.; Lin, T.; Lin, Y.; Qi, X.; Dai, Y.; et al. Cobalt carbide nanoprisms for direct production of lower olefins from syngas. Nature 2016, 538, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, D.; Yang, L.; Cen, J.; Xiao, K.; Yao, N.; Li, X. Formation Mechanism of the Co2C Nanoprisms Studied with the CoCe System in the Fischer–Tropsch to Olefin Reaction. ACS Catal. 2021, 11, 2746–2753. [Google Scholar] [CrossRef]
- Kim, K.Y.; Lee, H.; Noh, W.Y.; Shin, J.; Han, S.J.; Kim, S.K.; An, K.; Lee, J.S. Cobalt Ferrite Nanoparticles to Form a Catalytic Co–Fe Alloy Carbide Phase for Selective CO2 Hydrogenation to Light Olefins. ACS Catal. 2020, 10, 8660–8671. [Google Scholar] [CrossRef]
- Hwang, S.-M.; Han, S.J.; Park, H.-G.; Lee, H.; An, K.; Jun, K.-W.; Kim, S.K. Atomically Alloyed Fe–Co Catalyst Derived from a N-Coordinated Co Single-Atom Structure for CO2 Hydrogenation. ACS Catal. 2021, 11, 2267–2278. [Google Scholar] [CrossRef]
- Numpilai, T.; Witoon, T.; Chanlek, N.; Limphirat, W.; Bonura, G.; Chareonpanich, M.; Limtrakul, J. Structure–activity relationships of Fe-Co/K-Al2O3 catalysts calcined at different temperatures for CO2 hydrogenation to light olefins. Appl. Catal. A Gen. 2017, 547, 219–229. [Google Scholar] [CrossRef]
- Falkenhagen, J.P.; Maisonneuve, L.; Paalanen, P.P.; Coste, N.; Malicki, N.; Weckhuysen, B.M. Cobalt-Iron-Manganese Catalysts for the Conversion of End-of-Life-Tire-Derived Syngas into Light Terminal Olefins. Chem. A Eur. J. 2018, 24, 4597–4606. [Google Scholar] [CrossRef]
- Yuan, F.; Zhang, G.; Wang, M.; Zhu, J.; Zhang, M.; Ding, F.; Cheng, Z.; Song, C.; Guo, X. Boosting the Production of Light Olefins from CO2 Hydrogenation over Fe–Co Bimetallic Catalysts Derived from Layered Double Hydroxide. Ind. Eng. Chem. Res. 2023, 62, 8210–8221. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, B.; Gao, R.; Yao, S.; Zhai, P.; Li, S.; Yu, J.; Hou, Y.; Ma, D. Construction of Synergistic Fe5C2/Co Heterostructured Nanoparticles as an Enhanced Low Temperature Fischer–Tropsch Synthesis Catalyst. ACS Catal. 2017, 7, 5661–5667. [Google Scholar] [CrossRef]
- Zhang, L.; Dang, Y.; Zhou, X.; Gao, P.; Petrus van Bavel, A.; Wang, H.; Li, S.; Shi, L.; Yang, Y.; Vovk, E.I.; et al. Direct conversion of CO2 to a jet fuel over CoFe alloy catalysts. Innovation 2021, 2, 100170. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Wei, Y.; Lin, T.; Qi, X.; Sun, F.; Jiang, Z.; Zhong, L. Maximizing the Interface of Dual Active Sites to Enhance Higher Oxygenate Synthesis from Syngas with High Activity. ACS Catal. 2023, 13, 4533–4543. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Z.; Guan, T.; Guo, S.; Hu, Z.; Wang, J.; Rykov, A.; Lv, J.; Huang, S.; Wang, Y.; et al. CoFe alloy carbide catalysts for higher alcohols synthesis from syngas: Evolution of active sites and Na promoting effect. J. Catal. 2022, 405, 430–444. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Z.; Kang, L.; Han, X.; Qi, Z.; Guo, S.; Wang, J.; Rykov, A.; Lv, J.; Wang, Y.; et al. A Monodisperse ε′-(CoxFe1–x)2.2C Bimetallic Carbide Catalyst for Direct Conversion of Syngas to Higher Alcohols. ACS Catal. 2022, 12, 6016–6028. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Jacobs, G.; Hamdeh, H.H.; Shafer, W.D.; Liu, F.; Hopps, S.D.; Thomas, G.A.; Davis, B.H. Hydrogenation of Carbon Dioxide over Co–Fe Bimetallic Catalysts. ACS Catal. 2016, 6, 913–927. [Google Scholar] [CrossRef]
- Ismail, A.S.M.; Casavola, M.; Liu, B.; Gloter, A.; van Deelen, T.W.; Versluijs, M.; Meeldijk, J.D.; Stéphan, O.; de Jong, K.P.; de Groot, F.M.F. Atomic-Scale Investigation of the Structural and Electronic Properties of Cobalt–Iron Bimetallic Fischer–Tropsch Catalysts. ACS Catal. 2019, 9, 7998–8011. [Google Scholar] [CrossRef]
- Bezemer, G.L.; Radstake, P.B.; Falke, U.; Oosterbeek, H.; Kuipers, H.P.C.E.; van Dillen, A.J.; de Jong, K.P. Investigation of promoter effects of manganese oxide on carbon nanofiber-supported cobalt catalysts for Fischer–Tropsch synthesis. J. Catal. 2006, 237, 152–161. [Google Scholar] [CrossRef]
- Bezemer, G.L.; Bitter, J.H.; Kuipers, H.P.C.E.; Oosterbeek, H.; Holewijn, J.E.; Xu, X.; Kapteijn, F.; van Dillen, A.J.; de Jong, K.P. Cobalt Particle Size Effects in the Fischer−Tropsch Reaction Studied with Carbon Nanofiber Supported Catalysts. J. Am. Chem. Soc. 2006, 128, 3956–3964. [Google Scholar] [CrossRef]
- Xu, Y.; Zhai, P.; Deng, Y.; Xie, J.; Liu, X.; Wang, S.; Ma, D. Highly Selective Olefin Production from CO2 Hydrogenation on Iron Catalysts: A Subtle Synergy between Manganese and Sodium Additives. Angew. Chem. Int. Ed. 2020, 59, 21736–21744. [Google Scholar] [CrossRef]
- Xu, D.; Li, W.; Duan, H.; Ge, Q.; Xu, H. Reaction performance and characterization of Co/Al2O3 Fischer–Tropsch catalysts promoted with Pt, Pd and Ru. Catal. Lett. 2005, 102, 229–235. [Google Scholar] [CrossRef]
- Keyvanloo, K.; Mardkhe, M.K.; Alam, T.M.; Bartholomew, C.H.; Woodfield, B.F.; Hecker, W.C. Supported Iron Fischer–Tropsch Catalyst: Superior Activity and Stability Using a Thermally Stable Silica-Doped Alumina Support. ACS Catal. 2014, 4, 1071–1077. [Google Scholar] [CrossRef]
- Dry, M.E. The Fischer–Tropsch process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar] [CrossRef]
- Abelló, S.; Montané, D. Exploring iron-based multifunctional catalysts for Fischer-Tropsch synthesis: A review. ChemSusChem 2011, 4, 1538–1556. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Jiang, J.; Gong, Y.; Wu, P.; Wang, A.; Zhang, X.; Wang, T.; Meng, X.; Lin, Q.; Lv, Y.; et al. Recent Mechanistic Understanding of Fischer-Tropsch Synthesis on Fe-Carbide. Catalysts 2023, 13, 1052. [Google Scholar] [CrossRef]
- Guo, L.; Sun, J.; Ge, Q.; Tsubaki, N. Recent advances in direct catalytic hydrogenation of carbon dioxide to valuable C2+ hydrocarbons. J. Mater. Chem. A 2018, 6, 23244–23262. [Google Scholar] [CrossRef]
- Liu, S.; Sun, B.; Zhang, Y.; Li, J.; Resasco, D.E.; Nie, L.; Wang, L. The role of intermediate CoxMn1−xO (x = 0.6–0.85) nanocrystals in the formation of active species for the direct production of lower olefins from syngas. Chem. Commun. 2019, 55, 6595–6598. [Google Scholar] [CrossRef]
- Cheng, K.; Ordomsky, V.V.; Legras, B.; Virginie, M.; Paul, S.; Wang, Y.; Khodakov, A.Y. Sodium-promoted iron catalysts prepared on different supports for high temperature Fischer–Tropsch synthesis. Appl. Catal. A Gen. 2015, 502, 204–214. [Google Scholar] [CrossRef]
- An, X.; Wu, B.; Wan, H.; Li, T.; Tao, Z.; Xiang, H.; Li, Y. Comparative study of iron-based Fischer–Tropsch synthesis catalyst promoted with potassium or sodium. Catal. Commun. 2007, 8, 1957–1962. [Google Scholar] [CrossRef]
- van Steen, E.; Claeys, M.; Möller, K.P.; Nabaho, D. Comparing a cobalt-based catalyst with iron-based catalysts for the Fischer-Tropsch XTL-process operating at high conversion. Appl. Catal. A Gen. 2018, 549, 51–59. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, K.; Kang, J.; Zhou, C.; Subramanian, V.; Zhang, Q.; Wang, Y. New horizon in C1 chemistry: Breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels. Chem. Soc. Rev. 2019, 48, 3193–3228. [Google Scholar] [CrossRef]
- Ding, M.; Yang, Y.; Li, Y.; Wang, T.; Ma, L.; Wu, C. Impact of H2/CO ratios on phase and performance of Mn-modified Fe-based Fischer Tropsch synthesis catalyst. Appl. Energy 2013, 112, 1241–1246. [Google Scholar] [CrossRef]
- Dai, Y.; Zhao, Y.; Lin, T.; Li, S.; Yu, F.; An, Y.; Wang, X.; Xiao, K.; Sun, F.; Jiang, Z.; et al. Particle Size Effects of Cobalt Carbide for Fischer–Tropsch to Olefins. ACS Catal. 2019, 9, 798–809. [Google Scholar] [CrossRef]
- Molares, F.; de Groot, F.M.F.; Gijzeman, O.L.J.; Mens, A.; Stephan, O.; Weckhuysen, B.M. Mn promotion effects in Co/TiO2 Fischer–Tropsch catalysts as investigated by XPS and STEM-EELS. J. Catal. 2005, 230, 301–308. [Google Scholar] [CrossRef]
- Bezemer, G.L.; Radstake, P.B.; Koot, V.; van Dillen, A.J.; Geus, J.W.; de Jong, K.P. Preparation of Fischer–Tropsch cobalt catalysts supported on carbon nanofibers and silica using homogeneous deposition-precipitation. J. Catal. 2006, 237, 291–302. [Google Scholar] [CrossRef]
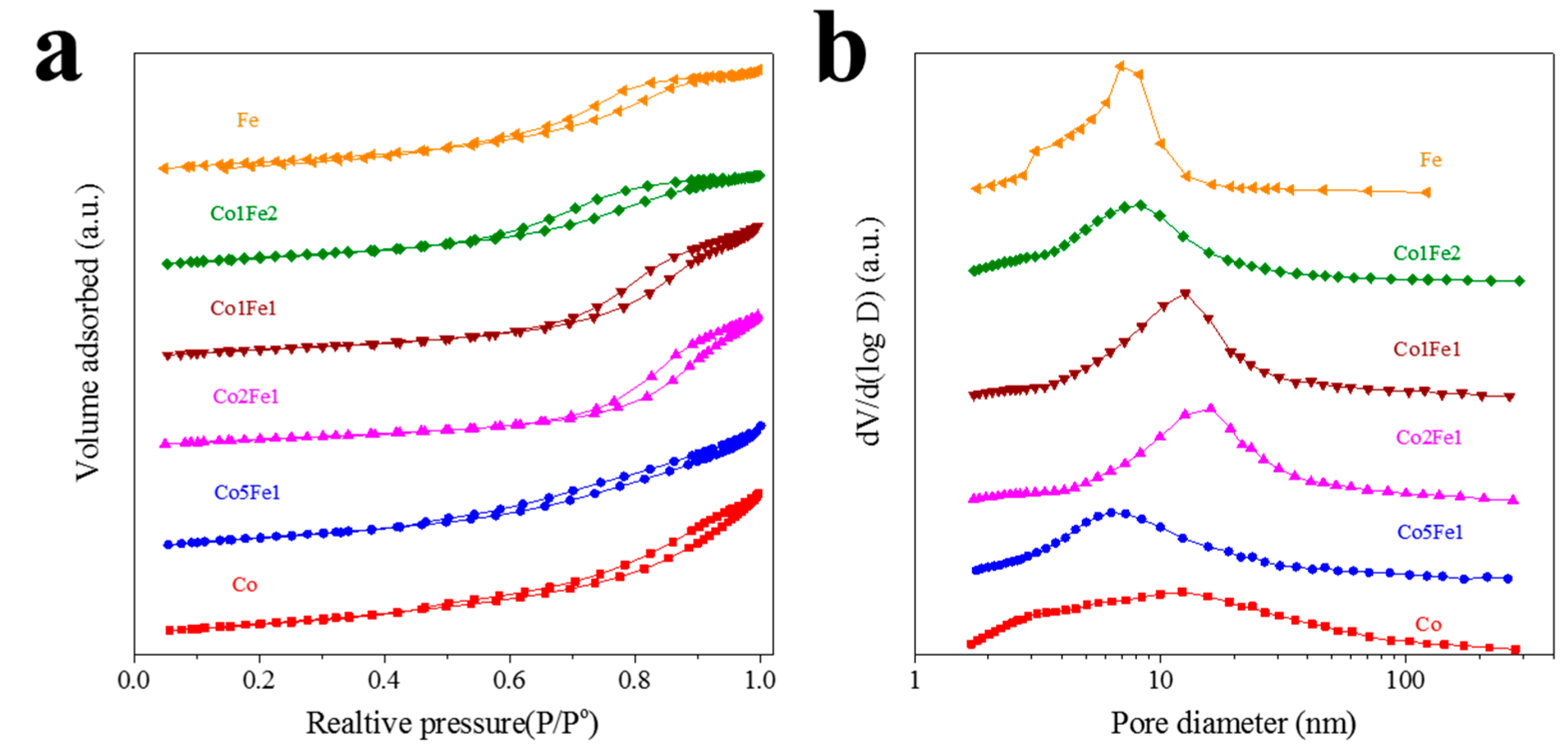


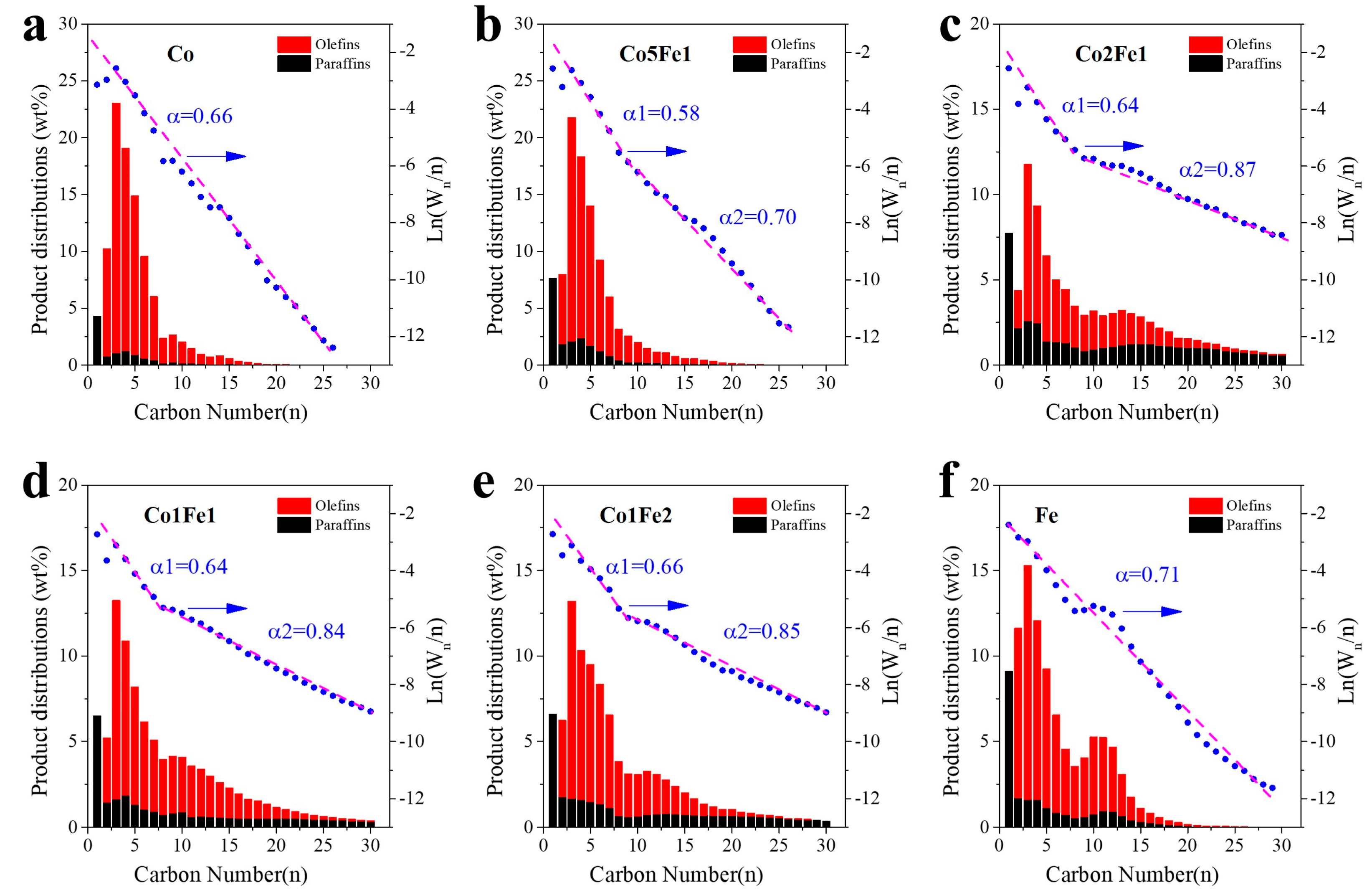

| Samples | Co a (wt.%) | Fe a (wt.%) | Na a (wt.%) | Co/Fe b | (Co + Fe)/Mn/Al b | SBET (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|---|---|---|---|---|
| Co | 26.6 | 0 | 3.8 | - | 1.0:1.0:1.2 | 222 | 0.49 | 8.8 |
| Co5Fe1 | 22.7 | 4.0 | 2.3 | 5.3 | 1.1:1.0:1.0 | 216 | 0.42 | 7.8 |
| Co2Fe1 | 17.5 | 8.2 | 3.5 | 2.0 | 1.0:1.0:0.9 | 141 | 0.44 | 12.4 |
| Co1Fe1 | 13.4 | 12.5 | 3.0 | 1.0 | 1.0:1.0:0.9 | 166 | 0.45 | 10.8 |
| Co1Fe2 | 8.7 | 16.1 | 3.8 | 0.5 | 0.6:1.0:1.0 | 184 | 0.33 | 7.2 |
| Fe | 0 | 26.6 | 3.6 | 0 | 1.1:1.0:0.8 | 178 | 0.36 | 8.0 |
| Catalysts | CO Conv. (C%) | CO2 Sele. (C%) | Product Sele. (C%, CO2-Free) | Olefin/Paraffin Ratio | Olefins Yield (C%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Olefins | Oxy. b | C2+ RH c | Olefins + Oxy. | CH4 | C2 | C3 | C4 | O/P | ||||
| Co | 13.5 | 47.5 | 67.3 | 25.0 | 4.9 | 92.3 | 2.9 | 12.4 | 20.4 | 14.2 | 8.7 | 9.1 |
| Co5Fe1 | 24.5 | 49.6 | 65.8 | 18.5 | 10.2 | 84.3 | 5.5 | 3.3 | 9.3 | 6.7 | 4.2 | 16.1 |
| Co2Fe1 | 37.5 | 46.1 | 66.1 | 17.1 | 12.5 | 83.2 | 4.3 | 3.0 | 8.8 | 6.0 | 3.9 | 24.8 |
| Co1Fe1 | 33.8 | 40.9 | 61.8 | 12.0 | 21.0 | 73.9 | 5.1 | 2.6 | 7.1 | 4.9 | 2.4 | 20.9 |
| Co1Fe2 | 25.0 | 35.0 | 62.3 | 7.9 | 24.4 | 70.2 | 5.4 | 2.5 | 6.9 | 5.3 | 2.1 | 15.6 |
| Fe | 13.6 | 49.2 | 70.1 | 10.2 | 12.5 | 80.3 | 7.2 | 5.8 | 8.5 | 6.5 | 3.6 | 9.5 |
| H2/CO | P (bar) | Temp. (°C) | XCO (C%) | CO2 Sele. (C%) | Product Selectivity (C%, CO2-Free) | Olefin/Paraffin Ratio | Olefins Yield (C%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Olefins | Oxy. b | C2+ RH c | Olefins + Oxy. | CH4 | C2 | C3 | C4 | O/P | ||||||
| 0.5 | 10 | 250 | 22.5 | 48.7 | 66.5 | 20.6 | 9.1 | 87.0 | 3.9 | 6.0 | 9.7 | 6.8 | 5.1 | 14.9 |
| 255 | 28.9 | 49.1 | 63.8 | 18.9 | 13.1 | 82.7 | 4.2 | 3.5 | 8.8 | 6.5 | 3.7 | 17.8 | ||
| 260 | 37.5 | 46.1 | 66.1 | 17.1 | 12.5 | 83.2 | 4.3 | 3.0 | 8.8 | 6.0 | 3.9 | 24.8 | ||
| 0.5 | 5 | 255 | 17.8 | 50.3 | 63.2 | 22.5 | 10.7 | 85.7 | 3.6 | 9.5 | 15.1 | 10.8 | 4.4 | 11.2 |
| 15 | 32.8 | 47.6 | 60.2 | 17.2 | 18.0 | 77.4 | 4.6 | 2.6 | 6.4 | 4.8 | 2.7 | 19.7 | ||
| 20 | 34.8 | 50.1 | 57.7 | 17.4 | 19.0 | 75.2 | 5.8 | 2.3 | 5.3 | 3.8 | 2.3 | 20.1 | ||
| 0.75 | 10 | 255 | 32.5 | 41.9 | 50.6 | 16.4 | 27.4 | 67.0 | 5.5 | 1.9 | 5.1 | 3.3 | 1.5 | 16.5 |
| 1 | 10 | 41.9 | 35.1 | 44.4 | 16.2 | 33.6 | 60.6 | 5.7 | 1.0 | 3.5 | 2.8 | 1.1 | 18.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Lin, T.; Lv, D.; An, Y.; Qi, X.; Gong, K.; Zhong, L. A CoFe Bimetallic Catalyst for the Direct Conversion of Syngas to Olefins. Catalysts 2023, 13, 1472. https://doi.org/10.3390/catal13121472
Wang X, Lin T, Lv D, An Y, Qi X, Gong K, Zhong L. A CoFe Bimetallic Catalyst for the Direct Conversion of Syngas to Olefins. Catalysts. 2023; 13(12):1472. https://doi.org/10.3390/catal13121472
Chicago/Turabian StyleWang, Xinxing, Tiejun Lin, Dong Lv, Yunlei An, Xingzhen Qi, Kun Gong, and Liangshu Zhong. 2023. "A CoFe Bimetallic Catalyst for the Direct Conversion of Syngas to Olefins" Catalysts 13, no. 12: 1472. https://doi.org/10.3390/catal13121472






