Optimizing Graphene Dopants for Direct Electrocatalytic Quantification of Small Molecules and Ions
Abstract
1. Introduction
2. Graphene as an Electrocatalyst
2.1. Properties of Graphene Relevant to Electrocatalysis
2.2. Limitations of Undoped Graphene in Electrocatalysis
2.3. Comparative Analysis with Other Catalytic Materials
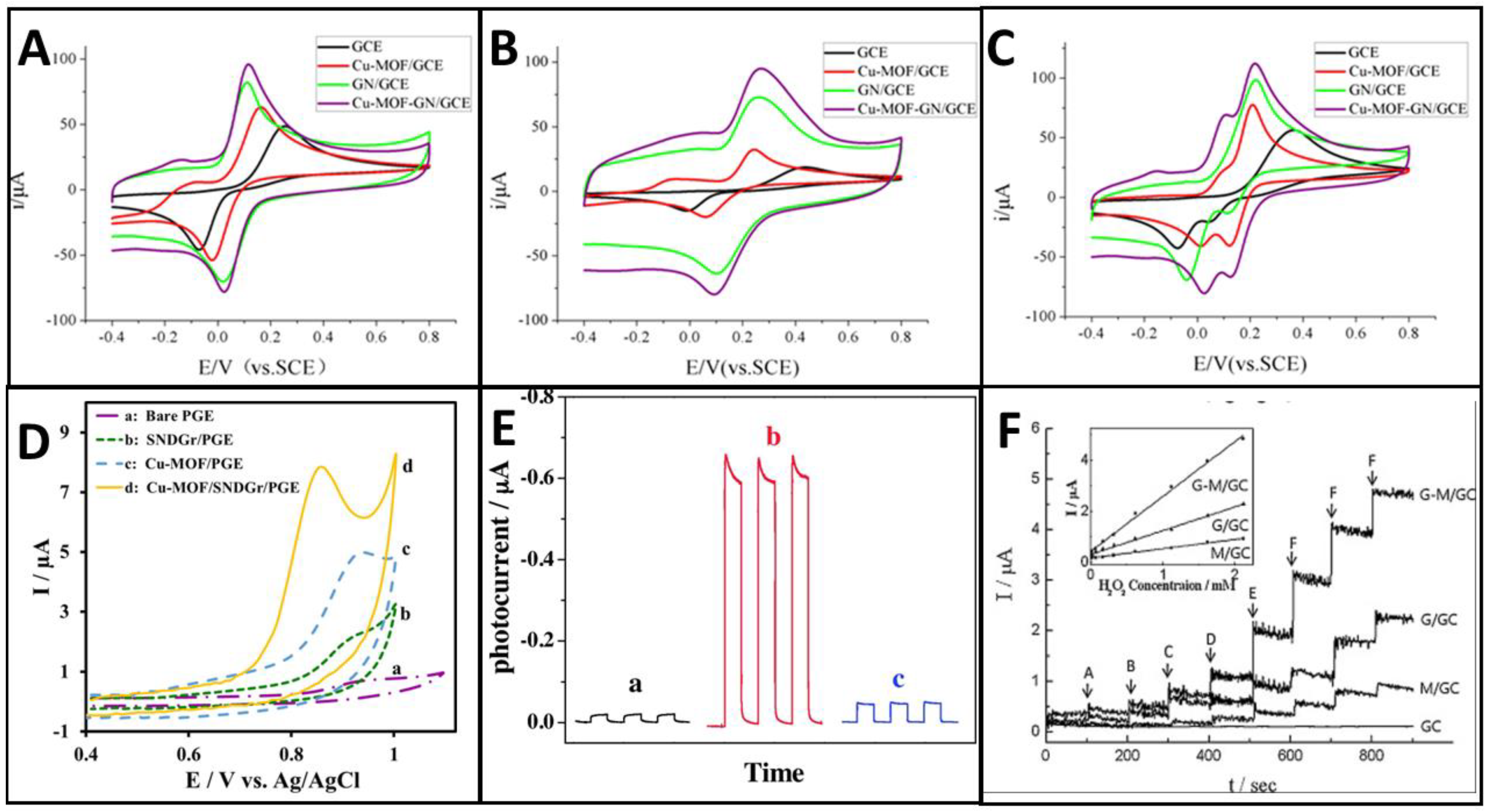
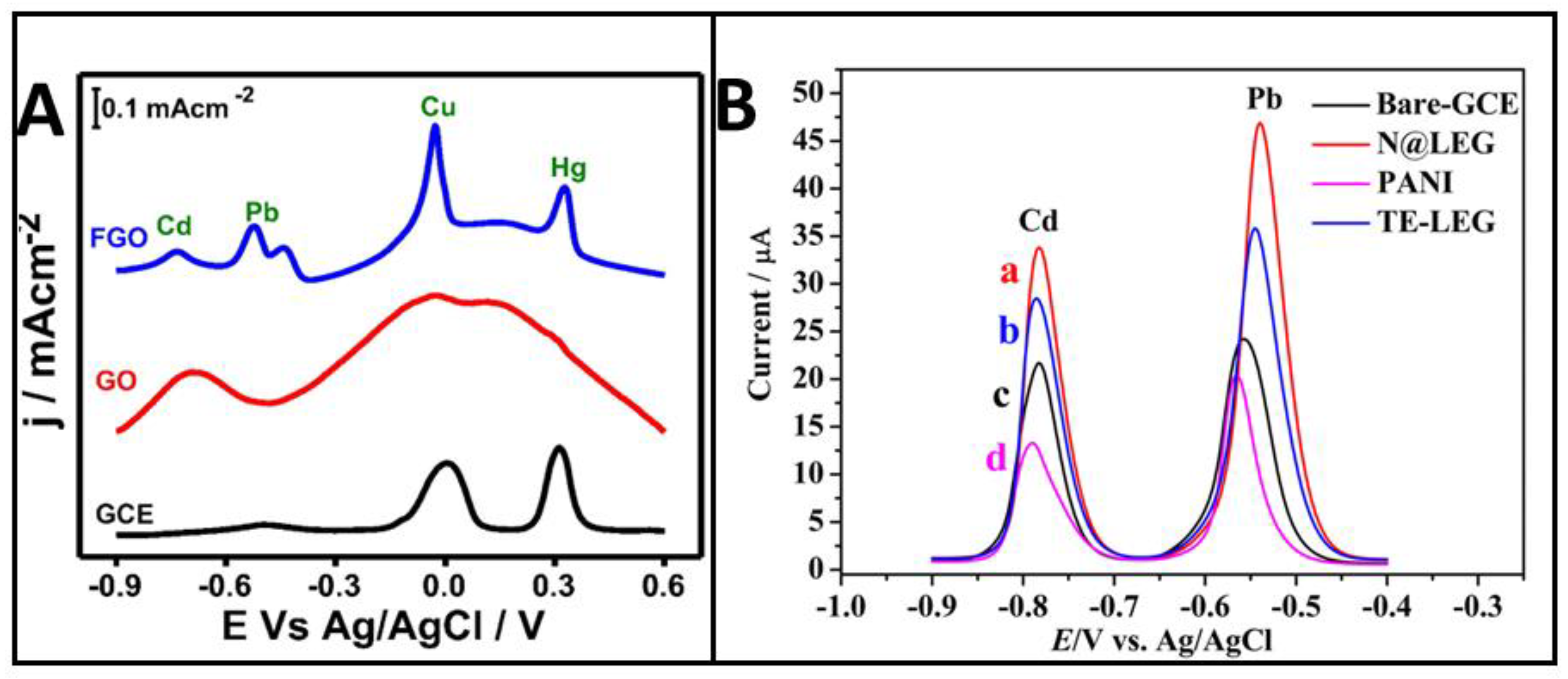
3. Dopants in Graphene and Mechanisms of Electrocatalysis
3.1. Methods of Graphene Doping
3.1.1. Substitutional Doping
3.1.2. Adsorption-Based Doping
3.2. Dynamic Doping Modulation Using External Stimuli
3.2.1. Light-Mediated Doping Modulation
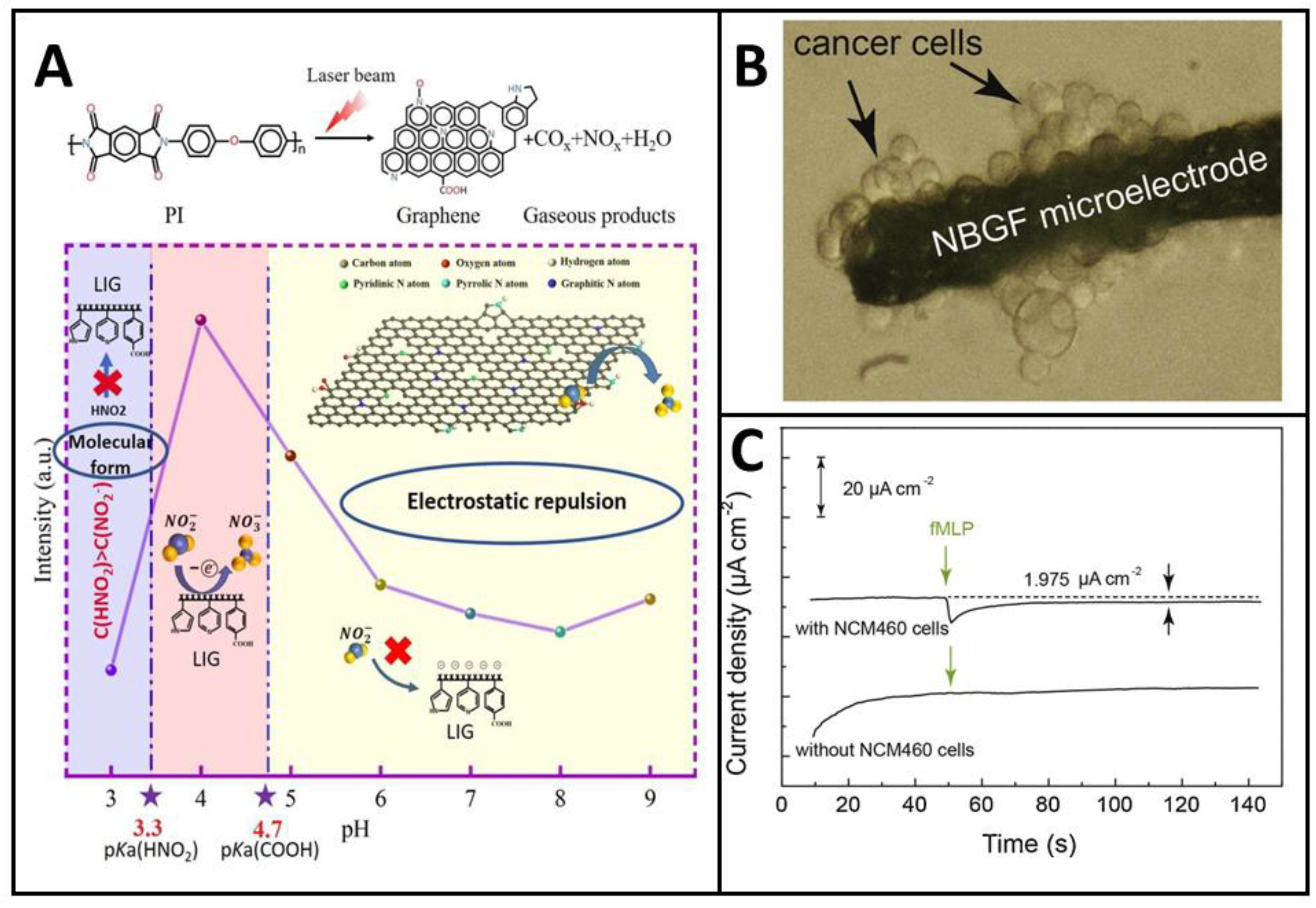
3.2.2. Chemical Cue-Responsive Doping Modulation
3.3. Influence of Dopant Type and Concentration on Electrocatalytic Activity
| Dopants | Doping Method | Analyte |
|---|---|---|
| N | CVD | DA [91,92], AA, UA, glucose [92]. |
| Thermally annealing | DA [93,94,95], AA [93,94], UA [93,94], hydroquinone [96], o-dihydroxybenzene [96]. | |
| Microwave synthesis | Acetaminophen [43], glucose [97]. | |
| Hydrothermal synthesis | H2O2 [98], glucose [97], salbutamol [99], parathion [45], adrenaline [100], nitrite [101], glycated hemoglobin [102], DA [103,104,105], AA [103], UA [103], daunorubicin [106], cholesterol [107], doxorubicin [108], rutin [109], chloramphenicol [110], diethylstilbestrol [110]. | |
| Wet chemical synthesis | Pb2+ [111], Cu2+ [111], Ha2+ [112],chloramphenicol [113], TNT [114], paraquat [115], bisphenol A [116], nicotine [117], AA [118], UA [118], DA [118,119], melatonin [119], tryptophan [119], glucose [120,121], 4-nitrophenol [122], amaranth [123], hydroquinone [124,125], catechol [124,125], acetaminophen [46]. | |
| Electrochemical exfoliation | Acetaminophen [126]. | |
| B | Atmospheric pressure carbothermal reaction | H2O2 [127]. |
| Bubbling method | Pb2+ [128]. | |
| Hydrothermal synthesis | Hydroquinone [129], catechol [129], HMX [130]. | |
| S | Thermally annealing | Hg2+ [131]. |
| Solvothermal synthesis | H2O2 [132], nilutamide [133]. | |
| Wet chemical synthesis | 8-hydroxy-2′-deoxyguanosine [134], Ha2+ [135]. | |
| F | Modified Hummers’ method | Caffeic acid [136], Cd2 + [48], Pb2 + [48], Cu2 + [48], vanillin [137]. |
| P | Hydrothermal synthesis | Acetaminophen [138], DA [139], H2O2 [140]. |
| Cl | Wet chemical synthesis | Chloramphenicol [141]. |
| I | Wet chemical synthesis | Bisphenol A [142]. |
| Ag | Wet chemical synthesis | H2O2 [143], Hg2+ [144]. |
4. Direct Electrocatalytic Quantification of Small Molecules
4.1. Importance of Detection of Small Molecules via Electrocatalytic Sensors
4.2. Techniques for Quantitative Analysis
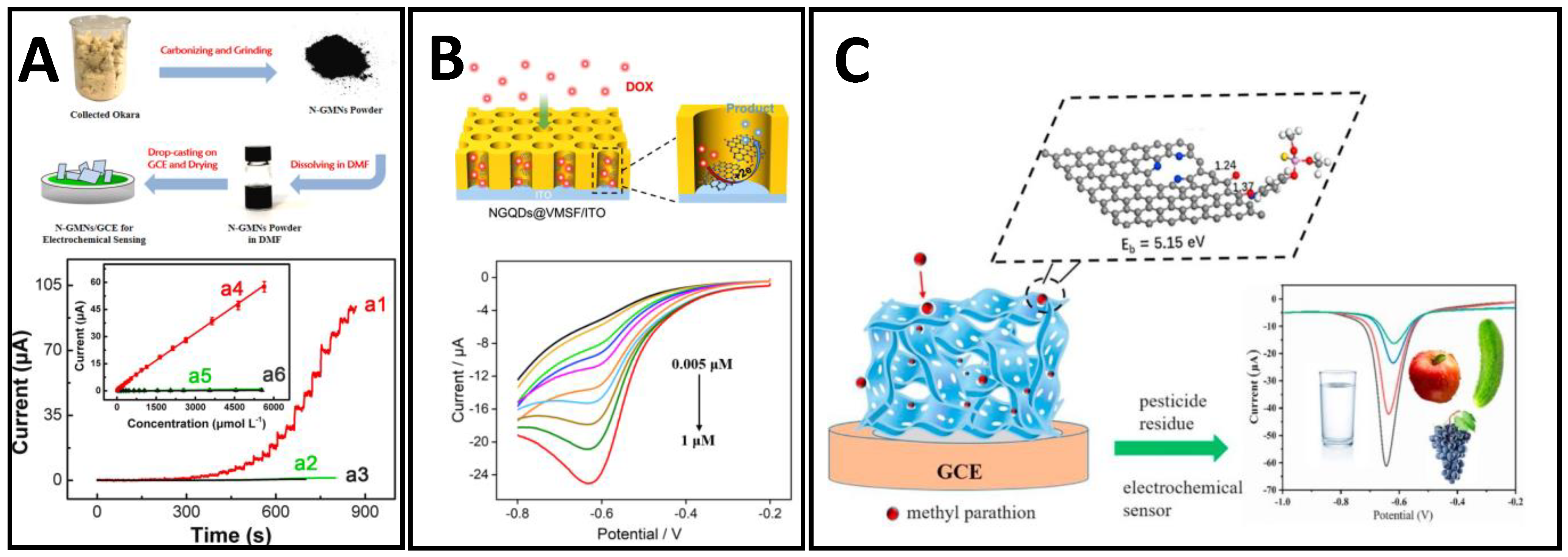
4.3. Case Studies from Current Research
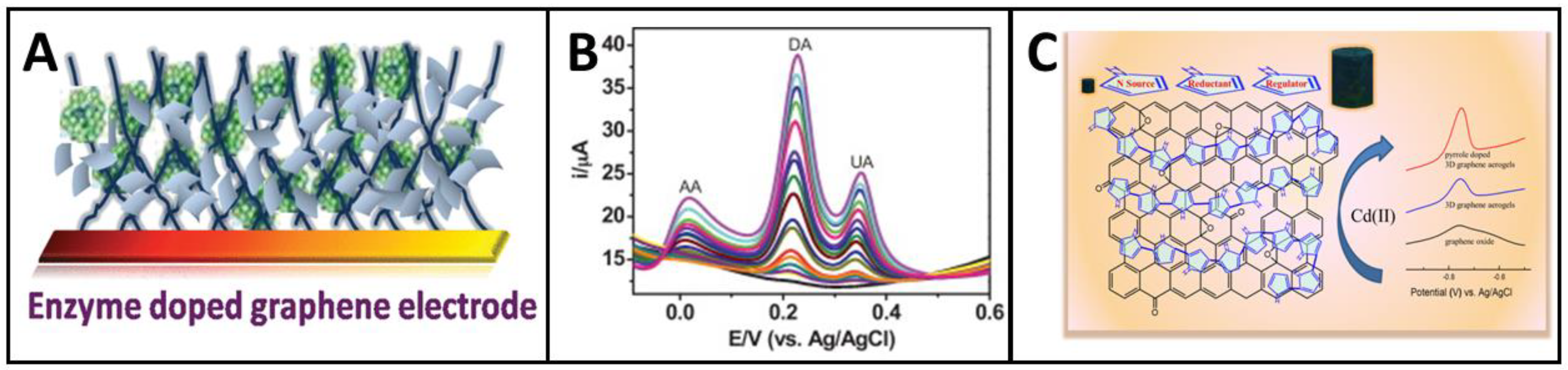
5. Computational Approaches
6. Challenges and Future Directions
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fu, L.; Mao, S.; Chen, F.; Zhao, S.; Su, W.; Lai, G.; Yu, A.; Lin, C.-T. Graphene-Based Electrochemical Sensors for Antibiotic Detection in Water, Food and Soil: A Scientometric Analysis in CiteSpace (2011–2021). Chemosphere 2022, 297, 134127. [Google Scholar] [CrossRef] [PubMed]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Graphene-Based Electrocatalysts: Hydrogen Evolution Reactions and Overall Water Splitting. Int. J. Hydrogen Energy 2021, 46, 21401–21418. [Google Scholar] [CrossRef]
- Farjadian, F.; Abbaspour, S.; Sadatlu, M.A.A.; Mirkiani, S.; Ghasemi, A.; Hoseini-Ghahfarokhi, M.; Mozaffari, N.; Karimi, M.; Hamblin, M.R. Recent Developments in Graphene and Graphene Oxide: Properties, Synthesis, and Modifications: A Review. ChemistrySelect 2020, 5, 10200–10219. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S.Z. Heteroatom-Doped Graphene-Based Materials for Energy-Relevant Electrocatalytic Processes. ACS Catal. 2015, 5, 5207–5234. [Google Scholar] [CrossRef]
- Fu, L.; Lai, G.; Jia, B.; Yu, A. Preparation and Electrocatalytic Properties of Polydopamine Functionalized Reduced Graphene Oxide-Silver Nanocomposites. Electrocatalysis 2015, 6, 72–76. [Google Scholar] [CrossRef]
- Han, F.; Li, H.; Yang, J.; Cai, X.; Fu, L. One-Pot Synthesis of Cuprous Oxide-Reduced Graphene Oxide Nanocomposite with Enhanced Photocatalytic and Electrocatalytic Performance. Phys. E Low-Dimens. Syst. Nanostruct. 2016, 77, 122–126. [Google Scholar] [CrossRef]
- Pham, T.S.H.; Fu, L.; Mahon, P.; Lai, G.; Yu, A. Fabrication of β-Cyclodextrin-Functionalized Reduced Graphene Oxide and Its Application for Electrocatalytic Detection of Carbendazim. Electrocatalysis 2016, 7, 411–419. [Google Scholar] [CrossRef]
- Xu, S.; Fu, L.; Pham, T.S.H.; Yu, A.; Han, F.; Chen, L. Preparation of ZnO Flower/Reduced Graphene Oxide Composite with Enhanced Photocatalytic Performance under Sunlight. Ceram. Int. 2015, 41, 4007–4013. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Z.; Peng, F.; Wang, A.; Cai, X.; Fu, L. Growth of Cu2O Nanoparticle on Reduced Graphene Sheets with High Photocatalytic Activity for Degradation of Rhodamine B. Fuller. Nanotub. Carbon Nanostruct. 2016, 24, 149–153. [Google Scholar] [CrossRef]
- Coroş, M.; Pruneanu, S.; Staden, R.-I.S. Review—Recent Progress in the Graphene-Based Electrochemical Sensors and Biosensors. J. Electrochem. Soc. 2019, 167, 037528. [Google Scholar] [CrossRef]
- Qazzazie, D.; Beckert, M.; Mülhaupt, R.; Yurchenko, O.; Urban, G. A Nitrogen-Doped Graphene Electrocatalyst for Selective Oxygen Reduction in Presence of Glucose and D-Gluconic Acid in pH-Neutral Media. Electrochim. Acta 2015, 186, 579–590. [Google Scholar] [CrossRef]
- Li, M.; Liu, C.; Zhao, H.; An, H.; Cao, H.; Zhang, Y.; Fan, Z. Tuning Sulfur Doping in Graphene for Highly Sensitive Dopamine Biosensors. Carbon 2015, 86, 197–206. [Google Scholar] [CrossRef]
- Feng, L.; Qin, Z.; Huang, Y.; Peng, K.; Wang, F.; Yan, Y.; Chen, Y. Boron-, Sulfur-, and Phosphorus-Doped Graphene for Environmental Applications. Sci. Total Environ. 2020, 698, 134239. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Liu, T.; Wang, Y.; Miao, P. Electrochemical Aptasensors for Detection of Small Molecules, Macromolecules, and Cells. Rev. Anal. Chem. 2016, 35, 201–211. [Google Scholar] [CrossRef]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical Glucose Sensors in Diabetes Management: An Updated Review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Lakard, S.; Pavel, I.-A.; Lakard, B. Electrochemical Biosensing of Dopamine Neurotransmitter: A Review. Biosensors 2021, 11, 179. [Google Scholar] [CrossRef]
- Piro, B.; Shi, S.; Reisberg, S.; Noël, V.; Anquetin, G. Comparison of Electrochemical Immunosensors and Aptasensors for Detection of Small Organic Molecules in Environment, Food Safety, Clinical and Public Security. Biosensors 2016, 6, 7. [Google Scholar] [CrossRef]
- Zheng, Y.; Mao, S.; Zhu, J.; Fu, L.; Moghadam, M. A Scientometric Study on Application of Electrochemical Sensors for Detection of Pesticide Using Graphene-Based Electrode Modifiers. Chemosphere 2022, 307, 136069. [Google Scholar] [CrossRef]
- Shi, H.; Fu, L.; Chen, F.; Zhao, S.; Lai, G. Preparation of Highly Sensitive Electrochemical Sensor for Detection of Nitrite in Drinking Water Samples. Environ. Res. 2022, 209, 112747. [Google Scholar] [CrossRef]
- Ziółkowski, R.; Jarczewska, M.; Górski, Ł.; Malinowska, E. From Small Molecules toward Whole Cells Detection: Application of Electrochemical Aptasensors in Modern Medical Diagnostics. Sensors 2021, 21, 724. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, Y. Designing Recognition Molecules and Tailoring Functional Surfaces for in Vivo Monitoring of Small Molecules in the Brain. Acc. Chem. Res. 2018, 51, 688–696. [Google Scholar] [CrossRef]
- Ramachandran, R.; Chen, T.-W.; Chen, S.-M.; Baskar, T.; Kannan, R.; Elumalai, P.; Raja, P.; Jeyapragasam, T.; Dinakaran, K. A Review of the Advanced Developments of Electrochemical Sensors for the Detection of Toxic and Bioactive Molecules. Inorg. Chem. Front. 2019, 6, 3418–3439. [Google Scholar] [CrossRef]
- Sarakhman, O.; Švorc, Ľ. A Review on Recent Advances in the Applications of Boron-Doped Diamond Electrochemical Sensors in Food Analysis. Crit. Rev. Anal. Chem. 2022, 52, 791–813. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Orooji, Y.; Karimi, F.; Alizadeh, M.; Baghayeri, M.; Rouhi, J.; Tajik, S.; Beitollahi, H.; Agarwal, S.; Gupta, V.K. A Critical Review on the Use of Potentiometric Based Biosensors for Biomarkers Detection. Biosens. Bioelectron. 2021, 184, 113252. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wu, M.; Shi, M.; Shi, P.; Zhao, N.; Zhu, Y.; Karimi-Maleh, H.; Ye, C.; Lin, C.-T.; Fu, L. An Overview to Molecularly Imprinted Electrochemical Sensors for the Detection of Bisphenol A. Sensors 2023, 23, 8656. [Google Scholar] [CrossRef] [PubMed]
- Fu, L. Book Review: “Emerging Freshwater Pollutants: Analysis, Fate and Regulations,” Tatenda Dalu, Nikita T. Tavengwa (Ed.), Elsevier, 391p., 2022, ISBN 978-0-12-822850-0, eBook ISBN: 978-0-32-390315-8. Environ. Process. 2023, 10, 39. [Google Scholar] [CrossRef]
- Rizzi, L.; Zienert, A.; Schuster, J.; Köhne, M.; Schulz, S.E. Electrical Conductivity Modeling of Graphene-Based Conductor Materials. ACS Appl. Mater. Interfaces 2018, 10, 43088–43094. [Google Scholar] [CrossRef]
- Velasco, A.; Ryu, Y.K.; Boscá, A.; Ladrón-de-Guevara, A.; Hunt, E.; Zuo, J.; Pedrós, J.; Calle, F.; Martinez, J. Recent Trends in Graphene Supercapacitors: From Large Area to Microsupercapacitors. Sustain. Energy Fuels 2021, 5, 1235–1254. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Liu, J.; Bao, C. Measuring the Specific Surface Area of Monolayer Graphene Oxide in Water. Mater. Lett. 2020, 261, 127098. [Google Scholar] [CrossRef]
- Głuchowski, P.; Tomala, R.; Jeżowski, A.; Szewczyk, D.; Macalik, B.; Smolina, I.; Kurzynowski, T.; Stręk, W. Preparation and Physical Characteristics of Graphene Ceramics. Sci. Rep. 2020, 10, 11121. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Guo, Z.; Xu, J.; Wang, H.; Zhai, K.; Zhuo, X. A Novel Hydrazine Electrochemical Sensor Based on the High Specific Surface Area Graphene. Microchim. Acta 2010, 169, 1–6. [Google Scholar] [CrossRef]
- Killedar, L.S.; Shanbhag, M.M.; Manasa, G.; Malode, S.J.; Veerapur, R.S.; Shetti, N.P.; Mascarenhas, R.J.; Kakarla, R.R. An Electrochemical Sensor Based on Graphene Oxide/Cholesterol Nanohybrids for the Sensitive Analysis of Cetirizine. J. Environ. Chem. Eng. 2022, 10, 108894. [Google Scholar] [CrossRef]
- Kang, X.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. A Graphene-Based Electrochemical Sensor for Sensitive Detection of Paracetamol. Talanta 2010, 81, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Pandikumar, A.; How, G.T.S.; Peik See, T.; Saiha Omar, F.; Jayabal, S.; Zangeneh Kamali, K.; Yusoff, N.; Jamil, A.; Ramaraj, R.; Abraham John, S.; et al. Graphene and Its Nanocomposite Material Based Electrochemical Sensor Platform for Dopamine. RSC Adv. 2014, 4, 63296–63323. [Google Scholar] [CrossRef]
- Baig, N.; Waheed, A.; Sajid, M.; Khan, I.; Kawde, A.-N.; Sohail, M. Porous Graphene-Based Electrodes: Advances in Electrochemical Sensing of Environmental Contaminants. Trends Environ. Anal. Chem. 2021, 30, e00120. [Google Scholar] [CrossRef]
- Yola, M.L. Development of Novel Nanocomposites Based on Graphene/Graphene Oxide and Electrochemical Sensor Applications. Curr. Anal. Chem. 2019, 15, 159–165. [Google Scholar] [CrossRef]
- Song, J.; Li, Y.; Yin, F.; Zhang, Z.; Ke, D.; Wang, D.; Yuan, Q.; Zhang, X.-E. Enhanced Electrochemical Impedance Spectroscopy Analysis of Microbial Biofilms on an Electrochemically In Situ Generated Graphene Interface. ACS Sens. 2020, 5, 1795–1803. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.; Ding, H.; Zhao, X.; Zhang, L.; Bai, J.; Liu, K. Bioelectronics-Related 2D Materials Beyond Graphene: Fundamentals, Properties, and Applications. Adv. Funct. Mater. 2020, 30, 2003732. [Google Scholar] [CrossRef]
- Kumunda, C.; Adekunle, A.S.; Mamba, B.B.; Hlongwa, N.W.; Nkambule, T.T.I. Electrochemical Detection of Environmental Pollutants Based on Graphene Derivatives: A Review. Front. Mater. 2021, 7, 616787. [Google Scholar] [CrossRef]
- Grajek, H.; Jonik, J.; Witkiewicz, Z.; Wawer, T.; Purchała, M. Applications of Graphene and Its Derivatives in Chemical Analysis. Crit. Rev. Anal. Chem. 2020, 50, 445–471. [Google Scholar] [CrossRef]
- Mackenzie, D.M.A.; Buron, J.D.; Whelan, P.R.; Caridad, J.M.; Bjergfelt, M.; Luo, B.; Shivayogimath, A.; Smitshuysen, A.L.; Thomsen, J.D.; Booth, T.J.; et al. Quality Assessment of Graphene: Continuity, Uniformity, and Accuracy of Mobility Measurements. Nano Res. 2017, 10, 3596–3605. [Google Scholar] [CrossRef]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Antonietti, M.; García, H. Active Sites on Graphene-Based Materials as Metal-Free Catalysts. Chem. Soc. Rev. 2017, 46, 4501–4529. [Google Scholar] [CrossRef] [PubMed]
- Anuar, N.S.; Basirun, W.J.; Ladan, M.; Shalauddin, M.; Mehmood, M.S. Fabrication of Platinum Nitrogen-Doped Graphene Nanocomposite Modified Electrode for the Electrochemical Detection of Acetaminophen. Sens. Actuators B Chem. 2018, 266, 375–383. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S. Graphene and Its Derivative-Based Sensing Materials for Analytical Devices. J. Mater. Chem. 2011, 21, 18503–18516. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Yang, Y.; Shi, X.; Zhang, L.; Jia, G. Hierarchical Nitrogen-Doped Holey Graphene as Sensitive Electrochemical Sensor for Methyl Parathion Detection. Sens. Actuators B Chem. 2021, 336, 129721. [Google Scholar] [CrossRef]
- Cao, Y.; Si, W.; Zhang, Y.; Hao, Q.; Lei, W.; Xia, X.; Li, J.; Wang, F. Nitrogen-Doped Graphene: Effect of Graphitic-N on the Electrochemical Sensing Properties towards Acetaminophen. FlatChem 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Zuo, Y.; Xu, J.; Zhu, X.; Duan, X.; Lu, L.; Yu, Y. Graphene-Derived Nanomaterials as Recognition Elements for Electrochemical Determination of Heavy Metal Ions: A Review. Microchim. Acta 2019, 186, 171. [Google Scholar] [CrossRef]
- Thiruppathi, A.R.; Sidhureddy, B.; Keeler, W.; Chen, A. Facile One-Pot Synthesis of Fluorinated Graphene Oxide for Electrochemical Sensing of Heavy Metal Ions. Electrochem. Commun. 2017, 76, 42–46. [Google Scholar] [CrossRef]
- Lin, X.; Lu, Z.; Dai, W.; Liu, B.; Zhang, Y.; Li, J.; Ye, J. Laser Engraved Nitrogen-Doped Graphene Sensor for the Simultaneous Determination of Cd(II) and Pb(II). J. Electroanal. Chem. 2018, 828, 41–49. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, D.-W.; Amal, R.; Dai, L. Carbon-Based Metal-Free Catalysts for Key Reactions Involved in Energy Conversion and Storage. Adv. Mater. 2019, 31, 1801526. [Google Scholar] [CrossRef]
- Song, H.; Zhang, X.; Liu, Y.; Su, Z. Developing Graphene-Based Nanohybrids for Electrochemical Sensing. Chem. Rec. 2019, 19, 534–549. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Hajian, A.; Rezaei, M.; Shirzadmehr, A. Composite of Cu Metal Nanoparticles-Multiwall Carbon Nanotubes-Reduced Graphene Oxide as a Novel and High Performance Platform of the Electrochemical Sensor for Simultaneous Determination of Nitrite and Nitrate. J. Hazard. Mater. 2017, 324, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, B.; Zhong, J.; Yan, B.; Zhang, K.; Zhai, C.; Shiraishi, Y.; Du, Y.; Yang, P. Dopamine and Uric Acid Electrochemical Sensor Based on a Glassy Carbon Electrode Modified with Cubic Pd and Reduced Graphene Oxide Nanocomposite. J. Colloid Interface Sci. 2017, 497, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.M.; Martins, P.R.; Rocha, D.P.; Matias, T.A.; Juliao, M.S.; Munoz, R.A.; Angnes, L. Recent Trends and Perspectives in Electrochemical Sensors Based on MOF-Derived Materials. J. Mater. Chem. C 2021, 9, 8718–8745. [Google Scholar] [CrossRef]
- Li, J.; Xia, J.; Zhang, F.; Wang, Z.; Liu, Q. An Electrochemical Sensor Based on Copper-Based Metal-Organic Frameworks-Graphene Composites for Determination of Dihydroxybenzene Isomers in Water. Talanta 2018, 181, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Habibi, B.; Pashazadeh, S.; Saghatforoush, L.A.; Pashazadeh, A. Direct Electrochemical Synthesis of the Copper Based Metal-Organic Framework on/in the Heteroatoms Doped Graphene/Pencil Graphite Electrode: Highly Sensitive and Selective Electrochemical Sensor for Sertraline Hydrochloride. J. Electroanal. Chem. 2021, 888, 115210. [Google Scholar] [CrossRef]
- Riaz, R.; Ali, M.; Anwer, H.; Ko, M.J.; Jeong, S.H. Highly Porous Self-Assembly of Nitrogen-Doped Graphene Quantum Dots over Reduced Graphene Sheets for Photo-Electrocatalytic Electrode. J. Colloid Interface Sci. 2019, 557, 174–184. [Google Scholar] [CrossRef]
- Vilian, A.E.; Rajkumar, M.; Chen, S.-M.; Hu, C.-C.; Piraman, S. A Promising Photoelectrochemical Sensor Based on a ZnO Particle Decorated N-Doped Reduced Graphene Oxide Modified Electrode for Simultaneous Determination of Catechol and Hydroquinone. RSC Adv. 2014, 4, 48522–48534. [Google Scholar] [CrossRef]
- Hallaj, R.; Haghighi, N. Photoelectrochemical Amperometric Sensing of Cyanide Using a Glassy Carbon Electrode Modified with Graphene Oxide and Titanium Dioxide Nanoparticles. Microchim. Acta 2017, 184, 3581–3590. [Google Scholar] [CrossRef]
- Azis, T.; Muzakkar, M.Z.; Nurwahida, A.T.; Dali, N.; Kadir, L.O.A.; Lestari, D.A.; Salim, L.O.A. ZnO-Enhanced Reduced Graphene Oxide Electrodes from Cocoa Shell: Nanoarchitectonics Platform for Photoelectrocatalytic Detection of Methylene Blue. J. Oleo Sci. 2023, 72, 1133–1140. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Q.; Du, X.; Qian, J.; Mao, H.; Wang, K. Visible Light Photoelectrochemical Sensor for Ultrasensitive Determination of Dopamine Based on Synergistic Effect of Graphene Quantum Dots and TiO2 Nanoparticles. Anal. Chim. Acta 2015, 853, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Beitollahi, H.; Movahedifar, F.; Tajik, S.; Jahani, S. A Review on the Effects of Introducing CNTs in the Modification Process of Electrochemical Sensors. Electroanalysis 2019, 31, 1195–1203. [Google Scholar] [CrossRef]
- Dumitrescu, I.; Unwin, P.R.; Macpherson, J.V. Electrochemistry at Carbon Nanotubes: Perspective and Issues. Chem. Commun. 2009, 45, 6886–6901. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Kim, Y.-R.; Chung, T.D.; Piao, Y.; Kim, H. Synthesis of a Graphene–Carbon Nanotube Composite and Its Electrochemical Sensing of Hydrogen Peroxide. Electrochim. Acta 2012, 59, 509–514. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-Doped Graphene by Chemical Vapor Deposition and Its Electrical Properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Zan, R.; Altuntepe, A. Nitrogen Doping of Graphene by CVD. J. Mol. Struct. 2020, 1199, 127026. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, W.; Zhao, Z.; Zhuang, P.; Zhan, L.; Zhou, Y.; Cai, W. CVD Synthesis of Nitrogen-Doped Graphene Using Urea. Sci. China Phys. Mech. Astron. 2015, 58, 107801. [Google Scholar] [CrossRef]
- Zan, R.; Altuntepe, A.; Erkan, S. Substitutional Boron Doping of Graphene Using Diborane in CVD. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 128, 114629. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Wu, D.; Liao, L.; Zhao, S.; Peng, H.; Liu, Z. Synthesis of Boron-Doped Graphene Monolayers Using the Sole Solid Feedstock by Chemical Vapor Deposition. Small 2013, 9, 1316–1320. [Google Scholar] [CrossRef]
- Gebhardt, J.; Koch, R.J.; Zhao, W.; Höfert, O.; Gotterbarm, K.; Mammadov, S.; Papp, C.; Görling, A.; Steinrück, H.-P.; Seyller, T. Growth and Electronic Structure of Boron-Doped Graphene. Phys. Rev. B 2013, 87, 155437. [Google Scholar] [CrossRef]
- Asen, P.; Shahrokhian, S.; Zad, A.I. Transition Metal Ions-Doped Polyaniline/Graphene Oxide Nanostructure as High Performance Electrode for Supercapacitor Applications. J. Solid State Electrochem. 2018, 22, 983–996. [Google Scholar] [CrossRef]
- Güneş, F.; Shin, H.-J.; Biswas, C.; Han, G.H.; Kim, E.S.; Chae, S.J.; Choi, J.-Y.; Lee, Y.H. Layer-by-Layer Doping of Few-Layer Graphene Film. ACS Nano 2010, 4, 4595–4600. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Li, X.; You, R.; Chen, M.; Yuan, R.; Huang, W.; Wu, X.; Chu, W.; Wu, C.; Xie, Y. Surface Immobilization of Transition Metal Ions on Nitrogen-Doped Graphene Realizing High-Efficient and Selective CO2 Reduction. Adv. Mater. 2018, 30, 1706617. [Google Scholar] [CrossRef] [PubMed]
- Paidi, V.K.; Jung, E.; Lee, J.; Lee, A.T.; Shepit, M.; Ihm, K.; Lee, B.-H.; van Lierop, J.; Hyeon, T.; Lee, K.-S. Robust Room Temperature Ferromagnetism In Cobalt Doped Graphene by Precision Control of Metal Ion Hybridization. Adv. Funct. Mater. 2023, 33, 2210722. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, Y.; Li, S.; Liu, W. Many-Body Dispersion Effects on the Binding of TCNQ and F4-TCNQ with Graphene. Carbon 2017, 111, 513–518. [Google Scholar] [CrossRef]
- Pinto, H.; Jones, R.; Goss, J.; Briddon, P. P-Type Doping of Graphene with F4-TCNQ. J. Phys. Condens. Matter 2009, 21, 402001. [Google Scholar] [CrossRef]
- Lee, H.; Paeng, K.; Kim, I.S. A Review of Doping Modulation in Graphene. Synth. Met. 2018, 244, 36–47. [Google Scholar] [CrossRef]
- Bagsican, F.R.; Winchester, A.; Ghosh, S.; Zhang, X.; Ma, L.; Wang, M.; Murakami, H.; Talapatra, S.; Vajtai, R.; Ajayan, P.M.; et al. Adsorption Energy of Oxygen Molecules on Graphene and Two-Dimensional Tungsten Disulfide. Sci. Rep. 2017, 7, 1774. [Google Scholar] [CrossRef]
- Luo, Z.; Pinto, N.J.; Davila, Y.; Charlie Johnson, A.T. Controlled Doping of Graphene Using Ultraviolet Irradiation. Appl. Phys. Lett. 2012, 100, 253108. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Khan, M.F.; Iqbal, M.W.; Eom, J. Tuning the Electrical Properties of Exfoliated Graphene Layers Using Deep Ultraviolet Irradiation. J. Mater. Chem. C 2014, 2, 5404–5410. [Google Scholar] [CrossRef]
- Döbbelin, M.; Ciesielski, A.; Haar, S.; Osella, S.; Bruna, M.; Minoia, A.; Grisanti, L.; Mosciatti, T.; Richard, F.; Prasetyanto, E.A.; et al. Light-Enhanced Liquid-Phase Exfoliation and Current Photoswitching in Graphene–Azobenzene Composites. Nat. Commun. 2016, 7, 11090. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.-R.; Jeon, E.K.; Kang, D.; Kim, G.; Kim, B.-S.; Kang, D.J.; Shin, H.S. Reversibly Light-Modulated Dirac Point of Graphene Functionalized with Spiropyran. ACS Nano 2012, 6, 9207–9213. [Google Scholar] [CrossRef] [PubMed]
- Austria, H.F.M.; Subrahmanya, T.M.; Setiawan, O.; Widakdo, J.; Chiao, Y.-H.; Hung, W.-S.; Wang, C.-F.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. A Review on the Recent Advancements in Graphene-Based Membranes and Their Applications as Stimuli-Responsive Separation Materials. J. Mater. Chem. A 2021, 9, 21510–21531. [Google Scholar] [CrossRef]
- Jang, S.K.; Jang, J.; Choe, W.-S.; Lee, S. Harnessing Denatured Protein for Controllable Bipolar Doping of a Monolayer Graphene. ACS Appl. Mater. Interfaces 2015, 7, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Hess, L.H.; Lyuleeva, A.; Blaschke, B.M.; Sachsenhauser, M.; Seifert, M.; Garrido, J.A.; Deubel, F. Graphene Transistors with Multifunctional Polymer Brushes for Biosensing Applications. ACS Appl. Mater. Interfaces 2014, 6, 9705–9710. [Google Scholar] [CrossRef] [PubMed]
- Akbari-Sharbaf, A.; Ezugwu, S.; Ahmed, M.S.; Cottam, M.G.; Fanchini, G. Doping Graphene Thin Films with Metallic Nanoparticles: Experiment and Theory. Carbon 2015, 95, 199–207. [Google Scholar] [CrossRef]
- Marinoiu, A.; Raceanu, M.; Carcadea, E.; Varlam, M.; Soare, A.; Stefanescu, I. Doped Graphene as Non-Metallic Catalyst for Fuel Cells. Mater. Sci. 2017, 23, 108–113. [Google Scholar] [CrossRef][Green Version]
- Megawati, M.; Chua, C.K.; Sofer, Z.; Klímová, K.; Pumera, M. Nitrogen-Doped Graphene: Effect of Graphite Oxide Precursors and Nitrogen Content on the Electrochemical Sensing Properties. Phys. Chem. Chem. Phys. 2017, 19, 15914–15923. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, J.; Ling, Y.; Yu, S.; Li, S.; Wu, X.; Zhang, Z. A Facile and Efficient Nitrite Electrochemical Sensor Based on N, O Co-Doped Porous Graphene Film. Microchem. J. 2022, 178, 107361. [Google Scholar] [CrossRef]
- Zhao, A.; She, J.; Xiao, C.; Xi, J.; Xu, Y.; Manoj, D.; Sun, Y.; Xiao, F. Green and Controllable Synthesis of Multi-Heteroatoms Co-Doped Graphene Fiber as Flexible and Biocompatible Microelectrode for in Situ Electrochemical Detection of Biological Samples. Sens. Actuators B Chem. 2021, 335, 129683. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Y.; Zhou, J.; Li, Y.; Chen, S.; Zhang, L.; Ma, Y.; Wang, L.; Yan, X. Three-Dimensional Nitrogen-Doped Graphene as an Ultrasensitive Electrochemical Sensor for the Detection of Dopamine. Nanoscale 2015, 7, 2427–2432. [Google Scholar] [CrossRef] [PubMed]
- Thanh, T.D.; Balamurugan, J.; Lee, S.H.; Kim, N.H.; Lee, J.H. Effective Seed-Assisted Synthesis of Gold Nanoparticles Anchored Nitrogen-Doped Graphene for Electrochemical Detection of Glucose and Dopamine. Biosens. Bioelectron. 2016, 81, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.-H.; Zheng, X.-Q.; Xu, J.-Y.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Electrochemical Sensor Based on Nitrogen Doped Graphene: Simultaneous Determination of Ascorbic Acid, Dopamine and Uric Acid. Biosens. Bioelectron. 2012, 34, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-M.; Yang, S.-Y.; Wang, Y.-S.; Lien, C.-H.; Tien, H.-W.; Hsiao, S.-T.; Liao, W.-H.; Tsai, H.-P.; Chang, C.-L.; Ma, C.-C.M.; et al. Controllable Synthesis of Nitrogen-Doped Graphene and Its Effect on the Simultaneous Electrochemical Determination of Ascorbic Acid, Dopamine, and Uric Acid. Carbon 2013, 59, 418–429. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Y.; Liu, Y.; Li, J. Selective Electrochemical Detection of Dopamine Using Nitrogen-Doped Graphene/Manganese Monoxide Composites. RSC Adv. 2015, 5, 85065–85072. [Google Scholar] [CrossRef]
- Juanjuan, Z.; Ruiyi, L.; Zaijun, L.; Junkang, L.; Zhiguo, G.; Guangli, W. Synthesis of Nitrogen-Doped Activated Graphene Aerogel/Gold Nanoparticles and Its Application for Electrochemical Detection of Hydroquinone and o-Dihydroxybenzene. Nanoscale 2014, 6, 5458–5466. [Google Scholar] [CrossRef] [PubMed]
- Rahsepar, M.; Foroughi, F.; Kim, H. A New Enzyme-Free Biosensor Based on Nitrogen-Doped Graphene with High Sensing Performance for Electrochemical Detection of Glucose at Biological pH Value. Sens. Actuators B Chem. 2019, 282, 322–330. [Google Scholar] [CrossRef]
- Ju, J.; Chen, W. In Situ Growth of Surfactant-Free Gold Nanoparticles on Nitrogen-Doped Graphene Quantum Dots for Electrochemical Detection of Hydrogen Peroxide in Biological Environments. Anal. Chem. 2015, 87, 1903–1910. [Google Scholar] [CrossRef]
- Li, J.; Xu, Z.; Liu, M.; Deng, P.; Tang, S.; Jiang, J.; Feng, H.; Qian, D.; He, L. Ag/N-Doped Reduced Graphene Oxide Incorporated with Molecularly Imprinted Polymer: An Advanced Electrochemical Sensing Platform for Salbutamol Determination. Biosens. Bioelectron. 2017, 90, 210–216. [Google Scholar] [CrossRef]
- Chen, S.; Shi, M.; Xu, Q.; Xu, J.; Duan, X.; Gao, Y.; Lu, L.; Gao, F.; Wang, X.; Yu, Y. Ti3C2Tx MXene/Nitrogen-Doped Reduced Graphene Oxide Composite: A High-Performance Electrochemical Sensing Platform for Adrenaline Detection. Nanotechnology 2021, 32, 265501. [Google Scholar] [CrossRef]
- Li, L.; Liu, D.; Wang, K.; Mao, H.; You, T. Quantitative Detection of Nitrite with N-Doped Graphene Quantum Dots Decorated N-Doped Carbon Nanofibers Composite-Based Electrochemical Sensor. Sens. Actuators B Chem. 2017, 252, 17–23. [Google Scholar] [CrossRef]
- Jain, U.; Chauhan, N. Glycated Hemoglobin Detection with Electrochemical Sensing Amplified by Gold Nanoparticles Embedded N-Doped Graphene Nanosheet. Biosens. Bioelectron. 2017, 89, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yu, L.; Yan, M.; Ye, J.; Huang, J.; Yang, X. Holey Nitrogen-Doped Graphene Aerogel for Simultaneously Electrochemical Determination of Ascorbic Acid, Dopamine and Uric Acid. Talanta 2021, 224, 121851. [Google Scholar] [CrossRef] [PubMed]
- Ai, S.; Chen, Y.; Liu, Y.; Zhang, Q.; Xiong, L.; Huang, H.; Li, L.; Yu, X.; Wei, L. Facile Synthesis of Nitrogen-Doped Graphene Aerogels for Electrochemical Detection of Dopamine. Solid State Sci. 2018, 86, 6–11. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, X.; Zheng, J. A Facile One-Step Synthesis of Fe2O3/Nitrogen-Doped Reduced Graphene Oxide Nanocomposite for Enhanced Electrochemical Determination of Dopamine. J. Alloys Compd. 2017, 709, 581–587. [Google Scholar] [CrossRef]
- Kong, F.-Y.; Yao, L.; Li, R.-F.; Li, H.-Y.; Wang, Z.-X.; Lv, W.-X.; Wang, W. Synthesis of Nitrogen-Doped Reduced Graphene Oxide Loading with Au-Ag Bimetallic Nanoparticles for Electrochemical Detection of Daunorubicin. J. Alloys Compd. 2019, 797, 413–420. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Doong, R. Functionalized N-Doped Graphene Quantum Dots for Electrochemical Determination of Cholesterol through Host-Guest Inclusion. Microchim. Acta 2018, 185, 526. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, X.; Yan, F.; Lin, J. N-Doped Graphene Quantum Dots Confined within Silica Nanochannels for Enhanced Electrochemical Detection of Doxorubicin. Molecules 2023, 28, 6443. [Google Scholar] [CrossRef]
- Zhen, Q.; Ma, H.; Jin, Z.; Zhu, D.; Liu, X.; Sun, Y.; Zhang, C.; Pang, H. Electrochemical Sensor for Rutin Detection Based on N-Doped Mesoporous Carbon Nanospheres and Graphene. New J. Chem. 2021, 45, 4986–4993. [Google Scholar] [CrossRef]
- Pakapongpan, S.; Poo-arporn, Y.; Tuantranont, A.; Poo-arporn, R.P. A Facile One-Pot Synthesis of Magnetic Iron Oxide Nanoparticles Embed N-Doped Graphene Modified Magnetic Screen Printed Electrode for Electrochemical Sensing of Chloramphenicol and Diethylstilbestrol. Talanta 2022, 241, 123184. [Google Scholar] [CrossRef]
- Lei, C.; Zhang, S.; Zhao, S. Synthesis of N-Doped Graphene for Simultaneous Electrochemical Detection of Lead and Copper in Water. Int. J. Electrochem. Sci. 2017, 12, 4856–4866. [Google Scholar] [CrossRef]
- Wen, G.-L.; Zhao, W.; Chen, X.; Liu, J.-Q.; Wang, Y.; Zhang, Y.; Huang, Z.-J.; Wu, Y.-C. N-Doped Reduced Graphene Oxide/MnO2 Nanocomposite for Electrochemical Detection of Hg2+ by Square Wave Stripping Voltammetry. Electrochim. Acta 2018, 291, 95–102. [Google Scholar] [CrossRef]
- Borowiec, J.; Wang, R.; Zhu, L.; Zhang, J. Synthesis of Nitrogen-Doped Graphene Nanosheets Decorated with Gold Nanoparticles as an Improved Sensor for Electrochemical Determination of Chloramphenicol. Electrochim. Acta 2013, 99, 138–144. [Google Scholar] [CrossRef]
- Cai, Z.; Li, F.; Wu, P.; Ji, L.; Zhang, H.; Cai, C.; Gervasio, D.F. Synthesis of Nitrogen-Doped Graphene Quantum Dots at Low Temperature for Electrochemical Sensing Trinitrotoluene. Anal. Chem. 2015, 87, 11803–11811. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lei, W.; Xu, Y.; Zhang, Y.; Xia, M.; Wang, F. Fabrication of Polypyrrole-Grafted Nitrogen-Doped Graphene and Its Application for Electrochemical Detection of Paraquat. Electrochim. Acta 2015, 174, 464–471. [Google Scholar] [CrossRef]
- Fan, H.; Li, Y.; Wu, D.; Ma, H.; Mao, K.; Fan, D.; Du, B.; Li, H.; Wei, Q. Electrochemical Bisphenol A Sensor Based on N-Doped Graphene Sheets. Anal. Chim. Acta 2012, 711, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, H.; Shi, L.; Zhu, X.; Lan, M.; Zhang, Q.; Hugh Fan, Z. Electrochemical Sensing of Nicotine Using Screen-Printed Carbon Electrodes Modified with Nitrogen-Doped Graphene Sheets. J. Electroanal. Chem. 2017, 784, 77–84. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S. Electrochemical Sensors Based on Nitrogen-Doped Reduced Graphene Oxide for the Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid. J. Alloys Compd. 2020, 842, 155873. [Google Scholar] [CrossRef]
- Tadayon, F.; Sepehri, Z. A New Electrochemical Sensor Based on a Nitrogen-Doped Graphene/CuCo2O4 Nanocomposite for Simultaneous Determination of Dopamine, Melatonin and Tryptophan. RSC Adv. 2015, 5, 65560–65568. [Google Scholar] [CrossRef]
- Felix, S.; Kollu, P.; Jeong, S.K.; Grace, A.N. A Novel CuO–N-Doped Graphene Nanocomposite-Based Hybrid Electrode for the Electrochemical Detection of Glucose. Appl. Phys. A 2017, 123, 620. [Google Scholar] [CrossRef]
- Reynoso-Soto, E.A.; Félix-Navarro, R.M.; Rivera-Lugo, Y.Y.; Lozano-Garcia, A.; Dominguez-Vargas, D.A.; Silva-Carrillo, C. Electrochemical Determination of Glucose Using Nitrogen-Doped Graphene. Top. Catal. 2022, 65, 1235–1243. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Lei, W.; Xia, X.; Xia, M.; Hao, Q. Electrochemical Determination of 4-Nitrophenol at Polycarbazole/N-Doped Graphene Modified Glassy Carbon Electrode. Electrochim. Acta 2014, 146, 568–576. [Google Scholar] [CrossRef]
- Moradpour, H.; Beitollahi, H.; Nejad, F.G.; Di Bartolomeo, A. Glassy Carbon Electrode Modified with N-Doped Reduced Graphene Oxide Sheets as an Effective Electrochemical Sensor for Amaranth Detection. Materials 2022, 15, 3011. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Zhou, P.; Xiao, F.; Tang, W.; Zhao, M.; Su, R.; He, P.; Yang, D.; Bian, L.; Tang, B. Electrochemical Sensor Based on Ni/N-Doped Graphene Oxide for the Determination of Hydroquinone and Catechol. Ionics 2023, 29, 1605–1615. [Google Scholar] [CrossRef]
- Wu, Y.; Lei, W.; Xia, M.; Wang, F.; Li, C.; Zhang, C.; Hao, Q.; Zhang, Y. Simultaneous Electrochemical Sensing of Hydroquinone and Catechol Using Nanocomposite Based on Palygorskite and Nitrogen Doped Graphene. Appl. Clay Sci. 2018, 162, 38–45. [Google Scholar] [CrossRef]
- Magerusan, L.; Pogacean, F.; Pruneanu, S. Enhanced Acetaminophen Electrochemical Sensing Based on Nitrogen-Doped Graphene. Int. J. Mol. Sci. 2022, 23, 14866. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.-H.; Li, Y.-S.; Chen, G.-L.; Lin, L.-Y.; Li, T.-J.; Chuang, H.-M.; Hsieh, C.-Y.; Lo, S.-C.; Chiang, W.-H.; Ho, K.-C. Facile Synthesis of Boron-Doped Graphene Nanosheets with Hierarchical Microstructure at Atmosphere Pressure for Metal-Free Electrochemical Detection of Hydrogen Peroxide. Electrochim. Acta 2015, 172, 52–60. [Google Scholar] [CrossRef]
- Pei, J.; Yu, X.; Zhang, Z.; Zhang, J.; Wei, S.; Boukherroub, R. In-Situ Graphene Modified Self-Supported Boron-Doped Diamond Electrode for Pb(II) Electrochemical Detection in Seawater. Appl. Surf. Sci. 2020, 527, 146761. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, R.; Luo, B.; Wang, L. Boron-Doped Graphene as High-Performance Electrocatalyst for the Simultaneously Electrochemical Determination of Hydroquinone and Catechol. Electrochim. Acta 2015, 156, 228–234. [Google Scholar] [CrossRef]
- Xu, Y.; Lei, W.; Han, Z.; Wang, T.; Xia, M.; Hao, Q. Boron-Doped Graphene for Fast Electrochemical Detection of HMX Explosive. Electrochim. Acta 2016, 216, 219–227. [Google Scholar] [CrossRef]
- Manna, B.; Raj, C.R. Nanostructured Sulfur-Doped Porous Reduced Graphene Oxide for the Ultrasensitive Electrochemical Detection and Efficient Removal of Hg(II). ACS Sustain. Chem. Eng. 2018, 6, 6175–6182. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Jin, Y.; Chen, G.; Zhang, X. Microwave-Assisted Solvothermal Synthesis of Sulfur-Doped Graphene for Electrochemical Sensing. J. Electroanal. Chem. 2015, 739, 172–177. [Google Scholar] [CrossRef]
- Sharma, T.S.K.; Hwa, K.-Y. Rational Design and Preparation of Copper Vanadate Anchored on Sulfur Doped Reduced Graphene Oxide Nanocomposite for Electrochemical Sensing of Antiandrogen Drug Nilutamide Using Flexible Electrodes. J. Hazard. Mater. 2021, 410, 124659. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Zaidi, S.A.; Koo, C.M. Highly Sensitive Electrochemical Sensor Based on Environmentally Friendly Biomass-Derived Sulfur-Doped Graphene for Cancer Biomarker Detection. Sens. Actuators B Chem. 2017, 241, 716–724. [Google Scholar] [CrossRef]
- Ran, Q.; Sheng, F.; Chang, G.; Zhong, M.; Xu, S. Sulfur-Doped Reduced Graphene Oxide@chitosan Composite for the Selective and Sensitive Electrochemical Detection of Hg2+ in Fish Muscle. Microchem. J. 2022, 175, 107138. [Google Scholar] [CrossRef]
- Manikandan, V.S.; Sidhureddy, B.; Thiruppathi, A.R.; Chen, A. Sensitive Electrochemical Detection of Caffeic Acid in Wine Based on Fluorine-Doped Graphene Oxide. Sensors 2019, 19, 1604. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, V.S.; Boateng, E.; Durairaj, S.; Chen, A. Electrochemical Sensing of Vanillin Based on Fluorine-Doped Reduced Graphene Oxide Decorated with Gold Nanoparticles. Foods 2022, 11, 1448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, K.-P.; Zhang, L.-N.; Zhang, Y.-C.; Shen, L. Phosphorus-Doped Graphene-Based Electrochemical Sensor for Sensitive Detection of Acetaminophen. Anal. Chim. Acta 2018, 1036, 26–32. [Google Scholar] [CrossRef]
- Chu, K.; Wang, F.; Tian, Y.; Wei, Z. Phosphorus Doped and Defects Engineered Graphene for Improved Electrochemical Sensing: Synergistic Effect of Dopants and Defects. Electrochim. Acta 2017, 231, 557–564. [Google Scholar] [CrossRef]
- Tian, Y.; Wei, Z.; Zhang, K.; Peng, S.; Zhang, X.; Liu, W.; Chu, K. Three-Dimensional Phosphorus-Doped Graphene as an Efficient Metal-Free Electrocatalyst for Electrochemical Sensing. Sens. Actuators B Chem. 2017, 241, 584–591. [Google Scholar] [CrossRef]
- Wang, K.-P.; Zhang, Y.-C.; Zhang, X.; Shen, L. Green Preparation of Chlorine-Doped Graphene and Its Application in Electrochemical Sensor for Chloramphenicol Detection. SN Appl. Sci. 2019, 1, 157. [Google Scholar] [CrossRef]
- Wang, K.-P.; Hu, J.-M.; Zhang, X. Sensitive Electrochemical Detection of Endocrine Disruptor Bisphenol A (BPA) in Milk Based on Iodine-Doped Graphene. Microchem. J. 2022, 173, 107047. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.; Ouyang, Z.; Zhang, M.; Lin, Z.; Li, J.; Su, Z.; Wei, G. Nanoscale Graphene Doped with Highly Dispersed Silver Nanoparticles: Quick Synthesis, Facile Fabrication of 3D Membrane-Modified Electrode, and Super Performance for Electrochemical Sensing. Adv. Funct. Mater. 2016, 26, 2122–2134. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, Q.; Zhu, J.; Shi, H.; Zhang, K.; Shen, Y. Rational Engineering of Ag-Doped Reduced Graphene Oxide as Electrochemical Sensor for Trace Mercury Ions Monitoring. Sens. Actuators B Chem. 2021, 345, 130383. [Google Scholar] [CrossRef]
- Rodbard, D. Continuous Glucose Monitoring: A Review of Successes, Challenges, and Opportunities. Diabetes Technol. Ther. 2016, 18, S2–S3. [Google Scholar] [CrossRef]
- Labouesse, M.A.; Cola, R.B.; Patriarchi, T. GPCR-Based Dopamine Sensors—A Detailed Guide to Inform Sensor Choice for in Vivo Imaging. Int. J. Mol. Sci. 2020, 21, 8048. [Google Scholar] [CrossRef]
- Jin, M.; Yuan, H.; Liu, B.; Peng, J.; Xu, L.; Yang, D. Review of the Distribution and Detection Methods of Heavy Metals in the Environment. Anal. Methods 2020, 12, 5747–5766. [Google Scholar] [CrossRef]
- Wei, Y.; Jiao, Y.; An, D.; Li, D.; Li, W.; Wei, Q. Review of Dissolved Oxygen Detection Technology: From Laboratory Analysis to Online Intelligent Detection. Sensors 2019, 19, 3995. [Google Scholar] [CrossRef]
- Sha, T.; Liu, J.; Sun, M.; Li, L.; Bai, J.; Hu, Z.; Zhou, M. Green and Low-Cost Synthesis of Nitrogen-Doped Graphene-like Mesoporous Nanosheets from the Biomass Waste of Okara for the Amperometric Detection of Vitamin C in Real Samples. Talanta 2019, 200, 300–306. [Google Scholar] [CrossRef]
- Niu, F.; Tao, L.-M.; Deng, Y.-C.; Wang, Q.-H.; Song, W.-G. Phosphorus Doped Graphene Nanosheets for Room Temperature NH3 Sensing. New J. Chem. 2014, 38, 2269–2272. [Google Scholar] [CrossRef]
- Jaiswal, M.; Kumar, R.; Mittal, J.; Jha, P. NO2 Gas Sensor Using Iodine Doped Graphene at Room Temperature with Electric Field Enhanced Recovery. IEEE Sens. J. 2022, 22, 6303–6310. [Google Scholar] [CrossRef]
- Alwarappan, S.; Liu, C.; Kumar, A.; Li, C.-Z. Enzyme-Doped Graphene Nanosheets for Enhanced Glucose Biosensing. J. Phys. Chem. C 2010, 114, 12920–12924. [Google Scholar] [CrossRef]
- Sajid, M.; Nazal, M.K.; Mansha, M.; Alsharaa, A.; Jillani, S.M.S.; Basheer, C. Chemically Modified Electrodes for Electrochemical Detection of Dopamine in the Presence of Uric Acid and Ascorbic Acid: A Review. TrAC Trends Anal. Chem. 2016, 76, 15–29. [Google Scholar] [CrossRef]
- Tukimin, N.; Abdullah, J.; Sulaiman, Y. Electrochemical Detection of Uric Acid, Dopamine and Ascorbic Acid. J. Electrochem. Soc. 2018, 165, B258. [Google Scholar] [CrossRef]
- Malik, M.; Narwal, V.; Pundir, C. Ascorbic Acid Biosensing Methods: A Review. Process Biochem. 2022, 118, 11–23. [Google Scholar] [CrossRef]
- Guo, X.; Cui, R.; Huang, H.; Li, Y.; Liu, B.; Wang, J.; Zhao, D.; Dong, J.; Sun, B. Insights into the Role of Pyrrole Doped in Three-Dimensional Graphene Aerogels for Electrochemical Sensing Cd(II). J. Electroanal. Chem. 2020, 871, 114323. [Google Scholar] [CrossRef]
- Ghanbari, M.H.; Norouzi, Z.; Ghanbari, M.M. Using a Nanocomposite Consist of Boron-Doped Reduced Graphene Oxide and Electropolymerized β-Cyclodextrin for Flunitrazepam Electrochemical Sensor. Microchem. J. 2020, 156, 104994. [Google Scholar] [CrossRef]
- Rohaizad, N.; Sofer, Z.; Pumera, M. Boron and Nitrogen Dopants in Graphene Have Opposite Effects on the Electrochemical Detection of Explosive Nitroaromatic Compounds. Electrochem. Commun. 2020, 112, 106660. [Google Scholar] [CrossRef]
- Niculae, A.-R.; Stefan-van Staden, R.-I.; van Staden, J.F.; Georgescu State, R. Sulfur-Doped Graphene-Based Electrochemical Sensors for Fast and Sensitive Determination of (R)-(+)-Limonene from Beverages. Sensors 2022, 22, 5851. [Google Scholar] [CrossRef]
- Tamilarasi, S.; Kumar, R.S.; Cho, K.-B.; Kim, C.-J.; Yoo, D.J. High-Performance Electrochemical Detection of Glucose in Human Blood Serum Using a Hierarchical NiO2 Nanostructure Supported on Phosphorus Doped Graphene. Mater. Today Chem. 2023, 34, 101765. [Google Scholar] [CrossRef]
- Kumatani, A.; Miura, C.; Kuramochi, H.; Ohto, T.; Wakisaka, M.; Nagata, Y.; Ida, H.; Takahashi, Y.; Hu, K.; Jeong, S.; et al. Chemical Dopants on Edge of Holey Graphene Accelerate Electrochemical Hydrogen Evolution Reaction. Adv. Sci. 2019, 6, 1900119. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Qi, L.; Huang, J.Y.; Li, J. Geometric and Electronic Structure of Graphene Bilayer Edges. Phys. Rev. B 2009, 80, 165407. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.; Woo, S.I.; Jung, Y. On the Mechanism of Enhanced Oxygen Reduction Reaction in Nitrogen-Doped Graphene Nanoribbons. Phys. Chem. Chem. Phys. 2011, 13, 17505–17510. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zheng, X.; Li, L.; Li, J.; Wei, Z. Influence of Phosphorus Configuration on Electronic Structure and Oxygen Reduction Reactions of Phosphorus-Doped Graphene. J. Phys. Chem. C 2017, 121, 19321–19328. [Google Scholar] [CrossRef]
- Lu, Z.; Li, S.; Liu, C.; He, C.; Yang, X.; Ma, D.; Xu, G.; Yang, Z. Sulfur Doped Graphene as a Promising Metal-Free Electrocatalyst for Oxygen Reduction Reaction: A DFT-D Study. RSC Adv. 2017, 7, 20398–20405. [Google Scholar] [CrossRef]
- Sun, X.; Li, K.; Yin, C.; Wang, Y.; He, F.; Bai, X.; Tang, H.; Wu, Z. The Oxygen Reduction Reaction Mechanism on Sn Doped Graphene as an Electrocatalyst in Fuel Cells: A DFT Study. RSC Adv. 2017, 7, 729–734. [Google Scholar] [CrossRef]
- Srivastava, M.; Srivastava, A. DFT Analysis of Nitrogen and Boron Doped Graphene Sheet as Lead Detector. Mater. Sci. Eng. B 2021, 269, 115165. [Google Scholar] [CrossRef]
- Khodadadi, Z. Evaluation of H2S Sensing Characteristics of Metals–Doped Graphene and Metals-Decorated Graphene: Insights from DFT Study. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 99, 261–268. [Google Scholar] [CrossRef]
- Salih, E.; Ayesh, A.I. Co-Doped Zigzag Graphene Nanoribbon Based Gas Sensor for Sensitive Detection of H2S: DFT Study. Superlattices Microstruct. 2021, 155, 106900. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Cao, T.; Wang, P. Screening for the Adsorption-Activated H2O2 and Peroxymonosulfate for High-Performance Heteroatom-Doped Graphene: Molecular Dynamics Simulation and DFT. J. Electroanal. Chem. 2023, 952, 117890. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Shi, M.; Wu, M.; Zhao, N.; Shi, P.; Zhu, Y.; Wang, A.; Ye, C.; Lin, C.-T.; Fu, L. Optimizing Graphene Dopants for Direct Electrocatalytic Quantification of Small Molecules and Ions. Catalysts 2024, 14, 8. https://doi.org/10.3390/catal14010008
Zhou Q, Shi M, Wu M, Zhao N, Shi P, Zhu Y, Wang A, Ye C, Lin C-T, Fu L. Optimizing Graphene Dopants for Direct Electrocatalytic Quantification of Small Molecules and Ions. Catalysts. 2024; 14(1):8. https://doi.org/10.3390/catal14010008
Chicago/Turabian StyleZhou, Qingwei, Mingjiao Shi, Mengfan Wu, Ningbin Zhao, Peizheng Shi, Yangguang Zhu, Aiwu Wang, Chen Ye, Cheng-Te Lin, and Li Fu. 2024. "Optimizing Graphene Dopants for Direct Electrocatalytic Quantification of Small Molecules and Ions" Catalysts 14, no. 1: 8. https://doi.org/10.3390/catal14010008
APA StyleZhou, Q., Shi, M., Wu, M., Zhao, N., Shi, P., Zhu, Y., Wang, A., Ye, C., Lin, C.-T., & Fu, L. (2024). Optimizing Graphene Dopants for Direct Electrocatalytic Quantification of Small Molecules and Ions. Catalysts, 14(1), 8. https://doi.org/10.3390/catal14010008











