Abstract
The acidity of supports can have a positive effect on their catalytic behaviors. Herein, the effects of HCl treatment of TiO2 on its acidic properties and catalytic activity were investigated. TiO2 was treated with various molar concentrations of HCl. Subsequently, Pd was deposited on the treated TiO2 via the deposition–precipitation method; here, the catalysts were denoted as Pd/xH-T, where X is the molar concentration of HCl. Evidently, the amount of strong acid in TiO2 increased with HCl treatment, whereas that in TiO2 treated with a high concentration (5 M) of HCl decreased. After Pd was supported, the amount of acid slightly decreased compared with that on the TiO2 support; however, the order of the acid amounts was similar. The strong acid density increased such that Pd/2H-T had the highest acid content, whereas Pd/5H-T had the lowest. The Pd/2H-T catalyst exhibited the highest selectivity for THFA (95.4%), thus confirming that the selectivity for THFA is correlated with the amount of strong acid. Thus, THFA selectivity is affected by the number of strongly acid sites.
1. Introduction
Owing to the continuous depletion of fossil resources, biorefineries that produce fuels using renewable biomass-derived chemicals are attracting considerable attention as replacements for existing fossil fuel resources. Furfural (FF), derived from lignocellulosic biomass, is an important starting material to produce furfuryl alcohol (FA), tetrahydrofurfural (THFF), tetrahydrofurfuryl alcohol (THFA), and tetrahydrofuran via hydrogenation or rearrangement [1]. Among these, THFA is widely used as a green solvent because of its low toxicity, high stability, and good degradability [2,3]. Additionally, it is an outstanding intermediate in the production of 1,2-pentanediol, which is used as a monomer to produce polyesters and low-toxicity microbicides [4]. THFA is based on a two-step process involving the hydrogenation of FF to THFF or FA. Direct hydrogenation of FF to THFA is more economically advantageous compared with the production of THFA using FA. Therefore, in the hydrogenation of FF to THFA, the hydrogenation of the C=C and C=O bonds must be controlled. Numerous supported catalysts such as Pd/Al2O3, Ni /SiO2, Cu/Al2O3, and Pt/C have been used for the FF hydrogenation reactions [5,6,7,8]. Among activated carbon, SiO2,, Al2O3, and TiO2 used as catalyst supports, TiO2 is widely used owing to its chemical resistance, high mechanical strength, and strong metal–support interactions (SMSI) [9].
The acid sites of the support affect the interaction between the metal particles and the support [10,11]. Previously, Parapat et al. investigated the influence of the surface properties of supports on their interactions during the metal deposition process; evidently, the greater the difference between the surface charges of the metal and support, the stronger the bond. Reportedly, the deposition yield of Ag nanoparticles (NPs) on the most acidic Al2O3, which had the greatest difference in the zeta potential of the Ag NPs, was the highest [10]. Additionally, Lashdaf et al.
reported that the higher the acidity of the beta zeolite support, the smaller the Ru particle size of the catalyst, thus indicating that the acidity of the support affects the size of the metal particles [11]. Studies on the effect of catalytic acid sites on catalytic performance have also been reported [12,13,14,15,16,17]. For instance, Song et al. reported that an additional hydrogen transfer through protons occurs at
the strong Brønsted acid site, thereby accelerating the hydrogenation and hydrolysis of acid sites near the metal [12]. Zhu et al. studied the effect of abundant surface acid–base sites on FF hydrogenation and reported that the acidic sites on ZrO2 can adsorb FF and activate the C=O bond, thus leading to improved conversion and selectivity for FA, which is the target product [13]. Feng et al. investigated the effect of the acid strength of the support on the hydrogenolysis of an α-methylbenzyl alcohol reaction. They reported that strong acid sites of the catalysts favor the formation of the byproduct acetophenone; thus, catalysts with few or no strong acid sites exhibit high selectivity for ethylbenzene [14]. Zhou et al. observed that the hydrogenation of cyclohexene to cyclohexane is enhanced as the number of acid sites increases, thus confirming that a ZrO2 support with a smaller number of acid sites is advantageous for higher selectivity to cyclohexene [15]. Lin et al. reported that the catalytic cracking pathway of 1-butene can be effectively controlled by the acid strength because the strong acidity of the catalyst reduces the activation energy of the reaction [16]. Previous studies have confirmed that the surface acidity of the support has a significant effect on catalyst performance by influencing the adsorption and desorption behaviors of reactants and products.
The transition metal TiO2 can adjust the acidity of the surface through the formation of oxygen defects and changes in the acid density of Ti. For example, doping TiO2 with metal oxides, such as SiO2, ZrO2, WO3, Al2O3, and V2O5, has been reported to increase the acidity of the surface and catalytic activity [18,19,20,21,22]. In addition, studies have reported the modification of the surface of TiO2 by introducing functional groups via chemical treatment such as PO43– and SO42– [23,24,25]. In addition, the surface properties of the support affect its interaction with the supported metal. The interaction between the metal and support is an important factor in catalysis. The high dispersion of the metal loaded on the support prevents sintering during the catalytic reaction, thereby stabilizing the metal particles [26]. TiO2 can promote electron migration through strong interactions with metal, thus leading to the generation of Ti3+ and oxygen defects via interfacial doping or modification [27].
In this study, the effect of the acid treatment of TiO2 on its catalyst surface properties was studied by synthesizing a catalyst with acid-treated TiO2 at different concentrations of hydrochloric acid. Pd/TiO2 catalysts were prepared using pre-treated supports via the deposition–precipitation method, and their catalytic activities for FF hydrogenation under mild conditions were compared. Subsequently, reaction conditions were optimized for Pd/TiO2 to obtain the highest THFA yield from FF. In addition, the contribution of support acidity to the activity and selectivity of the Pd/TiO2 catalyst for FF hydrogenation was explored.
2. Results
2.1. Characterization of Supports
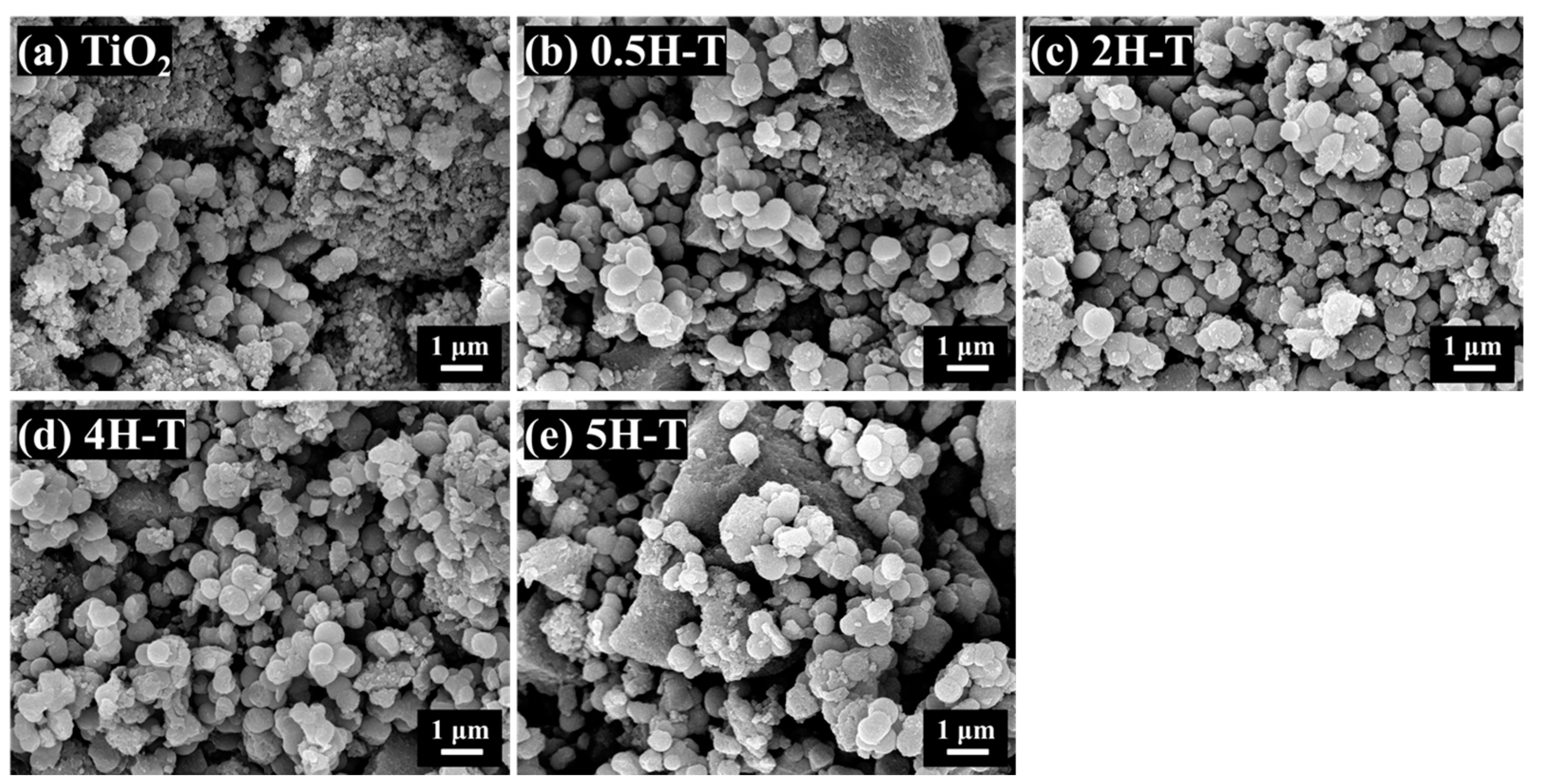
The sizes and morphologies of the TiO2 particles were studied using scanning electron microscopy (SEM). The SEM image of TiO2 shown in Figure 1 shows spherical particles with sizes of 300–600 nm. Among the HCl-treated TiO2, 2H-T and 4H-T were composed of uniformly sized spherical particles. TiO2 treated with the highest concentration of 5 M HCl resulted in slightly nonuniform particle shape and size distribution, which was attributed to the dissolution and aggregation of TiO2 particles owing to treatment with HCl, a strong acid [28].
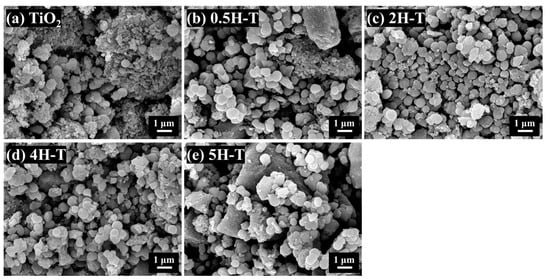
Figure 1.
FE-SEM images of TiO2: (a) pristine TiO2; (b) 0.5H-T; (c) 2H-T; (d) 4H-T; (e) 5H-T.
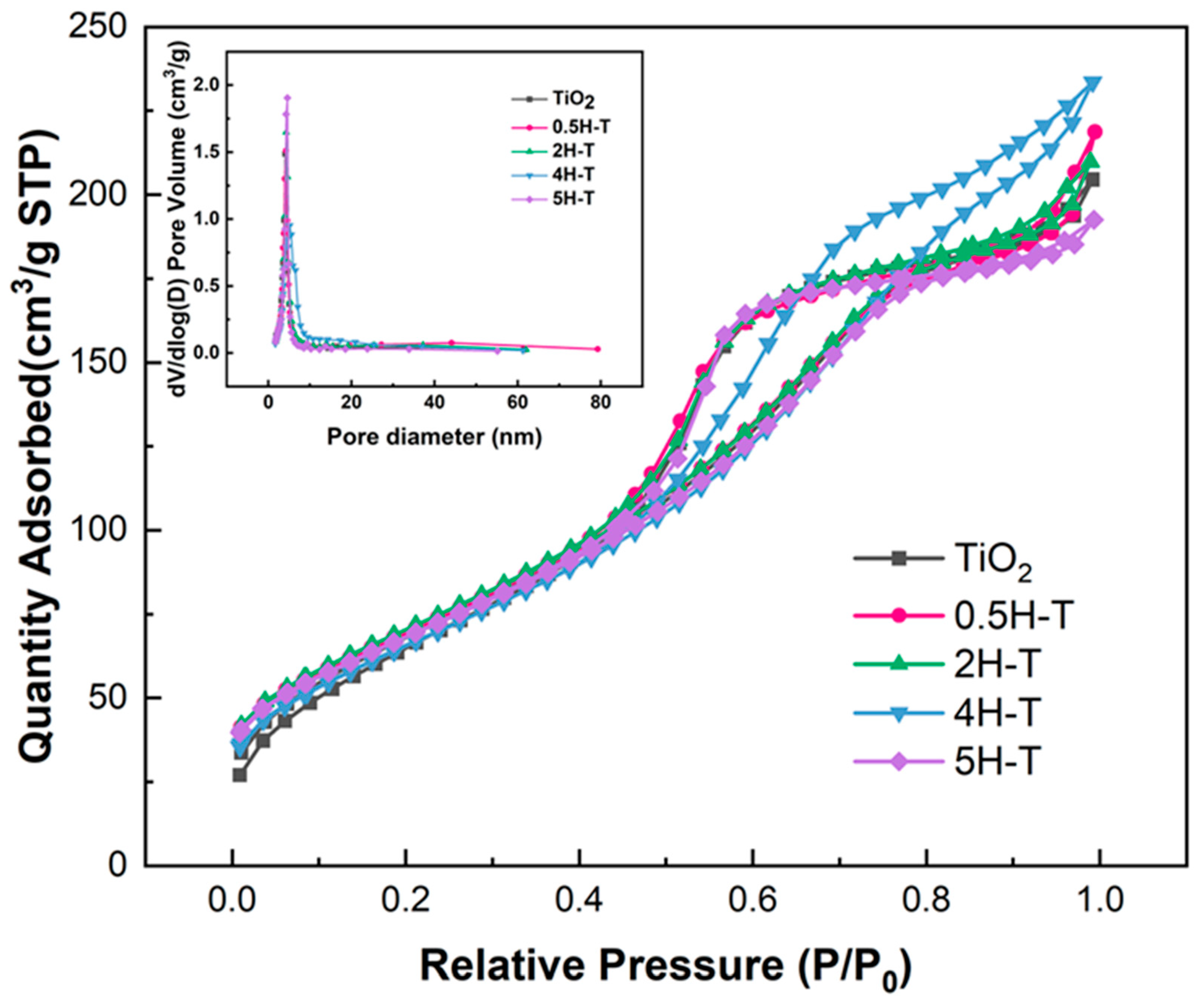
Figure 2 shows the N2 adsorption–desorption isotherms and pore size distributions of TiO2 treated with various concentrations of HCl. All isotherms of pristine TiO2 and pre-treated TiO2 belonged to type IV with a hysteresis loop of the IUPAC classification, thus indicating mesoporous structures [29,30]. The hysteresis loop of all samples was an H2 type, had an ink bottle-shaped pore shape, and capillary condensation occurred at a relative pressure (P/P0) of ~0.4. However, due to differences in the ink bottle-shaped neck size distribution and pore cavity size distribution, 4H-T was classified as type H2(b), and all samples except 4H-T were classified as type H2(a). When the pore cavity size distribution is wider than the neck size distribution, type H2(a) hysteresis with a steep desorption step occurs, and in the opposite case, type H2(b) hysteresis occurs [31]. Studies have reported that the size and shape of pores tend to change with acid treatment [32,33]. Therefore, it was considered that the pore size changed due to acid treatment, which affected the BET results. The textural properties of the supports are listed in Table 1. Evidently, acid-treated TiO2 had a slightly larger pore size compared with the pristine one owing to both the removal of organic matter from the TiO2 surface and surface erosion after the acid treatment [34].
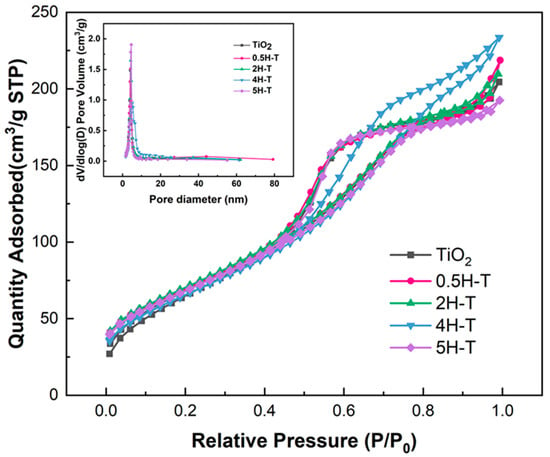
Figure 2.
N2 adsorption–desorption isotherms and pore-size distribution curve of TiO2 supports.

Table 1.
Physicochemical properties of TiO2 supports.
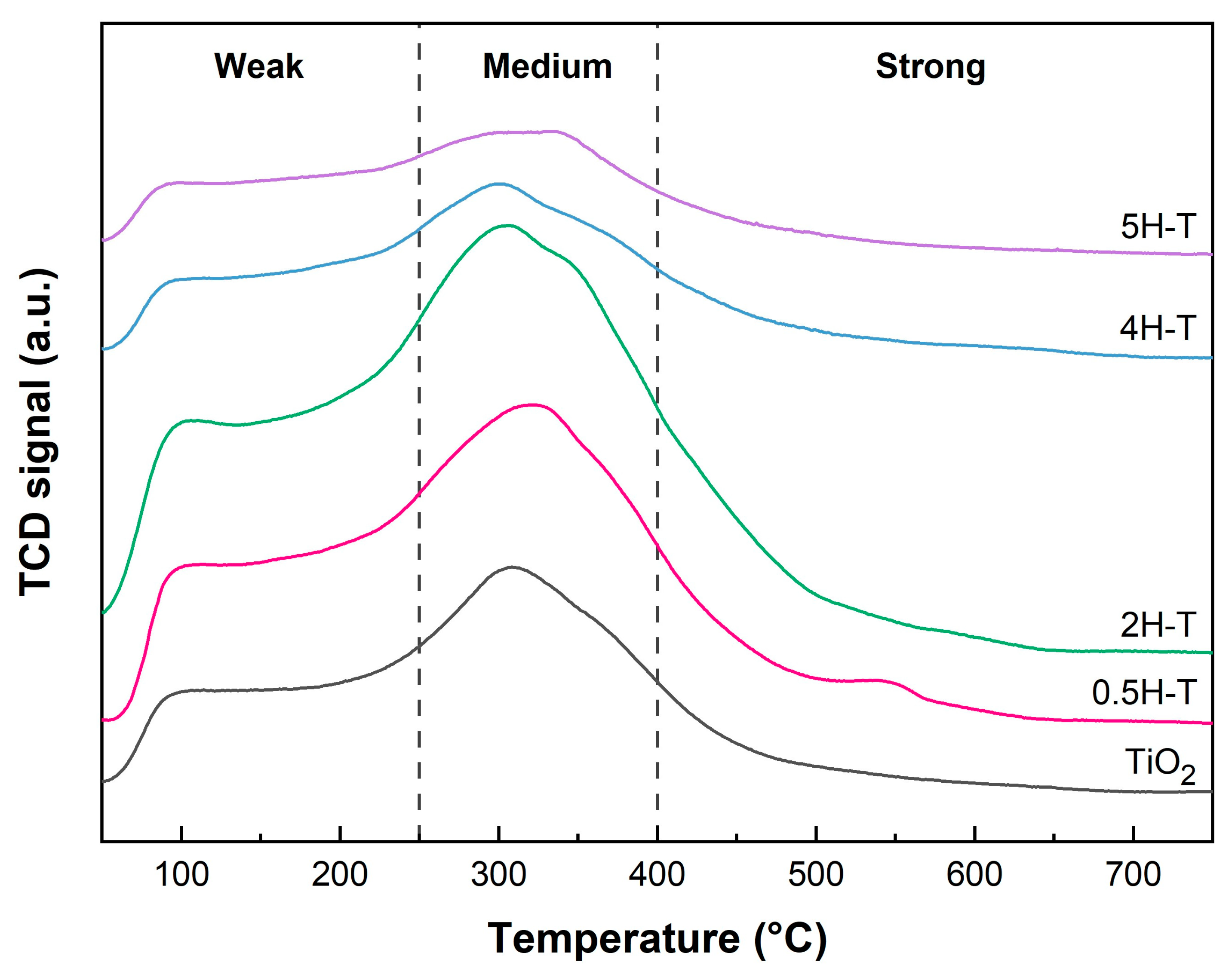
The acidic properties of the supports were determined by NH3-TPD. Figure 3 shows the NH3-TPD profiles of pristine and acid-treated TiO2. In general, the temperature of the desorption peak is related to the strength of the acid [35,36]. Herein, the acid strength could be divided into three regions of 50–250, 250–400, and >400 °C, essentially corresponding to weak acid, medium acid, and strong acid sites, respectively [37,38]. In the NH3-TPD profiles of all TiO2 supports, two desorption peaks were observed at different temperatures in the range of 50–500 °C. The desorption peaks below 300 °C corresponded to weak interactions or physical NH3 adsorption, whereas those above 300 °C corresponded to strong interactions between NH3 and acid sites. The peaks at about 100 and 305 °C indicate the weak acid and medium acid sites and correspond to the desorption of NH3 absorbed on weak Brønsted acid sites and strong Lewis acid sites, respectively [35,39]. The number of acidic sites was calculated according to the strength of the acidity by evaluating the area under the curve. A quantitative analysis of the NH3-TPD data is presented in Table 1. Evidently, the number of acid sites present on the TiO2 surface increased with the molar concentration of HCl, whereas the number of acid sites on TiO2 treated with 4 M HCl decreased as the surface hydroxyl groups were covered with chlorine. Further, the number of acid sites present on the TiO2 surface increased with the molar concentration of HCl, whereas the number of acid sites on TiO2 treated with concentrations above 4 M tended to decrease. The order of the highest number of strong acid sites was 2H-T > 0.5H-T > 4H-T > 5H-T > TiO2. Note that the presence of chlorine due to HCl treatment weakens the chemical bond between H and O of adjacent hydroxyl groups, thus increasing the proton transfer tendency and Brønsted acidity [40]. However, the presence of excess chlorine species can reduce the number of acid sites by covering the hydroxyl groups on the surface and inhibiting the adsorption of NH3. Therefore, herein, TiO2 treated with HCl concentrations above 4 M had a reduced number of acid sites owing to the presence of excessive chlorine species on the surface. Figure S1 (in the Supplementary Materials), due to the adsorption of Cl ion on the hydroxyl group, shows a decrease in two bands at 3690 cm−1 and 3630 cm−1 corresponding to the O-H stretching vibration [41]. From the XPS survey result shown in Table 2, the chlorine ion content varied slightly with different HCl-treated concentrations. The difference in chlorine content among the supports was only about 0.1%. This is close to the detection limit of XPS analysis, suggesting that such a small difference is not significant enough to affect the catalytic activity [42]. Therefore, we conclude that these minor variations in chlorine content do not remarkably influence the activity comparison of the catalysts.
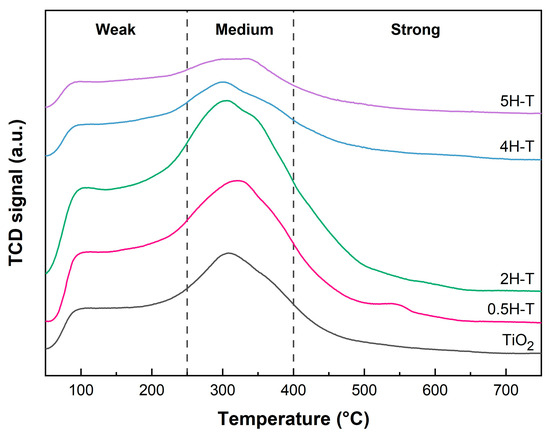
Figure 3.
NH3-TPD results of pristine and acid-treated TiO2 supports.

Table 2.
Atomic composition of HCl-treated TiO2 from XPS.
2.2. Characterization of Catalysts
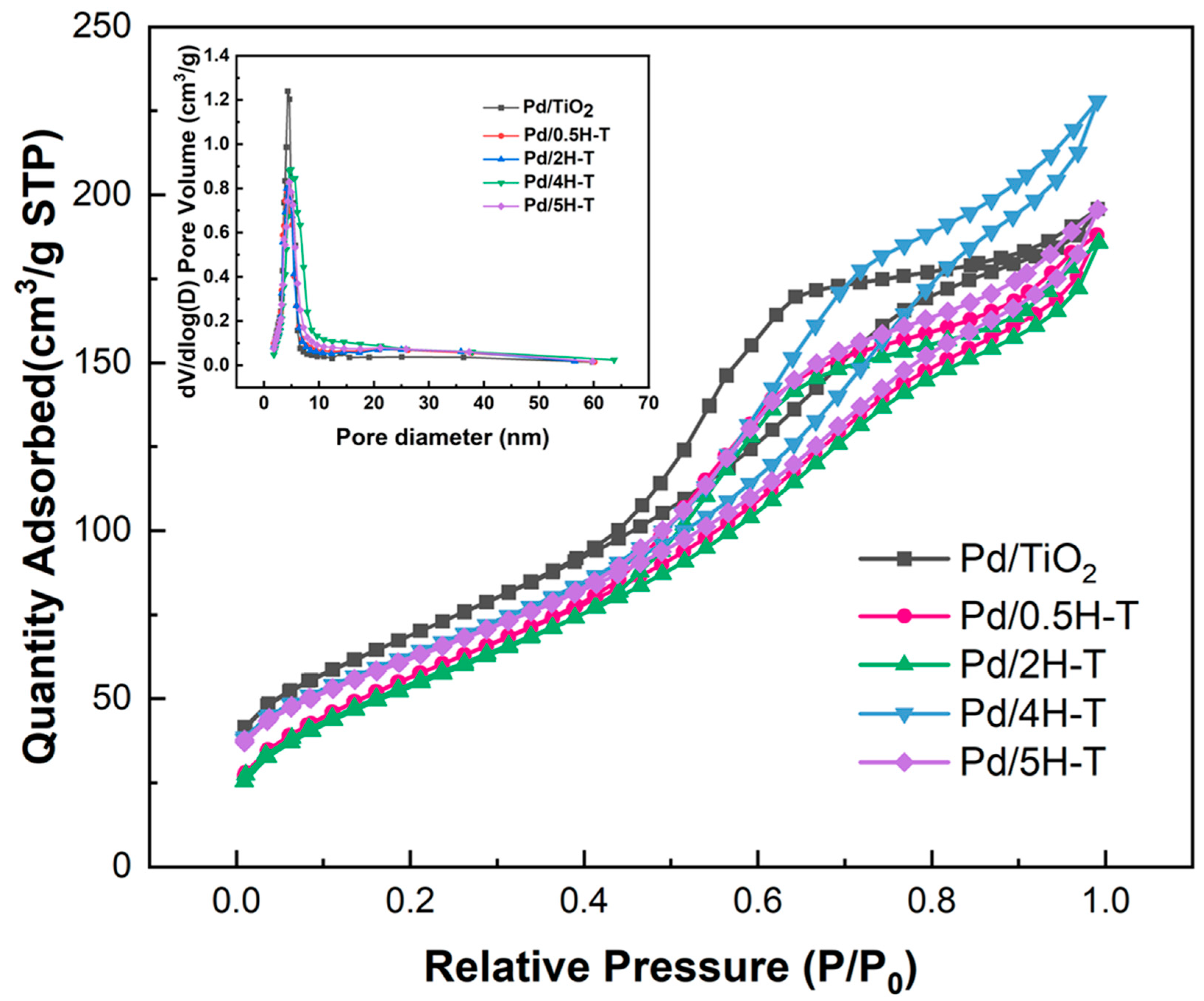
Figure 4 shows the N2 adsorption–desorption isotherms and pore-size distributions of the Pd/TiO2 catalysts. Evidently, the results of all the Pd/TiO2 catalysts were similar to the N2 adsorption–desorption isotherms of the catalyst support TiO2. As presented in Table 3, the specific surface area and pore volume decreased after Pd loading, thus indicating pore blockage. Despite the decrease in surface area, the pore size increased slightly. NaOH, which was used as a precipitation agent, affected the textural properties of the catalyst. It was deposited on the outer surface of the support and may fill the pores of TiO2, thereby resulting in a decrease in surface area [43,44]. A comparison of the specific surface areas of the supported TiO2 and Pd/TiO2 catalysts revealed that that the reduction in the specific surface area of the catalysts prepared with acid-treated TiO2 was greater. The specific surface area decreased as the ions remaining in the acid-treated TiO2 were removed.
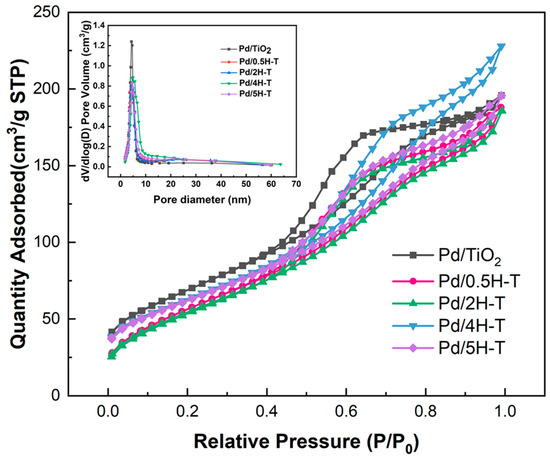
Figure 4.
N2 adsorption–desorption isotherms and pore-size distribution curve of Pd/TiO2 catalysts.

Table 3.
Physicochemical properties of Pd/TiO2 catalysts.
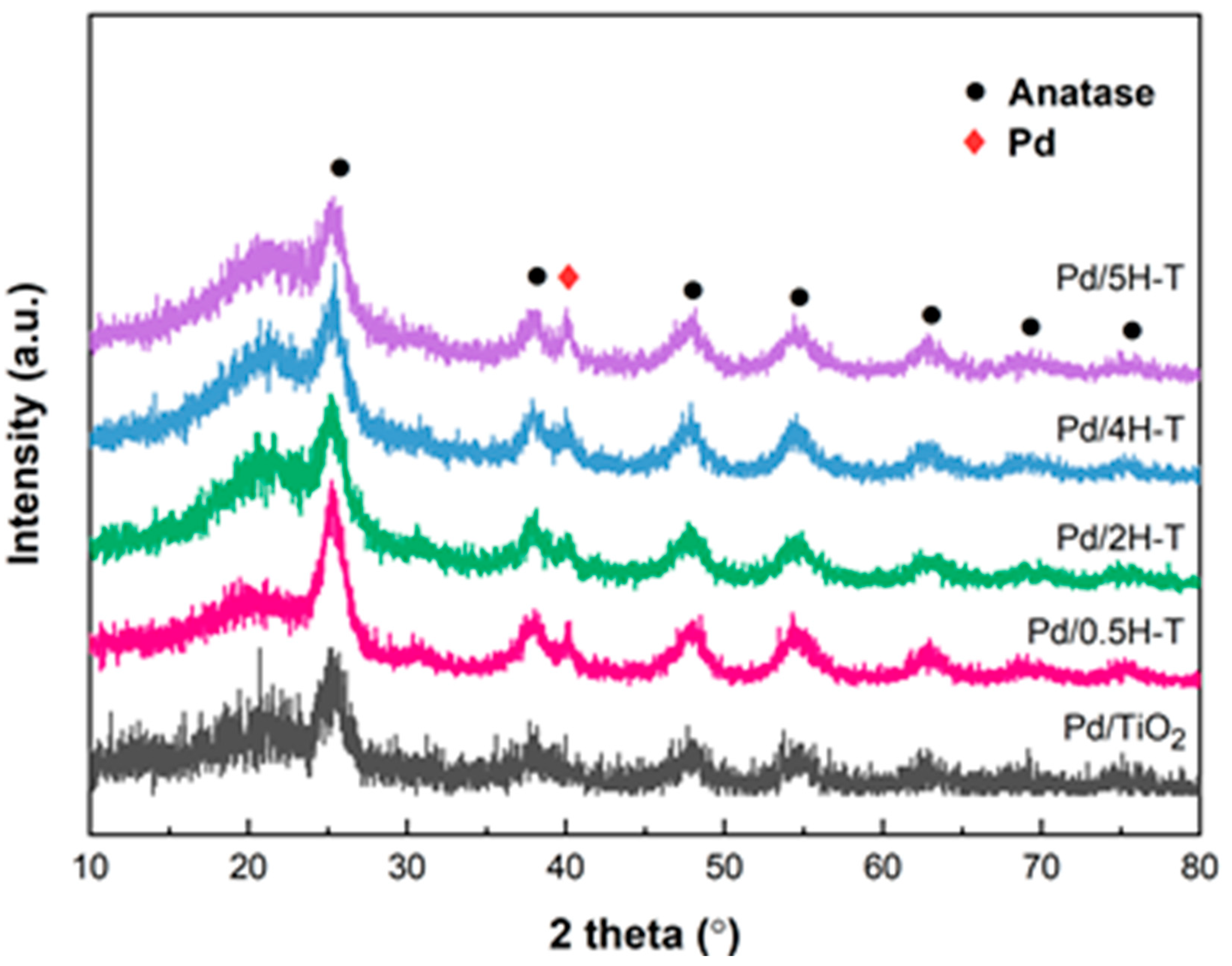
Figure 5 shows the X-ray diffraction (XRD) patterns of the Pd/TiO2 catalysts prepared using pristine and acid-treated TiO2 as supports. Evidently, all the catalysts presented typical anatase phase diffraction peaks at 2θ = 25.3°, 37.9°, 48.1°, 54.2°, 62.8°, 68.9°, and 75.3° (JCPDS card No. 78-2486). The broad peak at 19.4°–23.6° was attributed to the amorphous structure of TiO2 [45]. After HCL treatment, the intensities of the broad peak at 19.4–23.6° and the diffraction peak at 25° increased. It is important to note that TiO2, although exhibiting excellent chemical stability, is not an acid-insoluble substance [46,47]. Therefore, TiO2 treated with HCl, a strong acid, was partially dissolved, and Ti4+ ions were recrystallized, thus leading to a decrease in the particle size of anatase and an increase in the proportion of the amorphous state [48]. In addition, the XRD patterns of all the Pd/TiO2 catalysts exhibited a characteristic Pd peak at 40.1°, which corresponds to the (111) crystal plane of Pd (JCPDS card No. 05-0681). Table 3 presents the Pd average crystallite size calculated through the Scherrer formula using the diffraction peak at 2θ = 40.1°. The Pd particles dispersed in the Pd/5H-T catalyst were the largest (10.21 nm), whereas those dispersed in the Pd/2H-T catalyst were the smallest (7.04 nm). In the case of TiO2 treated with concentrations of >4 M of HCl, a catalyst with larger Pd particles was formed owing to a decrease in the size of the anatase crystalline phase and an increase in the amorphous phase.
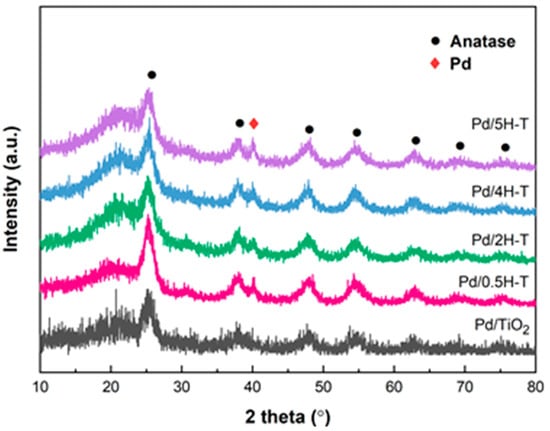
Figure 5.
XRD patterns of Pd/TiO2 catalysts.
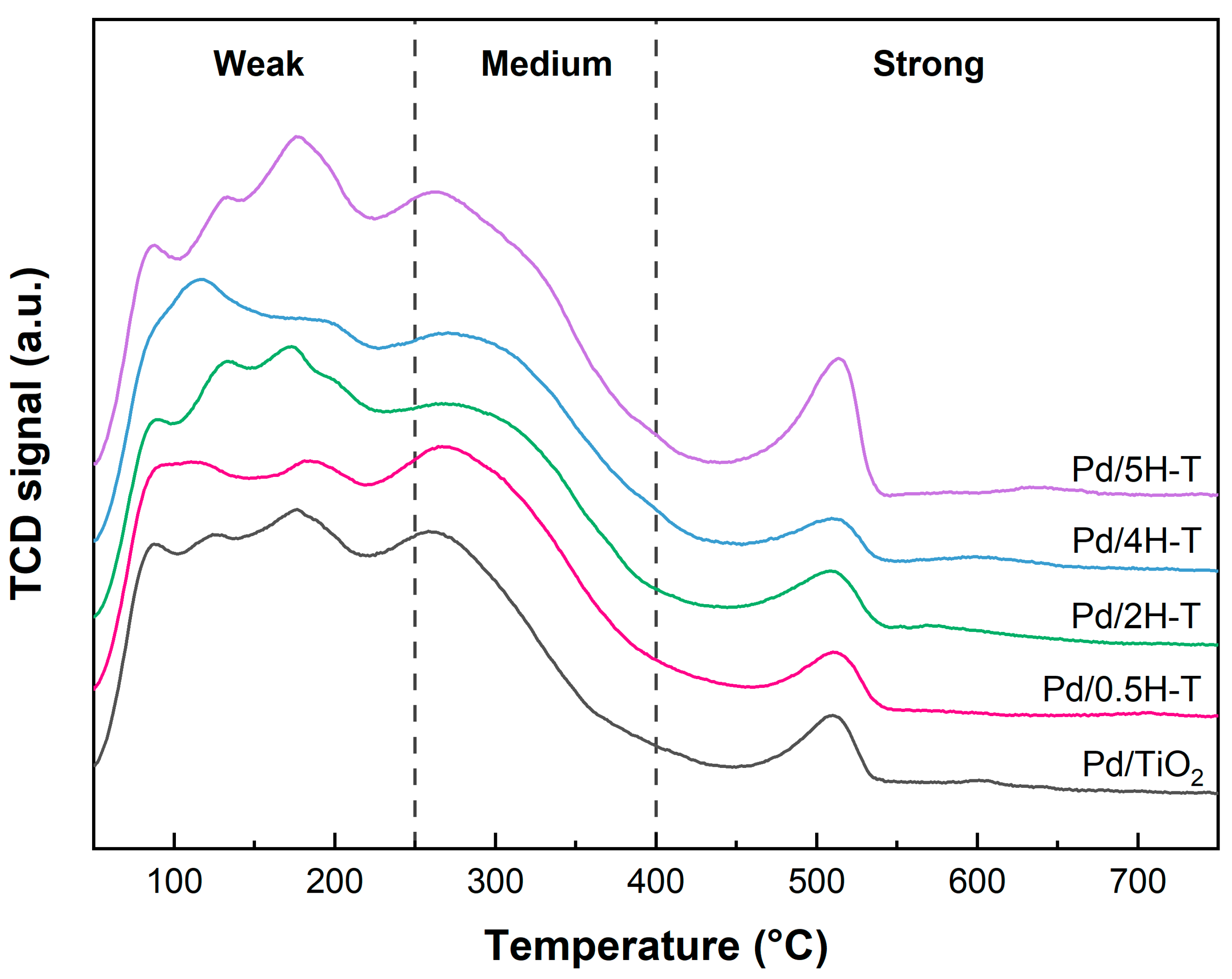
The NH3-TPD profile and quantitative analysis of the NH3-TPD data for Pd/TiO2 are presented in Figure 6 and Table 3, respectively. The acidity of the Pd catalysts decreased slightly compared with that of the TiO2 supports; this was attributed to the interaction with acid sites present on the surface of the support during the Pd-loading process. In addition, the order of the highest number of strong acid sites was Pd/2H-T > Pd/0.5H-T > Pd/4H-T > Pd/TiO2 > Pd/5H-T, which is similar to that of the supports.
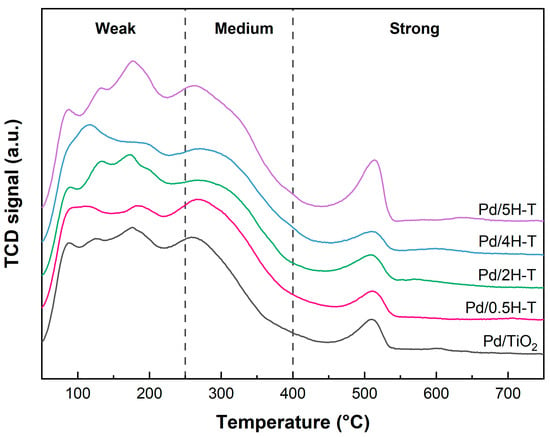
Figure 6.
NH3-TPD results of pristine and acid-treated Pd/TiO2 catalysts.
We performed SEM-EDS analysis to confirm the content of Pd loading. EDS was measured at 5 points for each catalyst, and the average values are shown in Table S1 (in the Supplementary Materials). All catalysts showed a Pd loading of 4.9 ± 0.1 wt %, as expected from the synthesis process. Furthermore, the dispersion and effective metal area of Pd for each catalyst were confirmed by CO chemisorption analysis using ASAP2920 equipment, and the results are shown in Table 4. The Pd/2H-T catalyst had the highest Pd dispersion of 25.0%, whereas the Pd/TiO2 catalyst had the lowest Pd dispersion of 17.7%. It was reported that the acid sites of the support act as anchoring sites for the nucleation and growth of metal during the catalyst-synthesis process [49,50]. X. Li et al. reported that metal dispersion was promoted in a support rich in Lewis acid sites and strong acid sites, thereby preparing a catalyst with a smaller active particle diameter of the metal [51]. This was the result of the Lewis acid separating the Bronsted acid sites and reducing the aggregation of metal species that prefer adsorption to the Brønsted acid. We suggested that Pd precursors can be deposited by accepting protons from acidic sites and that strong acid sites separate weak and medium acid sites, reducing the aggregation of Pd species. Therefore, the highest Pd dispersion was achieved in 2H-T, which had a large number of total acid sites and strong acid sites.

Table 4.
Results of CO chemisorption analysis for Pd/TiO2 catalysts.
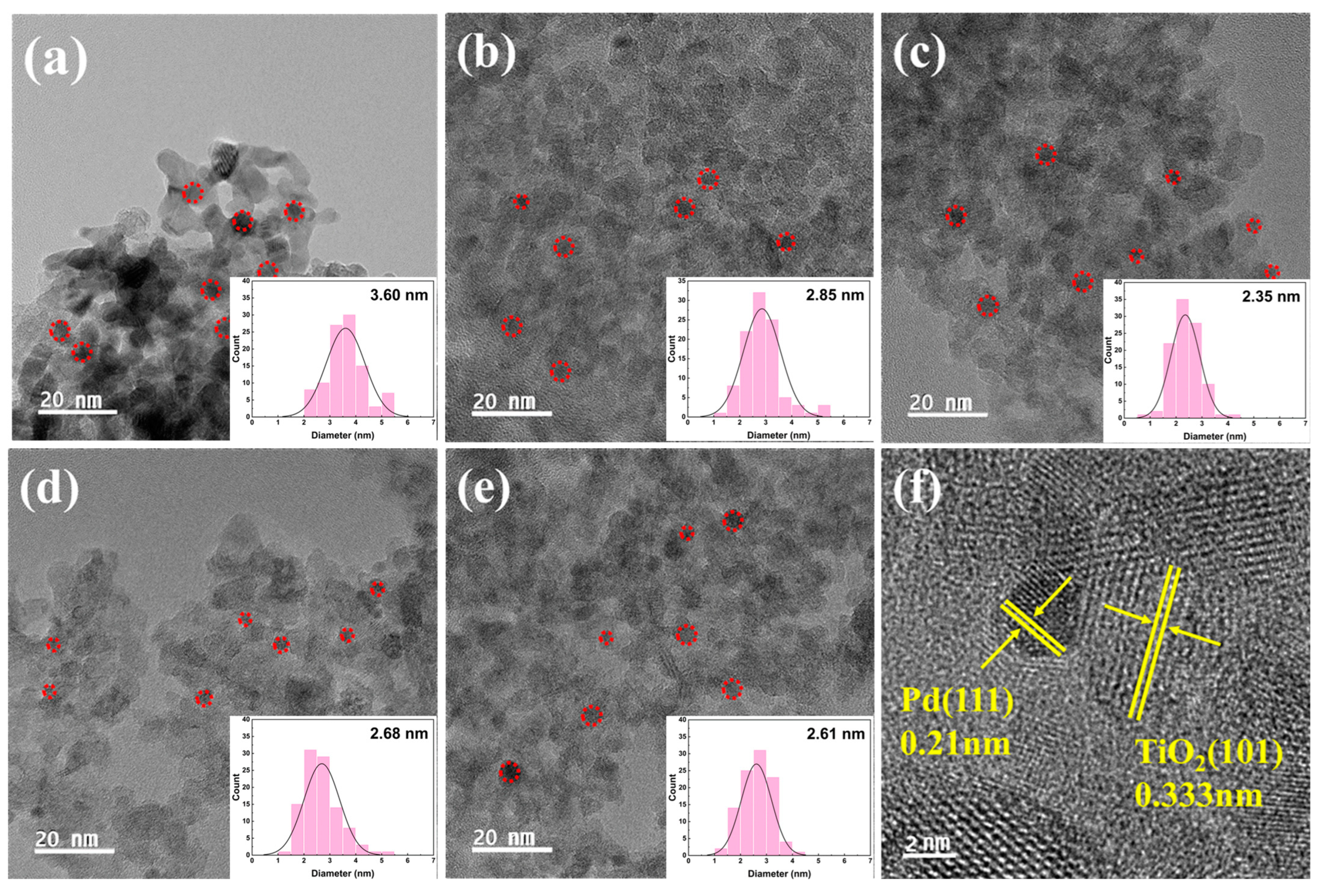
The size and dispersion of Pd nanoparticles (NPs) in the series of Pd/TiO2 catalysts were confirmed by FE-TEM analysis and are shown in Figure 7. The average particle size of Pd was calculated using 100 particles obtained from the TEM images. The TEM images show that the Pd NPs were evenly dispersed in all catalysts. Pd NPs with a small size of approximately 3 nm were dispersed in the catalyst using acid-treated TiO2 as the support. The smallest Pd NP particle size (2.35 nm) was observed in Pd/2H-T. Presumably, relatively little agglomeration occurred because of the acidic sites present on the surface of the support. As the amount of acid on the TiO2 surface increased and the surface was protonated by the HCl treatment, Pd NPs with smaller particle sizes were formed by interacting with the negatively charged Pd precursor [10,52].
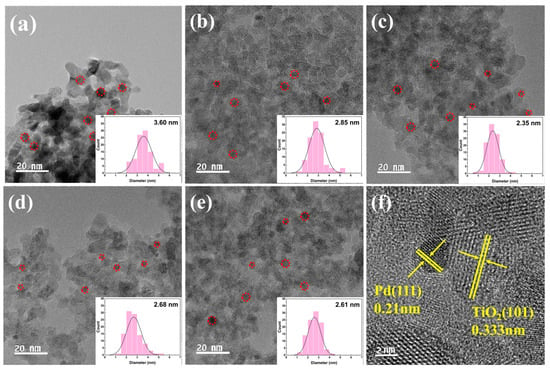
Figure 7.
FE-TEM images of (a) pristine TiO2, (b) 0.5H-T, (c) 2H-T, (d) 4H-T, (e) 5H-T, and (f) 0.5H-T (TEM image with obvious TiO2 lattice fringes). Red circles indicate Pd particles.
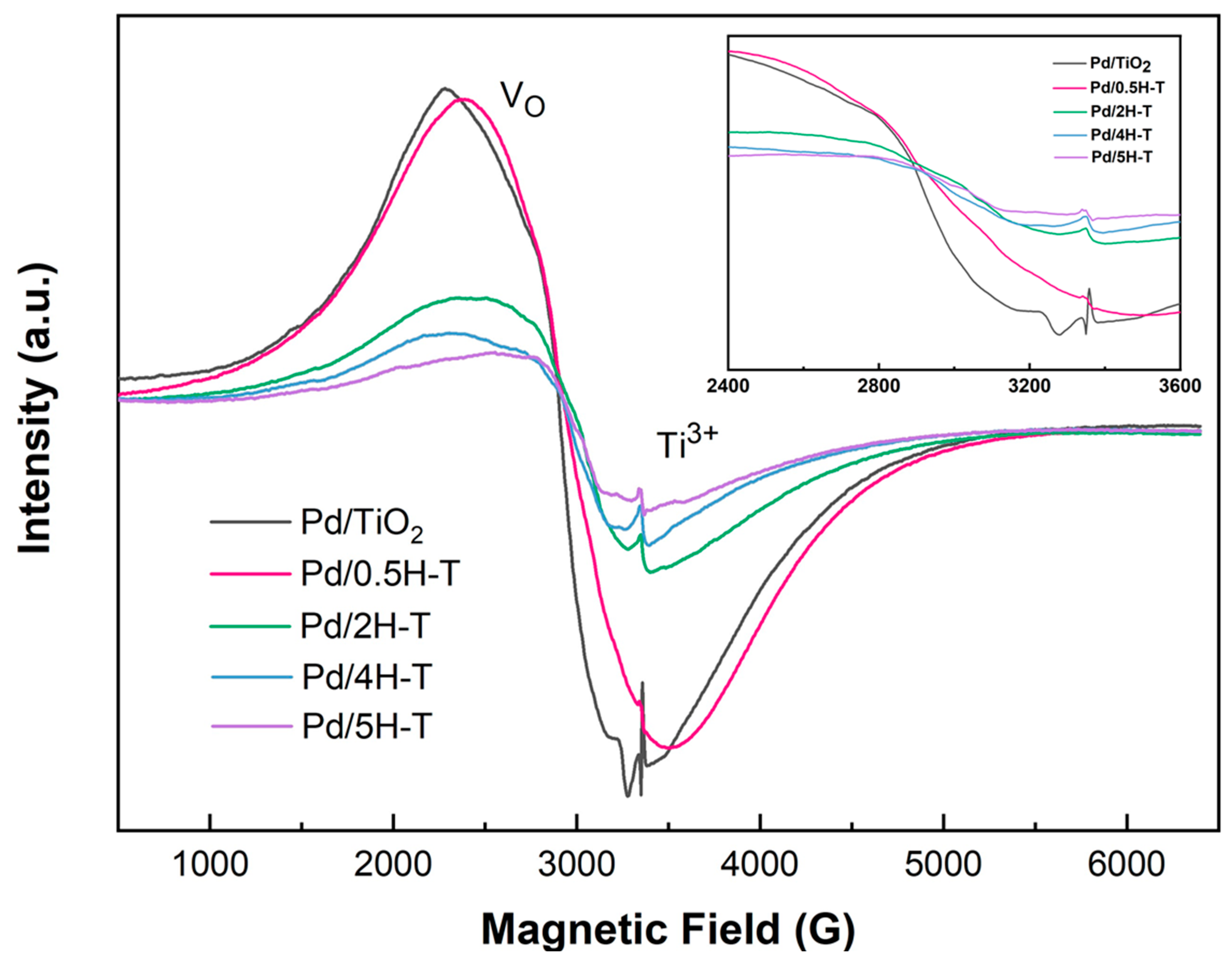
Figure 8 shows the ESR spectra of the Pd/TiO2 catalysts obtained to confirm the presence of defects on the catalyst surface. The analysis was conducted using the catalysts reduced to hydrogen. In all the spectra, strong signals near g = 2.313 and g = 2.007 were confirmed, which were due to oxygen vacancies (Ov) and Ti3+, respectively. Ov was generated when excess protons on the surface of TiO2 adsorbed by acid treatment were removed during the hydrogen-reduction process [53]. As the molar concentration of the treated HCl solution increased, the peak corresponding to the oxygen vacancies in the ESR graph shifted toward a low g value, whereas the peak corresponding to Ti3+ shifted toward a higher g value. This is attributed to the change in the crystal environment around Ti3+ and the formation of a Ti3+– Ov defect complex [54]. Essentially, excess electrons from Ov interact with various electron acceptors on the oxide surface. Thus, surface defects (Ov, Ti3+ species) facilitate the chemisorption of FF and activate the carbonyl groups to promote transitional hydrogenation [55].
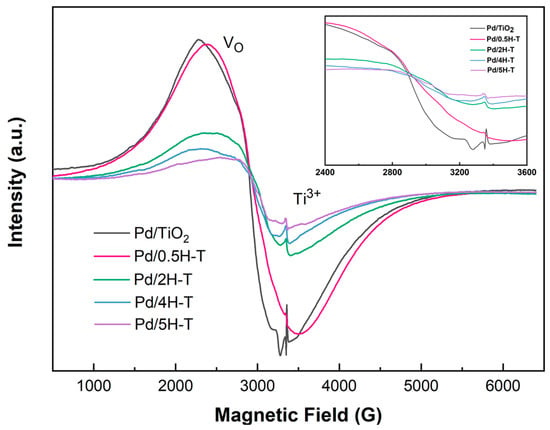
Figure 8.
ESR spectra of Pd/TiO2 catalysts obtained at room temperature.
2.3. FF Hydrogenation
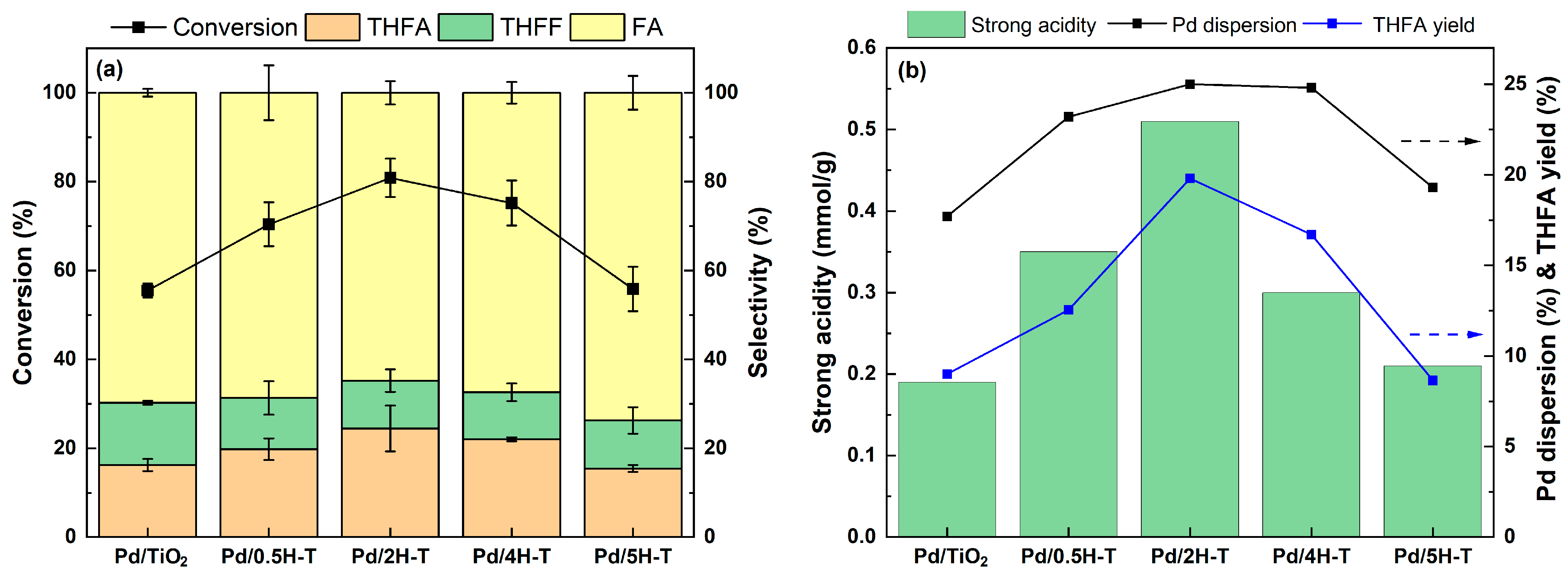
The effects of treated TiO2 with different concentrations of HCl on the conversion of FF to THFA were studied. The catalytic activity of the Pd/TiO2 catalyst was investigated via the FF hydrogenation reaction at 35 °C under 1 bar H2 in 2-propanol. The results are shown in Figure 9a with the standard deviation. The experimental error value was found to be less than 6.1, which is considered a reliable range. Regardless of experimental error, there was a clear difference in the FF conversion among the catalysts. The Pd/2H-T with a large number of strong acid sites showed a higher FF conversion of 80.8% among the catalysts, which is 25.3% different from Pd/TiO2. The Pd surface dissociates hydrogen molecules to form H atomic species, which attack the double bonds of the substrate adsorbed on the catalyst surface. Accordingly, highly dispersed Pd can provide more exposed Pd sites for hydrogenation. Nelson et al. reported that the activity increased with high Pd dispersion due to a strong support-Pd interaction [56]. According to Gelder et.al., catalysts with higher dispersion showed higher reaction rates and hydrogen uptake [57]. Kim et al. revealed that the catalytic activity increases as the particle size of Pd decreases. This was due to the small particle size not only providing more corner Pd atoms but also suppressing the diffusion of hydrogen atoms into the bulk with large particle size [58]. The catalytic activity of the Pd/ TiO2 catalyst under 20 bar H2 at 35 °C in 2-propanol is shown in Figure S3 (in the Supplementary Materials). Evidently, all catalysts exhibited more than 94% conversion. The main product was THFA, and the selectivity ranged from 70% to 90%, depending on the support treated with various concentrations of HCl. Compared to reaction results at 1 bar H2, there was no significant difference in the FF conversion of all catalysts, whereas there was a clear difference in THFA selectivity. Among the catalysts, the Pd/2H-T catalyst with the largest number of strong acid sites showed the highest THFA selectivity of 95.4%. Evidently, the selectivity for THFA increased with the strong acidity of TiO2. The strong acid sites of the catalyst can enhance the interaction with the aromatic ring and promote the nonuniform distribution of electrons to activate the aromatic ring [59]. In addition, the acidic sites of the support enhance the adsorption capacity of the C=O bond, thereby resulting in greater conversion of THFF to THFA [13]. Regarding the distinct activity of the prepared Pd/H-TiO2, we first hypothesized that acidic-driven reactant adsorption plays a key role in catalytic activity. As for O defects, it is known to readily activate molecular oxygen, which is the opposite of the hydrogenation reaction. Y. Nzuzo et al. reported that excess oxygen vacancies on the catalysts had a negative effect on the hydrogenation reaction when the H2 pressure increased from 40 to 50 bar [60]. In this study, although the presence of oxygen vacancies in the catalyst was confirmed by ESR analysis, no clear correlation between the oxygen vacancies and catalytic activity was found. However, the plot of particle size, Pd dispersion, and catalytic activity in Figure 9b showed a significant relationship. As small-sized Pd particles were formed, the dispersion increased, and thus, the catalytic activity also increased. As the metallic surface area increases, more hydrogen can be effectively adsorbed and dissociated, so catalysts with high Pd dispersion exhibit improved hydrogenation ability. Consequently, it was evident that factors such as strong acidic sites, high Pd dispersion, and large metallic surface area synergistically enhance the catalytic activity.
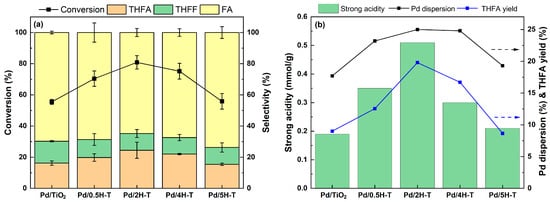
Figure 9.
(a) Furfural hydrogenation over Pd/TiO2 catalysts and (b) THFA yield the strong acidity and Pd dispersion of Pd/TiO2. Reaction conditions: 0.15 g of FF, 0.05 g of the catalyst, 20 mL of 2-propanol, reaction temperature of 35 °C, pressure of 1 bar H2, reaction time of 4 h, and stirring rate of 700 rpm.
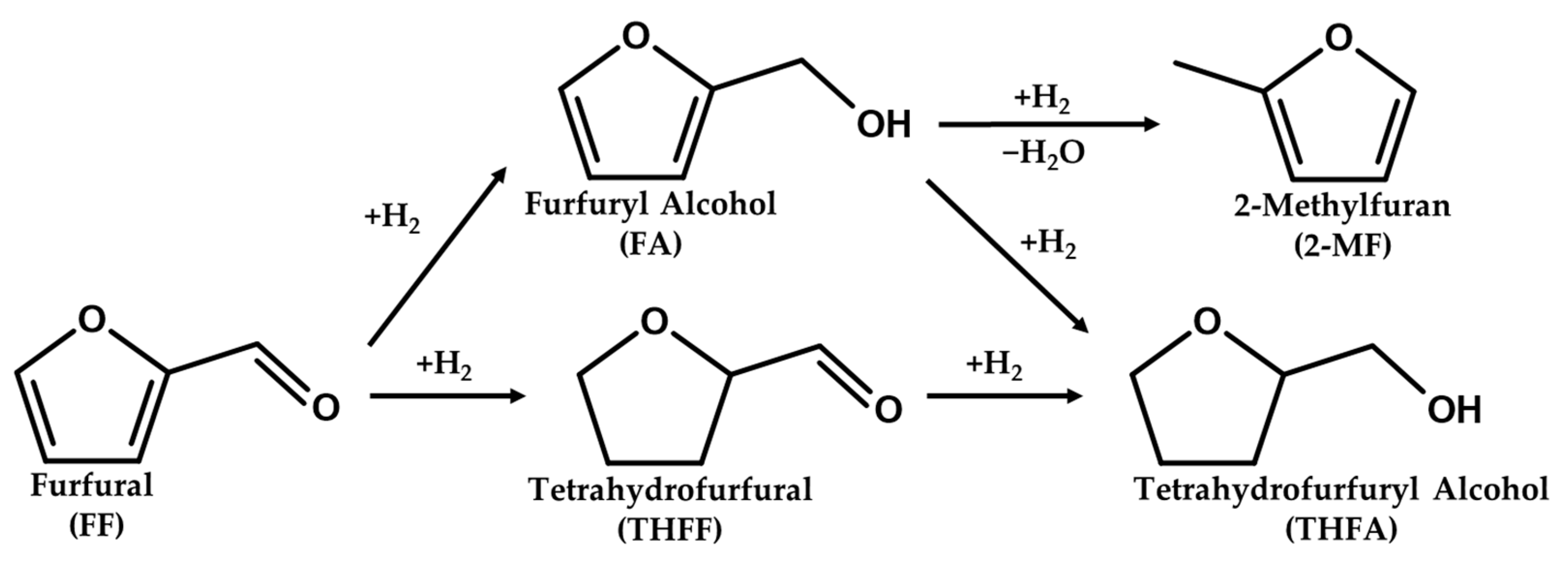
The reaction mechanism scheme of FF to THFA is reported in Figure 10. THFA is produced by complete hydrogenation of the C=O and C=C bonds of FF. In general, there are two pathways for the conversion of FF to THFA, consisting of two sequential steps: (i) a path to produce THFF as an intermediate via ring hydrogenation followed by C=O hydrogenation and (ii) a path to produce FA as an intermediate via ring hydrogenation following C=O hydrogenation. And 2-MF is formed by additional hydrogenolysis of FA. The acid sites on the catalyst surface can act as adsorption sites for reactants. In particular, strong acid sites improve the adsorption of both the aldehyde group and the furan ring of furfural [61]. Enhanced FF adsorption on strong acid sites was more advantageous for both ring hydrogenation and C=O hydrogenation, resulting in higher activity for converting FF to THFA.
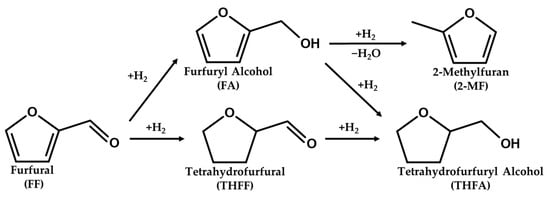
Figure 10.
Reaction pathway of furfural hydrogenation over Pd/TiO2.
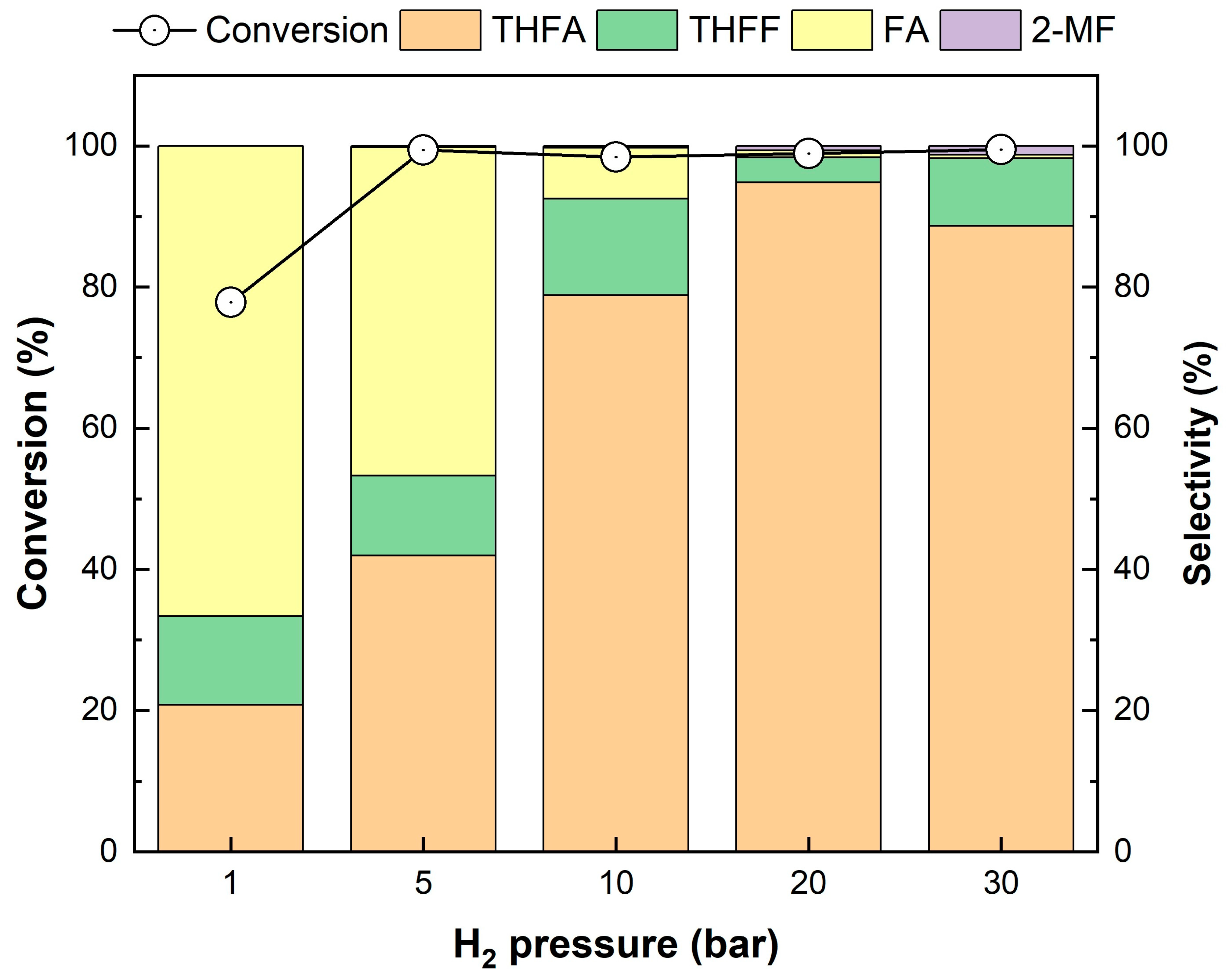
The effects of H2 pressure and reaction temperature on FF conversion were investigated using Pd/2H-T, which exhibited the highest selectivity for THFA. The effect of FF hydrogenation under different H2 pressures is shown in Figure 11. Evidently, the conversion of FF increased from 77.8% to 99.5% as the H2 pressure increased from 1 bar to 30 bar. The reaction at 1 bar H2 had the lowest conversion (77.8%), which is attributed to the fact that the chemical adsorption rate of hydrogen increases with increasing hydrogen partial pressure [62]. As the pressure changed, the distribution of the product changed. As the H2 pressure increased, the selectivity for THFA also increased, essentially reaching 95.4% when the H2 pressure increased to 20 bar. When the reaction was performed at a low H2 pressure, only partial hydrogenation of FF occurred in the same reaction time. Consequently, FA and THFF were produced the most. However, when the reaction vessel was pressurized to a higher H2 pressure, the chemisorption rate of hydrogen increased, thus allowing FA and THFF to be further converted into THFA through ring and C=O hydrogenation, respectively, within the same reaction time. Thus, the highest selectivity for THFA in the 20 bar H2 reaction was observed, and it decreased subsequently due to the increased conversion to 2-MF through cleavage of the C-OH bond at high pressure. Therefore, the optimal pressure was chosen as 20 bar H2.
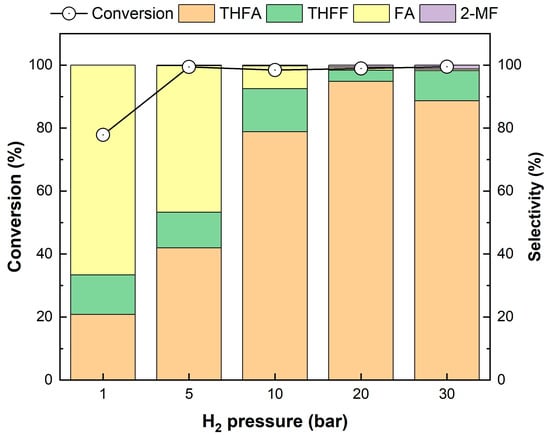
Figure 11.
Effect of H2 pressure on the hydrogenation of FF over the Pd/2H-T. Reaction conditions: reactants, 0.15 g of FF, 0.05 g of the catalyst, 20 mL of 2-propanol; reaction temperature, 35 °C; pressure, 1–30 bar H2; reaction time, 4 h; and stirring rate, 700 rpm.
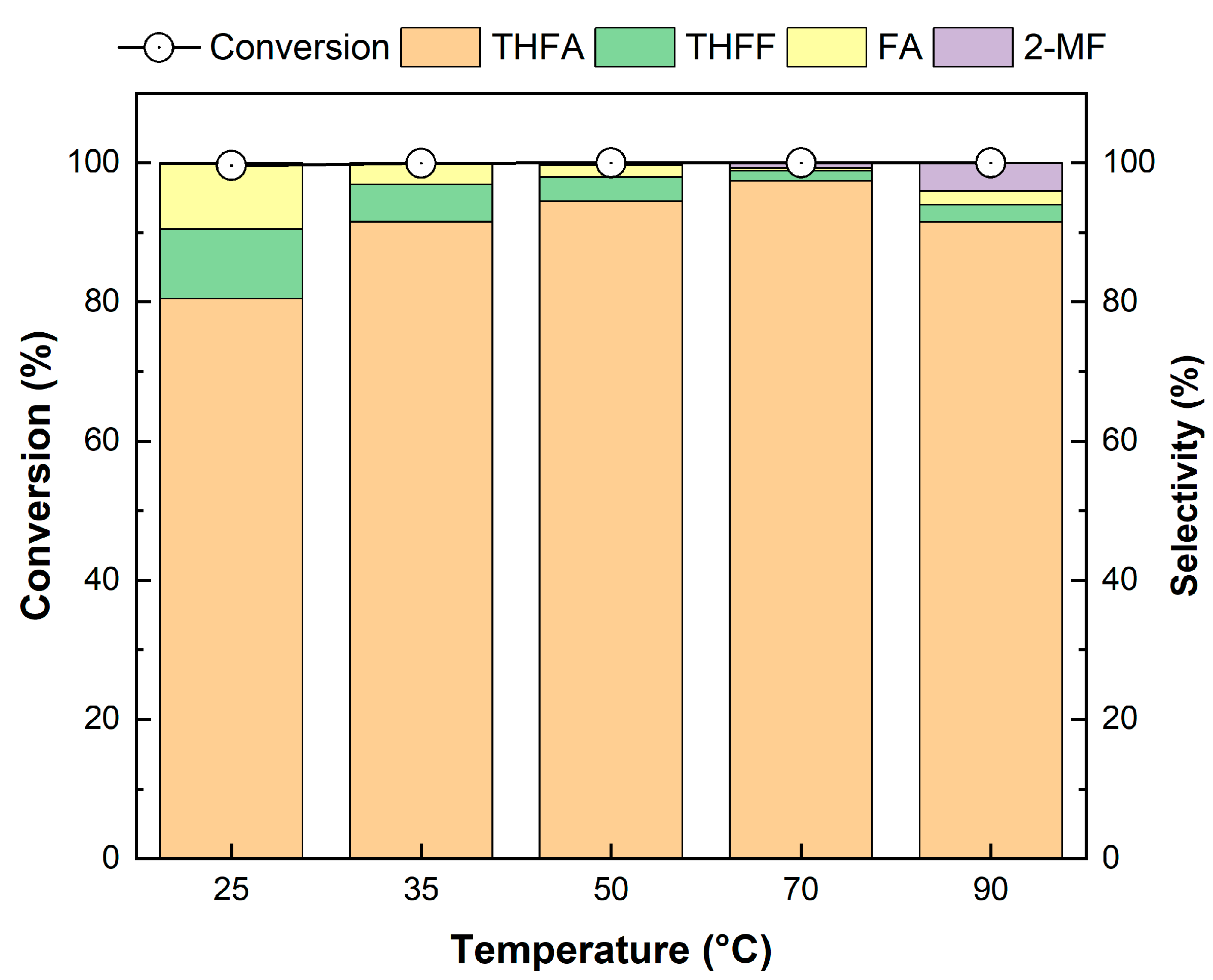
The effect of the reaction temperature on FF hydrogenation over the Pd/2H-T catalyst was investigated at reaction temperatures ranging from 25 to 90 °C, and the results are shown in Figure 12. The Pd/2H-T catalyst achieved a high FF conversion rate of over 99%, even at a low temperature of 25 °C. As the temperature increased from 25 °C to 70 °C, the selectivity of THFA slightly increased from 80.1% to 97.4%, whereas those of FA and THFF decreased. As the temperature increased, the cleavage rate of the C=C bond improved, thus resulting in an increase in THFA selectivity; in particular, 97.4% THFA selectivity was achieved at 70 °C. However, at a high temperature of 90 °C, the selectivity of THFA slightly decreased, which was attributed to the enhanced hydrogenation to 2-MF due to cleavage of the C-OH bond and the instability of THFA at high temperatures [63].
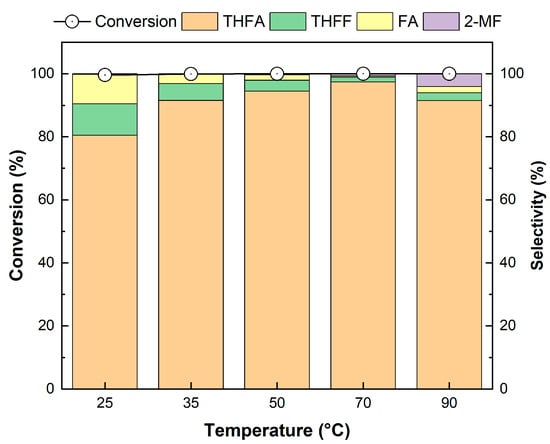
Figure 12.
Effect of temperature on the hydrogenation of FF over the Pd/2H-T. Reaction conditions: reactants, 0.15 g of FF, 0.05 g of the catalyst, 20 mL of 2-propanol; reaction temperature, 25–90 °C; pressure, 20 bar H2; reaction time, 4 h; and stirring rate, 700 rpm.
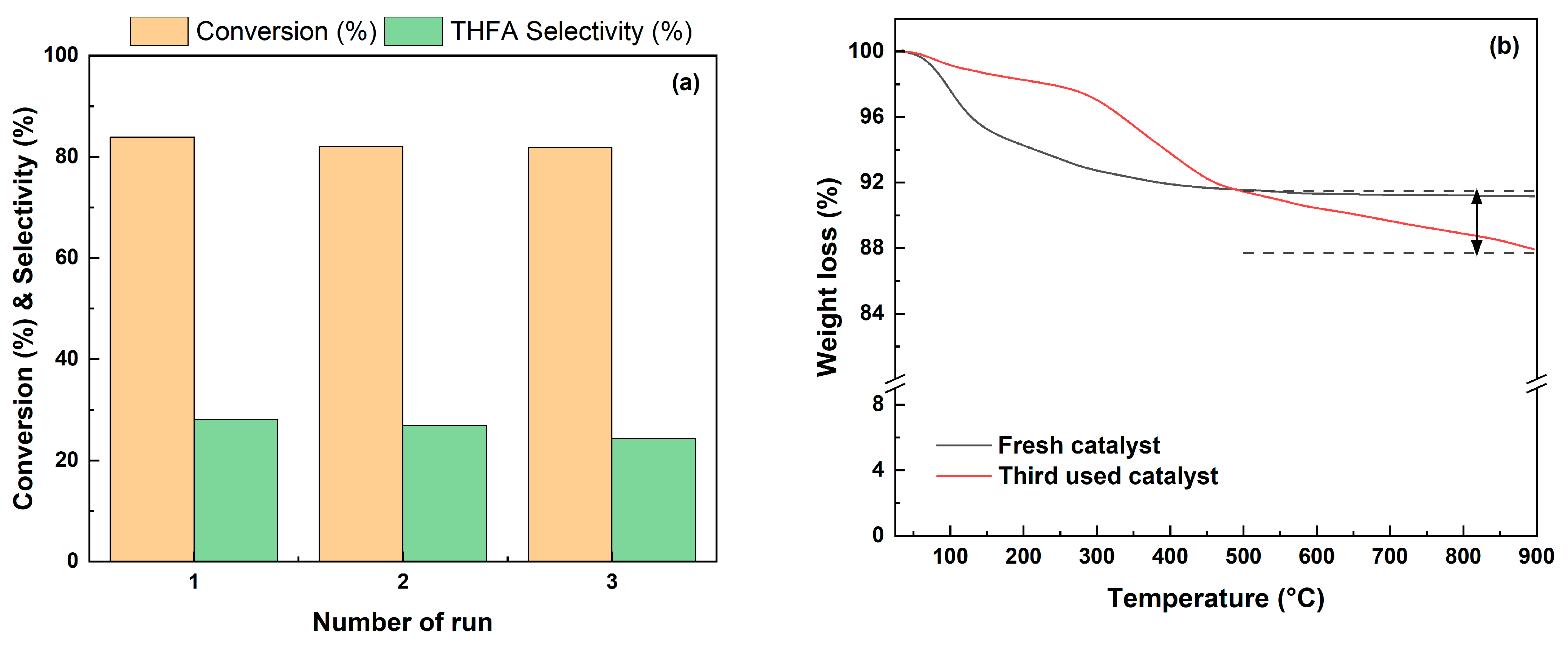
The stability of the catalyst was tested by performing three recycling tests at 35 °C for 4 h under 1 bar H2. The reused catalyst was washed with ethanol and reduced with hydrogen (4% H2 in N2, 20 ml/min) for 2 h at 250 °C prior to the catalytic activity test. The used catalyst was compared with the fresh catalyst through XRD and TGA analysis. There was no significant difference between the fresh catalyst and the used catalyst in the XRD pattern shown in Figure S3 (in the Supplementary Materials). Further, the results of TGA analysis to confirm the amount of coke formation are shown in Figure 13b, and it was confirmed that 3.8% of coke was generated after three reuses. For this reason, as shown in Figure 13a, Pd/2H-T showed a slight decrease in conversion from 83.9% to 81.8% and the selectivity of THFA from 28.1% to 24.3% after three recycles. As a result, Pd/2H-T was demonstrated to be an efficient and reusable heterogeneous catalyst in the hydrogenation of FF to THFA.
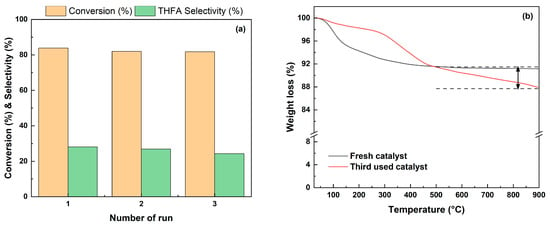
Figure 13.
(a) Effect of recycling on the catalytic activity of the Pd/2H-T catalyst and (b) TGA curve of the the fresh Pd/2H-T catalyst and the third Pd/2H-T catalyst used. Reaction conditions: reactants, 0.15 g of FF, 0.05 g of the catalyst, 20 mL of 2-propanol; reaction temperature, 35 °C; pressure, 1 bar H2; reaction time, 4 h; and stirring rate, 700 rpm.
3. Materials and Methods
3.1. Material
Titanium(IV) butoxide (reagent grade, 97%), HCl solution (38%), and sodium hydroxide (NaOH, 99.8%) were purchased from Sigma-Aldrich and used without further purification. Potassium tetrachloropalladate(ii) (K2PdCl4, 99.99%) and sodium formate (HCOONa, 99.0%) were purchased from Alfa Aesar. FF (99%, Sigma-Aldrich, St. Louis, MO, USA) was stored in a refrigerator to prevent oligomerization. FA (98%, Sigma-Aldrich), THFA (99%, Sigma-Aldrich), THFF (95%, Sigma-Aldrich), and 2-MF (98%, Sigma-Aldrich) were also used.
3.2. Preparation of TiO2 Supports (TiO2 NP)
TiO2 NPs were prepared using the sol-gel method [64,65]. Titanium (IV) butoxide (50 g) was dissolved in ethylene glycol (1 L). The solution was stirred at room temperature for 24 h. Subsequently, 3.4 L of acetone and deionized water (27 mL) were added and stirred for 1.5 h. The suspension was filtered, washed with ethanol, and dried at 80 °C overnight in an oven. Next, 10 g of the dried powder was suspended in 2 L of distilled water and stirred at 80 °C for 1 h to remove unreacted organic compounds. The suspension was filtered, washed with distilled water, and dried at 80 °C overnight.
3.3. Pre-Treatment of TiO2 Supports
TiO2 was pre-treated with different concentrations of HCl solution at room temperature for 6 h. Acid treatment was performed to modulate the surface’s acidic properties. Next, TiO2 was washed with distilled water until the filtrate reached pH 7. After filtration, TiO2 was dried at 80 °C overnight. The optimal concentration of HCl for TiO2 treatment was investigated. The HCl-treated TiO2 is denoted as xH-T, where x is the molar concentration of HCl. For example, TiO2 treated with 0.5 M HCl is denoted as 0.5H-T.
3.4. Preparation of Pd/TiO2 Catalysts
The 5 wt% Pd/TiO2 catalysts were prepared using the deposition–precipitation (DP) method. Briefly, 0.1 M K2PdCl4 5.64 mL was mixed in 120 mL distilled water and heated to 60 °C; subsequently, 1.14 g TiO2 was suspended in the solution. The pH value of the solution was adjusted to pH 11 using 0.25 M NaOH solution. After stirring for 3 h, 0.15 M HCOONa was added as reducing agent, and the temperature was raised to 80 °C. Subsequently, the mixture was stirred for 3 h. The suspension was filtered, washed with distilled water until the filtrate reached pH 7, and dried at 80 °C overnight.
3.5. Characterization
XRD was performed using a Rigaku D/MAX 2500-V/PC (Tokyo, Japan) instrument to confirm the crystal structures of the Pd/TiO2 catalysts. XRD patterns were recorded using Cu Kα radiation (1.5418 Å) at 40 kV, 100 mA, and 2θ ranging from 10° to 80°. The crystallite size of Pd was calculated by the Scherrer formula using the full width at half maximum (FWHM) of 2θ = 40.1°, which corresponds to the Pd (111) crystal plane. Liquid nitrogen adsorption was used to determine the specific surface area and pore volume of the support and catalyst via BET and BJH analysis on a Micromeritics ASAP 2020 instrument (Norcross, GA, USA). Prior to N2 adsorption, the samples were pre-treated for 12 h at 150 °C. To confirm the acidic properties of the samples, temperature-programmed desorption of NH3 (NH3-TPD) was performed using a Micromeritics AutoChem 2920 instrument (Norcross, GA, USA) equipped with a TCD detector. For 0.1 g of each sample, NH3 (10% NH3/He) was adsorbed at 50 °C for 30 min and then heated to 800 °C at 10 °C/min to confirm desorption behavior. The morphologies of the supports were determined by SEM performed on a JEOL JSM-6500 instrument (Tokyo, Japan) operated at 10 kV. The Pd particle size and distribution in the Pd/TiO2 catalyst were determined via transmission electron microscopy (TEM) performed on a JEOL JEM-F200 instrument (Tokyo, Japan). Fourier transform infrared spectroscopy (FT-IR) measurements were performed by Perkin Elmer (Waltham, MA, USA) in the 400–4000 cm–1 range. Electron spin resonance (ESR) spectra of the powder were obtained at microwave power (18.7 mW), microwave frequency (9.418 GHz), center field (340.1 mT), modulation frequency (100 kHz), and room temperature using a Bruker EMX plus-9.5/2.7 (Billerica, MA, USA).
3.6. FF Hydrogenation
FF hydrogenation was performed in 100 mL stainless-steel autoclave. Catalysts were reduced with hydrogen (4% H2 in N2, 20 ml/min) for 2 h at 250 °C prior to the catalytic activity test. Briefly, 20 mL of 2-propanol, 0.15 g of FF, and 0.05 g of catalyst were mixed and added to the reactor. The reactor was purged with N2 twice to remove air, and the H2 was then pressurized to 20 bar. Subsequently, the reactor was heated to 35 °C, stirred at 700 rpm, and the reaction was carried out for 4 h. After completion of the reaction, the products were separated from the catalyst using a polytetrafluoroethylene (PTFE) syringe filter with a pore diameter of 0.45 μm, and the product composition was analyzed by a gas chromatography (GC) instrument (7890A, Agilent, Santa Clara, CA, USA) equipped with a flame ionization detector (FID) and DB-Wax column (30 m × 0.32 mm × 0.25 µm). An external standard method was used to quantify the reaction products using analytical-grade FF, FA, THFF, THFA, and 2-MF without purification. The conversion, selectivity, and yield were calculated using the following equations:
4. Conclusions
This study investigated the effect of HCl treatment of TiO2 used as a catalyst support. The acidity of the treated TiO2 was confirmed by NH3-TPD, and the TiO2 treated with 2 M HCl had the highest acidity (3.66 mmol/g). After HCl treatment, numerous acidic sites were generated on the surface of TiO2 owing to the strong electron-induction effect and surface protonation. During the synthesis process, the Pd precursor was deposited by accepting protons from the acidic site, and the strong acidic site separated the acid sites from each other, thereby reducing the aggregation of Pd species. Therefore, the highest Pd dispersion (25.0%) and smallest Pd particle size were achieved in Pd/2H-T, which had a large number of total acid sites and strong acid sites. In addition, these acidic sites provide more surface adsorption sites for the reactants. The conversion of FF and yield of THFA varied depending on the strength and number of acid sites present on the TiO2 surface. The strong acidities imparted high reactivity on the TiO2 surface toward the adsorbing reactant. The FF conversion and THFA yield of the Pd/2H-T catalyst increased by about 25.3% and 10.7%, respectively, compared with Pd/TiO2 prepared with pristine TiO2. These results suggest that factors such as strong acid sites, high Pd dispersion, and large metallic surface area synergistically enhance the catalytic activity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13121481/s1, Figure S1: FT-IR spectra of pristine and acid treated TiO2 supports; Figure S2: Pore-size distribution curve of (a) TiO2 supports and (b) Pd/TiO2 catalysts; Figure S3: Furfural hydrogenation over Pd/TiO2 catalysts. Reaction conditions: 0.15 g of FF, 0.05 g of the catalyst, 20 mL of 2-propanol, reaction temperature 35 °C, pressure of 20 bar H2, reaction time 4 h, and stirring rate 700 rpm; Figure S4: XRD patterns of the fresh Pd/2H-T catalyst and the third used Pd/2H-T catalyst; Table S1: EDS results of Pd loading over Pd/TiO2 catalysts based on 5 regions for each catalyst.
Author Contributions
Investigation and writing—original draft preparation, H.J.S.; writing—review and editing, Y.E.K., J.J. and M.S.L.; supervision, M.S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Institute of Industrial Technology through Research and Development (EH230002, JF230004, JA230015), Ulsan Metropolitan City (IZ230061) grants.
Data Availability Statement
Data will be available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, X.; Jia, P.; Wang, T. Furfural: A promising platform compound for sustainable production of C4 and C5 chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Rodríguez-Padrón, D.; Len, C. Recent advances in catalytic hydrogenation of furfural. Catalysts 2019, 9, 796. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Hsu, C.-Y.; Chen, S.S.; Ahamad, T.; Alshehri, S.M.; Tsang, D.C.W.; Wu, K.C.-W. Selective hydrogenation of furfural to tetrahydrofurfuryl alcohol over a Rh-loaded carbon catalyst in aqueous solution under mild conditions. Sustain. Energy Fuels 2020, 4, 293–301. [Google Scholar] [CrossRef]
- Ma, R.; Wu, X.-P.; Tong, T.; Shao, Z.-J.; Wang, Y.; Liu, X.; Xia, Q.; Gong, X.-Q. The critical role of water in the ring opening of furfural alcohol to 1,2-pentanediol. ACS Catal. 2017, 7, 333–337. [Google Scholar] [CrossRef]
- Yan, K.; Jarvis, C.; Lafleur, T.; Qiao, Y.; Xie, X. Novel synthesis of Pd nanoparticles for hydrogenation of biomass-derived platform chemicals showing enhanced catalytic performance. RSC Adv. 2013, 3, 25865–25871. [Google Scholar] [CrossRef]
- Taylor, M.J.; Durndell, L.J.; Isaacs, M.A.; Parlett, C.M.A.; Wilson, K.; Lee, A.F.; Kyriakou, G. Highly selective hydrogenation of furfural over supported Pt nanoparticles under mild conditions. Appl. Cat. B 2016, 180, 580–585. [Google Scholar] [CrossRef]
- Lesiak, M.; Binczarski, M.; Karski, S.; Maniukiewicz, W.; Rogowski, J.; Szubiakiewicz, E.; Berlowska, J.; Dziugan, P.; Witońska, I. Hydrogenation of furfural over Pd−Cu/Al2O3 catalysts. The role of interaction between palladium and copper on determining catalytic properties. J. Mol. Cat. A Chem. 2014, 395, 337–348. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nakazawa, H.; Watanabe, H.; Tomishige, K. Total hydrogenation of furfural over a silica-supported nickel catalyst prepared by the reduction of a nickel nitrate precursor. ChemCatChem 2012, 4, 1791–1797. [Google Scholar] [CrossRef]
- Bagheri, S.; Muhd Julkapli, N.; Bee Abd Hamid, S. Titanium dioxide as a catalyst support in heterogeneous catalysis. Sci. World J. 2014, 2014, 727496. [Google Scholar] [CrossRef]
- Parapat, R.Y.; Saputra, O.H.I.; Ang, A.P.; Schwarze, M.; Schomäcker, R. Support effect in the preparation of supported metal catalysts via microemulsion. RSC Adv. 2014, 4, 50955–50963. [Google Scholar] [CrossRef]
- Lashdaf, M.; Tiitta, M.; Venäläinen, T.; Österholm, H.; Krause, A.O.I. Ruthenium on beta zeolite in cinnamaldehyde hydrogenation. Cat. Lett. 2004, 94, 7–14. [Google Scholar] [CrossRef]
- Song, W.; Liu, Y.; Baráth, E.; Zhao, C.; Lercher, J.A. Synergistic effects of Ni and acid sites for hydrogenation and C–O bond cleavage of substituted phenols. Green. Chem. 2015, 17, 1204–1218. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, L.; Ke, C.; Fan, G.; Yang, L.; Li, F. Highly efficient catalytic transfer hydrogenation of furfural over defect-rich amphoteric ZrO2 with abundant surface acid-base sites. Dalton Trans. 2021, 50, 2616–2626. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yang, C.; Zhang, D.; Wang, J.; Fu, H.; Chen, H.; Li, X. Catalytic transfer hydrogenolysis of α-methylbenzyl alcohol using palladium catalysts and formic acid. Appl. Cat. A 2009, 354, 38–43. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, J.; Tan, X.; Pei, Y.; Qiao, M.; Fan, K.; Zong, B. Effect of support acidity on liquid-phase hydrogenation of benzene to cyclohexene over Ru–B/ZrO2 catalysts. Ind. Eng. Chem. Res. 2012, 51, 12205–12213. [Google Scholar]
- Lin, L.; Qiu, C.; Zhuo, Z.; Zhang, D.; Zhao, S.; Wu, H.; Liu, Y.; He, M. Acid strength controlled reaction pathways for the catalytic cracking of 1-butene to propene over ZSM-5. J. Cat. 2014, 309, 136–145. [Google Scholar] [CrossRef]
- Byun, M.Y.; Park, D.-W.; Lee, M.S. Effect of oxide supports on the activity of Pd based catalysts for furfural hydrogenation. Catalysts 2020, 10, 837. [Google Scholar] [CrossRef]
- Wang, F.; Ma, J.; He, G.; Chen, M.; Wang, S.; Zhang, C.; He, H. Synergistic effect of TiO2−SiO2 in Ag/Si–Ti catalyst for the selective catalytic oxidation of ammonia. Ind. Eng. Chem. Res. 2018, 57, 11903–11910. [Google Scholar] [CrossRef]
- Fu, X.; Clark, L.A.; Yang, Q.; Anderson, M.A. Enhanced photocatalytic performance of titania-based binary metal oxides: TiO2/SiO2 and TiO2/ZrO2. Environ. Sci. Technol. 1996, 30, 647–653. [Google Scholar] [CrossRef]
- Tresatayawed, A.; Glinrun, P.; Jongsomjit, B. Ethanol dehydration over WO3/TiO2 catalysts using Titania derived from sol-gel and solvothermal methods. Int. J. Chem. Eng. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Camposeco, R.; Castillo, S.; Mejía-Centeno, I.; Navarrete, J.; Nava, N. Boosted surface acidity in TiO2 and Al2O3-TiO2 nanotubes as catalytic supports. Appl. Surf. Sci. 2015, 356, 115–123. [Google Scholar] [CrossRef]
- Wang, W.; Deng, S.; Tong, Q.; Zhang, X.; Wu, S.; Xu, B.; He, L.; Li, S.; Gong, J.; Fan, Y.; et al. The properties and SCR de-NOx application of supported V2O5/TiO2 catalysts with different polymerization state of VOx species controlled by the pH value of their precursors. ChemistrySelect 2020, 5, 12952–12959. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Yu, J.; Zhou, J.; Zhou, X.; Li, H.; He, Z.; Long, H.; Wang, Y.; Lu, P.; et al. Surface modification of metal materials for high-performance electrocatalytic carbon dioxide reduction. Matter 2021, 4, 888–926. [Google Scholar] [CrossRef]
- Songtawee, S.; Rungtaweevoranit, B.; Klaysom, C.; Faungnawakij, K. Tuning Brønsted and Lewis acidity on phosphated titanium dioxides for efficient conversion of glucose to 5-hydroxymethylfurfural. RSC Adv. 2021, 11, 29196–29206. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, Y.; Song, D.; An, C.; Wang, J. Catalysis of a nanometre solid super acid of SO42−/TiO2 on the thermal decomposition of ammonium nitrate. Nanomater. Nanotechnol. 2016, 6, 23. [Google Scholar] [CrossRef]
- Visser, N.L.; Verschoor, J.C.; Smulders, L.C.J.; Mattarozzi, F.; Morgan, D.J.; Meeldijk, J.D.; van der Hoeven, J.E.S.; Stewart, J.A.; Vandegehuchte, B.D.; de Jongh, P.E. Influence of carbon support surface modification on the performance of nickel catalysts in carbon dioxide hydrogenation. Cat. Today 2023, 418, 114071. [Google Scholar] [CrossRef]
- Hao, Z.; Liu, G.; Ma, N.; Zhang, H.; Li, Y.; Xia, Y.; Zhang, D.; Zhan, S. Oxygen-vacancy mediated acidity and redox properties on WOx/Cu-doped CeO2 for the removal of NOx. J. Environ. Chem. Eng. 2021, 9, 106024. [Google Scholar] [CrossRef]
- Lim, C.S.; Oh, W.-C. Reaction morphology depending on the amounts of HCl and NH₄OH and effect of pH on the preparation of TiO₂ nanopowder. Anal. Sci. Technol. 2007, 20, 302–307. [Google Scholar]
- Keluo, C.; Zhang, T.; Xiaohui, C.; Yingjie, H.; Liang, X. Model construction of micro-pores in shale: A case study of Silurian Longmaxi Formation shale in Dianqianbei area, SW China. Petrol. Explor. Dev. 2018, 45, 412–421. [Google Scholar] [CrossRef]
- Sych, N.V.; Trofymenko, S.I.; Poddubnaya, O.I.; Tsyba, M.M.; Sapsay, V.I.; Klymchuk, D.O.; Puziy, A.M. Porous structure and surface chemistry of phosphoric acid activated carbon from corncob. Appl. Surf. Sci. 2012, 261, 75–82. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Thommes, M. Progress in the physisorption characterization of nanoporous gas storage materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, H.; Ji, X.; Lan, Q.; Fan, Q. Activated Carbon Modified by Ester Hydrolysis of Ethyl Acetate for Water Vapor Adsorption Enhancement. Processes 2022, 10, 1527. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Zhang, T.; Ma, X.; Guo, J.; Wang, J.; Liu, F.; Li, S. Effect of acid/alkali treatment on the structure and catalytic performance of 3DOM CeCo0.7Mn0.3O3 catalyst. Environ. Sci. Pollut. Res. 2023, 30, 101358–101365. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.A.; Ali, A.F. Removal of organic matter from crude wet-process phosphoric acid. J. Chem. Technol. Biotechnol. 1992, 55, 205–208. [Google Scholar] [CrossRef]
- Marikutsa, A.; Rumyantseva, M.; Konstantinova, E.A.; Gaskov, A. The key role of active sites in the development of selective metal oxide sensor materials. Sensors 2021, 21, 2554. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, J.; Kang, M. Photodecomposition of concentrated ammonia over nanometer-sized TiO2, V-TiO~2, and Pt/V-TiO2 photocatalysts. Bull. Korean Chem. Soc. 2007, 28, 581–588. [Google Scholar] [CrossRef][Green Version]
- Shao, Y.; Sun, K.; Li, Q.; Liu, Q.; Zhang, S.; Liu, Q.; Hu, G.; Hu, X. Copper-based catalysts with tunable acidic and basic sites for the selective conversion of levulinic acid/ester to γ-valerolactone or 1,4-pentanediol. Green. Chem. 2019, 21, 4499–4511. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, T.; Sun, K.; Zhang, Z.; Zhang, L.; Li, Q.; Zhang, S.; Hu, G.; Hu, X. Competition between acidic sites and hydrogenation sites in Cu/ZrO2 catalysts with different crystal phases for conversion of biomass-derived organics. Green. Energy Environ. 2021, 6, 557–566. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, L.; Zhang, Y.; Du, W.; Zhang, Y. The study of C3H6 impact on selective catalytic reduction by ammonia (NH3-SCR) performance over Cu-SAPO-34 catalysts. Catalysts 2021, 11, 1327. [Google Scholar] [CrossRef]
- Yang, E.; Jang, E.J.; Lee, J.G.; Yoon, S.; Lee, J.; Musselwhite, N.; Somorjai, G.A.; Kwak, J.H.; An, K. Acidic effect of porous alumina as supports for Pt nanoparticle catalysts in n-hexane reforming. Catal. Sci. Technol. 2018, 8, 3295–3303. [Google Scholar] [CrossRef]
- Yuan, R.; Chen, T.; Fei, E.; Lin, J.; Ding, Z.; Long, J.; Zhang, Z.; Fu, X.; Liu, P.; Wu, L. Surface chlorination of TiO2-based photocatalysts: A way to remarkably improve photocatalytic activity in both UV and visible region. ACS Catal. 2011, 1, 200–206. [Google Scholar] [CrossRef]
- Schwoeble, A.S.; Strohmeier, B.R.; Bunker, K.L.; McAllister, D.R.; Marquis Jr, J.P.; Piasecki, J.D.; McAllister, N.M. Application of X-ray photoelectron spectroscopy (XPS) for the surface characterization of Gunshot Residue (GSR). Microsc. Today 2011, 19, 40–45. [Google Scholar] [CrossRef]
- Huang, H.; Leung, D.Y.C. Complete oxidation of formaldehyde at room temperature using TiO2 supported metallic Pd nanoparticles. ACS Catal. 2011, 1, 348–354. [Google Scholar] [CrossRef]
- Kim, Y.E.; Byun, M.Y.; Lee, K.Y.; Lee, M.S. Effects of chlorinated Pd precursors and preparation methods on properties and activity of Pd/TiO2 catalysts. RSC Adv. 2020, 10, 41462–41470. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, B.L.; Anderson, M.A. Peptization process in the sol-gel preparation of porous anatase (TiO2). Chem. Mater. 1995, 7, 1772–1778. [Google Scholar] [CrossRef]
- Bright, E.; Readey, D.W. Dissolution kinetics of TiO2 in HF-HC1 solutions. J. Am. Ceram. Soc. 1987, 70, 900–906. [Google Scholar] [CrossRef]
- Dai, S.; Wu, Y.; Sakai, T.; Du, Z.; Sakai, H.; Abe, M. Preparation of highly crystalline TiO(2) nanostructures by acid-assisted hydrothermal treatment of hexagonal-structured nanocrystalline titania/cetyltrimethyammonium bromide nanoskeleton. Nanoscale Res. Lett. 2010, 5, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Yu, J.; Sun, X.; Zhuang, J.; He, Q.; You, X.; Guo, J.; Tao, L. Hydrothermal treatment of a TiO2 film by hydrochloric acid for efficient dye-sensitized solar cells. New J. Chem. 2016, 40, 3233–3237. [Google Scholar] [CrossRef]
- Tran, S.B.T.; Choi, H.; Oh, S.; Park, J.Y. Influence of Support Acidity of Pt/Nb2O5 Catalysts on Selectivity of CO2 Hydrogenation. Catal. Lett. 2019, 149, 2823–2835. [Google Scholar] [CrossRef]
- Chen, P.; Wang, X.; Yu, R.; Gu, Y.; Lyu, Y.; Tian, Y.; Fu, J.; Liu, X. Enhancing metal dispersion over an Mo/ZSM-5 catalyst for methane dehydroaromatization. Inorg. Chem. Front. 2022, 9, 4642–4650. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Chen, G.; Zhang, J.; Liu, J. The relationship between acidity, dispersion of nickel, and performance of Ni/Al-SBA-15 catalyst on eugenol hydrodeoxygenation. Renew. Energy 2020, 149, 609–616. [Google Scholar] [CrossRef]
- Park, S.K.; Shin, H. Effect of HCl and H2SO4 treatment of TiO2 powder on the photosensitized degradation of aqueous rhodamine b under visible light. J. Nanosci. Nanotechnol. 2014, 14, 8122–8128. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Puigdollers, A.; Schlexer, P.; Tosoni, S.; Pacchioni, G. Increasing oxide reducibility: The role of metal/oxide interfaces in the formation of oxygen vacancies. ACS Catal. 2017, 7, 6493–6513. [Google Scholar] [CrossRef]
- Luhakhra, N.; Tiwari, S.K. Polaron and bipolaron mediated photocatalytic activity of polypyrrole nanoparticles under visible light. Colloids Surf. A Physicochem. Eng. Aspects 2023, 667, 131380. [Google Scholar] [CrossRef]
- Gao, Z.; Yang, L.; Fan, G.; Li, F. Promotional role of surface defects on carbon-supported ruthenium-based catalysts in the transfer hydrogenation of furfural. ChemCatChem 2016, 8, 3769–3779. [Google Scholar] [CrossRef]
- Nelson, N.C.; Manzano, J.S.; Sadow, A.D.; Overbury, S.H.; Slowing, I.I. Selective hydrogenation of phenol catalyzed by palladium on high-surface-area ceria at room temperature and ambient pressure. ACS Catal. 2015, 5, 2051–2061. [Google Scholar] [CrossRef]
- Gelder, E.A.; Jackson, S.D.; Lok, C.M. A study of nitrobenzene hydrogenation over palladium/carbon catalysts. Catal. Lett. 2002, 84, 205–208. [Google Scholar] [CrossRef]
- Kim, K.D.; Wang, Z.; Tao, Y.; Ling, H.; Yuan, Y.; Zhou, C.; Liu, Z.; Gaborieau, M.; Huang, J.; Yu, A. The comparative effect of particle size and support acidity on hydrogenation of aromatic ketones. ChemCatChem 2019, 11, 4810–4817. [Google Scholar] [CrossRef]
- Mao, C.; Zheng, J.; Matsagar, B.M.; Kankala, R.K.; Ahamad, T.; Yang, Y.; Wu, K.C.-W.; Zhang, X. Highly-efficient Ru/Al–SBA-15 catalysts with strong Lewis acid sites for the water-assisted hydrogenation of p-phthalic acid. Catal. Sci. Technol. 2020, 10, 2443–2451. [Google Scholar] [CrossRef]
- Nzuzo, Y.; Ntshibongo, S.; Matsinha, L.; Adeyinka, A.; Obodo, K.O.; Bingwa, N. Hydrogenation of furfural-to-furfuryl alcohol over La-based inorganic perovskites: A study of oxygen vacancies as catalytic descriptors. Catal. Commun. 2023, 181, 106717. [Google Scholar] [CrossRef]
- Hou, Q.; Cai, J.; Zuo, L.; Chen, H.; Fu, Y.; Shen, J. Selective hydrogenation of furfural over supported nickel and nickel phosphide catalysts. Appl. Surf. Sci. 2023, 619, 156738. [Google Scholar] [CrossRef]
- Vasiliades, M.A.; Govender, N.S.; Govender, A.; Crous, R.; Moodley, D.; Botha, T.; Efstathiou, A.M. The effect of H2 pressure on the carbon path of methanation reaction on Co/γ-Al2O3: Transient isotopic and operando methodology studies. ACS Catal. 2022, 12, 15110–15129. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, J.; Jia, X.; Du, Z.; Duan, Y.; Xu, J. Aqueous phase hydrogenation of furfural to tetrahydrofurfuryl alcohol on alkaline earth metal modified Ni/Al2O3. RSC Adv. 2016, 6, 51221–51228. [Google Scholar] [CrossRef]
- Cheng, G.; Akhtar, M.S.; Yang, O.-B.; Stadler, F.J. Structure modification of anatase TiO2 nanomaterials-based photoanodes for efficient dye-sensitized solar cells. Electrochim. Acta 2013, 113, 527–535. [Google Scholar] [CrossRef]
- Byun, M.Y.; Kim, Y.E.; Baek, J.H.; Jae, J.; Lee, M.S. Effect of surface properties of TiO2 on the performance of Pt/TiO2 catalysts for furfural hydrogenation. RSC Adv. 2021, 12, 860–868. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).