Abstract
Hydrogen production from natural gas or biogas, at different purity levels, has emerged as an important technology with continuous development and improvement in order to stand for sustainable and clean energy. Regarding biogas, which can be obtained from multiple sources, hydrogen production through the steam reforming of methane is one of the most important methods for its energy use. In that sense, the role of catalysts to make the process more efficient is crucial, normally contributing to a higher hydrogen yield under milder reaction conditions in the final product. The aim of this review is to cover the main points related to these catalysts, as every aspect counts and has an influence on the use of these catalysts during this specific process (from the feedstocks used for biogas production or the biodigestion process to the purification of the hydrogen produced). Thus, a thorough review of hydrogen production through biogas steam reforming was carried out, with a special emphasis on the influence of different variables on its catalytic performance. Also, the most common catalysts used in this process, as well as the main deactivation mechanisms and their possible solutions are included, supported by the most recent studies about these subjects.
Keywords:
methane; Ni-based catalysts; sintering; coking; poisoning; promoters; hydrogen; syngas; catalyst support 1. Introduction
1.1. Biogas: Production, Characteristics, and Upgrading
In a global context where sustainability, green chemistry, and a circular economy are highly demanded by society, companies, and governmental agencies (who are encouraging the implementation of facilities to produce biomethane, for instance), the implementation of green technologies is becoming more and more important to contribute to a lower dependency on geopolitical changes or the energy market.
Thus, regarding the replacement of petrochemical products, whose treatment and processing are equally unsustainable from an environmental point of view, the use of green technologies could be an interesting alternative. In a sense, these practices could counteract the abovementioned negative effects, influencing considerably the current energy and geopolitical scenarios, which are always in constant evolution [1,2,3].
In this respect, the role of anaerobic digestion is gaining more and more importance, as it is a very versatile technology where different feedstocks with different characteristics can be used under different operating conditions that could be easily adapted to current green policies and standards.
Indeed, a considerable increase in biogas production worldwide has taken place (it has quadrupled in the last two decades), with Europe as the leading region with more than 50% of biogas global production and Germany with the most facilities for this purpose, although there are still opportunities for the European biogas industry [4,5].
In any case, due to its versatility by treating different wastes, this technology presents a great opportunity for the implementation of green technologies all over the world, especially in developing countries, where the management of some wastes is a challenge and, equally, an opportunity.
In that sense, biogas production seems to be a promising and strategic sector in the future energy scenario, and its consolidation in developed countries, as well as its possible implementation in developing areas, is a clear example of the potential of this technology in the near future.
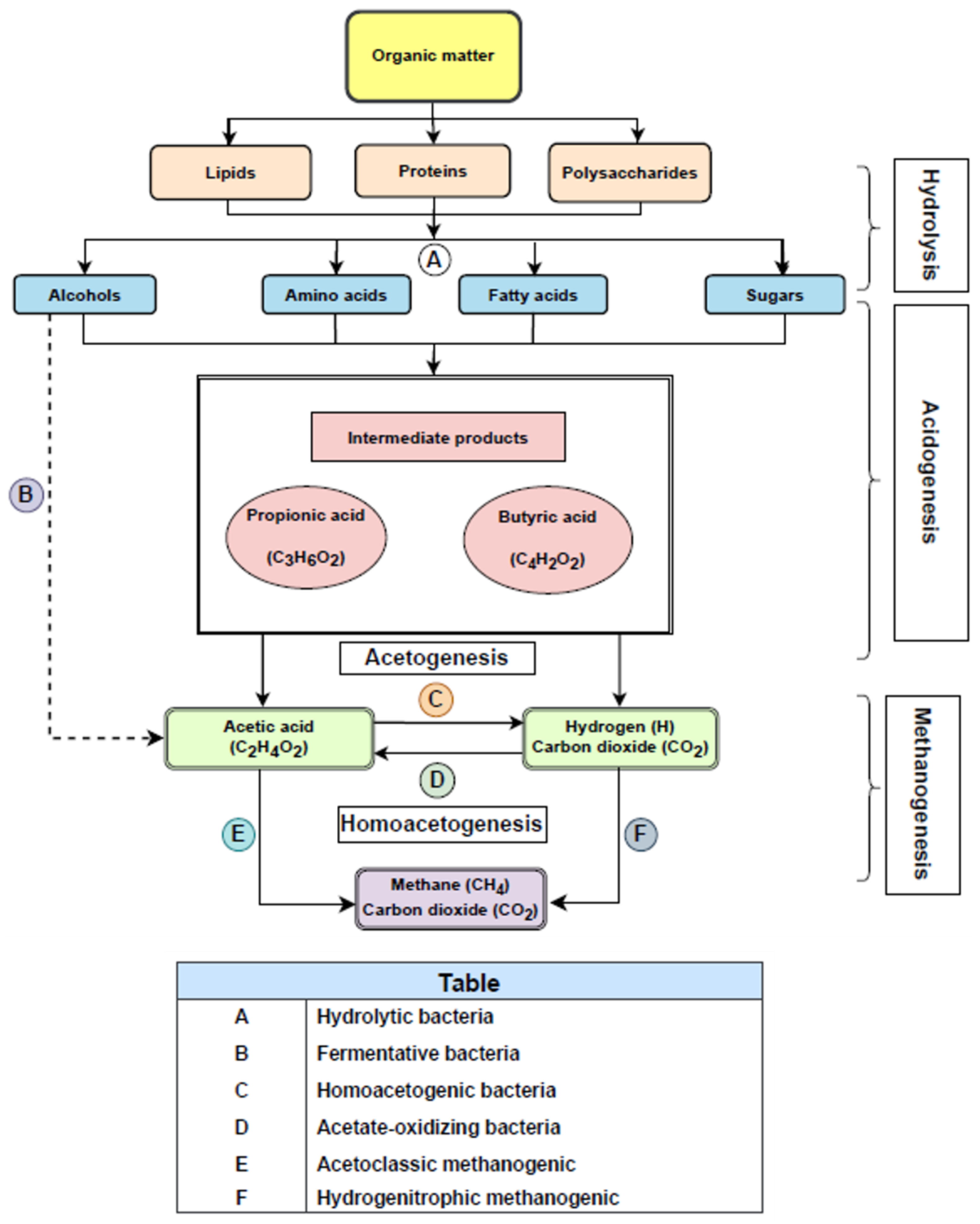
As can be seen from Figure 1, biogas is produced by anaerobic bacteria that degrade organic material to biogas in four steps: hydrolysis, acidification, the production of acetic acid, and the production of methane.
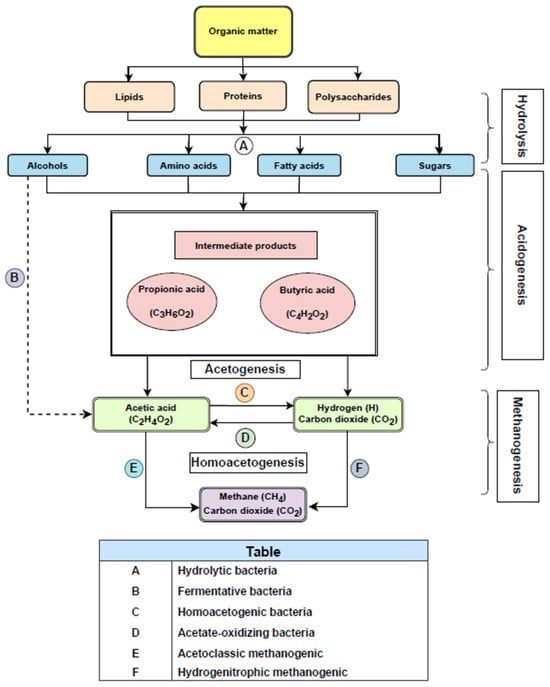
Figure 1.
Steps and products obtained during anaerobic digestion.
Initially, hydrolytic bacteria hydrolyze, that is, break down polymers (polysaccharides, proteins, and lipids) into monomers (fatty acids, amino acids, alcohols, and sugars); and solubilize the particulate material, and then fermenting bacteria ferment the resulting monomers into a wide range of end products. The end products of the acidogenic stage include acetic acid, hydrogen, and carbon dioxide. However, most of the products are volatile fatty acids (VFA) with higher carbon numbers, such as propionate, butyrate, and alcohols.
During acetogenesis, alcohols (ethanol) and volatile fatty acids (VFAs) like butyric acids (VFAs) are converted into acetate by acetate-producing bacteria, obtaining hydrogen and carbon dioxide as the main byproducts. This is an important process, as H2 and CO2 are reduced to acetate by homoacetogenic microorganisms, reducing excess hydrogen that may negatively affect the performance of acetogenic bacteria. Low hydrogen partial pressures (between 10.4 and 10.6 atm) are required for a suitable acetogenic reaction [6]. This is due to the fact that acetogenic bacteria can survive in a very-low-hydrogen-concentration environment.
Conversely, an increase in H2 partial pressure may result in a lower acetate production by acetogens. To ensure that low pressure is maintained during this stage, a mutually symbiotic relationship between the acetogens and the hydrogenotrophic methanogens should take place, so that acetogens can produce acetate that can be used as substrate by methanogens [7].
Methanogenesis is a critical stage in AD, as in the case of hydrolysis. It has a major impact on the AD process because approximately 70% of the methane used in AD is generated. In this stage, carbon-dioxide-reducing and hydrogen-oxidizing methanogens produce CH4 from H2 and CO2, whereas acetoclastic methanogens produce CH4 from acetate [7].
Methanogens (Archaea) mainly use acetate, H2, and CO2 (also methanol, methylamines, and formate to a lesser extent), to generate CH4 and CO2. These are the main substrates for methanogenic bacteria to produce biogas, which consists of 50–75% CH4, 50–25% CO2, and lower amounts of N2, H2, and H2S, which has a negative effect on the steam reforming of biogas, as explained in further sections.
In conclusion, methanogenesis indicates the extent of biological activity in an anaerobic system and the state of digestion. The more methane produced, the more stable and efficient the system is. Subsequently, in the context of this review, these steps are crucial to obtain a high-quality biogas, which should have a high methane percentage in order to carry out further upgrading processes in an optimized manner. Also, the presence of impurities (as previously mentioned) could worsen the performance of biogas steam reforming, from a catalytic point of view (for instance, through poisoning due to H2S) or the requirement of purification steps once the biogas is processed (in order to obtain high-purity hydrogen, for instance).
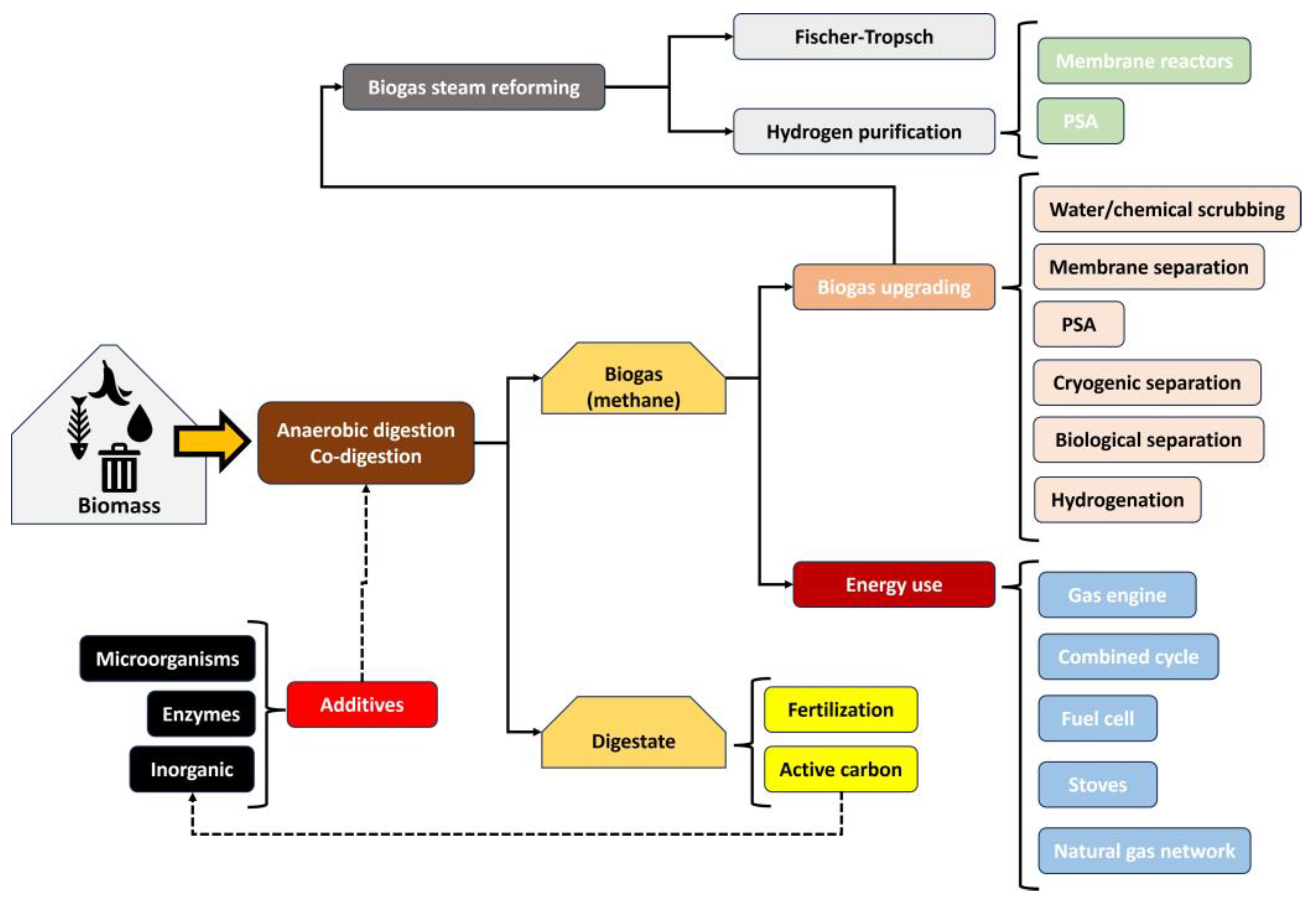
In a more complex context, biogas production is linked to multiple steps to valorize this product through upgrading or energy use. As observed in Figure 2, biogas is obtained through anaerobic digestion from different wastes, such as agricultural wastes, sewage sludge, solid municipal waste, and manure, etc. [8,9,10]. In some cases, depending on the properties of the feedstock, the co-digestion of several wastes can be recommended so that some properties in biodigesters (such as acidity, organic matter, and the presence of contaminants, etc.) are balanced to obtain a high biogas production [4,11,12]. In this stage, biogas and digestate are obtained and usually reused as fertilizers or in other processes like active carbon production through pyrolysis or hydrothermal carbonization. Depending on the quality of the biogas (that is, moisture levels, methane content, and the absence of hydrogen sulfide, etc.), this product can be used directly for energy purposes or undergo upgrading (for further treatments such as steam reforming). In any case, even for energy purposes (for instance, its direct use in stoves, gas engines, or its introduction in the natural gas grid), this biogas upgrading could be recommended, which includes steps such as drying (to remove moisture), depuration (H2S cleaning among other contaminants, such as siloxane, CO, or NH3), and CH4 separation from CO2 [13,14].
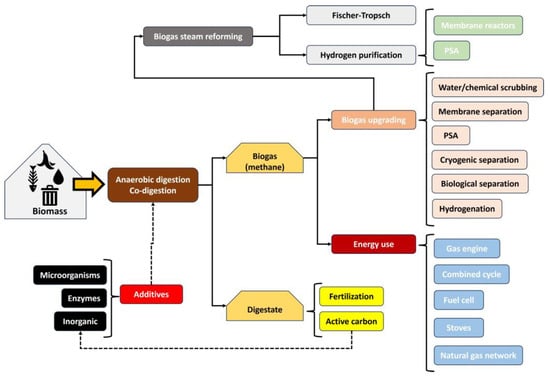
Figure 2.
Scheme of processing chain from biomass to commercial products through biodigestion.
Once biogas is upgraded, with good enough quality for its use in further steps, its treatment in processes such as methane steam reforming can be considered to produce a gas phase enriched with H2, typically between 50 and 80%. In order to generate a gas with a higher range of purity in hydrogen, a further purification step is needed, such as membrane reactors or pressure swing adsorption. If these are used, high-purity hydrogen (normally exceeding 98%) is obtained, with subsequent use in energy production or chemical synthesis (for instance, production of ammonia, methanol, hydrogen peroxide, acetic acid, aldehydes, dyes, hydrochloric acid, polyols, nylon, and polyurethane, etc.) [15]. Otherwise, a mixture of hydrogen with CO (which constitutes synthesis gas), among other components, can be obtained, which could be suitable for further processes such as Fischer–Tropsch synthesis to produce liquid fuels, among others [16,17]. It should be noted that biogas production and treatment offer a wide variety of opportunities (see Figure 2) for research and companies, including the aim of our review work, that is, biogas steam reforming and the use of catalysts.
It should be noted that the technologies applied to biogas are not limited to those observed in this figure, as further treatments of the derived products might be carried out. Regarding the subject of this review, two factors should be considered according to biogas processing:
- The role of catalysts is present in many aspects of biogas processing (specifically heterogeneous catalyst) when it comes to energy production (for instance in Fischer–Tropsch or steam reforming processes, the main topic of this review).
- In addition, many stages related to biogas can present an influence on catalytic performance in biogas steam reforming. For example, digestate can be transformed into active carbons, which can be used as additives for a better biogas production or biogas upgrading (with mercaptans or H2S removal, for instance, as they are toxic and corrosive components). In turn, it can present a positive effect on catalytic steam reforming (as H2S provokes poisoning and the subsequent deactivation of catalysts) [18,19]. Another example would be the use of membrane reactors to improve hydrogen yield during steam reforming, with the subsequent improvement in methane conversion. Many of these aspects (with a considerable influence on the catalytic steam reforming of biogas) will be covered in this review in following sections.
Considering the fact that, for every industrial process, the role of economics is essential, the valorization of some by-products or wastes during biogas production and treatment is important to make every process involved as efficient (and feasible) as possible, as it will be discussed in following sections.
1.2. Hydrogen Production from Methane (or Biogas) Steam Reforming
Hydrogen production is a perfect example of green chemistry, contributing to the transition to more renewable energies and the sustainable growth of population areas [20,21]. Pure hydrogen, as well as syngas (a mixture of hydrogen with CO), have been gaining importance recently, as they can be used as an energy carrier or in interesting industrial processes, like methanol synthesis (or more complex compounds) through Fischer–Tropsch reactions [22,23].
There are different chemical routes to produce hydrogen, such as thermochemical water decomposition, electrolysis, coal gasification, or fossil fuel reforming [21]. One of the main chemical routes to produce hydrogen or syngas from natural gas or biogas is methane steam reforming, which has been widely studied across the board, as explained in following sections. However, in many cases, it presents some advantages and disadvantages, as observed in Table 1 in a comparison with dry reforming. In the case of steam reforming, depending on feeding, operating conditions, or further/consecutive steps like the use of membrane reactors or pressure swing adsorption, different purity levels of hydrogen can be obtained [24].

Table 1.
Advantages and disadvantages of SRM and DRM [25,26,27].
From a chemical point of view, the steam reforming of methane (see Equation (1)) is an endothermic reaction that usually takes place at high temperatures, between 750 and 950 °C, and a wide range of pressure (5–20 bar) [25].
With an enthalpy of 206 kJ/mol, reforming is a highly endothermic process. Consequently, a significant amount of external energy is required to carry it out. For this reason, the most common process takes place in a tubular reactor inside a furnace that provides the energy required for the reaction, with the subsequent economic costs.
Equally, a water–gas shift reaction (WGS) (see Equation (2)) can simultaneously take place. This way, both chemical reactions contribute to a higher yield in hydrogen production and, subsequently, a higher hydrogen concentration in the resulting gas.
This exothermic reaction occurs at low rates in the reforming reactor, which explains the presence of CO2 at the outlet of the reforming process. High carbon dioxide levels are undesirable from an environmental point of view, and different aspects such as catalyst design and consecutive separation processes. There are other possible side reactions, like a direct reaction between CH4 and CO2, CH4 and CO, and methane decomposition, etc. These side reactions are more abundant if heterogeneous catalysts are used in the process [28,29]. The production of H2 depends on the equilibrium of the reforming and adjustment reactions (Equations (1) and (2)), and it can be maximized with low pressures, high temperatures in reforming, and a high excess of steam [30,31].
Regarding pressure, it usually presents two contrary effects. Thus, high pressure promotes the interaction of molecules, whereas high pressure would shift the chemical balance, especially in methane steam reforming (Equation (1)) towards the reagent generation. That is the reason why the optimization of this parameter is necessary, considering the rest of the chemical conditions.
On the other hand, the amount of steam to be added to the feed is quantified by the steam to carbon (S/C) ratio, which represents the moles of steam introduced per mole of carbon in the hydrocarbon stream. This ratio takes values between 2.5 and 6 depending on the feed and process optimization conditions. High values of the S/C ratio promote H2 formation while preventing catalyst deactivation due to carbon deposition, which is favored at low S/C ratios. However, there is a limitation on the maximum amount of steam that can be introduced, both from an energy penalty perspective, as it represents steam that will not generate power, and from an economic standpoint, as the investment cost increases. When the feed is natural gas, it is common to work with S/C ratios around 2.5–5.
Obviously, the physicochemical characteristics of biogas are essential for understanding the global performance of steam reforming. As observed in Table 2, depending on the kind of feedstock, the biogas composition might be different, although some general similarities are observed.

Table 2.
Biogas composition (from different feedstocks).
In general, biogas contains between 45 and 80% CH4 and around 20–60% CO2, including other secondary compounds such as H2O or N2 and residual percentages of H2S and H2, among others. Biogas composition might vary depending on many different factors (mainly due to feedstock heterogeneity on account of the sampling date, but also due to changing anaerobic digestion conditions), but the majority component is methane, which could be the starting point to produce hydrogen through steam reforming, among other processes.
Consequently, the methane content in the final biogas will determine its further uses or treatments. In that sense, as explained in the literature, the use of methane as a hydrogen carrier could be an interesting alternative for hydrogen storage, which is difficult or expensive (implying the use of costly technologies such as liquefaction and compression) [39]. In that sense, it is important to note that the catalyst for reforming can be deactivated by the presence of certain contaminants in the gases entering the reformer (sulfur, copper, vanadium, and lead, etc.).
In particular, hydrogen sulfide content deserves a special mention, as it will imply a negative effect on the catalytic performance of biogas steam reforming, drastically affecting the catalyst’s activity, even at very low concentrations (ppm). Therefore, it is common to use prior desulphurization systems based on activated carbons as adsorbents at room temperature.
1.3. Catalytic Biogas Steam Reforming in the Literature
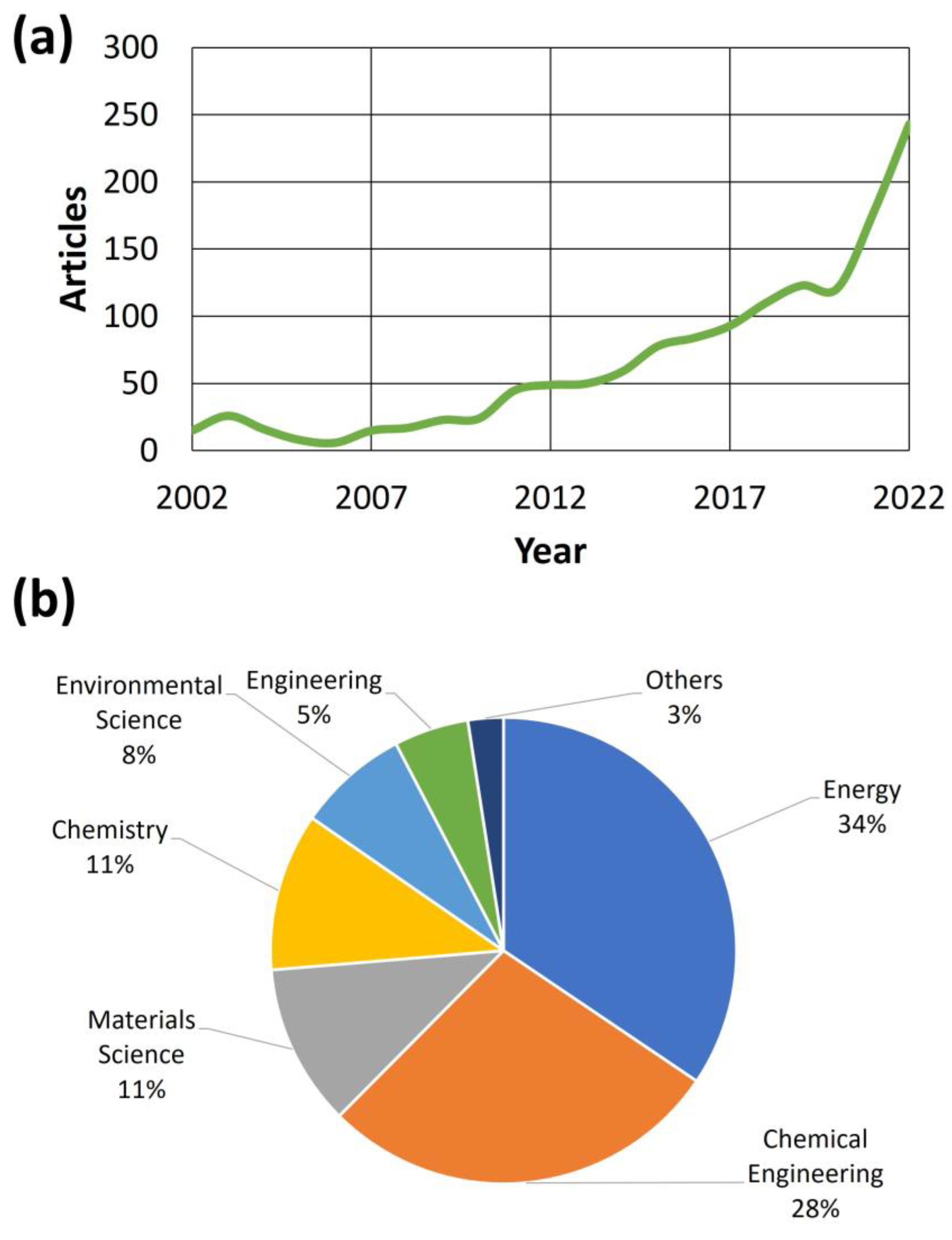
Apart from the obvious industrial development of methane steam reforming (SRM), including those gases (like biogas) with a considerable amount of this compound, there has been an increase in the scientific interest in this subject in the last two decades [40]. Figure 3 shows the main trends observed in the literature for the search criterion “biogas steam reforming catalyst”.
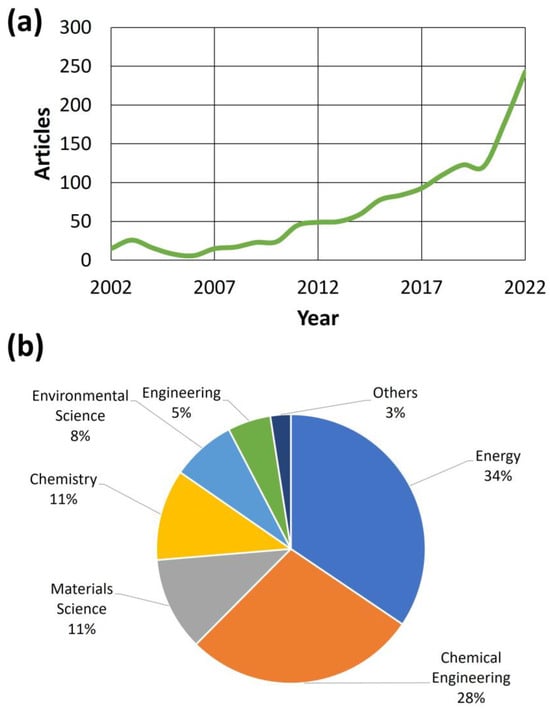
Figure 3.
Articles published over the past two decades (a) and the main scientific fields of the journals where they were published (b) [41]. The search keywords were the following: “Catalyst” and “steam” and“reforming” and “biogas”.
As observed in Figure 3a, there was an increasing trend in published articles related to the subject of this review, that is, the catalytic steam reforming of biogas. In that sense, especially from 2010, this increase was steady, whereas there was an exponential growth in the 2020–2022 stage, reaching up to 243 published articles in 2022. These figures confirm the increasing interest in this subject by the scientific community, where this subject has attracted the attention of diverse scientific fields (reflected in Figure 3b, including the main fields of the journals where articles devoted to catalytic biogas steam reforming were published). Thus, and as expected, energy was the field with most journals, pointing out the relevance of the products obtained during biogas steam reforming. Nevertheless, other fields like chemical engineering were equally important, as the implementation of new catalytic approaches at an industrial scale is vital in technologically advanced and mature industries. Also, materials science and chemistry are important in this sense, as the characterization of catalysts and the understanding of their main action mechanisms are important for understanding their effectiveness and durability during steam reforming. Finally, and not least, fields like environmental sciences and engineering (the latter related to the objectives explained for chemical engineering) are of interest in this review subject, as methane conversion to hydrogen and syngas could be a suitable way of reducing environmental impact (especially related to the greenhouse gas effect), pointing out the sustainability of this process.
There are numerous reviews on this subject among these articles published since 2000 [42,43,44], which have focused on several aspects such as methodologies, technologies, general processes, and catalysts, etc. Regarding research papers, according to Table 3, where the most cited articles about this subject are included, it should be noted that they are relatively new articles, proving the interest in this subject by the scientific community. In any case, only articles considering the main keywords in the title were included, as there are plenty of works (including reviews) that deal with this subject, requiring a stricter search criterion to obtain these results.

Table 3.
Top 10 cited articles with the following search criteria for the title: catalyst and biogas and steam and reforming (source: [41]).
If the most cited authors with research work about catalytic biogas steam reforming are considered (see Table 4), some interesting remarks can be made, like the following:

Table 4.
Top 10 cited authors with the following search criteria: catalyst and biogas and steam and reforming (source: [41]).
- They have contributed with at least 30 articles each about this subject, which proves the endless possibilities of catalytic biogas steam reforming.
- Most authors are reputed scientists, with a h index from 30 to 76 and many citations. The fact that such prestigious scientists have dealt with this field to a certain extent proves its relevance in the scientific community.
- As expected, these authors are equally focused on other subjects and fields (with a considerable percentage of published articles about the subject of this review), pointing out the multidisciplinarity of their research teams, which could enrich the research in this field.
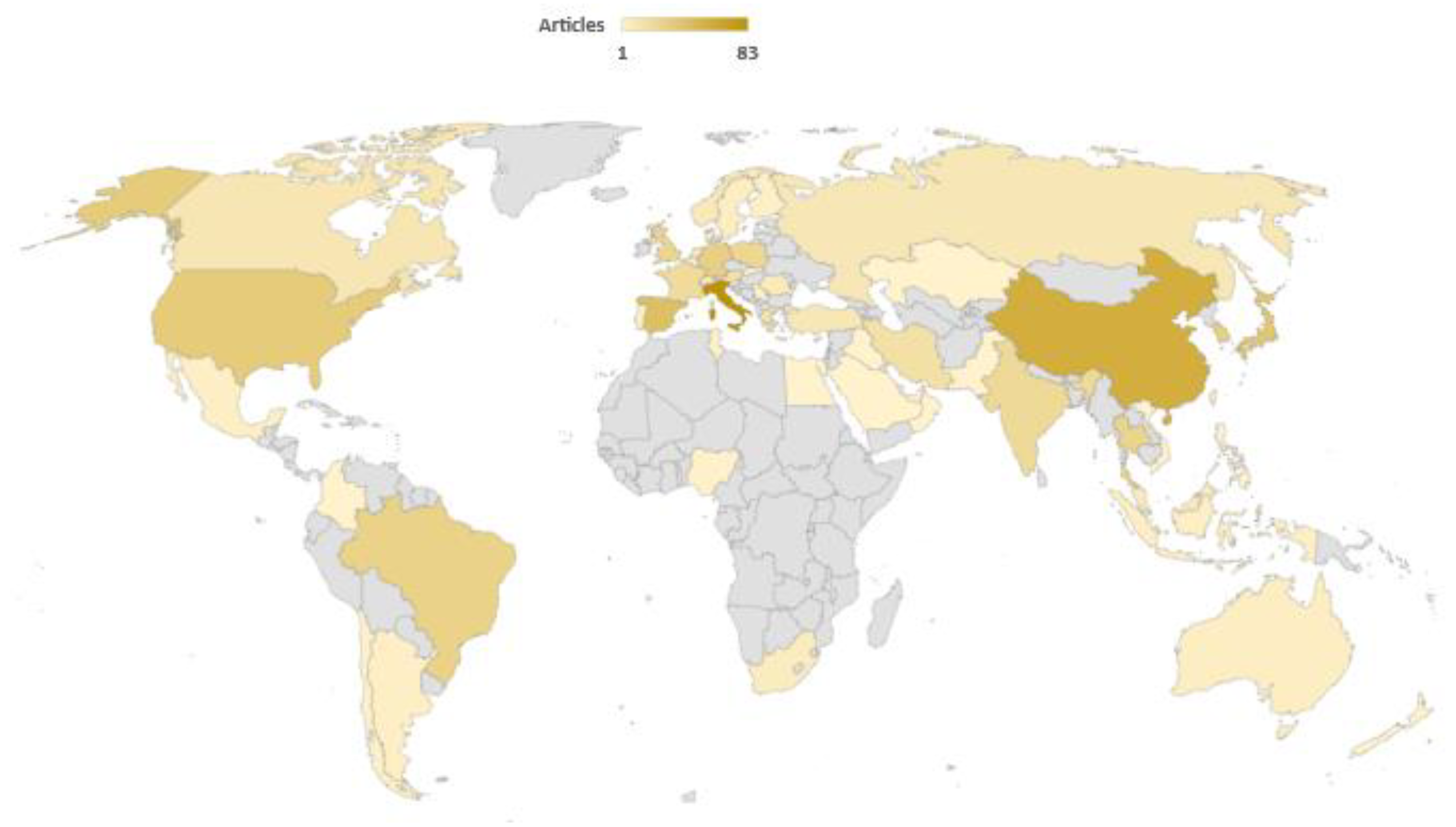
If the published articles about biogas steam reforming are arranged by country, an interesting outlook on scientific works can be found. Thus, as observed in Figure 4, there is a relatively homogeneous distribution of published articles about biogas steam reforming worldwide, with countries like Italy (83 articles) or China (53 articles) leading. Even though Europe has been traditionally focused on biogas upgrading, there are other regions like America and Asia where the role of this research is also representative. Equally, some works from African countries have been devoted to this subject, which could be an encouraging starting point for the implementation of these technologies on this continent.
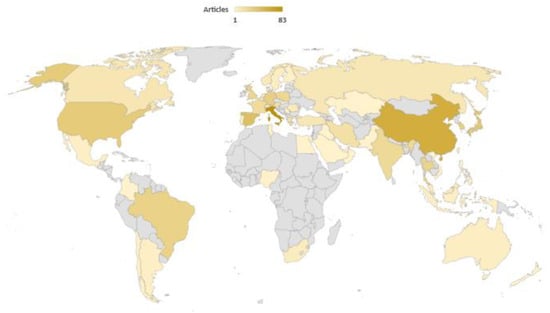
Figure 4.
Worldwide distribution of published articles about this subject. Search criteria: biogas and steam and reforming [41].
Finally, taking into account the relationship between the keywords of the works dealing with the use of catalysts in the steam reforming of biogas, interesting findings can be found, as observed in Figure 5:
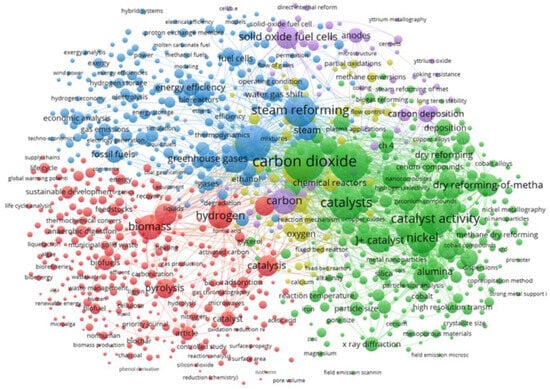
Figure 5.
Keyword co-occurrence map based on Scopus, obtained by VOS Viewer. Search criteria used: catalyst and biogas and steam and reforming (7688 documents in total) [41].
Thus, five interesting clusters can be found (represented in different colors), which cover every aspect intended to be included in this review work, like the following:
- Red cluster: it is mainly focused on feedstocks for biogas generation (biomass, municipal solid waste, and sewage sludge, etc.), as well as biogas production through anaerobic digestion.
- Yellow cluster: focused on reaction mechanisms, methane conversion, and flow control, etc. In that sense, this is the most interesting cluster for engineering purposes.
- Blue cluster: the main subjects included in this cluster are steam reforming, water gas shift, fuels, energy efficiency and storage, operating conditions, thermodynamics, gas emissions, and economic analysis, among others. It is similar to the previous cluster but focused on energy conversion.
- Green cluster: mainly devoted to catalysts, where the role of nickel (and cerium, among other promoters) as an active phase and alumina and silica as supports seems to be important. Also, some factors such as particle and pore size or reaction temperature are relevant, which are vital to increasing the useful life of catalysts (avoiding carbon deposition or sintering, terms included in this cluster too). In other words, this is the cluster where everything about catalysts is covered.
- Purple cluster: as in the previous case, it is mainly focused on catalysts and some inhibitory effects such as carbon deposition and the corresponding properties of the catalysts to avoid it to a certain extent (coking resistance). In that sense, the role of yttrium seems to be important.
In that sense, as explained in this figure, most of the main aspects covered by these clusters will be included in this review, which points out the interrelation of many subjects (from feedstock to catalyst characterization or from energy efficiency to catalyst activity, for instance) and proving the interest in this field.
1.4. Aim of This Review
According to the above, the aim of this review work was to carry out a review of biogas steam reforming, especially focused on catalytic conversion to obtain hydrogen. Thus, the following points will be covered:
- Biogas production in context, including the main feedstocks and innovative technologies for improving methane yield and quality, which is essential for better performances of catalysts during steam reforming.
- Biogas steam reforming and the main factors affecting its performance, which can be improved by the use of catalysts and, at the same time, can affect the performances of catalysts in some respects.
- The role of catalysts in biogas steam reforming, including the main catalysts used, the foundations, mechanisms, and main deactivation processes (and how to avoid them or, at least delay them).
- The main techno-economic analyses and patents carried out on this subject, paying attention to the role of catalysts.
Thus, a thorough review was carried out, which aims to clarify the role of catalysts in biogas steam reforming and all the details affecting their performance, paying attention to the research carried out in the last five years.
1.5. Scope and Bibliometric Analysis
In order to carry out this review work, Scopus was investigated for all entries in the literature on the topics of biogas (including keywords such as catalysts and steam reforming) for the last 20 years, with special attention to the last 5-year period (2018–2023), where there has been a considerable increase in published papers and innovative research in this field. The search, which was made from May to October 2023, returned 6968 results, from which up to 281 articles were considered for their inclusion in this work, including the information of about 148 published works (mainly research works, reviews, and, to a lesser extent, proceeding papers and patents) in the final paper.
2. Use of Catalysts in Biogas Steam Reforming
As briefly explained in the previous section, the role of catalysts in steam reforming in general, and in methane or biogas (whose majority compound is methane) in particular, is essential for the implementation of a competitive technology at an industrial level.
2.1. Main Considerations
In general, the catalyst is located inside a tubular reactor, which can be arranged as a fixed or fluidized bed. The most studied/used configuration is the fixed bed, due to its simplicity and reproducibility, However, it is possible that the deactivation in this configuration is higher than that of the fluidized bed [55], because carbon depositions (between catalyst particles/pellets) are less prevented as the catalyst is immobile. To highlight the recent appearance of new types of reactors in this type of reactions, the photo-thermal ones [56], which can reach H2 production velocities of 17.4 μmol s−1 with an STH efficiency of 22.5% and CO selectivity of 1% in the optimal design under concentrated light irradiance of 16 kW·m−2 in the lab, these reactors are positioned as an alternative to be developed to solve the great disadvantage of energy input.
When studying SMR, it is important to keep in mind that the diffusion of feed biogas and water is homogeneous. For this, the water feed can be performed in liquid form and vaporize inside the reactor, a fact that can lead to a gradient in the concentration of water along the catalytic bed. Another way to introduce water is in the vapor phase, a fact that implies having a vaporizer and a steam flow controller prior to entering the reforming reactor, in addition to avoiding condensation in the steam conduction.
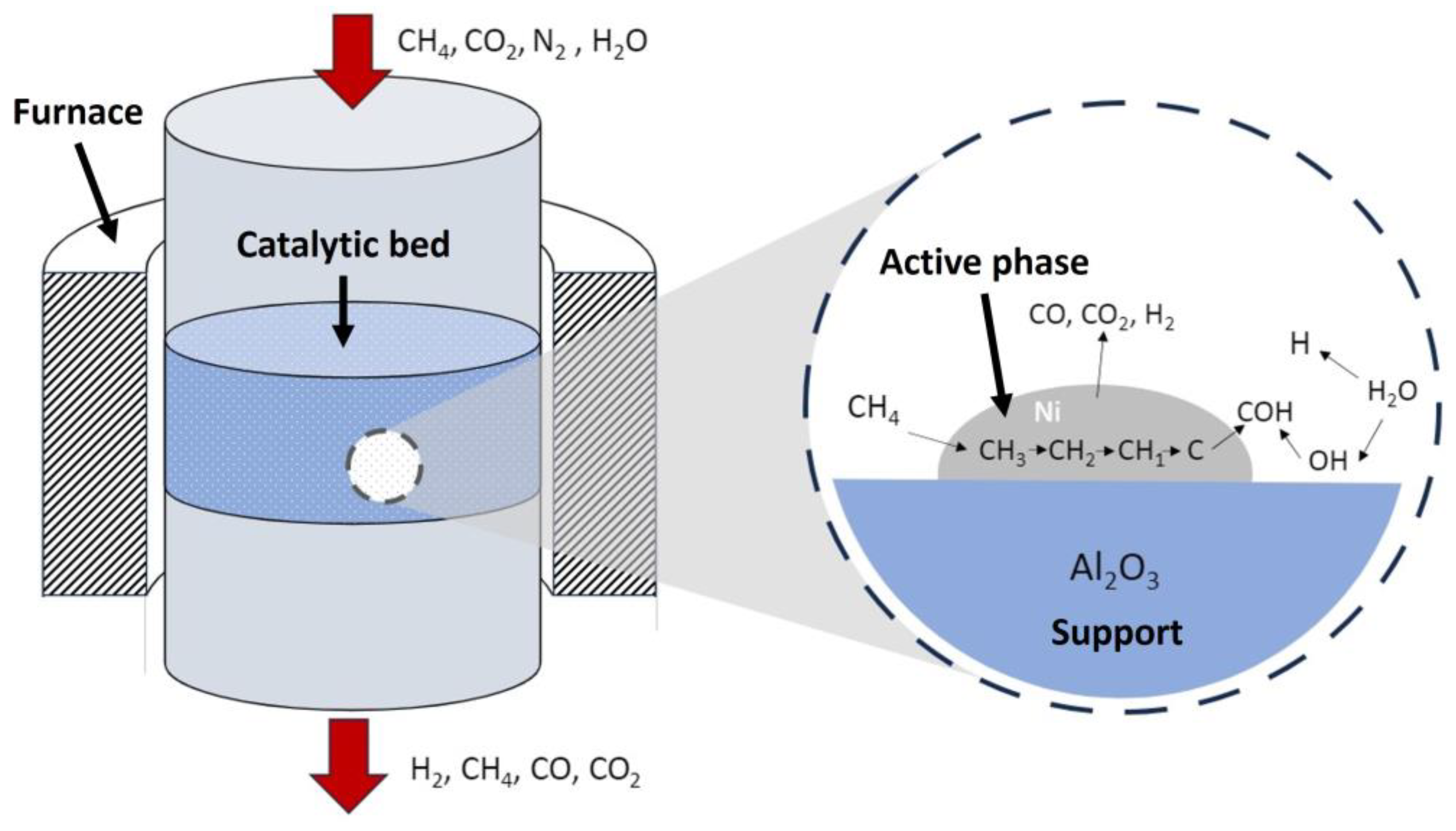
What is perhaps one of the most influential parameters in the effectiveness of the reforming process is the activity of the catalyst. The order of catalytic activities on active metals for SRM has been reported: Rh > Ru > Ni > Ir = Pd = Pt > Co > Fe. In addition, other studies have calculated the TOF in DRM for various metals and showed that the order differs for Al2O3 support and SiO2 support. The order of TOF of the methane reaction rate on each support is presented below [57]: Ni > Ru > Rh, Ir (SiO2 support); Rh > Ni > Ir > Pt, Ru > Co (Al2O3 support). Recent studies have assessed the activity of several composite structured catalysts, showing the following decreasing activity: Rh > Ru > Pt > Ni [58]. In any case, for SRM and DRM, it is common to use Ni catalysts, which are active and inexpensive, supported on metal oxides such as Al2O3, which have a high heat resistance.
Generally, the catalyst consists of an active phase dispersed on a support. The active phase in catalysts for biogas reforming is commonly composed of nickel, whereas the support is usually an aluminosilicate. These materials are normally used due to their catalytic activity, resistance to operating conditions, commercial availability, and versatility. Within aluminosilicates, many types can be found commercially, and in different forms, like powder, spheres of various sizes, and pellets, etc. This variety allows for a great adaptability to the type of reactor, since it is possible to adjust some parameters such as the contact time, charge loss within the reactor, and deactivation, etc. In the following sections, these aspects will be covered.
2.2. Kinds of Catalysts and Their Preparation
Different catalysts can be used in biogas steam reforming, like the following [59]:
- Monometallic catalysts: they are mainly Ni-based catalysts, which are very popular in the literature due to their great catalytic activity and relatively low cost compared to other equivalent catalysts. However, they have some negative effects (which will be explained in detail in following sections) during steam reforming, such as deactivation due to coke deposition or poisoning.
- Catalysts with promoters: the abovementioned catalysts can be considerably improved by adding promoters (such as B, Ir, La, or Mg) that can help to improve the global performance during SRM thanks to the improvement of metal–support interaction or the ability to promote a higher dispersion compared to traditional catalysts. Recent works point out the relevance of adding some promoters (La and Mg) to typical Ni/Al2O3 catalysts, in order to improve their catalytic performance. Thus, these additives improved the stability and dispersion of the active phase, with a better deactivation resistance [60].
- Bimetallic or polymetallic catalysts: to avoid deactivation derived from sintering or coke deposition, the use of combined metallic catalysts could present a positive effect. Bimetallic catalysts are mainly based on Ni or Co combined with noble metals, non-noble metals, or metalloids, whereas polymetallic catalysts are combinations of different metals, like: Ni, Cu, and Zn; Ni, Co, and Ce; and Ni, Ru, and Mg. These combinations can present not only additive, but synergistic effects [30,61].
The main characteristics of these catalysts will be explained in further detail in the following sections, paying attention to different factors such as the catalyst support, active phase, and the interaction between them, which will determine the catalytic performance during methane or biogas steam reforming. For instance, the activity of the resulting catalyst and its resistance to sintering will vary depending on the use of different promoters. In that sense, the preparation of a certain catalyst is vital to understanding some of the final properties of this product. There are different ways to prepare catalysts for this purpose, such as impregnation, co-precipitation, and the sol–gel method.
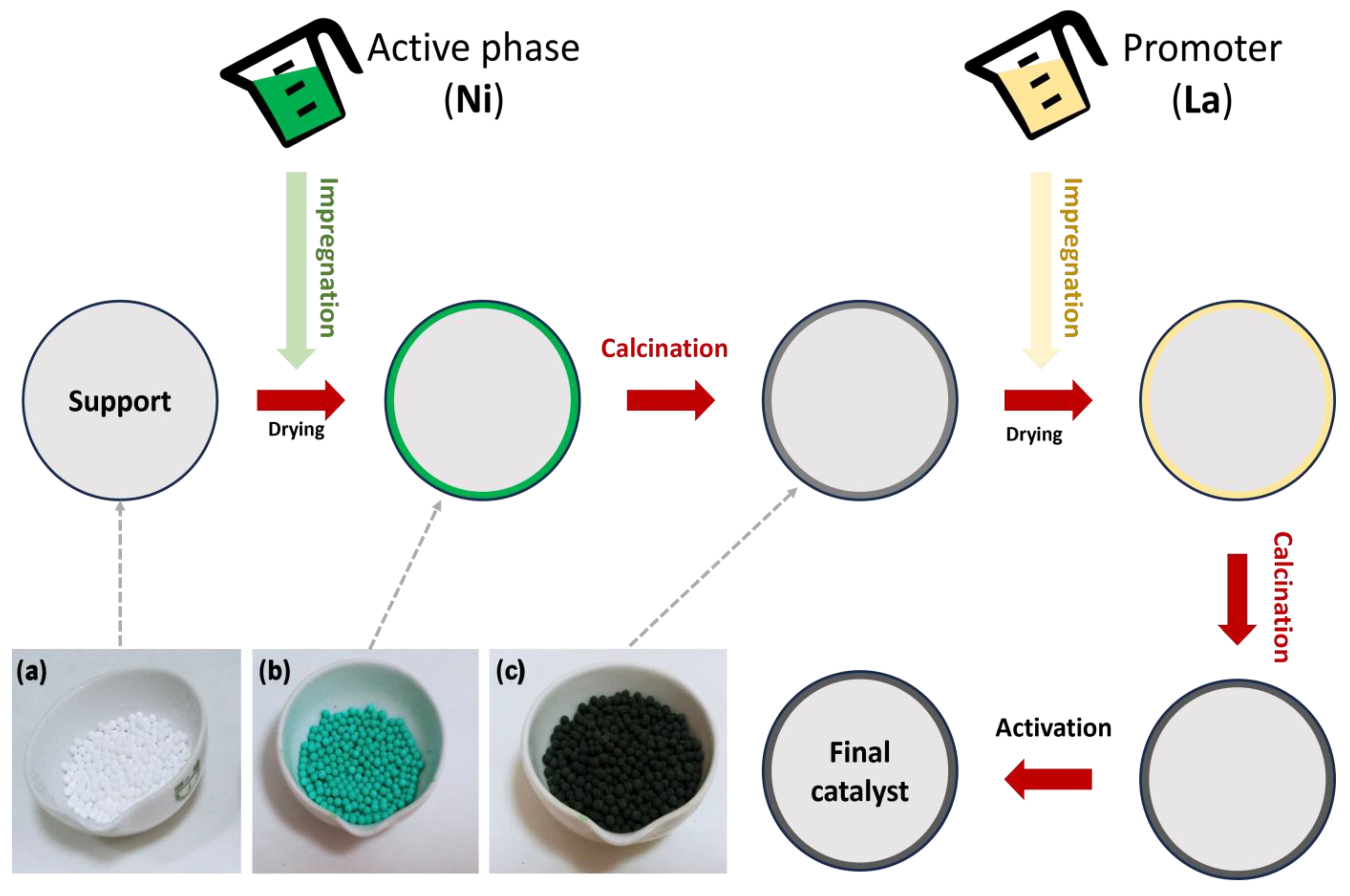
In the impregnation method (Figure 6 and Figure 7a), a precursor solution is combined with an active solid support phase, and then the solvent is removed by drying. In the application of this method, the solid and the solution are contacted in two ways: wet impregnation (WI) and incipient wet impregnation (IWI) (see Figure 6). In addition, there are dual mechanisms depending on the impregnation method used. WI involves a diffusion process, whereas IWI uses a capillary action method that allows the solution to penetrate pores in the support.
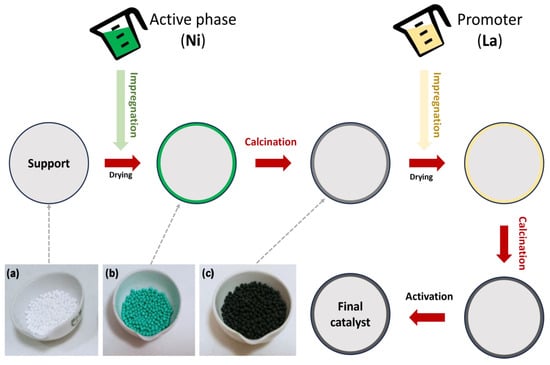
Figure 6.
Different stages for catalyst preparation, including images of the most representative ones: (a) catalyst support; (b) wet impregnation with a Ni solution; and (c) calcination.
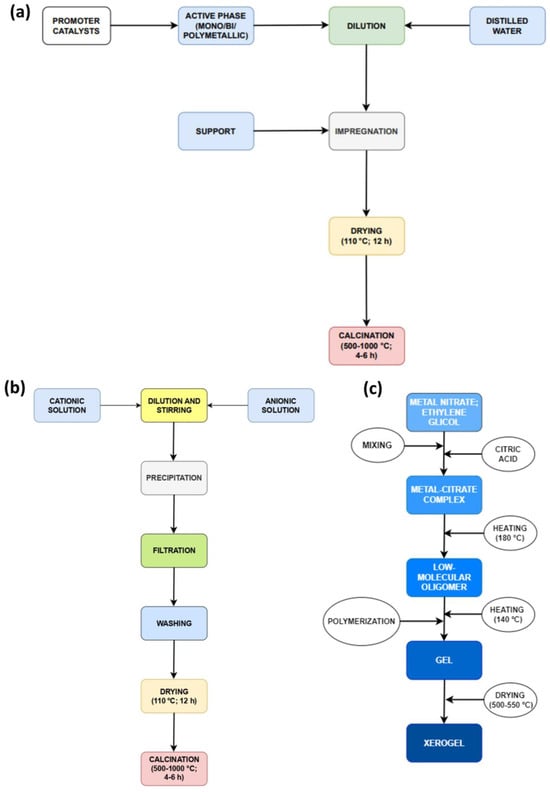
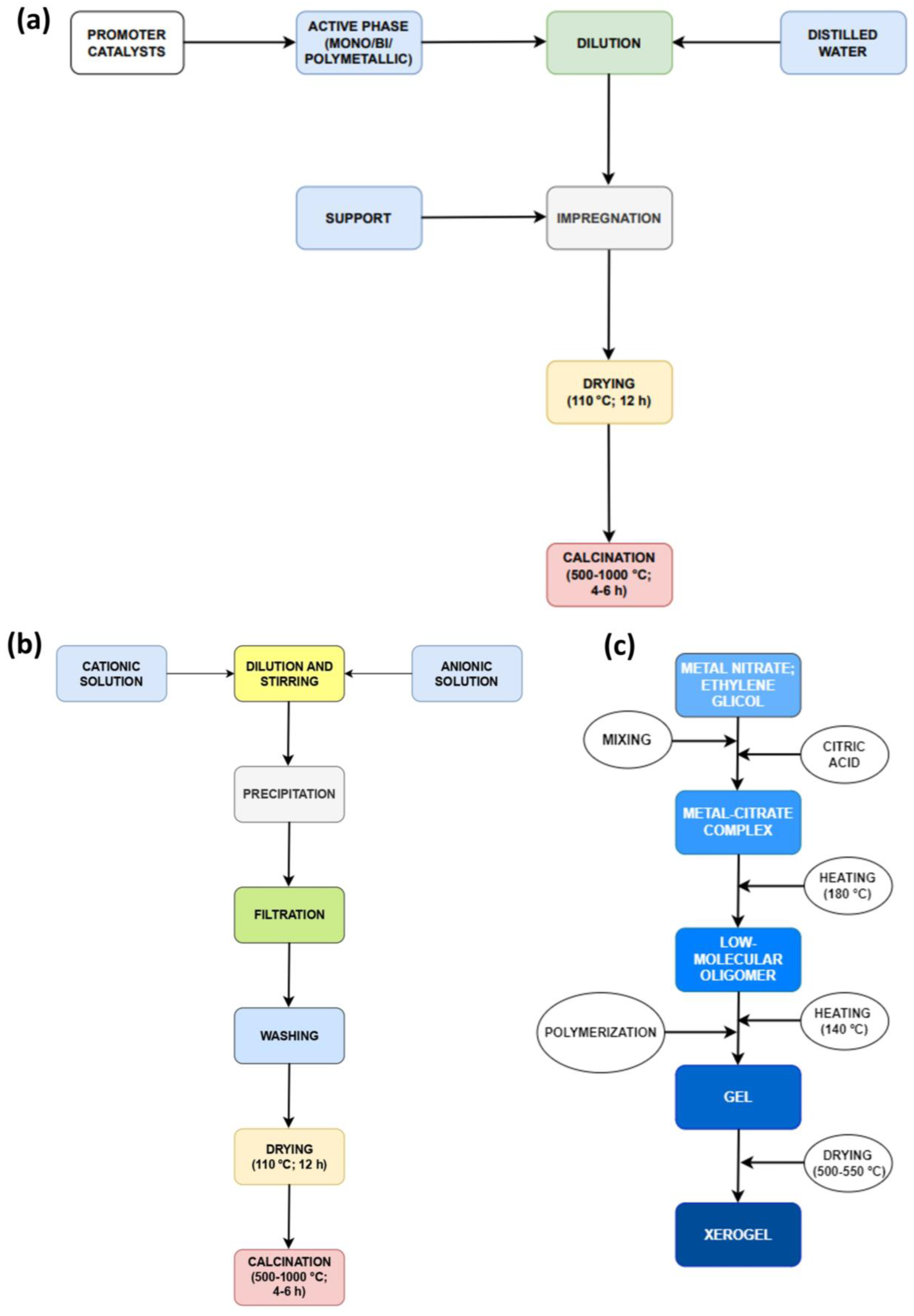
Figure 7.
Main steps for different catalyst developments: (a) impregnation; (b) precipitation; and (c) sol–gel.
In wet impregnation, an excess solution is used which is separated from the solid by drying for a certain period of time. During the diffusion process, the composition of the solution changes, forming a residue of impurities and releasing heat due to adsorption in a short time interval. The wet impregnation method is effective for the preparation of the metal catalysts used in methane reforming processes, achieving a high yield and catalyst microstructure. This is the reason why this is the majority catalyst preparation according to the literature. However, a drawback of this method is sintering, especially on catalysts with high oxide loading. In wet impregnation, the metals are dispersed on the surface because the precursor is distributed on the support. This results in high use rates but a low dosage of active metal on the surface, which can result in a non-uniform dispersion of the catalysts [62].
Impregnation by incipient wetting, also known as dry or capillary impregnation, is a method in which the pore volume of the active phase/support is approximately equal to or slightly larger than the volume of the solution. To explain the mechanism of the capillary action of the incipient wetting impregnation method, several reactions occur at different rates. The selective adsorption of charged or uncharged species occurs via H-bonds, van der Waals, or Coulomb forces. Ions are then exchanged between the electrolyte and the charged surfaces, resulting in the polymerization/depolymerization of the ions deposited on the surface. This is followed by the partial dilution of the solid on the surface. After the impregnation of the catalyst into the solid support/active phase, drying and calcination (at different temperatures according to the nature of the active phase) are performed to obtain the desired catalyst material. The products of the impregnation processes are highly dependent on the precursors used, and the parameters that can influence the final mixture include the pH of the solution, the type of solvent, the concentration, and the nature of the dissolved solids [63].
Co-precipitation (Figure 7b) is a versatile method that can be applied to the synthesis of simple, mixed, or supported catalysts [62,64]. Coprecipitation, often referred to as the “one-pot method”, is a conventional approach for synthesizing catalysts in the context of methane steam reforming. The formation of metallic precipitates occurs from oversaturated solutions of their salts. Consequently, all precipitation methods share common components, specifically a soluble source of divalent or trivalent cations and a strong base, such as sodium hydroxide (NaOH), which promotes the precipitation of ions. This process involves mixing metal salts in an aqueous phase with alkaline solutions, resulting in the formation of insoluble metal hydroxides and/or carbonates. During the combination stage, reaction parameters such as temperature, evaporation, salt concentration, and pH stimulate the precipitation process.
These parameters can modify the growth and size of crystallites. The precipitation method consists of five steps: dissolution, precipitation, filtration, drying, and calcination. During the dissolution stage, the active-phase precursors (in salt form) dissolve or hydrolyze in a medium (normally water) to obtain hydroxides in a homogeneous solution. Subsequently, filtration and drying steps are needed, allowing the solids to filter and dry at the boiling temperature of the medium. The dried sample is then crushed, and a binder is added. The appropriate binder is selected to promote easy conversion into vapor and CO2 during calcination and the subsequent activation. The calcination stage uses air (at an optimal temperature) to convert the material from its hydroxide or salt form into oxides. Several publications have resorted to the precipitation method to synthesize the catalyst support, and the catalyst for methane reforming processes [65,66,67,68].
The sol–gel method (Figure 7c) presents a different approach to prepare new materials. Conventional sol preparation involves the hydrolysis and condensation of metal precursors, resulting in a colloid suspension comprising various systems. Colloids are generated when one phase disperses into another, and the dispersed molecules have a dimension between 1 nm and 1 µm [62]. Depending on the kind of solvent, there are two pathways for using the sol–gel method: the aqueous sol–gel method, which refers to the use of water in the reaction, and the non-aqueous sol–gel method, which refers to the use of an organic solvent. In the aqueous method, O2 from water decomposition is necessary for metallic oxide formation.
This method is advantageous due to the high affinity of most precursors for water. However, the main reactions (hydrolysis, condensation, and drying) occur simultaneously, making it difficult to control the particle morphology and process reproducibility. However, this disadvantage is insignificant when preparing metal oxides in bulk.
Thus, the aqueous method can be utilized for preparing bulk metal oxides as opposed to small-scale preparation [69]. In the non-aqueous method, also known as the non-hydrolytic method, the required O2 is provided by solvents (like ketones and alcohols) or metal precursors. The organic solvent also contributes to modifying the process to refine the final properties of the material, such as the morphology, particle size, temperature, and humidity.
Most sol–gel processes use tetraethoxysilane (TEOS) in an aqueous solution, which forms SiO2. This hydrolytic medium is required for hydrolysis and condensation reactions to occur. Hydrolysis is a chemical reaction where silanol (Si-OH) is generated from the reaction between water and an alkoxide (Si-OR), such as TEOS. Si-OH and Si-OR are responsible for the subsequent condensation reactions in the process, resulting in the formation of siloxane in a complex system of competition between hydrolysis and condensation during the intermediate steps of the sol–gel process [70].
Furthermore, the influence of acidic and basic conditions should be considered, as they compete and have their respective peculiarities. The acidic route allows for the syntheses of more branched compounds, whereas the basic route allows for the production of more spherical and compact materials. These parameters are defined around the point of zero charge (PZC), which is determined by the material’s structure and porosity. The pH range of silica is between 1.5 and 4.5, and the condensation of silica species has a limited influence.
Pechini [71] patented a preparation method that adopted the principles used in the sol–gel method with modifications, which employs small molecules and chelating ligands. Initially, a homogeneous solution of metal/citrate complexes is formed in the method and the mixture is then converted into a covalently bonded polymeric matrix, thereby trapping the metal ions. The principle of the Pechini method is to slow down the thermal decomposition of the organic structure to control the resulting material. The primary reaction in this method is the transesterification that occurs between ethylene glycol and citric acid [72]. The Pechini method offers some benefits, including its simplicity, independence from process conditions due to the resulting material’s ion positivity, and the use of a low temperature for precursor treatment, resulting in complete sintering elimination. However, its drawbacks involve the use of toxic ethylene glycol and a significant amount of organic reagents per mass unit [62].
There are other preparation methods that provide interesting catalysts, such as ion exchange, plasma synthesis, or the combination of solution combustion synthesis (SCS) with the wetness impregnation (WI) technique, offering high activities in Rh-based catalysts under typical SR operating conditions [53].
It should be noted that catalytic performance is highly influenced by the preparation method, as the catalyst dispersion and interaction with the support depend on the corresponding procedure, as observed in dry reforming [73]. Consequently, these methods aim to obtain a catalyst with specific characteristics, as explained in the following subsection.
Firstly, the selection of a suitable catalyst support is essential due to its surface characteristics, but also on account of its thermal or mechanical resistance. Also, strong interactions with the active phase are desired to delay deactivation processes.
Second, the active phase will play an essential role in biogas steam reforming, promoting this reaction, as explained in detail in following sections. In that sense, the distribution of this phase on the catalyst support is important, which will be determined by the surface characteristics of the support (pore size distribution) and the preparation method. For instance, in impregnation processes, the concentration of the active-phase precursor in the dilution will determine the final distribution of the active phase to a greater extent, as high concentrations could promote the agglomeration of the active phase, obtaining bigger active sites that usually imply a decrease in the surface area of the final catalyst, with a subsequent lower CH4 conversion. On the other hand, lower concentrations would imply fewer active sites on the surface, which would decrease the hydrogen production. In a sense, an intermediate solution is suitable, taking into account the pore volume of the support and the concentration of the precursor required to cover the surface, avoiding agglomeration.
Finally, the use of bimetallic, trimetallic, or polymetallic catalysts is also advisable to complement the characteristics of typical active sites such as Ni. Also, the use of promoters (who are not directly involved in catalytic activity, but contribute to a suitable performance) is necessary, in order to promote a strong interaction between the active phase and the support. In this sense, the introduction of these components could change the development of the catalyst, as observed in Figure 6 in the case of impregnation, where successive steps should be carried out to introduce the promoter.
2.3. Characteristics of Catalysts
One of the key factors concerning the catalytic steam reforming of biogas is the main characteristics of the catalyst used. As in any field where catalysts are used, their properties should be perfectly adapted to the requirement of the corresponding conversion process. In this case, concepts such as the support (including shape or geometry), active phase (including the interaction with the support, which will determine the sintering or coke deposition resistance), or surface area should be taken into account, as observed in further subsections. It should be noted that the interaction between the active phase and the support is essential for understanding the catalytic performance during biogas steam reforming, as it will determine the resistance of the final catalyst to some factors such as poisoning, carbon deposition, or sintering, among others. Also, the combination of multiple metals in the active phase could improve some properties in the resulting heterogeneous catalyst, especially concerning some factors such as a longer useful life or selectivity towards hydrogen production.
2.3.1. Catalyst Support
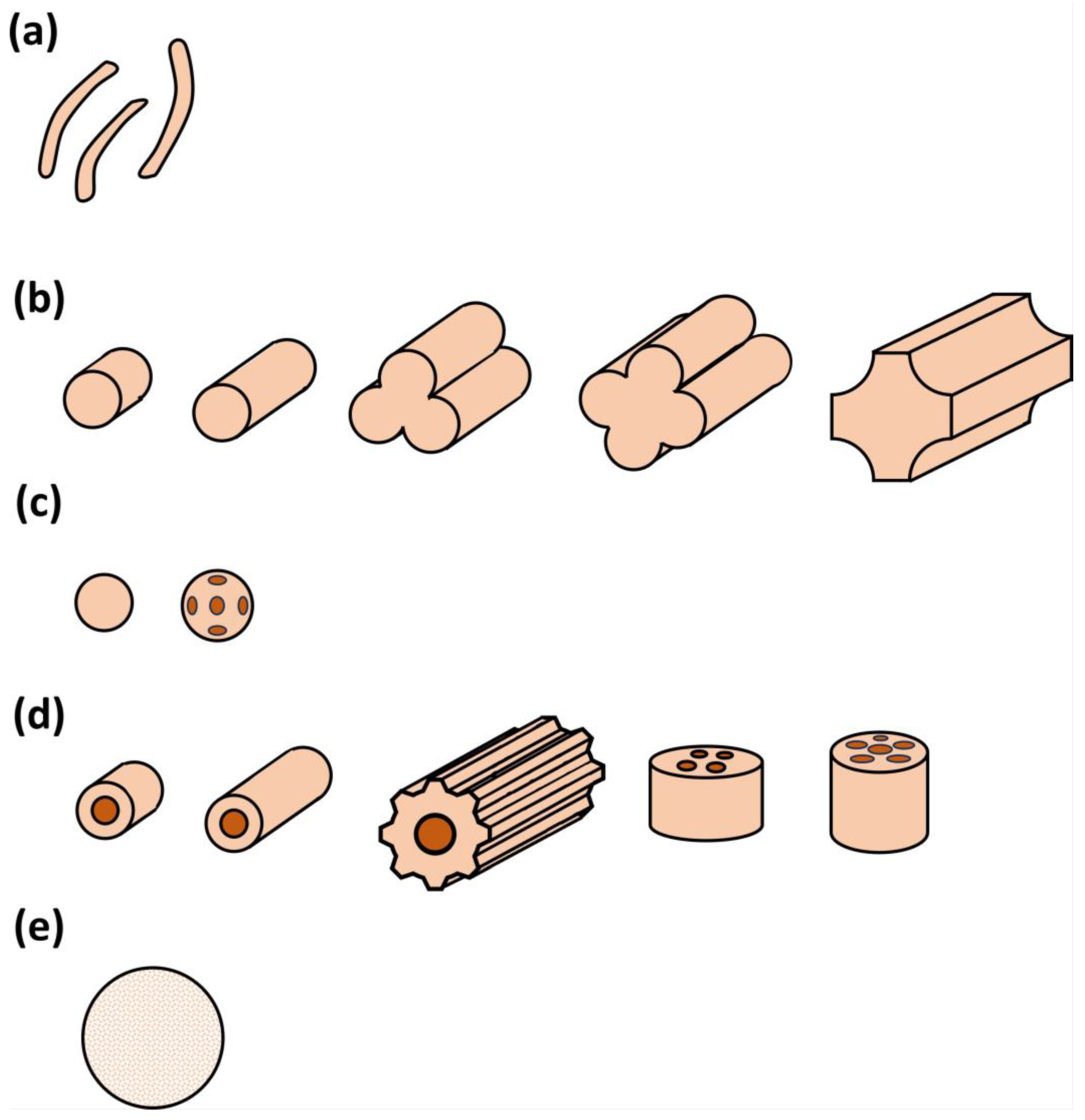
The support plays an important role in a suitable catalytic design, as it holds the active phase where the catalytic conversion will take place. In that sense, concerning biogas steam reforming, the nature of this support (normally alumina or silica), its porosity, mechanical resistance, and geometry will allow for a maximum interaction of the gas phase with the solid catalyst, depending on operating conditions such as flow rate and pressure, etc. Figure 8 shows the different shapes of the catalyst supports used for these purposes, with a great interest in spheres and hole catalysts, according to the literature. In any case, other shapes are equally used, proving the versatility of catalytic steam reforming.
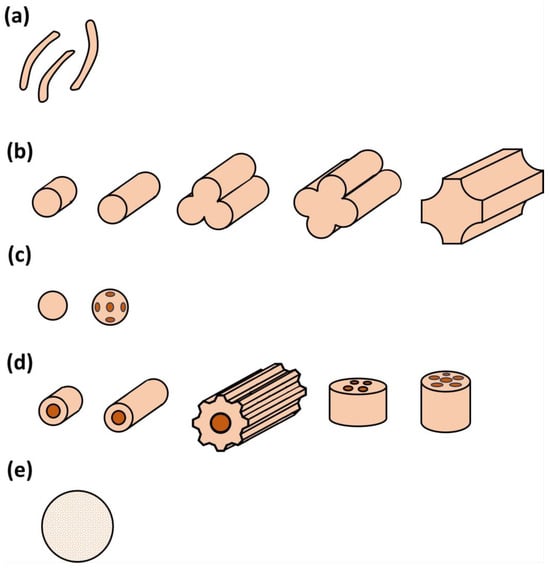
Figure 8.
Different shapes of catalyst supports: (a) amorphous pellets; (b) extrudates (solid, hollow, trifolium, and quadrifolium, etc.); (c) sphere carriers, including holes; (d) hollow supports, with single hole cylinders, ribbed cylinders or with multiple holes; and (e) ceramic foam.
As commented in previous subsections, and according to Table 5, impregnation and co-precipitation seem to be the most popular ways of preparing catalysts for methane steam reforming, offering a wide range of surface areas, from around 100 to 500 m2/g.

Table 5.
Catalysts for SRM, with typical supports and BET surface area.
Also, there are other alternative supports, like CeO2, which contribute to a better catalytic activity. Recent studies have carried out the combined steam and dry reforming of biogas using a Ni/CeO2-Al2O3 catalyst with a bimodal porous structure. When the CeO2 concentration was 5%, a great catalytic activity was found, thanks to the more intimate contact with alumina and the higher metal–support interaction, preserving it from carbon deposition by 70% [78].
These differences in shapes offer a wide range of SRM conditions. In any case, the maximum interaction between biogas and catalyst is highly desired, trying to avoid the free passage of gas as much as possible. Also, another aspect to be taken into account is the surface of the supports, as it plays a vital role both in catalyst preparation and their corresponding final performance.
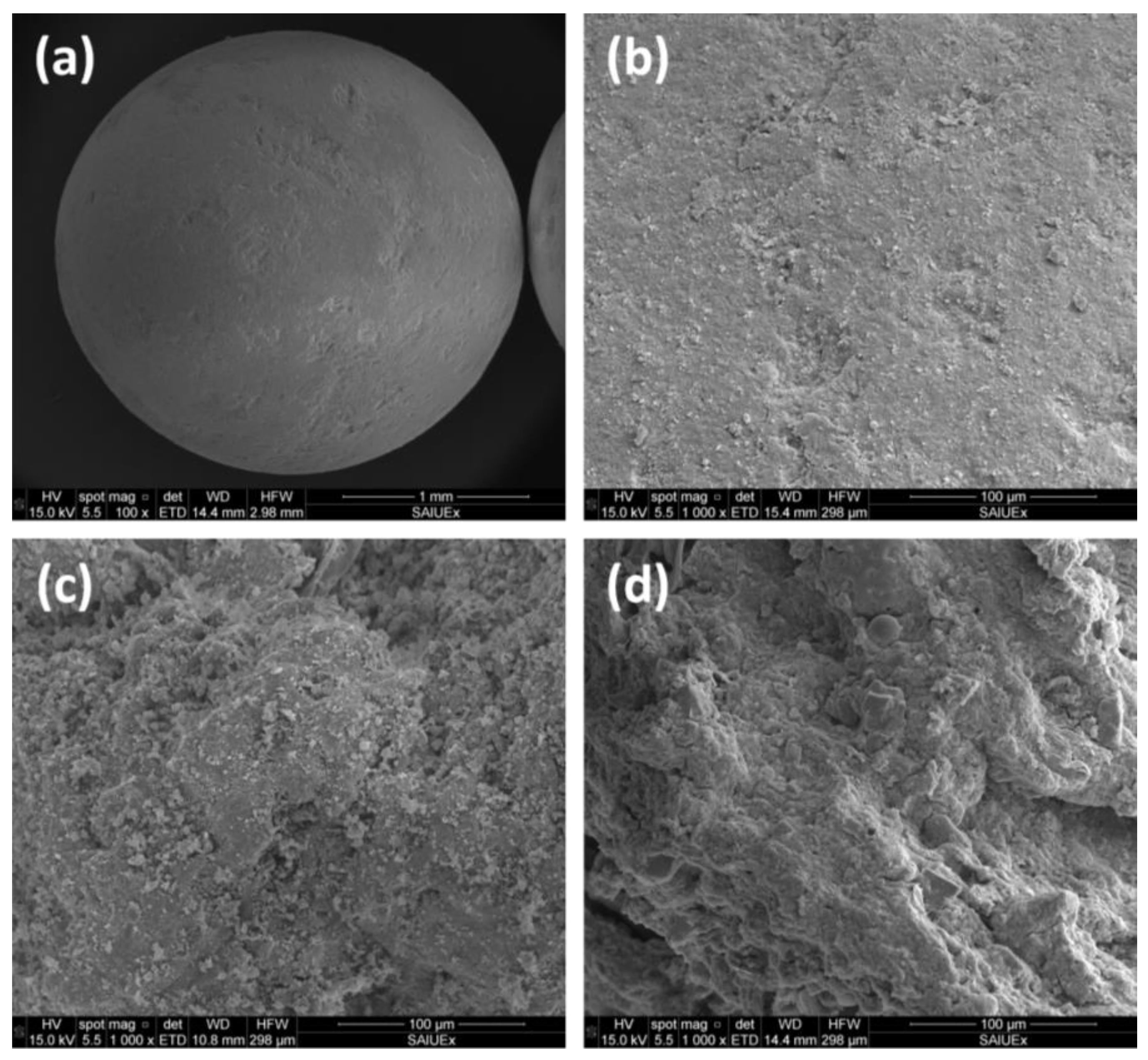
Thus, the pore size distribution of different supports (see Figure 9 for different examples in SEM images) can influence the impregnation of active phases during catalyst preparation, whereas the performance of the final catalysts and (including some processes such as coke deposition or sintering) is highly determined by the pore size, whose profile should be selected to favorize a long useful life of the catalyst.
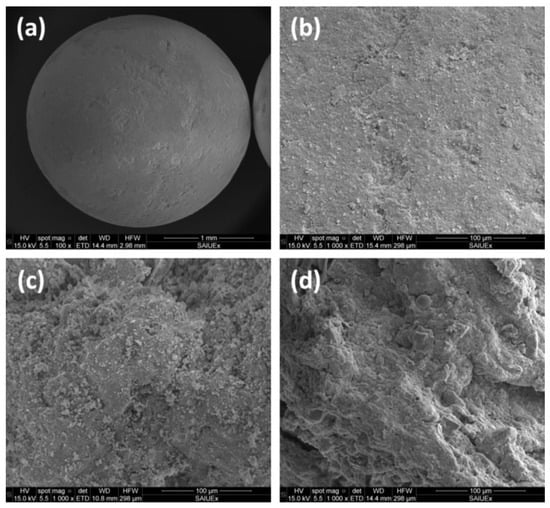
Figure 9.
SEM images of a sphere catalytic support (a) and its surface (b), and comparison with other different surfaces: dried sewage sludge (c) and pyrolyzed sewage sludge (d).
Equally, there are other characteristics of the support that should be considered, like its thermal and mechanical stabilities, which are essential in biogas steam reforming for different reasons. Firstly, due to the high temperatures taking place in this process, thermal stability is an ideal prerequisite to avoid surface or even structural changes in catalysts due to thermal shocks. Also, mechanical stability is important to avoid catalyst breakup due to different factors such as friction or high pressures, reducing the amount of detritus within the reactor and subsequent blockages. In that sense, the use of resistant materials like Al2O3 or SiO2 is common, whereas other catalysts based on carbonaceous materials could present some challenges in that regard.
In this regard, innovative works have been carried out where the role of the support is essential. For example, metal-foam-coated Pd–Rh catalysts with variable CeZrO2–Al2O3 support compositions were used in biogas steam reforming, resulting in higher CH4 conversion with the extent of CeZrO2 in the catalyst, a decreasing H2/CO ratio, suppressed coke deposition due to oxygen storage, and an improvement in oxygen reducibility, with an improvement in resistance to the deterioration of surface area, pore structure, and active-phase dispersion [79,80]. Mesoporous catalysts prepared via a reverse precipitation method, Ni2xCe1−xO2 (x = 0.05, 0.13, 0.2), were compared with a commercial catalyst (R67), obtaining a higher H2/CO ratio and excellent activity [81].
2.3.2. Active Phase
Generally, catalysts consist of an active phase, usually a noble metal or acid/base site, deposited and dispersed on a porous support such as alumina, silica, or other material. The solid catalyst’s active phase has a high affinity for molecules of specific reactants. Initially, the molecules chemically attach themselves to the active surface before reacting each other. This way, the activity of catalysts is normally proportional to the number of active sites on the surface. In the case of metal-supported catalysts, the active sites are represented by the exposed metal surface.
Nickel (a transition metal) is commonly used as an active phase in SRM processes due to its availability, low cost, and high activity. However, due to sintering and coke deposition, Ni-based catalysts are subject to rapid deactivation [82].
In addition to nickel, noble metals such as rhodium (Rh), ruthenium (Ru), palladium (Pd), platinum (Pt), and iridium (Ir) show promising potential as candidates for SMR due to their exceptional catalytic abilities and resistance to carbon deposition. Several experimental and numerical studies have reported that the catalytic activity of noble metals could be ordered as Rh∼Ru > Ir > Pt∼Pd [30].
It is important to consider different factors to make the addition of an active phase efficient. This way, catalysts with high activity, low concentrations of active phase, and subsequently high dispersion are desirable for carrying out high conversions in biogas upgrading into syngas. For this purpose, recent studies have proven different Ru-based catalysts (with different supports), with Ru/MgO showing an excellent catalytic performance in the bi-reforming of model biogas due to Ru dispersion with an ultra-small particle size [83]. Consequently, the use of nanoparticles seems to offer a promising outlook in this field. Also, the role of multi-metallic active phases is important for obtaining specific and interesting properties, as in the case of poisoning resistance. Thus, a catalyst (NiCeSnRh/Al2O3) was used in the bi-reforming of biogas, offering a high resistance to sulfur compounds [84].
2.3.3. Advantages and Disadvantages
There is such a wide range of catalysts that can be developed that it is difficult to cover the advantages and disadvantages related to their use. Nevertheless, there seems to be some common patterns, mainly to do with the active phase, which, in many cases, is the main limiting factor when it comes to producing an economically feasible catalyst. In that sense, the role of catalyst in techno-economic assessments in biogas steam reforming is important, as explained in the corresponding section. Essentially, it is a matter of cost–benefit analysis, paying attention to the benefits offered by a specific catalyst and its relative abundance. Regarding the active phase, Ni-based catalysts are popular for this reason, as they offer acceptable catalytic activity at a relatively low cost compared to other metals. However, there are other factors such as a higher propensity for deactivation processes, which could imply operational problems in the medium term, which could be solved with other more expensive catalysts based on Ru or the use of promoters such as La. In other words, life cycle assessments of catalysts for biogas steam reforming are, as in many other cases, essential for obtaining a cost–benefit balance.
Despite their advantages, noble-metal-based catalysts are limited due to their high prices. One way to keep the excellent performance of noble metals while maintaining a reasonable price is to combine two or more types of metals, using cheap transition metals (usually nickel or cobalt) as a base and noble metals as promoters, chemicals that are added to the catalyst in order to improve its catalytic properties. Bi/polymetallic catalysts have gained increasing attention in recent years, and the synergistic effect between commonly used metallic elements has been investigated experimentally and numerically. Numerical studies focus on the reaction pathway and the activation energies of certain reaction steps (specifically, C-H bond breaking during CH4 decomposition), as well as the adsorption energies of atomic or molecular species on the catalyst surface, which are indicators of the catalytic activity and stability of the material [30].
Cobalt is also considered to be a promising promoter in SRM due to its good activity for the WGS reaction, which helps shift the equilibrium towards H2 production. However, a problem related to the use of Co is its tendency to oxidize when the temperature and vapor partial pressure are in the range used for SMR. Alloying it with Ni is a possible solution to this problem while preserving the advantages of both elements [82].
Compared to the relatively simple mono and bimetallic systems, the application of catalysts containing three or more types of active metals in SMR has not been investigated in detail. The existing literature mainly examines Ni-based materials with the addition of two or three commonly used elements, such as Co, Cu, Pt, and Ru, etc. [30].
2.3.4. Catalytic Performance in Biogas Steam Reforming
Thus, after considering the relationship between the support and the active phase, the typical catalytic steam reforming of methane is explained in Figure 10, where the main steps that take place during the process are included.
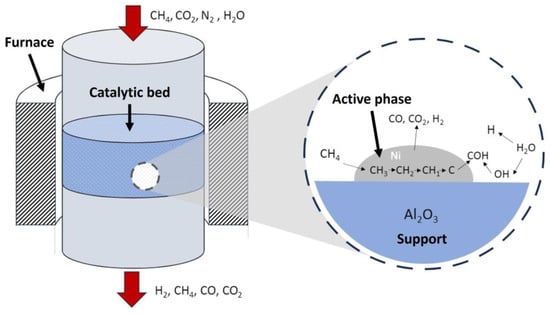
Figure 10.
Stages taking place during catalytic steam reforming in a reactor. Example for a Ni-Al2O3 catalyst.
Thus, the kinetic model of methane reforming, shown in Figure 8, is based on the following steps:
- H2O is adsorbed on the catalyst and dissociates, giving rise to adsorbed oxygen atoms and H2 in the gas phase.
- CH4 is adsorbed on the catalyst and dissociates, generating CH2 radicals and adsorbed H atoms.
- The adsorbed CH2 radicals and oxygen react, with bonds being formed and breaking at the same time, generating a transition state (CHO) and H2.
- The adsorbed CHO dissociates into adsorbed CO and H or reacts with adsorbed oxygen to produce CO2 and H in parallel (controlling stage).
- The adsorbed CO reacts with adsorbed oxygen to form CO2, or is desorbed to give gas-phase CO.
This way, and according to recent works found in the literature (see Table 6), different operating conditions with the subsequent methane conversion are included.

Table 6.
Catalysts for SRM, including operating conditions and methane conversion.
As observed in Table 6, the operating conditions allowed for methane conversions exceeding 90% in most cases, which usually implies a considerable hydrogen production in the obtained syngas. Ni and Pt catalysts are the most popular choices in these cases, adding, in some cases, promoters to improve the catalytic performance. In any case, these catalysts usually present long stabilities and a high coke resistance, with the possible softening of operating conditions within the range explained in previous sections. Regarding temperature, some studies have achieved very low values (under 800 °C), whereas pressure could be considerably decreases (up to 1 bar), with relatively low steam to carbon ratios, which could imply a considerable reduction in the fixed energy costs related to steam generation. All these improvements could mean an increase in the efficiency of biogas steam reforming. However, there are some challenges related to catalytic performance, mainly related to catalyst deactivation, which will be explained in the following section. Another emerging research line in catalytic steam reforming is the use of nano-catalysts supported on different materials. As previously explained, the dispersion of catalysts is essential to maximizing catalytic activity by increasing the effective active phase area, also avoiding negative effects like coke deposition. In that sense, as observed in Table 5, there are new research trends focused on the synthesis and performance of this kind of catalysts.
3. Catalytic Deactivation
One of the main aspects that should be taken into account for a suitable catalytic performance is the life cycle of catalysts during biogas steam reforming, especially in cases such as those with Ni-based catalysts and other heterogeneous catalysts. Indeed, deactivation can be caused by several factors due to mechanical, thermal, or chemical processes [101], that will be explained in following subsections. To a lesser extent, the durability of a catalyst can be affected by other factors, like its kind and shape, or operating conditions like steam to carbon ratio, pressure, or temperature [102].
3.1. Sintering
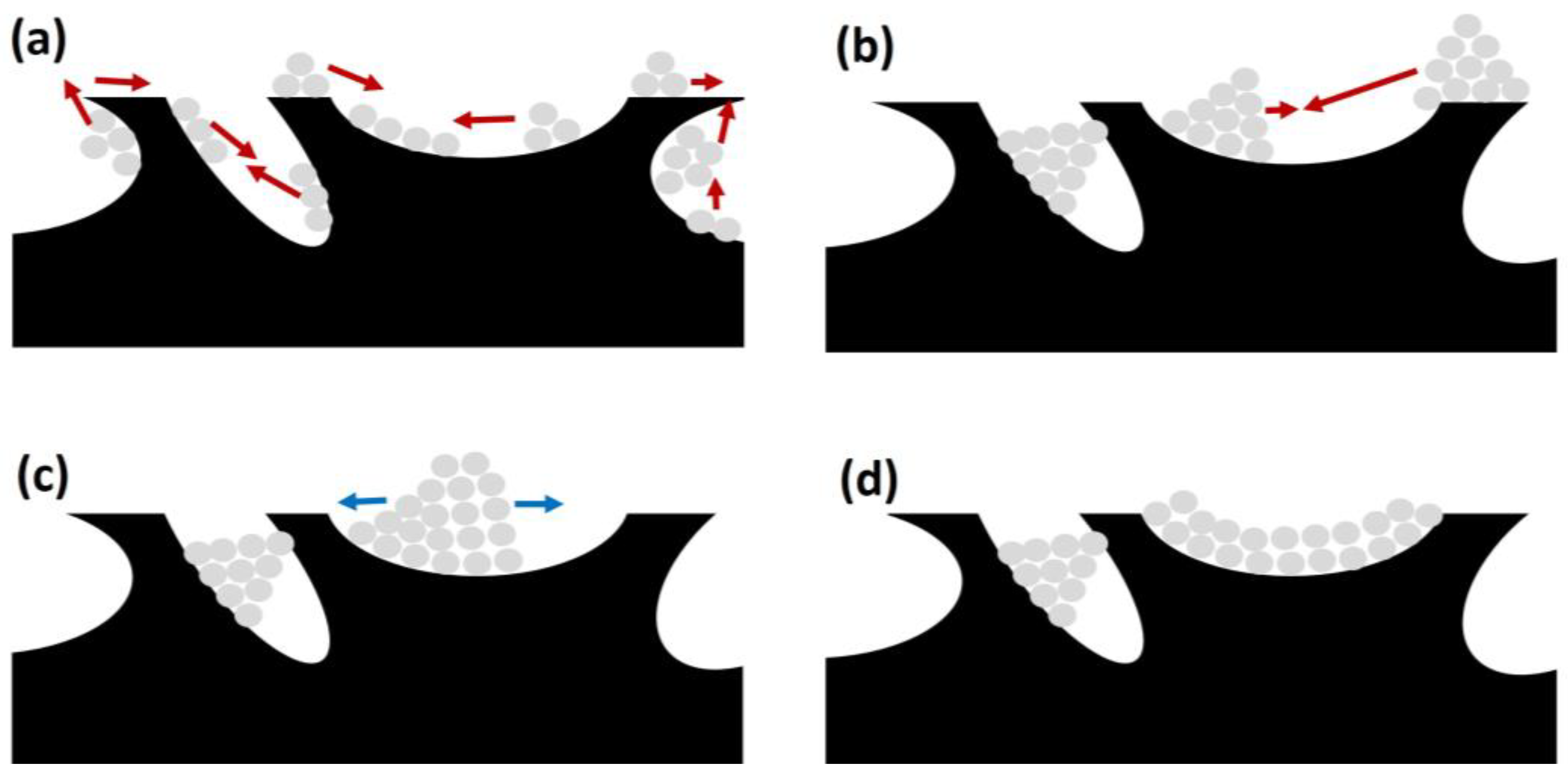
This is a process due to the agglomeration and growth of metal crystallites of the active phase, occurring at high temperatures. Considering that SRM usually requires high temperatures (above 600 °C), this is an event that should be considered. In that sense, Hüttig and Tamman temperatures determine the atom or crystallite migration for a certain metal, one third and one half of the melting point of the corresponding metal, respectively. Considering the typical operating conditions for biogas SR, the reaction temperatures for this process are high enough to provoke surface and bulk atom migration. Consequently, as included in Figure 11, there are three different stages in sintering on support’s surface.
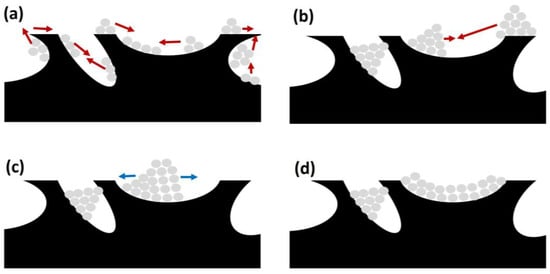
Figure 11.
Different stages during sintering of active phase on catalyst support (catalyst support in black and metal particles in grey): (a) migration (red arrows); (b) collision (red arrows); (c) spreading (blue arrows); and (d) blockage (also in (b,c)).
First, atomic migration takes place, with a detachment from the crystallites and migration on the support’s surface, generating bigger metal particles (Figure 11a). Afterwards, these bigger particles or crystallites can also migrate and collide, obtaining bigger particles (see Figure 11b). The process ends with particle spreading on the catalyst surface, as observed in Figure 11c, and blocking active sites.
This way, this phenomenon is related to a decrease in catalytic activity due to two main causes. First, sintering implies a decrease in the surface area of active sites, reducing the efficiency of the catalysts. Second, as observed in Figure 11d, crystallite growth can block pores on the catalyst support, containing further active sites that otherwise would be available for SRM [24,101].
Sintering can also be influenced by other aspects such as the catalyst structure and its porosity and metal–support interactions, promoting strong metal–support interactions that could decelerate the sintering [102].
3.2. Poisoning
Poisoning is due to a strong chemisorption of chemical species on catalytic sites, blocking them and avoiding SRM. There is a wide range of chemical products that can poison catalysts in biogas steam reforming. Among them, one of the most important ones is hydrogen sulfide (H2S), whose content in biogas after biodigestion processes is not negligible, ranging from few ppm up to 1%. High H2S content, apart from a poisoning effect, could be harmful or even deadly, promoting corrosion in industrial facilities, and decreasing the heating value of fuel gas. As a consequence, H2S removal before biogas steam reforming is necessary to avoid a decrease in the global yield [103,104].
Depending on the kind of catalyst, this negative effect could be more noticeable. For instance, Ni is very sensitive to poisoning (see Equation (3)), whereas other metals like Co seem to offer a lower affinity for sulfur, with possible and interesting uses in bimetallic catalysts. Equally, other metals can react with H2S, like Ag, Cu, Fe, Ru, or Pt [105].
where M can be any abovementioned metal. As a consequence of the interaction of hydrogen sulfide with the active site (through sulfidation), the catalyst cannot take part in steam reforming, partially or completely reducing its activity during the process.
In that sense, adsorption and absorption seem to be suitable techniques for removing hydrogen sulfide before biogas steam reforming, requiring low concentrations for this purpose (up to 5 ppm) before biogas processing in steam reforming facilities [102]. Specifically, the use of alkanolamines (such as methyl ethanolamine or methyl diethanolamine), alkaline salts, organic solvents, deep eutectic solvents, or ionic liquids for absorption, as well as zeolites, metal oxides, or carbon-based sorbents for adsorption, could be interesting treatments for removing H2S under ambient pressure and low operating temperatures [104,106,107,108]. It must be borne in mind that, as previously explained, sewage sludge reuse as an active carbon (obtained through pyrolysis and gasification processes) to adsorb H2S could be an interesting starting point for implementing a circular economy in wastewater treatment plants. Finally, recent studies have proposed a simplified heterogeneous fixed-bed reactor model to simulate the influence of H2S poisoning on Ni-Al2O3 catalysts for methane steam reforming, with a good agreement between the simulated and experimental experiences if an order of deactivation (n = 1) is assumed [109]. These kinds of simulations are quite useful, as they can be easily adapted to different H2S concentrations in biogas and GHSV. Also, the use of bimetallic catalysts to increase poisoning resistance is another interesting aspect to be taken into consideration, as explained in previous studies where the use of a Rh-Ni/Ce-Al2O3 catalyst showed a higher resistance to poisoning, being reversible by using regeneration processes, after which, the catalyst did not show selectivity to the reverse WGS reaction, allowing for high H2 yields [110].
3.3. Carbon Deposition
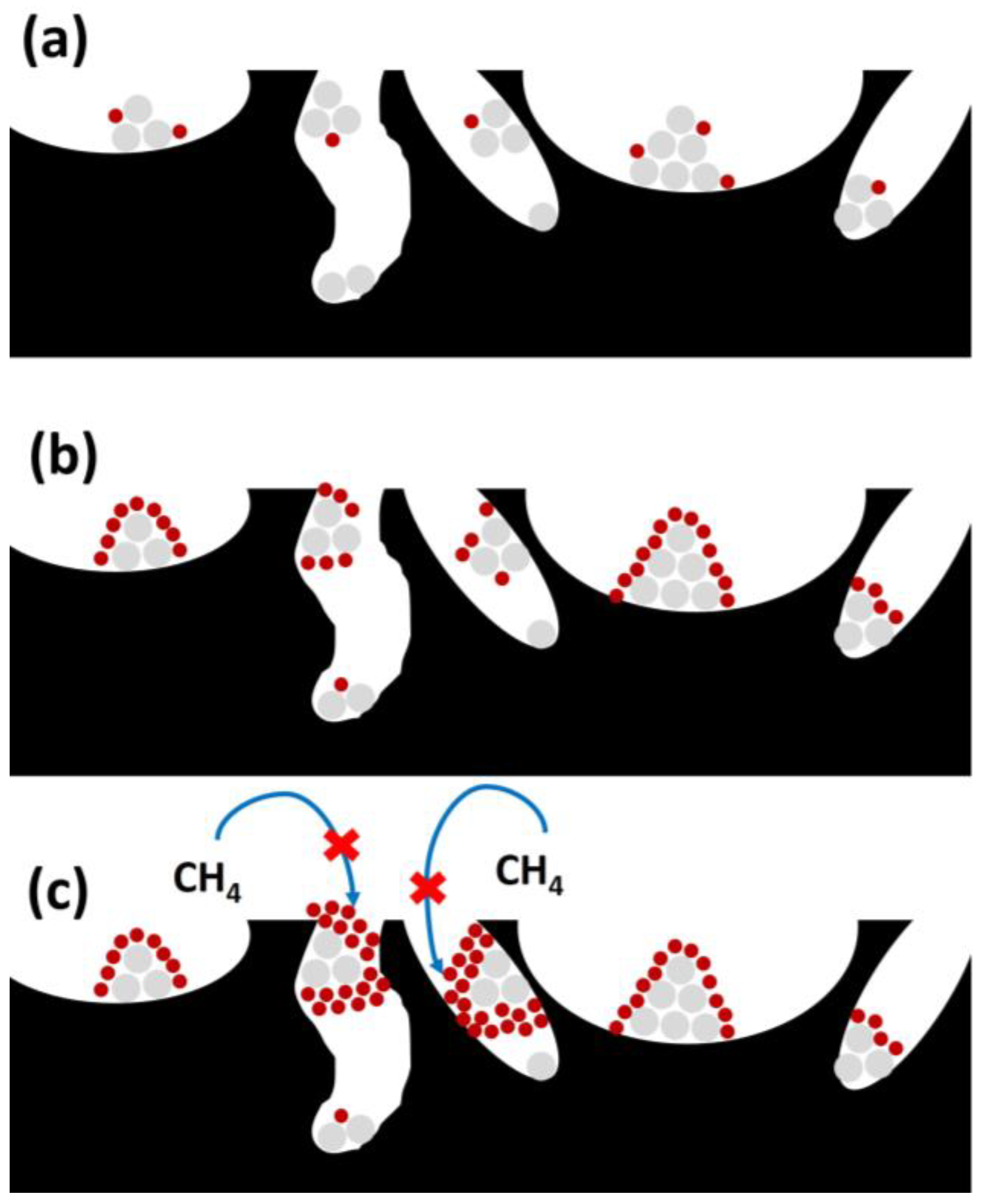
Coking could represent another negative factor, related to the physical formation of carbon deposits due to gas-phase chemical reactions like methane cracking or CO disproportionation [102]. Carbon deposition can imply the deactivation or blockage of active sites, which decreases the effectiveness of the active phase over reaction time. As observed in Figure 12, successive phases take place during carbon deposition, with different effects depending on its degree of severity.
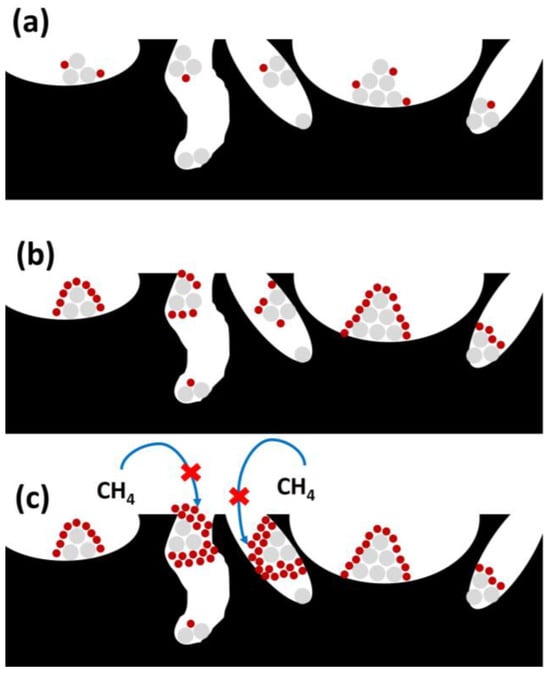
Figure 12.
Different stages during coke deposition (coke in red, active phase in grey, and support in black): (a) coke production and chemisorption; (b) coke spreading with the subsequent encapsulation; and (c) active sites and pore blockage.
This way, coke chemisorption or adsorption takes place on active sites (Figure 12a), reducing their access to reactants. In further stages, coke diffusion or dispersion to generate active site encapsulation occurs (as observed in Figure 12b), completely blocking the active sites to reactants, and pore blockage takes place (Figure 12c), hampering the SRM reaction on the available active sites. This fact takes place especially when the CO and CH4 decomposition is faster than the carbon removal.
To avoid this phenomenon, the use of promoters to strengthen the metal–support interactions could present a positive effect. Also, the particle size of the active phase could play an important role in controlling coke deposition [102].
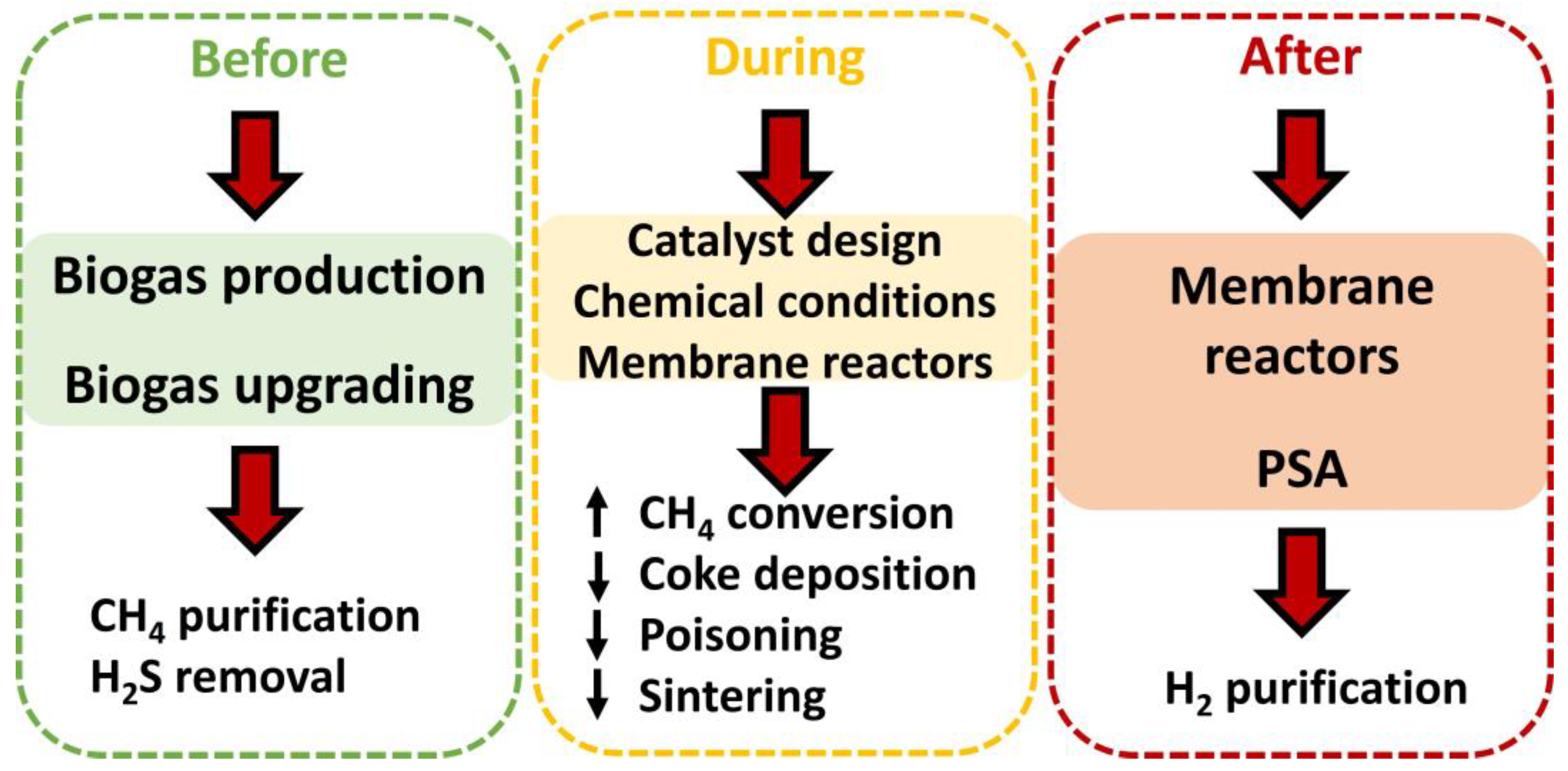
4. Key Points to Improve the Performance of Biogas Steam Reforming
Considering the previous section, it is essential to take steps to solve deactivation processes, which hinders a suitable catalytic performance (with high methane conversions) and long service life. These steps could be taken, as observed in Figure 13, before, during, or after biogas steam reforming. As mentioned earlier (as it will be discussed throughout this text), every detail counts when it comes to contributing to a better catalytic performance in this process. Each stage is explained in the following subsections. It should be noted that these steps directly affect the performances of catalysts during steam reforming, but other aspects related to this process could be equally improved, such as the membrane reactor performance (by reducing the coke deposition or hydrogen sulfide content) or the maintenance of steam reforming facilities (delaying corrosion with a decrease in H2S content). Nonetheless, other factors could be affected, implying efficiency loss and increases in costs.
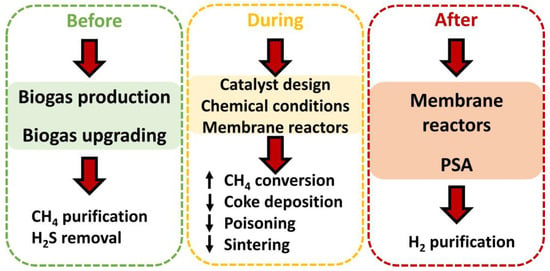
Figure 13.
Steps carried out to improve the performance (through better conversion or longer useful life) of catalysts in steam reforming.
4.1. Steps before Steam Reforming (Biogas Production and Upgrading)
Regarding the previous steps, there are many processes that could improve methane production during the anaerobic digestion of biomass, as well as biogas upgrading to increase methane concentration, avoiding undesirable compounds such as hydrogen sulfide. As explained in the introduction section, there are plenty of measures for increasing the efficiency of biogas production and quality. Thus, the use of additives (such as microorganisms, enzymes, or inorganic compounds) could facilitate different steps during anaerobic digestion by removing inhibitors (such as ammonia, long-chain fatty acids, and acidification caused by VFAs, etc.) and creating suitable conditions for microorganism proliferation [4]. In this sense, the use of active carbons obtained from digestates could be interesting (such as sewage sludge, whose active carbon obtained through hydrothermal carbonization could be a promising starting point for valorizing this waste, as explained in previous works [38]), as it could be an example of an applied circular economy during biodigestion. On the other hand, biogas upgrading is essential for removing H2S (which could deactivate catalysts through poisoning) and for increasing the CH4 concentration in biogas (which is desirable for carrying out a more efficient SRM). For the former, adsorption systems are usually selected (for instance, activated carbons or nanoparticles), although there are many techniques for retaining H2S, such as biological desulfurization, membrane separation (with polymeric membranes, normally capable of retaining CO2 and H2S [32]), or absorption with inorganic solutions (many of them based on iron) [103,104,111,112]. Also, some techniques such as PSA can be used to upgrade biogas by increasing methane content [113].
4.2. Steps during Steam Reforming (Catalyst Design, Chemical Conditions, Use of Membrane Reactors)
4.2.1. Catalyst Design (Promoters and Bi-Metallic Catalysts)
Regarding the catalyst design, if a certain catalyst is selected, with the aim of achieving the maximum conversion and stability, the catalyst should present as much dispersion of the active phase as possible, as there is not a minimum particle size from which a decrease in activity is found.
Another important factor is avoiding active-phase mobility and agglomeration, promoting that the active phase is dispersed enough to reduce the possibility of collision with other particles and the subsequent agglomeration. It is achieved by reducing the active phase concentration, whereas the number of active sites decrease. The typical Ni concentration in catalysts is 20% w/w, obtaining particle sizes from 10 to 100 nm.
In addition, feeding should be considered in catalyst design. In other words, catalyst deactivation by the pollutants included in biogas should be reduced. To reduce the effects related to coke deposition, the active phase surface on the support should be maximized, so that the diffusion of gas is enough to avoid a reducing atmosphere that allows for CO and CO2 decomposition, producing carbon which is placed on the active phase surface and generating carbide and coke.
The use of promoters in the active phase has been studied and reviewed, with some common additives such as alkalis (K and Na), transition metals (La, Zr, and Zn), and non-metals (Al and B) [114,115].
An interesting issue with room for improvement is the resistance of catalysts to poisoning due to H2S. This compound is usually removed through previous adsorption before steam reforming in a reactor [116], and few scientific articles have dealt with an increase in the resistance of catalysts to this pollutant. Some studies have focused on reviewing the influence of sulfur content in feeding for several processes [117]. As a conclusion, Ni seems to be a catalyst with a difficult direct protection from poisoning, with noble metals offering better results, except for Rh. On the other hand, there is a possibility of using metals from groups 4 to 6, through bifunctional catalysts, which could be a promising alternative.
4.2.2. Operating Conditions
There are several influences on catalytic performance depending on the operating conditions, mainly related to the promotion of deactivation processes [40]. Taking into account that methane steam reforming is an endothermic reaction (see Equation (1)), the effect of temperature is clear, with higher CH4 conversions with temperature (as observed in specific cases such as the catalytic steam reforming of biogas with a Rd catalyst) [31]. However, intermediate solutions should be achieved, as extra energy costs associated with keeping the reactor temperature should be avoided, as explained in following sections.
Concerning steam addition, high S/C ratios (at least 1.5, achieving excess of feed vapor) are recommended to avoid coke deposition, among other factors like high pressure [100,118]. However, from an economic point of view, the production of large quantities of superheated vapor would imply a considerable increase in costs [42]. Additionally, some studies have pointed out the possible catalyst deactivation on time-on-stream, especially at high temperatures, observing a direct correlation between deactivation rates and high S/C, mainly due to the steam-induced metal–support interaction, resulting in an inactive spinel phase and not due to metal reoxidation [89].
Also, as previously explained, temperature presents opposite effects. On the one hand, high temperatures would imply a sintering effect, whereas low temperatures could promote coke deposition. In the case of steam and temperature, optimization and intermediate steps should be considered, because intermediate conditions to meet both low energy costs and high methane conversions should be obtained (apart from the obvious effects on catalytic performance in biogas steam reforming).
Equally, the CH4/CO2 ratio in biogas seems to present an influence on catalytic performance. In that sense, according to recent studies using two different catalysts (4% Ni/NiAl2O4/Al2O3 and 3.1% Ru/Al2O3), an increase in CO2 implied a decrease in H2/CO ratio and H2 yield, finding an optimal CH4/CO2 ratio of 1.5/1 [119]. Therefore, CH4/CO2 ratios above 1 are advisable for a suitable catalytic performance, as explained in previous studies for Ni-based catalysts, possibly due to a CO2-promoted Boudouard reaction, implying further coke deposition [120].
At this point, it is essential to consider the optimization and modelling of steam reforming for any specific case, as will be discussed in future sections.
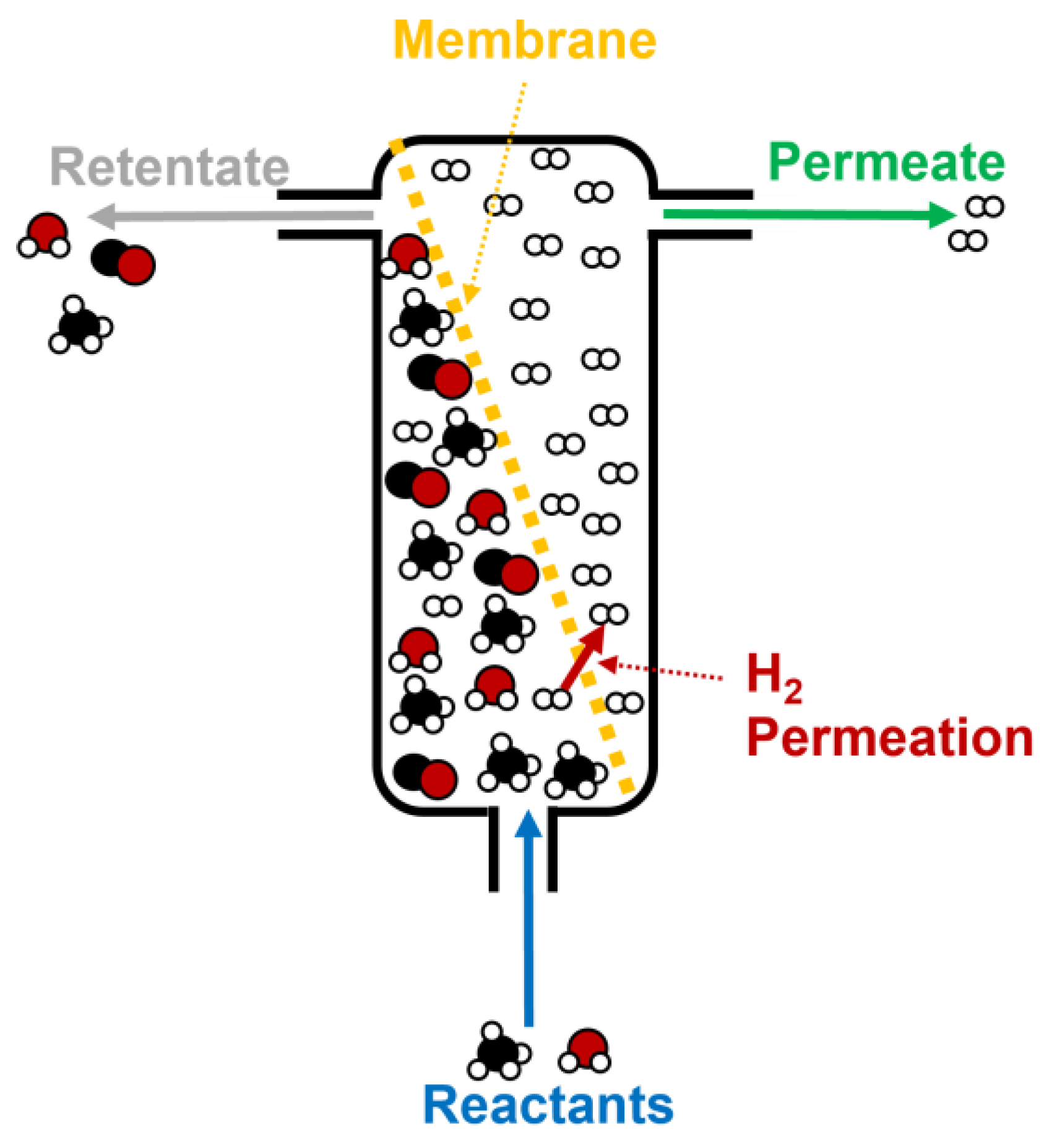
4.2.3. Membrane Reactors
The use of membrane reactors is another interesting starting point for improving the performance of hydrogen production from biogas through steam reforming. Thus, the aim of this technology is to purify the hydrogen obtained during steam reforming using thin membranes (the selective layer is usually made of Pd and Ag on different kinds of supports, like stainless steel or alumina) where H2 permeates, to obtain a final gas with a high purity (up to 95–99%) [121,122]. Figure 14 shows the different stages that take place during biogas (or methane, in a simplified form in this case), including the reactant inlet, H2 permeation through the membrane, and, finally, the retentate and permeate outlets [123].
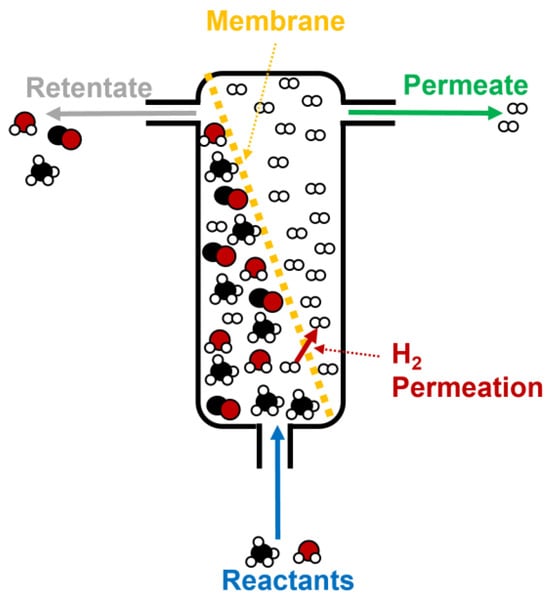
Figure 14.
Scheme of a standard membrane reactor for hydrogen production during methane steam reforming.
It should be noted that the catalyst is usually put into contact with the membrane, in order to assure that the chemical reactions (and the subsequent products, like H2) are as close as possible to the membrane.
This way, the chemical balance of the reactions observed in Equations (1) and (2) could be oriented towards product generation, as hydrogen is rapidly removed from the reaction medium to be delivered in a highly pure gas stream (permeate), whereas the rest of the products (mainly CO and CO2, along with unreacted CH4 and H2O) are separated in another stream (retentate), which can be further treated to obtain higher yields in hydrogen [124]. This fact is very interesting, as some chemical conditions could be softened to obtain a higher efficiency during biogas steam reforming.
In that sense, temperature and pressure could be lowered, whereas the catalyst design can vary (for instance, the active phase in catalysts could be reduced, with the subsequent savings for this process). Regarding temperature decrease, it could imply a positive aspect for catalyst deactivation, as sintering effects could be delayed at lower temperatures, and hydrogen recovery in a membrane reactor is usually improved [125].
However, coke deposition could be promoted at low temperatures, which could present a reverse impact in methane steam reforming [126]. As in the case of many catalysts, H2S present negative effects in membrane reactors, as Pd poisoning (the most popular element used in membranes) would provoke a progressive loss in separating performance [121,122].
4.3. Steps after Biogas Steam Reforming (Hydrogen Purification)
Even though there is not a clear and direct link between purification processes once biogas is converted into synthesis gas (a mixture of H2 and CO, among other compounds) and their influence on catalyst performance, some indirect positive effects could be found.
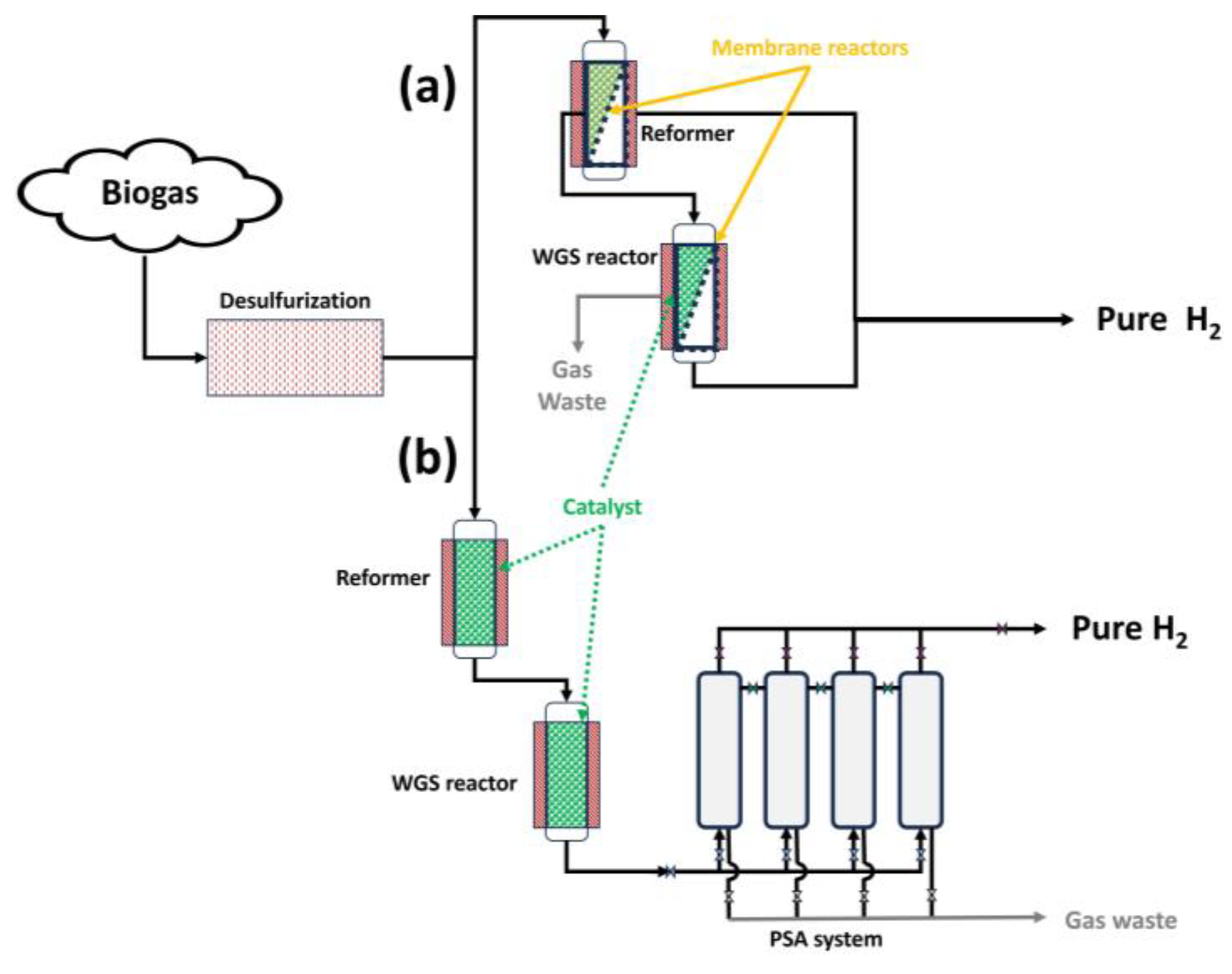
As observed in Figure 15, there are different biogas steam reforming configurations to carry out hydrogen production with a high purity. In that sense, considering the first route (Figure 15a), the use of membrane reactors in situ could contribute, as explained in the previous subsection, to shifting the reaction balance towards product generation, with the subsequent possibility of decreasing chemical conditions such as temperature and, consequently, a delay in deactivation processes such as sintering.
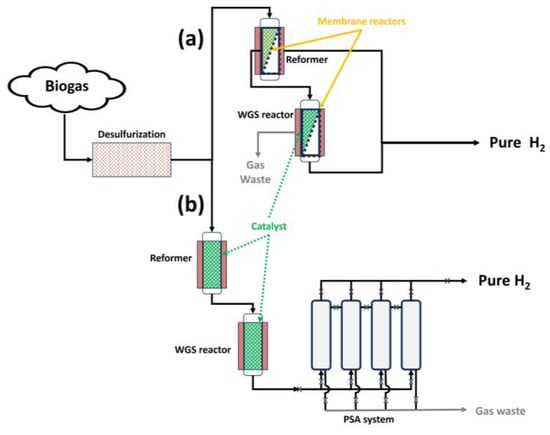
Figure 15.
Different routes for biogas steam reforming to obtain high-purity hydrogen: (a) through membrane reactors; and (b) through PSA.
However, in Figure 15b, the purification process (in this case, PSA, where an adsorbent is used to bind molecules depending on the gas component, type of adsorbent material, partial pressure, and operating temperature [25,127]) takes place after the products are obtained, with no direct effect on the chemical balance.
Also, other configurations (due to economic adaptations of previous steam reforming facilities) could couple membranes after steam reforming to obtain pure hydrogen, presenting a similar situation compared to the use of PSA.
In these situations, an improvement in chemical conditions can be equally found, considering the high-quality product obtained (normally >98% hydrogen, which is highly valuable in the energy market). In this context, lower reaction temperatures or catalyst concentrations could be used, offsetting the lower conversion with a valuable final product and with the possibility of recirculating gas waste in the same process or other processes. Also, biogas can be converted to synthesis gas, which can be used in other processes such as Fischer–Tropsch, where different H2/CO ratios (even at lower temperatures compared with normal steam reforming conditions to optimize hydrogen production) can be used to obtain, for instance, liquid fuels [128]. Under these circumstances, the catalyst could be less deactivated, especially if these steps are combined with the use of a suitable catalyst support or the use of promoters, which could equally improve the final conversion of methane in biogas.
5. Techno-Economic Analyses and Patents Derived from Catalytic Biogas Steam Reforming
As expected for such a mature technology, its implementation at an industrial level is highly extended, including research studies and patents for the application of steam reforming of methane (and biogas) about the feasibility of implementation (paying attention to techno-economic aspects) of this technology. In that sense, in general, there has been a considerable increase in hydrogen production patents focused on catalysts, especially at the beginning of this decade [26].
Firstly, the potential for the production of biogas, electricity, and heat from waste is important, as a wide range of electricity yields (from 52 to 850 kWh) could be obtained from 1 ton of biodegradable waste [35]. Previous studies recommend large-scale productions of biogas (at least 400 tons per day, and not recommending productions below 50 tons per day), especially for energy consumption due to steam generation [42]. It should be noted that, to make biogas steam reforming economically feasible, previous and further steps should be equally considered. As explained in the literature, biogas production and quality can be interrupted or reduced depending on some problems related to anaerobic digestion, such as over-acidification or foaming, which could lead to repeated and extended shutdowns of these units and the subsequent biogas production losses (up to 50%) [4]. Consequently, if biogas steam reforming is considered as a coupled technology related to biogas production (for instance, in WWTPs [38]), every aspect concerning the improvement of the stability and efficiency of biogas production will imply a better economic performance of biogas treatment on the whole. In that sense, the use of a circular economy (as seen in Figure 2, where the use of digestate to improve anaerobic digestion could be an interesting way of reusing waste with difficult management) could be resourceful, as well as the addition of macro, micronutrients enzymes, or carbon-based materials (for instance, hydrochar). Also, biogas upgrading should be considered, and an increase in the efficiency of contaminant removal, as well as an improvement in the service life of adsorbents (for instance), will have a positive repercussions for biogas steam reforming, allowing for the acquisition of more advanced equipment if global amortization takes place at an earlier economic stage.
Specifically, when it comes to the direct conversion of methane included in biogas, many factors are taken into account, especially the role of catalysts in this process, which will allow for softening some operating conditions (implying, for instance, a decrease in temperature reaction or steam addition) that could cheapen some permanent operating costs. Also, technological improvements in SRM are focused on the development of catalysts to contribute to an increase in hydrogen yield and a decrease in greenhouse gas emissions, with a reduction in energy consumption [26]. In other words, these works are devoted to minimize the main challenges related to SRM, like carbon dioxide production and energy costs [25].
Equally, regarding the treatment of gaseous gas obtained in biogas or methane steam reforming, there are plenty of choices to valorize biogas steam reforming. Obviously, in most cases, there must be a considerable initial investment, with the subsequent technological coupling or upgrading of biogas steam reforming facilities, as observed in Figure 2 in the case of Fischer–Tropsch synthesis, the use of membrane reactors, or PSA coupling (see also Figure 14 and Figure 15). Nevertheless, the final added-value product (liquid fuels in the case of Fischer–Tropsch or pure H2 in the case of membrane reactors and PSA) would offset this initial investment in the long run. Again, and even though these are technologies not directly related to biogas steam reforming by themselves, they can present a strong and positive influence, in a comprehensive manner, on the economic performance of this process.
Finally, a long, useful life of catalysts, as well as low concentrations (when possible), can positively contribute to an improvement in the economic performance of biogas steam reforming. As previously explained, the role of promoters is mainly focused on these purposes, as observed in previous studies where the use of nanosized Ni-Rh bimetallic clusters allowed for reducing the Ni content to 3% (as the size and distribution of the active phase was considerably improved compared to traditional Ni-based catalysts), with a considerable increase in catalytic stability and activity, which could contribute to a fixed cost reduction [129]. Thus, other works included in Table 7 follow a similar trend, where the use of promoters or even a change in the reactor disposition can make a reduction in catalyst addition (as well as other operating conditions like temperature) possible, with subsequent cost savings. As explained in previous sections, the role of some factors such as the use of membrane reactors can improve the economic performance of SR systems, including a reduction in catalyst amount or useful life. It should be noted that the works included in Table 7 are heterogeneous, dealing with facilities of different sizes (from the laboratory to semi-industrial level) and focusing on different aspects (from a direct improvement in catalysts through the use of promoters to the optimization of membrane reactors). In that sense, further studies about techno-economic assessments in this field will be really useful, enriching the current literature. Other works dealing with techno-economic research on biogas steam reforming are devoted to the use of PSA units [127,130,131], simulations and their comparisons with plant-scale experiences [132,133,134], or the whole process in general (in some cases. from biogas production to biogas steam reforming) [135,136], with an indirect role of the catalyst.

Table 7.
Main techno-economic studies related to biogas steam reforming.
Another interesting proof of the feasibility of this process at the industry level is the proliferation of patents about this subject. As observed in Table 8, there are plenty of patents whose main objective is the improvement of biogas (or methane) steam reforming performance, in many cases focused on the role of catalysts, which is to be expected, as they usually reduce the activation energy of the process, allowing for optimizing methane conversion into hydrogen. In any case, different aspects related to catalytic performance should be considered, such as their durability and stability, which would determine the subsequent stable and efficient hydrogen production. Also, other patents are focused on the abovementioned technologies to improve SRM performance, like the use of biogas upgrading through PSA to increase methane concentration, the use of membrane reactors or PSA for biogas purification or syngas upgrading (which can contribute to a reduced use of catalysts or an increase in stability, for instance), or possible alternatives for the produced syngas, like Fischer–Tropsch synthesis to obtain hydrocarbon products. As observed, practically all the techniques explained in previous sections are directly or indirectly applied in these patents, which proves the correlation between scientific works and applied research. On the other hand, these patents are focused on an increase in SRM efficiency, which usually implies an improvement in economic and energy costs, as well as, for instance, an improvement in catalytic performance with a higher stability.

Table 8.
Current patents focused on catalytic biogas steam reforming.
6. Conclusions and Future Trends
Biogas steam reforming is a reality for obtaining high yields of hydrogen from wastes with difficult environmental management such as sewage sludge, with multiple emerging techniques that might be promising in the near future. Indeed, according to the scientific interest from many different and multidisciplinary research teams, it represents the future and, also, the present of biogas and methane processing for obtaining hydrogen.
In that sense, the role of catalysts is vital to make this process more effective, efficient and, therefore, suitable at an industrial scale. High yields and selectivity values for hydrogen production have been obtained according to the literature, but there are some challenges to be solved, especially concerning the durability of these catalysts, which can be diminished by factors such as poisoning, coke deposition, or sintering.
This way, cutting-edge research works, focused on a wide range of aspects related to biogas steam reforming, have recently been carried out.
With regard to the catalytic steam reforming of biogas, the use of innovative catalyst formulations offers a wide range of possibilities, including the use of bi-metallic, multi-metallic catalysts, or promoters. In that sense, a resistance to coke deposition, sintering, and poisoning is essential by enhancing the interactions between the active phase and support surface or improving the catalytic performance of the active phase. Equally, the role of nano-catalysts, as well as innovative supports such as ceramic foams, are interesting, as increases in surface interaction and selectivity are usually found.
Also, the optimization of the process is essential, as many factors can influence on the catalytic performance. In that sense, temperature and steam optimization, as well as biogas quality control (especially with a low H2S content) should be taken into account.
Finally, concerning techno-economic assessments, recent studies seem to point out improvements to reduce costs related to catalyst addition (mainly reducing its amount and increasing its durability and useful life) and its performance (which could allow for softening chemical conditions, especially temperature). Equally, better hydrogen conversions could imply higher benefits (including softening of chemical conditions), as the final gas obtained (regardless of the subsequent purification processes) will be highly valued. Concurrently, patents are focused on similar topics, which points out the relevance of the catalytic steam reforming of biogas at industrial level.
Author Contributions
Conceptualization, S.N.-D.; methodology, S.N.-D.; investigation, S.N.-D., V.M. and C.M.Á.-M.; resources, J.F.G.; data curation, S.N.-D. and C.M.Á.-M.; writing—original draft preparation, S.N.-D., V.M. and C.M.Á.-M.; writing—review and editing, S.N.-D., V.M. and J.F.G.; visualization, S.N.-D., C.M.Á.-M. and J.F.G.; supervision, J.F.G.; project administration, J.F.G.; funding acquisition, V.M. and J.F.G. All authors have read and agreed to the published version of the manuscript.
Funding
Junta de Extremadura and FEDER funds through projects IB20042 and NextGeneration EU.
Acknowledgments
The authors thank the economic support to Junta de Extremadura and European FEDER funds through projects IB20042. S.N. and C.M. thank the NextGeneration EU funds, including investment lines C17.I1 “Planes complementarios con las Comunidades Autónomas”, within line 17 “Reforma institucional y fortalecimiento de las capacidades del sistema nacional de Ciencia, Tecnología e Innovación” within the Recovery, Transformation and Resilience Plan.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Abbreviation | Term |
| AD | Anaerobic digestion |
| BET | Brunauer–Emmett–Teller |
| CA | Chemical absorption |
| DRM | Dry reforming of methane |
| GHSV | Gas hourly space velocity |
| IWI | Incipient wet impregnation |
| PSA | Pressure swing adsorption |
| PZC | Point of zero charge |
| S/C | Steam to carbon ratio |
| SS | Sewage Sludge |
| SR | Steam reforming |
| SRM | Steam reforming of methane |
| VFAs | Volatile fatty acids |
| WGS | Water–gas shift |
| WI | Wet impregnation |
| WWTP | Wastewater treatment plant |
References
- Scholten, D.; Bosman, R. The Geopolitics of Renewables; Exploring the Political Implications of Renewable Energy Systems. Technol. Forecast. Soc. Chang. 2016, 103, 273–283. [Google Scholar] [CrossRef]
- Sharma, N.K.; Govindan, K.; Lai, K.K.; Chen, W.K.; Kumar, V. The Transition from Linear Economy to Circular Economy for Sustainability among SMEs: A Study on Prospects, Impediments, and Prerequisites. Bus. Strat. Environ. 2021, 30, 1803–1822. [Google Scholar] [CrossRef]
- Ramchuran, S.O.; O’Brien, F.; Dube, N.; Ramdas, V. An Overview of Green Processes and Technologies, Biobased Chemicals and Products for Industrial Applications. Curr. Opin. Green Sustain. Chem. 2023, 41, 100832. [Google Scholar] [CrossRef]
- Leca, E.; Zennaro, B.; Hamelin, J.; Carrère, H.; Sambusiti, C. Use of Additives to Improve Collective Biogas Plant Performances: A Comprehensive Review. Biotechnol. Adv. 2023, 65, 108129. [Google Scholar] [CrossRef] [PubMed]
- Bumharter, C.; Bolonio, D.; Amez, I.; García Martínez, M.J.; Ortega, M.F. New Opportunities for the European Biogas Industry: A Review on Current Installation Development, Production Potentials and Yield Improvements for Manure and Agricultural Waste Mixtures. J. Clean. Prod. 2023, 388, 135867. [Google Scholar] [CrossRef]
- Mccarty, P.L.; Smith, D.P. Anaerobic Wastewater Treatment. Environ. Sci. Technol. 1986, 20, 1200–1206. [Google Scholar] [CrossRef]
- Sawyerr, N.; Trois, C.; Workneh, T.; Okudoh, V. An Overview of Biogas Production: Fundamentals, Applications and Future Research. Int. J. Energy Econ. Policy 2019, 9, 105–116. [Google Scholar] [CrossRef]
- Khawer, M.U.B.; Naqvi, S.R.; Ali, I.; Arshad, M.; Juchelková, D.; Anjum, M.W.; Naqvi, M. Anaerobic Digestion of Sewage Sludge for Biogas & Biohydrogen Production: State-of-the-Art Trends and Prospects. Fuel 2022, 329, 125416. [Google Scholar] [CrossRef]
- Aromolaran, A.; Sartaj, M.; Abdallah, M. Supplemental Sewage Scum and Organic Municipal Solid Waste Addition to the Anaerobic Digestion of Thickened Waste Activated Sludge: Biomethane Potential and Microbiome Analysis. Fermentation 2023, 9, 237. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Wang, Y.; Turap, Y.; Wang, Z.; Zhang, Z.; Xia, Z.; Wang, W. Anaerobic Co-Digestion of Food Waste and Sewage Sludge in Anaerobic Sequencing Batch Reactors with Application of Co-Hydrothermal Pretreatment of Sewage Sludge and Biogas Residue. Bioresour. Technol. 2022, 364, 128006. [Google Scholar] [CrossRef]
- González, R.; Peña, D.C.; Gómez, X. Anaerobic Co-Digestion of Wastes: Reviewing Current Status and Approaches for Enhancing Biogas Production. Appl. Sci. 2022, 12, 8884. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Kumar, J.; Vu, M.T.; Mohammed, J.A.H.; Pathak, N.; Commault, A.S.; Sutherland, D.; Zdarta, J.; Tyagi, V.K.; Nghiem, L.D. Biomethane Production from Anaerobic Co-Digestion at Wastewater Treatment Plants: A Critical Review on Development and Innovations in Biogas Upgrading Techniques. Sci. Total Environ. 2021, 765, 142753. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on Research Achievements of Biogas from Anaerobic Digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Karrabi, M.; Ranjbar, F.M.; Shahnavaz, B.; Seyedi, S. A Comprehensive Review on Biogas Production from Lignocellulosic Wastes through Anaerobic Digestion: An Insight into Performance Improvement Strategies. Fuel 2023, 340, 127239. [Google Scholar] [CrossRef]
- Dragassi, M.-C.; Royon, L.; Redolfi, M.; Ammar, S. Hydrogen Storage as a Key Energy Vector for Car Transportation: A Tutorial Review. Hydrogen 2023, 4, 831–861. [Google Scholar] [CrossRef]
- Mazurova, K.; Miyassarova, A.; Eliseev, O.; Stytsenko, V.; Glotov, A.; Stavitskaya, A. Fischer–Tropsch Synthesis Catalysts for Selective Production of Diesel Fraction. Catalysts 2023, 13, 1215. [Google Scholar] [CrossRef]
- Al-Zuhairi, F.K.; Shakor, Z.M.; Hamawand, I. Maximizing Liquid Fuel Production from Reformed Biogas by Kinetic Studies and Optimization of Fischer–Tropsch Reactions. Energies 2023, 16, 7009. [Google Scholar] [CrossRef]
- Kumar, M.; Dutta, S.; You, S.; Luo, G.; Zhang, S.; Show, P.L.; Sawarkar, A.D.; Singh, L.; Tsang, D.C.W. A Critical Review on Biochar for Enhancing Biogas Production from Anaerobic Digestion of Food Waste and Sludge. J. Clean. Prod. 2021, 305, 127143. [Google Scholar] [CrossRef]
- Liu, M.; Wei, Y.; Leng, X. Improving Biogas Production Using Additives in Anaerobic Digestion: A Review. J. Clean. Prod. 2021, 297, 126666. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Dehghanimadvar, M.; Shirmohammadi, R.; Sadeghzadeh, M.; Aslani, A.; Ghasempour, R. Hydrogen Production Technologies: Attractiveness and Future Perspective. Int. J. Energy Res. 2020, 44, 8233–8254. [Google Scholar] [CrossRef]
- Al-Qahtani, A.M. A Comprehensive Review in Microwave Pyrolysis of Biomass, Syngas Production and Utilisation. Energies 2023, 16, 6876. [Google Scholar] [CrossRef]
- Parente, M.; Soria, M.A.; Madeira, L.M. Hydrogen and/or Syngas Production through Combined Dry and Steam Reforming of Biogas in a Membrane Reactor: A Thermodynamic Study. Renew. Energy 2020, 157, 1254–1264. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Hu, Y.H. Steam Reforming of Methane: Current States of Catalyst Design and Process Upgrading. Renew. Sustain. Energy Rev. 2021, 149, 111330. [Google Scholar] [CrossRef]
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen Production from Biomasses and Wastes: A Technological Review. Int. J. Hydrogen. Energy 2021, 46, 33756–33781. [Google Scholar] [CrossRef]
- Arsad, S.R.; Ker, P.J.; Hannan, M.A.; Tang, S.G.H.; Norhasyima, R.S.; Chau, C.F.; Mahlia, T.M.I. Patent Landscape Review of Hydrogen Production Methods: Assessing Technological Updates and Innovations. Int. J. Hydrogen Energy, 2023; in press. [Google Scholar]
- Gangadharan, P.; Kanchi, K.C.; Lou, H.H. Evaluation of the Economic and Environmental Impact of Combining Dry Reforming with Steam Reforming of Methane. Chem. Eng. Res. Des. 2012, 90, 1956–1968. [Google Scholar] [CrossRef]
- Navarro, R.M.; Peña, M.A.; Fierro, J.L.G. Hydrogen Production Reactions from Carbon Feedstocks: Fossil Fuels and Biomass. Chem. Rev. 2007, 107, 3952–3991. [Google Scholar] [CrossRef]
- Richter, J.; Rachow, F.; Israel, J.; Roth, N.; Charlafti, E.; Günther, V.; Flege, J.I.; Mauss, F. Reaction Mechanism Development for Methane Steam Reforming on a Ni/Al2O3 Catalyst. Catalysts 2023, 13, 884. [Google Scholar] [CrossRef]
- Wang, S.; Nabavi, S.A.; Clough, P.T. A Review on Bi/Polymetallic Catalysts for Steam Methane Reforming. Int. J. Hydrogen Energy 2023, 48, 15879–15893. [Google Scholar] [CrossRef]
- Ahmed, S.; Lee, S.H.D.; Ferrandon, M.S. Catalytic Steam Reforming of Biogas—Effects of Feed Composition and Operating Conditions. Int. J. Hydrogen Energy 2015, 40, 1005–1015. [Google Scholar] [CrossRef]
- Roozitalab, A.; Hamidavi, F.; Kargari, A. A Review of Membrane Material for Biogas and Natural Gas Upgrading. Gas Sci. Eng. 2023, 114, 204969. [Google Scholar] [CrossRef]
- Czekała, W.; Jasiński, T.; Dach, J. Profitability of the Agricultural Biogas Plants Operation in Poland, Depending on the Substrate Use Model. Energy Rep. 2023, 9, 196–203. [Google Scholar] [CrossRef]
- Guerrero, F.; Espinoza, L.; Ripoll, N.; Lisbona, P.; Arauzo, I.; Toledo, M. Syngas Production From the Reforming of Typical Biogas Compositions in an Inert Porous Media Reactor. Front. Chem. 2020, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Szyba, M.; Mikulik, J. Energy Production from Biodegradable Waste as an Example of the Circular Economy. Energies 2022, 15, 1269. [Google Scholar] [CrossRef]
- Chen, X.Y.; Vinh-Thang, H.; Ramirez, A.A.; Rodrigue, D.; Kaliaguine, S. Membrane Gas Separation Technologies for Biogas Upgrading. RSC Adv. 2015, 5, 24399–24448. [Google Scholar] [CrossRef]
- Abanades, S.; Abbaspour, H.; Ahmadi, A.; Das, B.; Ehyaei, M.A.; Esmaeilion, F.; El Haj Assad, M.; Hajilounezhad, T.; Hmida, A.; Rosen, M.A.; et al. A Conceptual Review of Sustainable Electrical Power Generation from Biogas. Energy Sci. Eng. 2022, 10, 630–655. [Google Scholar] [CrossRef]
- González, J.F.; Álvez-Medina, C.M.; Nogales-Delgado, S. Biogas Steam Reforming in Wastewater Treatment Plants: Opportunities and Challenges. Energies 2023, 16, 6343. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Q.; Yu, D. The Future of Hydrogen Energy: Bio-Hydrogen Production Technology. Int. J. Hydrogen Energy 2022, 47, 33677–33698. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Pal, A. Overview of Hydrogen Production from Biogas Reforming: Technological Advancement. Int. J. Hydrogen Energy 2022, 47, 34831–34855. [Google Scholar] [CrossRef]
- Scopus. Scopus. Available online: https://www.scopus.com/home.uri (accessed on 15 November 2023).
- Boscherini, M.; Storione, A.; Minelli, M.; Miccio, F.; Doghieri, F. New Perspectives on Catalytic Hydrogen Production by the Reforming, Partial Oxidation and Decomposition of Methane and Biogas. Energies 2023, 16, 6375. [Google Scholar] [CrossRef]
- Abou Rjeily, M.; Gennequin, C.; Pron, H.; Abi-Aad, E.; Randrianalisoa, J.H. Pyrolysis-Catalytic Upgrading of Bio-Oil and Pyrolysis-Catalytic Steam Reforming of Biogas: A Review. Environ. Chem. Lett. 2021, 19, 2825–2872. [Google Scholar] [CrossRef]
- Nahar, G.; Mote, D.; Dupont, V. Hydrogen Production from Reforming of Biogas: Review of Technological Advances and an Indian Perspective. Renew. Sustain. Energy Rev. 2017, 76, 1032–1052. [Google Scholar] [CrossRef]
- Angeli, S.D.; Turchetti, L.; Monteleone, G.; Lemonidou, A.A. Catalyst Development for Steam Reforming of Methane and Model Biogas at Low Temperature. Appl. Catal. B Environ. 2016, 181, 34–46. [Google Scholar] [CrossRef]
- Ashrafi, M.; Pfeifer, C.; Pröll, T.; Hofbauer, H. Experimental Study of Model Biogas Catalytic Steam Reforming: 2. Impact of Sulfur on the Deactivation and Regeneration of Ni-Based Catalysts. Energy Fuels 2008, 22, 4190–4195. [Google Scholar] [CrossRef]
- Appari, S.; Janardhanan, V.M.; Bauri, R.; Jayanti, S. Deactivation and Regeneration of Ni Catalyst during Steam Reforming of Model Biogas: An Experimental Investigation. Int. J. Hydrogen Energy 2014, 39, 297–304. [Google Scholar] [CrossRef]
- Appari, S.; Janardhanan, V.M.; Bauri, R.; Jayanti, S.; Deutschmann, O. A Detailed Kinetic Model for Biogas Steam Reforming on Ni and Catalyst Deactivation Due to Sulfur Poisoning. Appl. Catal. A Gen. 2014, 471, 118–125. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Fabiano, C.; Laganà, M.; Pino, L. Influence of Ce-Precursor and Fuel on Structure and Catalytic Activity of Combustion Synthesized Ni/CeO2 Catalysts for Biogas Oxidative Steam Reforming. Mater. Chem. Phys. 2015, 163, 337–347. [Google Scholar] [CrossRef]
- Phromprasit, J.; Powell, J.; Wongsakulphasatch, S.; Kiatkittipong, W.; Bumroongsakulsawat, P.; Assabumrungrat, S. H2 Production from Sorption Enhanced Steam Reforming of Biogas Using Multifunctional Catalysts of Ni over Zr-, Ce- and La-Modified CaO Sorbents. Chem. Eng. J. 2017, 313, 1415–1425. [Google Scholar] [CrossRef]
- Phromprasit, J.; Powell, J.; Wongsakulphasatch, S.; Kiatkittipong, W.; Bumroongsakulsawat, P.; Assabumrungrat, S. Activity and Stability Performance of Multifunctional Catalyst (Ni/CaO and Ni/Ca12Al14O33—CaO) for Bio-Hydrogen Production from Sorption Enhanced Biogas Steam Reforming. Int J Hydrogen Energy 2016, 41, 7318–7331. [Google Scholar] [CrossRef]
- Italiano, C.; Vita, A.; Fabiano, C.; Laganà, M.; Pino, L. Bio-Hydrogen Production by Oxidative Steam Reforming of Biogas over Nanocrystalline Ni/CeO2 Catalysts. Int. J. Hydrogen Energy 2015, 40, 11823–11830. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Ashraf, M.A.; Pino, L.; Specchia, S. Syngas Production by Steam and Oxy-Steam Reforming of Biogas on Monolith-Supported CeO2-Based Catalysts. Int. J. Hydrogen Energy 2018, 43, 11731–11744. [Google Scholar] [CrossRef]
- Roy, P.S.; Park, C.S.; Raju, A.S.K.; Kim, K. Steam-Biogas Reforming over a Metal-Foam-Coated (Pd–Rh)/(CeZrO2–Al2O3) Catalyst Compared with Pellet Type Alumina-Supported Ru and Ni Catalysts. J. CO2 Util. 2015, 12, 12–20. [Google Scholar] [CrossRef]
- Abbas, H.F.; Wan Daud, W.M.A. Hydrogen Production by Methane Decomposition: A Review. Int. J. Hydrogen Energy 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- Li, D.; Sun, J.; Zhang, Z.; Ma, R.; Wei, J. High-Efficient Sunlight-Driven Hydrogen Production from Methanol Steam Reforming on a Novel Photo-Thermo-Catalysis and Thermo-Catalysis Dual-Bed Reactor. Fuel 2024, 357, 129895. [Google Scholar] [CrossRef]
- Ferreira-Aparicio, P.; Rodríguez-Ramos, I.; Anderson, J.A.; Guerrero-Ruiz, A. Mechanistic Aspects of the Dry Reforming of Methane over Ruthenium Catalysts. Appl. Catal. A Gen. 2000, 202, 183–196. [Google Scholar] [CrossRef]
- Ruban, N.V.; Rogozhnikov, V.N.; Stonkus, O.A.; Emelyanov, V.A.; Pakharukova, V.P.; Svintsitskiy, D.A.; Zazhigalov, S.V.; Zagoruiko, A.N.; Snytnikov, P.V.; Sobyanin, V.A.; et al. A Comparative Investigation of Equimolar Ni-, Ru-, Rh- and Pt-Based Composite Structured Catalysts for Energy-Efficient Methane Reforming. Fuel 2023, 352, 128973. [Google Scholar] [CrossRef]
- Schiaroli, N.; Battisti, M.; Benito, P.; Fornasari, G.; Di Gisi, A.G.; Lucarelli, C.; Vaccari, A. Catalytic Upgrading of Clean Biogas to Synthesis Gas. Catalysts 2022, 12, 109. [Google Scholar] [CrossRef]
- Dan, M.; Mihet, M.; Borodi, G.; Lazar, M.D. Combined Steam and Dry Reforming of Methane for Syngas Production from Biogas Using Bimodal Pore Catalysts. Catal. Today 2021, 366, 87–96. [Google Scholar] [CrossRef]
- Saeidi, S.; Sápi, A.; Khoja, A.H.; Najari, S.; Ayesha, M.; Kónya, Z.; Asare-Bediako, B.B.; Tatarczuk, A.; Hessel, V.; Keil, F.J.; et al. Evolution Paths from Gray to Turquoise Hydrogen via Catalytic Steam Methane Reforming: Current Challenges and Future Developments. Renew. Sustain. Energy Rev. 2023, 183, 113392. [Google Scholar] [CrossRef]
- Osazuwa, O.U.; Abidin, S.Z.; Fan, X.; Amenaghawon, A.N.; Azizan, M.T. An Insight into the Effects of Synthesis Methods on Catalysts Properties for Methane Reforming. J. Environ. Chem. Eng. 2021, 9, 105052. [Google Scholar] [CrossRef]
- Zhao, X.; Joseph, B.; Kuhn, J.; Ozcan, S. Biogas Reforming to Syngas: A Review. iScience 2020, 23, 101082. [Google Scholar] [CrossRef]
- Perego, C.; Villa, P. Catalyst Preparation Methods. Catal. Today 1997, 34, 281–305. [Google Scholar] [CrossRef]
- Rotaru, C.G.; Postole, G.; Florea, M.; Matei-Rutkovska, F.; Pârvulescu, V.I.; Gelin, P. Dry Reforming of Methane on Ceria Prepared by Modified Precipitation Route. Appl. Catal. A Gen. 2015, 494, 29–40. [Google Scholar] [CrossRef]
- Świrk Da Costa, K.; Zhang, H.; Li, S.; Chen, Y.; Rønning, M.; Motak, M.; Grzybek, T.; Da Costa, P. Carbon-Resistant NiO-Y2O3-Nanostructured Catalysts Derived from Double-Layered Hydroxides for Dry Reforming of Methane. Catal. Today 2021, 366, 103–113. [Google Scholar] [CrossRef]
- Mallikarjun, G.; Sagar, T.V.; Swapna, S.; Raju, N.; Chandrashekar, P.; Lingaiah, N. Hydrogen Rich Syngas Production by Bi-Reforming of Methane with CO2 over Ni Supported on CeO2-SrO Mixed Oxide Catalysts. Catal. Today 2020, 356, 597–603. [Google Scholar] [CrossRef]
- Florea, M.; Matei-Rutkovska, F.; Postole, G.; Urda, A.; Neatu, F.; Pârvulescu, V.I.; Gelin, P. Doped Ceria Prepared by Precipitation Route for Steam Reforming of Methane. Catal Today 2018, 306, 166–171. [Google Scholar] [CrossRef]
- Niederberger, M. Nonaqueous Sol–Gel Routes to Metal Oxide Nanoparticles. Acc. Chem. Res. 2007, 40, 793–800. [Google Scholar] [CrossRef]
- Rex, A.; dos Santos, J.H.Z. The Use of Sol–Gel Processes in the Development of Supported Catalysts. J. Sol-Gel Sci. Technol. 2023, 105, 30–49. [Google Scholar] [CrossRef]
- Pechini, M.P. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to Form a Capacitor. U.S. Patent 3,330,697, 11 July 1967. [Google Scholar]
- Lin, J.; Yu, M.; Lin, C.; Liu, X. Multiform Oxide Optical Materials via the Versatile Pechini-Type Sol−Gel Process: Synthesis and Characteristics. J. Phys. Chem. C 2007, 111, 5835–5845. [Google Scholar] [CrossRef]
- Moogi, S.; Hyun Ko, C.; Hoon Rhee, G.; Jeon, B.H.; Ali Khan, M.; Park, Y.K. Influence of Catalyst Synthesis Methods on Anti-Coking Strength of Perovskites Derived Catalysts in Biogas Dry Reforming for Syngas Production. Chem. Eng. J. 2022, 437, 135348. [Google Scholar] [CrossRef]
- Zolghadri, S.; Honarvar, B.; Rahimpour, M.R. Synthesis, Application, and Characteristics of Mesoporous Alumina as a Support of Promoted Ni-Co Bimetallic Catalysts in Steam Reforming of Methane. Fuel 2023, 335, 127005. [Google Scholar] [CrossRef]
- Meshksar, M.; Farsi, M.; Rahimpour, M.R. Hollow Sphere Ni-Based Catalysts Promoted with Cerium for Steam Reforming of Methane. J. Energy Inst. 2022, 105, 342–354. [Google Scholar] [CrossRef]
- Zarei-Jelyani, F.; Salahi, F.; Farsi, M.; Reza Rahimpour, M. Synthesis and Application of Ni-Co Bimetallic Catalysts Supported on Hollow Sphere Al2O3 in Steam Methane Reforming. Fuel 2022, 324, 124785. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, B.; Lv, J.; Wang, Y.; Wu, Y.; Yang, H.; Hu, H. In-Situ Catalytic Upgrading of Tar from Integrated Process of Coal Pyrolysis with Steam Reforming of Methane over Carbon Based Ni Catalyst. J. Fuel Chem. Technol. 2022, 50, 129–142. [Google Scholar] [CrossRef]
- Dan, M.; Mihet, M.; Barbu-Tudoran, L.; Lazar, M.D. Biogas Upgrading to Syngas by Combined Reforming Using Ni/CeO2–Al2O3 with Bimodal Pore Structure. Microporous Mesoporous Mater. 2022, 341, 112082. [Google Scholar] [CrossRef]
- Roy, P.S.; Kang, M.S.; Kim, K. Effects of Pd-Rh Composition and CeZrO2-Modification of Al2O3 on Performance of Metal-Foam-Coated Pd-Rh/Al2O3 Catalyst for Steam Reforming of Model Biogas. Catal. Lett. 2014, 144, 2021–2032. [Google Scholar] [CrossRef]
- Roy, P.S.; Song, J.; Kim, K.; Kim, J.M.; Park, C.S.; Raju, A.S.K. Effects of CeZrO2–Al2O3 Support Composition of Metal-Foam-Coated Pd–Rh Catalysts for the Steam-Biogas Reforming Reaction. J. Ind. Eng. Chem. 2018, 62, 120–129. [Google Scholar] [CrossRef]
- Lin, K.H.; Chang, H.F.; Chang, A.C.C. Biogas Reforming for Hydrogen Production over Mesoporous Ni2xCe1-XO2 Catalysts. Int. J. Hydrogen Energy 2012, 37, 15696–15703. [Google Scholar] [CrossRef]
- Arbag, H.; Yasyerli, S.; Yasyerli, N.; Dogu, G.; Dogu, T. Enhancement of Catalytic Performance of Ni Based Mesoporous Alumina by Co Incorporation in Conversion of Biogas to Synthesis Gas. Appl. Catal. B 2016, 198, 254–265. [Google Scholar] [CrossRef]
- Zhong, J.; Han, B.; Zhang, Z.; Bi, G.; Xie, J. Bi-Reforming of Model Biogas to Syngas over Ru Nano-Catalysts Supported on Mg-Al Oxide Derived from Layered Double Hydroxides. Fuel 2023, 343, 127941. [Google Scholar] [CrossRef]
- Poggio-Fraccari, E.; Mariño, F.; Herrera, C.; Larrubia-Vargas, M.Á.; Alemany, L. Bi-Reforming of Biogas for Hydrogen Production with Sulfur-Resistant Multimetallic Catalyst. Chem. Eng. Technol. 2023, 46, 1176–1184. [Google Scholar] [CrossRef]
- Park, D.; Lee, C.; Moon, D.J.; Kim, T. Design, Analysis, and Performance Evaluation of Steam-CO2 Reforming Reactor for Syngas Production in GTL Process. Int. J. Hydrogen Energy 2015, 40, 11785–11790. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Chen, X.; Liang, X.; Zou, X.; Lu, X. Combined Steam and CO2 Reforming of Methane over One-Pot Prepared Ni/La-Si Catalysts. Int. J. Hydrogen Energy 2019, 44, 4780–4793. [Google Scholar] [CrossRef]
- Ren, P.; Zhao, Z. Unexpected Coke-Resistant Stability in Steam-CO2 Dual Reforming of Methane over the Robust Mo2C-Ni/ZrO2 Catalyst. Catal. Commun. 2019, 119, 71–75. [Google Scholar] [CrossRef]
- Schiaroli, N.; Lucarelli, C.; Sanghez de Luna, G.; Fornasari, G.; Vaccari, A. Ni-Based Catalysts to Produce Synthesis Gas by Combined Reforming of Clean Biogas. Appl. Catal. A Gen. 2019, 582, 117087. [Google Scholar] [CrossRef]
- Ponugoti, P.V.; Pathmanathan, P.; Rapolu, J.; Gomathi, A.; Janardhanan, V.M. On the Stability of Ni/γ-Al2O3 Catalyst and the Effect of H2O and O2 during Biogas Reforming. Appl. Catal. A Gen. 2023, 651, 119033. [Google Scholar] [CrossRef]
- Kolbitsch, P.; Pfeifer, C.; Hofbauer, H. Catalytic Steam Reforming of Model Biogas. Fuel 2008, 87, 701–706. [Google Scholar] [CrossRef]
- Azevedo, I.R.; da Silva, A.A.A.; Xing, Y.T.; Rabelo-Neto, R.C.; Luchters, N.T.J.; Fletcher, J.C.Q.; Noronha, F.B.; Mattos, L.V. Long-Term Stability of Pt/Ce0.8Me0.2O2-γ/Al2O3 (Me = Gd, Nb, Pr, and Zr) Catalysts for Steam Reforming of Methane. Int. J. Hydrogen Energy 2022, 47, 15624–15640. [Google Scholar] [CrossRef]
- Raju, A.S.K.; Park, C.S.; Norbeck, J.M. Synthesis Gas Production Using Steam Hydrogasification and Steam Reforming. Fuel Process. Technol. 2009, 90, 330–336. [Google Scholar] [CrossRef]
- Dong, W.-S.; Roh, H.-S.; Liu, Z.-W.; Jun, K.-W.; Park, S.-E. Hydrogen Production from Methane Reforming Reactions over Ni/MgO Catalyst. Bull. Korean Chem. Soc. 2001, 22, 1323–1327. [Google Scholar]
- Díez-Ramírez, J.; Dorado, F.; Martínez-Valiente, A.; García-Vargas, J.M.; Sánchez, P. Kinetic, Energetic and Exergetic Approach to the Methane Tri-Reforming Process. Int. J. Hydrogen Energy 2016, 41, 19339–19348. [Google Scholar] [CrossRef]
- Chouhan, K.; Sinha, S.; Kumar, S.; Kumar, S. Utilization of Biogas from Different Substrates for SOFC Feed via Steam Reforming: Thermodynamic and Exergy Analyses. J. Environ. Chem. Eng. 2019, 7, 103018. [Google Scholar] [CrossRef]
- Bkour, Q.; Marin-Flores, O.G.; Graham, T.R.; Ziaei, P.; Saunders, S.R.; Norton, M.G.; Ha, S. Nickel Nanoparticles Supported on Silica for the Partial Oxidation of Isooctane. Appl Catal A Gen 2017, 546, 126–135. [Google Scholar] [CrossRef]
- Bej, B.; Pradhan, N.C.; Neogi, S. Production of Hydrogen by Steam Reforming of Methane over Alumina Supported Nano-NiO/SiO2 Catalyst. Catal. Today 2013, 207, 28–35. [Google Scholar] [CrossRef]
- Shinde, V.M.; Madras, G. Catalytic Performance of Highly Dispersed Ni/TiO2 for Dry and Steam Reforming of Methane. RSC Adv. 2014, 4, 4817–4826. [Google Scholar] [CrossRef]
- Dang, C.; Xia, H.; Yuan, S.; Wei, X.; Cai, W. Green Hydrogen Production from Sorption-Enhanced Steam Reforming of Biogas over a Pd/Ni–CaO-Mayenite Multifunctional Catalyst. Renew. Energy 2022, 201, 314–322. [Google Scholar] [CrossRef]
- Park, M.-J.; Kim, H.-M.; Gu, Y.-J.; Jeong, D.-W. Optimization of Biogas-Reforming Conditions Considering Carbon Formation, Hydrogen Production, and Energy Efficiencies. Energy 2023, 265, 126273. [Google Scholar] [CrossRef]
- Santamaria, L.; Lopez, G.; Fernandez, E.; Cortazar, M.; Arregi, A.; Olazar, M.; Bilbao, J. Progress on Catalyst Development for the Steam Reforming of Biomass and Waste Plastics Pyrolysis Volatiles: A Review. Energy Fuels 2021, 35, 17051–17084. [Google Scholar] [CrossRef]
- Farooqi, A.S.; Yusuf, M.; Mohd Zabidi, N.A.; Saidur, R.; Sanaullah, K.; Farooqi, A.S.; Khan, A.; Abdullah, B. A Comprehensive Review on Improving the Production of Rich-Hydrogen via Combined Steam and CO2 Reforming of Methane over Ni-Based Catalysts. Int. J. Hydrogen Energy 2021, 46, 31024–31040. [Google Scholar] [CrossRef]
- Chan, Y.H.; Lock, S.S.M.; Wong, M.K.; Yiin, C.L.; Loy, A.C.M.; Cheah, K.W.; Chai, S.Y.W.; Li, C.; How, B.S.; Chin, B.L.F.; et al. A State-of-the-Art Review on Capture and Separation of Hazardous Hydrogen Sulfide (H2S): Recent Advances, Challenges and Outlook. Environ. Pollut. 2022, 314, 120219. [Google Scholar] [CrossRef]
- Pudi, A.; Rezaei, M.; Signorini, V.; Andersson, M.P.; Baschetti, M.G.; Mansouri, S.S. Hydrogen Sulfide Capture and Removal Technologies: A Comprehensive Review of Recent Developments and Emerging Trends. Sep. Purif. Technol. 2022, 298, 121448. [Google Scholar] [CrossRef]
- Capa, A.; González-Vázquez, M.P.; Chen, D.; Rubiera, F.; Pevida, C.; Gil, M.V. Effect of H2S on Biogas Sorption Enhanced Steam Reforming Using a Pd/Ni-Co Catalyst and Dolomite as a Sorbent. Chem. Eng. J. 2023, 476, 146803. [Google Scholar] [CrossRef]
- Rahim, D.A.; Fang, W.; Wibowo, H.; Hantoko, D.; Susanto, H.; Yoshikawa, K.; Zhong, Y.; Yan, M. Review of High Temperature H2S Removal from Syngas: Perspectives on Downstream Process Integration. Chem. Eng. Process.-Process Intensif. 2023, 183, 109258. [Google Scholar] [CrossRef]
- Ozekmekci, M.; Salkic, G.; Fellah, M.F. Use of Zeolites for the Removal of H2S: A Mini-Review. Fuel Process. Technol. 2015, 139, 49–60. [Google Scholar] [CrossRef]
- Secco, C.; Fuziki, M.E.K.; Tusset, A.M.; Lenzi, G.G. Reactive Processes for H2S Removal. Energies 2023, 16, 1759. [Google Scholar] [CrossRef]
- Fabrik, M.; Salama, A.; Ibrahim, H. Modeling of Catalyst Poisoning during Hydrogen Production via Methane Steam and Dry Reforming. Fuel 2023, 347, 128429. [Google Scholar] [CrossRef]
- Izquierdo, U.; García-García, I.; Gutierrez, Á.M.; Arraibi, J.R.; Barrio, V.L.; Cambra, J.F.; Arias, P.L. Catalyst Deactivation and Regeneration Processes in Biogas Tri-Reforming Process. The Effect of Hydrogen Sulfide Addition. Catalysts 2018, 8, 12. [Google Scholar] [CrossRef]
- Cattaneo, C.R.; Muñoz, R.; Korshin, G.V.; Naddeo, V.; Belgiorno, V.; Zarra, T. Biological Desulfurization of Biogas: A Comprehensive Review on Sulfide Microbial Metabolism and Treatment Biotechnologies. Sci. Total Environ. 2023, 893, 164689. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Cornacchia, M.; Pagliero, M.; Fabiano, B.; Vocciante, M.; Reverberi, A. Pietro Hydrogen Sulfide Adsorption by Iron Oxides and Their Polymer Composites: A Case-Study Application to Biogas Purification. Materials 2020, 13, 4725. [Google Scholar] [CrossRef]
- Abd, A.A.; Othman, M.R.; Majdi, H.S.; Helwani, Z. Green Route for Biomethane and Hydrogen Production via Integration of Biogas Upgrading Using Pressure Swing Adsorption and Steam-Methane Reforming Process. Renew. Energy 2023, 210, 64–78. [Google Scholar] [CrossRef]
- Wang, X.; Su, X.; Zhang, Q.; Hu, H. Effect of Additives on Ni-Based Catalysts for Hydrogen-Enriched Production from Steam Reforming of Biomass. Energy Technol. 2020, 8, 2000136. [Google Scholar] [CrossRef]
- Torimoto, M.; Sekine, Y. Effects of Alloying for Steam or Dry Reforming of Methane: A Review of Recent Studies. Catal. Sci. Technol. 2022, 12, 3387–3411. [Google Scholar] [CrossRef]
- Becker, C.M.; Marder, M.; Junges, E.; Konrad, O. Technologies for Biogas Desulfurization—An Overview of Recent Studies. Renew. Sustain. Energy Rev. 2022, 159, 112205. [Google Scholar] [CrossRef]
- Hulteberg, C. Sulphur-Tolerant Catalysts in Small-Scale Hydrogen Production, a Review. Int. J. Hydrogen Energy 2012, 37, 3978–3992. [Google Scholar] [CrossRef]
- Pashchenko, D.; Makarov, I. Carbon Deposition in Steam Methane Reforming over a Ni-Based Catalyst: Experimental and Thermodynamic Analysis. Energy 2021, 222, 119993. [Google Scholar] [CrossRef]
- Tuna, C.E.; Silveira, J.L.; da Silva, M.E.; Boloy, R.M.; Braga, L.B.; Pérez, N.P. Biogas Steam Reformer for Hydrogen Production: Evaluation of the Reformer Prototype and Catalysts. Int. J. Hydrogen Energy 2018, 43, 2108–2120. [Google Scholar] [CrossRef]
- Papurello, D.; Chiodo, V.; Maisano, S.; Lanzini, A.; Santarelli, M. Catalytic Stability of a Ni-Catalyst towards Biogas Reforming in the Presence of Deactivating Trace Compounds. Renew. Energy 2018, 127, 481–494. [Google Scholar] [CrossRef]
- Iulianelli, A.; Manisco, M.; Bion, N.; Le Valant, A.; Epron, F.; Colpan, C.O.; Esposito, E.; Jansen, J.C.; Gensini, M.; Caravella, A. Sustainable H2 Generation via Steam Reforming of Biogas in Membrane Reactors: H2S Effects on Membrane Performance and Catalytic Activity. Int. J. Hydrogen Energy 2021, 46, 29183–29197. [Google Scholar] [CrossRef]
- Amiri, T.Y.; Ghasemzageh, K.; Iulianelli, A. Membrane Reactors for Sustainable Hydrogen Production through Steam Reforming of Hydrocarbons: A Review. Chem. Eng. Process. Process Intensif. 2020, 157, 108148. [Google Scholar] [CrossRef]
- Arratibel Plazaola, A.; Pacheco Tanaka, D.A.; Van Sint Annaland, M.; Gallucci, F. Recent Advances in Pd-Based Membranes for Membrane Reactors. Molecules 2017, 22, 51. [Google Scholar] [CrossRef]
- Iulianelli, A.; Alavi, M.; Bagnato, G.; Liguori, S.; Wilcox, J.; Rahimpour, M.R.; Eslamlouyan, R.; Anzelmo, B.; Basile, A. Supported Pd-Au Membrane Reactor for Hydrogen Production: Membrane Preparation, Characterization and Testing. Molecules 2016, 21, 581. [Google Scholar] [CrossRef] [PubMed]
- Ben-Mansour, R.; Haque, M.A.; Habib, M.A.; Paglieri, S.; Harale, A.; Mokheimer, E.M.A. Effect of Temperature and Heat Flux Boundary Conditions on Hydrogen Production in Membrane-Integrated Steam-Methane Reformer. Appl. Energy 2023, 346, 121407. [Google Scholar] [CrossRef]
- Jokar, S.M.; Farokhnia, A.; Tavakolian, M.; Pejman, M.; Parvasi, P.; Javanmardi, J.; Zare, F.; Gonçalves, M.C.; Basile, A. The Recent Areas of Applicability of Palladium Based Membrane Technologies for Hydrogen Production from Methane and Natural Gas: A Review. Int. J. Hydrogen Energy 2023, 48, 6451–6476. [Google Scholar] [CrossRef]
- Di Marcoberardino, G.; Vitali, D.; Spinelli, F.; Binotti, M.; Manzolini, G. Green Hydrogen Production from Raw Biogas: A Techno-Economic Investigation of Conventional Processes Using Pressure Swing Adsorption Unit. Processes 2018, 6, 19. [Google Scholar] [CrossRef]
- Al-Zuhairi, F.K.; Azeez, R.A.; Mahdi, S.A. Synthesis of Long-Chain Hydrocarbons from Syngas over Promoted Co/SiO2 Catalysts Using Fischer–Tropsch Reaction. AIP Conf. Proc. 2022, 2443, 030028. [Google Scholar] [CrossRef]
- Schiaroli, N.; Lucarelli, C.; Iapalucci, M.C.; Fornasari, G.; Crimaldi, A.; Vaccari, A. Combined Reforming of Clean Biogas over Nanosized Ni–Rh Bimetallic Clusters. Catalysts 2020, 10, 1345. [Google Scholar] [CrossRef]
- He, X.; Lei, L.; Dai, Z. Green Hydrogen Enrichment with Carbon Membrane Processes: Techno-Economic Feasibility and Sensitivity Analysis. Sep. Purif. Technol. 2021, 276, 119346. [Google Scholar] [CrossRef]
- Cherif, A.; Nebbali, R.; Sen, F.; Sheffield, J.W.; Doner, N.; Nasseri, L. Modeling and Simulation of Steam Methane Reforming and Methane Combustion over Continuous and Segmented Catalyst Beds in Autothermal Reactor. Int. J. Hydrogen Energy 2022, 47, 9127–9138. [Google Scholar] [CrossRef]
- Kenkel, P.; Wassermann, T.; Zondervan, E. Biogas Reforming as a Precursor for Integrated Algae Biorefineries: Simulation and Techno-Economic Analysis. Processes 2021, 9, 1348. [Google Scholar] [CrossRef]
- Udemu, C.; Font-Palma, C. Modelling of Sorption-Enhanced Steam Reforming (SE-SR) Process in Fluidised Bed Reactors for Low-Carbon Hydrogen Production: A Review. Fuel 2023, 340, 127588. [Google Scholar] [CrossRef]
- Avraam, D.G.; Halkides, T.I.; Liguras, D.K.; Bereketidou, O.A.; Goula, M.A. An Experimental and Theoretical Approach for the Biogas Steam Reforming Reaction. Int. J. Hydrogen Energy 2010, 35, 9818–9827. [Google Scholar] [CrossRef]
- Cormos, C.C.; Cormos, A.M.; Petrescu, L.; Dragan, S. Techno-Economic Assessment of Decarbonized Biogas Catalytic Reforming for Flexible Hydrogen and Power Production. Appl. Therm. Eng. 2022, 207, 118218. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, J.H.; Lee, Y.H.; Kim, H.M.; Jeong, D.W. System Optimization for Effective Hydrogen Production via Anaerobic Digestion and Biogas Steam Reforming. Int. J. Hydrogen Energy 2020, 45, 30188–30200. [Google Scholar] [CrossRef]
- Frontera, P.; Malara, A.; Macario, A.; Miceli, M.; Bonaccorsi, L.; Boaro, M.; Pappacena, A.; Trovarelli, A.; Antonucci, P.L. Performance and Stability of Doped Ceria–Zirconia Catalyst for a Multifuel Reforming. Catalysts 2023, 13, 165. [Google Scholar] [CrossRef]
- Matus, E.; Kerzhentsev, M.; Ismagilov, I.; Nikitin, A.; Sozinov, S.; Ismagilov, Z. Hydrogen Production from Biogas: Development of an Efficient Nickel Catalyst by the Exsolution Approach. Energies 2023, 16, 2993. [Google Scholar] [CrossRef]
- Pajak, M.; Brus, G.; Kimijima, S.; Szmyd, J.S. Enhancing Hydrogen Production from Biogas through Catalyst Rearrangements. Energies 2023, 16, 4058. [Google Scholar] [CrossRef]
- Wodołażski, A.; Magdziarczyk, M.; Smoliński, A. Techno-Economic Analysis of Hydrogen Production from Swine Manure Biogas via Steam Reforming in Pilot-Scale Installation. Energies 2023, 16, 6389. [Google Scholar] [CrossRef]
- Ongis, M.; Di Marcoberardino, G.; Baiguini, M.; Gallucci, F.; Binotti, M. Optimization of Small-Scale Hydrogen Production with Membrane Reactors. Membranes 2023, 13, 331. [Google Scholar] [CrossRef]
- Ongis, M.; Di Marcoberardino, G.; Manzolini, G.; Gallucci, F.; Binotti, M. Membrane Reactors for Green Hydrogen Production from Biogas and Biomethane: A Techno-Economic Assessment. Int. J. Hydrogen Energy 2023, 48, 19580–19595. [Google Scholar] [CrossRef]
- Yan, P.; Cheng, Y. Design and Operational Considerations of Packed-Bed Membrane Reactor for Distributed Hydrogen Production by Methane Steam Reforming. Int. J. Hydrogen Energy 2022, 47, 36493–36503. [Google Scholar] [CrossRef]
- Lo Basso, G.; Pastore, L.M.; Mojtahed, A.; de Santoli, L. From Landfill to Hydrogen: Techno-Economic Analysis of Hybridized Hydrogen Production Systems Integrating Biogas Reforming and Power-to-Gas Technologies. Int. J. Hydrogen Energy 2023, 48, 37607–37624. [Google Scholar] [CrossRef]
- Minutillo, M.; Perna, A.; Sorce, A. Green Hydrogen Production Plants via Biogas Steam and Autothermal Reforming Processes: Energy and Exergy Analyses. Appl. Energy 2020, 277, 115452. [Google Scholar] [CrossRef]
- Braga, L.B.; Silveira, J.L.; Da Silva, M.E.; Tuna, C.E.; Machin, E.B.; Pedroso, D.T. Hydrogen Production by Biogas Steam Reforming: A Technical, Economic and Ecological Analysis. Renew. Sustain. Energy Rev. 2013, 28, 166–173. [Google Scholar] [CrossRef]
- Bo, G.; Li, F.; Chauhan, B.S.; Ghaebi, H. Multi-Objective Optimization and 4E Analyses to Simultaneous Production of Electricity, Freshwater, H2 and Cooling Based on Biogas-Steam Reforming. Process Saf. Environ. Prot. 2023, 177, 1307–1320. [Google Scholar] [CrossRef]
- He, J.; Han, N.; Xia, M.; Sun, T.; Ghaebi, H. Multi-Objective Optimization and Exergoeconomic Analysis of a Multi-Generation System Based on Biogas-Steam Reforming. Int. J. Hydrogen Energy 2023, 48, 21161–21175. [Google Scholar] [CrossRef]
- Farhang, B.; Ghaebi, H.; Javani, N. Techno-Economic Modeling of a Novel Poly-Generation System Based on Biogas for Power, Hydrogen, Freshwater, and Ammonia Production. J. Clean. Prod. 2023, 417, 137907. [Google Scholar] [CrossRef]
- Chouhan, K.; Sinha, S.; Kumar, S.; Kumar, S. Simulation of Steam Reforming of Biogas in an Industrial Reformer for Hydrogen Production. Int. J. Hydrogen Energy 2021, 46, 26809–26824. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, S.; Zhou, H. Method for Preparing Hydrogen by Utilizing Biogas. China Patent CN113772628 (A), 10 December 2021. [Google Scholar]
- Xu, S.; Pan, X.; Pehowa, P.; Lillabrig, A. Method for Producing Hydrogen from Biogas. China Patent CN113460963 (A), 1 October 2021. [Google Scholar]
- Mortensen, P.M.; Aasberg-Petersen, K.; Nielsen, C.S. Biogas Conversion to Synthesis Gas for Producing Hydrocarbons. United States Patent A/S US 2023/0012800 A1, 19 January 2023. [Google Scholar]
- Zhong, Y.; Liu, K.; Zhong, Y.; Cai, Y.; Chen, Y.; Mou, S. Method for Producing Hydrogen from Biogas Biomass. China Patent CN107758617 (A), 6 March 2018. [Google Scholar]
- Xu, S.; Pan, X.; Bazan, M.; Pechova, P. Apparatus for Producing Biomethane and Syngas from Biogas Stream with Means for Regulating Quality Biogas. China Patent CN202210209672 20220304, 23 September 2022. [Google Scholar]
- Allidieres, L. Method for Producing Hydrogen from Biogas. France Patent US2014186258 (A1), 3 July 2014. [Google Scholar]
- Guenther, L. Method and Plant for the Production of Synthesis Gas from Biogas. Germany Patent US2011175032 (A1), 21 July 2011. [Google Scholar]
- Offerman, J.D. Efficient Use of Biogas Carbon Dioxide in Liquid Fuel Synthesis. Germany Patent US2008220489 (A1), 11 September 2008. [Google Scholar]
- Su, B.; Tang, S.; Huang, S. Method and System for Coupling Biogas Reforming with Natural Gas Combined Cycle Based on Solar Energy Driving. China Patent CN114836480 (A), 2 August 2022. [Google Scholar]
- Han, W.; Su, B.; Jin, H. Biogas and Solar Energy Complementary Two-Stage Preparation System and Method for Synthesis Gas. China Patent CN110436413 (A), 12 November 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).