The Role of Alcohols in the Hexene-1 Hydroalkoxycarbonylation Reaction with Catalysts Based on Palladium Complexes
Abstract
:1. Introduction
2. Results and Discussion
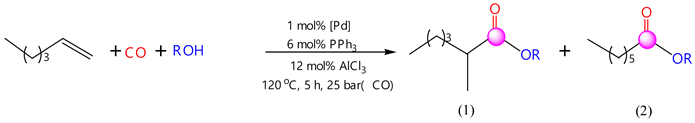
2.1. Performance of Hexene-1 Hydroalkoxycarbonylation Reaction Using PdCl2(PPh3)2-PPh3-AlCl3 Catalytic System
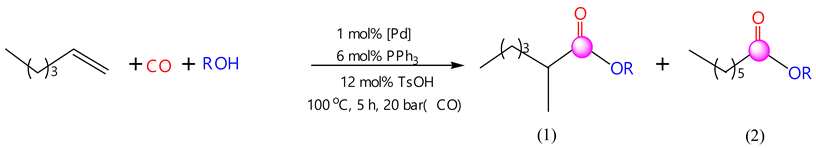
2.2. Performance of Hexene-1 Hydroalkoxycarbonylation Reaction Using Pd(PPh3)4-PPh3-TsOH Catalytic System
2.3. Determination of the Influence of Parameters for the Hydroalkoxycarbonylation Reaction of Hexene-1 with Propanol-1 and Butanol-1
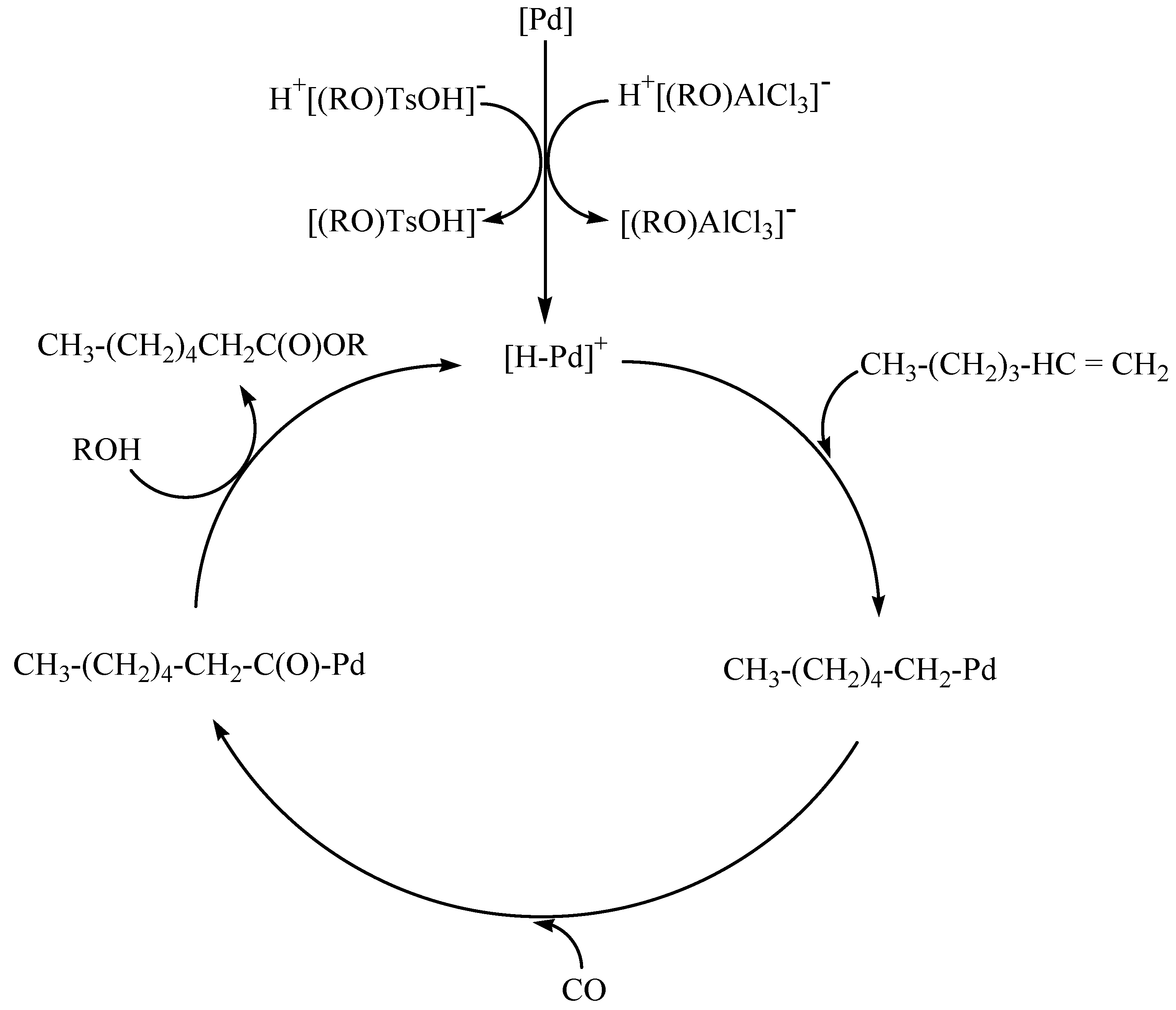
2.4. Mechanism of Hexene-1 Hydroalkoxycarbonylation Reaction in the Presence of PdCl2(PPh3)2-PPh3-AlCl3 and Pd(PPh3)4-PPh3-TsOH Catalytic System
3. Materials and Methods
3.1. Initial Reagents
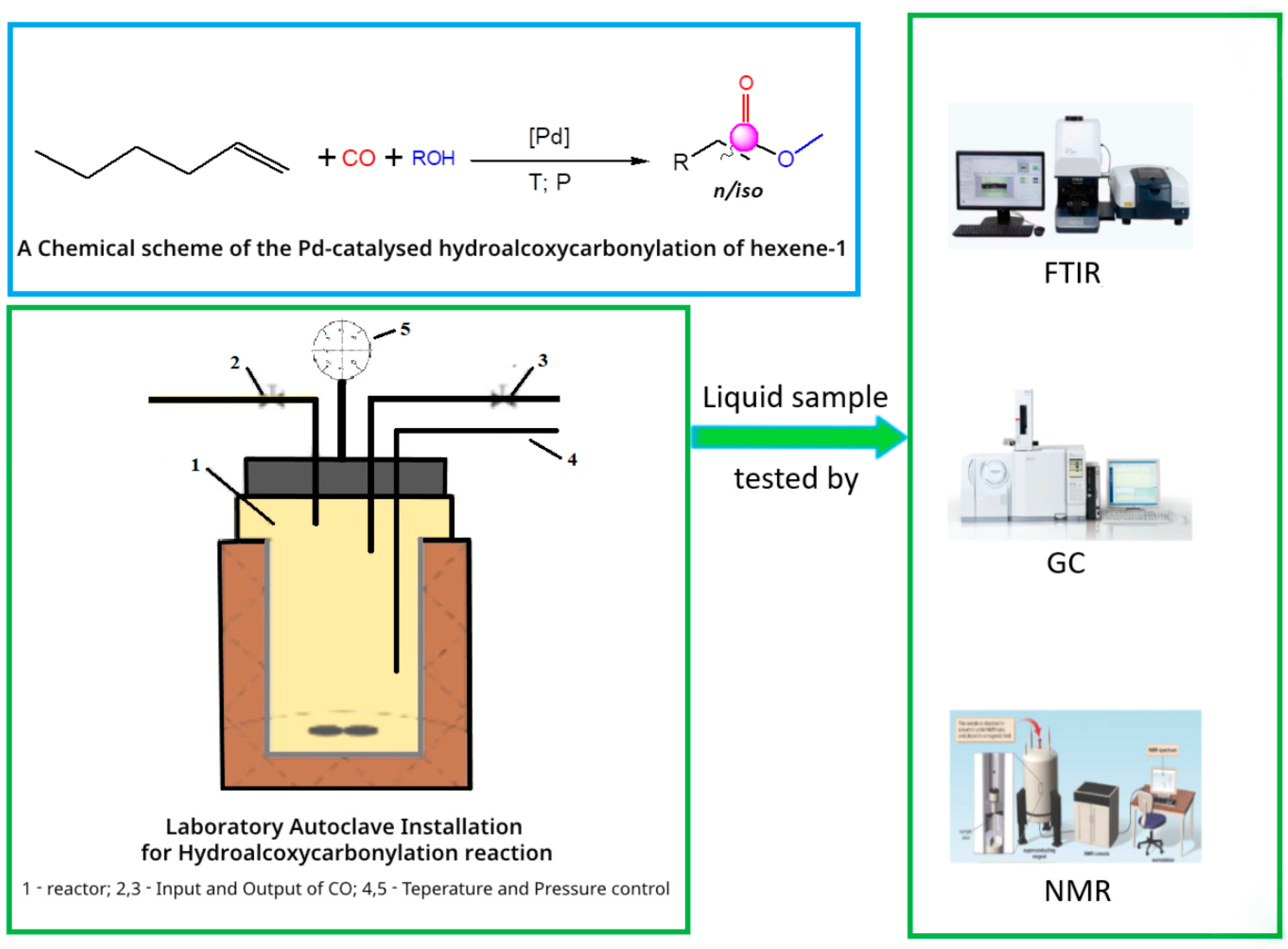
3.2. Methodology of the Experiment
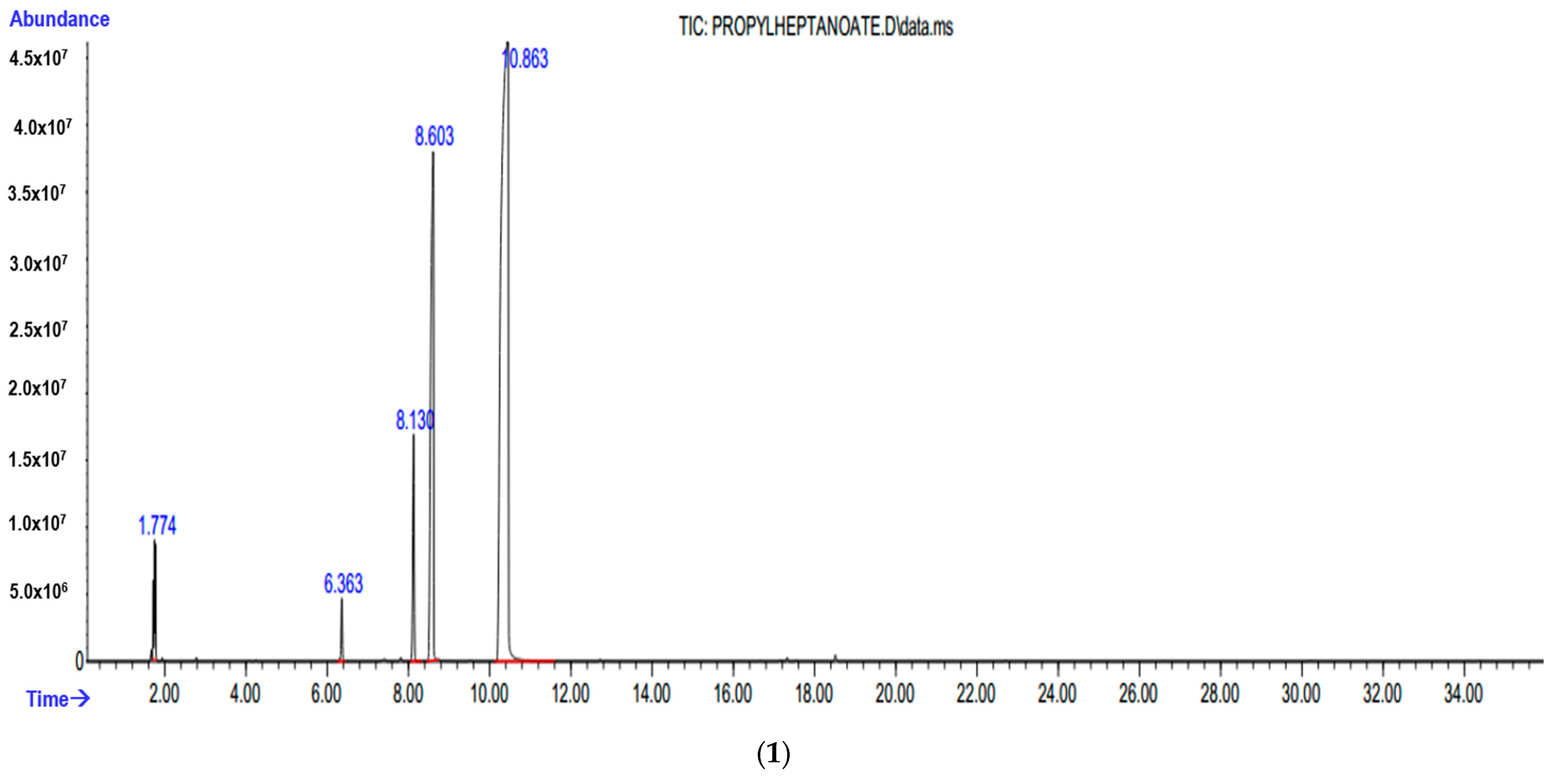
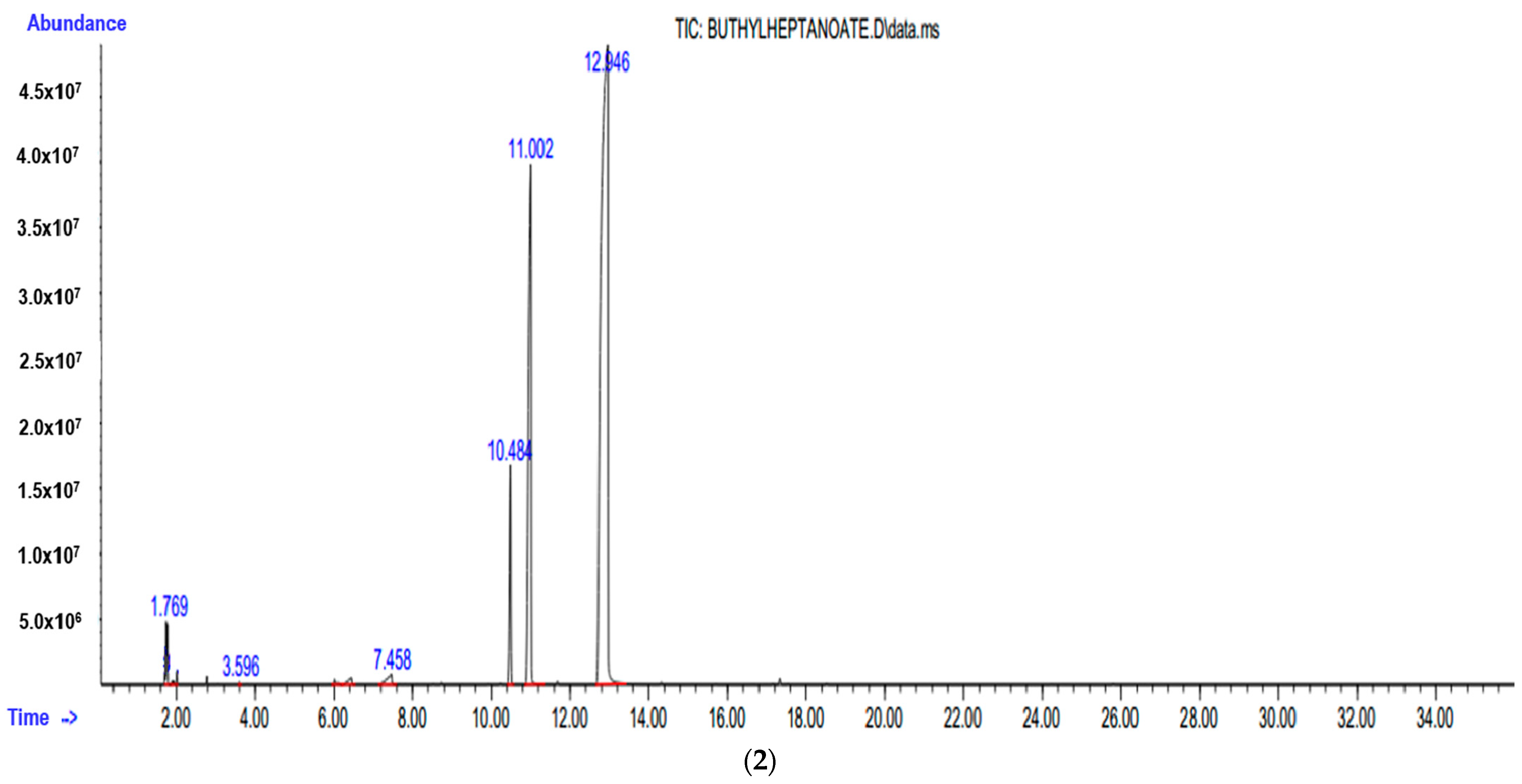
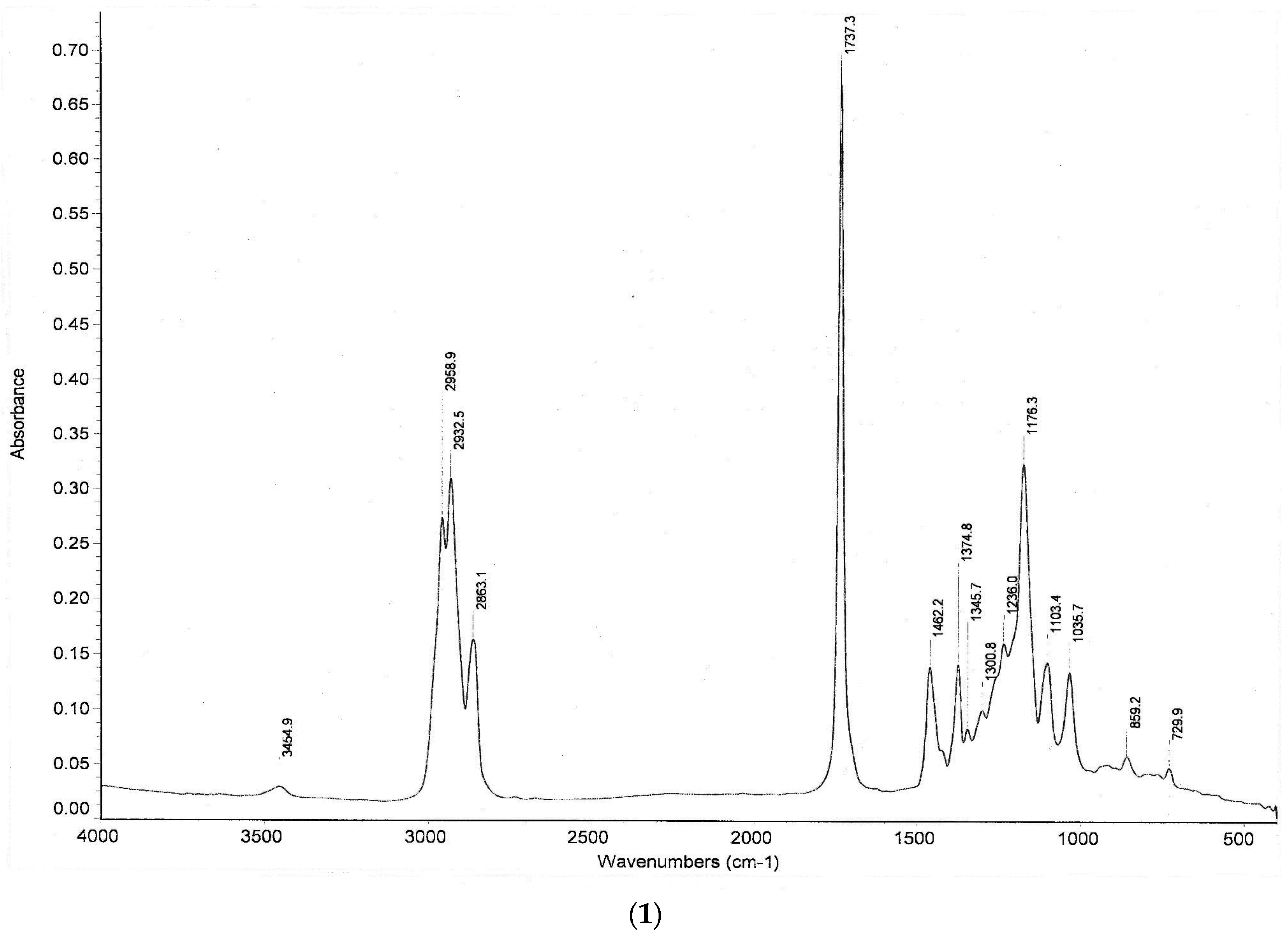
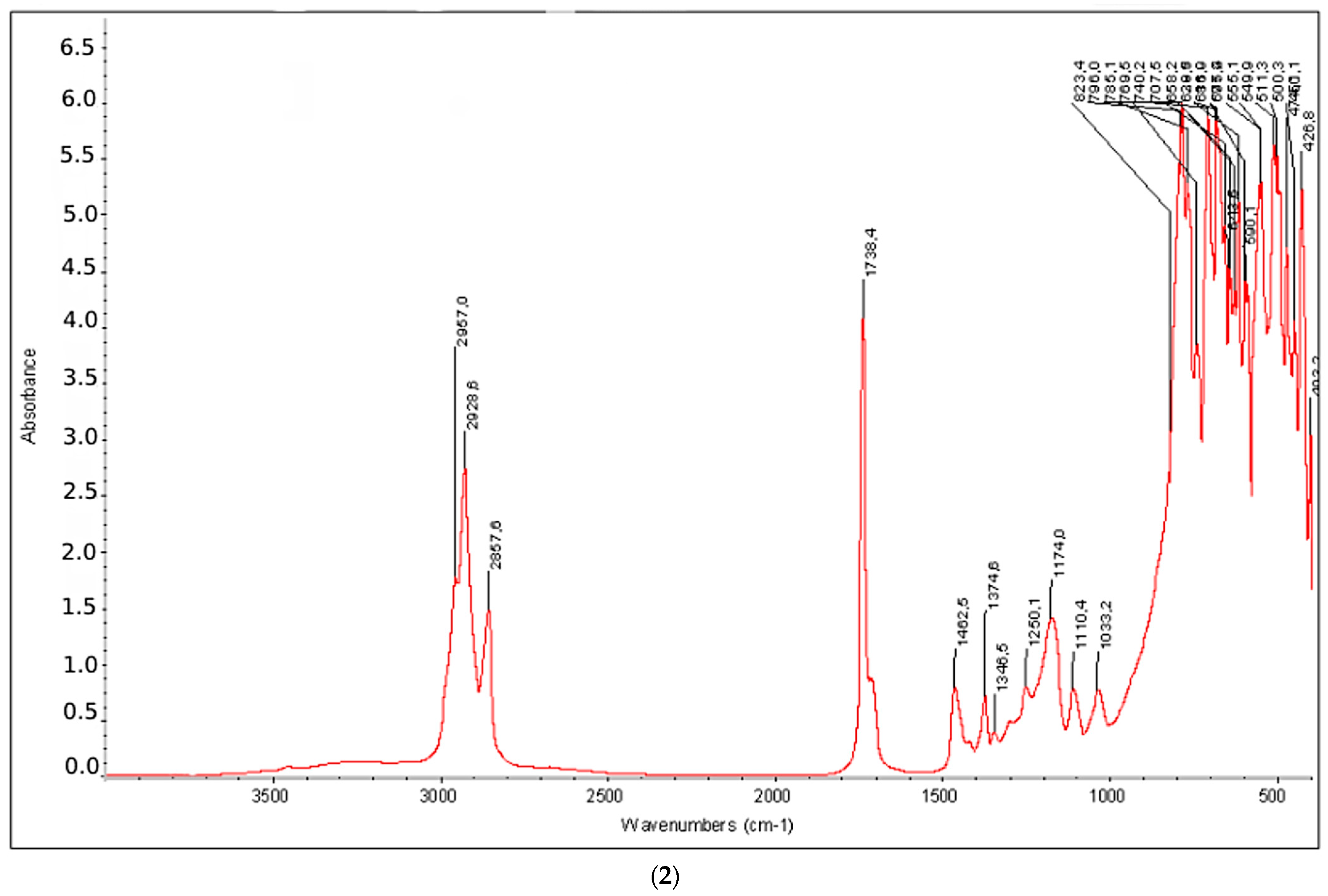
3.3. Instrumental Analyses of the Obtained Products
4. Conclusions
- In the carbonylation reaction of hexene-1 with carbon monoxide and alcohols (ethanol, propanol, butanol-1, isobutanol, pentanol-1 and isoamyl alcohol), the three-component catalytic system PdCl2(PPh3)2-PPh3-AlCl3 exhibited high activity. As a result, under optimal conditions ([C6H12]:[ROH]:[PdCl2(PPh3)2]:[PPh3]:[AlCl3] = 690:435:1:6:8, T = 120 °C, Pco = 2.5 MPa and τ = 5 h), the following yields of products were obtained: in hydroethoxycarbonylation, 89.1%; in hydropropoxycarbonylation, 91.8%; in hydrobutoxycarbonylation, 91.5%; in hydroisoalyoxycarbonylation, 46.6%; hydroisobutoxycarbonylation, 88.3%; and in hydropentoxycarbonylation, 67.6%. The reactions with allyl and tertbutyl alcohols showed no activity.
- In the carbonylation reaction of hexene-1 with carbon monoxide and alcohols (ethanol, propanol, isopropanol, butanol-1, isobutanol, cyclohexanol, menthol and benzyl alcohol), the three-component catalytic system Pd(PPh3)4-PPh3-TsOH showed high activity. Under the found optimal conditions ([C6H12]:[ROH]:[Pd(PPh3)4]:[PPh3]:[TsOH] = 550:435:1:6:12, PCO = 2.0 MPa, T = 100 °C and τ = 5 h), the yields of the products were 80.7% in hydroethoxycarbonylation; 80.5% in hydropropoxycarbonylation; 67.8% in hydroisopropoxycarbonylation; 79.0% in hydrobutoxycarbonylation; 75.0% in hydroisobutoxycarbonylation; 83.1% in hydrocyclohexoxycarbonylation; 89.1% in hydromentoxycarbonylation; and 75.9% in hydrobenzyloxycarbonylation.
- The influence of the process duration, temperature and carbon monoxide pressure on the yield of target products were also investigated in detail in the hydropropoxycarbonylation and hydrobutoxycarbonylation of hexene-1 at a particular ratio of starting reagents and components of the catalytic system ([C6H12]:[C3H7OH/C4H9OH]:[PdCl2(PPh3)2]:[PPh3]:[AlCl3] = 690:435:1:6:8), and the total yields of the target products were 91.8% and 91.5%, respectively.
- IR, NMR and GC-MS spectra of the synthesized products were taken, and the structures were studied and analyzed.
- The mechanism of hexene-1 hydroalkoxycarbonylation in the presence of two catalytic systems was proposed.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, L.; Liu, Q.; Jackstell, R.; Beller, M. Carbonylations of alkenes with CO surrogates. Angew. Chem. Int. Ed. 2014, 53, 6310–6320. [Google Scholar] [CrossRef]
- Wu, X.F.; Neumann, H. Ruthenium and Rhodium-Catalyzed Carbonylation Reactions. ChemCatChem 2012, 4, 447–458. [Google Scholar] [CrossRef]
- Sole, R.; Scrivanti, A.; Alam, M.M.; Beghetto, V. The intriguing methoxycarbonylation of trimethylsilylacetylene in the presence of Drent’s catalytic system. Appl. Organomet. Chem. 2021, 35, e6391. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.; Neumann, H.; Franke, R.; Jackstell, R.; Beller, M. Direct synthesis of adipic acid esters via palladium-catalyzed carbonylation of 1,3-dienes. Science 2019, 366, 1514–1517. [Google Scholar] [CrossRef] [PubMed]
- Geitner, R.; Gurinov, A.; Huang, T.; Kupfer, S.; Gräfe, S.; Weckhuysen, B.M. Reaction Mechanism of Pd-Catalyzed “CO-Free” Carbonylation Reaction Uncovered by In Situ Spectroscopy: The Formyl Mechanism. Angew. Chem. Int. Ed. 2021, 60, 3422–3427. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque, D.Y.; Teixeira, W.K.O.; Narayanaperumal, S.; Schwab, R.S. Recent Developments on Palladium-Catalyzed Carbonylation Reactions in Renewable Solvents. J. Braz. Chem. Soc. 2022, 33, 637–663. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.; Ge, Y.; Huang, W.; Neumann, H.; Jackstell, R.; Beller, M. Direct and Selective Synthesis of Adipic and Other Dicarboxylic Acids by Palladium-Catalyzed Carbonylation of Allylic Alcohols. Angew. Chem. Int. Ed. 2020, 59, 20394–20398. [Google Scholar] [CrossRef]
- Sang, R.; Kucmierczyk, P.; Dühren, R.; Razzaq, R.; Dong, K.; Liu, J.; Franke, R.; Jackstell, R.; Beller, M. Synthesis of Carboxylic Acids by Palladium-Catalyzed Hydroxycarbonylation. Angew. Chem. Int. Ed. 2019, 58, 14365–14373. [Google Scholar] [CrossRef] [PubMed]
- Brezny, A.C.; Landis, C.R. Recent Developments in the Scope, Practicality, and Mechanistic Understanding of Enantioselective Hydroformylation. Acc. Chem. Res. 2018, 51, 2344–2354. [Google Scholar] [CrossRef]
- Arderne, C.; Guzei, L.A.; Holzapfel, C.W.; Bredenkamp, T. Branched Selectivity in the Pd-Catalysed Methoxycarbonylation of 1-Alkenes. ChemCatChem 2016, 8, 1084–1093. [Google Scholar] [CrossRef]
- Bredenkamp, T.; Holzapfel, C. The Pd-catalysed hydromethoxycarbonylation of aliphatic internal alkenes with minimal double bond isomerisation. Catal. Commun. 2017, 96, 74–78. [Google Scholar] [CrossRef]
- Kiss, G. Palladium-catalyzed reppe carbonylation. Chem. Rev. 2001, 101, 3435–3456. [Google Scholar] [CrossRef] [PubMed]
- Akiri, S.O.; Ojwach, S.O. Structural studies and applications of water soluble (phenoxy)imine palladium(II) complexes as catalysts in biphasic methoxycarbonylation of 1-hexene. J. Organomet. Chem. 2021, 942, 121812. [Google Scholar] [CrossRef]
- Holzapfel, C.; Bredenkamp, T. An Empirical Study of Phosphine Ligands for the Methoxycarbonylation of Medium-Chain Alkenes. ChemCatChem 2015, 7, 2598–2606. [Google Scholar] [CrossRef]
- Ahmad, S.; Lockett, A.; Shuttleworth, T.A.; Miles-Hobbs, A.M.; Pringle, P.G.; Bühl, M. Palladium-catalysed alkyne alkoxycarbonylation with P,N-chelating ligands revisited: A density functional theory study. Phys. Chem. Chem. Phys. 2019, 21, 8543–8552. [Google Scholar] [CrossRef] [PubMed]
- Zolezzi, S.; Moya, S.A.; Valdebenito, G.; Abarca, G.; Parada, J.; Aguirre, P. Methoxycarbonylation of olefins catalyzed by palladium(II) complexes containing naphthyl(diphenyl)phosphine ligands. Appl. Organomet. Chem. 2014, 28, 364–371. [Google Scholar] [CrossRef]
- Cavinato, G.; Toniolo, L.; Vavasori, A. Carbonylation of ethene in methanol catalysed by cationic phosphine complexes of Pd(II): From polyketones to monocarbonylated products. Top. Organomet. Chem. 2006, 18, 125–164. [Google Scholar] [CrossRef]
- Peng, J.B.; Geng, H.Q.; Wu, X.F. The Chemistry of CO: Carbonylation. Chem 2019, 5, 526–552. [Google Scholar] [CrossRef]
- Cavinato, G.; Toniolo, L. Carbonylation of ethene catalysed by Pd(II)-Phosphine complexes. Molecules 2014, 19, 15116–15161. [Google Scholar] [CrossRef]
- Lanke, V.; Marek, I. Stereospecific nucleophilic substitution at tertiary and quaternary stereocentres. Chem. Sci. 2020, 11, 9378–9385. [Google Scholar] [CrossRef]
- Gehrtz, P.H.; Hirschbeck, V.; Fleischer, I. A recyclable CO surrogate in regioselective alkoxycarbonylation of alkenes: Indirect use of carbon dioxide. Chem. Commun. 2015, 51, 12574–12577. [Google Scholar] [CrossRef] [PubMed]
- Franke, R.; Selent, D.; Börner, A. Applied hydroformylation. Chem. Rev. 2012, 112, 5675–5732. [Google Scholar] [CrossRef] [PubMed]
- Suerbaev, K.A.; Kudaibergenov, N.Z.; Vavasori, A. Hydroethoxycarbonylation of α-olefins at low pressure of carbon(II) oxide in the presence of the PdCl2(PPh3)2–PPh3–AlCl3 system. Russ. J. Gen. Chem. 2017, 87, 707–712. [Google Scholar] [CrossRef]
- Turkbenov, T.K.; Siromakha, L.N.; Zhunusova, K.Z.; Eshchanova, Z.R.; Bakina, E.V.; Abdrakhmanova, A.K.; Turkbenova, D.S. Syntheses on the carbon oxides. Hexene-1 hydroethoxycarbonylation in the presence of the system Pd(PPh3)4-PPh3-TsOH. Bull. Kazn. Chem. Ser. 2011, 4, 203–206. [Google Scholar] [CrossRef]
- Wu, X.; Fang, X.; Wu, L.; Jackstell, R.; Neumann, H.; Beller, M. Transition-Metal-Catalyzed Carbonylation Reactions of Olefins and Alkynes: A Personal Account. Acc. Chem. Res. 2014, 47, 1041–1053. [Google Scholar] [CrossRef]
- Ahmad, S.; Bühl, M. Computational modelling of Pd-catalysed alkoxycarbonylation of alkenes and alkynes. Phys. Chem. Chem. Phys. 2021, 23, 15869–15880. [Google Scholar] [CrossRef]
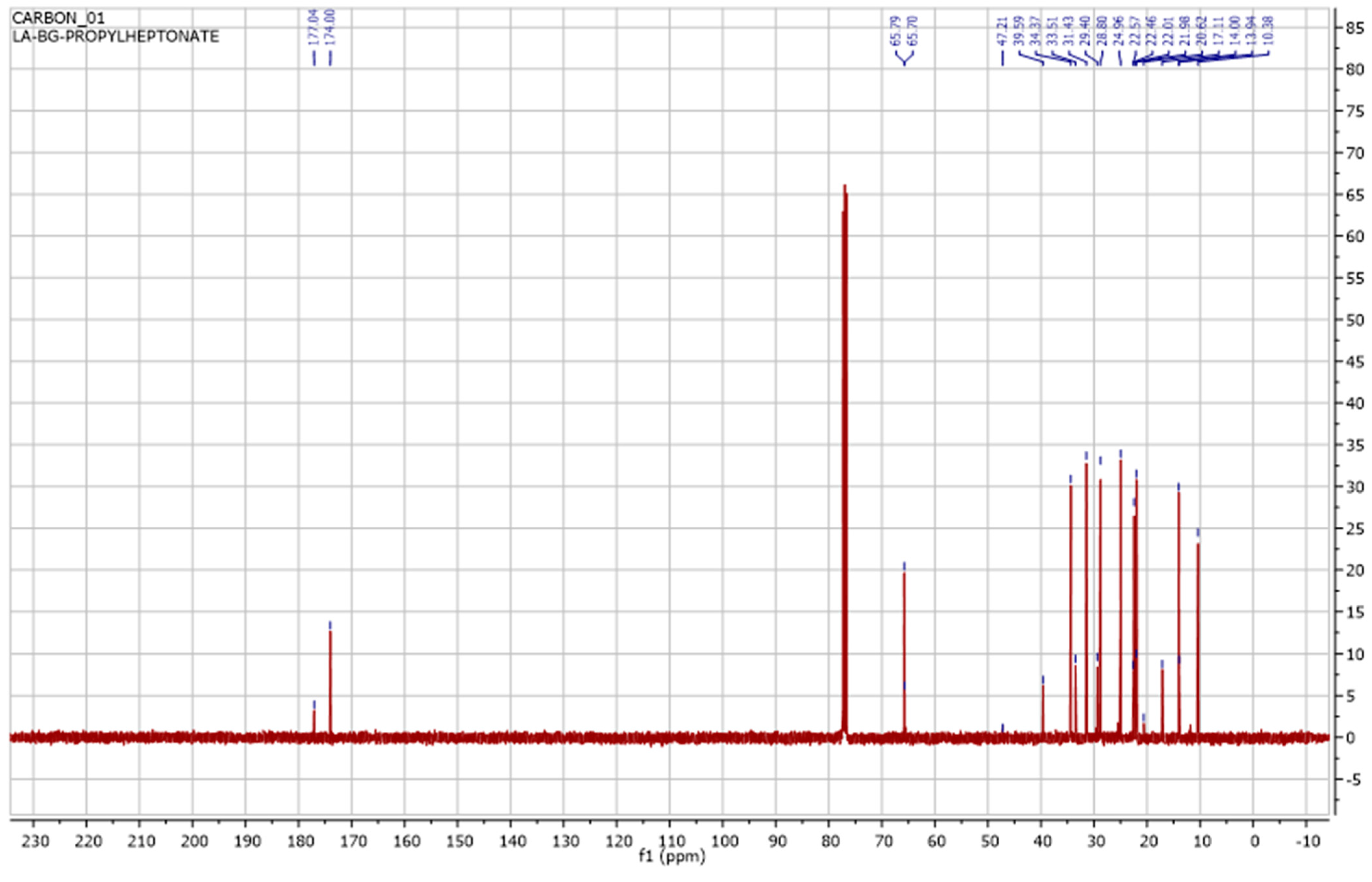
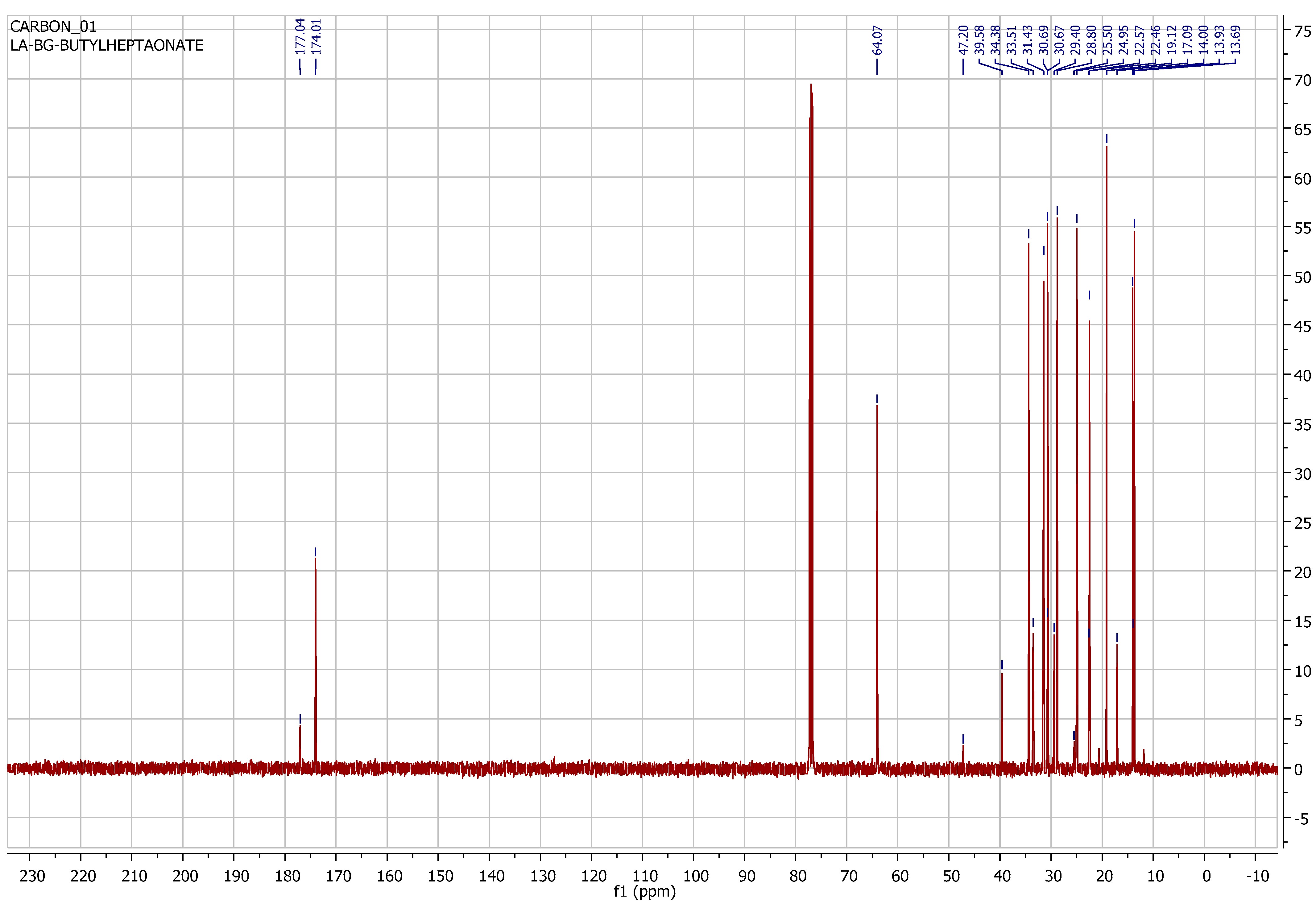
| Entry | [C6H12]:[ROH] | Product Yield, % | Selectivity iso:n |
|---|---|---|---|
| 1 | Ethanol | 89.1 | 1:2.5 |
| 2 | Propanol-1 | 91.8 | 1:2.8 |
| 3 | Butanol-1 | 91.5 | 1:2.7 |
| 4 | Isoamyl alcohol | 46.6 | 1:2.35 |
| 5 | Isobutanol | 88.3 | |
| 6 | Pentanol-1 | 67.6 | |
| 7 | Allyl alcohol | - | |
| 8 | Tert butyl alcohol | - |
| Entry | [C6H12]:[ROH] | Product Yield, % | Selectivity iso:n |
|---|---|---|---|
| 1 | Ethanol | 80.7 | 1:39 |
| 2 | Propanol-1 | 80.5 | 1:5.0 |
| 3 | Isopropanol | 67.8 | 1:3.6 |
| 4 | Butanol-1 | 79.0 | 1:5.3 |
| 5 | Isobutanol | 75.0 | 1:4.8 |
| 6 | Cyclohexanol | 83.1 | - |
| 7 | Mentol | 89.1 | - |
| 8 | Benzyl alcohol | 75.9 | - |
| Exp. No. | [C6H12]:[C3H7OH]: [PdCl2(PPh3)2]:[PPh3]:[AlCl3] | T, °C | PCO, MPa | τ, h | Products Yield, (PEEA + PE2-MCA)/ (BEEA + BE2-MCA), % |
|---|---|---|---|---|---|
| 1 | 690:435:1:6:8 | 120 | 2.5 | 3 | 79.1/86.9 |
| 2 | 690:435:1:6:8 | 120 | 2.5 | 4 | 82.3/90.1 |
| 3 | 690:435:1:6:8 | 120 | 2.5 | 5 | 91.8/91.5 |
| 4 | 690:435:1:6:8 | 120 | 2.5 | 6 | 54.5/87.1 |
| 5 | 690:435:1:6:8 | 120 | 2.5 | 7 | 35.9/86.5 |
| 6 | 690:435:1:6:8 | 100 | 2.5 | 5 | 29.5/86.2 |
| 7 | 690:435:1:6:8 | 110 | 2.5 | 5 | 59.2/90.1 |
| 8 | 690:435:1:6:8 | 120 | 2.5 | 5 | 91.8/91.5 |
| 9 | 690:435:1:6:8 | 130 | 2.5 | 5 | 54.5/85.0 |
| 10 | 690:435:1:6:8 | 140 | 2.5 | 5 | 31.0/83.9 |
| 11 | 690:435:1:6:8 | 120 | 1.5 | 5 | 40.7/86.2 |
| 12 | 690:435:1:6:8 | 120 | 2.0 | 5 | 55.5/90.6 |
| 13 | 690:435:1:6:8 | 120 | 2.5 | 5 | 91.8/91.5 |
| 14 | 690:435:1:6:8 | 120 | 3.0 | 5 | 63.3/85.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhaksylykova, G.; Shalmagambetov, K.; Kanapiyeva, F.; Kudaibergenov, N.; Bulybayev, M.; Zykai, M.; Abyzbekova, G.; Balykbayeva, G. The Role of Alcohols in the Hexene-1 Hydroalkoxycarbonylation Reaction with Catalysts Based on Palladium Complexes. Catalysts 2023, 13, 1507. https://doi.org/10.3390/catal13121507
Zhaksylykova G, Shalmagambetov K, Kanapiyeva F, Kudaibergenov N, Bulybayev M, Zykai M, Abyzbekova G, Balykbayeva G. The Role of Alcohols in the Hexene-1 Hydroalkoxycarbonylation Reaction with Catalysts Based on Palladium Complexes. Catalysts. 2023; 13(12):1507. https://doi.org/10.3390/catal13121507
Chicago/Turabian StyleZhaksylykova, Gulbanu, Kairzhan Shalmagambetov, Fatima Kanapiyeva, Nurbolat Kudaibergenov, Marat Bulybayev, Meruyert Zykai, Gulmira Abyzbekova, and Gulzhan Balykbayeva. 2023. "The Role of Alcohols in the Hexene-1 Hydroalkoxycarbonylation Reaction with Catalysts Based on Palladium Complexes" Catalysts 13, no. 12: 1507. https://doi.org/10.3390/catal13121507







