Sodium Promoted FeZn@SiO2-C Catalysts for Sustainable Production of Low Olefins by CO2 Hydrogenation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Fe-Based Catalysts
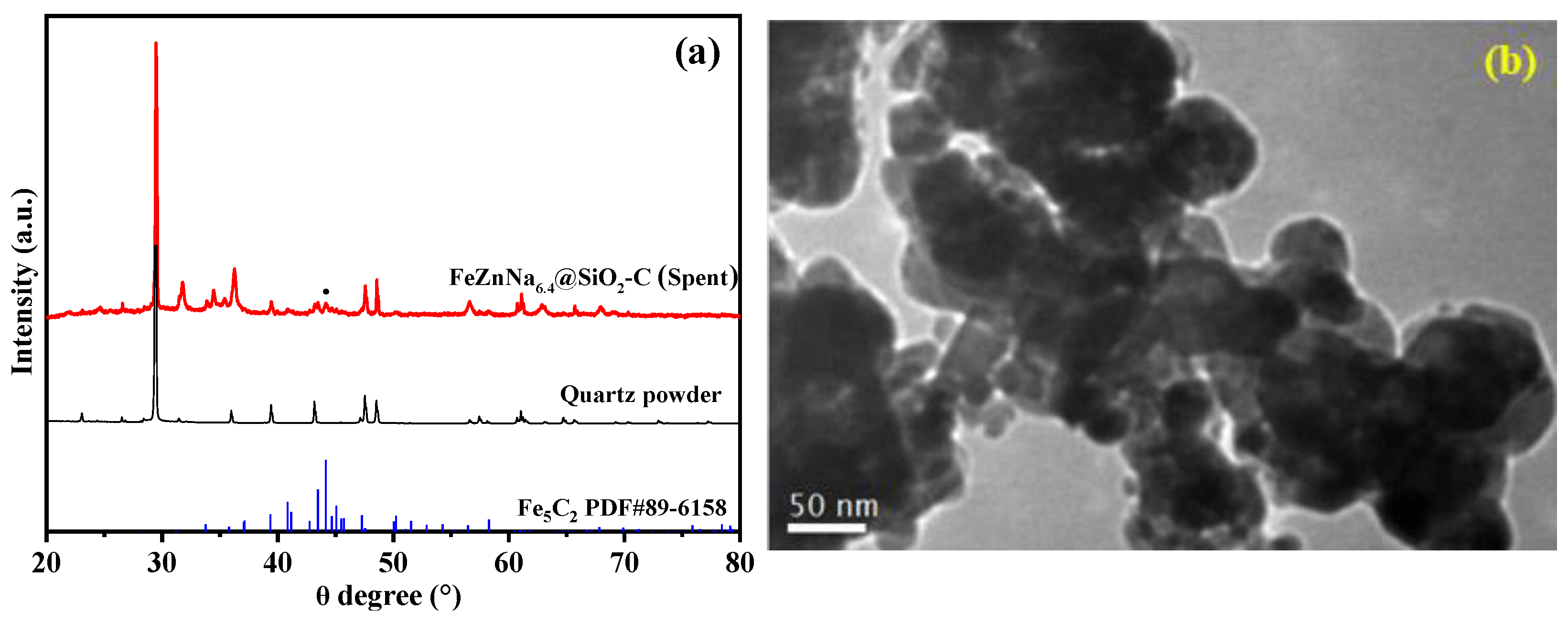
2.1.1. Textural Property, Structure and Morphology
2.1.2. XPS Results
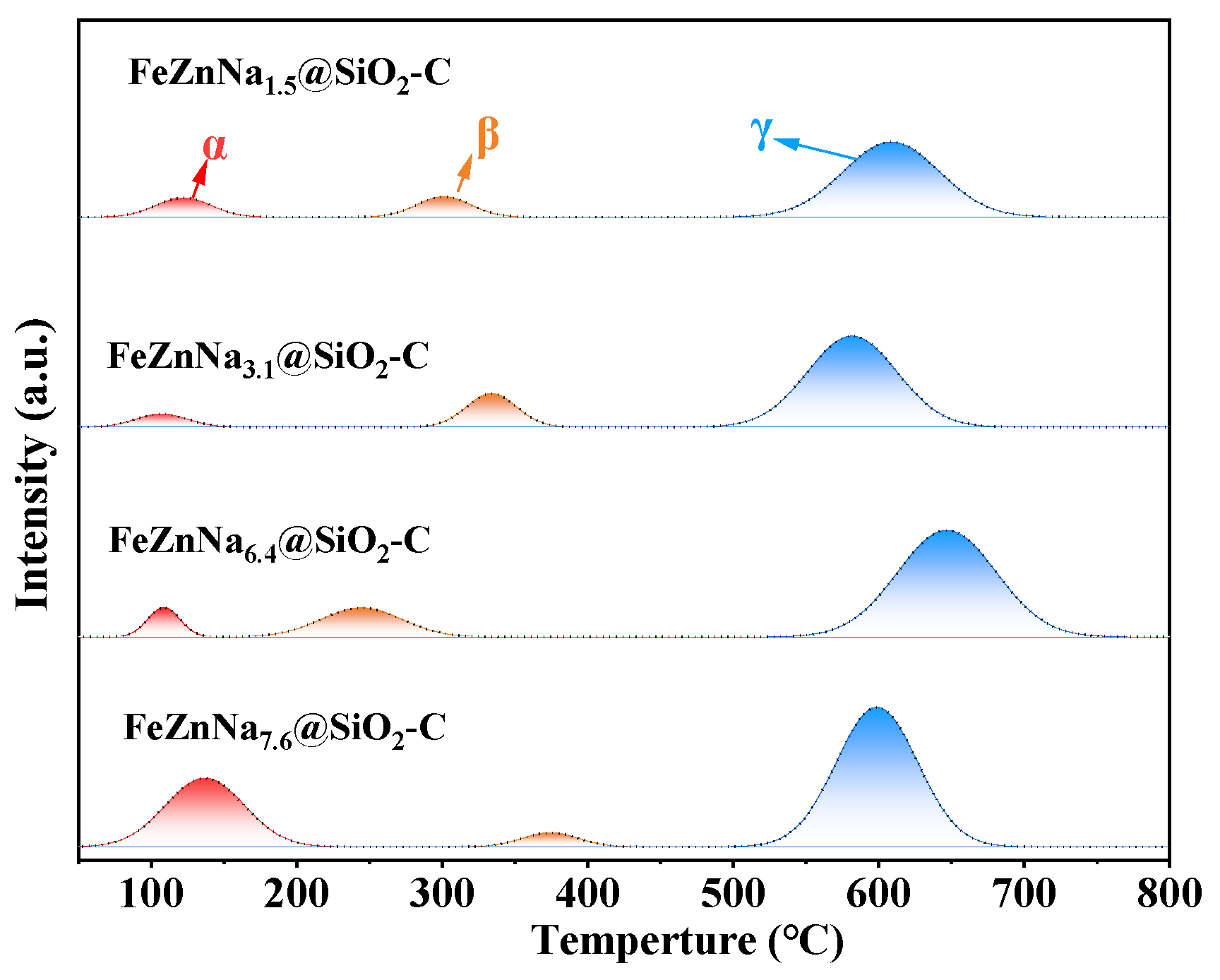
2.1.3. Reduction Behavior
2.1.4. Chemisorption Behavior
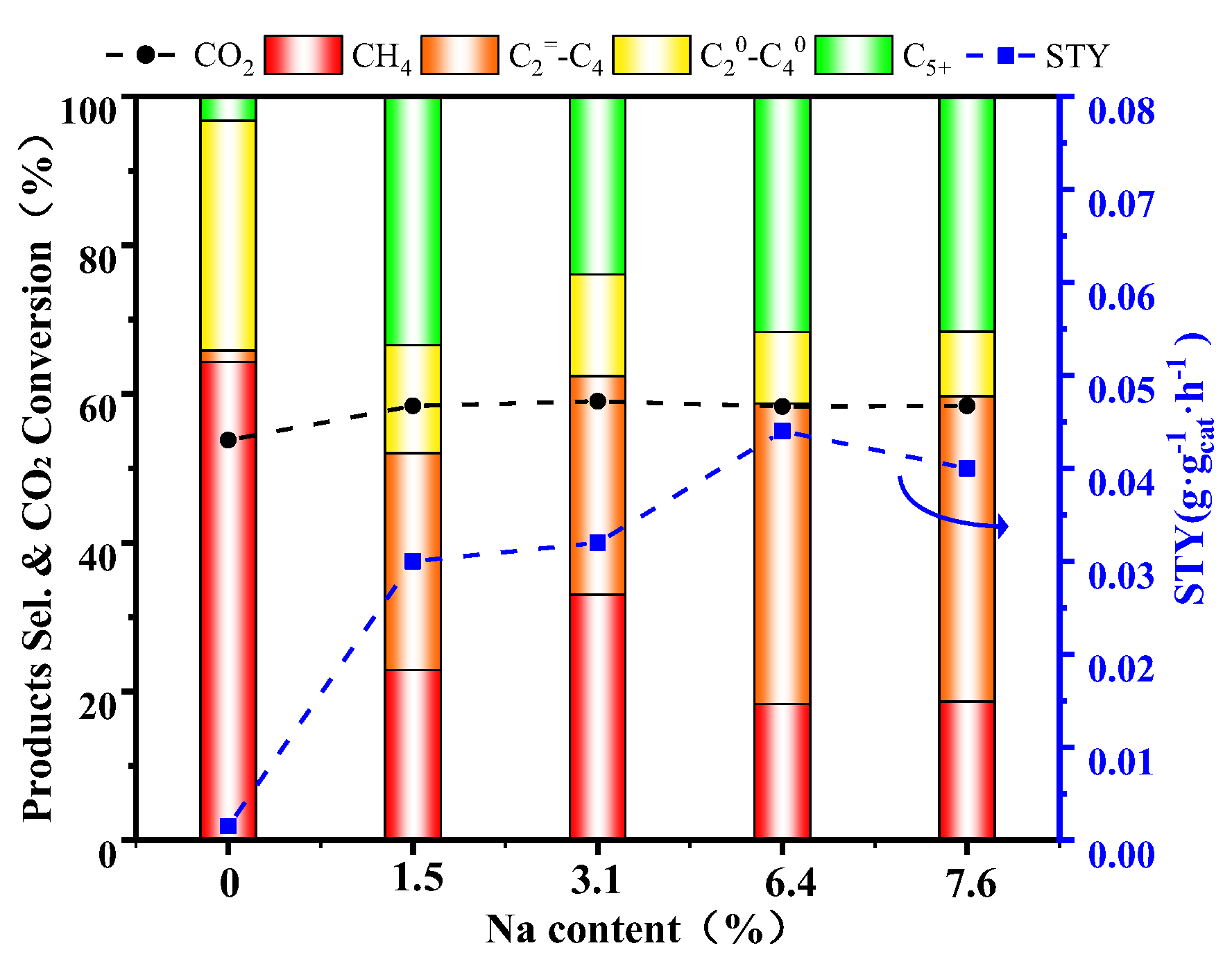
2.2. Catalytic Performances for CO2 Hydrogenation
2.3. Effect of Different Na Doping on Final Catalytic Performance
3. Experimental
3.1. Catalyst Preparation
3.2. Nanocomposite Characterization
3.3. Catalytic Reaction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peter, S.C. Reduction of CO2 to Chemicals and Fuels: A Solution to Global Warming and Energy Crisis. ACS Energy Lett. 2018, 3, 1557–1561. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, W.; Li, W. Recent advances in the catalytic conversion of CO2 to chemicals and demonstration projects in China. Mol. Catal. 2023, 541, 113093. [Google Scholar] [CrossRef]
- Gao, D.; Li, W.; Wang, H.; Wang, G.; Cai, R. Heterogeneous Catalysis for CO2 Conversion into Chemicals and Fuels. Trans. Tianjin Univ. 2022, 28, 245–264. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, L.; Li, S.; Zhou, Z.; Sun, Y. Novel Heterogeneous Catalysts for CO2 Hydrogenation to Liquid Fuels. ACS Cent. Sci. 2020, 6, 1657–1670. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Guo, S.; Qin, Z.; Dong, M.; Wang, J.; Fan, W. Effective conversion of CO2 into light olefins along with generation of low amounts of CO. J. Catal. 2022, 413, 923–933. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Ma, G.; Lin, J.; Wang, H.; Zhang, C.; Ding, M. Directly Converting Syngas to Linear alpha-Olefins over Core-Shell Fe3O4@MnO2 Catalysts. ACS Appl. Mater. Interfaces 2018, 10, 43578–43587. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, S.; Malhi, H.S.; Gao, X.; Zhang, Z.; Tu, W. Hydrogenation of CO2 to Olefins over Iron-Based Catalysts: A Review. Catalysts 2022, 12, 1432. [Google Scholar] [CrossRef]
- Liu, G.; Liu, P.; Meng, D.; Zhao, T.; Qian, X.; He, Q.; Guo, X.; Qi, J.; Peng, L.; Xue, N.; et al. CO(x) hydrogenation to methanol and other hydrocarbons under mild conditions with Mo3S4@ZSM-5. Nat. Commun. 2023, 14, 513. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, C.; Jun, K.-W.; Kim, S.K.; Park, H.-G.; Zhao, T.; Wang, L.; Wan, H.; Guan, G. Transformation of CO2 into liquid fuels and synthetic natural gas using green hydrogen: A comparative analysis. Fuel 2021, 291, 120111. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Quaranta, E. State of the art and perspectives in catalytic processes for CO2 conversion into chemicals and fuels: The distinctive contribution of chemical catalysis and biotechnology. J. Catal. 2016, 343, 2–45. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, G.; Tang, X.; Yin, H.; Kang, J.; Zhang, Q.; Wang, Y. Zn and Na promoted Fe catalysts for sustainable production of high-valued olefins by CO2 hydrogenation. Fuel 2022, 309, 122105. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Yin, H.; Hu, J.; Cheng, K.; Kang, J.; Zhang, Q.; Wang, Y. Tandem Catalysis for Hydrogenation of CO and CO2 to Lower Olefins with Bifunctional Catalysts Composed of Spinel Oxide and SAPO-34. ACS Catal. 2020, 10, 8303–8314. [Google Scholar] [CrossRef]
- Shekari, A.; Labrecque, R.; Larocque, G.; Vienneau, M.; Simoneau, M.; Schulz, R. Conversion of CO2 by reverse water gas shift (RWGS) reaction using a hydrogen oxyflame. Fuel 2023, 344, 127947. [Google Scholar] [CrossRef]
- Ma, Z.; Porosoff, M.D. Development of Tandem Catalysts for CO2 Hydrogenation to Olefins. ACS Catal. 2019, 9, 2639–2656. [Google Scholar] [CrossRef]
- Zhu, J.; Mu, M.; Liu, Y.; Zhang, M.; Zhang, G.; Cheng, Z.; Yin, B.H.; Yip, A.C.K.; Song, C.; Guo, X. Unveiling the promoting effect of potassium on the structural evolution of iron catalysts during CO2 hydrogenation. Chem. Eng. Sci. 2023, 282, 119228. [Google Scholar] [CrossRef]
- Zhai, P.; Xu, C.; Gao, R.; Liu, X.; Li, M.; Li, W.; Fu, X.; Jia, C.; Xie, J.; Zhao, M.; et al. Highly Tunable Selectivity for Syngas-Derived Alkenes over Zinc and Sodium-Modulated Fe5C2 Catalyst. Angew. Chem. Int. Ed. Engl. 2016, 55, 9902–9907. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Jia, L.; Sun, C.; Chen, B.; Liu, R.; Tan, Y.; Tu, W. Effects of the reducing gas atmosphere on performance of FeCeNa catalyst for the hydrogenation of CO2 to olefins. Chem. Eng. J. 2022, 428, 131388. [Google Scholar] [CrossRef]
- Xu, Y.; Zhai, P.; Deng, Y.; Xie, J.; Liu, X.; Wang, S.; Ma, D. Highly Selective Olefin Production from CO2 Hydrogenation on Iron Catalysts: A Subtle Synergy between Manganese and Sodium Additives. Angew. Chem. Int. Ed. Engl. 2020, 59, 21736–21744. [Google Scholar] [CrossRef]
- Wu, H.C.; Chen, T.C.; Wu, J.H.; Pao, C.W.; Chen, C.S. Influence of sodium-modified Ni/SiO2 catalysts on the tunable selectivity of CO2 hydrogenation: Effect of the CH4 selectivity, reaction pathway and mechanism on the catalytic reaction. J. Colloid Interface Sci. 2021, 586, 514–527. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Ma, H.; Qian, W.; Sun, Q.; Ying, W. Effect of alkalis (Li, Na, and K) on precipitated iron-based catalysts for high-temperature Fischer-Tropsch synthesis. Fuel 2022, 326, 125090. [Google Scholar] [CrossRef]
- Zeng, F.; Mebrahtu, C.; Xi, X.; Liao, L.; Ren, J.; Xie, J.; Heeres, H.J.; Palkovits, R. Catalysts design for higher alcohols synthesis by CO2 hydrogenation: Trends and future perspectives. Appl. Catal. B Environ. 2021, 291, 120073. [Google Scholar] [CrossRef]
- Kangvansura, P.; Chew, L.M.; Saengsui, W.; Santawaja, P.; Poo-arporn, Y.; Muhler, M.; Schulz, H.; Worayingyong, A. Product distribution of CO2 hydrogenation by K- and Mn-promoted Fe catalysts supported on N -functionalized carbon nanotubes. Catal. Today 2016, 275, 59–65. [Google Scholar] [CrossRef]
- Yang, H.; Dang, Y.; Cui, X.; Bu, X.; Li, J.; Li, S.; Sun, Y.; Gao, P. Selective synthesis of olefins via CO2 hydrogenation over transition-metal-doped iron-based catalysts. Appl. Catal. B Environ. 2023, 321, 122050. [Google Scholar] [CrossRef]
- Shadravan, V.; Kennedy, E.; Stockenhuber, M. An experimental investigation on the effects of adding a transition metal to Ni/Al2O3 for catalytic hydrogenation of CO and CO2 in presence of light alkanes and alkenes. Catal. Today 2018, 307, 277–285. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, J.; Xu, Y.; Ma, G.; Wang, J.; Ding, M. Transition Metals Modified Ni−M (M=Fe, Co, Cr and Mn) Catalysts Supported on Al2O3−ZrO2 for Low-Temperature CO2 Methanation. ChemCatChem 2020, 12, 3553–3559. [Google Scholar] [CrossRef]
- Liang, J.; Wang, X.-Y.; Gao, X.-H.; Tian, J.-M.; Duan, B.; Zhang, W.; Jiang, Y.-J.; Reubroycharoen, P.; Zhang, J.-L.; Zhao, T.-S. Effect of Na promoter and reducing atmosphere on phase evolution of Fe-based catalyst and its CO2 hydrogenation performance. J. Fuel Chem. Technol. 2022, 50, 1573–1580. [Google Scholar] [CrossRef]
- Wei, C.; Tu, W.; Jia, L.; Liu, Y.; Lian, H.; Wang, P.; Zhang, Z. The evolutions of carbon and iron species modified by Na and their tuning effect on the hydrogenation of CO2 to olefins. Appl. Surf. Sci. 2020, 525, 146622. [Google Scholar] [CrossRef]
- Xu, D.; Ding, M.; Hong, X.; Liu, G.; Tsang, S.C.E. Selective C2+ Alcohol Synthesis from Direct CO2 Hydrogenation over a Cs-Promoted Cu-Fe-Zn Catalyst. ACS Catal. 2020, 10, 5250–5260. [Google Scholar] [CrossRef]
- Ni, Z.; Qin, H.; Kang, S.; Bai, J.; Wang, Z.; Li, Y.; Zheng, Z.; Li, X. Effect of graphitic carbon modification on the catalytic performance of Fe@SiO2-GC catalysts for forming lower olefins via Fischer-Tropsch synthesis. J. Colloid Interface Sci. 2018, 516, 16–22. [Google Scholar] [CrossRef]
- Ni, Z.; Zhang, X.; Bai, J.; Wang, Z.; Li, X.; Zhang, Y. Potassium promoted core–shell-structured FeK@SiO2-GC catalysts used for Fischer–Tropsch synthesis to olefins without further reduction. New J. Chem. 2020, 44, 87–94. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Jinda, A.; Ge, H.; Hamahara, K.; Yamashita, H. CO2 Hydrogenation to Methanol over a Pt-Loaded Molybdenum Suboxide Nanosheet with Abundant Surface Oxygen Vacancies. J. Phys. Chem. C 2023, 127, 4942–4952. [Google Scholar] [CrossRef]
- Chen, B.; Dong, M.; Liu, S.; Xie, Z.; Yang, J.; Li, S.; Wang, Y.; Du, J.; Liu, H.; Han, B. CO2 Hydrogenation to Formate Catalyzed by Ru Coordinated with a N,P-Containing Polymer. ACS Catal. 2020, 10, 8557–8566. [Google Scholar] [CrossRef]
- Nasiri, S.; Rabiei, M.; Palevicius, A.; Janusas, G.; Vilkauskas, A.; Nutalapati, V.; Monshi, A. Modified Scherrer equation to calculate crystal size by XRD with high accuracy, examples Fe2O3, TiO2 and V2O5. Nano Trends 2023, 3, 100015. [Google Scholar] [CrossRef]
- Xiong, H.; Motchelaho, M.A.; Moyo, M.; Jewell, L.L.; Coville, N.J. Effect of Group I alkali metal promoters on Fe/CNT catalysts in Fischer–Tropsch synthesis. Fuel 2015, 150, 687–696. [Google Scholar] [CrossRef]
- Liang, B.; Duan, H.; Sun, T.; Ma, J.; Liu, X.; Xu, J.; Su, X.; Huang, Y.; Zhang, T. Effect of Na Promoter on Fe-Based Catalyst for CO2 Hydrogenation to Alkenes. ACS Sustain. Chem. Eng. 2018, 7, 925–932. [Google Scholar] [CrossRef]
- Cui, X.; Gao, P.; Li, S.; Yang, C.; Liu, Z.; Wang, H.; Zhong, L.; Sun, Y. Selective Production of Aromatics Directly from Carbon Dioxide Hydrogenation. ACS Catal. 2019, 9, 3866–3876. [Google Scholar] [CrossRef]
- Barrios, A.J.; Peron, D.V.; Chakkingal, A.; Dugulan, A.I.; Moldovan, S.; Nakouri, K.; Thuriot-Roukos, J.; Wojcieszak, R.; Thybaut, J.W.; Virginie, M.; et al. Efficient Promoters and Reaction Paths in the CO2 Hydrogenation to Light Olefins over Zirconia-Supported Iron Catalysts. ACS Catal. 2022, 12, 3211–3225. [Google Scholar] [CrossRef]

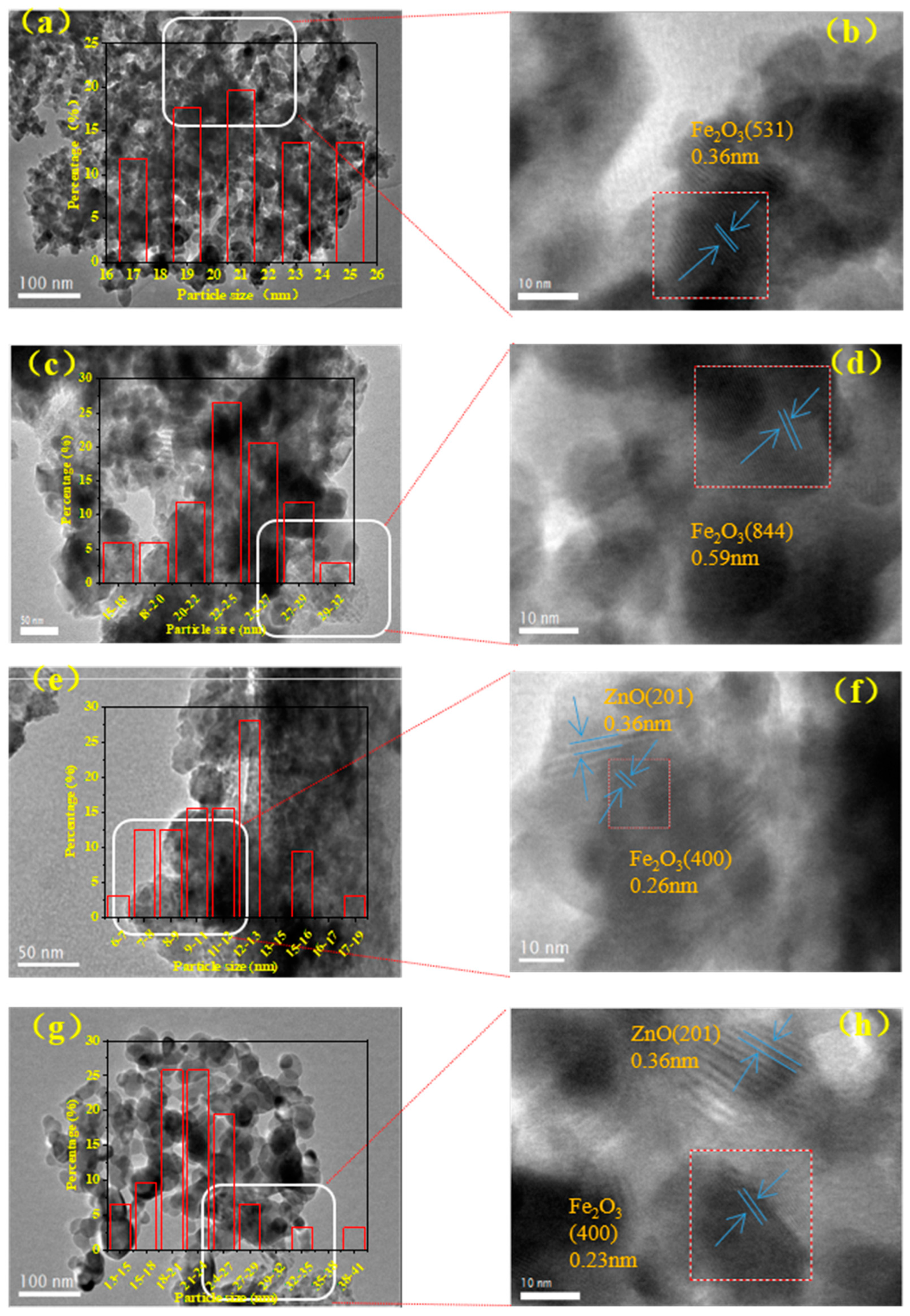
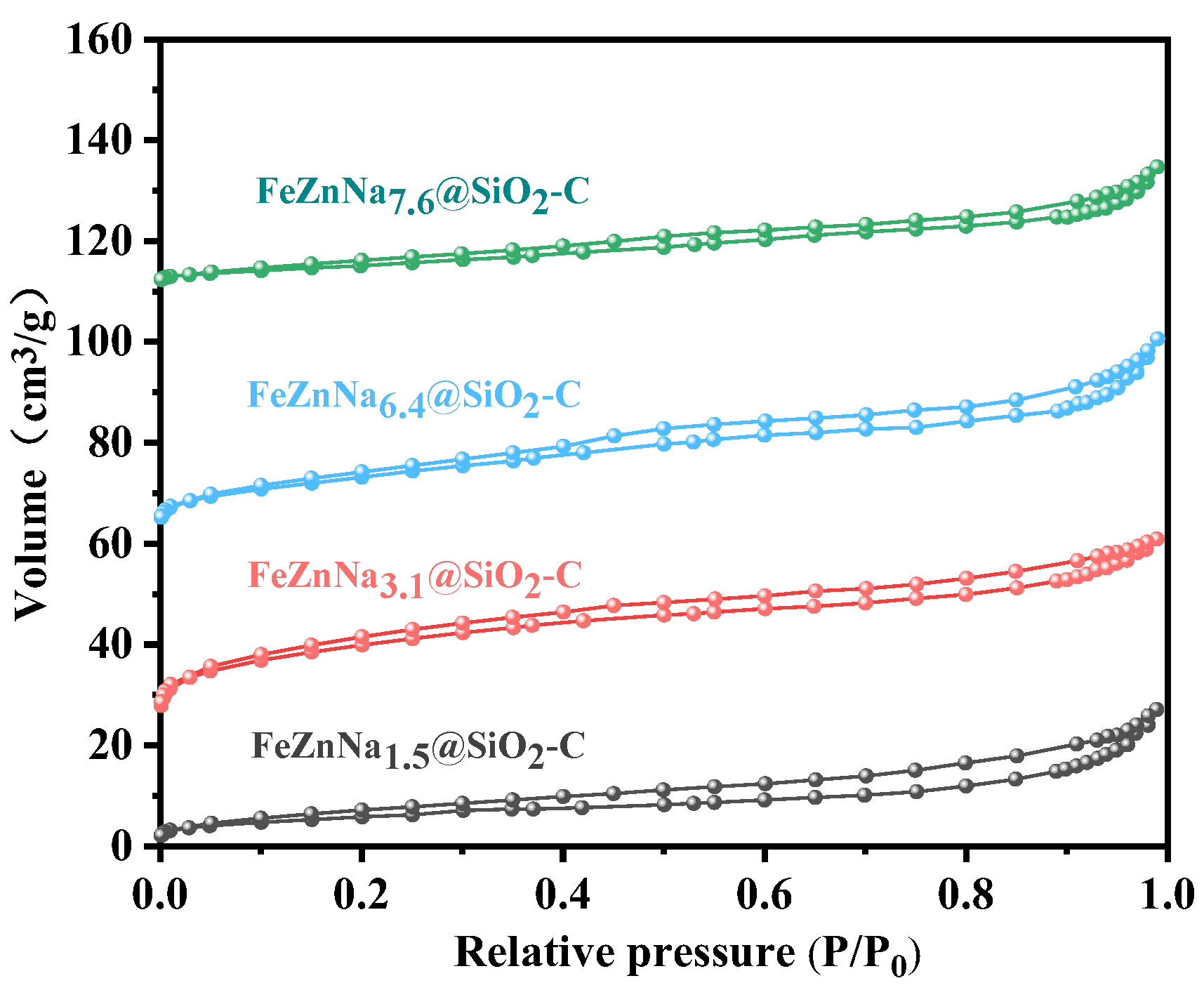



| Catalyst | Fe Loading (wt%) a | Na Loading (wt%) a | Total CO Chemisorbed (μmol/g) b | % Fe Dispersion b | Fe2O3 Size (nm) c | SSA (m2/g) d | PV (cm3/g) d | APS (nm) d |
|---|---|---|---|---|---|---|---|---|
| Fe@SiO2-C | 25.4 | 0 | 472 | 5.2 | 23 | 75.5 | 0.087 | 3.19 |
| FeNa@SiO2-C | 24.2 | 1.5 | 570 | 6.6 | 33 | 78.4 | 0.111 | 4.18 |
| FeZn@SiO2-C | 25.3 | 0 | 551 | 6.1 | 15 | 48.5 | 0.144 | 4.19 |
| FeZnNa1.5@SiO2-C | 23.7 | 1.5 | 576 | 6.8 | 21 | 9.2 | 0.035 | 4.46 |
| FeZnNa3.1@SiO2-C | 23.5 | 3.1 | 596 | 7.1 | 23 | 16.4 | 0.035 | 2.95 |
| FeZnNa6.4@SiO2-C | 22.1 | 6.4 | 576 | 7.3 | 16 | 20.5 | 0.047 | 2.95 |
| FeZnNa7.6@SiO2-C | 23.4 | 7.6 | 568 | 6.8 | 18 | 14.6 | 0.035 | 2.95 |
| Catalysts | The Amount of CO2 Desorbed (μmol/g) | |||
|---|---|---|---|---|
| α Peak | β Peak | γ Peak | Total | |
| FeZnNa1.5@SiO2-C | 58.5 | 60.4 | 396.4 | 515.3 |
| FeZnNa3.1@SiO2-C | 36.6 | 91.3 | 446.0 | 573.9 |
| FeZnNa6.4@SiO2-C | 51.4 | 128.6 | 576.7 | 756.6 |
| FeZnNa7.6@SiO2-C | 295.4 | 43.1 | 621.7 | 960.2 |
| Catalyst | CO2 Con (%) | Selectivity a (%) | O/P b | TOF c (10−3 s−1) | STY (g/gcat·h) | |||
|---|---|---|---|---|---|---|---|---|
| CH4 | ||||||||
| Fe@SiO2-C | 52.17 | 83.06 | 5.93 | 5.59 | 5.45 | 1.06 | 0.56 | 0.0057 |
| FeZn@SiO2-C | 53.78 | 64.32 | 1.54 | 30.87 | 3.29 | 0.05 | 0.12 | 0.0015 |
| FeNa@SiO2-C | 52.26 | 66.56 | 6.48 | 20.23 | 8.33 | 0.32 | 0.53 | 0.0090 |
| FeZnNa1.5@SiO2-C | 58.41 | 22.89 | 29.15 | 14.50 | 33.42 | 2.01 | 2.53 | 0.0315 |
| FeZnNa3.1@SiO2-C | 59.03 | 33.00 | 29.40 | 13.67 | 23.83 | 2.15 | 2.49 | 0.0321 |
| FeZnNa6.4@SiO2-C | 58.32 | 18.30 | 40.40 | 9.60 | 31.69 | 4.21 | 3.50 | 0.0436 |
| FeZnNa7.6@SiO2-C | 58.4 | 18.63 | 41.07 | 8.68 | 31.72 | 4.73 | 3.61 | 0.0400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, Z.; Cai, M.; Zhong, S.; Chen, X.; Shen, H.; Su, L. Sodium Promoted FeZn@SiO2-C Catalysts for Sustainable Production of Low Olefins by CO2 Hydrogenation. Catalysts 2023, 13, 1508. https://doi.org/10.3390/catal13121508
Ni Z, Cai M, Zhong S, Chen X, Shen H, Su L. Sodium Promoted FeZn@SiO2-C Catalysts for Sustainable Production of Low Olefins by CO2 Hydrogenation. Catalysts. 2023; 13(12):1508. https://doi.org/10.3390/catal13121508
Chicago/Turabian StyleNi, Zhijiang, Mingxing Cai, Shiyu Zhong, Xiaoyu Chen, Hanyu Shen, and Lin Su. 2023. "Sodium Promoted FeZn@SiO2-C Catalysts for Sustainable Production of Low Olefins by CO2 Hydrogenation" Catalysts 13, no. 12: 1508. https://doi.org/10.3390/catal13121508
APA StyleNi, Z., Cai, M., Zhong, S., Chen, X., Shen, H., & Su, L. (2023). Sodium Promoted FeZn@SiO2-C Catalysts for Sustainable Production of Low Olefins by CO2 Hydrogenation. Catalysts, 13(12), 1508. https://doi.org/10.3390/catal13121508






