Green Synthesis of Mixed ZnO-SnO2 Nanoparticles for Solar-Assisted Degradation of Synthetic Dyes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Nanophotocatalysts
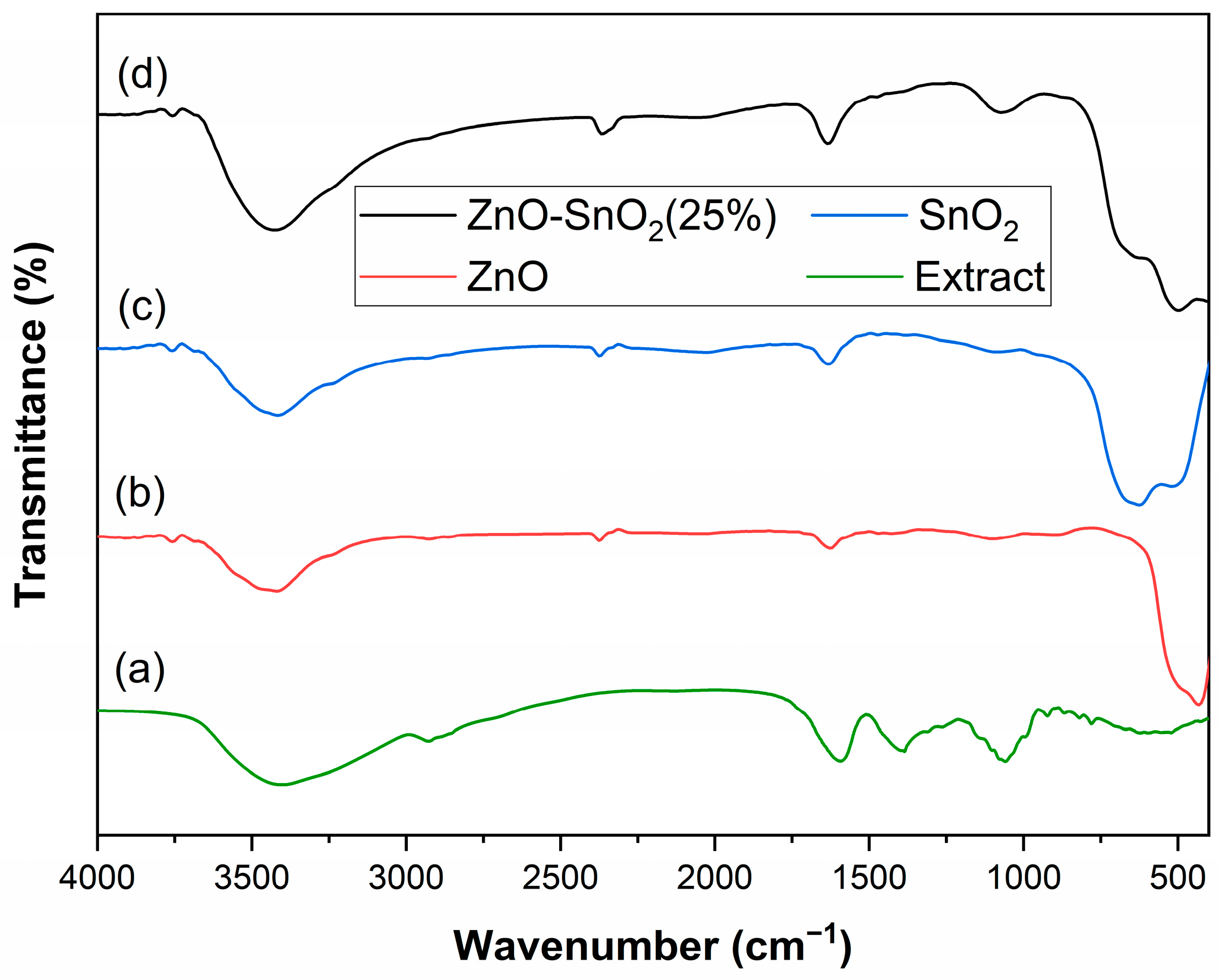
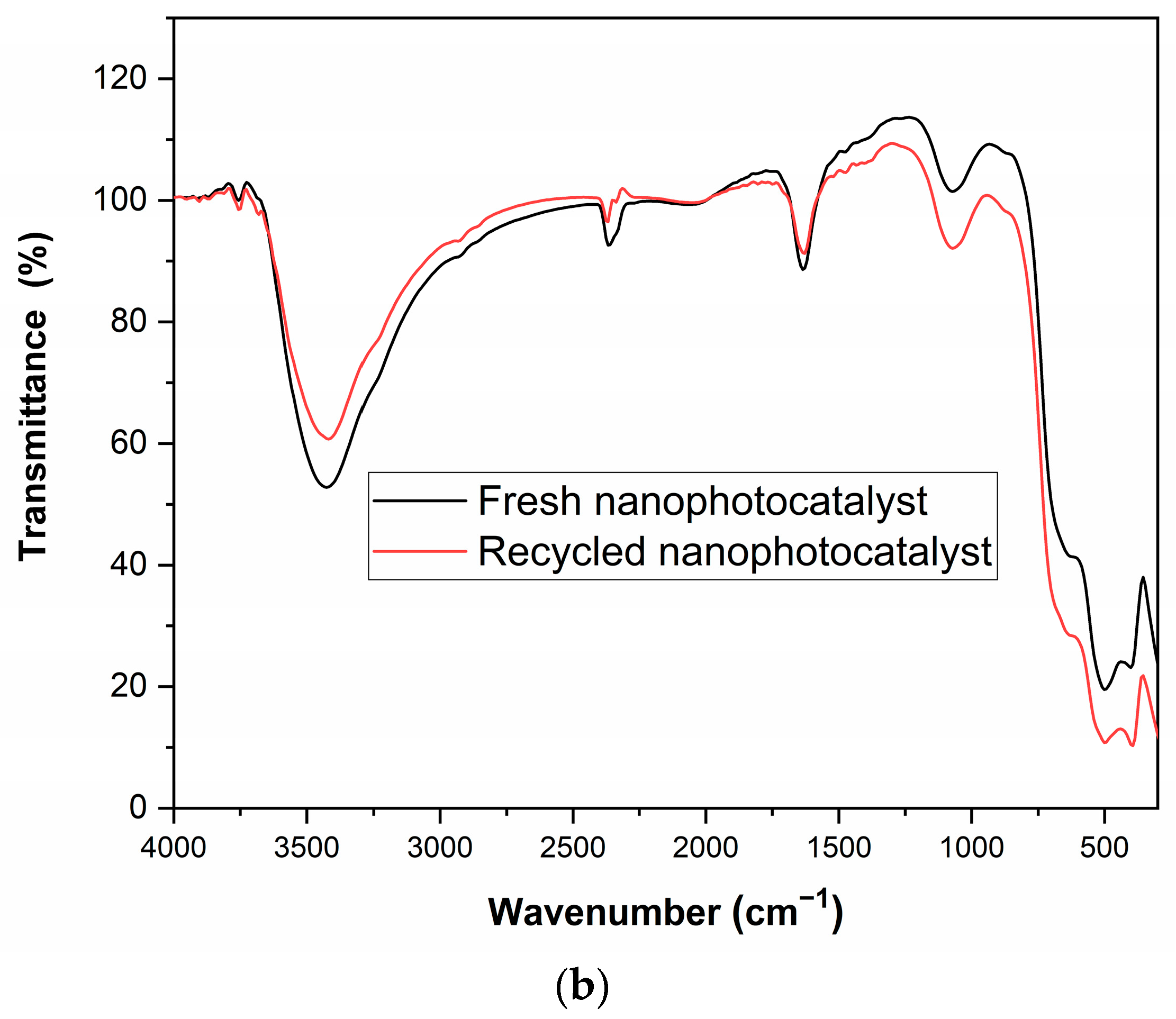
2.1.1. FTIR Analysis
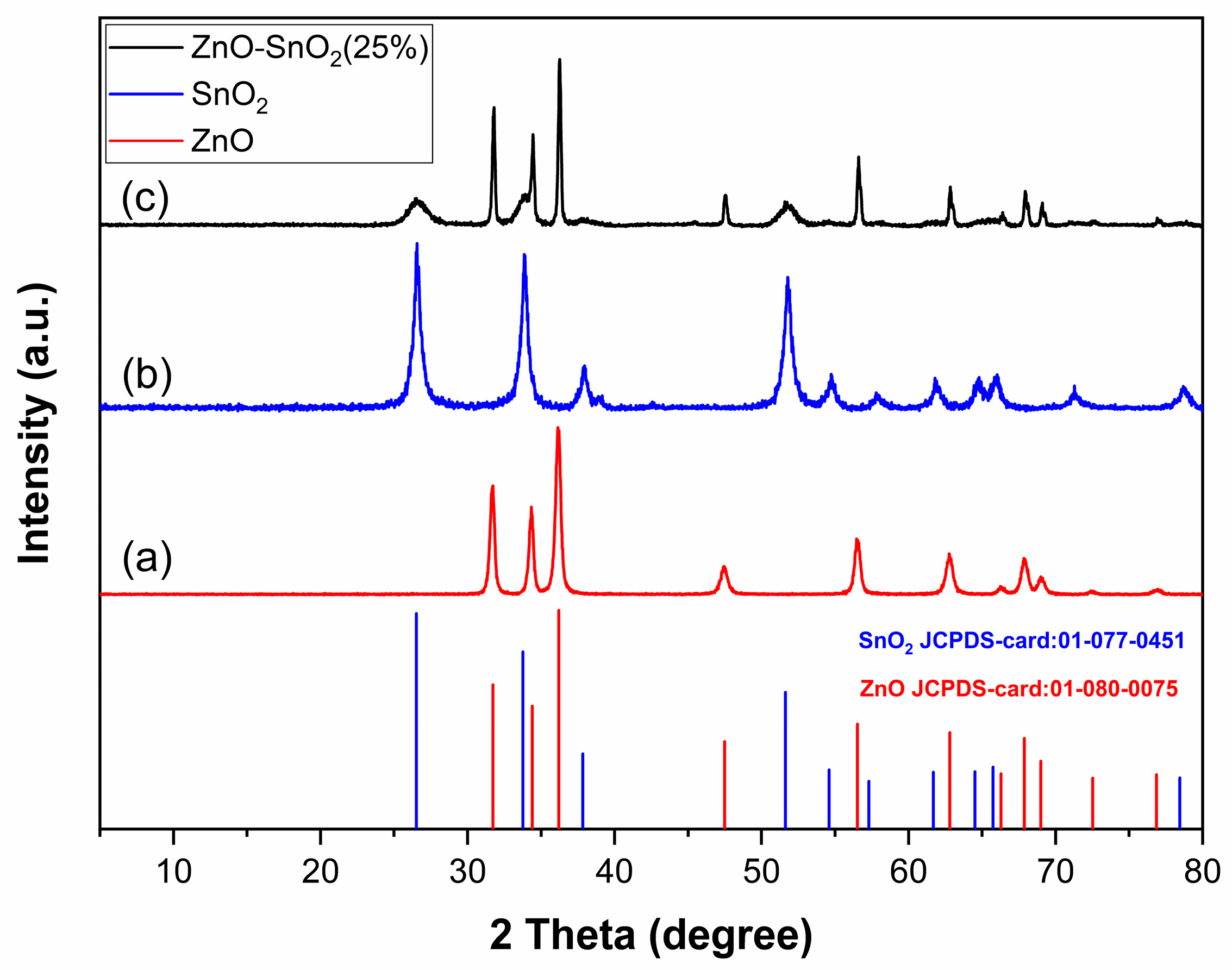
2.1.2. XRD Analysis
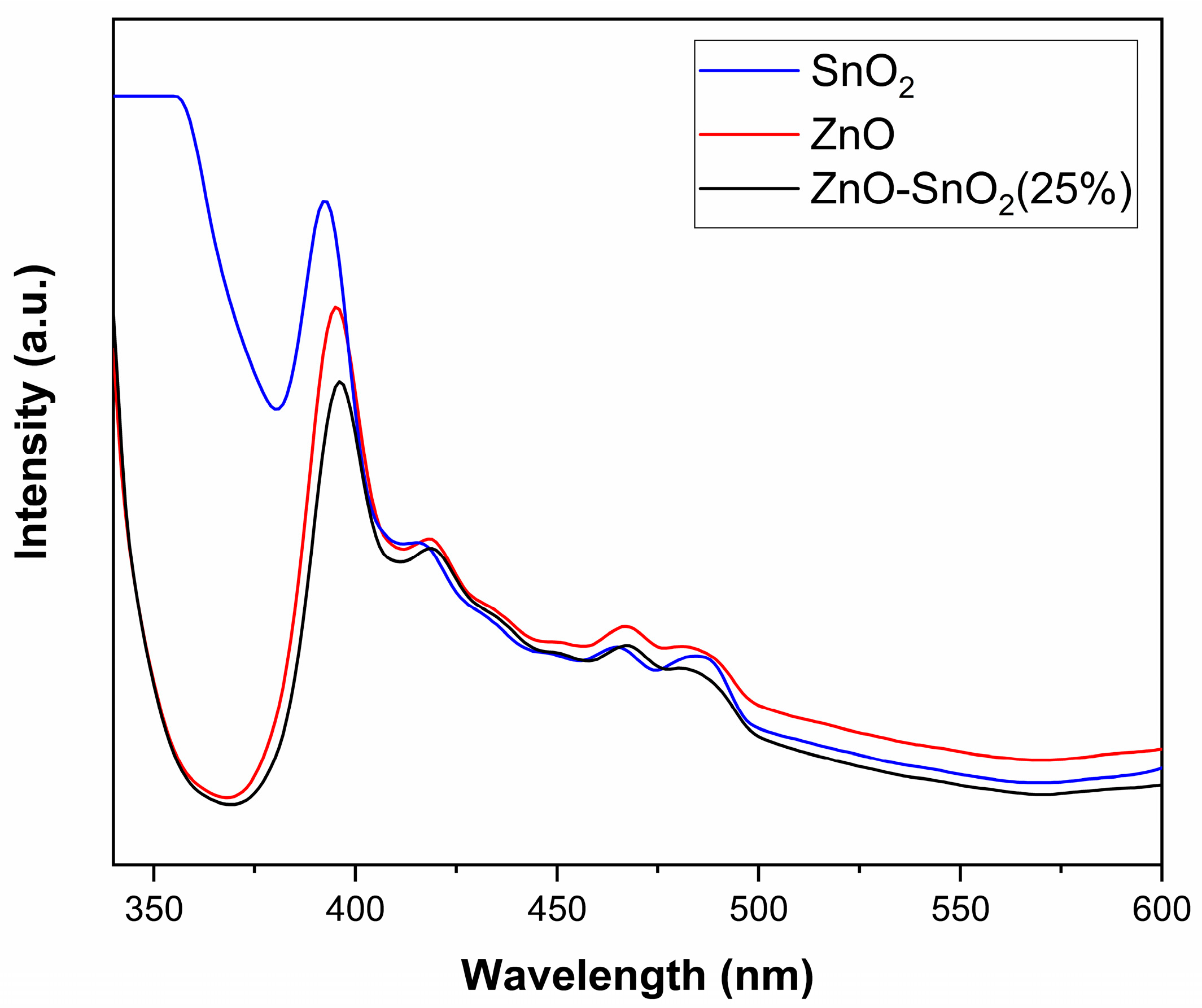
2.1.3. Optical Analysis—Band Gap Determination
2.1.4. Photoluminescence (PL) Measurements
2.1.5. Morphology Analysis
2.1.6. BET/BJH Analyses
2.1.7. EDS Analysis
2.1.8. TGA Analysis
2.2. Photocatalytic Activities
2.2.1. Comparative Analysis
2.2.2. Effect of Irradiation Time
2.2.3. Mechanism of Photocatalytic Degradation
- (i)
- The electron transfer from ZnO to SnO2 and the hole traders from SnO2 to ZnO until a stable equilibrium state is achieved.
- (ii)
- The hole at VB of ZnO2 is trapped by physically adsorbed water molecules or hydroxyl groups (OH−) at the ZnO surface, and hydroxyl radicals () are generated, whereas the electron at the CB band of SnO2 is captured by dissolved O2 molecules, and superoxide ions () are produced.
- (iii)
- The reactive hydroxyl radicals () and superoxide ions () degrade organic contaminants (dyes) in the aqueous media due to their potent oxidizing and reducing ability.
2.2.4. Photocatalytic Kinetics
2.2.5. Reusability of ZnO-SnO2(25%) Nanophotocatalyst
3. Materials and Methods
3.1. Materials
3.2. Preparation of Aqueous Banana Peel Extract (ABPE)
3.3. Synthesis of Nanophotocatalysts
3.3.1. Synthesis of Zinc Oxide Nanoparticles (ZnO NPs)
3.3.2. Synthesis of Tin Oxide Nanoparticles (SnO2 NPs)
3.3.3. Synthesis of Mixed ZnO-SnO2(25%) Nanoparticles
3.4. Photocatalysis
3.5. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saxena, S.; Asm, R. Natural Dyes: Sources, Chemistry, Application and Sustainability Issues. In Roadmap to Sustainable Textiles and Clothing: Eco-Friendly Raw Materials, Technologies, and Processing Methods; Springer: Singapore, 2014; pp. 37–80. [Google Scholar]
- Vivek, M.; Joshi, K.; Sharma, K.; Dave, A. A Brief Review on: Extraction of Natural Dyes from Barks of Mangrove and Walnut Tree and their Applications. Int. J. Sci. Res. (IJSR) 2020, 9, 669–673. [Google Scholar] [CrossRef]
- Jamee, R.; Siddique, R. Biodegradation of Synthetic Dyes of Textile Effluent by Microorganisms: An Environmentally and Economically Sustainable Approach. Eur. J. Microbiol. Immunol. 2019, 9, 114–118. [Google Scholar] [CrossRef]
- Pandey, A.; Sarada, D.; Kumar, A. Microbial Decolorization and Degradation of Reactive Red 198 Azo Dye by a Newly Isolated Alkaligenes Species. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 86, 805–815. [Google Scholar] [CrossRef]
- Ben Slama, H.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golinska, P.; Lassaad, B. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Nazri, M.K.H.M.; Sapawe, N. A short review on photocatalytic toward dye degradation. Mater. Today Proc. 2020, 31, A42–A47. [Google Scholar] [CrossRef]
- Yaseen, D.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2018, 16, 1193–1226. [Google Scholar] [CrossRef]
- Khan, I.; Sadiq, M.; Khan, I.; Saeed, K. Manganese dioxide nanoparticles/activated carbon composite as efficient UV and visible-light photocatalyst. Env. Sci. Pollut. Res. Int. 2019, 26, 5140–5154. [Google Scholar] [CrossRef] [PubMed]
- Hsuan, C.; Chang, T.-F.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Naseem, K.; Farooqi, Z.H.; Begum, R.; Wu, W.; Irfan, A.; Al-Sehemi, A.G. Silver Nanoparticles Engineered Polystyrene-Poly(N-isopropylmethacrylamide-acrylic acid) Core Shell Hybrid Polymer Microgels for Catalytic Reduction of Congo Red. Macromol. Chem. Phys. 2018, 219, 1800211. [Google Scholar] [CrossRef]
- Bal, G.; Thakur, A. Distinct approaches of removal of dyes from wastewater: A review. Mater. Today Proc. 2022, 50, 1575–1579. [Google Scholar] [CrossRef]
- Alsukaibi, A. Various Approaches for the Detoxification of Toxic Dyes in Wastewater. Processes 2022, 10, 1968. [Google Scholar] [CrossRef]
- Damiri, F.; Andra, S.; Kommineni, N.; Balu, S.K.; Bulusu, R.; Boseila, A.; Akamo, D.; Ahmad, Z.; Khan, F.; Rahman, M.; et al. Recent Advances in Adsorptive Nanocomposite Membranes for Heavy Metals Ion Removal from Contaminated Water: A Comprehensive Review. Materials 2022, 15, 5392. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Hariani, P.; Said, M.; Salni, S.; Aprianti, N.; Naibaho, Y. High Efficient Photocatalytic Degradation of Methyl Orange Dye in an Aqueous Solution by CoFe2O4-SiO2-TiO2 Magnetic Catalyst. J. Ecol. Eng. 2022, 23, 118–128. [Google Scholar] [CrossRef]
- Iqbal, A.; Yusaf, A.; Usman, M.; Bokhari, T.; Mansha, A. Insight into the degradation of different classes of dyes by advanced oxidation processes: A detailed review. Int. J. Environ. Anal. Chem. 2023, 1–35. [Google Scholar] [CrossRef]
- Lee, D.-E.; Kim, M.-K.; Danish, M.; Jo, W. State-of-the-art review on photocatalysis for efficient wastewater treatment: Attractive approach in photocatalyst design and parameters affecting the photocatalytic degradation. Catal. Commun. 2023, 183, 106764. [Google Scholar] [CrossRef]
- Jayasankari, S.; Pramothkumar, A.; Mani, P. Facile synthesis and characterization of ZnO NPs, ZnO/CdO and ZnO/SnO2 nanocomposites for photocatalytic degradation of Eosin Yellow and Direct Blue 15 under UV light irradiation. J. Mater. Sci. Mater. Electron. 2022, 33, 9858–9874. [Google Scholar] [CrossRef]
- Zarei, S.; Hasheminiasari, M.; Masoudpanah, S.M.; Javadpour, J. Photocatalytic properties of ZnO/SnO2 nanocomposite films: Role of morphology. J. Mater. Res. Technol. 2022, 17, 2305–2312. [Google Scholar] [CrossRef]
- Groeneveld, I.; Kanelli, M.; Ariese, F.; Bommel, M.R. Parameters that affect the photodegradation of dyes and pigments in solution and on substrate—An overview. Dye. Pigment. 2022, 210, 110999. [Google Scholar] [CrossRef]
- Shafiq, A.; Chen, S.-Q.; Nazar, M. A Comprehensive Review on Adsorption, Photocatalytic and Chemical Degradation of Dyes and Nitro-Compounds over Different Kinds of Porous and Composite Materials. Molecules 2023, 28, 1081. [Google Scholar] [CrossRef]
- Bekele, M. Photocatalytic degradation of organic pollutants in the presence of selected transition metal nanoparticles: Review. J. Plant Sci. Phytopathol. 2022, 6, 115–125. [Google Scholar] [CrossRef]
- Navidpour, A.H.; Abbasi, S.; Li, D.; Mojiri, A.; Zhou, J.L. Investigation of advanced oxidation process in the presence of TiO2 semiconductor as photocatalyst: Property, principle, kinetic analysis, and photocatalytic activity. Catalysts 2023, 13, 232. [Google Scholar] [CrossRef]
- Munawar, T.; Yasmeen, S.; Hussain, F.; Mahmood, K.; Hussain, A.; Asghar, M.; Iqbal, F. Synthesis of novel heterostructured ZnO-CdO-CuO nanocomposite: Characterization and enhanced sunlight driven photocatalytic activity. Mater. Chem. Phys. 2020, 249, 122983. [Google Scholar] [CrossRef]
- Khan, S.; Noor, A.; Khan, I.; Muhammad, M.; Sadiq, M.; Muhammad, N. Photocatalytic Degradation of Organic Dyes Contaminated Aqueous Solution Using Binary CdTiO2 and Ternary NiCdTiO2 Nanocomposites. Catalysts 2022, 13, 44. [Google Scholar] [CrossRef]
- Ali, A. Review on the Synthesis Method of Nanocomposites and Approach to Making Semiconductors Visible Light Active. 2022. Available online: https://www.longdom.org/open-access/review-on-the-synthesis-method-of-nano-composites-and-approach-to-making-semiconductors-visible-light-active-96837.html#ai (accessed on 1 October 2023).
- Ibrahim, H.M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef]
- Mandavgane, S.; Pathak, P.; Kulkarni, B. Fruit Peel Waste: Characterization and its Potential Uses. Curr. Sci. 2017, 113, 444–454. [Google Scholar] [CrossRef]
- Feumba, D.R. Chemical composition of some selected fruit peels. Eur. J. Food Sci. Technol. 2016, 4, 12–21. [Google Scholar]
- Zaini, H.; Roslan, J.; Saallah, S.; Munsu, E.; Sulaiman, N.; Pindi, W. Banana peels as a bioactive ingredient and its potential application in the food industry. J. Funct. Foods 2022, 92, 105054. [Google Scholar] [CrossRef]
- Kapadia, S.P.; Pudakalkatti, P.S.; Shivanaikar, S. Detection of antimicrobial activity of banana peel (Musa paradisiaca L.) on Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans: An in vitro study. Contemp. Clin. Dent. 2015, 6, 496–499. [Google Scholar] [CrossRef]
- Aburto, J.; Moran, M.; Galano, A.; Torres-García, E. Non-isothermal pyrolysis of pectin: A thermochemical and kinetic approach. J. Anal. Appl. Pyrolysis 2015, 112, 94–104. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.G.; Sun, H.J.; Wei, Z.J. Pectin from Abelmoschus esculentus: Optimization of extraction and rheological properties. Int. J. Biol. Macromol. 2014, 70, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Dmochowska, A.; Czajkowska, J.; Jędrzejewski, R.; Stawiński, W.; Migdał, P.; Fiedot-Toboła, M. Pectin based banana peel extract as a stabilizing agent in zinc oxide nanoparticles synthesis. Int. J. Biol. Macromol. 2020, 165, 1581–1592. [Google Scholar] [CrossRef]
- Ferreira, N.S.; Sasaki, J.M.; Silva, R.S., Jr.; Attah-Baah, J.M.; Macêdo, M.A. Visible-Light-Responsive Photocatalytic Activity Significantly Enhanced by Active [V(Zn)+V(O)(+)] Defects in Self-Assembled ZnO Nanoparticles. Inorg. Chem. 2021, 60, 4475–4496. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Pal, U.; Serrano, J.; Ucer, K.B.; Williams, R.T. Photoluminescence and FTIR study of ZnO nanoparticles: The impurity and defect perspective. Phys. Status Solidi C 2006, 3, 3577–3581. [Google Scholar] [CrossRef]
- Wasly, D.-H.; Abd El-sadek, M.S.; Henini, M. Influence of reaction time and synthesis temperature on the physical properties of ZnO nanoparticles synthesized by the hydrothermal method. Appl. Phys. A 2018, 124, 76. [Google Scholar] [CrossRef]
- Luque, P.; Nava, O.; Soto-Robles, C.A.; Garrafa Galvez, H.; Martinez-Rosas, M.; Chinchillas-Chinchillas, M.J.; Vilchis-Nestor, A.; Castro-Beltrán, A. SnO2 nanoparticles synthesized with Citrus aurantifolia and their performance in photocatalysis. J. Mater. Sci. Mater. Electron. 2020, 31, 16859–16866. [Google Scholar] [CrossRef]
- Paramarta, V.; Taufik, A.; Saleh, R. Better adsorption capacity of SnO2 nanoparticles with different graphene addition. J. Phys. Conf. Ser. 2016, 776, 012039. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Surendhiran, S.; Kumar, P.; Kumar, E.; Khadar, Y.; Balamurugan, A. Green synthesis of SnO2 nanoparticles using Delonix elata leaf extract: Evaluation of its structural, optical, morphological and photocatalytic properties. SN Appl. Sci. 2020, 2, 1735. [Google Scholar] [CrossRef]
- Mahalakshmi, S.; Hema, N.; Vijaya, P.P. In Vitro Biocompatibility and Antimicrobial activities of Zinc Oxide Nanoparticles (ZnO NPs) Prepared by Chemical and Green Synthetic Route—A Comparative Study. BioNanoScience 2019, 10, 112–121. [Google Scholar] [CrossRef]
- Elango, G.; Roopan, S.M. Efficacy of SnO2 nanoparticles toward photocatalytic degradation of methylene blue dye. J. Photochem. Photobiol. B Biol. 2016, 155, 34–38. [Google Scholar] [CrossRef]
- Obaidullah, M.; Furusawa, T.; Siddiquey, I.; Bahadur, N.; Sato, M.; Suzuki, N. A fast and facile microwave irradiation method for the synthesis of ZnO@ZrO2 core-shell nanocomposites and the investigation of their optical properties. Adv. Powder Technol. 2018, 29, 1804–1811. [Google Scholar] [CrossRef]
- Ishwarya, R.; Vaseeharan, B.; Kalyani, S.; Banumathi, B.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Al-Anbr, M.N.; Khaled, J.M.; Benelli, G. Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J. Photochem. Photobiol. B Biol. 2018, 178, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Rad, S.S.; Sani, A.M.; Mohseni, S. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Mentha pulegium (L.). Microb. Pathog. 2019, 131, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef]
- Arakha, M.; Saleem, M.; Mallick, B.C.; Jha, S. The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci. Rep. 2015, 5, 9578. [Google Scholar] [CrossRef]
- Wongsaprom, K.; Winyayong, A.; Maensiri, S. Synthesis and room-temperature ferromagnetism in flower-like SnO2 nanostructures. J. Phys. Conf. Ser. 2018, 1144, 012042. [Google Scholar] [CrossRef]
- Lamdhade, G.; Raghuwanshi, F.C.; Agrawal, R.M.; Balkhande, V.M.; Shripathi, T. SnO2 Nanoparticles Synthesis Via Liquid-Phase Co-Precipitation Technique. Adv. Mater. Lett. 2015, 6, 738–742. [Google Scholar] [CrossRef]
- Chen, S.; Sun, Z.; Zhang, L.; Xie, H. Photodegradation of Gas Phase Benzene by SnO2 Nanoparticles by Direct Hole Oxidation Mechanism. Catalysts 2020, 10, 117. [Google Scholar] [CrossRef]
- Luque, P.A.; Nava, O.; Soto-Robles, C.A.; Chinchillas-Chinchillas, M.J.; Garrafa-Galvez, H.E.; Baez-Lopez, Y.A.; Valdez-Núñez, K.P.; Vilchis-Nestor, A.R.; Castro-Beltrán, A. Improved photocatalytic efficiency of SnO2 nanoparticles through green synthesis. Optik 2020, 206, 164299. [Google Scholar] [CrossRef]
- Petrov, V.; Sysoev, V.; Ivanishcheva, A.; Volkova, M.; Kalazhokov, Z.; Storozhenko, V.; Khubezhov, S.; Bayan, E. Synthesis, Characterization and Gas Sensing Study of ZnO-SnO2 Nanocomposite Thin Films. Chemosensors 2021, 9, 124. [Google Scholar] [CrossRef]
- Alfaro Cruz, M.R.; Saldaña-Ramírez, A.; Juárez-Ramírez, I.; Torres-Martínez, L.M. Development of SnO2–ZnO thin films as a photocatalyst for obtaining alternative fuels through photocatalytic reactions. Solid. State Sci. 2023, 137, 107112. [Google Scholar] [CrossRef]
- Karthika, K.; Ravichandran, K. Tuning the Microstructural and Magnetic Properties of ZnO Nanopowders through the Simultaneous Doping of Mn and Ni for Biomedical Applications. J. Mater. Sci. Technol. 2015, 31, 1111–1117. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.; Olivier, J.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Ikim, M.; Gromov, V.; Gerasimov, G.; Spiridonova, E.; Erofeeva, A.; Kurmangaleev, K.; Polunin, K.; Ilegbusi, O.; Trakhtenberg, L. Structure, Conductivity, and Sensor Properties of Nanosized ZnO-In2O3 Composites: Influence of Synthesis Method. Micromachines 2023, 14, 1685. [Google Scholar] [CrossRef] [PubMed]
- Kadam, A.N.; Bhopate, D.P.; Kondalkar, V.V.; Majhi, S.M.; Bathula, C.D.; Tran, A.-V.; Lee, S.-W. Facile synthesis of Ag-ZnO core–shell nanostructures with enhanced photocatalytic activity. J. Ind. Eng. Chem. 2018, 61, 78–86. [Google Scholar] [CrossRef]
- Peng, S.; Hong, P.; Li, Y.; Xing, X.; Yang, Y.; Wang, Z.; Zou, T.; Wang, Y. Pt decorated SnO2 nanoparticles for high response CO gas sensor under the low operating temperature. J. Mater. Sci. Mater. Electron. 2019, 30, 3921–3932. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z. Facile Synthesis of Quasi Spherical ZnO Nanoparticles with Excellent Photo catalytic Activity. J. Clust. Sci. 2014, 26, 1187–1201. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Talinungsang; Upadhaya, D.; Kumar, P.; Dhar Purkayastha, D. Superhydrophilicity of photocatalytic ZnO/SnO2 heterostructure for self-cleaning applications. J. Sol-Gel Sci. Technol. 2019, 92, 575–584. [Google Scholar] [CrossRef]
- Szczepanik, B.; Slomkiewicz, P.; Widel, D.; Czaplicka, M.; Frydel, L. Kinetics and Mechanism of Aniline and Chloroanilines Degradation Photocatalyzed by Halloysite-TiO2 and Halloysite-Fe2O3 Nanocomposites. Catalysts 2021, 11, 1548. [Google Scholar] [CrossRef]
- Hai, T.; Nguyen, D.; Do, P.; Tran, U. Kinetics of photocatalytic degradation of organic compounds: A mini-review and new approach. RSC Adv. 2023, 13, 16915–16925. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Iqbal Rokon, M.Z.; Rahim, M.A.; Hossain, M.I.; Islam, M.S.; Ali, M.R.; Bacchu, M.S.; Waizumi, H.; Komeda, T.; Hossain Khan, M.Z. Enhanced photocatalytic activity of Cu and Ni-doped ZnO nanostructures: A comparative study of methyl orange dye degradation in aqueous solution. Heliyon 2023, 9, e16506. [Google Scholar] [CrossRef]
- Khan, M.; Ware, P.; Shimpi, N. Synthesis of ZnO nanoparticles using peels of Passiflora foetida and study of its activity as an efficient catalyst for the degradation of hazardous organic dye. SN Appl. Sci. 2021, 3, 528. [Google Scholar] [CrossRef]

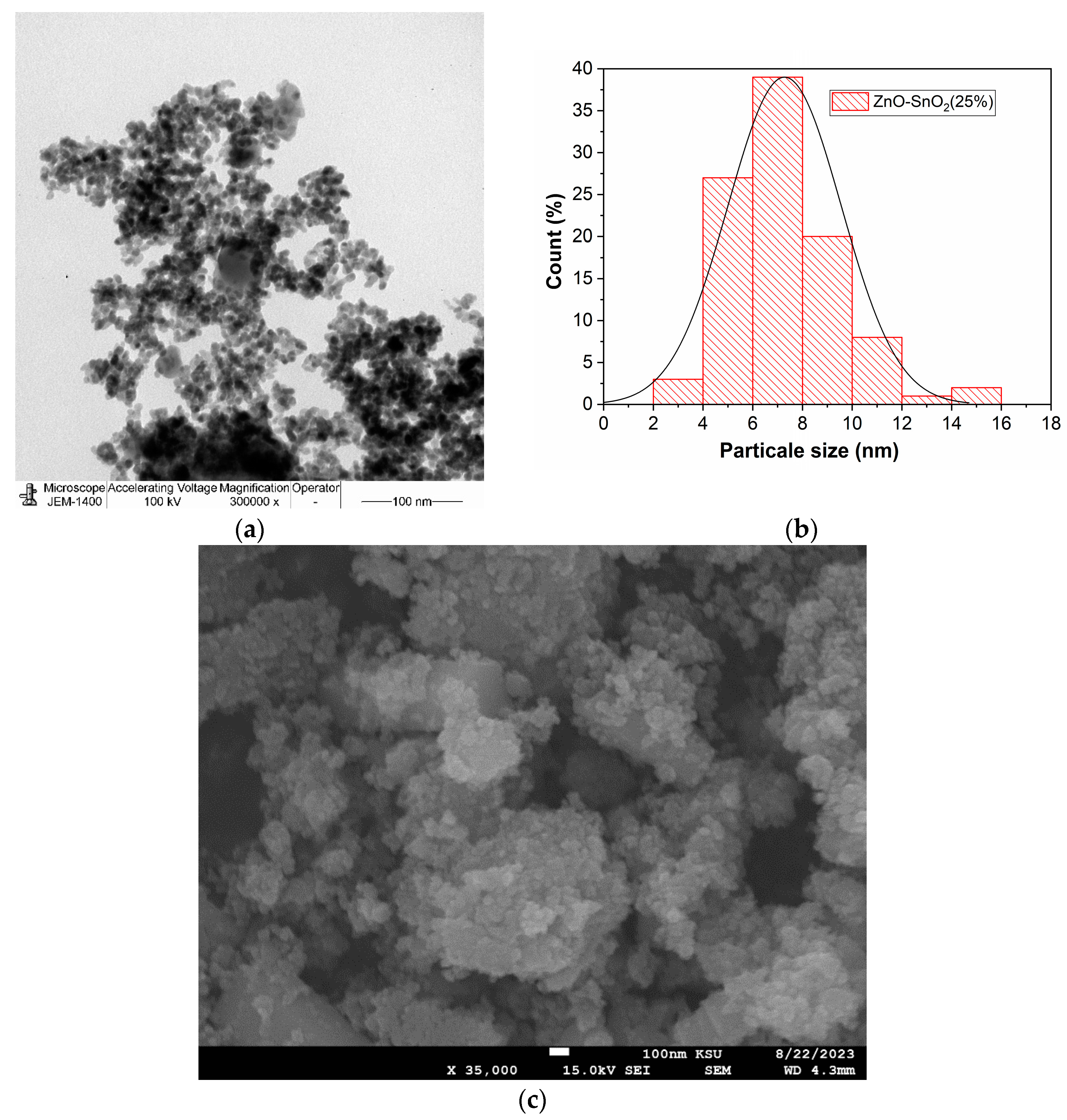
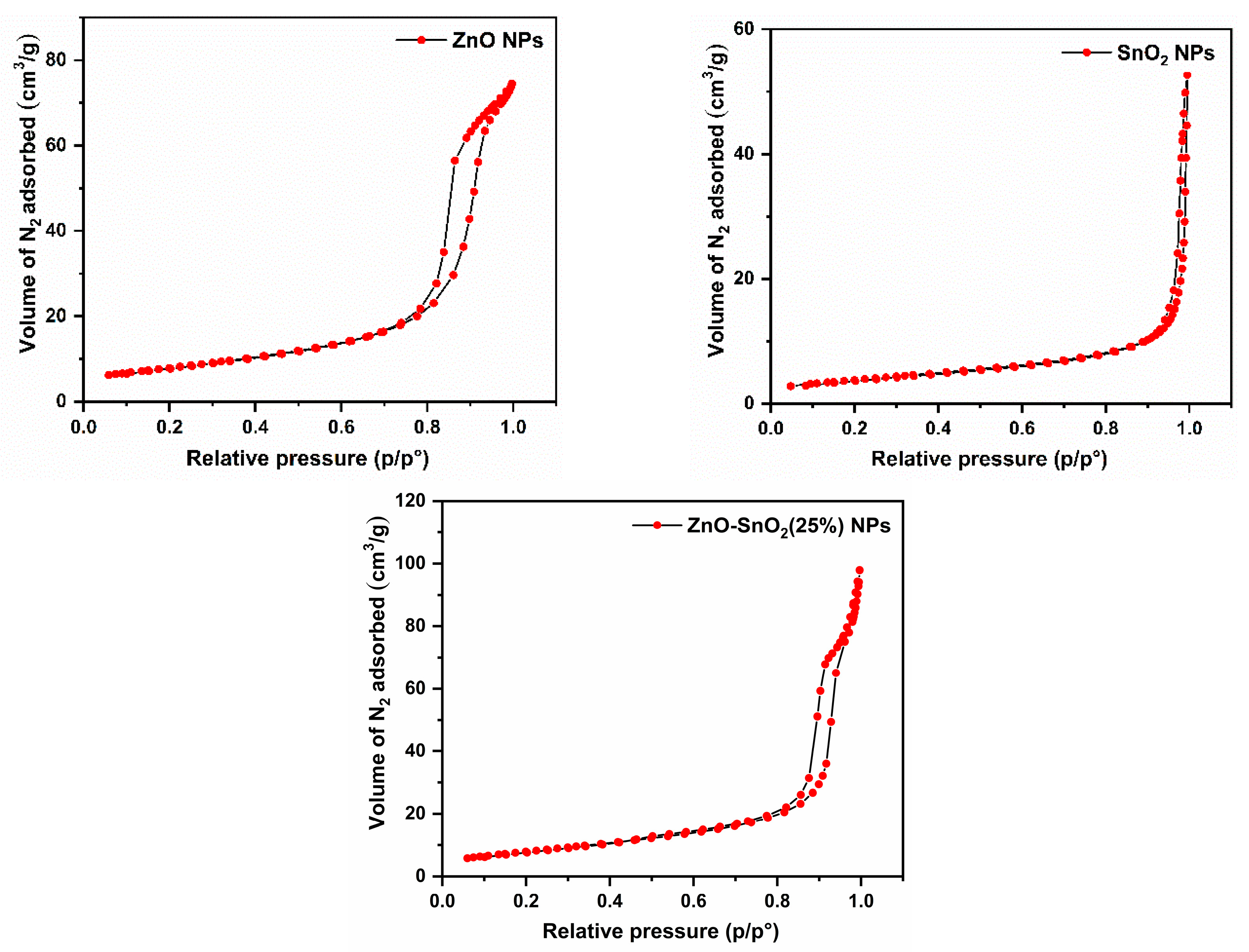


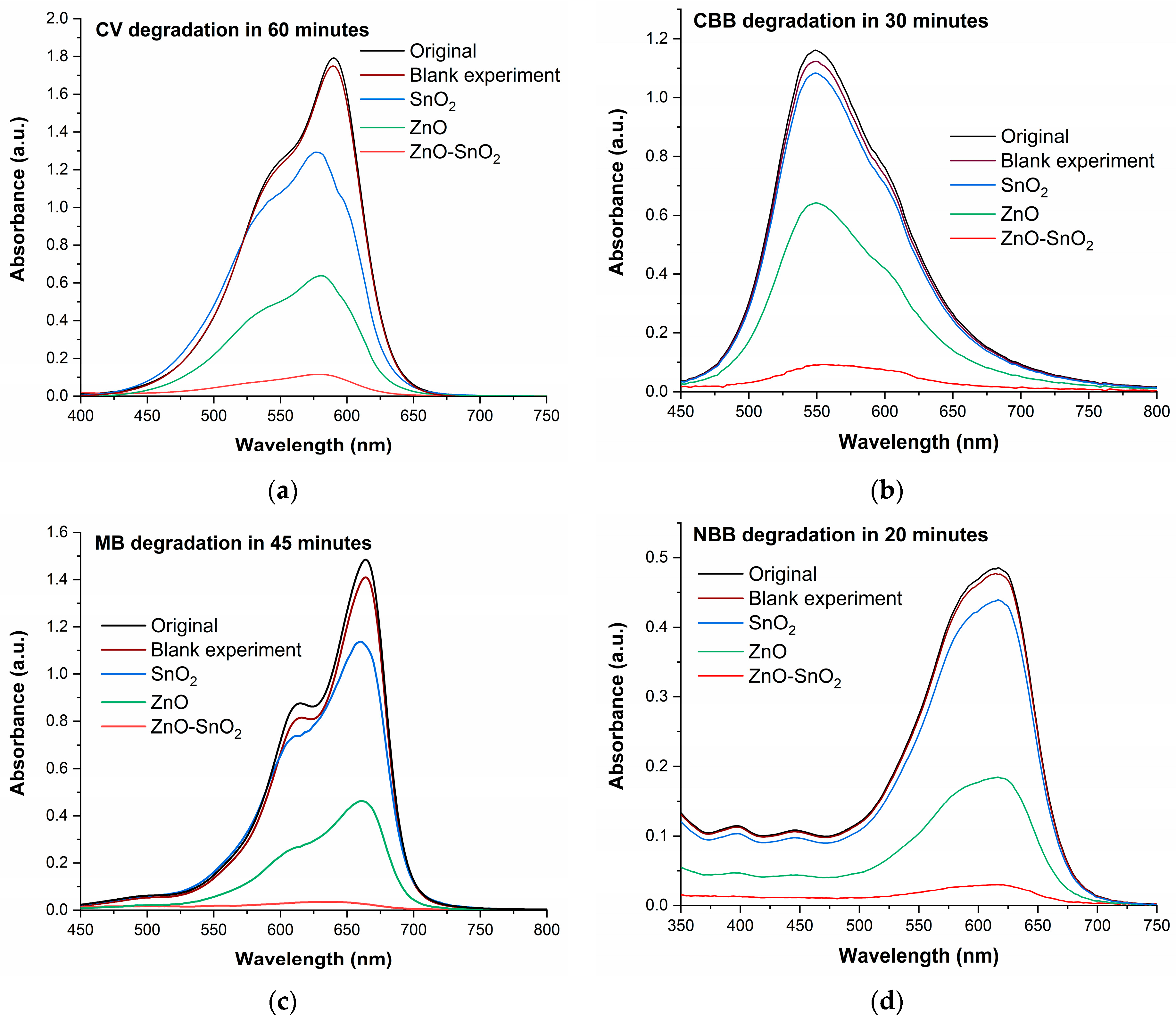


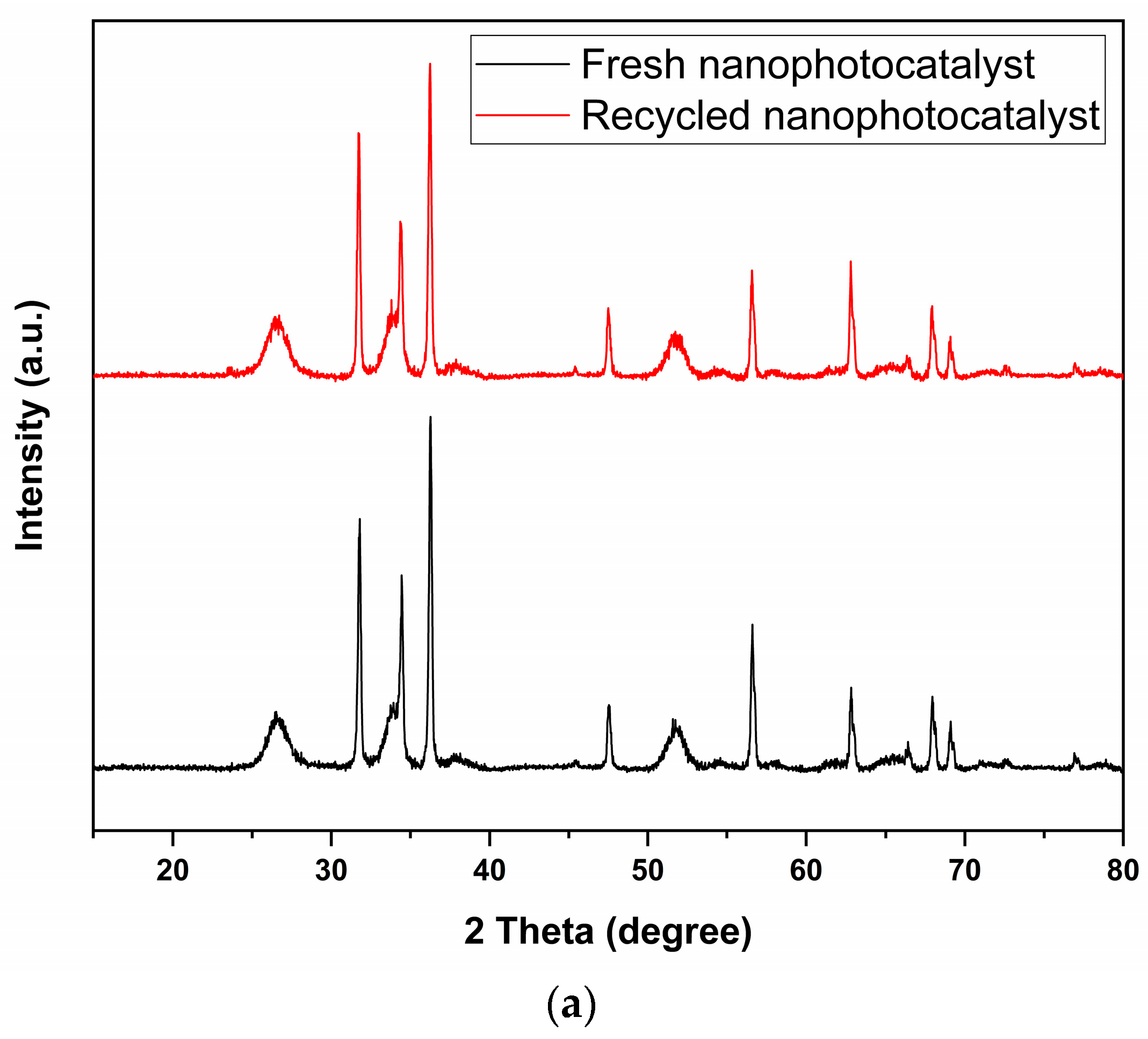
| Nanophotocatalyst | Total Surface Area (m2/g) | Average Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| ZnO | 27.8 | 0.12 | 15.9 |
| SnO2 | 13.3 | 0.07 | 23.2 |
| ZnO-SnO2(25%) | 28.2 | 0.15 | 19.7 |
| Degradation (%) | ||||
|---|---|---|---|---|
| Nanophotocatalyst | CV (in 60 min) | CBB (in 30 min) | MB (in 45 min) | NBB (in 20 min) |
| ZnO-SnO2(25%) | 94.1 | 92.2 | 98.0 | 93.8 |
| ZnO | 67.4 | 45.2 | 66.6 | 62.0 |
| SnO2 | 36.0 | 7.6 | 17.8 | 9.5 |
| Organic Pollutant | Initial Concentration (M) | Rate Constant, k (min−1) | Correlation Coefficient (R2) | Degradation (%) (in × Minute) |
|---|---|---|---|---|
| CV | 2.45 × 10−3 | 0.0481 | 0.9985 | 92.2% (in 60 min) |
| CBB | 1.21 × 10−3 | 0.0856 | 0.9781 | 94.1% (in 30 min) |
| MB | 3.13 × 10−3 | 0.1119 | 0.9925 | 96.2% (in 30 min) |
| NBB | 1.62 × 10−3 | 0.131 | 0.9832 | 93.8% (in 20 min) |
| Dye | First Use | Second Use | Third Use | Fourth Use | Lost Activity (%) after Four Uses |
|---|---|---|---|---|---|
| MB | 96.2 | 94.2 | 91.4 | 89.2 | 7.3 |
| CV | 94.2 | 93.5 | 92.9 | 91.1 | 3.3 |
| NBB | 93.8 | 91.1 | 90.3 | 88.4 | 5.8 |
| CBB | 92.2 | 91.7 | 88.6 | 88.3 | 4.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algarni, T.S.; Al-Mohaimeed, A.M.; Abduh, N.A.Y.; Habab, R.A.; Alqahtani, S.M. Green Synthesis of Mixed ZnO-SnO2 Nanoparticles for Solar-Assisted Degradation of Synthetic Dyes. Catalysts 2023, 13, 1509. https://doi.org/10.3390/catal13121509
Algarni TS, Al-Mohaimeed AM, Abduh NAY, Habab RA, Alqahtani SM. Green Synthesis of Mixed ZnO-SnO2 Nanoparticles for Solar-Assisted Degradation of Synthetic Dyes. Catalysts. 2023; 13(12):1509. https://doi.org/10.3390/catal13121509
Chicago/Turabian StyleAlgarni, Tahani Saad, Amal M. Al-Mohaimeed, Naaser A. Y. Abduh, Reem Abdulrahman Habab, and Saad Mohammed Alqahtani. 2023. "Green Synthesis of Mixed ZnO-SnO2 Nanoparticles for Solar-Assisted Degradation of Synthetic Dyes" Catalysts 13, no. 12: 1509. https://doi.org/10.3390/catal13121509
APA StyleAlgarni, T. S., Al-Mohaimeed, A. M., Abduh, N. A. Y., Habab, R. A., & Alqahtani, S. M. (2023). Green Synthesis of Mixed ZnO-SnO2 Nanoparticles for Solar-Assisted Degradation of Synthetic Dyes. Catalysts, 13(12), 1509. https://doi.org/10.3390/catal13121509







