Abstract
The reductive cyclization of different organic nitro compounds by carbon monoxide, catalyzed by transition metal complexes, is a very efficient and clean strategy for the synthesis of many N-heterocycles. However, its use requires the use of autoclaves and pressurized CO lines. In this perspective, the authors will present the results obtained in their laboratories on the use of phenyl formate as a convenient CO surrogate, able to liberate carbon monoxide under the reaction conditions and allowing the use of a cheap glass pressure tube as a reaction vessel. In most cases, yields were better than those previously reported by the use of pressurized CO, proving that the use of CO surrogates can be a viable alternative to the gaseous reagent.
1. Introduction
Nitrogen heterocycles are privileged structures in pharmaceutical chemistry [1] and an enormous effort is continuously being made to improve their synthesis [2]. Among the numerous possible synthetic approaches, the reductive cyclization of nitroarenes and nitroalkenes by carbon monoxide, catalyzed by transition metal complexes, appears to have some highly desirable features: (1) nitroarenes are usually the entry point for the introduction of nitrogen-containing groups on the aromatic ring and nitroalkenes can also be often prepared easily, e.g., by an Henry reaction; (2) carbon monoxide is cheap with respect to virtually any other reducing agent except for dihydrogen, which however affords anilines and not heterocyclic compounds in most cases; (3) the only stoichiometric byproduct is CO2, which spontaneously separates from the reaction products at the end of the reaction, thus simplifying the work-up. This is a clear advantage with respect to reactions employing phosphites or phosphines as reductants (e.g., the classical Cadogan reactions), whose oxidized product usually needs a chromatographic purification to be completely eliminated; (4) selectivities in the desired heterocycles are often very high and almost quantitative in several cases; (5) low catalyst loading is possible, up to 0.1 mol % or even lesser in some cases [3,4,5,6,7,8,9]. Given these features, it may appear surprising that such a synthetic approach has not become widespread in synthetic organic chemistry laboratories or even in industrial practice. The main reasons for this are clearly technical: performing these reactions requires the use of high-pressure equipment and pressurized CO lines. The latter, in particular, are not present in the overwhelming majority of chemical laboratories. The problem is also common to other carbonylation reactions and, in the last decade, different solid or liquid substances able to liberate CO under the reaction conditions have been developed. The field has been reviewed several times [10,11,12,13,14]. However, several of these so-called CO surrogates are quite expensive, highly toxic, or require the use of a two-chamber reactor to be employed. Several years ago, we started to investigate the use of CO surrogates in the field of reductive cyclization reactions of organic nitro compounds and selected formate esters as reagents because they are cheap, non- or little-toxic and because the stoichiometric byproduct, an alcohol or phenol, is unlikely to interfere with the reaction course. In this account, our results in the field are summarized. For the sake of completeness, it should be mentioned that other groups have also employed Mo(CO)6 [15,16,17], Co2(CO)8 [18,19], and a triformate ester [20,21] as CO surrogates for related reductive cyclization reactions of nitroarenes to give N-heterocycles.
2. Discussion
2.1. General Aspects
Before discussing the synthesis of the individual heterocycles, we need to summarize some general trends in the reactivity of nitro compounds with CO and on the use of CO surrogates, which have been evidenced in numerous previous studies.
- The initial activation of the nitro compound, at least when late transition metal catalysts are employed, is always an electron transfer from the metal to the nitro group [22,23,24,25,26,27,28,29,30,31]. For this reason, low-valent metal complexes need to be used. However, due to the high sensitivity of the latter, metal complexes in higher oxidation states are often used as precatalysts, which are reduced by CO under the operating conditions. By the same token, nitroarene and to a lesser extent nitroalkenes are suitable substrates, but nitroalkanes have higher oxidation potentials and are unreactive in these systems.
- Palladium, ruthenium and rhodium compounds have all been employed as catalysts, but the best results have been obtained by the use of palladium and in the last decade the other two metals have only rarely been used.
- Phosphines have been used as ligands for palladium in many cases, but it has been shown that they are oxidized to phosphine oxides during the reaction [32]. Since we aim at developing a catalytic system that may also be applied at an industrial level, we prefer to avoid using them. No successful use of N-heterocyclic carbenes as ligands in this field has ever been reported. The best ligands in terms of activity and stability of the catalytic system are phenanthroline and its substituted derivatives [33,34,35,36].
- Aryl formates can be decomposed to CO and phenols even by weak organic bases. Alkyl formates are cheaper, but they are activated only by very strong bases, which would not be compatible with most reactions. Alternatively, they can be decomposed by the action of a ruthenium-based catalytic system [37].
- When using CO surrogates, the features of the vessel in which the reaction is performed are important for the success of the reaction and for safety reasons. We have discussed the pros and cons of different kinds of “pressure tubes” in a previous paper, thus we will not do it here again [38].
2.2. Synthesis of Indoles from O-Nitrostyrenes
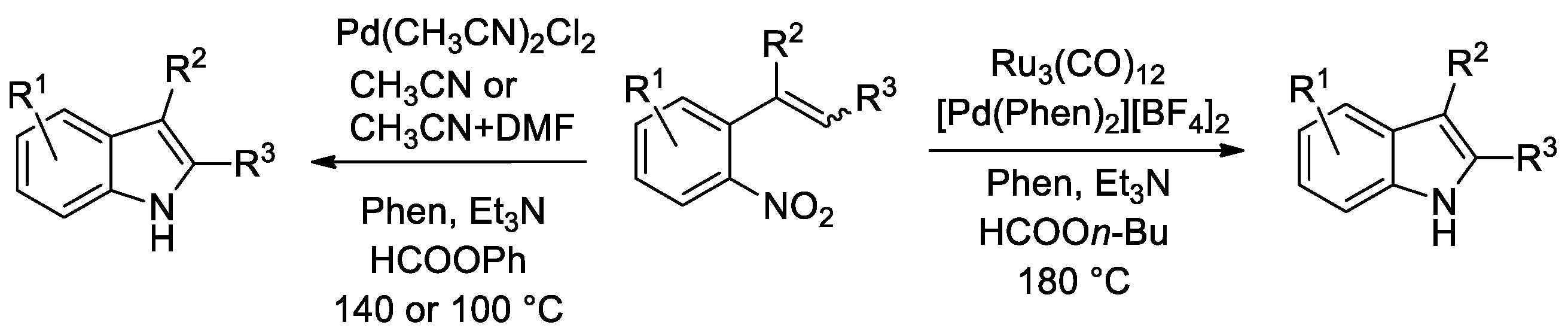
The first reaction we tried to accomplish using formate esters as CO surrogates was the synthesis of indoles from o-nitrostyrenes (Scheme 1).
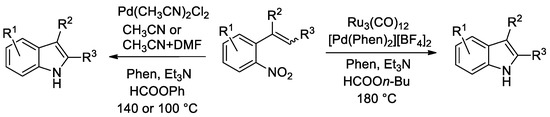
Scheme 1.
Synthesis of indoles from o-nitrostyrenes.
Initially, alkyl formates were attempted as CO surrogates (Scheme 1, right side). A palladium catalyst alone was inactive, as no formate decomposition occurs in its presence when a weak base is employed. Ru3(CO)12 has long been known to be a catalyst for reductive carbonylation reactions of nitroarenes [39,40] and, in the presence of phenanthroline, it is also known to activate alkyl formates [41]. Indeed, some indole was formed when this cluster was employed as a catalyst in the absence of any palladium compound. However, substrate conversion was very low. Best results were obtained with a bimetallic Ru3(CO)12/[Pd(Phen)2][BF4]2/Phen catalytic system (Phen = 1,10-phenanthroline). Yet, a high temperature of 180 °C is required for efficient formate decomposition and the synthetic results were not satisfactory, with a maximum indole yield of around 70% [42] (Conditions A in Figure 1).
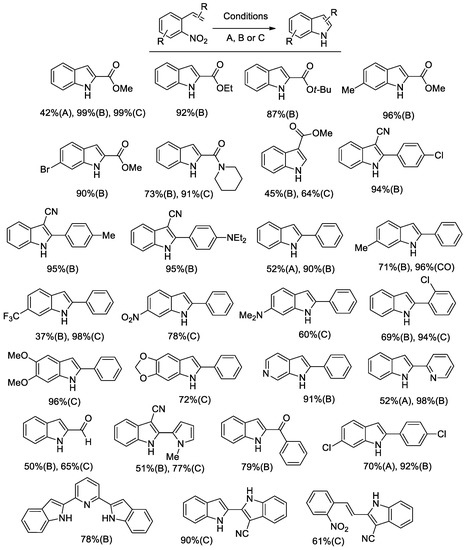
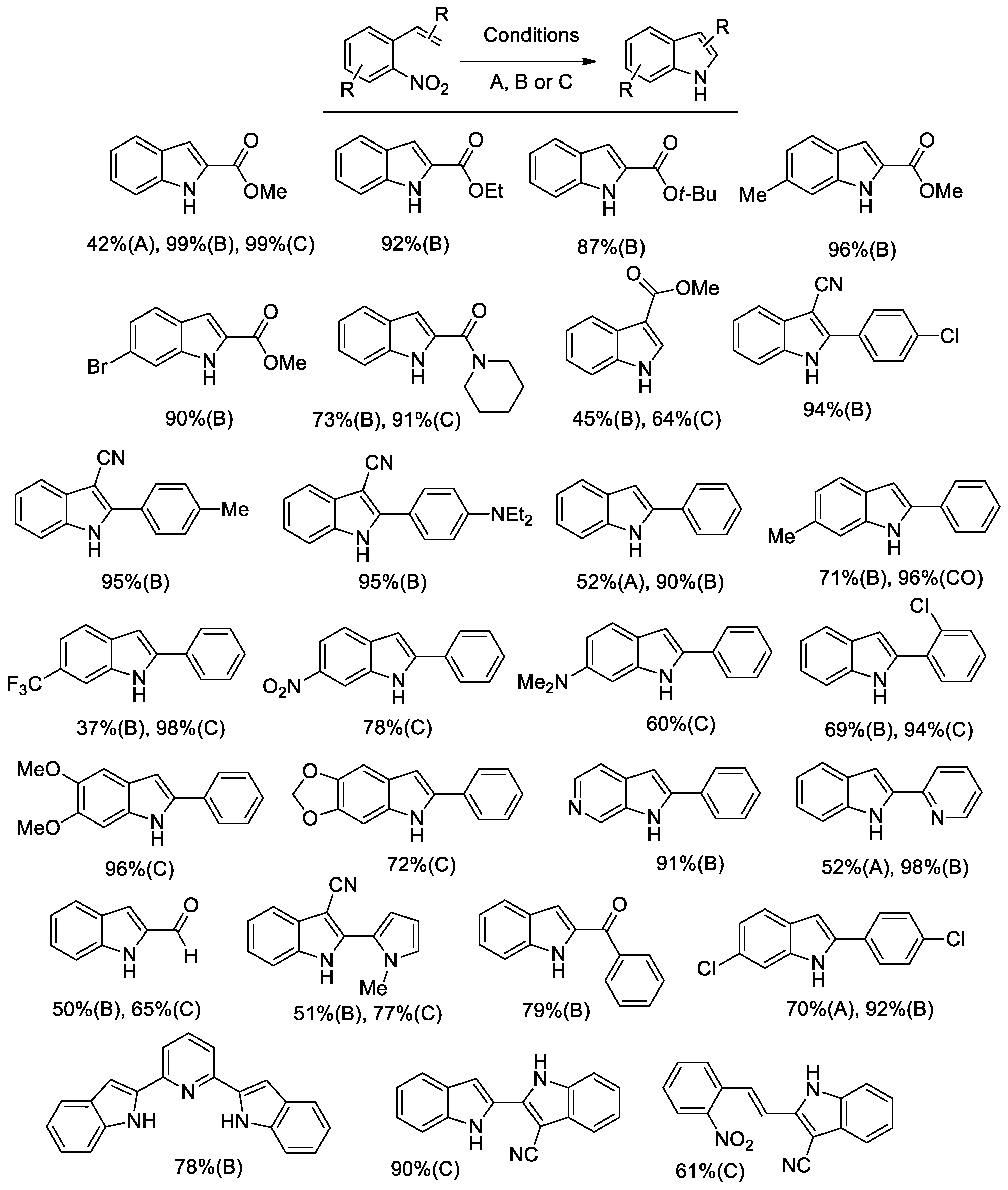
Figure 1.
Indoles from o-nitrostyrenes. Conditions A: 0.27 mmol nitrostyrene, mol. 1 mol % [Pd(Phen)2][BF4]2, 1 mol % Ru3(CO)12, 20 mol % Phen; mol. 40 µL (0.29 mmol) Et3N; in butyl formate (10 mL), at 180 °C for 10 h. Conditions B: 0.54 mmol nitrostyrene, 1 mol % Pd(CH3CN)2Cl2, 2.5 mol % Phen, 240 µL (2.2 mmol) HCOOPh, 40 µL (0.29 mmol) Et3N, in CH3CN (10 mL), 140 °C for 3 h (unless otherwise noted). Conditions C: 0.54 mmol nitrostyrene, 1 mol % Pd(CH3CN)2Cl2, 5 mol % Phen, 260 µL (2.38 mmol) HCOOPh, 100 µL (0.72 mmol) Et3N, in CH3CN + DMF (9+1 mL), at 100 °C for 6 h.
The use of aryl formates, the best phenyl formate, allowed us to achieve much better results and to employ just palladium as a catalyst, thus simplifying the catalytic system (Scheme 1, left side). This catalytic system was subjected to two optimization rounds. During the first one [42], the temperature was set at 140 °C because the rate appeared to be too slow at lower temperatures and substrate conversion was not complete. Almost quantitative yields could be achieved in several cases, but substrates bearing sensitive groups such as an aldehyde still gave unsatisfactory results (Conditions B in Figure 1). Thus, we engaged in a second round of optimization of the reaction conditions and we succeeded in finding a set of experimental conditions, which allow us to work at 100 °C with as little as 0.2 mol % palladium and obtain good yields even for indoles bearing a pyrrolyl or an aldehydic group [38] (Conditions C in Figure 1). Quite surprisingly, it was found that the best single reaction solvent is CH3CN, but the use of a 9:1 CH3CN/DMF mixture gave even better results.
The main synthetic results are reported in Figure 1, where the yields obtained under different conditions are also compared. It should be noted that, whenever a comparison is possible, the obtained yields are in most cases higher than the best previously reported yields for the same reaction employing pressurized CO as a reductant.
Notably, when two nitro groups are present in the substrate in the ortho and para position with respect to the vinyl group, a selective reaction of the ortho group could be achieved, suggesting that the double bond coordinates to the metal. Indeed, the para nitro group should be more reactive if only steric effects were present, as happens in the case of 2,4-dinitrotoluene [43]. Moreover, when a double cyclization is possible, the reaction could be stopped at the first step or run to completion simply by changing the reaction time. Only in a few cases the reaction failed to yield an isolable indole, and in each case there is an explanation for that. For example, a free amino group on the aryl ring was not tolerated because anilines easily react with nitroarenes under similar reaction conditions to give diarylureas [44,45].
2.3. Synthesis of Indoles from β-Nitrostyrenes
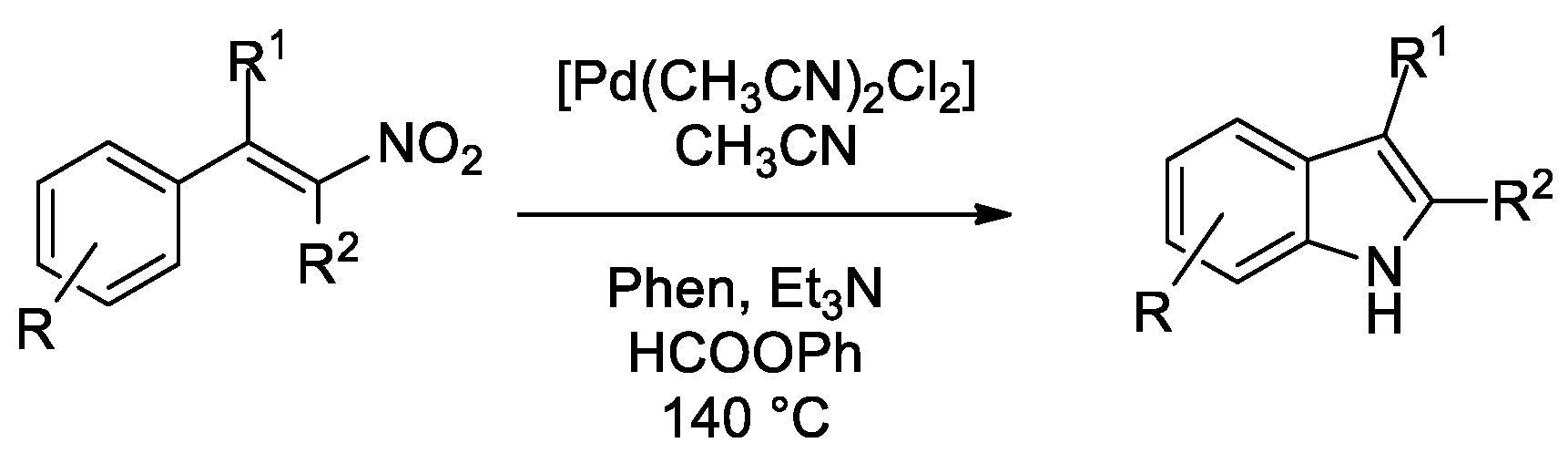
The reactions described in the previous paragraph are very selective, but the synthesis of the starting o-nitrostyrene is not always high yielding. In some cases, synthesizing a β-nitrostyrene, where the nitro group is on the olefin moiety, is more straightforward. Moreover, when indoles polysubstituted at the phenyl ring are targeted, the use of β-nitrostyrenes as substrates allows the replacement of two functional groups (nitro and vinyl) with just one. Reductive cyclization of β-nitrostyrenes was first reported by Dong [46], but only for the more reactive α-aryl-β-nitrostyrenes. We later were able to extend the reaction to substrates lacking the second aryl ring [47] and even to the synthesis of thienopyrroles [48] from thienyl-substituted nitroalkenes and to that of pyrroles from nitrodienes [49]. We thus decided to test the use of phenyl formate as a CO surrogate for this reaction (Scheme 2).
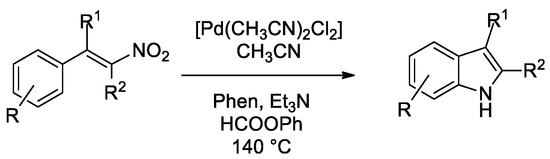
Scheme 2.
Synthesis of indoles from β-nitrostyrenes.
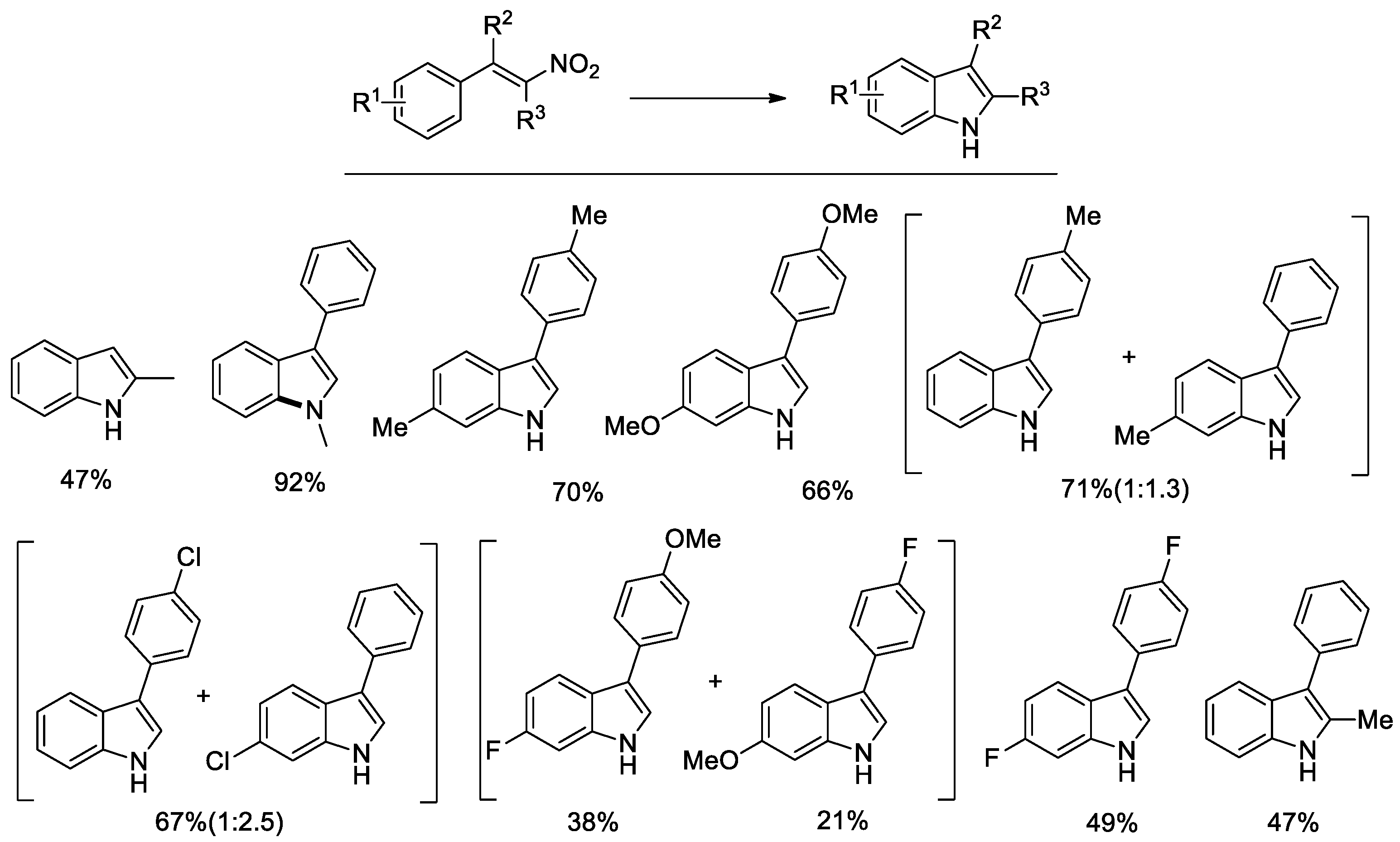
Despite some effort in optimizing the experimental conditions, only fair yields could be obtained in the case of β-nitrostyrenes lacking any substituent in the alpha position [50]. Investigation of the reasons for this failure revealed that bases catalyze the oligo/polymerization of the nitrostyrene itself and this reaction occurs at a competitive rate with respect to the cyclization. The only partially successful solution was to increase the phenyl formate amount so that the higher generated CO pressure accelerates the cyclization reaction with respect to polymerization. However, the amount of formate can be increased only to a small extent not to exceed the safety pressure limits of the employed apparatus (ca. 10 bar). Retrospectively, the cyclization of β-nitrostyrenes is the reaction that needed the highest CO pressures to obtain good results [47] among those here investigated and the reason is clearly the same.
Better results were obtained with the more reactive and less prone to polymerization α-aryl-β-nitrostyrenes. The substrate scope is shown in Figure 2.
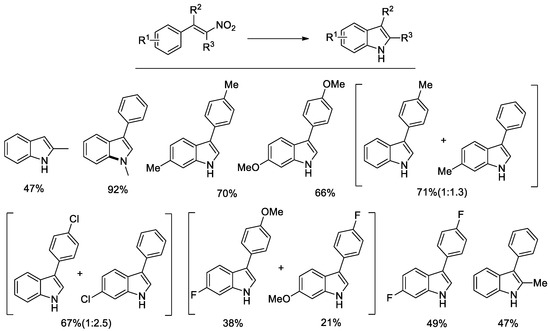
Figure 2.
Synthesis of indoles from β-nitrostyrenes. Experimental conditions: 0.54 mmol nitrostyrene, 1 mol % PdCl2(CH3CN)2, 5 mol % Phen, 260 μL PhOC(O)H, 120 μL Et3N, in CH3CN (10 mL) at 140 °C for 4 h.
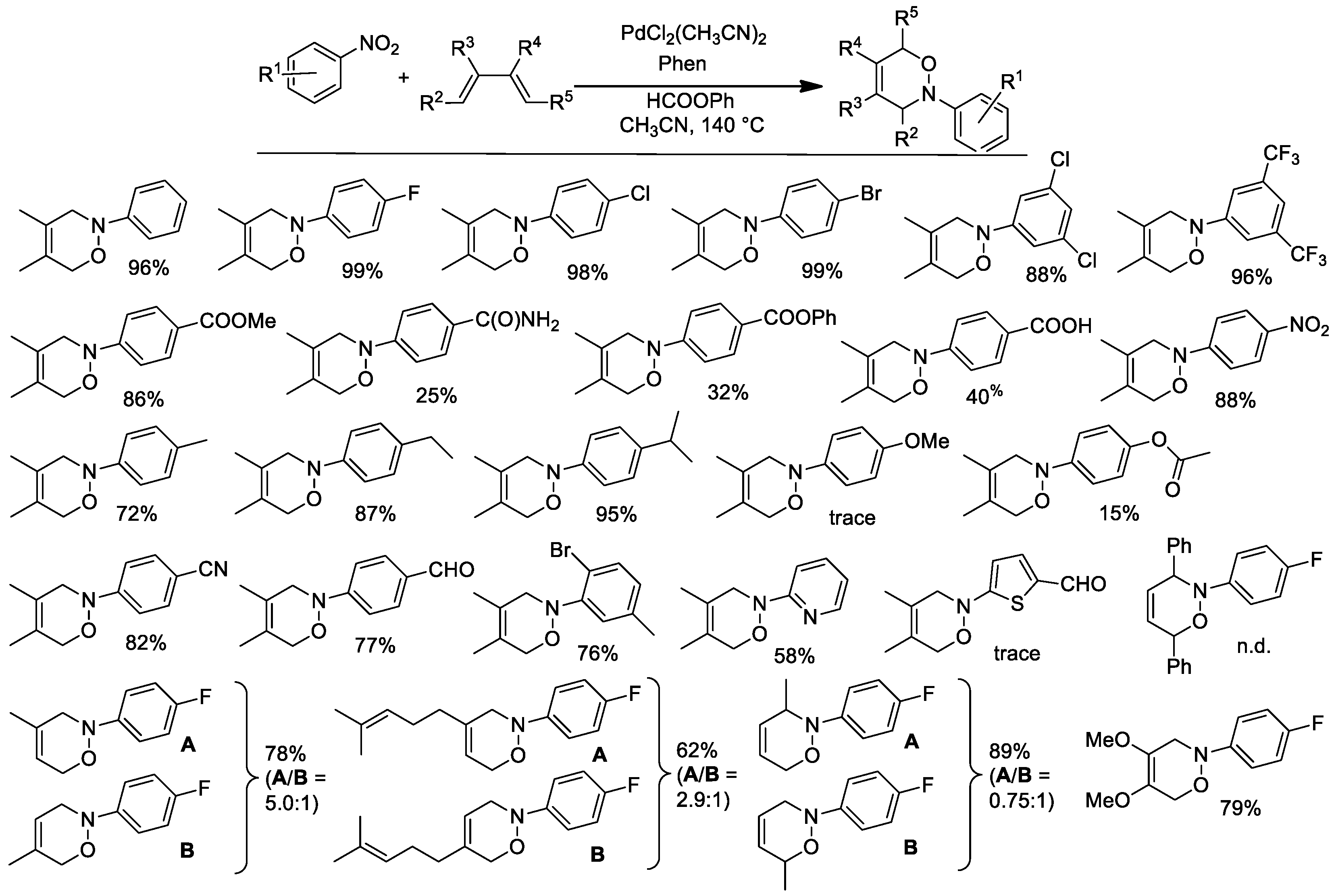
2.4. Synthesis of 3,6-dihydro-2H-[1,2]-oxazines from Nitroarenes and Conjugated Dienes
Free nitrosoarenes quickly react with conjugated dienes in a hetero Diels-Alder reaction to give 3,6-dihydro-2H-[1,2]-oxazines [51,52,53]. However, the synthesis of nitrosoarenes is not straightforward and nitrosoarenes themselves are not indefinitely stable molecules if stored in the air at room temperature. Trapping of nitrosoarenes intermediately formed during the reduction of nitroarenes is an effective strategy to synthesize oxazines [54,55]. The experimental conditions initially optimized for the synthesis of indoles using phenyl formate as a CO surrogate proved to also be suitable for the synthesis of oxazines [56].
The synthetic results are shown in Figure 3. Excellent results were obtained in many cases. The reaction only failed when the nitroarene bears strongly electron-donating substituents (e.g., a para-methoxy group), because in these cases, the corresponding nitrosoarene is a poor dienophile, or when both the terminal positions of the diene are substituted (e.g., 1,4-diphenylbutadiene), because in this case, the formation of the oxazine is reversible at high temperature.
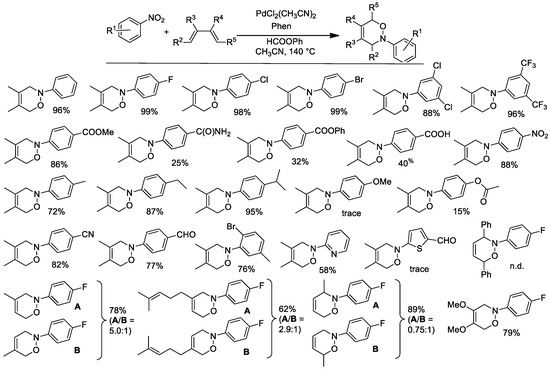
Figure 3.
Synthesis of oxazines from nitroarenes and conjugated dienes. Experimental conditions: nitroarene 0.54 mmol, Pd(CH3CN)2Cl2 1 mol %, Phen 2.5 mol %, diene 4 equiv, HCOOPh 2.2 mmol, Et3N 0.27 mmol, in CH3CN (10 mL) at 140 °C.
When the diene is not symmetrical, a mixture of the two possible regioisomers is obtained. Notably, the relative amounts of the two isomers were the same whether the oxazines were obtained from a catalytic reaction starting from the nitroarene or when they were obtained from an uncatalyzed reaction of the nitrosoarene with the diene in the same solvent and at the same temperature. This observation strongly indicates that the formation of the oxazine occurs outside the coordination sphere of palladium.
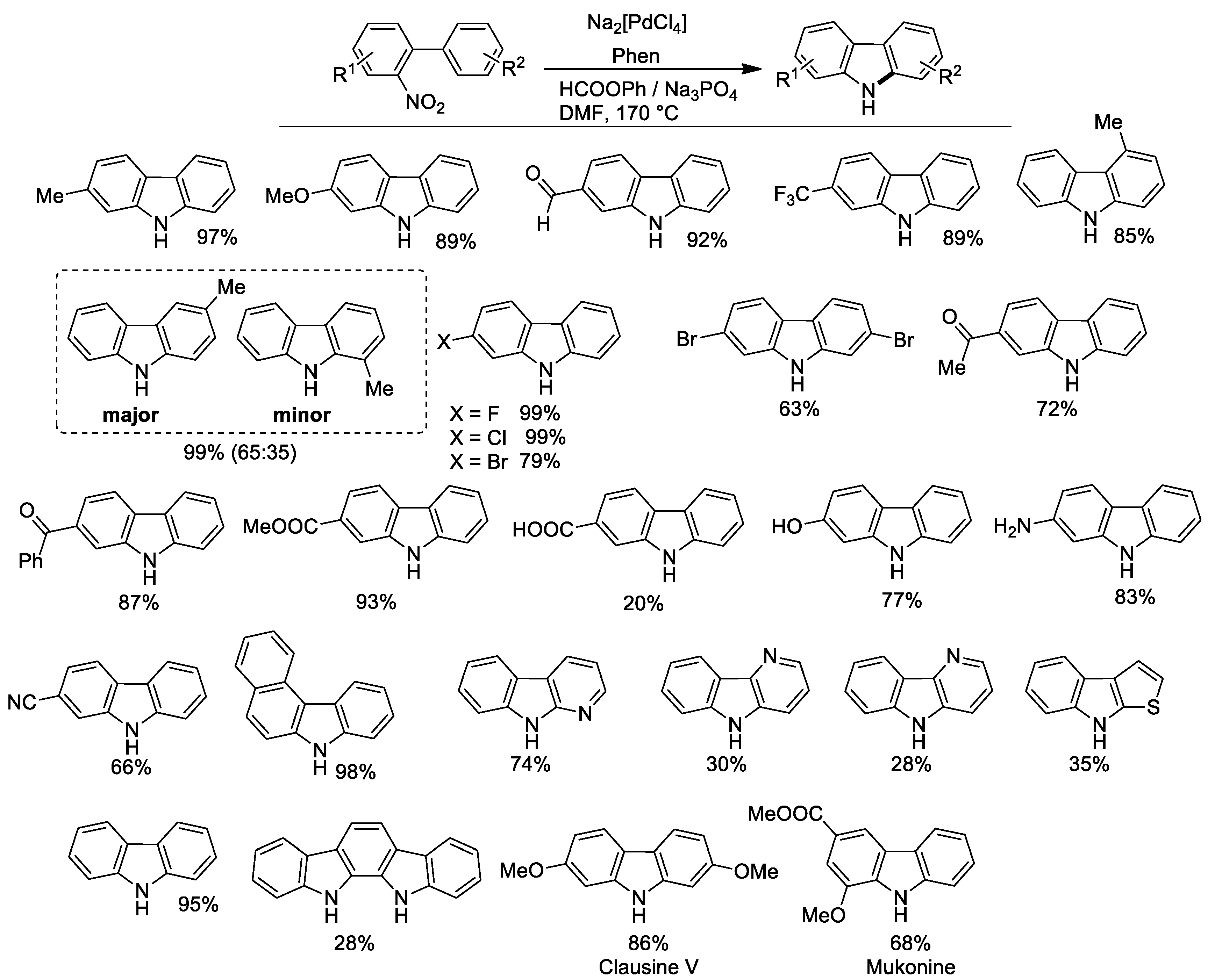
2.5. Synthesis of Carbazoles from o-Nitrobiphenyls
Cyclization of o-nitrobiphenyls to carbazoles under the reducing action of carbon monoxide has long been known [57,58], but has been little developed with respect to the synthesis of indoles because it usually requires harsher conditions and affords lower yields of the desired heterocycle. Wishing to solve the problem, we decided to apply our phenyl formate protocol to this interesting reaction. Initial attempts were disappointing and only low yields of carbazole, accompanied by larger amounts of o-aminobiphenyl, were obtained. However, extensive optimization of the reaction conditions allowed us to reach high yields of the desired products [59]. Key points for success proved to be the use of DMF as a solvent in place of acetonitrile and the substitution of triethylamine with an inorganic base, the best Na3PO4. Employing Na2[PdCl4] as a catalyst precursor instead of PdCl2(CH3CN)2 also improved the stability of the catalytic system.
Synthetic results are shown in Figure 4.
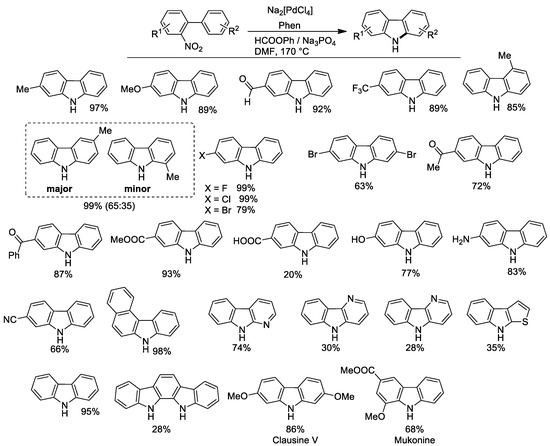
Figure 4.
Synthesis of carbazoles from o-nitrobiphenyls. Experimental conditions: nitrobiphenyl (0.54 mmol), Na2[PdCl4] 1 mol %, Phen 5 mol %, HCOOPh 2.4 mmol, Na3PO4 7.3 × 10−2 mmol in DMF (10 mL), at 170 °C for 5 h.
Good results were in general obtained both when the substituent was present on the nitro-containing ring or on the other. However, if a substituent is present in the 3’ position (meta to the nitrophenyl ring) a mixture of isomers was obtained. As for other syntheses described in this paper, a free aldehydic group was well tolerated. Surprisingly, the synthesis was successful even in the presence of potentially reactive groups, such as amino and hydroxy. The presence of a free carboxylic group lowered the yield considerably, but this carbazole can be more effectively obtained by hydrolysis of the corresponding methyl ester, which is instead obtained in a 93% yield. The protocol could also be employed for the synthesis of the natural products Clausine V and Mukonine. Yields are in general higher than those previously obtained by the use of pressurized CO.
2.6. Future Perspectives
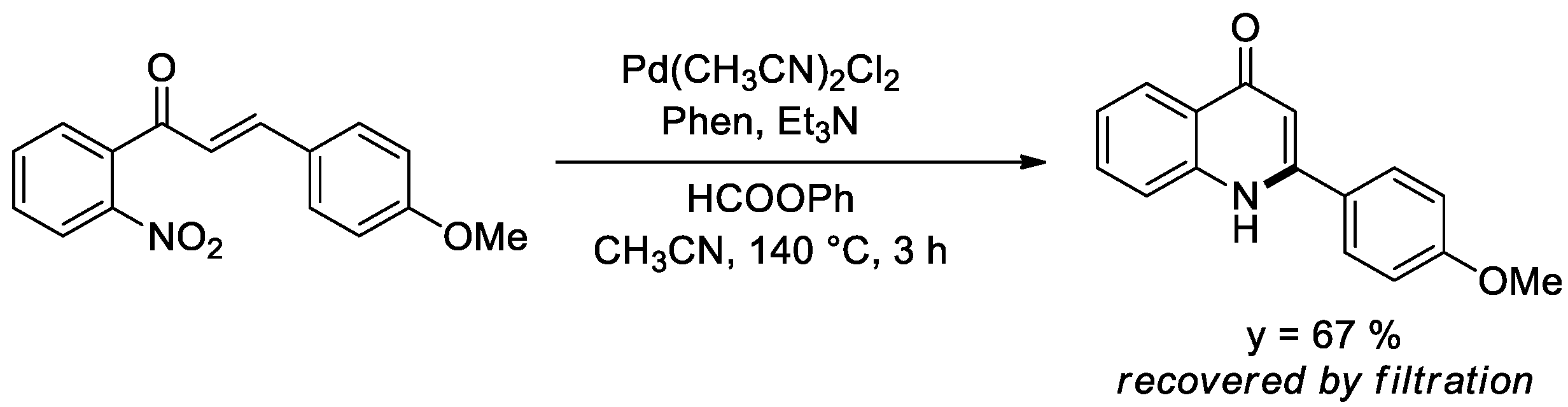
The reactions described in this account do not exhaust the possibilities offered by the use of phenyl formate as a CO surrogate. We already reported a single example of reductive cyclization of an o-nitrochalcone to give a quinolone in which phenyl formate [38] successfully replaced pressurized CO [60,61] (Scheme 3).
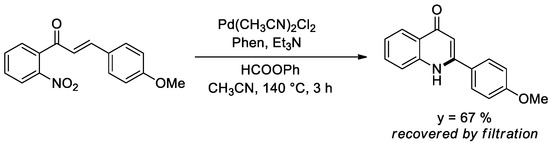
Scheme 3.
Synthesis of a quinolone from a nitrochalcone.
An optimization and a more general investigation of this reaction have not yet been accomplished. However, this is not the only reductive cyclization reaction that may be amenable to be investigated and many others may be successfully performed, for which the use of pressurized CO has been described and that are mentioned in the reviews cited at the beginning of this paper.
In addition, the development of different CO surrogates which do not release phenol as a stoichiometric byproduct is a very stimulating task. Promising results have already been obtained in our group on the use of the HCOOH/Ac2O mixture [62].
3. Conclusions
The use of CO as a reductant for nitroarenes and nitroalkenes presents many advantages from a synthetic point of view, but is operationally complex for many research groups. In this brief account, we have presented our results on the use of formate esters as convenient CO surrogates. In particular, phenyl formate can be activated even by weak bases, which do not interfere with the reactions or even have a beneficial role for them. Notably, in most cases, the isolated yield in the desired heterocycle was higher than those previously obtained for the same reaction when gaseous CO or even other reductant had been employed. The only exception is the cyclization of β-nitrostyrenes to indoles. The reason is that in order to give good results, this reaction requires too high CO pressures to be sustained by a glass pressure tube. The reason for the higher selectivity in the other cases may reside just in a more extensive optimization of the reaction conditions, but the slow generation of CO during the reaction may also play a role. In any case, the high yields obtained in the other cases clearly show that the use of CO surrogates should not necessarily be considered as a second choice when the use of pressurized CO is not possible, but may represent the best available option in any case.
Author Contributions
Methodology, F.F. and M.A.F.; investigation, F.F. and M.A.F.; writing—original draft preparation, F.R.; supervision, F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Università degli Studi di Milano, PSR2021 “Design and synthesis of high added-value fine-chemicals by sustainable catalytic approaches”.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article. Refer to the original papers for data availability.
Acknowledgments
We thank the former Master’s and PhD students who made part of the experimental work described in this account and whose names can be found in the references.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Ameta, K.L.; Kant, R.; Penoni, A.; Maspero, A.; Scapinello, L. (Eds.) N-Heterocycles; Springer: Singapore, 2022. [Google Scholar]
- Soderberg, B.C.G. Synthesis of heterocycles via intramolecular annulation of nitrene intermediates. Curr. Org. Chem. 2000, 4, 727–764. [Google Scholar] [CrossRef]
- Cenini, S.; Ragaini, F. Catalytic Reductive Carbonylation of Organic Nitro Compounds; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Ragaini, F.; Cenini, S.; Gallo, E.; Caselli, A.; Fantauzzi, S. Fine chemicals by reductive carbonylation of nitroarenes, catalyzed by transition metal complexes. Curr. Org. Chem. 2006, 10, 1479–1510. [Google Scholar] [CrossRef]
- Ferretti, F.; Formenti, D.; Ragaini, F. The reduction of organic nitro compounds by carbon monoxide as an effective strategy for the synthesis of N-heterocyclic compounds: A personal account. Rend. Fis. Acc. Lincei 2017, 28, 97–115. [Google Scholar] [CrossRef]
- Afanasyev, O.I.; Chusov, D. Carbon Monoxide Related Reductions. Ineos Open 2020, 3, 133–139. [Google Scholar] [CrossRef]
- Tsygankov, A.A.; Makarova, M.; Chusov, D. Carbon monoxide as a selective reducing agent in organic chemistry. Mendeleev Commun. 2018, 28, 113–122. [Google Scholar] [CrossRef]
- Ferretti, F.; Ramadan, D.R.; Ragaini, F. Transition Metal Catalyzed Reductive Cyclization Reactions of Nitroarenes and Nitroalkenes. ChemCatChem 2019, 11, 4450–4488. [Google Scholar] [CrossRef]
- Peng, J.B.; Qi, X.X.; Wu, X.F. Recent Achievements in Carbonylation Reactions: A Personal Account. Synlett 2017, 28, 175–194. [Google Scholar]
- Wu, L.; Liu, Q.; Jackstell, R.; Beller, M. Carbonylations of Alkenes with CO Surrogates. Angew. Chem. Int. Ed. 2014, 53, 6310–6320. [Google Scholar] [CrossRef]
- Konishi, H.; Manabe, K. Formic Acid Derivatives as Practical Carbon Monoxide Surrogates for Metal-Catalyzed Carbonylation Reactions. Synlett 2014, 25, 1971–1986. [Google Scholar] [CrossRef]
- Gautam, P.; Bhanage, B.M. Recent advances in the transition metal catalyzed carbonylation of alkynes, arenes and aryl halides using CO surrogates. Catal. Sci. Technol. 2015, 5, 4663–4702. [Google Scholar] [CrossRef]
- Friis, S.D.; Lindhardt, A.T.; Skrydstrup, T. The Development and Application of Two-Chamber Reactors and Carbon Monoxide Precursors for Safe Carbonylation Reactions. Acc. Chem. Res. 2016, 49, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, D.-S.; Driver, T.G. Palladium-Catalyzed Formation of N-Heteroarenes from Nitroarenes using Molybdenum Hexacarbonyl as the Source of Carbon Monoxide. Adv. Synth. Catal. 2015, 357, 3463–3468. [Google Scholar] [CrossRef]
- Su, Z.; Liu, B.; Liao, H.; Lin, H.-W. Synthesis of N-Heterocycles by Reductive Cyclization of Nitroalkenes Using Molybdenum Hexacarbonyl as Carbon Monoxide Surrogate. Eur. J. Org. Chem. 2020, 2020, 4059–4066. [Google Scholar] [CrossRef]
- Jana, N.; Zhou, F.; Driver, T.G. Promoting Reductive Tandem Reactions of Nitrostyrenes with Mo(CO)6 and a Palladium Catalyst To Produce 3H-Indoles. J. Am. Chem. Soc. 2015, 137, 6738–6741. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wei, P.; Ying, J.; Wu, X.-F. Nickel-catalyzed carbonylative domino cyclization of arylboronic acid pinacol esters with 2-alkynyl nitroarenes toward N-aroyl indoles. Org. Chem. Front. 2022, 9, 2685–2689. [Google Scholar] [CrossRef]
- Yao, L.; Ying, J.; Wu, X.-F. Nickel-catalyzed cascade carbonylative synthesis of N-benzoyl indoles from 2-nitroalkynes and aryl iodides. Org. Chem. Front. 2021, 8, 6541–6545. [Google Scholar] [CrossRef]
- Qi, X.; Zhou, R.; Peng, J.-B.; Ying, J.; Wu, X.-F. Selenium-Catalyzed Carbonylative Synthesis of 2-Benzimidazolones from 2-Nitroanilines with TFBen as the CO Source. Eur. J. Org. Chem. 2019, 2019, 5161–5164. [Google Scholar] [CrossRef]
- Zhou, R.; Qi, X.; Wu, X.-F. Selenium-Catalyzed Carbonylative Synthesis of 3,4-Dihydroquinazolin-2(1H)-one Derivatives with TFBen as the CO Source. ACS Comb. Sci. 2019, 21, 573–577. [Google Scholar] [CrossRef]
- Berman, R.S.; Kochi, J.K. Kinetics and mechanism of oxygen atom transfer from nitro compounds mediated by nickel(0) complexes. Inorg. Chem. 1980, 19, 248–254. [Google Scholar] [CrossRef]
- Skoog, S.J.; Gladfelter, W.L. Activation of Nitroarenes in the Homogenous Catalytic Carbonylation of Nitroaromatics via an Oxygen-Atom-Transfer Mechanism Induced by Inner Sphere Electron Transfer. J. Am. Chem. Soc. 1997, 119, 11049–11060. [Google Scholar] [CrossRef]
- Kunin, A.J.; Noirot, M.D.; Gladfelter, W.L. In situ FTIR spectroscopy at elevated carbon monoxide pressure. Evidence for single electron transfer in the catalytic carbonylation of nitroaromatics. J. Am. Chem. Soc. 1989, 111, 2739–2741. [Google Scholar] [CrossRef]
- Belousov, Y.A. Radical chemistry of iron carbonyls. Russ. Chem. Rev. 2007, 76, 41–58. [Google Scholar] [CrossRef]
- Belousov, Y.A.; Kolosova, T.A. Electron-spin resonance study of the reaction of iron carbonyls with nitro and nitrosoparaffines—A mechanism of the reductive carbonylation of nitrocompounds. Polyhedron 1987, 6, 1959–1970. [Google Scholar] [CrossRef]
- Ragaini, F.; Song, J.S.; Ramage, D.L.; Geoffroy, G.L.; Yap, G.A.P.; Rheingold, A.L. Radical Processes in the Reduction of Nitrobenzene Promoted by Iron Carbonyl Clusters—X-ray Crystal-Structures of [Fe3(CO)9(μ3-NPh)]2−, [HFe3(CO)9(μ3-NPh)]−, and the Radical-Anion [Fe3(CO)11]−. Organometallics 1995, 14, 387–400. [Google Scholar] [CrossRef]
- Ragaini, F. Mechanistic study of the phase-transfer-catalyzed reduction of nitrobenzene to aniline by iron carbonyl complexes. Role of the radical anion [Fe3(CO)11)]−. Organometallics 1996, 15, 3572–3578. [Google Scholar] [CrossRef]
- Liu, P.H.; Liao, H.Y.; Cheng, C.H. Electro-transfer from tetracarbonylrhodate(1-) to nitro aromatics—Novel interaction of nitro radical-anions with a rhodium(I) center. J. Chem. Soc. Chem. Commun. 1995, 23, 2441–2442. [Google Scholar] [CrossRef]
- Ragaini, F.; Cenini, S.; Demartin, F. Mechanistic Studies of the Carbonylation of Nitrobenzene Catalyzed by the [Rh(CO)4]−/Bipy System—X-ray Structure of [PPN][Rh(CO)2ON[C6H3Cl2)C(O)O] [PPN+=(PPh3)2N+-Bipy = 2,2’-Bipyridyl]. J. Chem. Soc. Chem. Commun. 1992, 1467–1468. [Google Scholar] [CrossRef]
- Ragaini, F.; Cenini, S.; Demartin, F. Mechanistic Study of the Carbonylation of Nitrobenzene Catalyzed by the [Rh(CO)4]− Nitrogen Base System—X-ray Structure of [PPN][Rh(CO)2ON(C6H3Cl2)C(O)O]. Organometallics 1994, 13, 1178–1189. [Google Scholar] [CrossRef]
- Wehman, P.; van Donge, H.M.A.; Hagos, A.; Kamer, P.C.J.; van Leeuwen, P.W.N.M. Influence of various P/N and P/P ligands on the palladium-catalysed reductive carbonylation of nitrobenzene. J. Organomet. Chem. 1997, 535, 183–193. [Google Scholar] [CrossRef]
- Bontempi, A.; Alessio, E.; Chanos, G.; Mestroni, G. Reductive carbonylation of nitroaromatic compounds to urethanes catalyzed by (di-1,10-phenanthroline)palladium bis(hexafluorophosphate) and related complexes. J. Mol. Catal. 1987, 42, 67–80. [Google Scholar] [CrossRef]
- Wehman, P.; Kaasjager, V.E.; Delange, W.G.J.; Hartl, F.; Kamer, P.C.J.; van Leeuwen, P.W.N.M.; Fraanje, J.; Goubitz, K. Subtle Balance between Various Phenanthroline Ligands and Anions in the Palladium-Catalyzed Reductive Carbonylation of Nitrobenzene. Organometallics 1995, 14, 3751–3761. [Google Scholar] [CrossRef]
- Ferretti, F.; Gallo, E.; Ragaini, F. Nitrogen ligands effects in the palladium-catalyzed carbonylation reaction of nitrobenzene to give N-methyl phenylcarbamate. J. Organomet. Chem. 2014, 771, 59–67. [Google Scholar] [CrossRef]
- Ferretti, F.; Ragaini, F.; Lariccia, R.; Gallo, E.; Cenini, S. New Nonsymmetric Phenanthrolines as Very Effective Ligands in the Palladium-Catalyzed Carbonylation of Nitrobenzene. Organometallics 2010, 29, 1465–1471. [Google Scholar] [CrossRef]
- Jenner, G.; Nahmed, E.M.; Leismann, H. Homogeneous Activation of th C-H Bond in Formates—Decarbonylation of Formates to Alcohols. J. Organomet. Chem. 1990, 387, 315–321. [Google Scholar] [CrossRef]
- Fouad, M.A.; Ferretti, F.; Formenti, D.; Milani, F.; Ragaini, F. Synthesis of Indoles by Reductive Cyclization of Nitro Compounds Using Formate Esters as CO Surrogates. Eur. J. Org. Chem. 2021, 2021, 4876–4894. [Google Scholar] [CrossRef]
- Cenini, S.; Crotti, C.; Pizzotti, M.; Porta, F. Ruthenium carbonyl catalyzed reductive carbonylation of aromatic nitro compounds. A selective route to carbamates. J. Org. Chem. 1988, 53, 1243–1250. [Google Scholar] [CrossRef]
- Cenini, S.; Pizzotti, M.; Crotti, C.; Ragaini, F.; Porta, F. Effects of neutral ligands in the reductive carbonylation of nitrobenzene catalyzed by Ru3(CO)12 and Rh6(CO)16. J. Mol. Catal. 1988, 49, 59–69. [Google Scholar] [CrossRef]
- Ben Taleb, A.; Jenner, G. Catalytic reduction of nitrobenzene to aniline with aqueous methyl formate. J. Organomet. Chem. 1993, 456, 263–269. [Google Scholar] [CrossRef]
- Formenti, D.; Ferretti, F.; Ragaini, F. Synthesis of N-Heterocycles by Reductive Cyclization of Nitro Compounds using Formate Esters as Carbon Monoxide Surrogates. ChemCatChem 2018, 10, 148–152. [Google Scholar] [CrossRef]
- Gasperini, M.; Ragaini, F.; Cazzaniga, C.; Cenini, S. Carbonylation of dinitrotoluene to dimethyl toluenedicarbamate; high efficiency of phosphorus acids as promoters for the palladium-phenanthroline catalytic system. Adv. Synth. Catal. 2005, 347, 105–120. [Google Scholar] [CrossRef]
- Oh, J.S.; Lee, S.M.; Yeo, J.K.; Lee, C.W.; Lee, J.S. Palladium-Catalyzed Synthesis of N,N′-Diphenylurea from Nitrobenzene, Aniline, and Carbon Monoxide. Ind. Eng. Chem. Res. 1991, 30, 1456–1461. [Google Scholar] [CrossRef]
- Gasperini, M.; Ragaini, F.; Remondini, C.; Caselli, A.; Cenini, S. The palladium-phenanthroline catalyzed carbonylation of nitroarenes to diarylureas: Effect of chloride and diphenylphosphinic acid. J. Organomet. Chem. 2005, 690, 4517–4529. [Google Scholar] [CrossRef]
- Hsieh, T.H.H.; Dong, V.M. Indole synthesis: Palladium-catalyzed C-H bond amination via reduction of nitroalkenes with carbon monoxide. Tetrahedron 2009, 65, 3062–3068. [Google Scholar] [CrossRef]
- Ferretti, F.; El-Atawy, M.A.; Muto, S.; Hagar, M.; Gallo, E.; Ragaini, F. Synthesis of Indoles by Palladium-Catalyzed Reductive Cyclization of β-Nitrostyrenes with Carbon Monoxide as the Reductant. Eur. J. Org. Chem. 2015, 2015, 5712–5715. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Ferretti, F.; Ragaini, F. Palladium-Catalyzed Intramolecular Cyclization of Nitroalkenes: Synthesis of Thienopyrroles. Eur. J. Org. Chem. 2017, 2017, 1902–1910. [Google Scholar] [CrossRef]
- EL-Atawy, M.A.; Ferretti, F.; Ragaini, F. A Synthetic Methodology for Pyrroles from Nitrodienes. Eur. J. Org. Chem. 2018, 2018, 4818–4825. [Google Scholar] [CrossRef]
- Ferretti, F.; Fouad, M.A.; Ragaini, F. Synthesis of Indoles by Palladium-Catalyzed Reductive Cyclization of Nitrostyrenes with Phenyl Formate as a CO Surrogate. Catalysts 2022, 12, 106. [Google Scholar] [CrossRef]
- Boger, D.L.; Weinreb, S.M. Hetero Diels-Alder Methodology in Organic Synthesis; Academic Press: New York, NY, USA, 1987. [Google Scholar]
- Carosso, S.; Miller, M.J. Nitroso Diels-Alder (NDA) Reaction as an Efficient Tool for the Functionalization of Diene-Containing Natural Products. Org. Biomol. Chem. 2014, 12, 7445–7468. [Google Scholar] [CrossRef] [PubMed]
- Maji, B.; Yamamoto, H. Catalytic Enantioselective Nitroso Diels-Alder Reaction. J. Am. Chem. Soc. 2015, 137, 15957–15963. [Google Scholar] [CrossRef]
- Ragaini, F.; Cenini, S.; Brignoli, D.; Gasperini, M.; Gallo, E. Synthesis of oxazines and N-arylpyrroles by reaction of unfunctionalized dienes with nitroarenes and carbon monoxide, catalyzed by palladium-phenanthroline complexes. J. Org. Chem. 2003, 68, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Ragaini, F.; Cenini, S.; Borsani, E.; Dompe, M.; Gallo, E.; Moret, M. Synthesis of N-arylpyrroles, hetero-Diels-Alder adducts, and allylic amines by reaction of unfunctionalized dienes with nitroarenes and carbon monoxide, catalyzed by Ru(CO)3(Ar-BIAN). Organometallics 2001, 20, 3390–3398. [Google Scholar] [CrossRef]
- EL-Atawy, M.A.; Formenti, D.; Ferretti, F.; Ragaini, F. Synthesis of 3,6-Dihydro-2H-[1,2]-Oxazines from Nitroarenes and Conjugated Dienes, Catalyzed by Palladium/Phenanthroline Complexes and Employing Phenyl Formate as a CO Surrogate. ChemCatChem 2018, 10, 4707–4717. [Google Scholar] [CrossRef]
- Crotti, C.; Cenini, S.; Bassoli, A.; Rindone, B.; Demartin, F. Synthesis of carbazole by triruthenium dodecacarbonyl catalyzed reductive carbonylation of 2-nitrobiphenyl: The crystal and molecular structure of Ru3(μ3-NC6H4-o-C6H5)2(CO)9. J. Mol. Catal. 1991, 70, 175–187. [Google Scholar] [CrossRef]
- Smitrovich, J.H.; Davies, I.W. Catalytic C-H functionalization driven by CO as a stoichiometric reductant: Application to carbazole synthesis. Org. Lett. 2004, 6, 533–535. [Google Scholar] [CrossRef]
- Ramadan, D.R.; Ferretti, F.; Ragaini, F. Catalytic reductive cyclization of 2-nitrobiphenyls using phenyl formate as CO surrogate: A robust synthesis of 9H-carbazoles. J. Catal. 2022, 409, 41–47. [Google Scholar] [CrossRef]
- Cenini, S.; Bettettini, E.; Fedele, M.; Tollari, S. Intramolecular amination catalyzed by ruthenium and palladium. Synthesis of 2-acyl indoles and 2-aryl quinolines by carbonylation of 2-nitrochalcones. J. Mol. Catal. A Chem. 1996, 111, 37–41. [Google Scholar] [CrossRef]
- Ragaini, F.; Sportiello, P.; Cenini, S. Investigation of the possible role of arylamine formation in the ortho-substituted nitroarenes reductive cyclization reactions to afford heterocycles. J. Organomet. Chem. 1999, 577, 283–291. [Google Scholar] [CrossRef]
- Fouad, M.A.; Ferretti, F.; Ragaini, F. Formic Acid as Carbon Monoxide Source in the Palladium-Catalyzed N-Heterocyclization of o-Nitrostyrenes to Indoles. J. Org. Chem. 2023, in press. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).