H2O2-Based Selective Oxidations Catalyzed by Supported Polyoxometalates: Recent Advances
Abstract
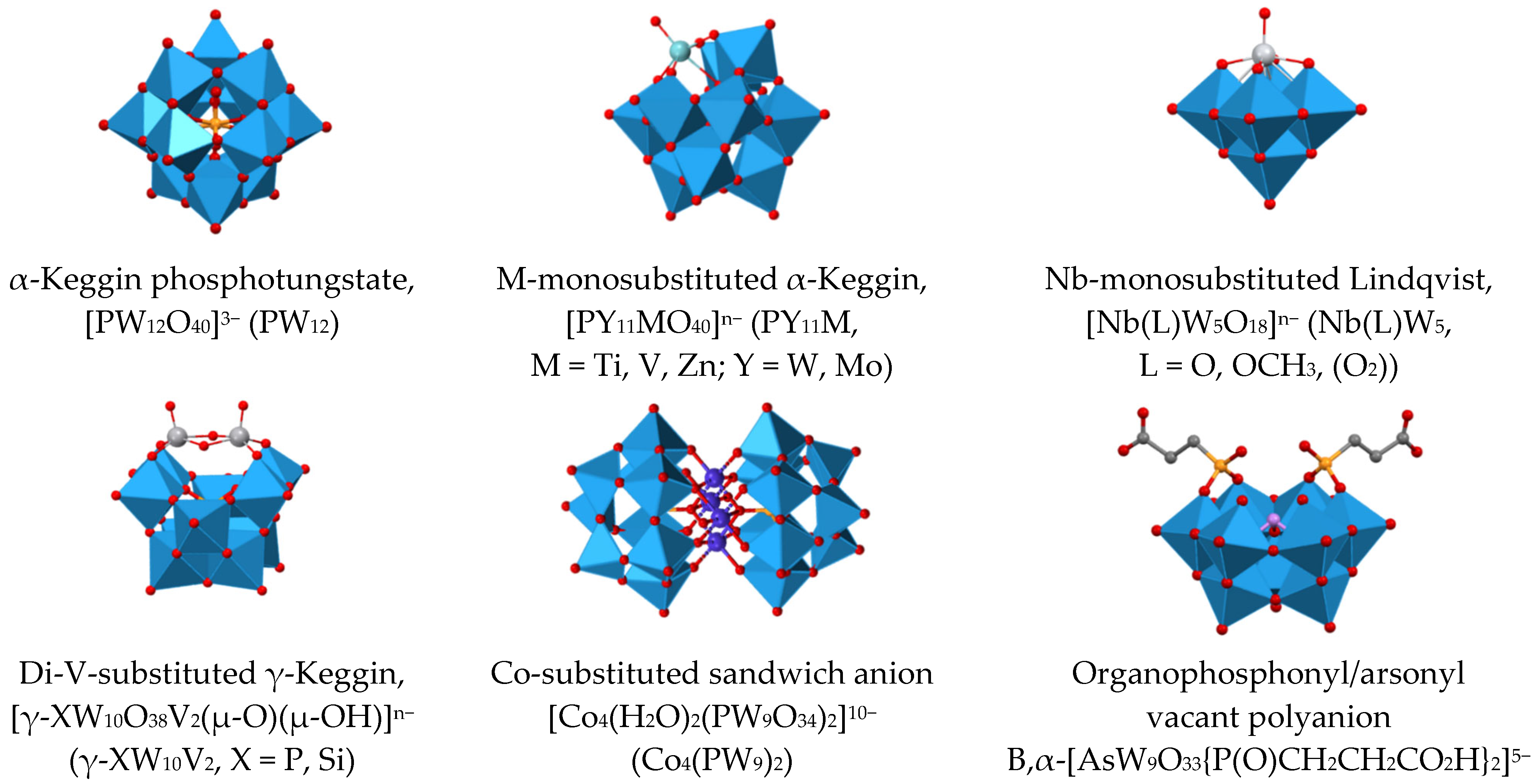
:1. Introduction
2. Supported POMs as Heterogeneous Catalysts for Selective Oxidations with H2O2
2.1. Embedding via Template Synthesis
2.2. Electrostatic Binding via Anion-Exchange
2.3. Inclusion in Metal–Organic Frameworks
| POM | MOF | Synthesis Method | Substrate/Conv. % | Product/ Selectivity, % | Nature of Catalysis a | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| PW12 | MIL-101(Cr) | Ads. b |  | 55 |  | 89 | Heterog. | [129] |
 | 50 |  | 61 | |||||
 | 72 |  | 76 | |||||
| PW4 | MIL-101(Cr) | Ads. |  | 75 |  | 77 | Heterog. | [129] |
| PW12 | MIL-101(Cr) | Solv. c |  | n.d. d |  | 92 e | Heterog. | [135] |
| IL-PW12 | MIL-101(Cr) | Post-synth. f |  | n.d. |  | 78 e | n.d. | [142] |
| PW11Ti | MIL-101(Cr) | Ads. |  | 88 |  | 100 | Heterog. g | [128] |
| Co4(PW9)2 | MIL-101(Cr) | Ads. |  | ˃99 |  | ˃99 | n.d. | [137] |
 | 57 |  | ˃99 | |||||
| PW9 | MIL-101(Cr) | Imp. i |  | 93 |  | 50 h | Heterog. | [138] |
 | ˃99 |  | ˃99 | |||||
| PMo11V | rho-ZIF | Mechanochem. |  | 83 |  | 89 | Heterog. | [141] |
 | 97 |  | 96 | |||||
| PW12 | UiO-66 | Solv. |  | 95 |  | 83 | n.d. | [147] |
| Co-PMo12 j | UiO-67 | Solv. |  | 82 |  | ˃99 | Heterog. | [149] |
| PMo12 | UiO-67 | Solv. | 75 | ˃99 | n.d. | |||
| POM | MOF | Synthesis Method | O/S, mol. Ratio | Sulfur Content, ppm | Desulfurization % | Nature of Catalysis a | Ref. |
|---|---|---|---|---|---|---|---|
| Tb(PW11)2 | MIL-101(Cr) | Imp. b | 21 | 1500 | ˃99 | Heterog. | [136] |
| PW9 | MIL-101(Cr) | Imp. | 21 | 1700 | ˃99 | Heterog. | [138] |
| PW4 | MIL-101(Cr) | Imp. | 5 | 2000 | ˃99 | n.d. c | [139] |
| EuW10 | ZIF-8 | Imp. | 1500 | 95 | n.d. | [140] | |
| PW12 | MOF-808 | Solv. d | 5 | 1000 e | 100 | Heterog. | [145] |
| PMo12 | MOF-808 | Solv. | 21 | 1500 | 92 | n.d. | [146] |
| PMo12 | UiO-66 | Solv. | 3 | 500 | 100 | n.d. | [150] |
| (mim(CH2)3COO)-PW12 f | UiO-66 | Solv. | 5 | 1000 e | 100 | n.d. | [143] |
| PW12 | UiO-66-D g | Solv. | 7 | 800 | 100 | n.d. | [151] |
| PW12 | UiO-66 | Mechanochem. | 5 | 1000 e | 100 | Heterog. | [144] |
| PW12 | UiO-67 | Solv. | 13 | 1000 | 100 | Heterog. | [148] |
| BMIM-PMo12 h | ZIF-8 | Solv. | 16 | 2000 | 92 | n.d. | [152] |
2.4. Covalent Anchoring in Hybrid Materials
2.5. Encapsulation within Supramolecular Complexes
2.6. Immobilization on Carbon Materials
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sheldon, R.; Arends, I.W.C.E.; Hanefeld, U. Green Chemistry and Catalysis; Wiley VCH: Weinheim, Germany, 2007. [Google Scholar]
- Clerici, M.G.; Kholdeeva, O.A. (Eds.) Liquid Phase Oxidation via Heterogeneous Catalysis: Organic Synthesis and Industrial Applications; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Duprez, D.; Cavani, F. (Eds.) Handbook of Advanced Methods and Processes in Oxidation Catalysis; Imperial College Press: London, UK, 2014. [Google Scholar]
- Jones, C.W. Application of Hydrogen Peroxide and Derivatives; Royal Society of Chemistry: Cambridge, UK, 1999. [Google Scholar]
- Strukul, G. (Ed.) Catalytic Oxidations with Hydrogen Peroxide as Oxidant; Kluwer: Dordrecht, The Netherlands, 1992. [Google Scholar]
- Strukul, G.; Scarso, A. Environmentally benign oxidants. In Liquid Phase Oxidation via Heterogeneous Catalysis: Organic Synthesis and Industrial Applications; Clerici, M.G., Kholdeeva, O.A., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 1–20. [Google Scholar]
- Strukul, G.; Menegazzo, F. (Eds.) Catalysts 2019, Special Issue on Direct Synthesis of Hydrogen Peroxide. Available online: https://www.mdpi.com/si/catalysts/hydrogen_peroxide (accessed on 10 December 2022).
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L.G. Hydrogen peroxide synthesis: An outlook beyond the anthraquinone process. Angew. Chem. Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.K.; Freakley, S.J.; Lewis, R.J.; Pritchard, J.C.; Hutchings, G.J. Advances in the direct synthesis of hydrogen peroxide from hydrogen and oxygen. Catal. Today 2015, 248, 3–9. [Google Scholar] [CrossRef]
- Ciriminna, R.; Albanese, L.; Meneguzzo, F.; Pagliaro, M. Hydrogen peroxide: A key chemical for today’s sustainable development. ChemSusChem 2016, 9, 3374–3381. [Google Scholar] [CrossRef]
- Ranganathan, S.; Sieber, V. Recent advances in the direct synthesis of hydrogen peroxide using chemical catalysis—A review. Catalysts 2018, 8, 379. [Google Scholar] [CrossRef]
- Menegazzo, F.; Signoretto, M.; Ghedini, E.; Strukul, G. Looking for the “dream catalyst” for hydrogen peroxide production from hydrogen and oxygen. Catalysts 2019, 9, 251. [Google Scholar] [CrossRef]
- Taramasso, M.; Perego, G.; Notari, B. Preparation of Porous Crystalline Synthetic Material Comprised of Silicon and Titanium Oxides. US Patent Application 4 410 501, 18 December 1983. [Google Scholar]
- Clerici, M.G.; Domine, M.E. Oxidation reactions catalyzed by transition-metal-substituted zeolites. In Liquid Phase Oxidation via Heterogeneous Catalysis: Organic Synthesis and Industrial Applications; Clerici, M.G., Kholdeeva, O.A., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 21–126. [Google Scholar]
- Romano, U.; Ricci, M. The hydroxylation of phenol to hydroquinone and catechol. In Liquid Phase Oxidation via Heterogeneous Catalysis: Organic Synthesis and Industrial Applications; Clerici, M.G., Kholdeeva, O.A., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 451–462. [Google Scholar]
- Rivetti, F.; Buzzoni, R. The greening of nylon: The ammoximation process. In Liquid Phase Oxidation via Heterogeneous Catalysis: Organic Synthesis and Industrial Applications; Clerici, M.G., Kholdeeva, O.A., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 462–474. [Google Scholar]
- Forlin, A.; Bergamo, M.; Lindner, J. Production of propylene oxide. In Liquid Phase Oxidation via Heterogeneous Catalysis: Organic Synthesis and Industrial Applications; Clerici, M.G., Kholdeeva, O.A., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 474–496. [Google Scholar]
- Cavani, F.; Teles, J.H. Sustainability in catalytic oxidation: An alternative approach or a structural evolution? ChemSusChem 2009, 2, 508–534. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.-S. Ordered mesoporous materials with improved stability and catalytic activity. Top. Catal. 2005, 35, 9–24. [Google Scholar] [CrossRef]
- Kholdeeva, O.A.; Mel’gunov, M.S.; Shmakov, A.N.; Trukhan, N.N.; Kriventsov, V.V.; Zaikovskii, V.I.; Malyshev, M.E.; Romannikov, V.N. A new mesoporous titanium-silicate Ti-MMM-2: A highly active and hydrothermally stable catalyst for H2O2-based selective oxidations. Catal. Today 2004, 91–92, 205–209. [Google Scholar] [CrossRef]
- Ivanchikova, I.D.; Kovalev, M.K.; Mel’gunov, M.S.; Shmakov, A.N.; Kholdeeva, O.A. User-friendly synthesis of highly selective and recyclable mesoporous titanium-silicate catalysts for the clean production of substituted p-benzoquinones. Catal. Sci. Technol. 2014, 4, 200–207. [Google Scholar] [CrossRef]
- Kholdeeva, O.A. Selective oxidations catalyzed by mesoporous metal silicates. In Liquid Phase Oxidation via Heterogeneous Catalysis: Organic Synthesis and Industrial Applications; Clerici, M.G., Kholdeeva, O.A., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 127–219. [Google Scholar]
- Kholdeeva, O.A. Recent developments in liquid-phase selective oxidation using environmentally benign oxidants and mesoporous metal silicates. Catal. Sci. Technol. 2014, 4, 1869–1889. [Google Scholar] [CrossRef]
- Bisio, C.; Carniato, F.; Guidotti, M. The control of the coordination chemistry for the genesis of heterogeneous catalytically active sites in oxidation reactions. Angew. Chem. Int. Ed. 2022, 61, e202209894. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, M.; Batonneau-Gener, I.; Gianotti, E.; Marchese, L.; Mignard, S.; Psaro, R.; Sgobba, M.; Ravasio, N. The effect of silylation on titanium-containing silica catalysts for the epoxidation of functionalised molecules. Micropor. Mesopor. Mater. 2008, 111, 39–47. [Google Scholar] [CrossRef]
- Guidotti, M.; Psaro, R.; Batonneau-Gener, I.; Gavrilova, E. Heterogeneous catalytic epoxidation: High limonene oxide yields by surface silylation of Ti-MCM-41. Chem. Eng. Technol. 2011, 34, 1924–1927. [Google Scholar] [CrossRef]
- Gallo, A.; Tiozzo, C.; Psaro, R.; Carniato, F.; Guidotti, M. Niobium metallocenes deposited onto mesoporous silica via dry impregnation as catalysts for selective epoxidation of alkenes. J. Catal. 2013, 298, 77–83. [Google Scholar] [CrossRef]
- Ivanchikova, I.D.; Maksimchuk, N.V.; Skobelev, I.Y.; Kaichev, V.V.; Kholdeeva, O.A. Mesoporous niobium-silicates prepared by evaporation-induced self-assembly as catalysts for selective oxidations with aqueous H2O2. J. Catal. 2015, 332, 138–148. [Google Scholar] [CrossRef]
- Kholdeeva, O.A.; Ivanchikova, I.D.; Maksimchuk, N.V.; Skobelev, I.Y. H2O2-based selective oxidations: Nb(V) versus Ti(IV). Catal. Today 2019, 333, 63–70. [Google Scholar] [CrossRef]
- Thornburg, N.E.; Thompson, A.B.; Notestein, J.M. Periodic trends in highly dispersed groups IV and V supported metal oxide catalysts for alkene epoxidation with H2O2. ACS Catal. 2015, 5, 5077–5088. [Google Scholar] [CrossRef]
- Hill, C.L.; Prosser-McCartha, C.M. Homogeneous catalysis by transition metal oxygen anion clusters. Coord. Chem. Rev. 1995, 143, 407–455. [Google Scholar] [CrossRef]
- Neumann, R. Polyoxometalates as catalysts for oxidation with hydrogen peroxide and molecular oxygen. In Transition Metals for Organic Synthesis, 2nd ed.; Beller, M., Bolm, C., Eds.; Wiley-VCH: Weinheim, Germany, 2004; Volume 2, pp. 415–426. [Google Scholar]
- Hill, C.L. Polyoxometalates: Reactivity. In Comprehensive Coordination Chemistry II; Wedd, A.G., Ed.; Elsevier Science: New York, NY, USA, 2004; Volume 4, pp. 679–759. [Google Scholar]
- Pope, M.T. Polyoxo anions: Synthesis and structure. In Comprehensive Coordination Chemistry II; Wedd, A.G., Ed.; Elsevier Science: New York, NY, USA, 2004; Volume 4, pp. 635–678. [Google Scholar]
- Mizuno, N.; Kamata, K.; Uchida, S.; Yamaguchi, K. Liquid-phase oxidations with hydrogen peroxide and molecular oxygen catalyzed by polyoxometalate-based compounds. In Modern Heterogeneous Oxidation Catalysis: Design, Reactions and Characterization; Mizuno, N., Ed.; WILEY-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2009; pp. 185–216. [Google Scholar]
- Neumann, R. Activation of molecular oxygen, polyoxometalates, and liquid-phase catalytic oxidation. Inorg. Chem. 2010, 49, 3594–3601. [Google Scholar] [CrossRef]
- Mizuno, N.; Yamaguchi, K.; Kamata, K. Epoxidation of olefins with hydrogen peroxide catalyzed by polyoxometalates. Coord. Chem. Rev. 2005, 249, 1944–1956. [Google Scholar] [CrossRef]
- Hill, C.L.; Kholdeeva, O.A. Selective liquid phase oxidations in the presence of supported polyoxometalates. In Liquid Phase Oxidation via Heterogeneous Catalysis: Organic Synthesis and Industrial Applications; Clerici, M.G., Kholdeeva, O.A., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 263–319. [Google Scholar]
- Sun, M.; Zhang, J.; Putaj, P.; Caps, V.; Lefebvre, F.; Pelletier, J.; Basset, J.-M. Catalytic oxidation of light alkanes (C1−C4) by heteropoly compounds. Chem. Rev. 2014, 114, 981–1019. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Yang, G.-Y. Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, I.A.; Schreiber, R.E.; Neumann, R. Dioxygen in polyoxometalate mediated reactions. Chem. Rev. 2018, 118, 2680–2717. [Google Scholar] [CrossRef]
- Carabineiro, H.; Villanneau, R.; Carrier, X.; Herson, P.; Lemos, F.; Ribeiro, F.R.; Proust, A.; Che, M. Zirconium-substituted isopolytungstates: Structural models for zirconia-supported tungsten catalysts. Inorg. Chem. 2006, 45, 1915–1923. [Google Scholar] [CrossRef]
- Kholdeeva, O.A. Titanium-monosubstituted polyoxometalates: Relation between homogeneous and heterogeneous Ti-single-site-based catalysis. Top. Catal. 2006, 40, 229–243. [Google Scholar] [CrossRef]
- Kholdeeva, O.A.; Maksimovskaya, R.I. Titanium- and Zirconium-monosubstituted polyoxometalates as molecular models for studying mechanisms of oxidation catalysis. J. Mol. Catal. A Chem. 2007, 262, 7–24. [Google Scholar] [CrossRef]
- Kholdeeva, O.A. Hydrogen peroxide activation over Ti(IV): What have we learned from studies on Ti-containing polyoxometalates? Eur. J. Inorg. Chem. 2013, 2013, 1595–1605. [Google Scholar] [CrossRef]
- Zhang, T.; Mazaud, L.; Chamoreau, L.-M.; Paris, C.; Guillemot, G.; Proust, A. Unveiling the active surface sites in heterogeneous titanium-based silicalite epoxidation catalysts: Input of silanol-functionalized polyoxotungstates as soluble analogues. ACS Catal. 2018, 8, 2330–2342. [Google Scholar] [CrossRef]
- Solé-Daura, A.; Zhang, T.; Fouilloux, H.; Robert, C.; Thomas, C.M.; Chamoreau, L.-M.; Carbó, J.J.; Proust, A.; Guillemot, G.; Poblet, J.M. Catalyst design for alkene epoxidation by molecular analogues of heterogeneous titanium-silicalite catalysts. ACS Catal. 2020, 10, 4737–4750. [Google Scholar] [CrossRef]
- Pope, M.T.; Muüller, A. (Eds.) Polyoxometalate Chemistry: From Topology via Self-Assembly to Applications; Kluwer: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Kozhevnikov, I.V. Catalysis by Polyoxometalates; Wiley: Chichester, UK, 2002. [Google Scholar]
- Hill, C.L. (Ed.) Special Issue on Polyoxometalates in Catalysis. J. Mol. Catal. A Chem. 2007, 262, 1–242. [Google Scholar]
- Weinstock, I.A. (Ed.) Thematic issue on Frontiers in Metal Oxide Cluster Science. Isr. J. Chem. 2011, 51, 169–302. [Google Scholar]
- Cronin, L.; Muüller, A. (Eds.) Themed collection on Polyoxometalate Cluster Science. Chem. Soc. Rev. 2012, 41, 7325–7648. [Google Scholar]
- Carraro, M.; Fiorani, G.; Sartorel, A.; Bonchio, M. Polyoxometalates catalysts for sustainable oxidations and energy applications. In Handbook of Advanced Methods and Processes in Oxidation Catalysis; Duprez, D., Cavani, F., Eds.; Imperial College Press: London, UK, 2014; pp. 586–630. [Google Scholar]
- Wu, L. Organically encapsulated polyoxometalate catalysts: Supramolecular composition and synergistic catalysis. In Encapsulated Catalysts; Sadjadi, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–33. [Google Scholar]
- Kholdeeva, O.A. Recent progress in selective oxidations with hydrogen peroxide catalyzed by polyoxometalates. In Frontiers of Green Catalytic Selective Oxidations. Green Chemistry and Sustainable Technology; Bryliakov, K.P., Ed.; Springer: Singapore, 2019; pp. 61–91. [Google Scholar]
- Enferadi-Kerenkan, A.; Do, T.-O.; Kaliaguine, S. Heterogeneous catalysis by tungsten-based heteropoly compounds. Catal. Sci. Technol. 2018, 8, 2257–2284. [Google Scholar] [CrossRef]
- Evtushok, V.Y.; Lopatkin, V.A.; Podyacheva, O.Y.; Kholdeeva, O.A. Immobilization of polyoxometalates on carbon nanotubes: Tuning catalyst activity, selectivity and stability in H2O2-based oxidations. Catalysts 2022, 12, 472. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Wallau, M.; Arends, I.W.C.E.; Schuchardt, U. Heterogeneous catalysts for liquid-phase oxidations: Philosophers’ stones or Trojan horses? Acc. Chem. Res. 1998, 31, 485–493. [Google Scholar] [CrossRef]
- Izumi, Y. Recent advances in immobilization of heteropolyacids. Res. Chem. Intermediat. 1998, 24, 461–471. [Google Scholar] [CrossRef]
- Vazylyev, M.; Sloboda-Rozner, D.; Haimov, A.; Maayan, G.; Neumann, R. Strategies for oxidation catalyzed by polyoxometalates at the interface of homogeneous and heterogeneous catalysis. Top. Catal. 2005, 34, 93–99. [Google Scholar] [CrossRef]
- Mizuno, N.; Kamata, K.; Yamaguchi, K. Liquid-phase oxidations catalyzed by polyoxometalates. In Surface and Nanomolecular Catalysis; Richards, R., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2006; pp. 463–492. [Google Scholar]
- Proust, A.; Thouvenot, R.; Gouzerh, P. Functionalization of polyoxometalates: Towards advanced applications in catalysis and materials science. Chem. Commun. 2008, 2008, 1837–1852. [Google Scholar] [CrossRef]
- Mizuno, N.; Kamata, K.; Yamaguchi, K. Green oxidation reactions by polyoxometalate-based catalysts: From molecular to solid catalysts. Top. Catal. 2010, 53, 876–893. [Google Scholar] [CrossRef]
- Dolbecq, A.; Dumas, E.; Mayer, C.R.; Mialane, P. Hybrid organic−inorganic polyoxometalate compounds: From structural diversity to applications. Chem. Rev. 2010, 110, 6009–6048. [Google Scholar] [CrossRef]
- Kholdeeva, O.A.; Maksimchuk, N.V.; Maksimov, G.M. Polyoxometalate-based heterogeneous catalysts for liquid phase selective oxidations: Comparison of different strategies. Catal. Today 2010, 157, 107–113. [Google Scholar] [CrossRef]
- Proust, A.; Matt, B.; Villanneau, R.; Guillemot, G.; Gouzerh, P.; Izzet, G. Functionalization and post-functionalization: A step towards polyoxometalate-based materials. Chem. Soc. Rev. 2012, 41, 7605–7622. [Google Scholar] [CrossRef]
- Song, Y.F.; Tsunashima, R. Recent advances on polyoxometalate-based molecular and composite materials. Chem. Soc. Rev. 2012, 41, 7384–7402. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, G.; Long, Z.; Wang, J. Recent advances in polyoxometalate-based heterogeneous catalytic materials for liquid-phase organic transformations. RSC Adv. 2014, 4, 42092–42113. [Google Scholar] [CrossRef]
- Rafiee, E.; Eavani, S. Heterogenization of heteropoly compounds: A review of their structure and synthesis. RSC Adv. 2016, 6, 46433–46466. [Google Scholar] [CrossRef]
- Cherevan, A.S.; Nandan, S.P.; Roger, I.; Liu, R.; Streb, C.; Eder, D. Polyoxometalates on functional substrates: Concepts, synergies, and future perspectives. Adv. Sci. 2020, 7, 1903511. [Google Scholar] [CrossRef] [PubMed]
- Rhule, J.T.; Neiwert, W.A.; Hardcastle, K.I.; Do, B.T.; Hill, C.L. Ag5PMo10V2O40, a heterogeneous catalyst for air-based selective oxidation at ambient temperature. J. Am. Chem. Soc. 2001, 123, 12101–12102. [Google Scholar] [CrossRef] [PubMed]
- Okuhara, T. Water-tolerant solid acid catalysts. Chem. Rev. 2002, 102, 3641–3666. [Google Scholar] [CrossRef] [PubMed]
- Plault, L.; Hauseler, A.; Nlate, S.; Astruc, D.; Ruiz, J.; Gatard, S.; Neumann, R. Synthesis of dendritic polyoxometalate complexes assembled by ionic bonding and their function as recoverable and reusable oxidation catalysts. Angew. Chem. Int. Ed. 2004, 43, 2924–2928. [Google Scholar] [CrossRef]
- Mizuno, N.; Uchida, S.; Kamata, K.; Ishimoto, R.; Nojima, S.; Yonehara, K.; Sumida, Y. A flexible nonporous heterogeneous catalyst for size-selective oxidation through a bottom-up approach. Angew. Chem. Int. Ed. 2010, 49, 9972–9976. [Google Scholar] [CrossRef]
- Mirante, F.; Dias, L.; Silva, M.; Ribeiro, S.O.; Corvo, M.C.; de Castro, B.; Granadeiro, C.M.; Balula, S.S. Efficient heterogeneous polyoxometalate-hybrid catalysts for the oxidative desulfurization of fuels. Catal. Commun. 2018, 104, 1–8. [Google Scholar] [CrossRef]
- Kholdeeva, O.A.; Vanina, M.P.; Timofeeva, M.N.; Maksimovskaya, R.I.; Trubitsina, T.A.; Melgunov, M.S.; Burgina, E.B.; Mrowiec-Bialon, J.; Jarzebski, A.B.; Hill, C.L. Co-containing polyoxometalate-based heterogeneous catalysts for the selective aerobic oxidation of aldehydes under ambient conditions. J. Catal. 2004, 226, 363–371. [Google Scholar] [CrossRef]
- Maksimchuk, N.V.; Melgunov, M.S.; Mrowiec-Białoń, J.; Jarzębski, A.B.; Kholdeeva, O.A. H2O2-based allylic oxidation of α-pinene over different single site catalysts. J Catal. 2005, 235, 175–183. [Google Scholar] [CrossRef]
- Dufaud, V.; Lefebvre, F.; Niccolai, G.P.; Aouine, M. New insights into the encapsulation and stabilization of heteropolyacids inside the pore walls of mesostructured silica materials. J. Mater. Chem. 2009, 19, 1142–1150. [Google Scholar] [CrossRef]
- Sousa, J.L.C.; Santos, I.C.M.S.; Simões, M.M.Q.; Cavaleiro, J.A.S.; Nogueira, H.I.S.; Cavaleiro, A.M.V. Iron(III)-substituted polyoxotungstates immobilized on silica nanoparticles: Novel oxidative heterogeneous catalysts. Catal. Commun. 2011, 12, 459–463. [Google Scholar] [CrossRef]
- Jalkh, C.; Ghazaly, C.; El-Rassy, H. Entrapment vs. immobilization of polyoxometalates into silica and titania aerogels: Application in heterogeneous oxidation catalysis. Mater. Chem. Phys. 2020, 252, 123296. [Google Scholar] [CrossRef]
- Yun, S.K.; Pinnavaia, T.J. Layered double hydroxides intercalated by polyoxometalate anions with Keggin (α-H2W12O406−), Dawson (α-P2W18O626−), and Finke (Co4(H2O)2(PW9O34)210−) structures. Inorg. Chem. 1996, 35, 6853–6860. [Google Scholar] [CrossRef]
- Jana, S.K.; Kubota, Y.; Tatsumi, T. Cobalt-substituted polyoxometalate pillared hydrotalcite: Synthesis and catalysis in liquid-phase oxidation of cyclohexanol with molecular oxygen. J. Catal. 2008, 255, 40–47. [Google Scholar] [CrossRef]
- Omwoma, S.; Chen, W.; Tsunashima, R.; Song, Y.F. Recent advances on polyoxometalates intercalated layered double hydroxides: From synthetic approaches to functional material applications. Coord. Chem. Rev. 2014, 258–259, 58–71. [Google Scholar] [CrossRef]
- Li, T.; Miras, H.N.; Song, Y.-F. Polyoxometalate (POM)-layered double hydroxides (LDH) composite materials: Design and catalytic applications. Catalysts 2017, 7, 260. [Google Scholar] [CrossRef]
- Neumann, R.; Miller, H.J. Alkene oxidation in water using hydrophobic silica particles derivatized with polyoxometalates as catalysts. J. Chem. Soc. Chem. Commun. 1995, 1995, 2277–2278. [Google Scholar] [CrossRef]
- Kamada, M.; Kominami, H.; Kera, Y. Deposition and interaction of phosphododecatungstate on a silica gel surface modified with a silane coupling agent having anilino groups. J. Colloid Interface Sci. 1996, 182, 297–300. [Google Scholar] [CrossRef]
- Okun, N.M.; Anderson, T.M.; Hill, C.L. [(FeIII(OH2)2)3(A-α-PW9O34)2]9− on cationic silica nanoparticles, a new type of material and efficient heterogeneous catalyst for aerobic oxidations. J. Am. Chem. Soc. 2003, 125, 3194–3195. [Google Scholar] [CrossRef] [PubMed]
- Kasai, J.; Nakagawa, Y.; Uchida, S.; Yamaguchi, K.; Mizuno, N. [γ-1,2-H2SiV2W10O40] immobilized on surface-modified SiO2 as a heterogeneous catalyst for liquid-phase oxidation with H2O2. Chem. Eur. J. 2006, 12, 4176–4184. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, A.; Lefebvre, F.; Halligudi, S.B. Selective oxidation of anthracene using inorganic–organic hybrid materials based on molybdovanadophosphoric acids. J. Catal. 2007, 247, 166–175. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yoshida, C.; Uchida, S.; Mizuno, N. Peroxotungstate immobilized on ionic liquid-modified silica as a heterogeneous epoxidation catalyst with hydrogen peroxide. J. Am. Chem. Soc. 2005, 127, 530–531. [Google Scholar] [CrossRef]
- Kato, C.N.; Tanabe, A.; Negishi, S.; Goto, K.; Nomiya, K. An efficient PMo11VVO404−/silica material having cationic ammonium moiety: Synthesis, characterization, and catalytic performance for oxidation of alcohols with dioxygen. Chem. Lett. 2005, 34, 238–239. [Google Scholar] [CrossRef]
- Maksimchuk, N.V.; Melgunov, M.S.; Chesalov, Y.A.; Mrowiec-Białoń, J.; Jarzebski, A.B.; Kholdeeva, O.A. Aerobic oxidations of α-pinene over cobalt-substituted polyoxometalate supported on amino-modified mesoporous silicates. J. Catal. 2007, 246, 241–248. [Google Scholar] [CrossRef]
- Inumaru, K.; Ishihara, T.; Kamiya, Y.; Okuhara, T.; Yamanaka, S. Water-tolerant, highly active solid acid catalysts composed of the Keggin-type polyoxometalate H3PW12O40 immobilized in hydrophobic nanospaces of organomodified mesoporous silica. Angew. Chem. Int. Ed. 2007, 46, 7625–7676. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, K.; Bi, L.-H.; Suchopar, A.; Reicke, M.; Mathys, G.; Jaensch, H.; Kortz, U.; Richards, R.M. Solvent-free aerobic oxidation of n-alkane by iron(iii)-substituted polyoxotungstates immobilized on SBA-15. Inorg. Chem. 2007, 46, 8457–8459. [Google Scholar] [CrossRef]
- Panchenko, V.N.; Borbáth, I.; Timofeeva, M.N.; Góbölös, S. Amine-modified silica NH2–(CH2)x–SiO2 (x = 0, 2, 3) as support for cobalt-substituted polyoxometalate TBA4HPW11CoO39: Effect of the nature of the support on the oxidation activity. J. Mol. Catal. A Chem. 2010, 319, 119–125. [Google Scholar] [CrossRef]
- Kovalchuk, T.V.; Sfihi, H.; Zaitsev, V.N.; Fraissard, J. Preparation and characterization of catalysts based on oniumsilica-immobilized Keggin acids. Catal. Today 2011, 169, 138–149. [Google Scholar] [CrossRef]
- Balula, S.S.; Cunha-Silva, L.; Santos, I.C.M.S.; Estrada, A.C.; Fernandes, A.C.; Cavaleiro, J.A.S.; Pires, J.; Freirea, C.; Cavaleiro, A.M.V. Mono-substituted silicotungstates as active catalysts for sustainable oxidations: Homo- and heterogeneous performance. New J. Chem. 2013, 37, 2341–2350. [Google Scholar] [CrossRef]
- Mirante, F.; Ribeiro, S.O.; de Castro, B.; Granadeiro, C.M.; Balula, S.S. Sustainable desulfurization processes catalyzed by titanium-polyoxometalate@TM-SBA-15. Top. Catal. 2017, 60, 1140–1150. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Granadeiro, C.M.; Almeida, P.L.; Pires, J.; Valenca, R.; Campos-Martin, J.M.; Ribeiro, J.C.; de Castro, B.; Balula, S.S. Effective zinc-substituted Keggin composite to catalyze the removal of sulfur from real diesels under a solvent-free system. Ind. Eng. Chem. Res. 2019, 58, 18540–18549. [Google Scholar] [CrossRef]
- Johnson, B.J.S.; Stein, A. Surface modification of mesoporous, macroporous, and amorphous silica with catalytically active polyoxometalate clusters. Inorg. Chem. 2001, 40, 801–808. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.; Hu, C.; Wang, Y.; Wang, E. Preparation of surface modifications of mesoporous titania with monosubstituted Keggin units and their catalytic performance for organochlorine pesticide and dyes under UV irradiation. Appl. Catal. A General 2004, 273, 201–210. [Google Scholar] [CrossRef]
- Errington, R.J.; Petkar, S.S.; Horrocks, B.R.; Houlton, A.; Lie, L.H.; Patole, S.N. Covalent immobilization of a TiW5 polyoxometalate on derivatized silicon surfaces. Angew. Chem. Int. Ed. 2005, 44, 1254–1257. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao, R.; Yue, B.; Gu, M.; Pei, S.; He, H. Synthesis, characterization and catalytic application of SBA-15 immobilized rare earth metal sandwiched polyoxometalates. J. Mol. Catal. A Chemical 2007, 270, 50–55. [Google Scholar] [CrossRef]
- Joo, N.; Renaudineau, S.; Delapierre, G.; Bidan, G.; Chamoreau, L.-M.; Thouvenot, R.; Gouzerh, P.; Proust, A. Organosilyl/-germyl polyoxotungstate hybrids for covalent grafting onto silicon surfaces: Towards molecular memories. Chem. Eur. J. 2010, 16, 5043–5051. [Google Scholar] [CrossRef]
- Luo, X.; Yang, C. Photochromic ordered mesoporous hybrid materials based on covalently grafted polyoxometalates. Phys. Chem. Chem. Phys. 2011, 13, 7892–7902. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhou, L.; Guo, Z.; Du, X.; Lei, J. Preparation of ordered meso/macroporous HPW/titania–silica catalyst for efficient oxidative desulfurization of model fuel. J. Porous Mater. 2019, 26, 1069–1077. [Google Scholar] [CrossRef]
- Yan, X.-M.; Mei, P.; Xiong, L.; Gao, L.; Yang, Q.; Gong, L. Mesoporous titania–silica–polyoxometalate nanocomposite materials for catalytic oxidation desulfurization of fuel oil. Catal. Sci. Technol. 2013, 3, 1985–1992. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Ferreira, R.A.S.; Soares-Santos, P.C.R.; Carlos, L.D.; Trindadea, T.; Nogueira, H.I.S. Lanthanopolyoxotungstates in silica nanoparticles: Multi-wavelength photoluminescent core/shell materials. J. Mater. Chem. 2010, 20, 3313–3318. [Google Scholar] [CrossRef]
- Gao, H.; Wu, X.; Sun, D.; Niu, G.; Guan, J.; Meng, X.; Liu, C.; Xia, W.; Song, X. Preparation of core–shell PW12@TiO2 micro-spheres and oxidative desulfurization performance. Dalton Trans. 2019, 48, 5749–5755. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, S.; Song, Y.-F. An efficient heterogeneous catalyst based on highly dispersed Na7H2LaW10O36·32H2O nanoparticles on mesoporous silica for deep desulfurization. Appl. Catal. A Gen. 2013, 466, 307–314. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Nogueira, L.S.; Gago, S.; Almeida, P.L.; Corvd, M.C.; de Castro, B.; Granadeiro, C.M.; Balula, S.S. Desulfurization process conciliating heterogeneous oxidation and liquid extraction: Organic solvent or centrifugation/water? Appl. Catal. A Gen. 2017, 542, 359–367. [Google Scholar] [CrossRef]
- Leng, Y.; Zhang, W.; Wang, J.; Jiang, P. A novel heteropolyanion-based amino-containing cross-linked ionic copolymer catalyst for epoxidation of alkenes with H2O2. Appl. Catal. A Gen. 2012, 445–446, 306–311. [Google Scholar] [CrossRef]
- Doherty, S.; Knight, J.G.; Ellison, J.R.; Weekes, D.; Harrington, R.W.; Hardacre, C.; Manya, H. An efficient recyclable peroxometalate-based polymer-immobilised ionic liquid phase (PIILP) catalyst for hydrogen peroxide-mediated oxidation. Green Chem. 2012, 14, 925–929. [Google Scholar] [CrossRef]
- Romanenko, I.; Lechner, M.; Wendler, F.; Horenz, C.; Streb, C.; Schacher, F.H. POMbranes: Polyoxometalate-functionalized block copolymer membranes for oxidation catalysis. J. Mater. Chem. A 2017, 5, 15789–15796. [Google Scholar] [CrossRef]
- Li, T.; Wang, Z.; Chen, W.; Miras, H.N.; Song, Y.-F. Rational design of a polyoxometalate intercalated layered double hydroxide: Highly efficient catalytic epoxidation of allylic alcohols under mild and solvent-free conditions. Chem. Eur. J. 2017, 23, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.K.; Férey, G.; Lee, U.H.; Chang, J.S. Liquid phase oxidation of organic compounds by metal-organic frameworks. In Liquid Phase Oxidation via Heterogeneous Catalysis: Organic Synthesis and Industrial Applications; Clerici, M.G., Kholdeeva, O.A., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 371–409. [Google Scholar]
- Yu, R.; Kuang, X.-F.; Wua, X.-Y.; Lua, C.-Z.; Donahue, J.P. Stabilization and immobilization of polyoxometalates in porous coordination polymers through host–guest interactions. Coord. Chem. Rev. 2009, 253, 2872–2890. [Google Scholar] [CrossRef]
- Maksimchuk, N.V.; Kholdeeva, O.A.; Kovalenko, K.A.; Fedin, V.P. MIL-101 Supported polyoxometalates: Synthesis, characterization, and catalytic applications in selective liquid-phase oxidation. Isr. J. Chem. 2011, 51, 281–289. [Google Scholar] [CrossRef]
- Du, D.Y.; Qin, J.S.; Li, S.L.; Su, Z.M.; Lan, Y.Q. Recent advances in porous polyoxometalate based metal–organic framework materials. Chem. Soc. Rev. 2014, 43, 4615–4632. [Google Scholar] [CrossRef]
- Kholdeeva, O.A. Liquid-phase selective oxidation catalysis with metal-organic frameworks. Catal. Today 2016, 278, 22–29. [Google Scholar] [CrossRef]
- Freire, C.; Fernandes, D.M.; Nunes, M.; Abdelkader, V.K. POM & MOF-based electrocatalysts for energy-related reactions. Chem. Cat. Chem. 2018, 10, 1703–1730. [Google Scholar]
- Samaniyan, M.; Mirzaei, M.; Khajavian, R.; Eshtiagh-Hosseini, H.; Streb, C. Heterogeneous catalysis by polyoxometalates in metal−organic frameworks. ACS Catal. 2019, 9, 10174–10191. [Google Scholar] [CrossRef]
- Buru, C.T.; Farha, O.K. Strategies for incorporating catalytically active polyoxometalates in metal−organic frameworks for organic transformations. ACS Appl. Mater. Interfaces 2020, 12, 5345–5360. [Google Scholar] [CrossRef]
- Piscopo, C.G.; Granadeiro, C.M.; Balula, S.S.; Bošković, D. Metal-organic framework-based catalysts for oxidative desulfurization. ChemCatChem 2020, 12, 4721–4731. [Google Scholar] [CrossRef]
- Sun, J.; Abednatanzi, S.; Van Der Voort, P.; Liu, Y.-Y.; Leus, K. POM@MOF hybrids: Synthesis and applications. Catalysts 2020, 10, 578. [Google Scholar] [CrossRef]
- Mialane, P.; Mellot-Draznieks, C.; Gairola, P.; Duguet, M.; Benseghir, Y.; Oms, O.; Dolbecq, A. Heterogenisation of polyoxometalates and other metal-based complexes in metal–organic frameworks: From synthesis to characterisation and applications in catalysis. Chem. Soc. Rev. 2021, 50, 6152–6220. [Google Scholar] [CrossRef] [PubMed]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Maksimchuk, N.V.; Timofeeva, M.N.; Melgunov, M.S.; Shmakov, A.N.; Chesalov, Y.A.; Dybtsev, D.N.; Fedin, V.P.; Kholdeeva, O.A. Heterogeneous selective oxidation catalysts based on coordination polymer MIL-101 and transition metal substituted polyoxometalates. J. Catal. 2008, 257, 315–323. [Google Scholar] [CrossRef]
- Maksimchuk, N.V.; Kovalenko, K.A.; Arzumanov, S.S.; Chesalov, Y.A.; Stepanov, A.G.; Fedin, V.P.; Kholdeeva, O.A. Hybrid polyoxotungstate/MIL-101 materials: Synthesis, characterization, and catalysis of H2O2-based alkene epoxidation. Inorg. Chem. 2010, 49, 2920–2930. [Google Scholar] [CrossRef] [PubMed]
- Aubry, C.; Chottard, G.; Platzer, N.; Bregeault, J.-M.; Thouvenot, R.; Chauveau, F.; Huet, C.; Ledon, H. Reinvestigation of epoxidation using tungsten-based precursors and hydrogen peroxide in a biphase medium. Inorg. Chem. 1991, 30, 4409–4415. [Google Scholar] [CrossRef]
- Duncan, D.C.; Chambers, R.C.; Hecht, E.; Hill, C.L. Mechanism and dynamics in the H3[PW12O40]-catalyzed selective epoxidation of terminal olefins by H2O2. Formation, reactivity, and stability of {PO4[WO(O2)2] 4}3−. J. Am. Chem. Soc. 1995, 117, 681–691. [Google Scholar] [CrossRef]
- Bailey, A.J.; Griffith, W.P.; Parkin, B.C.; Bailey, A.J.; Griffith, W.P.; Parkin, B.C. Heteropolyperoxo- and isopolyperoxo-tungstates and-molybdates as catalysts for the oxidation of tertiary amines, alkenes and alcohols. J. Chem. Soc. Dalton Trans. 1995, 1995, 1833–1837. [Google Scholar] [CrossRef]
- Bregeault, J.-M. Transition-metal complexes for liquid-phase catalytic oxidation: Some aspects of industrial reactions and of emerging technologies. Dalton Trans. 2003, 2003, 3289–3302. [Google Scholar] [CrossRef]
- Küsgens, P.; Rose, M.; Senkovska, I.; Fröde, H.; Henschel, A.; Siegle, S.; Kaskel, S. Characterization of metal-organic frameworks by water adsorption. Micropor. Mesopor. Mater. 2009, 120, 325–330. [Google Scholar] [CrossRef]
- Bromberg, L.; Diao, Y.; Wu, H.; Speakman, T.A.; Hatton, S.A. Chromium(III) terephthalate metal organic framework (MIL-101): HF-free synthesis, structure, polyoxometalate composites, and catalytic properties. Chem. Mater. 2012, 24, 1664–1675. [Google Scholar] [CrossRef]
- Ribeiro, S.; Granadeiro, C.M.; Silva, P.; Paz, F.A.A.; de Biani, F.F.; Cunha-Silva, L.; Balula, S.S. An efficient oxidative desulfurization process using terbium-polyoxometalate@MIL-101(Cr). Catal. Sci. Technol. 2013, 3, 2404–2414. [Google Scholar] [CrossRef]
- Balula, S.S.; Granadeiro, C.M.; Barbosa, A.D.S.; Santos, I.C.M.S.; Cunha-Silva, L. Multifunctional catalyst based on sandwich-type polyoxotungstate and MIL-101 for liquid phase oxidations. Catal. Today 2013, 210, 142–148. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Barbosa, A.D.S.; Ribeiro, S.; Santos, I.C.M.S.; de Castro, B.; Cunha-Silva, L.; Balula, S.S. Oxidative catalytic versatility of a trivacant polyoxotungstate incorporated into MIL-101(Cr). Catal. Sci. Technol. 2014, 4, 1416–1425. [Google Scholar] [CrossRef]
- Gao, Y.; Mirante, F.; de Castro, B.; Zhao, J.; Cunha-Silva, L.; Balula, S.S. An effective hybrid heterogeneous catalyst to desulfurize diesel: Peroxotungstate@metal–organic framework. Molecules 2020, 25, 5494. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Flores, D.; Silva, D.; Santos-Vieira, I.; Mirante, F.; Granadeiro, C.M.; Balula, S.S. Lindqvist@nanoporous MOF-based catalyst for effective desulfurization of fuels. Nanomaterials 2022, 12, 2887. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Duan, Y.; Yang, F.; Wei, W.; Xu, Y.; Hu, C. Efficient mechanochemical synthesis of polyoxometalate⊂ZIF complexes as reusable catalysts for highly selective oxidation. Inorg. Chem. 2017, 56, 14506–14512. [Google Scholar] [CrossRef]
- Wang, L.; Lub, J.; Wang, Y.; Wang, H.; Wang, J.; Ren, T. Preparation and characterization of novel cyclohexene-to-adipic acid catalyst with ionic liquid phosphotungstate immobilized on MIL-101 nanocages based on Cr-N coordination. J. Mol. Struct. 2023, 1271, 133973. [Google Scholar] [CrossRef]
- Qi, Z.; Huang, Z.; Wang, H.; Li, L.; Ye, C.; Qiu, T. In situ bridging encapsulation of a carboxyl-functionalized phosphotungstic acid ionic liquid in UiO-66: A remarkable catalyst for oxidative desulfurization. Chem. Eng. Sci. 2020, 225, 115818. [Google Scholar] [CrossRef]
- Wu, C.; Sun, Z.; Ye, C.; Qi, Z.; Chen, J.; Huang, Z.; Qiu, T. Encapsulation of HPW and preparation of composites rich in Zr-defects by manual grinding: Synergistic catalysis for efficient oxidative desulfurization at room temperature. Chem. Eng. J. 2023, 451, 138906. [Google Scholar] [CrossRef]
- Lin, Z.-J.; Zheng, H.-Q.; Chen, J.; Zhuang, W.-E.; Lin, Y.-X.; Su, J.-W.; Huang, Y.-B.; Cao, R. Encapsulation of phosphotungstic acid into metal−organic frameworks with tunable window sizes: Screening of PTA@MOF catalysts for efficient oxidative desulfurization. Inorg. Chem. 2018, 57, 13009–13019. [Google Scholar] [CrossRef]
- Fernandes, S.C.; Viana, A.M.; de Castro, B.; Cunha-Silva, L.; Balula, S.S. Synergistic combination of the nanoporous system of MOF-808 with a polyoxomolybdate to design an effective catalyst: Simultaneous oxidative desulfurization and denitrogenation processes. Sustain. Energy Fuels 2021, 5, 4032–4040. [Google Scholar] [CrossRef]
- Yang, X.-L.; Qiao, L.-M.; Dai, W.-L. Phosphotungstic acid encapsulated in metal-organic framework UiO-66: An effective catalyst for the selective oxidation of cyclopentene to glutaraldehyde. Micropor. Mesopor. Mater. 2015, 211, 73–81. [Google Scholar] [CrossRef]
- Peng, Y.-L.; Liu, J.; Zhang, H.-F.; Luo, D.; Li, D. A size-matched POM@MOF composite catalyst for highly efficient and recyclable ultra-deep oxidative fuel desulfurization. Inorg. Chem. Front. 2018, 5, 1563–1569. [Google Scholar] [CrossRef]
- Song, X.; Hu, D.; Yang, X.; Zhang, H.; Zhang, W.; Li, J.; Jia, M.; Yu, J. Polyoxomolybdic cobalt encapsulated within Zr-based metal−organic frameworks as efficient heterogeneous catalysts for olefins epoxidation. ACS Sustain. Chem. Eng. 2019, 7, 3624–3631. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Zhang, B.; Yang, X.; Chang, X.; Zhou, Z.; Wang, D.-H.; Zhang, M.-H.; Bu, X.-H. Synergistic effect of Zr-MOF on phosphomolybdic acid promotes efficient oxidative desulfurization. Appl. Catal. B Environ. 2019, 256, 117804. [Google Scholar] [CrossRef]
- Ma, Y.; Li, A.; Wang, C.; Ge, X. Preparation of HPW@UiO-66 catalyst with defects and its application in oxidative desulfurization. Chem. Eng. J. 2021, 404, 127062. [Google Scholar] [CrossRef]
- Silva, D.F.; Viana, A.M.; Santos-Vieira, I.; Balula, S.S.; Cunha-Silva, L. Ionic liquid-based polyoxometalate incorporated at ZIF-8: A sustainable catalyst to combine desulfurization and denitrogenation processes. Molecules 2022, 27, 1711. [Google Scholar] [CrossRef]
- Miras, H.N.; Vilá-Nadal, L.; Cronin, L. Polyoxometalate based open-frameworks (POM-OFs). Chem. Soc. Rev. 2014, 43, 5679–5699. [Google Scholar] [CrossRef]
- Song, J.; Luo, Z.; Britt, D.K.; Furukawa, H.; Yaghi, O.M.; Hardcastle, K.I.; Hill, C.L. A multiunit catalyst with synergistic stability and reactivity: A polyoxometalatemetal organic framework for aerobic decontamination. J. Am. Chem. Soc. 2011, 133, 6839–16846. [Google Scholar] [CrossRef]
- Zou, C.; Zhang, Z.; Xu, X.; Gong, Q.; Li, J.; Wu, C.-D. A multifunctional organic–inorganic hybrid structure based on MnIII−porphyrin and polyoxometalate as a highly effective dye scavenger and heterogenous catalyst. J. Am. Chem. Soc. 2012, 134, 87–90. [Google Scholar] [CrossRef]
- Han, Q.; He, C.; Zhao, M.; Qi, B.; Niu, J.; Duan, C. Engineering chiral polyoxometalate hybrid metal−organic frameworks for asymmetric dihydroxylation of olefins. J. Am. Chem. Soc. 2013, 135, 10186–10189. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, Q.; Li, Y.; Qiu, Y.; Ma, P.; Niu, J.; Wang, J. A stable polyoxometalate-based metal−organic framework as highly efficient heterogeneous catalyst for oxidation of alcohols. Inorg. Chem. 2019, 58, 4945–4953. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, B.; Wang, Y.; Wang, H.; Hu, X.; Ma, P.; Niu, J.; Wang, J. Polyoxometalate-incorporated framework as a heterogeneous catalyst for selective oxidation of C−H bonds of alkylbenzenes. Inorg. Chem. 2021, 60, 7753–7761. [Google Scholar] [CrossRef] [PubMed]
- Gouzerh, P.; Proust, A. Main-group element, organic, and organometallic derivatives of polyoxometalates. Chem. Rev. 1998, 98, 77–112. [Google Scholar] [CrossRef] [PubMed]
- Villanneau, R.; Marzouk, A.; Wang, Y.; Djamaa, A.B.; Laugel, G.; Proust, A.; Launay, F. Covalent grafting of organic−inorganic polyoxometalates hybrids onto mesoporous SBA-15: A key step for new anchored homogeneous catalysts. Inorg. Chem. 2013, 52, 2958–2965. [Google Scholar] [CrossRef]
- Bentaleb, F.; Makrygenni, O.; Brouri, D.; Diogo, C.C.; Mehdi, A.; Proust, A.; Launay, F.; Villanneau, R. Efficiency of polyoxometalate-based mesoporous hybrids as covalently anchored catalysts. Inorg. Chem. 2015, 54, 7607–7616. [Google Scholar] [CrossRef]
- Makrygenni, O.; Brouri, D.; Proust, A.; Launay, F.; Villanneau, R. Immobilization of polyoxometalate hybrid catalysts onto mesoporous silica supports using phenylene diisothiocyanate as a cross-linking agent. Micropor. Mesopor. Mater. 2019, 278, 314–321. [Google Scholar] [CrossRef]
- Cravena, M.; Xiao, D.; Kunstmann-Olsena, C.; Kozhevnikova, E.F.; Blanca, F.; Steiner, A.; Kozhevnikov, I.V. Oxidative desulfurization of diesel fuel catalyzed by polyoxometalate immobilized on phosphazene-functionalized silica. Appl. Catal. B Environ. 2018, 231, 82–91. [Google Scholar] [CrossRef]
- Liu, Y.; Zuo, P.; Wang, R.; Liu, Y.; Jiao, W. Covalent immobilization of Dawson polyoxometalates on hairy particles and its catalytic properties for the oxidation desulfurization of tetrahydrothiophene. J. Cleaner Production 2020, 274, 122774. [Google Scholar] [CrossRef]
- Nisar, A.; Zhuang, J.; Wang, X. Construction of amphiphilic polyoxometalate mesostructures as a highly efficient desulfurization catalyst. Adv. Mater. 2011, 23, 1130–1135. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, S.; Ji, Y.; Song, Y.-F. Deep desulfurization by amphiphilic lanthanide-containing polyoxometalates in ionic-liquid emulsion systems under mild conditions. Chem. Eur. J. 2013, 19, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Li, H.; Wu, L. A novel, luminescent, silica-sol–gel hybrid basedon surfactant-encapsulated polyoxometalates. Adv. Mater. 2007, 19, 1983–1987. [Google Scholar] [CrossRef]
- Qi, W.; Wu, L. Polyoxometalate/polymer hybrid materials: Fabrication and properties. Polym. Int. 2009, 58, 1217–1225. [Google Scholar] [CrossRef]
- Qi, W.; Wang, Y.; Li, W.; Wu, L. Surfactant-encapsulated polyoxometalates as immobilized supramolecular catalysts for highly efficient and selective oxidation reactions. Chem. Eur. J. 2010, 16, 1068–1078. [Google Scholar] [CrossRef]
- Yan, Y.; Wu, L. Polyoxometalate-incorporated supramolecular self assemblies: Structures and functional properties. Isr. J. Chem. 2011, 51, 181–190. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Y.Z.; Li, B.; Wu, L.X. Polyoxometalate complexes for oxidative kinetic resolution of secondary alcohols: Unique effects of chiral environment, immobilization and aggregation. Dalton Trans. 2014, 43, 9177–9188. [Google Scholar] [CrossRef]
- Nogueira, L.S.; Ribeiro, S.; Granadeiro, C.M.; Pereira, E.; Feio, G.; Cunha-Silva, L.; Balula, S.S. Novel polyoxometalate silica nano-sized spheres: Efficient catalysts for olefin oxidation and the deep desulfurization process. Dalton Trans. 2014, 43, 9518–9528. [Google Scholar] [CrossRef]
- Chen, X.; Yang, A.; Wang, G.; Wei, M.; Liu, N.; Li, B.; Wu, L. Reinforced catalytic oxidation of polyoxometalate@charge transfer complex by on-site heating from photothermal conversion. Chem. Eng. J. 2022, 446, 137134. [Google Scholar] [CrossRef]
- Izumi, Y.; Urabe, K. Catalysis of heteropoly acids entrapped in activated carbon. Chem. Lett. 1981, 10, 663–666. [Google Scholar] [CrossRef]
- Schwegler, M.A.; Vinke, P.; van der Eijk, M.; van Bekkum, H. Activated carbon as a support for heteropolyanion catalysts. Appl. Catal. 1992, 80, 41–57. [Google Scholar] [CrossRef]
- Evtushok, V.Y.; Suboch, A.N.; Podyacheva, O.Y.; Stonkus, O.A.; Zaikovskii, V.I.; Chesalov, Y.A.; Kibis, L.S.; Kholdeeva, O.A. Highly efficient catalysts based on divanadium-substituted polyoxometalate and N-doped carbon nanotubes for selective oxidation of alkylphenols. ACS Catal. 2018, 8, 1297–1307. [Google Scholar] [CrossRef]
- Eder, D. Carbon nanotube−inorganic hybrids. Chem. Rev. 2010, 110, 1348–1385. [Google Scholar] [CrossRef]
- Shearer, C.J.; Cherevan, A.; Eder, D. Application of functional hybrids incorporating carbon nanotubes or graphene. In Carbon Nanotubes and Graphene; Tanaka, K., Iijima, S., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Chapter 16; pp. 387–433. [Google Scholar]
- Shearer, C.J.; Cherevan, A.; Eder, D. Application and future challenges of functional nanocarbon hybrids. Adv. Mater. 2014, 26, 2295–2318. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Huang, L.; Hu, J.; Streb, C.; Song, Y.F. Polyoxometalate-functionalized nanocarbon materials for energy conversion, energy storage and sensor systems. Energy Environ. Sci. 2015, 8, 776–789. [Google Scholar] [CrossRef]
- Toma, F.M.; Sartorel, A.; Iurlo, M.; Carraro, M.; Parisse, P.; Maccato, C.; Rapino, S.; Gonzalez, B.R.; Amenitsch, H.; Da Ros, T.; et al. Efficient water oxidation at carbon nanotube–polyoxometalate electrocatalytic interfaces. Nat. Chem. 2010, 2, 826–831. [Google Scholar] [CrossRef]
- Akter, T.; Hu, K.; Lian, K. Investigations of multilayer polyoxometalates-modified carbon nanotubes for electrochemical capacitors. Electrochim. Acta 2011, 56, 4966–4971. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, R.; Zhang, G.; Zheng, Y.; Zhao, J. Oxidative desulfurization of model fuel in the presence of molecular oxygen over polyoxometalate based catalysts supported on carbon nanotubes. Fuel 2018, 224, 261–270. [Google Scholar] [CrossRef]
- Jawale, D.V.; Fossard, F.; Miserque, F.; Geertsen, V.; Teillout, A.L.; de Oliveira, P.; Mbomekalle, I.M.; Gravel, E.; Doris, E. Carbon nanotube-polyoxometalate nanohybrids as efficient electro-catalysts for the hydrogen evolution reaction. Carbon 2022, 188, 523–532. [Google Scholar] [CrossRef]
- Ma, D.; Liang, L.; Chen, W.; Liu, H.; Song, Y.F. Covalently tethered polyoxometalate-pyrene hybrids for noncovalent side-wall functionalization of single-walled carbon nanotubes as high-performance anode material. Adv. Funct. Mater. 2013, 23, 6100–6105. [Google Scholar] [CrossRef]
- Chen, W.; Huang, L.; Hu, J.; Li, T.; Jia, F.; Song, Y.F. Connecting carbon nanotubes to polyoxometalate clusters for engineering high-performance anode materials. Phys. Chem. Chem. Phys. 2014, 16, 19668–19673. [Google Scholar] [CrossRef]
- Kawasaki, N.; Wang, H.; Nakanishi, R.; Hamanaka, S.; Kitaura, R.; Shinohara, H.; Yokoyama, T.; Yoshikawa, H.; Awaga, K. Nanohybridization of polyoxometalate clusters and single-wall carbon nanotubes: Applications in molecular cluster batteries. Angew. Chem. Int. Ed. 2011, 50, 3471–3474. [Google Scholar] [CrossRef]
- Ji, Y.; Hu, J.; Huang, L.; Chen, W.; Streb, C.; Song, Y.F. Covalent attachment of Anderson-type polyoxometalates to single-walled carbon nanotubes gives enhanced performance electrodes for lithium ion batteries. Chem. Eur. J. 2015, 21, 6469–6474. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Al-Oweini, R.; Friedl, J.; Lee, C.Y.; Li, L.; Kortz, U.; Stimming, U.; Srinivasan, M. A novel SWCNT-polyoxometalate nanohybrid material as an electrode for electrochemical supercapacitors. Nanoscale 2015, 7, 7934–7941. [Google Scholar] [CrossRef]
- Hu, J.; Ji, Y.; Chen, W.; Streb, C.; Song, Y.F. “Wiring” redox-active polyoxometalates to carbon nanotubes using a sonication-driven periodic functionalization strategy. Energy Environ. Sci. 2016, 9, 1095–1101. [Google Scholar] [CrossRef]
- Hajian, R.; Alghour, Z. Selective oxidation of alcohols with H2O2 catalyzed by zinc polyoxometalate immobilized on multi-wall carbon nanotubes modified with ionic liquid. Chin. Chem. Lett. 2017, 28, 971–975. [Google Scholar] [CrossRef]
- Salavati, H.; Tangestaninejad, S.; Moghadam, M.; Mirkhani, V.; Mohammadpoor-Baltork, I. Sonocatalytic epoxidation of alkenes by vanadium-containing polyphosphomolybdate immobilized on multi-wall carbon nanotubes. Ultrason. Sonochemistry 2010, 17, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yu, F.; Zhang, G.; Zhao, H. Performance evaluation of the carbon nanotubes supported Cs2.5H0.5PW12O40 as efficient and recoverable catalyst for the oxidative removal of dibenzothiophene. Catal. Today 2010, 150, 37–41. [Google Scholar] [CrossRef]
- Evtushok, V.Y.; Ivanchikova, I.D.; Podyacheva, O.Y.; Stonkus, O.A.; Suboch, A.N.; Chesalov, Y.A.; Zalomaeva, O.V.; Kholdeeva, O.A. Carbon Nanotubes modified by Venturello complex as highly efficient catalysts for alkene and thioethers oxidation with hydrogen peroxide. Front. Chem. 2019, 7, 858. [Google Scholar] [CrossRef]
- Evtushok, V.Y.; Podyacheva, O.Y.; Suboch, A.N.; Maksimchuk, N.V.; Stonkus, O.A.; Kibis, L.S.; Kholdeeva, O.A. H2O2-based selective oxidations by divanadium-substituted polyoxotungstate supported on nitrogen-doped carbon nanomaterials. Catal. Today 2020, 354, 196–203. [Google Scholar] [CrossRef]
- Evtushok, V.Y.; Ivanchikova, I.D.; Lopatkin, V.A.; Maksimchuk, N.V.; Podyacheva, O.Y.; Suboch, A.N.; Stonkus, O.A.; Kholdeeva, O.A. Heterolytic alkene oxidation with H2O2 catalyzed by Nb-substituted Lindqvist tungstates immobilized on carbon nanotubes. Catal. Sci. Technol. 2021, 11, 3198–3207. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Shen, J.-J.; Wu, J.; Liang, X.-Y.; Xu, J.; Liu, P.; Xue, B.; Li, Y.-X. An amphiphilic graphene oxide-immobilized polyoxometalate-based ionic liquid: A highly efficient triphase transfer catalyst for the selective oxidation of alcohols with aqueous H2O2. Mol. Catal. 2017, 443, 262–269. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Modarres, M. Clicked graphene oxide supported Venturello catalyst: A new hybrid nanomaterial as catalyst for the selective epoxidation of olefins. Mater. Chem. Phys. 2017, 199, 522–527. [Google Scholar] [CrossRef]
- Gan, M.; Yang, G.; Wang, Z.; Sui, X.; Hou, Y. Highly efficient oxidative desulfurization catalyzed by a polyoxometalate/carbonized cellulose nanofiber composite. Energy Fuels 2020, 34, 778–786. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Li, Y.; Wang, S.; Wang, X. Polyoxometalate immobilized on graphene via click reaction for simultaneous dismutation of H2O2 and oxidation of sulfur mustard simulant. ACS Appl. Nano Mater. 2019, 2, 6971–6981. [Google Scholar] [CrossRef]
- Podyacheva, O.Y.; Ismagilov, Z.R. Nitrogen-doped carbon nanomaterials: To the mechanism of growth, electrical conductivity and application in catalysis. Catal. Today 2015, 249, 12–22. [Google Scholar] [CrossRef]
- Arrigo, R.; Schuster, M.E.; Xie, Z.; Yi, Y.; Wowsnick, G.; Sun, L.L.; Hermann, K.E.; Friedrich, M.; Kast, P.; Hävecker, M.; et al. Nature of the N-Pd interaction in nitrogen-doped carbon nanotube catalysts. ACS Catal. 2015, 5, 2740–2753. [Google Scholar] [CrossRef]
- Cao, Y.; Mao, S.; Li, M.; Chen, Y.; Wang, Y. Metal/porous carbon composites for heterogeneous catalysis: Old catalysts with improved performance promoted by N-doping. ACS Catal. 2017, 7, 8090–8112. [Google Scholar] [CrossRef]
- Skobelev, I.Y.; Evtushok, V.Y.; Kholdeeva, O.A.; Maksimchuk, N.V.; Maksimovskaya, R.I.; Ricart, J.M.; Poblet, J.M.; Carbó, J.J. Understanding the regioselectivity of aromatic hydroxylation over divanadium-substituted γ-Keggin polyoxotungstate. ACS Catal. 2017, 7, 8514–8523. [Google Scholar] [CrossRef]
- Wang, Y.; Kamata, K.; Ishimoto, R.; Ogasawara, Y.; Suzuki, K.; Yamaguchi, K.; Mizuno, N. Composites of [γ-H2PV2W10O40]3− and [α-SiW12O40]4− supported on Fe2O3 as heterogeneous catalysts for selective oxidation with aqueous hydrogen peroxide. Catal. Sci. Technol. 2015, 5, 2602–2611. [Google Scholar] [CrossRef]
- Kholdeeva, O.A.; Zalomaeva, O.V. Recent advances in transition-metal-catalyzed selective oxidation of substituted phenols and methoxyarenes with environmentally benign oxidants. Coord. Chem. Rev. 2016, 306, 302–330. [Google Scholar] [CrossRef]
- Maksimchuk, N.V.; Maksimov, G.M.; Evtushok, V.Y.; Ivanchikova, I.D.; Chesalov, Y.A.; Maksimovskaya, R.I.; Kholdeeva, O.A.; Sole-Daura, A.; Poblet, J.M.; Carbo, J.J. Relevance of protons in heterolytic activation of H2O2 over Nb(V): Insights from model studies on Nb-substituted polyoxometalates. ACS Catal. 2018, 8, 9722–9737. [Google Scholar] [CrossRef]
- Zalomaeva, O.V.; Maksimchuk, N.V.; Maksimov, G.M.; Kholdeeva, O.A. Thioether oxidation with H2O2 catalyzed by Nb-substituted polyoxotungstates: Mechanistic insights. Eur. J. Inorg. Chem. 2019, 2019, 410–416. [Google Scholar] [CrossRef]








| Catalyst | Substrate/Conversion, % | Epoxide Selectivity, % | |
|---|---|---|---|
| AsW9-P(O)COOH |  | 96 | ˃99 |
| AsW9-P(O)COOH@SBA-NH2 | 76 | ˃99 | |
| PW9-As(O)NH2 | 97 | ˃99 | |
| PW9-As(O)NH2@SBA-COOH | 19 | ˃99 | |
| AsW9-P(O)COOH@MCF-NH2 | 41 | ˃99 | |
| AsW9-P(O)COOH |  | 73 | 81 |
| AsW9-P(O)COOH@SBA-NH2 | 75 | 94 | |
| POM | Support | H+ Added, Equiv. a | Substrate | Substrate Conv. % | Epoxide Selectivity, b % | Ref. |
|---|---|---|---|---|---|---|
| PW4 | CNTs | 0.2 |  | 66 | 79 | [194] |
| PW10V2 c | CNTs | 0 | 82 | 79 (100) | [195] | |
| HNb(O2)W5 | CNTs | 0 | 32 | 39 (93) | [196] | |
| HNb(O2)W5 | CNTs | 2 | 59 | 15 (90) | [196] | |
| HNb(O2)W5 | N-CNTs | 2 | 30 | 23 (81) | [196] | |
| PW4 | CNTs | 2 |  | 93 | 97 | [194] |
| HNb(O2)W5 | CNTs | 2 | 94 | 98 | [196] | |
| HNb(O2)W5 | N-CNTs | 2 | 70 | 97 | [196] | |
| PW4 | CNTs | 2 |  | 100 | 65 | [194] |
| HNb(O2)W5 | CNTs | 2 | 100 | 55 (100) | [196] | |
| HNb(O2)W5 | CNTs | 0 | 100 | 60 (99) | [196] | |
| PW4 | CNTs | 0 |  | 50 | 80 | [194] |
| HNb(O2)W5 | CNTs | 2 | 80 | 25 (77) | [196] | |
| PW4 | CNTs | 0 |  | 85 | 85 | [194] |
| HNb(O2)W5 | CNTs | 0 | 80 | 67 (94) | [196] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksimchuk, N.V.; Kholdeeva, O.A. H2O2-Based Selective Oxidations Catalyzed by Supported Polyoxometalates: Recent Advances. Catalysts 2023, 13, 360. https://doi.org/10.3390/catal13020360
Maksimchuk NV, Kholdeeva OA. H2O2-Based Selective Oxidations Catalyzed by Supported Polyoxometalates: Recent Advances. Catalysts. 2023; 13(2):360. https://doi.org/10.3390/catal13020360
Chicago/Turabian StyleMaksimchuk, Nataliya V., and Oxana A. Kholdeeva. 2023. "H2O2-Based Selective Oxidations Catalyzed by Supported Polyoxometalates: Recent Advances" Catalysts 13, no. 2: 360. https://doi.org/10.3390/catal13020360
APA StyleMaksimchuk, N. V., & Kholdeeva, O. A. (2023). H2O2-Based Selective Oxidations Catalyzed by Supported Polyoxometalates: Recent Advances. Catalysts, 13(2), 360. https://doi.org/10.3390/catal13020360







