Composition and Morphology Modulation of Bimetallic Nitride Nanostructures on Nickel Foams for Efficient Oxygen Evolution Electrocatalysis
Abstract
:1. Introduction
2. Results and Discussion
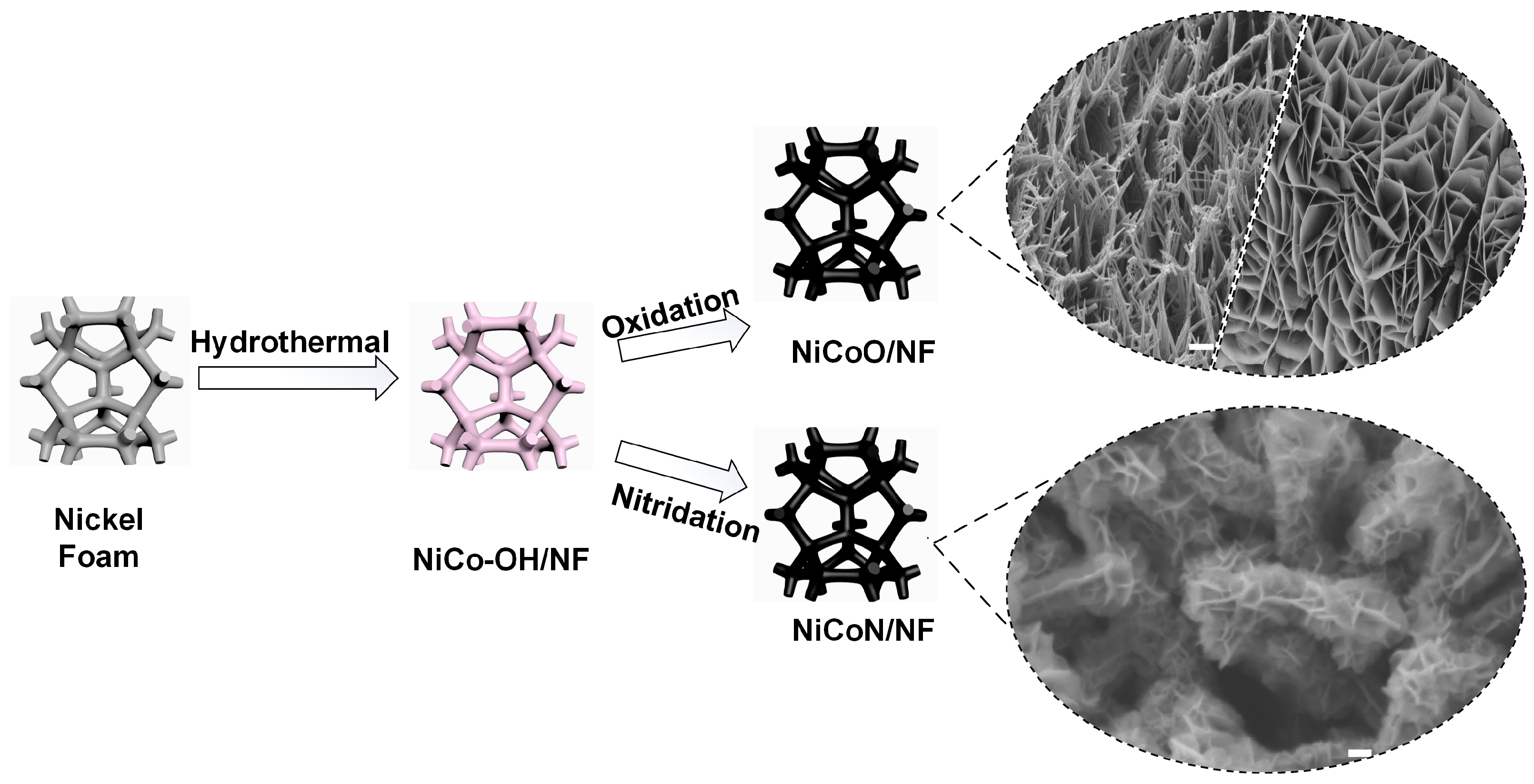
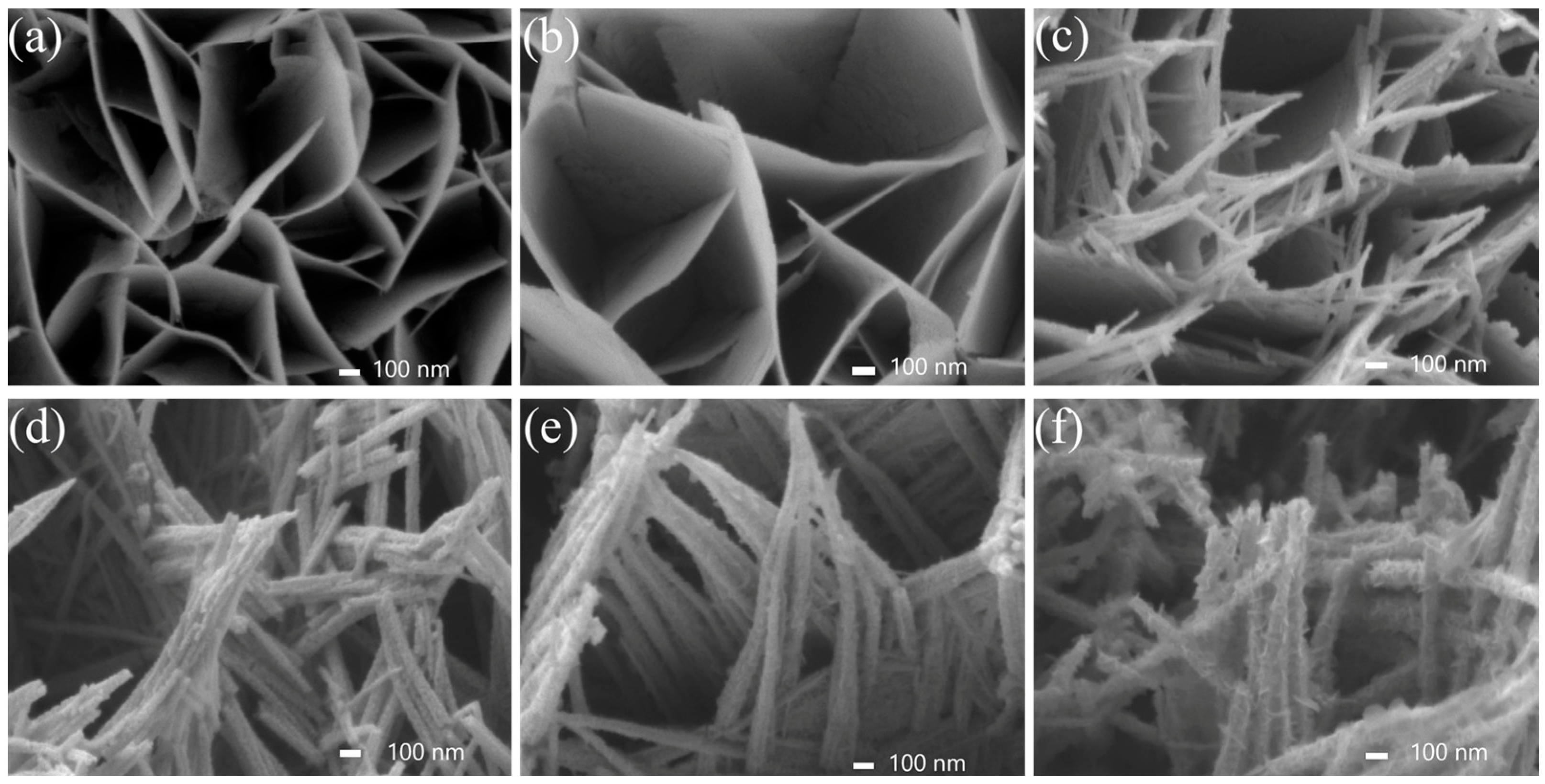
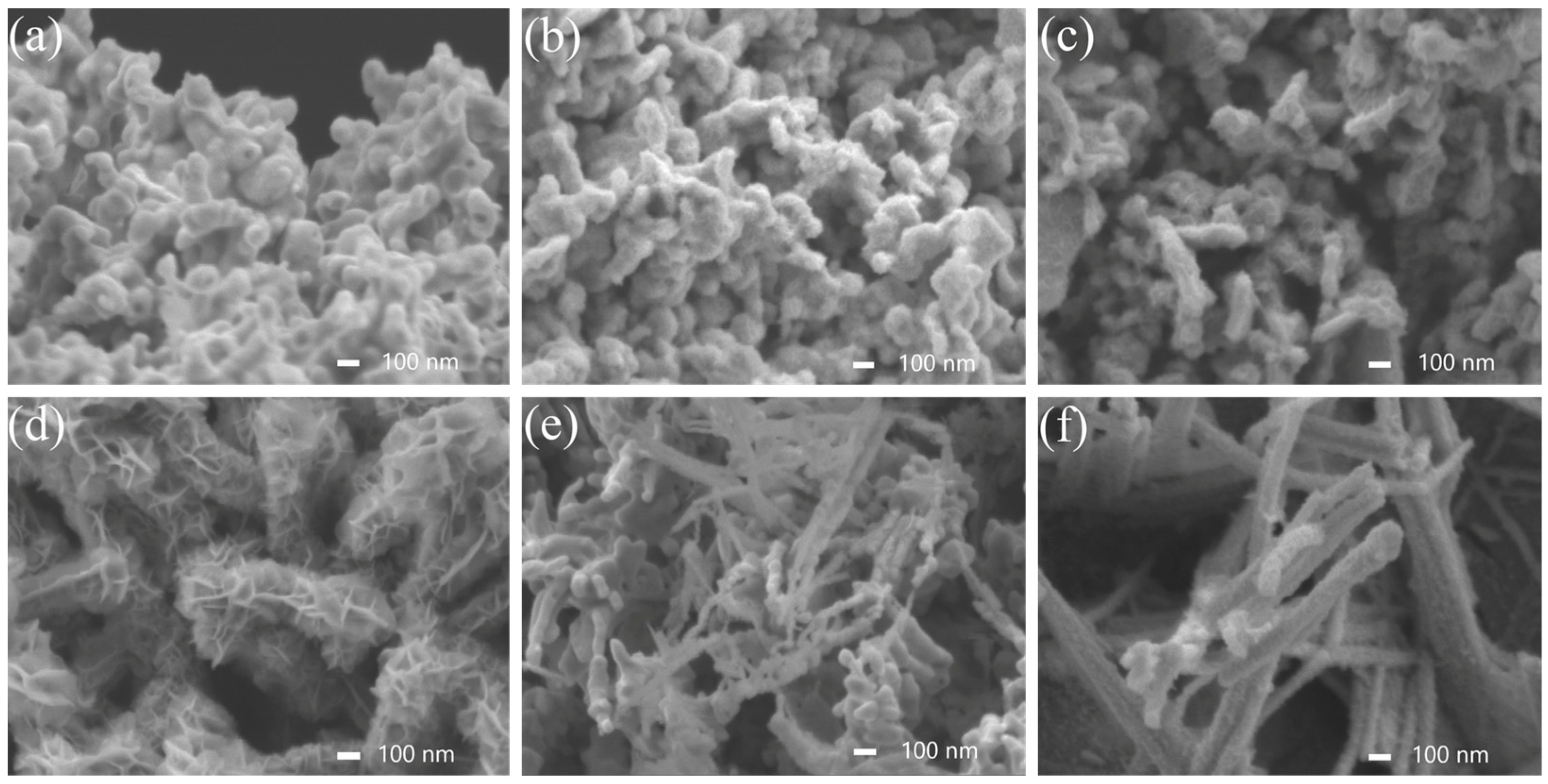
2.1. Morphology
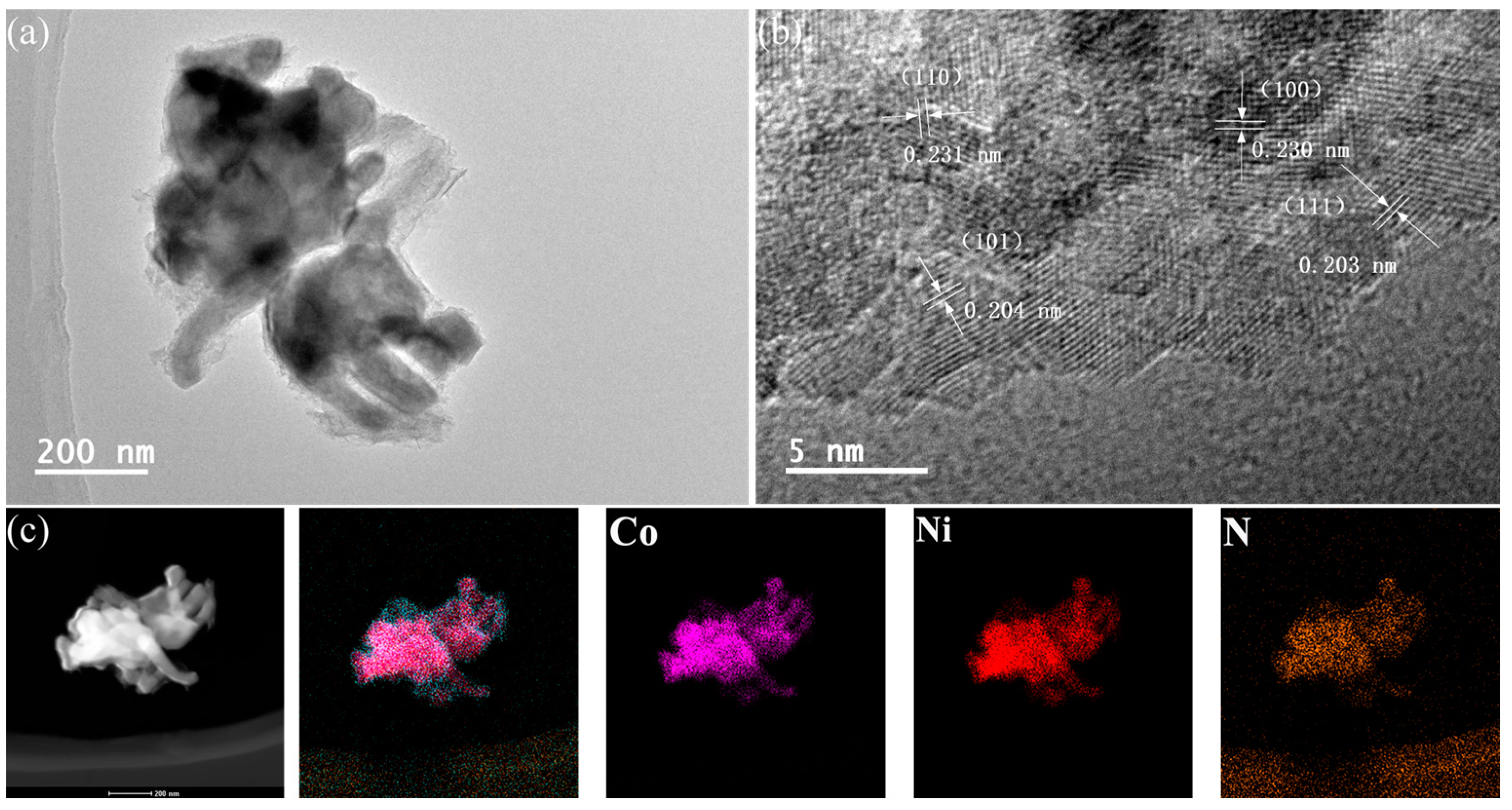
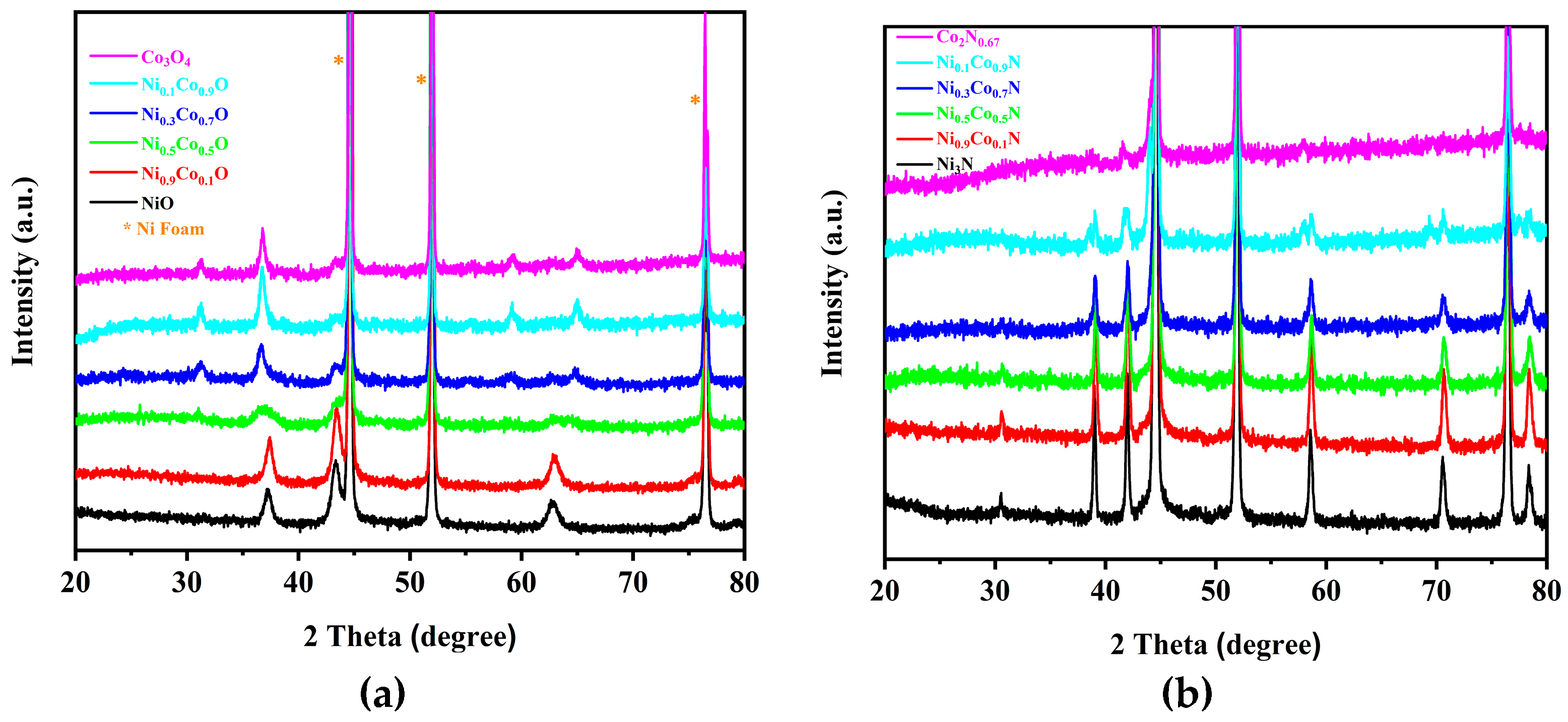
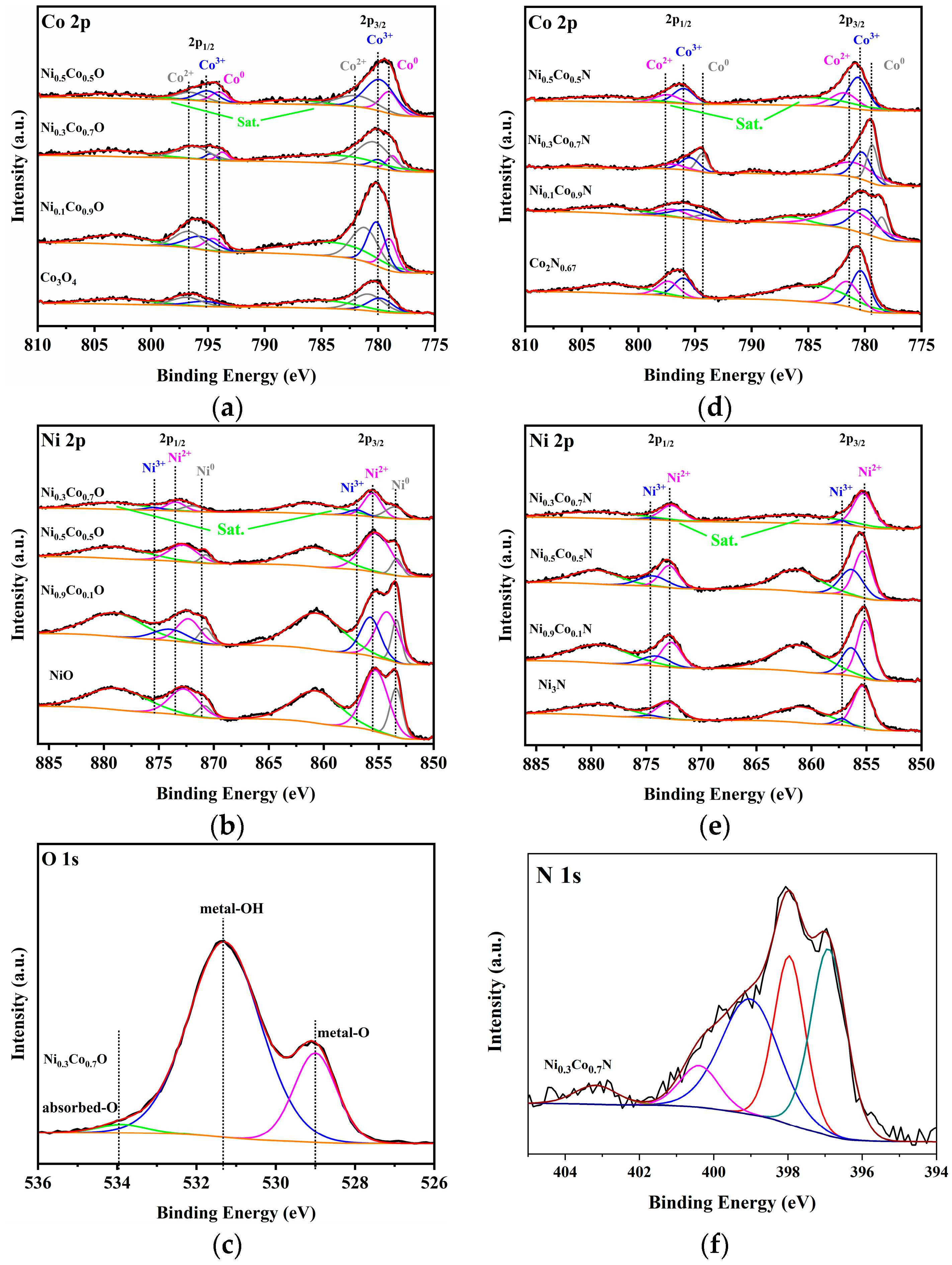
2.2. Crystal Structure and Composition
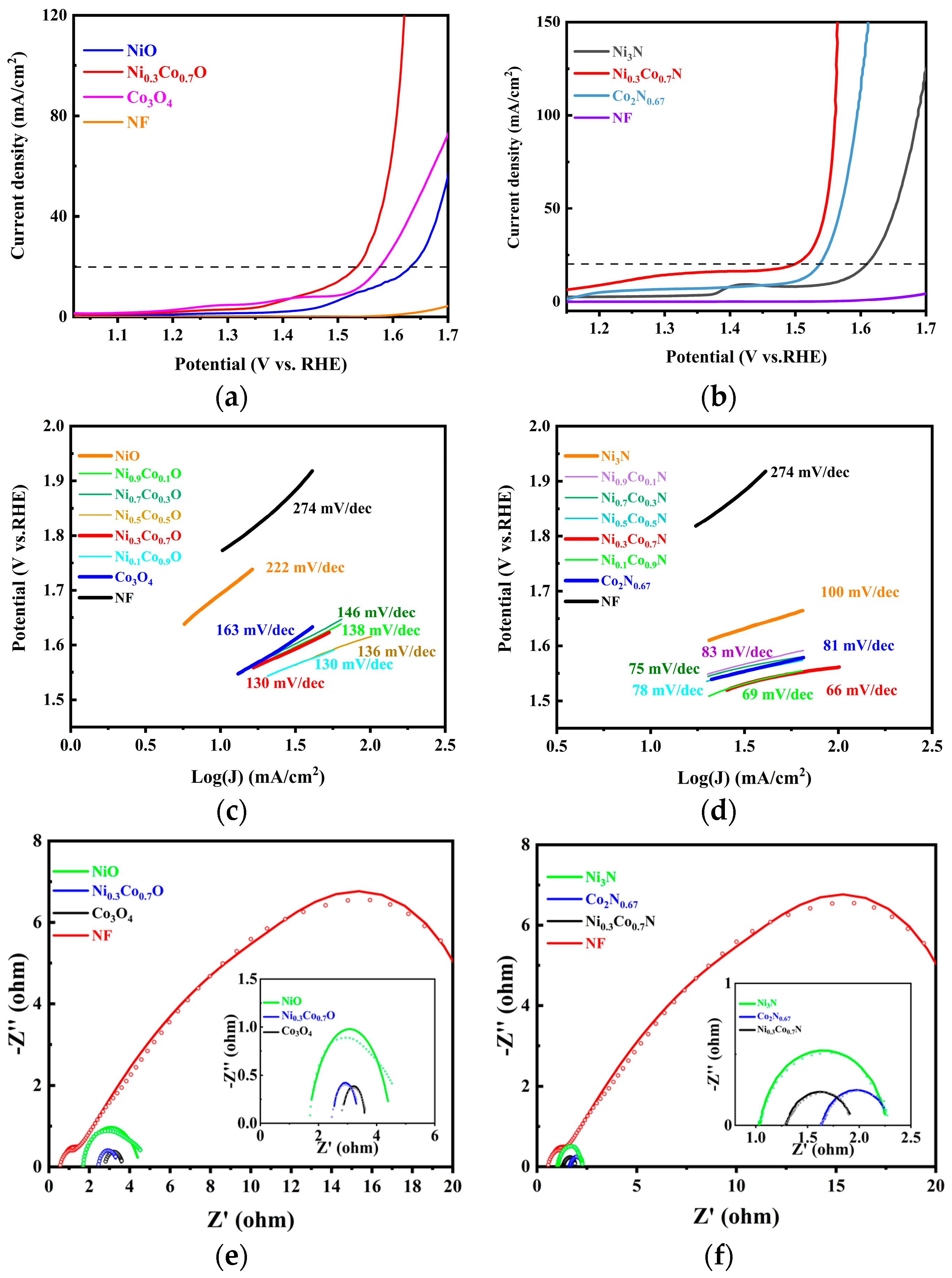
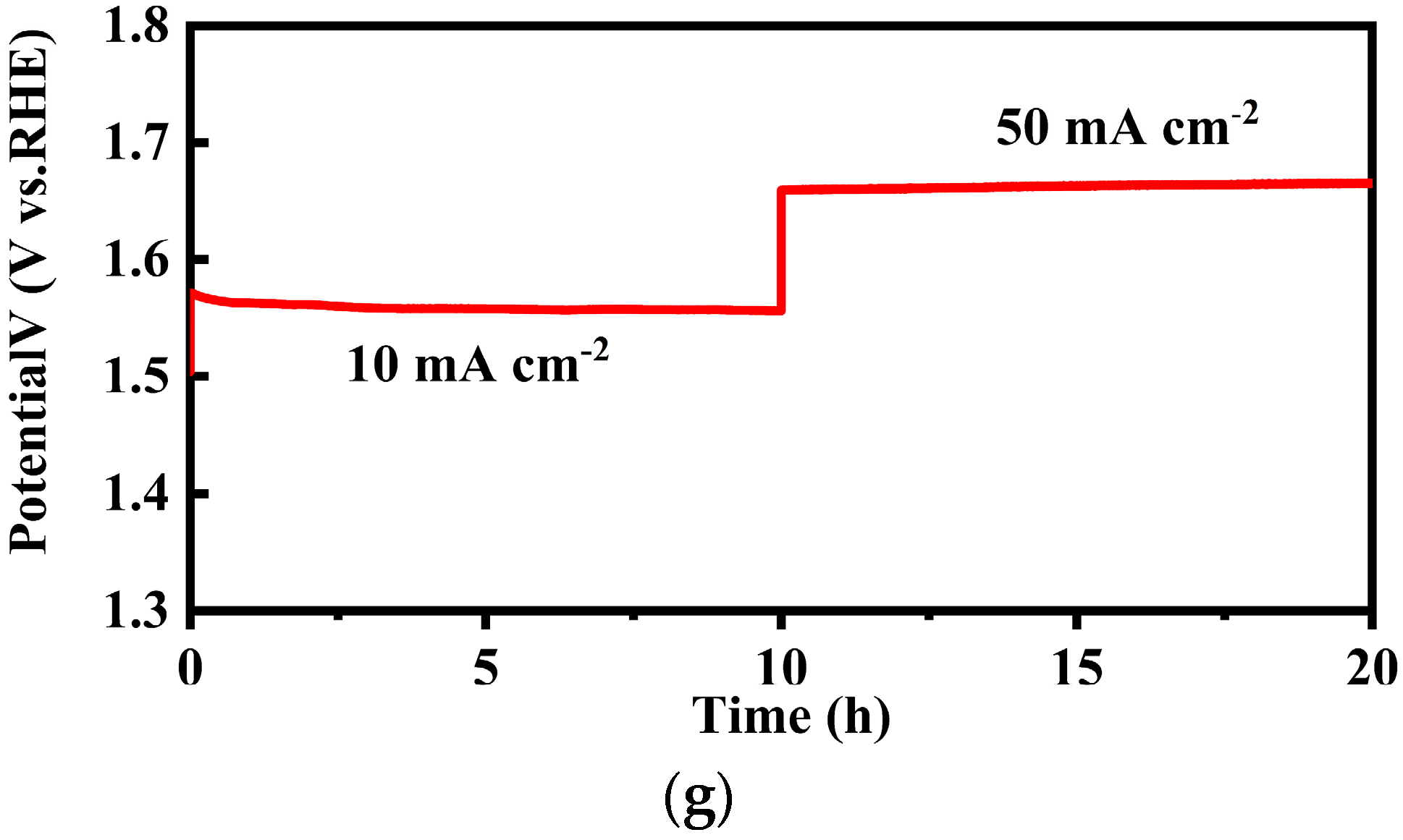
2.3. Electrochemical Analysis
3. Materials and Methods
3.1. Synthesis of NixCo1−xO/NF and NixCo1−xN/NF
3.2. Structural Characterization
3.3. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Glenk, G.; Reichelstein, S. Economics of converting renewable power to hydrogen. Nat. Energy 2019, 4, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Meng, Z.; Zheng, S.; Luo, R.; Tang, H.; Wang, R.; Zhang, R.; Tian, T.; Tang, H. Transition Metal Nitrides for Electrocatalytic Application: Progress and Rational Design. Nanomaterials 2022, 12, 2660. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.E.; Wahid, M.A. Hydrogen from solar energy, a clean energy carrier from a sustainable source of energy. Int. J. Energy Res. 2020, 44, 4110–4131. [Google Scholar] [CrossRef]
- Ding, H.; Liu, H.; Chu, W.; Wu, C.; Xie, Y. Structural Transformation of Heterogeneous Materials for Electrocatalytic Oxygen Evolution Reaction. Chem. Rev. 2021, 121, 13174–13212. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self-Supported Transition-Metal-Based Electrocatalysts for Hydrogen and Oxygen Evolution. Adv. Mater. 2020, 32, 1806326. [Google Scholar] [CrossRef] [PubMed]
- Tariq, I.; Asghar, M.A.; Ali, A.; Badshah, A.; Abbas, S.M.; Iqbal, W.; Zubair, M.; Haider, A.; Zaman, S. Surface Reconstruction of Cobalt-Based Polyoxometalate and CNT Fiber Composite for Efficient Oxygen Evolution Reaction. Catalysts 2022, 12, 1242. [Google Scholar] [CrossRef]
- Liu, C.; Bai, G.; Tong, X.; Wang, Y.; Lv, B.; Yang, N.; Guo, X.Y. Mesoporous and ultrathin arrays of cobalt nitride nanosheets for electrocatalytic oxygen evolution. Electrochem. Commun. 2019, 98, 87–91. [Google Scholar] [CrossRef]
- Hou, J.; Wu, Y.; Zhang, B.; Cao, S.; Li, Z.; Sun, L. Rational Design of Nanoarray Architectures for Electrocatalytic Water Splitting. Adv. Funct. Mater. 2019, 29, 1808367. [Google Scholar] [CrossRef]
- Ren, B.; Li, D.; Jin, Q.; Cui, H.; Wang, C. In-situ Tailoring Cobalt Nickel Molybdenum Oxide Components for Overall Water-Splitting at High Current Densities. ChemElectroChem 2019, 6, 413–420. [Google Scholar] [CrossRef]
- Bergmann, A.; Jones, T.E.; Martinez Moreno, E.; Teschner, D.; Chernev, P.; Gliech, M.; Reier, T.; Dau, H.; Strasser, P. Unified structural motifs of the catalytically active state of Co(oxyhydr)oxides during the electrochemical oxygen evolution reaction. Nat. Catal. 2018, 1, 711–719. [Google Scholar] [CrossRef]
- Wu, X.; Lee, H.; Liu, H.; Lu, L.; Wu, X.; Sun, L. NiCo/Ni/CuO nanosheets/nanowires on copper foam as an efficient and durable electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 2020, 45, 21354–21363. [Google Scholar] [CrossRef]
- Rani, B.J.; Nivedha, K.; Ravi, G.; Yuvakkumar, R. Electrochemical Water Oxidation of NiCo2O4 and CoNi2S4 Nanospheres Supported on Ni Foam Substrate. ChemistrySelect 2019, 4, 10122–10132. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, X.-J.; Cao, X.; Huang, X.; Tan, C.; Tian, J.; Liu, H.; Wang, J.; Zhang, H. Ni3S2 nanorods/Ni foam composite electrode with low overpotential for electrocatalytic oxygen evolution. Energy Environ. Sci. 2013, 6, 2921–2924. [Google Scholar] [CrossRef]
- Huang, R.; Chen, W.; Zhang, Y.; Huang, Z.; Dai, H.; Zhou, Y.; Wu, Y.; Lv, X. Well-designed cobalt-nickel sulfide microspheres with unique peapod-like structure for overall water splitting. J. Colloid Interface Sci. 2019, 556, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Zou, Z.; Du, J.; Zhao, Y.; Zhou, B.; Xu, C. Coupling FeSe2 with CoSe: An effective strategy to create stable and efficient electrocatalysts for water oxidation. Chem. Commun. 2018, 54, 11140–11143. [Google Scholar] [CrossRef]
- Xu, K.; Ding, H.; Jia, K.; Lu, X.; Chen, P.; Zhou, T.; Cheng, H.; Liu, S.; Wu, C.; Xie, Y. Solution-Liquid-Solid Synthesis of Hexagonal Nickel Selenide Nanowire Arrays with a Nonmetal Catalyst. Angew. Chem. Int. Ed. 2016, 55, 1710–1713. [Google Scholar] [CrossRef]
- Xu, X.; Song, F.; Hu, X. A nickel iron diselenide-derived efficient oxygen-evolution catalyst. Nat. Commun. 2016, 7, 12324. [Google Scholar] [CrossRef]
- Peng, X.; Pi, C.; Zhang, X.; Li, S.; Huo, K.; Chu, P.K. Recent progress of transition metal nitrides for efficient electrocatalytic water splitting. Sustain. Energy Fuels 2019, 3, 366–381. [Google Scholar] [CrossRef]
- Liu, H.; Lu, X.; Hu, Y.; Chen, R.; Zhao, P.; Wang, L.; Zhu, G.; Ma, L.; Jin, Z. CoxFeyN nanoparticles decorated on graphene sheets as high-performance electrocatalysts for the oxygen evolution reaction. J. Mater. Chem. A 2019, 7, 12489–12497. [Google Scholar] [CrossRef]
- Saad, A.; Cheng, Z.; Zhang, X.; Liu, S.; Shen, H.; Thomas, T.; Wang, J.; Yang, M. Ordered Mesoporous Cobalt–Nickel Nitride Prepared by Nanocasting for Oxygen Evolution Reaction Electrocatalysis. Adv. Mater. Interfaces 2019, 6, 1900960. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Pan, W.; Ma, H.; Zhang, J. 3 D Porous Nickel–Cobalt Nitrides Supported on Nickel Foam as Efficient Electrocatalysts for Overall Water Splitting. ChemSusChem 2017, 10, 4170–4177. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xing, Y.; Yang, R.; Wen, T.; Jiang, D.; Shi, W.; Yuan, S. Holey Cobalt-Iron Nitride Nanosheet Arrays as High-Performance Bifunctional Electrocatalysts for Overall Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 29253–29263. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wei, M.; Zaman, S.; Ali, A.; Xia, B.Y. Well-connection of micro-platinum and cobalt oxide flower array with optimized water dissociation and hydrogen recombination for efficient overall water splitting. Chem. Eng. J. 2020, 398, 125669. [Google Scholar] [CrossRef]
- Bruno, L.; Battiato, S.; Scuderi, M.; Priolo, F.; Terrasi, A.; Mirabella, S. Physical insights into alkaline overall water splitting with NiO microflowers electrodes with ultra-low amount of Pt catalyst. Int. J. Hydrogen Energy 2022, 47, 33988–33998. [Google Scholar] [CrossRef]
- Gao, X.; Liu, X.; Zang, W.; Dong, H.; Pang, Y.; Kou, Z.; Wang, P.; Pan, Z.; Wei, S.; Mu, S.; et al. Synergizing in-grown Ni3N/Ni heterostructured core and ultrathin Ni3N surface shell enables self-adaptive surface reconfiguration and efficient oxygen evolution reaction. Nano Energy 2020, 78, 105355. [Google Scholar] [CrossRef]
- Yang, H.; Guo, H.; Pang, K.; Fan, P.; Li, X.; Ren, W.; Song, R. An amorphous carbon nitride/NiO/CoN-based composite: A highly efficient nonprecious electrode for supercapacitors and the oxygen evolution reaction. Nanoscale 2020, 12, 7024–7034. [Google Scholar] [CrossRef]
- Ray, C.; Lee, S.C.; Jin, B.; Kundu, A.; Park, J.H.; Chan, J.S. Conceptual design of three-dimensional CoN/Ni3N-coupled nanograsses integrated on N-doped carbon to serve as efficient and robust water splitting electrocatalysts. J. Mater. Chem. A 2018, 6, 4466–4476. [Google Scholar] [CrossRef]
- Ji, S.; Chen, W.; Zhao, Z.; Yu, X.; Park, H.S. Molybdenum oxynitride nanoparticles on nitrogen-doped CNT architectures for the oxygen evolution reaction. Nanoscale Adv. 2020, 2, 5659–5665. [Google Scholar] [CrossRef]
- Zaman, S.; Wang, M.; Liu, H.; Sun, F.; Yu, Y.; Shui, J.; Chen, M.; Wang, H. Carbon-based catalyst supports for oxygen reduction in proton-exchange membrane fuel cells. Trends Chem. 2022, 4, 886–906. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, B.; Xu, J.; Jia, G.; Chen, S.; Rawat, R.S.; Fan, H.J. Rapid Synthesis of Cobalt Nitride Nanowires: Highly Efficient and Low-Cost Catalysts for Oxygen Evolution. Angew. Chem.-Int. Ed. 2016, 55, 8670–8674. [Google Scholar] [CrossRef]
- Xue, Z.; Kang, J.; Guo, D.; Zhu, C.; Li, C.; Zhang, X.; Chen, Y. Self-supported cobalt nitride porous nanowire arrays as bifunctional electrocatalyst for overall water splitting. Electrochim. Acta 2018, 273, 229–238. [Google Scholar] [CrossRef]
- Chen, Z.; Song, Y.; Cai, J.; Zheng, X.; Han, D.; Wu, Y.; Zang, Y.; Niu, S.; Liu, Y.; Zhu, J.; et al. Tailoring the d-Band Centers Enables Co4N Nanosheets to Be Highly Active for Hydrogen Evolution Catalysis. Angew. Chem.-Int. Ed. 2018, 57, 5076–5080. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Jeong, D.I.; Woo, S.; Bin Kwon, S.; Wu, S.; Kim, J.H.; Yang, W.S.; Kang, B.K.; Lim, B.; Yoon, D.H. Morphology adjustable CoxN with 3D mesoporous structure and amorphous N-doped carbon for overall water splitting. Appl. Surf. Sci. 2020, 529, 147177. [Google Scholar] [CrossRef]
- Yan, H.; Xie, Y.; Wu, A.; Cai, Z.; Wang, L.; Tian, C.; Zhang, X.; Fu, H. Anion-Modulated HER and OER Activities of 3D Ni–V-Based Interstitial Compound Heterojunctions for High-Efficiency and Stable Overall Water Splitting. Adv. Mater. 2019, 31, 1901174. [Google Scholar] [CrossRef]
- Zeng, F.; Mebrahtu, C.; Liao, L.; Beine, A.K.; Palkovits, R. Stability and deactivation of OER electrocatalysts: A review. J. Energy Chem. 2022, 69, 301–329. [Google Scholar] [CrossRef]
- Yoon, K.R.; Shin, K.; Park, J.; Cho, S.-H.; Kim, C.; Jung, J.-W.; Cheong, J.Y.; Byon, H.R.; Lee, H.M.; Kim, I.-D. Brush-Like Cobalt Nitride Anchored Carbon Nanofiber Membrane: Current Collector-Catalyst Integrated Cathode for Long Cycle LiO2 Batteries. ACS Nano 2018, 12, 128–139. [Google Scholar] [CrossRef]
- Chen, P.; Xu, K.; Tong, Y.; Li, X.; Tao, S.; Fang, Z.; Chu, W.; Wu, X.; Wu, C. Cobalt nitrides as a class of metallic electrocatalysts for the oxygen evolution reaction. Inorg. Chem. Front. 2016, 3, 236–242. [Google Scholar] [CrossRef]
- Chen, P.; Xu, K.; Fang, Z.; Tong, Y.; Wu, J.; Lu, X.; Peng, X.; Ding, H.; Wu, C.; Xie, Y. Metallic Co4N porous nanowire arrays activated by surface oxidation as electrocatalysts for the oxygen evolution reaction. Angew. Chem.-Int. Ed. 2015, 54, 14710–14714. [Google Scholar] [CrossRef]
- Xu, K.; Chen, P.; Li, X.; Tong, Y.; Ding, H.; Wu, X.; Chu, W.; Peng, Z.; Wu, C.; Xie, Y. Metallic nickel nitride nanosheets realizing enhanced electrochemical water oxidation. J. Am. Chem. Soc. 2015, 137, 4119–4125. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Han, C.; Gao, J.; Pan, L.; Wu, J.; Zhu, X.-D.; Zou, J.-J. NiCo-Based Electrocatalysts for the Alkaline Oxygen Evolution Reaction: A Review. ACS Catal. 2021, 11, 12485–12509. [Google Scholar] [CrossRef]
- Maarisetty, D.; Hang, D.-R.; Chou, M.M.C.; Parida, S. Tuning the Ni/Co Ratios and Surface Concentration of Reduced Molybdenum States for Enhanced Electrocatalytic Performance in Trimetallic Molybdates: OER, HER, and MOR Activity. ACS Appl. Energy Mater. 2022, 5, 14059–14070. [Google Scholar] [CrossRef]
- Liu, X.; Zang, W.; Guan, C.; Zhang, L.; Qian, Y.; Elshahawy, A.M.; Zhao, D.; Pennycook, S.J.; Wang, J. Ni-Doped Cobalt-Cobalt Nitride Heterostructure Arrays for High-Power Supercapacitors. ACS Energy Lett. 2018, 3, 2462–2469. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Y.; Liang, Q.; Pang, Y.; Miao, L.; Liu, X.; Kou, Z.; He, J.; Pennycook, S.J.; Mu, S.; et al. Surface nitridation of nickel-cobalt alloy nanocactoids raises the performance of water oxidation and splitting. Appl. Catal. B Environ. 2020, 270, 118889. [Google Scholar] [CrossRef]
- Wan, X.; Su, J.; Guo, L. Enhanced Photoelectrochemical Water Oxidation on BiVO4 with Mesoporous Cobalt Nitride Sheets as Oxygen-Evolution Cocatalysts. Eur. J. Inorg. Chem. 2018, 2018, 2557–2563. [Google Scholar] [CrossRef]
- Tong, Y.; Liu, J.; Wang, L.; Su, B.-J.; Wu, K.-H.; Juang, J.-Y.; Hou, F.; Yin, L.; Dou, S.X.; Liu, J.; et al. Carbon-Shielded Single-Atom Alloy Material Family for Multi-Functional Electrocatalysis. Adv. Funct. Mater. 2022, 32, 2205654. [Google Scholar] [CrossRef]
- Lee, D.U.; Kim, B.J.; Chen, Z. One-pot synthesis of a mesoporous NiCo2O4 nanoplatelet and graphene hybrid and its oxygen reduction and evolution activities as an efficient bi-functional electrocatalyst. J. Mater. Chem. A 2013, 1, 4754–4762. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, X.; Wang, X.; Lu, D.; Xu, Y.; Liu, G.; Fu, Y.; Shui, T.; Wang, H.; Cheng, Z. Composition and Morphology Modulation of Bimetallic Nitride Nanostructures on Nickel Foams for Efficient Oxygen Evolution Electrocatalysis. Catalysts 2023, 13, 230. https://doi.org/10.3390/catal13020230
Wan X, Wang X, Lu D, Xu Y, Liu G, Fu Y, Shui T, Wang H, Cheng Z. Composition and Morphology Modulation of Bimetallic Nitride Nanostructures on Nickel Foams for Efficient Oxygen Evolution Electrocatalysis. Catalysts. 2023; 13(2):230. https://doi.org/10.3390/catal13020230
Chicago/Turabian StyleWan, Xiaokang, Xianyun Wang, Dashun Lu, Yunbo Xu, Gezhong Liu, Yanming Fu, Taotao Shui, Haitao Wang, and Zude Cheng. 2023. "Composition and Morphology Modulation of Bimetallic Nitride Nanostructures on Nickel Foams for Efficient Oxygen Evolution Electrocatalysis" Catalysts 13, no. 2: 230. https://doi.org/10.3390/catal13020230
APA StyleWan, X., Wang, X., Lu, D., Xu, Y., Liu, G., Fu, Y., Shui, T., Wang, H., & Cheng, Z. (2023). Composition and Morphology Modulation of Bimetallic Nitride Nanostructures on Nickel Foams for Efficient Oxygen Evolution Electrocatalysis. Catalysts, 13(2), 230. https://doi.org/10.3390/catal13020230








