Research Progress on Application in Energy Conversion of Silicon Carbide-Based Catalyst Carriers
Abstract
:1. Introduction
2. Preparation and Raw Material Variation in Silicon Carbide (SiC)-Based Catalyst Carriers
2.1. Carbothermic Reduction Method
2.2. Other Methods
2.2.1. Sol-Gel Method
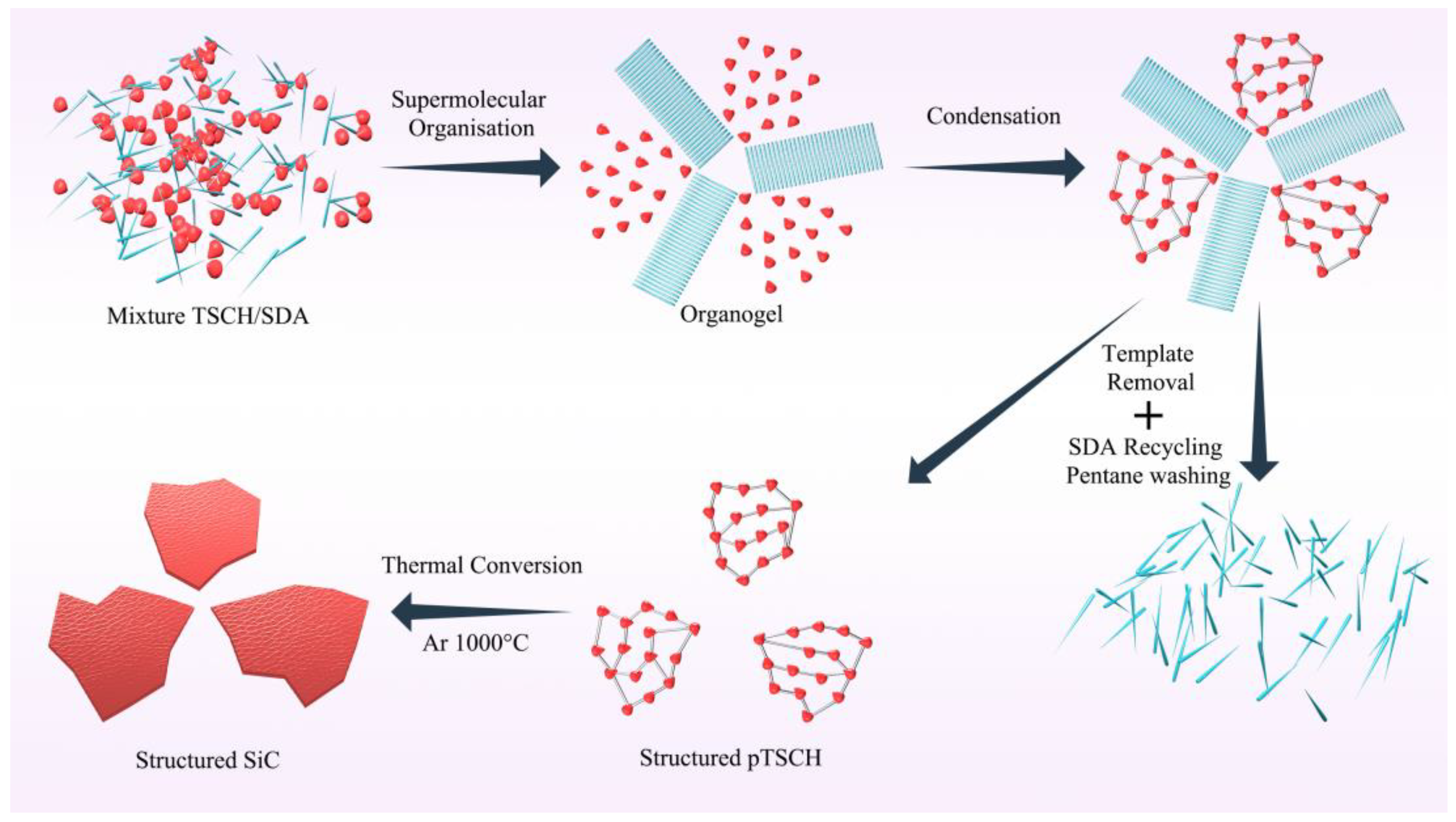
2.2.2. Shape Memory Synthesis (SMS)
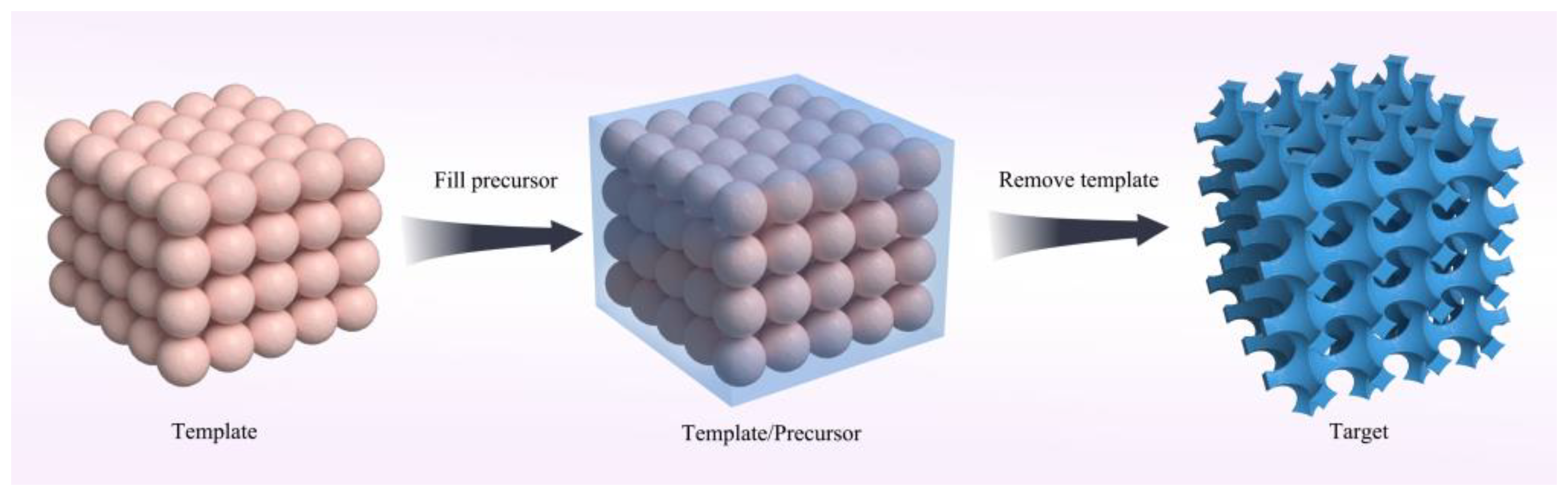
2.2.3. Nanocasting Method
2.3. Raw Material Variation for SiC Preparation
3. Catalytic Mechanism of SiC-Based Catalyst Carriers
4. Application of SiC-Based Catalyst Carriers
4.1. Surface Modification
4.2. Construction of Structures
4.3. Preparation of Composite Materials
5. Conclusions and Prospects
- (1)
- The preparation of SiC-based catalyst carriers using waste as the raw material not only saves costs but also conforms to the concept of reutilization of resources and ecological environmental protection. However, the specific surface area of SiC prepared by using waste is relatively low, due to which it can meet the requirements of a catalyst carrier only to a certain extent, and the overall performance of the catalyst is still limited. Therefore, the use of waste to prepare SiC-based catalyst carriers with a high specific surface area has very broad prospects, and therefore is one of the developmental directions of this field in the future.
- (2)
- The preparation of SiC-based catalyst carriers at low temperatures can also greatly reduce the production cost. The preparation temperatures of SiC are generally high. If SiC-based catalyst carriers can be prepared at low temperatures by improving and innovating the present technical means, their commercial value can be directly increased with a more extensive range of applications.
- (3)
- In the construction of porous SiC nanostructures and the use of waste to prepare SiC-based catalyst carriers at low temperatures in combination with the first two innovations, it was found that the specific surface area of SiC nanomaterials with a porous structure was large, and the performance of the catalyst loaded on such carriers was considerably enhanced.
- (4)
- The synergistic effect of SiC with its supported metal active phase needs to be further explored.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Acheson, E.G. Production of Artificial Crystalline Carbonaceous Materials. US Patent US0492767, 28 February 1893. [Google Scholar]
- Wang, Z.J.; Fu, Q.; Bao, X.H. Silicon Carbide as a Novel Support for Heterogeneous Catalysis. Prog. Chem. 2014, 26, 502–511. [Google Scholar]
- Tuci, G.; Liu, Y.F.; Rossin, A.; Guo, X.Y.; Pham, C.; Giambastiani, G.; Cuong, P.H. Porous Silicon Carbide (SiC): A Chance for Improving Catalysts or Just Another Active-Phase Carrier? Chem. Rev. 2021, 121, 10559–10665. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.R.; Velisoju, V.K.; Tavares, F.; Dikhtiarenko, A.; Gascon, J.; Castano, P. Silicon carbide in catalysis: From inert bed filler to catalytic support and multifunctional material. Catal. Rev. Sci. Eng. 2022, 65, 174–237. [Google Scholar] [CrossRef]
- Wu, R.B.; Zhou, K.; Yue, C.Y.; Wei, J.; Pan, Y. Recent progress in synthesis, properties and potential applications of SiC nanomaterials. Prog. Mater. Sci. 2015, 72, 1–60. [Google Scholar] [CrossRef]
- Okada, K.; Kato, H.; Nakajima, K.J. Preparation of silicon-carbide fiber from activated carbon-fiber and gaseous silicon monoxide. J. Am. Ceram. Soc. 1994, 77, 1691–1693. [Google Scholar] [CrossRef]
- Pesant, L.; Matta, J.; Garin, F.; Ledoux, M.J.; Bernhardt, P.; Pham, C.; Pham-Huu, C. A high-performance Pt/β-SiC catalyst for catalytic combustion of model carbon particles (CPs). Appl. Catal. A Gen. 2004, 266, 21–27. [Google Scholar] [CrossRef]
- Kizling, M.B.; Stenius, P.; Andersson, S.; Frestad, A. Characterization and catalytic activity of silicon-carbide powder as catalyst support in exhaust catalysts. Appl. Catal. B Environ. 1992, 1, 149–168. [Google Scholar] [CrossRef]
- Ledoux, M.J.; Pham-Huu, C.; Keller, N.; Nougayrede, J.B.; Savin-Poncet, S.; Bousquet, J. Selective oxidation of H2S in Claus tail-gas over SiC supported NiS2 catalyst. Catal. Today 2000, 61, 157–163. [Google Scholar] [CrossRef]
- Pham-Huu, C.; Bouchy, C.; Dintzer, T.; Ehret, G.; Estournes, C.; Ledoux, M.J. High surface area silicon carbide doped with zirconium for use as catalyst support. Preparation, characterization and catalytic application. Appl. Catal. A Gen. 1999, 180, 385–397. [Google Scholar] [CrossRef]
- Pham-Huu, C.; Keller, N.; Ledoux, M.J. Silicon carbide: A novel catalyst support for heterogeneous catalysis. Actual. Chim. 2002, 10, 8–18. [Google Scholar]
- Keller, N.; Pham-Huu, C.; Ledoux, M.J. Continuous process for selective oxidation of H2S over SiC-supported iron catalysts into elemental sulfur above its dewpoint. Appl. Catal. A Gen. 2001, 217, 205–217. [Google Scholar] [CrossRef]
- Keller, N.; Pham-Huu, C.; Estournès, C.; Ledoux, M.J. Low temperature use of SiC-supported NiS2-based catalysts for selective H2S oxidation: Role of SiC surface heterogeneity and nature of the active phase. Appl. Catal. A Gen. 2002, 234, 191–205. [Google Scholar] [CrossRef]
- Wine, G.; Tessonnier, J.P.; Pham-Huu, C.; Ledoux, M.J. Beta zeolite supported on a macroscopic pre-shaped SiC as a high performance catalyst for liquid-phase benzoylation. Chem. Commun. 2002, 2, 2418–2419. [Google Scholar] [CrossRef] [PubMed]
- Wine, G.; Matta, J.; Tessonnier, J.P.; Pham-Huu, C.; Ledoux, M.J. Beta zeolite supported on silicon carbide for Friedel-Crafts fixed-bed reactions. Chem. Commun. 2003, 4, 530–531. [Google Scholar] [CrossRef]
- Yessimova, O.; Kumargaliyeva, S.; Kerimkulova, M.; Mussabekov, K.; Toktarbay, Z. Wetting ability of a phytopreparation and their associates with polyelectrolytes. Rasayan J. Chem. 2020, 13, 481–487. [Google Scholar] [CrossRef]
- Mansurov, Z.A.; Jandosov, J.M.; Kerimkulova, A.R.; Azat, S.; Zhubanova, A.A.; Digel, I.E.; Savistkaya, I.S.; Akimbekov, N.S.; Kistaubaeva, A.S. Nanostructured carbon materials for biomedical use. Eurasian Chem. Technol. J. 2013, 15, 209–217. [Google Scholar] [CrossRef]
- Li, M.X.; Su, G.J.; Zhang, P. Synthesis and Characterization of High Surface Area Nano-silicon Carbide. Bull. Chin. Ceram. Soc. 2007, 26, 800–803. [Google Scholar]
- Yao, J.F.; Wang, H.T.; Zhang, X.Y.; Zhu, W.; Wei, J.P.; Cheng, Y.B. Role of pores in the carbothermal reduction of carbon-silica nanocomposites into silicon carbide nanostructures. J. Phys. Chem. C 2007, 111, 636–641. [Google Scholar] [CrossRef]
- Vix-guterl, C.; Ehrburger, P. Effect of the properties of a carbon substrate on its reaction with silica for silicon carbide formation. Carbon 1997, 35, 1587–1592. [Google Scholar] [CrossRef]
- Vix-guterl, C.; McEnaney, B.; Ehrburger, P. SiC material produced by carbothermal reduction of a freeze gel silica-carbon artefact. J. Eur. Ceram. Soc. 1999, 19, 427–432. [Google Scholar] [CrossRef]
- Liang, C.H.; Meng, G.W.; Zhang, L.D.; Wu, Y.C.; Cui, Z. Large-scale synthesis of beta-SiC nanowires by using mesoporous silica embedded with Fe nanoparticles. Chem. Phys. Lett. 2000, 329, 323–328. [Google Scholar] [CrossRef]
- Zhang, L.D.; Mou, J.M. Nanomaterials and Nanostructures, 2nd ed.; Science Press: Beijing, China, 2001; p. 15. [Google Scholar]
- Samane, M.; Mohannad, M.; Sahajwalla, V. Novel Synthesis of Silicon Carbide Nanowires from e-Waste. ACS Sustain. Chem. Eng. 2017, 5, 4171–4178. [Google Scholar]
- Kudrenko, E.; Roddatis, V.; Zhokhov, A.; Zverkova, I.; Khodos, I.; Emelchenko, G. Morphology of SiC nanowires grown on the surface of carbon fibers. RSC Adv. 2012, 2, 4913–4919. [Google Scholar] [CrossRef]
- Gao, Y.H.; Bando, Y.; Kurashima, K.; Sato, T. SiC nanorods prepared from SiO and activated carbon. J. Biol. Chem. 2002, 37, 2023–2029. [Google Scholar]
- Alexander, A.C. Modern Crystallography III; Springer: Berlin/Heidelberg, Germany, 1984; pp. 104–158. [Google Scholar]
- Pamplin, B.R. Crystal Growth, 2nd ed.; Pergamon Press: Oxford, UK, 1980. [Google Scholar]
- Dong, L.L.; Wang, Y.W.; Tong, X.L.; Lei, T.Z. Silicon carbide encapsulated graphite nanocomposites supported Pt nanoparticles as high-performance catalyst for methanol and ethanol oxidation reaction. Diam. Relat. Mater. 2020, 104. [Google Scholar] [CrossRef]
- Chen, Y.; Ola, O.; Liu, G.S.; Han, L.; Hussain, M.Z.; Thummavichai, K.; Wen, J.H.; Zhang, L.Y.; Wang, N.N.; Xia, Y.D.; et al. Multifunctional porous SiC nanowire scaffolds. J. Eur. Ceram. Soc. 2021, 41, 3970–3979. [Google Scholar] [CrossRef]
- Dong, L.L.; Tong, X.L.; Wang, Y.Y.; Guo, X.N.; Jin, G.Q.; Guo, X.Y. Promoting performance and CO tolerance of Pt nanocatalyst for direct methanol fuel cells by supporting on high-surface-area silicon carbide. J. Solid State Electrochem. 2014, 18, 929–934. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, Y.F.; Qu, Y.N.; Wu, J.M.; Zhang, S.E.; Yang, J.L. Three-Dimensional Reticulated, Spongelike, Resilient Aerogels Assembled by SiC/Si3N4 Nanowires. Nano Lett. 2021, 21, 4167–4175. [Google Scholar] [CrossRef]
- Jin, G.Q.; Guo, X.Y. Synthesis and characterization of mesoporous silicon carbide. Microporous Mesoporous Mater. 2003, 60, 207–212. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, Y.; Lin, L.X.; Ni, J.; Wei, K.M. Synthesis of a novel mesoporous silicon carbide with a thorn-ball-like shape. Scr. Mater. 2006, 55, 883–886. [Google Scholar] [CrossRef]
- Worsley, M.A.; Kuntz, J.D.; Satcher, J.H., Jr.; Baumann, T.F. Synthesis and characterization of monolithic, high surface area SiO2/C and SiC/C composites. J. Mark. Commun. 2010, 20, 4840–4844. [Google Scholar] [CrossRef]
- Wang, D.H.; Fu, X. Synthesis of High Surface Area Porous Silicon Carbide by a Modified Sol-Gel Method. In Proceedings of the 2nd International Conference on Manufacturing Science and Engineering, Guilin, China, 9–11 April 2011. [Google Scholar]
- Yang, D.J.; Li, J.P.; Xu, Y.; Wu, D.; Sun, Y.H.; Zhu, H.Y.; Deng, F. Direct formation of hydrophobic silica-based micro/mesoporous hybrids from polymethylhydrosiloxane and tetraethoxysilane. Microporous Mesoporous Mater. 2006, 95, 180–186. [Google Scholar] [CrossRef]
- Wu, X.D.; Shao, G.F.; Shen, X.D.; Cui, S.; Chen, X.B. Evolution of the novel C/SiO2/SiC ternary aerogel with high specific surface area and improved oxidation resistance. Chem. Eng. J. 2017, 330, 1022–1034. [Google Scholar] [CrossRef]
- Nhut, J.M.; Vieira, R.; Pesant, L.; Tessonnier, J.P.; Keller, N.; Ehret, G.; Pham-Huu, C.; Ledoux, M.J. Synthesis and catalytic uses of carbon and silicon carbide nanostructures. Catal. Today 2002, 76, 11–32. [Google Scholar] [CrossRef]
- Hasegawa, G.; Kanamori, K.; Nakanishi, K.; Hanada, T. Fabrication of macroporous silicon carbide ceramics by intramolecular carbothermal reduction of phenyl-bridged polysilsesquioxane. J. Mark. Commun. 2009, 19, 7716–7720. [Google Scholar] [CrossRef]
- Lee, H.Y.; Tsui, F.C.; Lee, Y.C. Structural and microstructural studies on SiC-SiO2 ceramic composites. Mater. Chem. Phys. 2019, 233, 203–212. [Google Scholar] [CrossRef]
- Krawiec, P.; Kaskel, S. Thermal stability of high surface area silicon carbide materials. J. Solid State Chem. 2006, 179, 2281–2289. [Google Scholar] [CrossRef]
- Ledoux, M.J.; Hantzer, S.; HuuC, P.; Guille, J.; Desaneaux, M.P. New synthesis and uses of high-specific-surface SiC as a catalytic support that is chemically inert and has high thermal-resistance. J. Catal. 1988, 114, 176–185. [Google Scholar] [CrossRef]
- Wang, K.; Wang, H.T.; Cheng, Y.B. Synthesis of nanostructured silicon carbide spheres from mesoporous C-SiO2 nanocomposites. Chem. Commun. 2010, 46, 303–305. [Google Scholar] [CrossRef]
- Keller, N.; Reiff, O.; Keller, V.; Ledoux, M.J. High surface area submicrometer-sized β-SiC particles grown by shape memory synthesis method. Diam. Relat. Mater. 2005, 14, 1353–1360. [Google Scholar] [CrossRef]
- Ledoux, M.J.; Pham-huu, C. Silicon carbide: A novel catalyst support for heterogeneous catalysis. Cattech 2001, 5, 226–246. [Google Scholar] [CrossRef]
- Keller, N.; Pham-huu, C.; Roy, S. Influence of the preparation conditions on the synthesis of high surfacearea SiC for use as a heterogeneous catalyst support. J. Mater. Sci. 1999, 34, 3189–3202. [Google Scholar] [CrossRef]
- Yan, J.; Wang, A.J.; Kim, D.P. Preparation of ordered mesoporous SiC from preceramic polymer templatedby nanoporous silica. J. Phys. Chem. B 2006, 110, 5429–5433. [Google Scholar] [CrossRef]
- Shi, Y.F.; Meng, Y.; Chen, D.H. Highly ordered mesoporous silicon carbide ceramics with large surface areas and high stability. Adv. Funct. Mater. 2006, 16, 561–567. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, J.Y.; Hyeon, T.H. Recent progress in the synthesis of porous carbon materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Park, K.H.; Sung, I.K.; Kim, D.P. A facile route to prepare high surface area mesoporous SiC from SiO2 sphere templates. J. Mark. Commun. 2004, 14, 3436–3439. [Google Scholar] [CrossRef]
- Krawiec, P.; Weidenthaler, C.; Kaskel, S. SiC/MCM-48 and SiC/SBA-15 nanocomposite materials. Chem. Mater. 2004, 16, 2869–2880. [Google Scholar] [CrossRef]
- Krawiec, P.; Geiger, D.; Kaskel, S. Ordered mesoporous silicon carbide (OM-SiC) via polymer precursor nanocasting. Chem. Commun. 2006, 2469–2470. [Google Scholar] [CrossRef] [PubMed]
- Krawiec, P.; Schrage, C.; Kockrick, E.; Kaskel, S. Tubular and Rodlike Ordered Mesoporous Silicon (Oxy)carbide Ceramics and their Structural Transformations. Chem. Mater. 2008, 20, 5421–5433. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Lue, J.W.; Yan, X.N.; Hu, L.T.; Xue, Q.J. Preparation of ordered mesoporous silicon carbide monoliths via preceramic polymer nanocasting. Microporous Mesoporous Mater. 2011, 142, 754–758. [Google Scholar] [CrossRef]
- Hoffmann, C.; Biemelt, T.; Seifert, A.; Pinkert, K.; Gemming, T.; Spange, S.; Kaskel, S. Polymer-derived nanoporous silicon carbide with monodisperse spherical pores. J. Mark. Commun. 2012, 22, 24841–24847. [Google Scholar] [CrossRef]
- Xue, F.D.; Zhou, K.C.; Wu, N.; Hang, L.; Wang, X.F.; Zhou, X.F.; Ya, Z.N.; Abrahams, I.; Zhang, D. Porous SiC ceramics with dendritic pore structures by freeze casting from chemical cross-linked polycarbosilane. Ceram. Int. 2018, 44, 6293–6299. [Google Scholar] [CrossRef]
- Ferraro, C.; Garcia-Tunon, E.; Barg, S.; Miranda, M.; Ni, N.; Bell, R.; Saiz, E. SiC porous structures obtained with innovative shaping technologies. J. Eur. Ceram. Soc. 2018, 38, 823. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, X.H.; Zhang, D.Y.; Sun, B.Q.; Yan, L.W.; Luo, X.G. Strong effect of atmosphere on the microstructure and microwave absorption properties of porous SiC ceramics. J. Eur. Ceram. Soc. 2018, 38, 29–39. [Google Scholar] [CrossRef]
- Amirthan, G.; Udayakumar, A.; Prasad, V.B.; Balasubramanian, M. Synthesis and characterization of Si/SiC ceramics prepared using cotton fabric. Ceramurg. Int. 2009, 35, 967–973. [Google Scholar] [CrossRef]
- Czapski, M.; Stora, T.; Tardivat, C.; Deville, S.; Augusto, R.S.; Leloup, J.; Bouville, F.; Fernandes, L.R. Porous silicon carbide and aluminum oxide with unidirectional open porosity as model target materials for radioisotope beam production. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2013, 317, 385–388. [Google Scholar] [CrossRef] [Green Version]
- Tu, H.L.; Ma, F.; Zhang, S.R.; Li, T.F.; Zhao, H.B. Current Status and Future Insight of New Materials Industry in China. Chin. J. Rare Met. 2019, 43, 1121–1130. [Google Scholar]
- Hoffmann, C.; Reinhardt, B.; Enke, D.; Kaskel, S. Inverse silicon carbide replica of porous glasses. Microporous Mesoporous Mater. 2014, 184, 1–6. [Google Scholar] [CrossRef]
- Lu, A.H.; Schmidt, W.; Kiefer, W.; Schuth, F. High surface area mesoporous SiC synthesized via nanocasting and carbothermal reduction process. J. Mater. Sci. 2005, 40, 5091–5093. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Sun, J.Y.; Li, Q.Z.; Stucky, G.D. Morphological control of highly ordered mesoporous silica SBA-15. Chem. Mater. 2000, 12, 275–279. [Google Scholar] [CrossRef]
- Tueysuez, H.; Lehmann, C.W.; Bongard, H.; Tesche, B.; Schmidt, R.; Schueth, F. Direct imaging of surface topology and pore system of ordered mesoporous silica (MCM-41, SBA-15, and KIT-6) and nanocast metal oxides by high resolution scanning electron microscopy. J. Am. Chem. Soc. 2008, 130, 11510–11517. [Google Scholar] [CrossRef]
- Kleitz, F.; Berube, F.; Guillet-Nicolas, R.; Yang, C.M.; Thommes, M. Probing Adsorption, Pore Condensation, and Hysteresis Behavior of Pure Fluids in Three-Dimensional Cubic Mesoporous KIT-6 Silica. J. Phys. Chem. C 2010, 114, 9344–9355. [Google Scholar] [CrossRef]
- Sayari, A. Catalysis by crystalline mesoporous molecular sieves. Chem. Mater. 1996, 8, 1840–1852. [Google Scholar] [CrossRef]
- Clark, J.H.; Macquarrie, D.J.; Tavener, S.J. The application of modified mesoporous silicas in liquid phase catalysis. Dalton Trans. 2006, 4297–4309. [Google Scholar] [CrossRef]
- Nardin, T.; Gouze, B.; Cambedouzou, J.; Bauduin, P.; Man, M.W.C.; Deschanels, X.; Bourgeois, D.; Meyer, D.; Diat, O. Elaboration of porous silicon carbide by soft templating molecular precursors with semi-fluorinated alkanes. J. Mater. Chem. A 2015, 3, 3082–3090. [Google Scholar] [CrossRef]
- Krafft, M.P.; Riess, J.G. Chemistry, Physical Chemistry, and Uses of Molecular Fluorocarbon-Hydrocarbon Diblocks, Triblocks, and Related Compounds-Unique “Apblar” Components for Self-Assembled Colloid and Interface Engineering. Chem. Rev. 2009, 109, 1714–1792. [Google Scholar] [CrossRef]
- Broniatowski, M.; Dynarowicz-Latka, P. Semifluorinated alkanes—Primitive surfactants of fascinating properties. Adv. Colloid Interface Sci. 2008, 138, 63–83. [Google Scholar] [CrossRef]
- Lo Nostro, P. Aggregates from semifluorinated n-alkanes: How incompatibility determines self-assembly. Curr. Opin. Colloid Interface Sci. 2003, 8, 223–226. [Google Scholar] [CrossRef]
- Russell, T.P.; Rabolt, J.F.; Twieg, R.J.; Siemens, R.L.; Farmer, B.L. Structural Characterization Of Semifluorinated Normal-Alkanes. 2. Solid Solid Transition Behavior. Macromolecules 1986, 19, 1135–1143. [Google Scholar] [CrossRef]
- Song, K.; Twieg, R.J.; Rabolt, J.F. Low-Temperature Crystal Crystal Phase-Transitions in Triblock Semifluorinated N-Alkanes. Macromolecules 1990, 23, 3712–3714. [Google Scholar] [CrossRef]
- Laversanne, R. Polymerization of acrylamide in lamellar, hexagonal, and cubic lyotropic phases. Macromolecules 1992, 25, 489–491. [Google Scholar] [CrossRef]
- Meng, S.; Wang, D.H.; Jin, G.Q.; Wang, Y.Y.; Guo, X.Y. Preparation of SiC nanoparticles from plastic wastes. Mater. Lett. 2010, 64, 2731–2734. [Google Scholar] [CrossRef]
- Wang, D.H. Preparation SiC nanomaterials using disposable chopsticks waste. New Chem. Mater. 2015, 43, 67–69. [Google Scholar]
- Qian, Y.T.; Ju, Y.Z.; Ma, X.J. A Method for Preparing Cubic Silicon Carbide Ultrafine Powder at Low Temperature from Waste Plastic. CHN Patent CN101525134, 9 September 2009. [Google Scholar]
- Qin, Y.X.; Li, R.Y.; Mi, W.; Shi, W.; Lu, B.Y.; Tong, X.L. Phenol hydrogenation to cyclohexanol on a novel Pd7P3/SiC catalyst with high activity and selectivity. Diam. Relat. Mater. 2021, 111. [Google Scholar] [CrossRef]
- Hao, C.H.; Guo, X.N.; Sankar, M.; Yang, H.; Ma, B.; Zhang, Y.F.; Tong, X.L.; Jin, G.Q.; Guo, X.Y. Synergistic Effect of Segregated Pd and Au Nanoparticles on Semiconducting SiC for Efficient Photocatalytic Hydrogenation of Nitroarenes. ACS Appl. Mater. Interfaces 2018, 10, 23029–23036. [Google Scholar] [CrossRef]
- Roland, U.; Braunschweig, T.; Roessner, F. On the nature of spilt-over hydrogen. Mol. Catal. 1997, 127, 61–84. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 8th ed.; Chemical Industry Press: Beijing, China, 1979; pp. 344–347. [Google Scholar]
- Boronat, M.; Concepcion, P.; Corma, A.; Gonzalez, S.; Illas, F.; Serna, P. A molecular mechanism for the chemoselective hydrogenation of substituted nitroaromatics with nanoparticles of gold on TiO2 catalysts: A cooperative effect between gold and the support. J. Am. Chem. Soc. 2007, 129, 16230–16237. [Google Scholar] [CrossRef]
- Jiao, Z.F.; Guo, X.N.; Zhai, Z.Y.; Jin, G.Q.; Wang, X.M.; Guo, X.Y. The enhanced catalytic performance of Pd/SiC for the hydrogenation of furan derivatives at ambient temperature under visible light irradiation. Catal. Sci. Technol. 2014, 8, 2494–2498. [Google Scholar] [CrossRef]
- Li, X.H.; Baar, M.; Blechert, S.; Antonietti, M. Facilitating room-temperature Suzuki coupling reaction with light: Mott-Schottky photocatalyst for C-C-coupling. Electron. J. Stat. 2013, 3, 1743. [Google Scholar] [CrossRef] [Green Version]
- Li, X.H.; Antonietti, M. Metal nanoparticles at mesoporous N-doped carbons and carbon nitrides: Functional Mott-Schottky heterojunctions for catalysis. Chem. Soc. Rev. 2013, 42, 6593–6604. [Google Scholar] [CrossRef]
- Wang, J.R.; Wang, Y.Y.; Tong, X.L.; Wang, Y.W.; Jin, G.Q.; Guo, X.Y. Highly active Ir/SiC catalyst for aqueous hydrogenation of levulinic acid to γ-valerolactone. Catal. Commun. 2020, 139, 105971. [Google Scholar] [CrossRef]
- Han, C.; Wang, B.; Wu, N.; Shen, S.J.; Wang, Y.D. Deep and selective photoreduction of CO2 to CH4 over ultrafine Pt nanoparticles-decorated SiC nanosheets. Appl. Surf. Sci. 2020, 515. [Google Scholar] [CrossRef]
- Jiao, Z.F.; Zhai, Z.Y.; Guo, X.N.; Guo, X.Y. Visible-Light-Driven Photocatalytic Suzuki-Miyaura Coupling Reaction on Mott-Schottky-type Pd/SiC Catalyst. J. Phys. Chem. C 2015, 119, 3238–3243. [Google Scholar] [CrossRef]
- Hao, C.H.; Guo, X.N.; Pan, Y.T.; Chen, S.; Jiao, Z.F.; Yang, H.; Guo, X.Y. Visible-Light-Driven Selective Photocatalytic Hydrogenation of Cinnamaldehyde over Au/SiC Catalysts. J. Am. Chem. Soc. 2016, 138, 9361–9364. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, Z.X.; Ding, L.J.; Chen, J.J. Facile fabrication and efficient photoelectrochemical water-splitting activity of electrodeposited nickel/SiC nanowires composite electrode. Catal. Commun. 2017, 96, 46–49. [Google Scholar] [CrossRef]
- Song, J.T.; Mashiko, H.; Kamiya, M.; Nakamine, Y.; Ohtomo, A.; Iwasaki, T.; Hatano, M. Improved visible light driven photoelectrochemical properties of 3C-SiC semiconductor with Pt nanoparticles for hydrogen generation. Appl. Phys. Lett. 2013, 103, 213901. [Google Scholar] [CrossRef]
- Zou, T.; Xie, C.S.; Liu, Y.; Zhang, S.S.; Zou, Z.J.; Zhang, S.P. Full mineralization of toluene by photocatalytic degradation with porous TiO2/SiC nanocomposite film. J. Alloys Compd. 2013, 552, 504–510. [Google Scholar] [CrossRef]
- Li, H.J.; Yan, Y.F.; Feng, S.; Zhu, Y.L.; Chen, Y.R.; Fan, H.; Zhang, L.; Yang, Z.Q. Transition metal tuned semiconductor photocatalyst CuCo/beta-SiC catalyze hydrolysis of ammonia borane to hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 8307–8314. [Google Scholar] [CrossRef]
- Wang, A.Q.; Liu, X.Y.; Mou, C.Y.; Zhang, T. Understanding the synergistic effects of gold bimetallic catalysts. J. Catal. 2013, 308, 258–271. [Google Scholar] [CrossRef]
- Zhang, H.J.; Watanabe, T.; Okumura, M.; Haruta, M.; Toshima, N. Catalytically highly active top gold atom on palladium nanocluster. Nat. Mater. 2012, 11, 49–52. [Google Scholar] [CrossRef]
- Chen, M.S.; Kumar, D.; Yi, C.W.; Goodman, D.W. The promotional effect of gold in catalysis by palladium-gold. Science 2005, 310, 291–293. [Google Scholar] [CrossRef] [Green Version]
- Enache, D.I.; Knight, D.W.; Hutchings, G.J. Solvent-free oxidation of primary alcohols to aldehydes using Au-Pd/TiO2 catalysts. Science 2006, 311, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.S.; Bando, Y.; Gautam, U.K.; Ye, C.H.; Golberg, D. Inorganic semiconductor nanostructures and their field-emission applications. J. Mark. Commun. 2008, 18, 509–522. [Google Scholar] [CrossRef]
- Chen, S.L.; Ying, P.Z.; Wang, L.; Wei, G.D.; Zheng, J.J.; Gao, F.M.; Su, S.B.; Yang, W.Y. Growth of flexible N-doped SiC quasialigned nanoarrays and their field emission properties. J. Mater. Chem. C 2013, 1, 4779–4784. [Google Scholar] [CrossRef]
- Xiao, Q.; Sarina, S.; Jaatinen, E.; Jia, J.F.; Arnold, D.P.; Liu, H.W.; Zhu, H.Y. Efficient photocatalytic Suzuki cross-coupling reactions on Au-Pd alloy nanoparticles under visible light irradiation. Green Chem. 2014, 16, 4272–4285. [Google Scholar] [CrossRef]
- Guo, X.Y. High Specific Surface Area Silicon Carbide; Chemical Industry Press: Beijing, China, 2020; pp. 49–74. [Google Scholar]
- Li, M.Y.; Lu, W.D.; He, L.; Schueth, F.; Lu, A.H. Tailoring the Surface Structure of Silicon Carbide Support for Copper Catalyzed Ethanol Dehydrogenation. Chemcatchem 2019, 11, 481–487. [Google Scholar] [CrossRef]
- Noh, Y.S.; Lee, K.Y.; Moon, D.J. Hydrogen production by steam reforming of methane over nickel based structured catalysts supported on calcium aluminate modified SiC. Int. J. Hydrogen Energy 2019, 44, 21010–21019. [Google Scholar] [CrossRef]
- Pathak, S.; Saini, S.; Kondamudi, K.; Upadhyayula, S.; Bhattacharya, S. Insights into enhanced stability and activity of silica modified SiC supported iron oxide catalyst in sulfuric acid decomposition. Appl. Catal. B Environ. 2021, 284. [Google Scholar] [CrossRef]
- Pan, B.; Chen, J.H.; Zhang, F.; Zhang, B.W.; Li, D.Y.; Zhong, Z.X.; Xing, W.H. Porous TiO2 aerogel-modified SiC ceramic membrane supported MnOx catalyst for simultaneous removal of NO and dust. J. Membr. Sci. 2020, 611. [Google Scholar] [CrossRef]
- Manfred, N.; Steffen, H.; Marius, H.; Georg, S. Catalytic activation of ceramic filter elements for combined particle separation, NOx removal and VOC total oxidation. Appl. Catal. B Environ. 2007, 70, 370–376. [Google Scholar]
- Damma, D.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. A Review of Low Temperature NH3-SCR for Removal of NOx. Catalysts 2019, 9, 349. [Google Scholar] [CrossRef] [Green Version]
- Li, H.T.; Qiu, Y.; Wang, C.Z.; Huang, X.; Xiao, T.C.; Zhao, Y.X. Nickel catalysts supported on ordered mesoporous SiC materials for CO2 reforming of methane. Catal. Today 2018, 317, 76–85. [Google Scholar] [CrossRef]
- Wang, H.Y.; Ruckenstein, E. Carbon dioxide reforming of methane to synthesis gas over supported rhodium catalysts: The effect of support. Appl. Catal. A Gen. 2000, 204, 143–152. [Google Scholar] [CrossRef]
- Chen, X.W.; Xiao, T.C.; Sergio, L.G.; Malcolm, L.H. Methane Dry Reforming over Alumina Supported Co Catalysts. Chem. Res. Chin. Univ. 2004, 20, 457–461. [Google Scholar]
- Xu, L.L.; Song, H.L.; Chou, L.J. Mesoporous nanocrystalline ceria-zirconia solid solutions supported nickel based catalysts for CO2 reforming of CH4. Int. J. Hydrogen Energy 2012, 37, 18001–18020. [Google Scholar] [CrossRef]
- Li, S.R.; Gong, J.L. Strategies for improving the performance and stability of Ni-based catalysts for reforming reactions. Chem. Soc. Rev. 2014, 43, 7245–7256. [Google Scholar] [CrossRef]
- Li, D.; Zeng, L.; Li, X.Y.; Wang, X.; Ma, H.Y.; Assabumrungrat, S.; Gong, J.L. Ceria-promoted Ni/SBA-15 catalysts for ethanol steam reforming with enhanced activity and resistance to deactivation. Appl. Catal. B Environ. 2015, 176, 532–541. [Google Scholar] [CrossRef]
- Wang, C.Z.; Sun, N.N.; Kang, M.; Wen, X.; Zhao, N.; Xiao, F.K.; Wei, W.; Zhao, T.J.; Sun, Y.H. The bi-functional mechanism of CH4 dry reforming over a Ni-CaO-ZrO2 catalyst: Further evidence via the identification of the active sites and kinetic studies. Catal. Sci. Technol. 2013, 3, 2435–2443. [Google Scholar] [CrossRef]
- Huang, X.; Jia, C.C.; Wang, C.Z.; Xiao, F.K.; Zhao, N.; Sun, N.N.; Wei, W.; Sun, Y.H. Ordered mesoporous CoO-NiO-Al2O3 bimetallic catalysts with dual confinement effects for CO2 reforming of CH4. Catal. Today 2017, 281, 241–249. [Google Scholar] [CrossRef]
- Alotaibi, R.; Alenazey, F.; Alotaibi, F.; Wei, N.; Al-Fatesh, A.; Fakeeha, A. Ni catalysts with different promoters supported on zeolite for dry reforming of methane. Appl. Petrochem. Res. 2015, 5, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Ying, J.Y.; Mehnert, C.P.; Wong, M.S. Synthesis and applications of supramolecular-templated mesoporous materials. Angew. Chem. Int. Ed. 1999, 38, 56–77. [Google Scholar] [CrossRef]
- Jiang, R.Z.; Jiao, Y.L.; Xie, Y.P.; Yang, Z.M.; Zhang, J.S. SiC foam based structured catalyst for process intensification in oxidative dehydrogenation of 1-butene to butadiene. Chem. Eng. Process. 2019, 137, 108–115. [Google Scholar] [CrossRef]
- White, W.C. Butadiene production process overview. Chem. Biol. Interact. 2007, 166, 10–14. [Google Scholar] [CrossRef]
- Hong, E.; Park, J.H.; Shin, C.H. Oxidative Dehydrogenation of n-Butenes to 1,3-Butadiene over Bismuth Molybdate and Ferrite Catalysts: A Review. Catal. Surv. Asia 2016, 20, 23–33. [Google Scholar] [CrossRef]
- Makshina, E.V.; Dusselier, M.; Janssens, W.; Degreve, J.; Jacobs, P.A.; Sels, B.F. Review of old chemistry and new catalytic advances in the on-purpose synthesis of butadiene. Chem. Soc. Rev. 2014, 43, 7917–7953. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.L.; Tong, X.L.; Wang, Y.Y.; Jin, G.Q.; Guo, X.Y. Boron-doped silicon carbide supported Pt catalyst for methanol electrooxidation. J. Fuel Chem. Technol. 2014, 42, 845–850. [Google Scholar] [CrossRef]
- Kriener, M.; Muranaka, T.; Kato, J.Y.; Ren, Z.A.; Akimitsu, J.; Maeno, Y. Superconductivity in heavily boron-doped silicon carbide. Sci. Technol. Adv. Mater. 2008, 9, 044205. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.G.; Zhao, G.F.; Bi, G.C.; Guo, Y.Y.; Xie, J. Monolithic SiC-foam supported Ni-La2O3 composites for dry reforming of methane with enhanced carbon resistance. Fuel Process. Technol. 2021, 212. [Google Scholar] [CrossRef]
- Nguyen, P.; Pham, C. Innovative porous SiC-based materials: From nanoscopic understandings to tunable carriers serving catalytic needs. Appl. Catal. A Gen. 2011, 391, 443–454. [Google Scholar] [CrossRef]
- Dama, S.; Ghodke, S.R.; Bobade, R.; Gurav, H.R.; Chilukuri, S. Active and durable alkaline earth metal substituted perovskite catalysts for dry reforming of methane. Appl. Catal. B Environ. 2018, 224, 146–158. [Google Scholar] [CrossRef]
- Wang, Y.J.; Xu, Y.; Liu, Q.K.; Sun, J.W.; Ji, S.F.; Wang, Z.J. Enhanced low-temperature activity for CO2 methanation over NiMgAl/SiC composite catalysts. J. Chem. Technol. Biotechnol. 2019, 94, 3780–3786. [Google Scholar] [CrossRef]
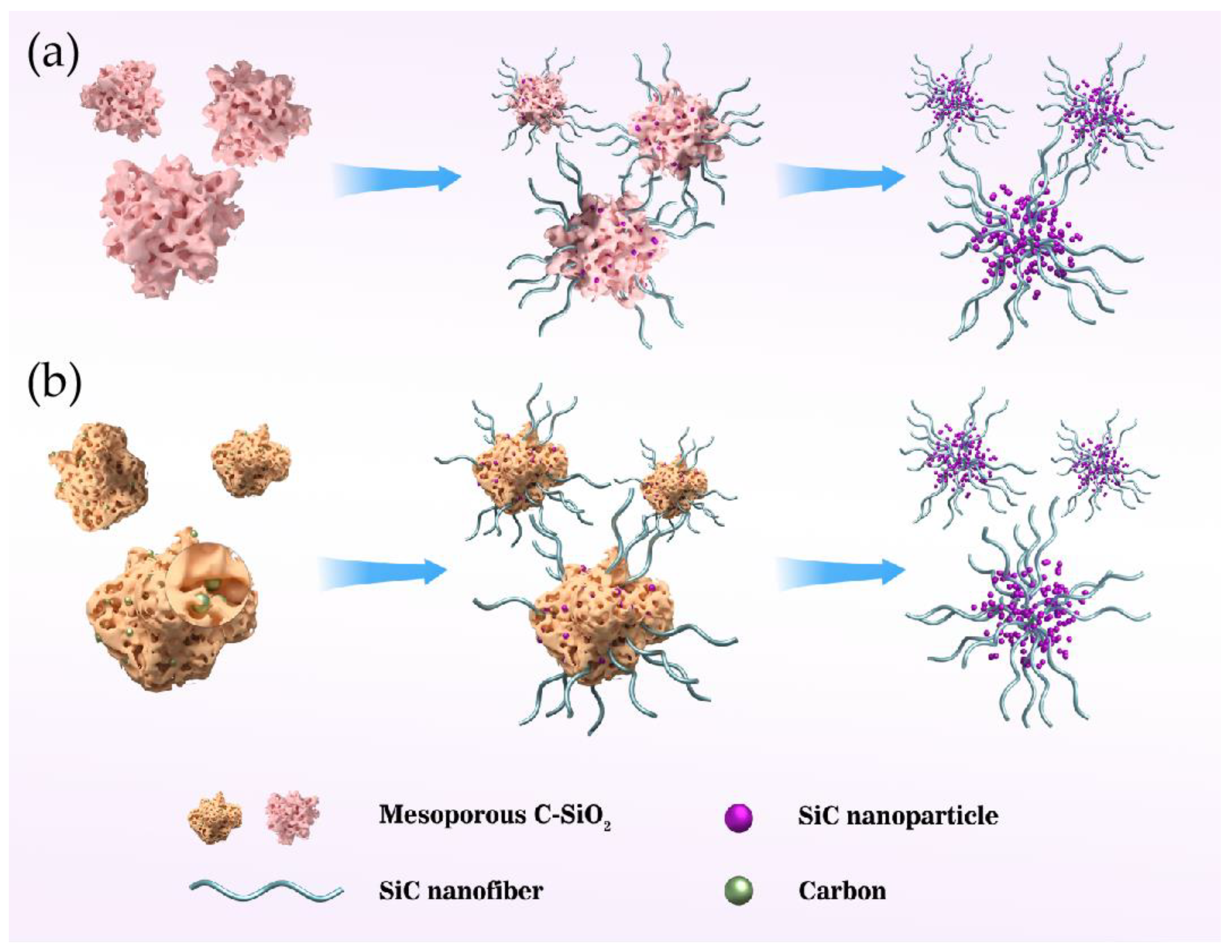

| Precursor | Temperature | Time | SiC Structure | SiC Specific Surface Area (m2/g) | Ref. |
|---|---|---|---|---|---|
| Mesoporous C-SiO2 nanocomposites | 1450 °C | 5 h | Nanofibers and nanoparticle | 76.7–83.0 | [19] |
| Expanded graphite (EG) and silica sol | 1300 °C | 6 h | Nanobelts | 97.4 | [29] |
| SiO2–sugar slurries | 1500 °C | 3 h | 3D SiCNW scaffolds | - | [30] |
| Polymer sponge with reactive particles | 1600 °C | 3 h | 3D porous structure with interwoven nanofibers | 28.26 | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, Y.; Liu, D.; Li, Q.; Bai, X.; Song, Y. Research Progress on Application in Energy Conversion of Silicon Carbide-Based Catalyst Carriers. Catalysts 2023, 13, 236. https://doi.org/10.3390/catal13020236
Teng Y, Liu D, Li Q, Bai X, Song Y. Research Progress on Application in Energy Conversion of Silicon Carbide-Based Catalyst Carriers. Catalysts. 2023; 13(2):236. https://doi.org/10.3390/catal13020236
Chicago/Turabian StyleTeng, Yingyue, Dingze Liu, Qiang Li, Xue Bai, and Yinmin Song. 2023. "Research Progress on Application in Energy Conversion of Silicon Carbide-Based Catalyst Carriers" Catalysts 13, no. 2: 236. https://doi.org/10.3390/catal13020236





