Synthesis of Green Magnetite/Carbonized Coffee Composite from Natural Pyrite for Effective Decontamination of Congo Red Dye: Steric, Synergetic, Oxidation, and Ecotoxicity Studies
Abstract
:1. Introduction
2. Results and Discussion
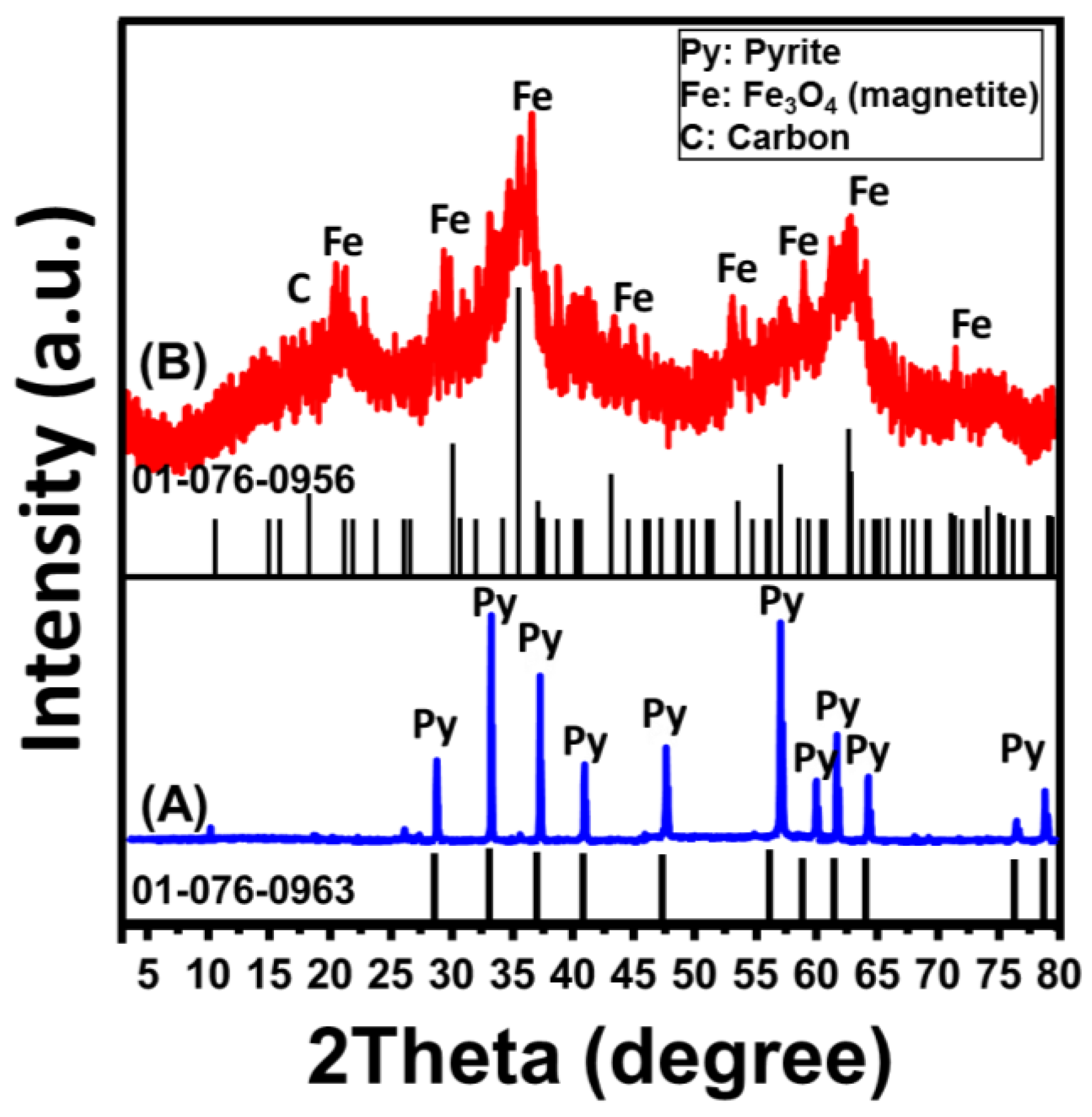
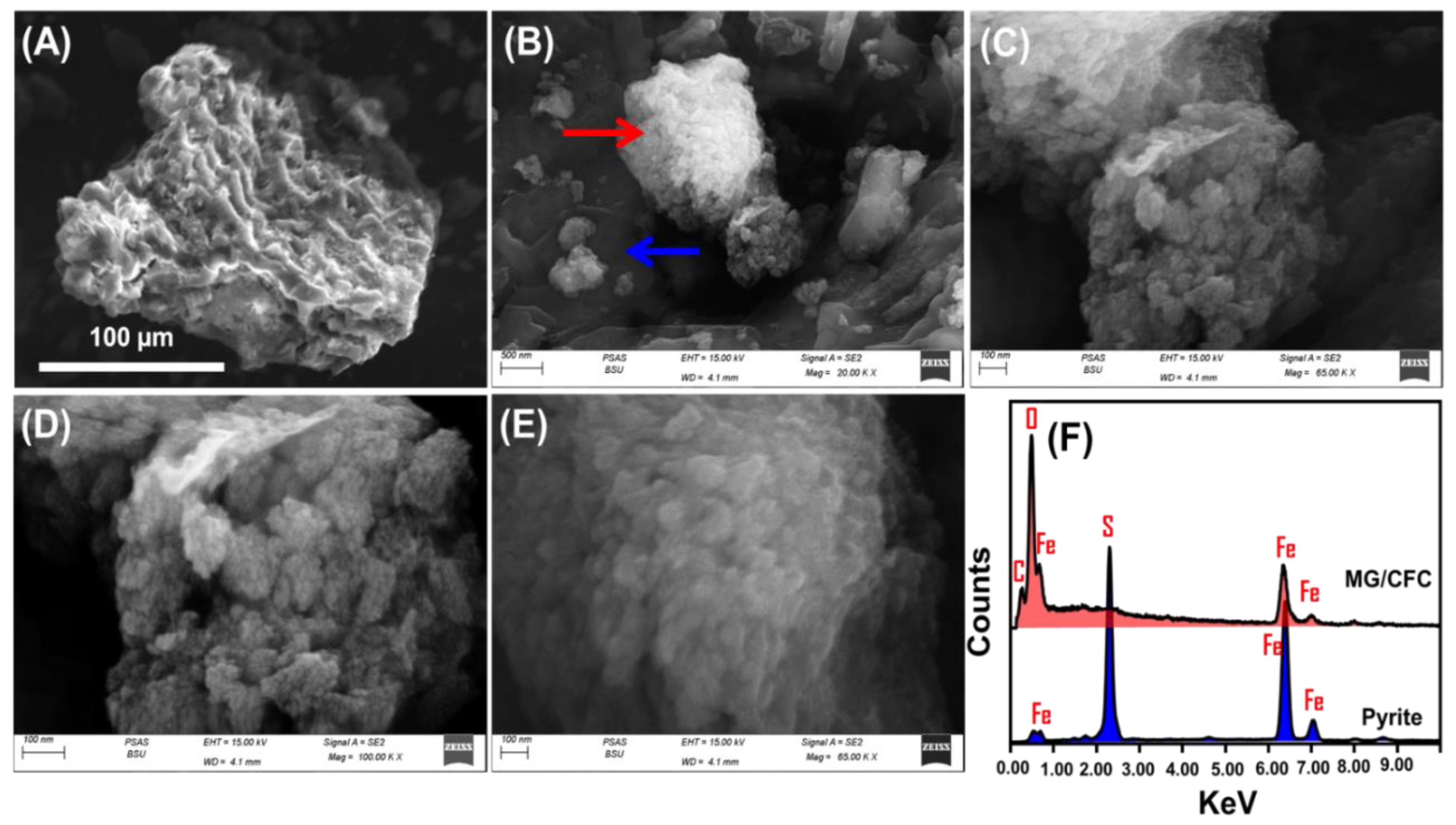
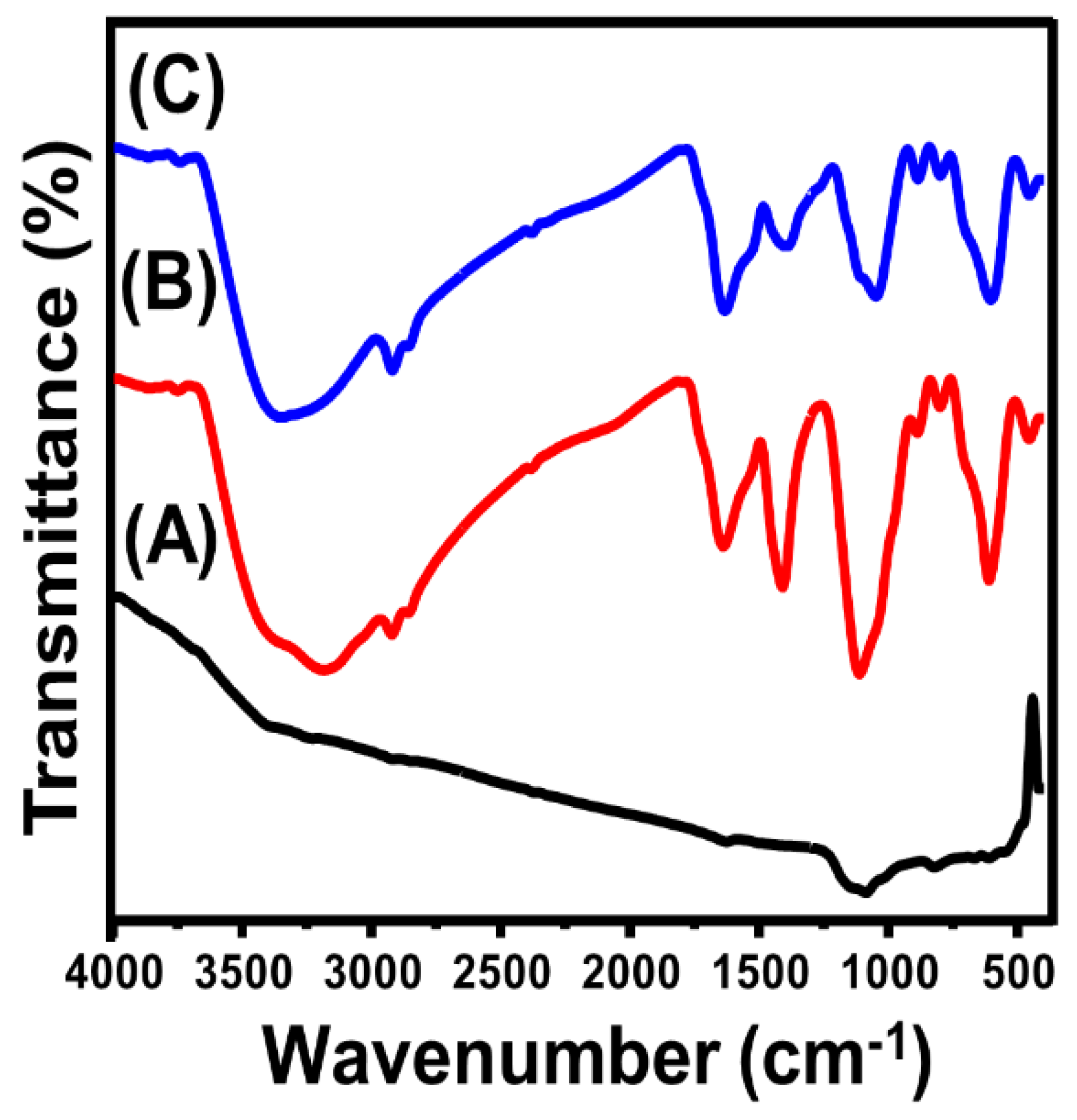
2.1. Characterization of the MG/CFC Catalyst
2.2. Adsorption Studies
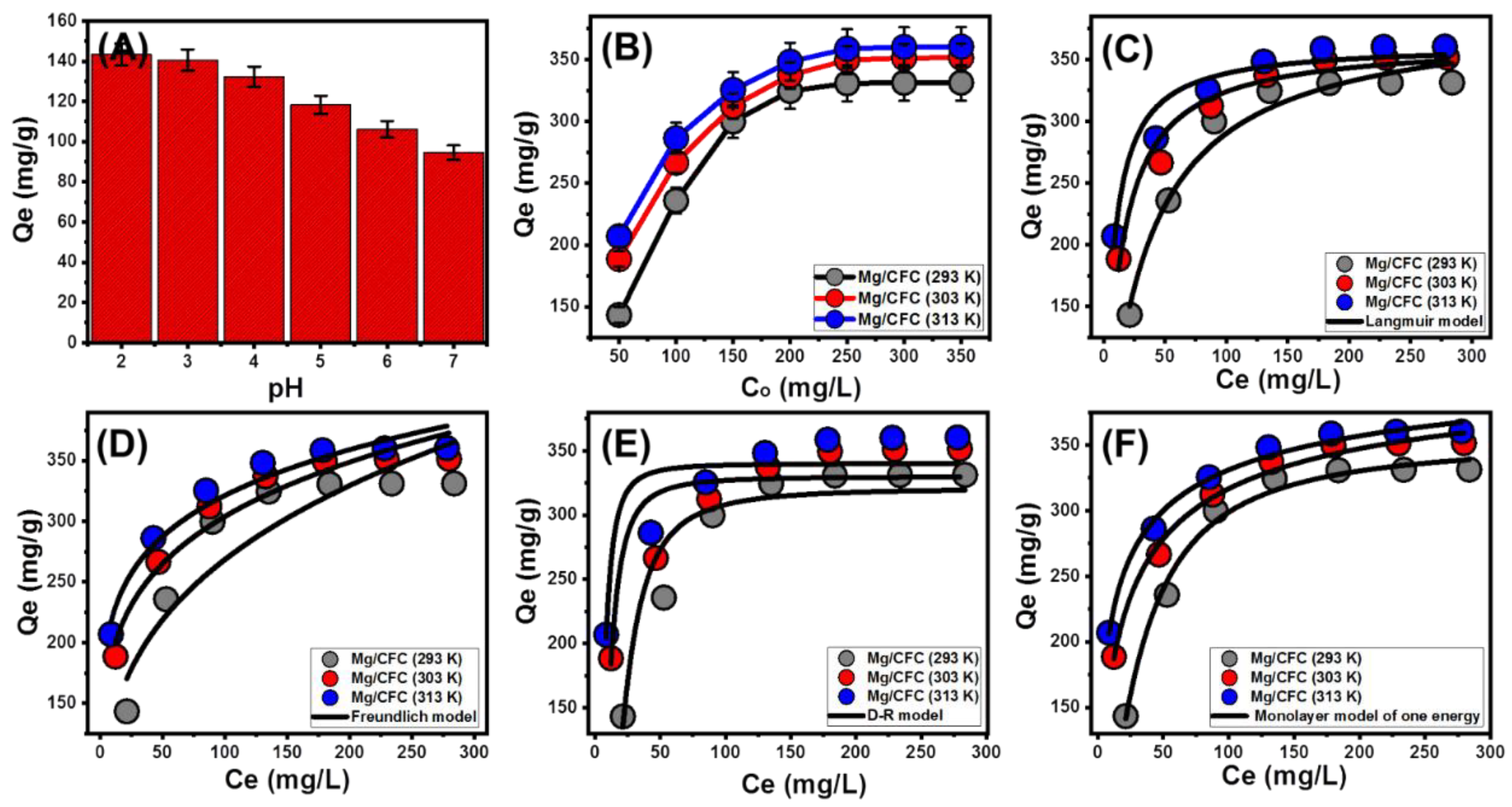
2.2.1. Effect of pH
2.2.2. Equilibrium Studies
Effect of C.R Concentrations
Classic Isotherm Models
Advanced Isotherm Models
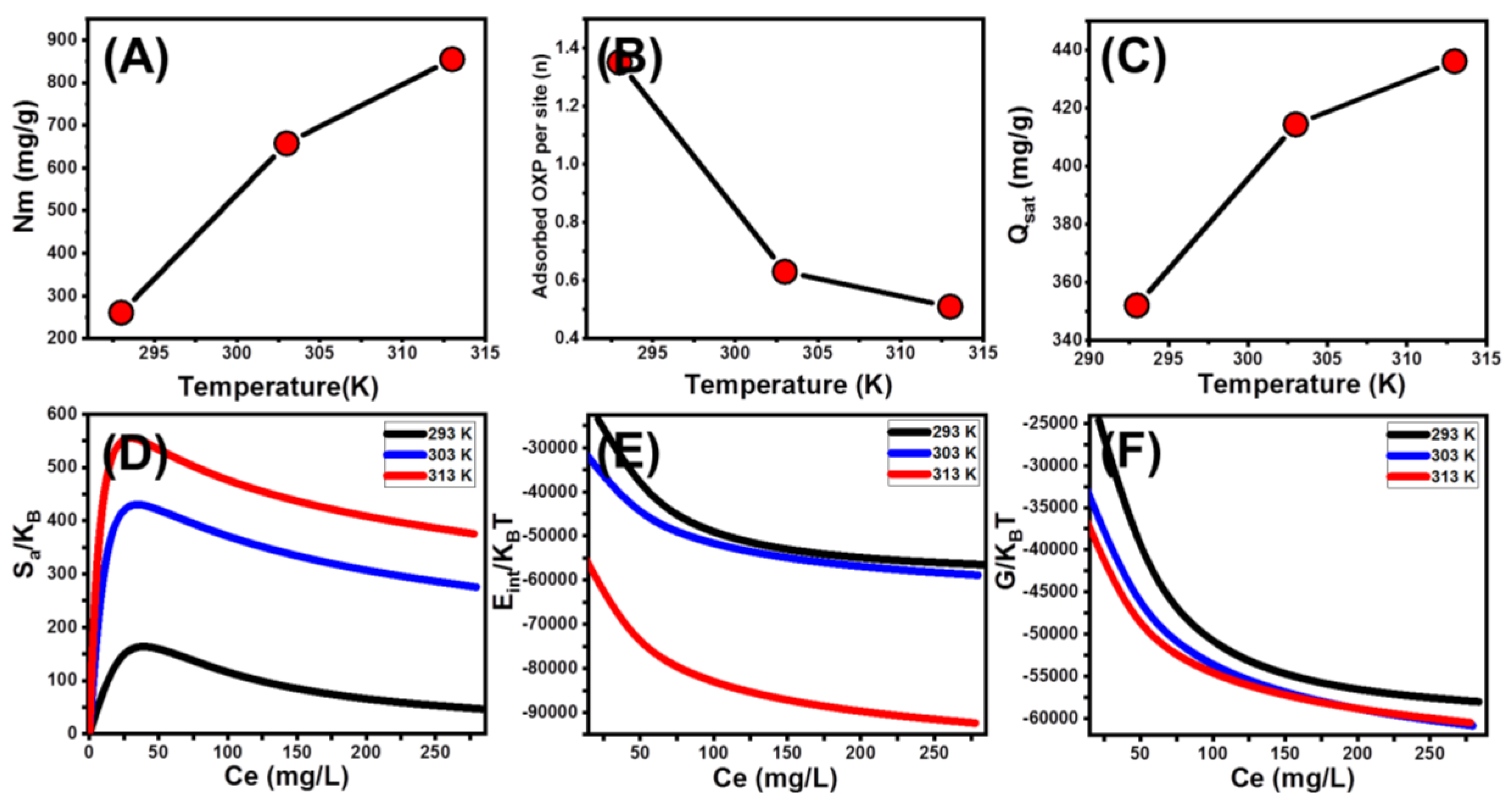
Steric Parameters
Energetic Properties
- Adsorption energy
- Thermodynamic functions
2.3. Photo-Fenton Oxidation of C.R
2.3.1. Effect of Oxidation Parameters
Effect of pH
Effect of C.R Concentrations at Different Oxidation Intervals
Effect of Catalyst Dosage at Different Oxidation Intervals
2.3.2. Mineralization Efficiency
2.3.3. Synergetic Properties of Oxidation System
- H2O2 (0.1 mL) without catalyst and a light source (Figure 6E)
- CFC, MG, and MG/CFC without H2O2 or a light source as a separated test (adsorption) (Figure 6E)
- H2O2 + catalyst (CFC, MG, and MG/CFC) without the light source (Fenton’s oxidation) (Figure 6E)
- visible light source without catalyst or H2O2 (Figure 6F)
- visible light source+ H2O2 (0.1 mL) (Figure 6F)
- visible light source+ catalyst (CFC, MG, and MG/CFC) (photocatalytic oxidation) (Figure 6F)
- visible light source+ catalyst (CFC, MG, and MG/CFC) +H2O2 (photo-Fenton’s oxidation) (Figure 6F)
2.3.4. Kinetic and Quantum Yield Studies
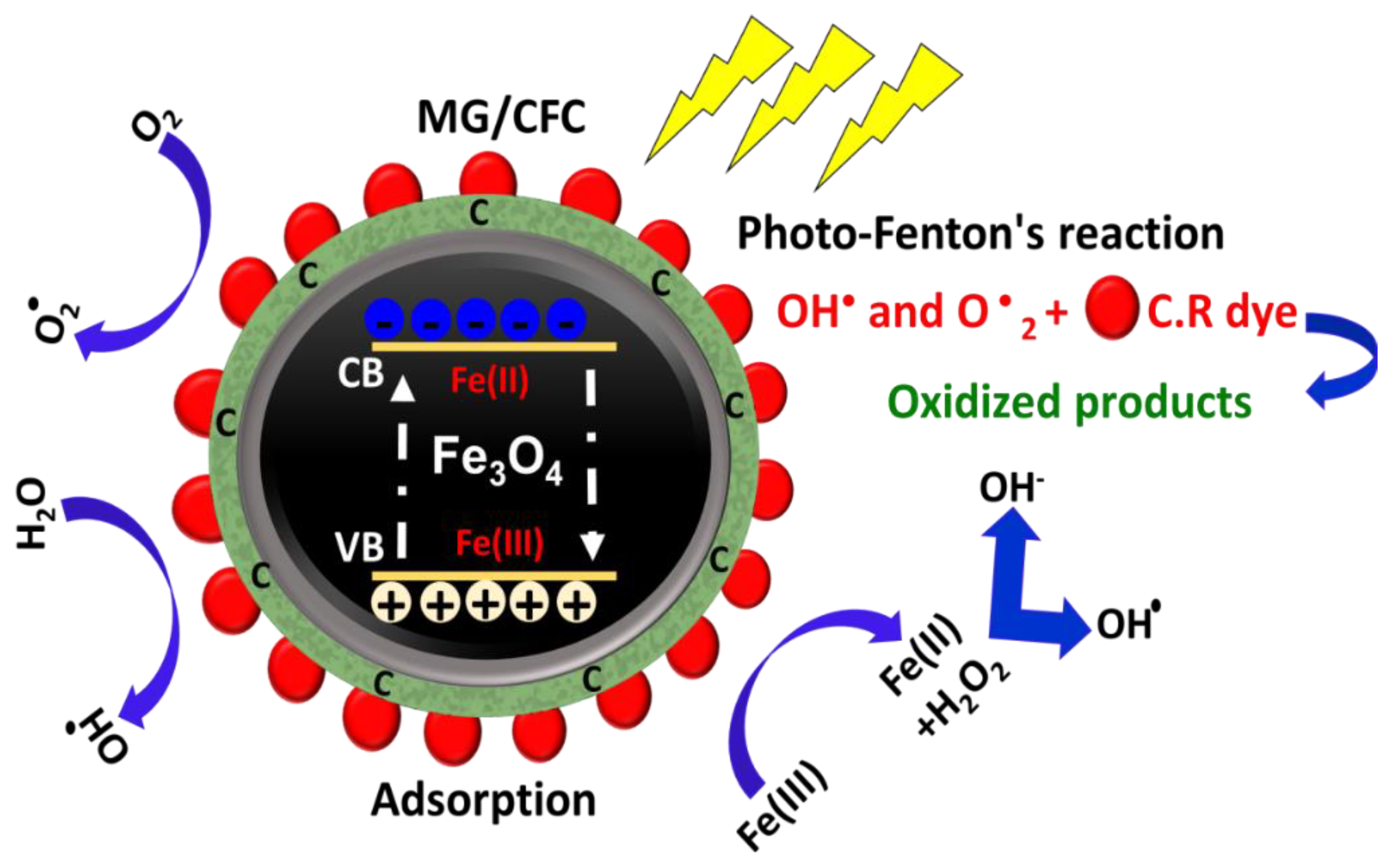
2.3.5. Suggested Oxidation Mechanism
Effective Oxidizing Species
General Oxidation Mechanism
2.4. Recyclability
2.5. Ecotoxicity Properties
2.6. Comparison Study
3. Methodology
3.1. Materials
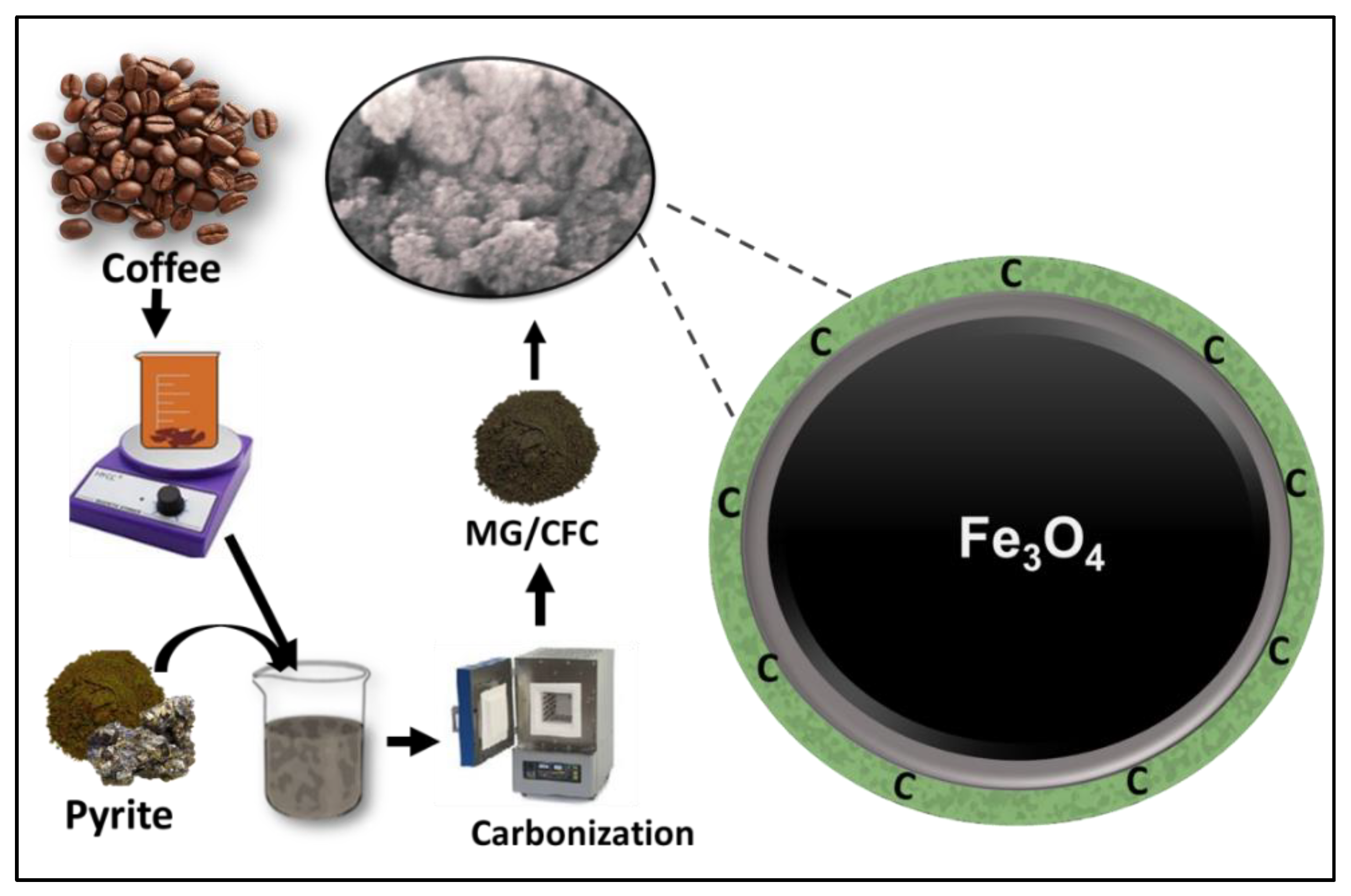
3.2. Synthesis of MG/CFC Green Nanocomposite
3.3. Characterization
3.4. Batch Adsorption Studies of C.R Dye
3.5. Photo-Fenton’s Oxidation of C.R Dye
3.6. Ecotoxicity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bilińska, L.; Blus, K.; Gmurek, M.; Ledakowicz, S. Coupling of electrocoagulation and ozone treatment for textile wastewater reuse. Chem. Eng. J. 2018, 358, 992–1001. [Google Scholar]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Ferreira, L.F.R.; Hussain, C.M.; Mulla, S.I.; Bharagava, R.N. Degradation mechanism and toxicity reduction of methyl orange dye by a newly isolated bacterium Pseudomonas aeruginosa MZ520730. J. Water Process. Eng. 2021, 43, 102300. [Google Scholar] [CrossRef]
- Wekoye, J.N.; Wanyonyi, W.C.; Wangila, P.T.; Tonui, M.K. Kinetic and equilibrium studies of Congo red dye adsorption on cabbage waste powder. Environ. Chem. Ecotoxicol. 2020, 2, 24–31. [Google Scholar] [CrossRef]
- Masalvad, S.K.S.; Sakare, P.K. Application of photo Fenton process for treatment of textile Congo-red dye solution. Mater. Today Proc. 2020, 46, 5291–5297. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; El-Meligy, M.A.; El-Sherbeeny, A.M. Evaluation and characterization of Egyptian ferruginous kaolinite as adsorbent and heterogeneous catalyst for effective removal of safranin-O cationic dye from water. Arab. J. Geosci. 2020, 13, 169. [Google Scholar] [CrossRef]
- Adly, E.R.; Shaban, M.S.; El-Sherbeeny, A.M.; Al Zoubi, W.; Abukhadra, M.R. Enhanced Congo Red Adsorption and Photo-Fenton Oxidation over an Iron-Impeded Geopolymer from Ferruginous Kaolinite: Steric, Energetic, Oxidation, and Synergetic Studies. ACS Omega 2022, 7, 31218–31232. [Google Scholar] [CrossRef]
- Navia-Mendoza, J.M.; Filho, O.A.E.; Zambrano-Intriago, L.A.; Maddela, N.R.; Duarte, M.M.M.B.; Quiroz-Fernández, L.S.; Baquerizo-Crespo, R.J.; Rodríguez-Díaz, J.M. Advances in the application of nanocatalysts in photocatalytic processes for the treatment of food dyes: A review. Sustainability 2021, 13, 11676. [Google Scholar] [CrossRef]
- Harja, M.; Buema, G.; Bucur, D. Recent advances in removal of Congo Red dye by adsorption using an industrial waste. Sci. Rep. 2022, 12, 1–18. [Google Scholar] [CrossRef]
- Han, M.; Wang, S.; Chen, X.; Liu, H.; Gao, H.; Zhao, X.; Wang, F.; Yang, H.; Yi, Z.; Fang, L. Spinel CuB2O4 (B = Fe, Cr, and Al) oxides for selective adsorption of Congo red and photocatalytic removal of antibiotics. ACS Appl. Nano Mater. 2022, 5, 11194–11207. [Google Scholar] [CrossRef]
- Al-Salihi, S.; Jasim, A.M.; Fidalgo, M.M.; Xing, Y. Removal of Congo red dyes from aqueous solutions by porous γ-alumina nanoshells. Chemosphere 2021, 286, 131769. [Google Scholar] [CrossRef]
- Kumar, N.; Narayanasamy, S. Toxicological assessment and adsorptive removal of lead (Pb) and Congo red (CR) from water by synthesized iron oxide/activated carbon (Fe3O4/AC) nanocomposite. Chemosphere 2022, 294, 133758. [Google Scholar]
- Kang, X.; Cheng, Y.; Wen, Y.; Qi, J.; Li, X. Bio-inspired co-deposited preparation of GO composite loose nanofiltration membrane for dye contaminated wastewater sustainable treatment. J. Hazard. Mater. 2020, 400, 123121. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Dwivedi, S.K. Biosorptive and Biodegradative Mechanistic Approach for the Decolorization of Congo Red Dye by Aspergillus Species. Bull. Environ. Contam. Toxicol. 2021, 108, 457–467. [Google Scholar] [CrossRef]
- Kaur, H.; Jaryal, N. Utilization of biogenic tea waste silver nanoparticles for the reduction of organic dyes. Mater. Res. Express 2018, 5, 055402. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Z.; Luo, S.-Y.; Zong, M.-H.; Lou, W.-Y. Multi-functional magnetic hydrogels based on Millettia speciosa Champ residue cellulose and Chitosan: Highly efficient and reusable adsorbent for Congo red and Cu2+ removal. Chem. Eng. J. 2021, 423, 130198. [Google Scholar] [CrossRef]
- Mudhoo, A.; Sillanpää, M. Magnetic nanoadsorbents for micropollutant removal in real water treatment: A review. Environ. Chem. Lett. 2021, 19, 4393–4413. [Google Scholar] [CrossRef]
- Indira, K.; Shanmugam, S.; Hari, A.; Vasantharaj, S.; Sathiyavimal, S.; Brindhadevi, K.; El Askary, A.; Elfasakhany, A.; Pugazhendhi, A. Photocatalytic degradation of congo red dye using nickel–titanium dioxide nanoflakes synthesized by Mukia madrasapatna leaf extract. Environ. Res. 2021, 202, 111647. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, G.E.; de Freitas, R.A.; Rodríguez-Díaz, J.M.; da Silva, P.M.; Napoleão, T.H.; Duarte, M.M.M.B. Degradation of the residual textile mixture cetyltrimethylammonium bromide/remazol yellow gold RNL-150%/reactive blue BF-5G: Evaluation photo-peroxidation and photo-Fenton processes in LED and UV-C photoreactors. Environ. Sci. Pollut. Res. 2021, 28, 64630–64641. [Google Scholar] [CrossRef]
- Gorozabel-Mendoza, M.L.; Filho, O.A.E.; Zambrano-Intriago, L.A.; Baquerizo-Crespo, R.J.; Giler-Molina, J.M.; Rodríguez-Díaz, J.M. Degradation of Blue 1 and Yellow 6 Dyes in Binary Mixture Using Photo-Fenton/Sunlight System: Optimization by Factorial Designs. Water Air Soil Pollut. 2021, 232, 1–10. [Google Scholar] [CrossRef]
- Tan, X.; Li, H.; Li, X.; Sun, W.; Jin, C.; Chen, L.; Wei, H.; Sun, C. A novel isophorone wastewater treatment technology-wet electrocatalytic oxidation and its degradation mechanism study. J. Hazard. Mater. 2020, 389, 122035. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R.; Ibrahim, S.S.; Shahien, M. Photocatalytic degradation and photo-Fenton oxidation of Congo red dye pollutants in water using natural chromite—Response surface optimization. Appl. Water Sci. 2017, 7, 4743–4756. [Google Scholar] [CrossRef] [Green Version]
- Abukhadra, M.R.; Saad, I.; Othman, S.I.; Katowah, D.F.; Ajarem, J.S.; Alqarni, S.A.; Allam, A.A.; Al Zoubi, W.; Ko, Y.G. Characterization of Fe0@ Chitosan/Cellulose structure as effective green adsorbent for methyl Parathion, malachite Green, and levofloxacin Removal: Experimental and theoretical studies. J. Mol. Liq. 2022, 368, 120730. [Google Scholar] [CrossRef]
- Wu, J.; Wang, B.; Cagnetta, G.; Huang, J.; Wang, Y.; Deng, S.; Yu, G. Nanoscale zero valent iron-activated persulfate coupled with Fenton oxidation process for typical pharmaceuticals and personal care products degradation. Sep. Purif. Technol. 2020, 239, 116534. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M. Synthesis and application of zero-valent iron nanoparticles in water treatment, environmental remediation, catalysis, and their biological effects. Nanomaterials 2020, 10, 917. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Lu, J.; Ye, J.; Zhou, Y. The effects and mechanisms of zero-valent iron on anaerobic digestion of solid waste: A mini-review. J. Clean. Prod. 2020, 278, 123567. [Google Scholar] [CrossRef]
- Tang, H.; Wang, J.; Zhang, S.; Pang, H.; Wang, X.; Chen, Z.; Li, M.; Song, G.; Qiu, M.; Yu, S. Recent advances in nanoscale zero-valent iron-based materials: Characteristics, environmental remediation and challenges. J. Clean. Prod. 2021, 319, 128641. [Google Scholar] [CrossRef]
- Paz, C.B.; Araújo, R.S.; Oton, L.F.; Oliveira, A.C.; Soares, J.M.; Medeiros, S.N.; Rodríguez-Castellón, E.; Rodríguez-Aguado, E. Acid red 66 dye removal from aqueous solution by Fe/C-based composites: Adsorption, kinetics and thermodynamic studies. Materials 2020, 13, 1107. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.F.; Mofijur, M.; Rafa, N.; Chowdhury, A.T.; Chowdhury, S.; Nahrin, M.; Islam, A.S.; Ong, H.C. Green approaches in synthesising nanomaterials for environmental nanobioremediation: Technological advancements, applications, benefits and challenges. Environ. Res. 2021, 204, 111967. [Google Scholar] [CrossRef]
- He, P.; Zhu, J.; Chen, Y.; Chen, F.; Zhu, J.; Liu, M.; Zhang, K.; Gan, M. Pyrite-activated persulfate for simultaneous 2, 4-DCP oxidation and Cr (VI) reduction. Chem. Eng. J. 2020, 406, 126758. [Google Scholar] [CrossRef]
- Li, W.; Yang, S.; Wang, W.; Liu, Q.; He, J.; Li, B.; Cai, Z.; Chen, N.; Fang, H.; Sun, S. Simultaneous removal of Cr (VI) and acid orange 7 from water in pyrite-persulfate system. Environ. Res. 2020, 189, 109876. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Huang, R.; Cao, Y. A novel strategy for enhancing heterogeneous Fenton degradation of dye wastewater using natural pyrite: Kinetics and mechanism. Chemosphere 2021, 272, 129883. [Google Scholar] [CrossRef] [PubMed]
- Rickard, D. How long does it take a pyrite framboid to form? Earth Planet. Sci. Lett. 2019, 513, 64–68. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, J.; Long, X.; Li, J.; Xiang, Q.; Zhang, J.; Hao, J. Genesis and evolution of framboidal pyrite and its implications for the ore-forming process of Carlin-style gold deposits, southwestern China. Ore Geol. Rev. 2018, 102, 426–436. [Google Scholar] [CrossRef]
- Du, M.; Kuang, H.; Zhang, Y.; Zeng, X.; Yi, C.; Hussain, I.; Hung, S.; Zhao, S. Enhancement of ball-milling on pyrite/zero-valent iron for persulfate activation on imidacloprid removal in aqueous solution: A mechanistic study. J. Environ. Chem. Eng. 2021, 9, 105647. [Google Scholar] [CrossRef]
- Liu, A.; Liu, J.; Pan, B.; Zhang, W.X. Formation of lepidocrocite (γ-FeOOH) from oxidation of nanoscale zero-valent iron (nZVI) in oxygenated water. RSC Adv. 2014, 4, 57377–57382. [Google Scholar] [CrossRef]
- Rahimi, F.; van der Hoek, J.P.; Royer, S.; Javid, A.; Mashayekh-Salehi, A.; Sani, M.J. Pyrite nanoparticles derived from mine waste as efficient catalyst for the activation of persulfates for degradation of tetracycline. J. Water Process. Eng. 2020, 40, 101808. [Google Scholar] [CrossRef]
- Khabbaz, M.; Entezari, M. Simple and versatile one-step synthesis of FeS2 nanoparticles by ultrasonic irradiation. J. Colloid Interface Sci. 2016, 470, 204–210. [Google Scholar] [CrossRef]
- Gong, B.; Li, D.; Niu, Z.; Liu, Y.; Dang, Z. Inhibition of pyrite oxidation using PropS-SH/sepiolite composite coatings for the source control of acid mine drainage. Environ. Sci. Pollut. Res. 2020, 28, 11090–11105. [Google Scholar] [CrossRef]
- Silva, C.A.; Silva, R.L.; Figueiredo, A.T.D.; Alves, V.N. Magnetic solid-phase microextraction for lead detection in aqueous samples using magnetite nanoparticles. J. Braz. Chem. Soc. 2020, 31, 109–115. [Google Scholar] [CrossRef]
- Azizi, A. Green synthesis of Fe3O4 nanoparticles and its application in preparation of Fe3O4/cellulose magnetic nanocomposite: A suitable proposal for drug delivery systems. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3552–3561. [Google Scholar] [CrossRef]
- Eslami, S.; Ebrahimzadeh, M.A.; Biparva, P. Green synthesis of safe zero valent iron nanoparticles by Myrtus communis leaf extract as an effective agent for reducing excessive iron in iron-overloaded mice, a thalassemia model. RSC Adv. 2018, 8, 26144–26155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, L.; Wang, P.; Valiyaveettil, S. Successive extraction of As (V), Cu (II) and P (V) ions from water using spent coffee powder as renewable bioadsorbents. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.; Do, Q.C.; Kim, D.; Kim, J.; Kang, S. Urchin-like structured magnetic hydroxyapatite for the selective separation of cerium ions from aqueous solutions. J. Hazard. Mater. 2022, 430, 128488. [Google Scholar] [CrossRef]
- Abdel Salam, M.; Mokhtar, M.; Albukhari, S.M.; Baamer, D.F.; Palmisano, L.; Jaremko, M.; Abukhadra, M.R. Synthesis and Characterization of Green ZnO@ polynaniline/Bentonite Tripartite Structure (G. Zn@ PN/BE) as Adsorbent for As (V) Ions: Integration, Steric, and Energetic Properties. Polymers 2022, 14, 2329. [Google Scholar] [CrossRef]
- Albukhari, S.M.; Salam, M.A.; Abukhadra, M.R. Effective retention of inorganic Selenium ions (Se (VI) and Se (IV)) using novel sodalite structures from muscovite; characterization and mechanism. J. Taiwan Inst. Chem. Eng. 2021, 120, 116–126. [Google Scholar] [CrossRef]
- Sayed, I.R.; Farhan, A.M.; AlHammadi, A.A.; El-Sayed, M.I.; El-Gaied, I.M.A.; El-Sherbeeny, A.M.; Al Zoubi, W.; Ko, Y.G.; Abukhadra, M.R. Synthesis of novel nanoporous zinc phosphate/hydroxyapatite nano-rods (ZPh/HPANRs) core/shell for enhanced adsorption of Ni2+ and Co2+ ions: Characterization and application. J. Mol. Liq. 2022, 360, 119527. [Google Scholar] [CrossRef]
- Dawodu, F.A.; Akpomie, G.K.; Abuh, M.A. Equilibrium Isotherm Studies on the Batch Sorption of Copper (II) ions from Aqueous Solution unto Nsu Clay. Int. J. Sci. Eng. Res. 2012, 3, 1–7. [Google Scholar]
- Amrhar, O.; El Gana, L.; Mobarak, M. Calculation of adsorption isotherms by statistical physics models: A review. Environ. Chem. Lett. 2021, 19, 4519–4547. [Google Scholar] [CrossRef]
- Dhaouadi, F.; Sellaoui, L.; Badawi, M.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Jaime-Leal, J.E. Statistical physics interpretation of the adsorption mechanism of Pb2+, Cd2+ and Ni2+ on chicken feathers. J. Mol. Liq. 2020, 319, 114168. [Google Scholar] [CrossRef]
- Ali, R.A.; Mobarak, M.; Badawy, A.M.; Lima, E.C.; Seliem, M.K.; Ramadan, H. New insights into the surface oxidation role in enhancing Congo red dye uptake by Egyptian ilmenite ore: Experiments and physicochemical interpretations. Surf. Interfaces 2021, 26, 101316. [Google Scholar] [CrossRef]
- Ashraf, M.-T.; AlHammadi, A.A.; El-Sherbeeny, A.M.; Alhammadi, S.; Al Zoubi, W.; Ko, Y.G.; Abukhadra, M.R. Synthesis of cellulose fibers/Zeolite-A nanocomposite as an environmental adsorbent for organic and inorganic selenium ions; Characterization and advanced equilibrium studies. J. Mol. Liq. 2022, 360, 119573. [Google Scholar] [CrossRef]
- Dhaouadi, F.; Sellaoui, L.; Reynel-Ávila, H.E.; Landín-Sandoval, V.; Mendoza-Castillo, D.I.; Jaime-Leal, J.E.; Lima, E.C.; Bonilla-Petriciolet, A.; Lamine, A.B. Adsorption mechanism of Zn2+, Ni2+, Cd2+, and Cu2+ ions by carbon-based adsorbents: Interpretation of the adsorption isotherms via physical modelling. Environ. Sci. Pollut. Res. 2021, 28, 30943–30954. [Google Scholar] [CrossRef] [PubMed]
- Sellaoui, L.; Ali, J.; Badawi, M.; Bonilla-Petriciolet, A.; Chen, Z. Understanding the adsorption mechanism of Ag+ and Hg2+ on functionalized layered double hydroxide via statistical physics modeling. Appl. Clay Sci. 2020, 198, 105828. [Google Scholar] [CrossRef]
- Sellaoui, L.; Guedidi, H.; SarraWjihi, S.; Reinert, L.; Knani, S.; Duclaux, L.; Ben Lamine, A. Experimental and theoretical studies of adsorption of ibuprofen on raw and two chemically modified activated carbons: New physicochemical interpretations. RSC Adv. 2016, 6, 12363–12373. [Google Scholar] [CrossRef]
- Abdullah, R.R.; Shabeed, K.M.; Alzubaydi, A.B.; Alsalhy, Q.F. Novel photocatalytic polyether sulphone ultrafiltration (UF) membrane reinforced with oxygen-deficient Tungsten Oxide (WO2. 89) for Congo red dye removal. Chem. Eng. Res. Des. 2022, 177, 526–540. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, Q.; Yang, G.; Li, X.; Du, W.; Leong, Y.K.; Chang, J.-S. Enhanced chlortetracycline removal by iron oxide modified spent coffee grounds biochar and persulfate system. Chemosphere 2022, 301, 134654. [Google Scholar] [CrossRef]
- Salam, M.A.; AbuKhadra, M.R.; Mohamed, A.S. Effective oxidation of methyl parathion pesticide in water over recycled glass based-MCM-41 decorated by green Co3O4 nanoparticles. Environ. Pollut. 2020, 259, 113874. [Google Scholar] [CrossRef]
- Saad, A.M.; Abukhadra, M.R.; Ahmed, S.A.-K.; Elzanaty, A.M.; Mady, A.H.; Betiha, M.A.; Shim, J.-J.; Rabie, A.M. Photocatalytic degradation of malachite green dye using chitosan supported ZnO and Ce–ZnO nano-flowers under visible light. J. Environ. Manag. 2020, 258, 110043. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; AlHammadi, A.A.; Khim, J.S.; Ajarem, J.S.; Allam, A.A. Enhanced decontamination of Levofloxacin residuals from water using recycled glass based a green zinc oxide/mesoporous silica nanocomposite; adsorption and advanced oxidation studies. J. Clean. Prod. 2022, 356, 131836. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.-T.; Teng, Y.; Zhao, J.; Sun, X. Heterogeneous activation of persulfate by carbon nanofiber supported Fe3O4@ carbon composites for efficient ibuprofen degradation. J. Hazard. Mater. 2020, 401, 123428. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; El-Sherbeeny, A.M.; AlHammadi, A.A.; Park, W.-H.; Abukhadra, M.R. Insight into the adsorption and oxidation activity of a ZnO/piezoelectric quartz core-shell for enhanced decontamination of ibuprofen: Steric, energetic, and oxidation studies. Chem. Eng. J. 2021, 431, 134312. [Google Scholar] [CrossRef]
- Fard, S.G.; Haghighi, M.; Shabani, M. Facile one-pot ultrasound-assisted solvothermal fabrication of ball-flowerlike nanostructured (BiOBr) x (Bi7O9I3) 1-x solid-solution for high active photodegradation of antibiotic levofloxacin under sun-light. Appl. Catal. B Environ. 2019, 248, 320–331. [Google Scholar] [CrossRef]
- Sun, Q.; Hu, X.; Zheng, S.; Zhang, J.; Sheng, J. Effect of calcination on structure and photocatalytic property of N-TiO2/g-C3N4@ diatomite hybrid photocatalyst for improving reduction of Cr (Ⅵ). Environ. Pollut. 2019, 245, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Alsamhary, K.; Al-Enazi, N.M.; Alhomaidi, E.; Alwakeel, S. Spirulina platensis mediated biosynthesis of Cuo Nps and photocatalytic degradation of toxic azo dye Congo red and kinetic studies. Environ. Res. 2022, 207, 112172. [Google Scholar] [CrossRef] [PubMed]
- Abukhadra, M.R.; Adlii, A.; Bakry, B.M. Green fabrication of bentonite/chitosan@cobalt oxide composite (BE/CH@Co) of enhanced adsorption and advanced oxidation removal of Congo red dye and Cr (VI) from water. Int. J. Biol. Macromol. 2019, 126, 402–413. [Google Scholar] [CrossRef]
- Khan, S.; Khan, A.; Ali, N.; Ahmad, S.; Ahmad, W.; Malik, S. Degradation of Congo red dye using ternary metal selenide-chitosan microspheres as robust and reusable catalysts. Environ. Technol. Innov. 2021, 22, 101402. [Google Scholar] [CrossRef]
- Zhou, H.; Qiu, Y.; Yang, C.; Zang, J.; Song, Z.; Yang, T.; Li, J.; Fan, Y.; Dang, F.; Wang, W. Efficient Degradation of Congo Red in Water by UV-Vis Driven CoMoO4/PDS Photo-Fenton System. Molecules 2022, 27, 8642. [Google Scholar] [CrossRef]
- Said, M.; Rizki, W.T.; Asri, W.R.; Desnelli, D.; Rachmat, A.; Hariani, P.L. SnO2–Fe3O4 nanocomposites for the photodegradation of the Congo red dye. Heliyon 2022, 8, e09204. [Google Scholar] [CrossRef]
- Rohilla, S. Rietveld refinement and structural characterization of TiO2/CoFe2O4 nanocomposites. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 872, p. 012171. [Google Scholar]
- Taher, T.; Putra, R.; Palapa, N.R.; Lesbani, A. Preparation of magnetite-nanoparticle-decorated NiFe layered double hydroxide and its adsorption performance for congo red dye removal. Chem. Phys. Lett. 2021, 777, 138712. [Google Scholar] [CrossRef]
- Gao, L.; Gao, T.; Zhang, Y.; Hu, T. A bifunctional 3D porous Zn-MOF: Fluorescence recognition of Fe3+ and adsorption of congo red/methyl orange dyes in aqueous medium. Dye Pigment. 2021, 197, 109945. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Wen, H.-Y.; Gollakota, A.R.; Wen, J.-C.; Shu, C.-M.; Lin, K.-Y.A.; Tian, Z.; Wen, J.-H.; Reddy, G.M.; Zyryanov, G.V. Magnetic Fe3O4 nanoparticles loaded papaya (Carica papaya L.) seed powder as an effective and recyclable adsorbent material for the separation of anionic azo dye (Congo Red) from liquid phase: Evaluation of adsorption properties. J. Mol. Liq. 2021, 345, 118255. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Mondal, M.K. Green synthesis of Fe3O4-onion peel biochar nanocomposites for adsorption of Cr(VI), methylene blue and congo red dye from aqueous solutions. J. Mol. Liq. 2022, 349, 118161. [Google Scholar] [CrossRef]



| Parameters of the Classic Isotherm Models | |||||
|---|---|---|---|---|---|
| 293 K | 303 K | 313 K | |||
| MG/CFC | Langmuir model | Qmax (mg/g) | 362.7 | 364.8 | 388.07 |
| b(L/mg) | 0.14 | 0.079 | 0.029 | ||
| R2 | 0.95 | 0.97 | 0.98 | ||
| X2 | 0.53 | 0.49 | 0.58 | ||
| Freundlich model | 1/n | 0.16 | 0.19 | 0.29 | |
| kF (mg/g) | 153.5 | 122 | 68.9 | ||
| R2 | 0.95 | 0.95 | 0.88 | ||
| X2 | 0.67 | 0.81 | 3.6 | ||
| D-R model | β (mol2/KJ2) | 0.0166 | 0.0384 | 0.0169 | |
| Qm (mg/g) | 321 | 330.1 | 340.2 | ||
| R2 | 0.92 | 0.85 | 0.85 | ||
| X2 | 1.96 | 2.8 | 2.3 | ||
| E (KJ/mol) | 1.73 | 3.61 | 5.43 | ||
| Steric and energetic parameters of the advanced isotherm model | |||||
| MG/CFC | R2 X2 n Nm (mg/g) QSat (mg/g) C1/2 (mg/L) ∆E (kJ/mol) | 0.99 0.29 1.53 260.6 351.8 28.8 8.2 | 0.99 0.17 0.63 657.7 414.3 17.3 7.3 | 0.99 0.12 0.51 855.1 436.1 10.79 6.2 | |
| First Order | Second Order | Quantum Yield (ɸ) | ||||
|---|---|---|---|---|---|---|
| R2 | K1 | R2 | K2 | |||
| Dosage | 0.2 g/L | 0.83 | 0.052 | 0.75 | 0.0092 | 5.34 × 10−8 |
| 0.3 g/L | 0.87 | 0.0652 | 0.66 | 0.0428 | 6.73 × 10−8 | |
| 0.4 g/L | 0.81 | 0.0739 | 0.70 | 0.0569 | 7.63 × 10−8 | |
| Concentration | 5 mg/L | 0.83 | 0.052 | 0.75 | 0.0092 | 5.34 × 10−8 |
| 10 mg/L | 0.80 | 0.034 | 0.63 | 0.0065 | 3.51 × 10−8 | |
| 15 mg/L | 0.81 | 0.029 | 0.63 | 0.0040 | 2.99 × 10−8 | |
| 20 mg/L | 0.84 | 0.024 | 0.66 | 0.0032 | 2.47 × 10−8 | |
| 25 mg/L | 0.93 | 0.018 | 0.72 | 0.0025 | 1.859 × 10−8 | |
| Compound | Fish (LC50) | Green Algae (EC50) | Fish (ChV) | Green Algae (ChV) |
|---|---|---|---|---|
| C.R control | 0.534 | 0.311 | 0.077 | 0.812 |
| 30 min | 87.8 | 71.4 | 3.86 | 6.4 |
| 60 min | 486.9 | 213.6 | 211.3 | 153.7 |
| 90 min | 4754.4 | 566.2 | 1341.6 | 387.4 |
| 120 min | 10320.2 | 924.3 | 1672.8 | 934.5 |
| Catalysts | Dosage | Conc., | Light Source | Oxidation (%) | References |
|---|---|---|---|---|---|
| Ni–TiO2 | 0.02 g/L | 10 mg/L | 450 W Xe lamp | 180 min, ca.92.3% | [17] |
| CuO NPs | 0.01 g/L | 5 × 10− 5 M | 100 W electric bulb | 120 min, ca. 91% | [64] |
| BE/CH@Co3O4 | 0.02 g/L | 25 mg/L | visible light source | 240 min, ca. 98.8% | [65] |
| ZBiSe-NPs | 0.225 g/L | 40 mg/L | UV-1602 double beam | 120 min, ca. 99.6% | [66] |
| CoMoO4/PDS | 0.8 g/L | 100 mg/L | 500 W xenon lamp, PDS (0.5 mmol/L) | 35 min, ca. 96.9% | [67] |
| SnO2–Fe3O4 | 0.03 g/L | 18 mg/L | 14 W UV lamp | 90 min, ca. 50.76% | [68] |
| TiO2-CoFe2O4 | 0.08 g/L | 100 mg/L | 150 W metal Halide lamp | 250 min, ca. 97% | [69] |
| MG/CFC | 0.5 g/L | 5 mg/L | metal Halide lamp, 0.1 mL H2O2 | 45 min, ca. 100% | This study |
| MG/CFC | 0.2 g/L | 5 mg/L | metal Halide lamp, 0.1 mL H2O2 | 105 min, ca. 100% | This study |
| MG/CFC | 0.2 g/L | 10 mg/L | metal Halide lamp, 0.1 mL H2O2 | 120 min, ca. 100% | This study |
| MG/CFC | 0.2 g/L | 20 mg/L | metal Halide lamp, 0.1 mL H2O2 | 180 min, ca. 100% | This study |
| Adsorption | |||||
| Adsorbents | Adsorption capacity (mg/g) | References | |||
| MNPs@NiFe LDH | 79.6 | [70] | |||
| Zn-MOF | 355.16 | [71] | |||
| Fe3O4-OPBC-2 NCs | 299.82 | [72] | |||
| Fe3O4- OPBC-1 NCs | 317.33 | [73] | |||
| polyacrolein (PA-1) | 140.8 | [72] | |||
| Pyrite | 123.4 | This study | |||
| MG/CFC | 436.1 | This study | |||
| Classic Isotherm Models | ||
|---|---|---|
| Model | Equation | Parameters |
| Langmuir | Ce is the rest ions concentrations (mg/L), Qmax is the maximum adsorption capacity (mg/g), and b is Langmuir constant (L/mg) | |
| Freundlich | KF (mg/g) is the constant of Freundlich model related to the adsorption capacity and n is the constant of Freundlich model related to the adsorption intensities | |
| Dubinin–Radushkevich | β (mol2/KJ2) is the D-R constant, ɛ (KJ2/mol2) is the polanyil potential, and Qm is the adsorption capacity (mg/g) | |
| Advanced isotherm models | ||
| Model | Equation | Parameters |
| Monolayer model with one energy site (Model 1) | Q is the adsorbed quantities in mg/g n is the number of adsorbed ion per site Nm is the density of the effective receptor sites (mg/g) Qo is the adsorption capacity at the saturation state in mg/g C1/2 is the concentration of the ions at half saturation stage in mg/L C1 and C2 are the concentrations of the ions at the half saturation stage for the first active sites and the second active sites, respectively n1 and n2 are the adsorbed ions per site for the first active sites and the second active sites, respectively | |
| Monolayer model with two energy sites (Model 2) | ||
| Double layer model with one energy site (Model 3) | ||
| Double layer model with two energy sites (Model 3) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shemy, M.H.; Othman, S.I.; Alfassam, H.E.; Al-Waili, M.A.; Alqhtani, H.A.; Allam, A.A.; Abukhadra, M.R. Synthesis of Green Magnetite/Carbonized Coffee Composite from Natural Pyrite for Effective Decontamination of Congo Red Dye: Steric, Synergetic, Oxidation, and Ecotoxicity Studies. Catalysts 2023, 13, 264. https://doi.org/10.3390/catal13020264
Shemy MH, Othman SI, Alfassam HE, Al-Waili MA, Alqhtani HA, Allam AA, Abukhadra MR. Synthesis of Green Magnetite/Carbonized Coffee Composite from Natural Pyrite for Effective Decontamination of Congo Red Dye: Steric, Synergetic, Oxidation, and Ecotoxicity Studies. Catalysts. 2023; 13(2):264. https://doi.org/10.3390/catal13020264
Chicago/Turabian StyleShemy, Marwa H., Sarah I. Othman, Haifa E. Alfassam, Maha A. Al-Waili, Haifa A. Alqhtani, Ahmed A. Allam, and Mostafa R. Abukhadra. 2023. "Synthesis of Green Magnetite/Carbonized Coffee Composite from Natural Pyrite for Effective Decontamination of Congo Red Dye: Steric, Synergetic, Oxidation, and Ecotoxicity Studies" Catalysts 13, no. 2: 264. https://doi.org/10.3390/catal13020264








